
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr. , 09 December 2021
Sec. Nutrition Methodology
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.784164
This article is part of the Research Topic Clinical Safety of Natural Products, An Evidence-Based Approach View all 10 articles
Background: Gastric cancer (GC) is one of the most common digestive tract cancers and ranks fifth in the incidence of malignant tumors worldwide. Brucea javanica oil emulsion injection (BJOEI), a Chinese patent medicine extracted from Brucea javanica (Yadanzi in Chinese Pinyin), is widely used as an adjuvant treatment for GC in China. This systematic review and meta-analysis aimed to evaluate the available data on the efficacy and safety of BJOEI in the treatment of GC and assess the quality of the synthesized evidence.
Methods: A comprehensive search was performed on PubMed, EMBASE, CENTRAL, Web of Science, Chinese Biomedical Literature Database (CBM), China National Knowledge Infrastructure (CNKI), Wanfang database and Chinese Scientific Journals Database (VIP database), and other potential resources, such as the Chinese Clinical Trial Registry (ChiCTR) and ClinicalTrials.gov from their inception to July 31, 2021. Randomized controlled trials (RCTs) comparing the therapeutic effects of BJOEI combined with conventional therapy to those of conventional therapy alone were included. We used RevMan 5.3 for data analysis and quality evaluation of the included studies and assessed the evidence quality based on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria.
Results: Eighteen RCTs involving 1,210 patients were included, and the meta-analysis results demonstrated that compared with the control group (conventional therapy), the experimental group (BJOEI combined with conventional therapy) showed a significantly improved overall response rate (ORR) (risk ratio [RR] = 1.52, 95% CI: 1.36–1.69, P < 0.00001), clinical benefit rate (CBR) (RR = 1.17, 95% CI: 1.11–1.23, P < 0.00001), performance status (RR = 1.72, 95% CI: 1.46–2.01, P < 0.00001), and reduced incidence of the following adverse drug reactions (ADRs): neutropenia, leukopenia, nausea and vomiting, diarrhea, liver damage, hand-foot syndrome, and peripheral sensory nerve toxicity. Subgroup analysis showed that the BJOEI intervention could significantly improve the ORR and CBR in patients with GC when combined with FOLFOX4, XELOX, and other chemotherapeutics.
Conclusion: The evidence presented in this study supports the fact that BJOEI combined with conventional chemotherapy provides a statistically significant and clinically important effect in the improvement of ORR, CBR, performance status, and ADR reduction in patients with GC. To further support this conclusion, more rigorously designed, large-scale, and multicenter RCTs are needed in the future.
Gastric cancer (GC) is one of the most common malignant tumors of the digestive tract and ranks fifth in incidence worldwide (1). There were ~1.089 million new cases of GC worldwide, of which 43.9% were reported in China, in 2020 (2). Due to the lack of specific symptoms in early GC, the diagnosis is often made at an advanced disease stage, and the mortality rate is high (3). At present, radical resection is still the main GC treatment, but most patients experience recurrence within 3 years after surgery. The postoperative recurrence rate of patients with locally advanced GC is as high as 50–80%. Once patients experience recurrence and metastasis after the operation, even if palliative chemotherapy is administered again, the 5-year survival rate remains low (4–7). Moreover, molecularly targeted therapy and immunotherapy of GC lag behind those of many other tumor types, and better survival benefits are still being explored (8).
Traditional Chinese Medicine (TCM) has a long historical tradition and currently attracts extensive attention because of its potential treatment benefits in the field of oncology. Our team has been committed to investigating the preventive and therapeutic values of TCM for many years (9–11). Brucea javanica oil emulsion injection (BJOEI) is a Chinese patent medicine extracted from Brucea javanica (Yadanzi in Chinese Pinyin). Its main active component is quassinoid sand fatty acids, which exert anticancer effects through multiple mechanisms (12). Studies have shown the synergistic effects of BJOEI combined with chemoradiotherapy on tumor attenuation, such as reversal of chemotherapy resistance, reduction of the recurrence and metastasis rates, and improvement of the quality of life (13–16). Although several existing systematic reviews have been conducted to evaluate the clinical efficacy of BJOEI in GC, none of them assessed the quality of the synthesized evidence and arrived at definitive conclusions (13, 17–19). The most recent one was reported by Wu et al. in 2018, in which the retrieval deadline was January 2017 (17). With the growing number of studies on the value of BJOEI in GC treatment, more randomized controlled trials (RCTs) have been published in recent years (20–23). Therefore, we conducted a systematic review to evaluate all available evidence of the efficacy and safety of BJOEI in the treatment of GC and assessed the quality of the synthesized evidence.
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines, and readers can access the protocol of this systematic review in the International Prospective Register of Systematic Reviews (CRD42021265646).
Studies that met the following criteria were included: (1) the study design was limited to RCTs, whether it was blinding or not; (2) the studies needed to meet the diagnostic criteria for GC by biopsy or postoperative pathological examination; and (3) studies provided the experimental group with BJOEI in combination with the same interventions provided to the control group.
Studies were excluded if any of the following reasons were involved: (1) duplicate studies; (2) inappropriate interventions; (3) incomplete data; and (4) irrelevance to outcome indicators.
Primary outcome measures included the overall response rate (ORR) and clinical benefit rate (CBR). The secondary outcome measure was the performance status. Safety outcome measures included the occurrence of adverse drug reactions (ADRs).
We searched the following relevant databases from inception to July 31, 2021: PubMed, EMBASE, CENTRAL, Web of Science, the Chinese Biomedical Literature Database (CBM), the China National Knowledge Infrastructure (CNKI), Wanfang database, and Chinese Scientific Journals Database (VIP database), and other potential resources, such as the Chinese Clinical Trial Registry (ChiCTR) and ClinicalTrials.gov for more study records. The combination of MeSH terms and text words was applied to study retrieval. “Stomach Neoplasms” was regarded as the MeSH term. All the strategies were adapted from different databases. The search strategies used in PubMed were as follows:
#1 “Stomach Neoplasms” [MeSH]
#2 “Stomach Neoplasms*” [Title/Abstract] OR “Gastric Cancer*” [Title/Abstract] OR “Gastric Carcinoma” [Title/Abstract] OR “Gastric Neoplasm*” [Title/Abstract] OR “Cancer of Stomach” [Title/Abstract] OR “Stomach Cancer*” [Title/Abstract]
#3 #1 OR #2
#4 “Javanica oil emulsion injection” [Title/Abstract] OR “Yadanzi” [Title/Abstract] OR “Brucea javanica oil emulsion” [Title/Abstract] OR “Brucea javanica” [Title/Abstract]
#5 #3 AND #4
The search results were imported into Excel 2003. After removing duplicates, the titles and abstracts were screened for potential studies. Then, the full articles were checked to determine whether the studies met the inclusion criteria. The study selection process was independently performed by two investigators.
All data were independently extracted by two investigators, and any discrepancies between the reviewers were resolved by the intercessor (JL) until consensus was reached. Data retrieved from the publications included author name, year of publication, number of patients, average age, gender, details about dosage and course of treatment, and outcome data. When necessary and feasible, the corresponding authors of the selected studies were contacted to obtain missing or incomplete data.
In terms of bias, the articles were evaluated as low risk, high risk, and unclear risk according to the following quality items: randomization generation, allocation concealment, subject blinding, outcome assessment, incomplete outcome data, and selective outcome reporting.
Quantitative synthesis was conducted for outcomes reported in more than one homogeneous RCT. The systematic review was performed using the RevMan 5.3 software. Random-effects or fixed-effects models were chosen based on the analysis of heterogeneity. Randomized individuals were considered as unit-of-analysis issues. If a meta-analysis was not appropriate because of clinical/methodological issues or statistical heterogeneity, a narrative summary of the findings or relevant subgroup analyses were used. The RR was used to evaluate dichotomous outcomes, while the mean difference (MD) was used to assess continuous variables. Each outcome numerical value was presented with 95% CIs. Funnel plots were used to test the risk of publication bias. The heterogeneity between RCTs was analyzed using the chi-square test and estimated using I2. Results of P ≥ 0.1 and I2 ≤ 50% suggested a lack of significant heterogeneity, and a fixed-effects model was used accordingly; otherwise, the random-effects model was used. When conducting the meta-analysis, several subgroup analyses were performed to identify subpopulations that might be associated with differences in efficacy. The results of the sensitivity analysis were reported.
Quality assessment of the synthesized evidence was performed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach (24). This assessment of evidence quality includes the risk of bias, heterogeneity, indirectness, imprecision, and publication bias. The quality of the evidence was classified as high, moderate, low, or very low.
A total of 458 clinical studies were identified based on the retrieval strategy. After screening based on the inclusion/exclusion criteria, 18 articles were selected for further analysis (Figure 1).
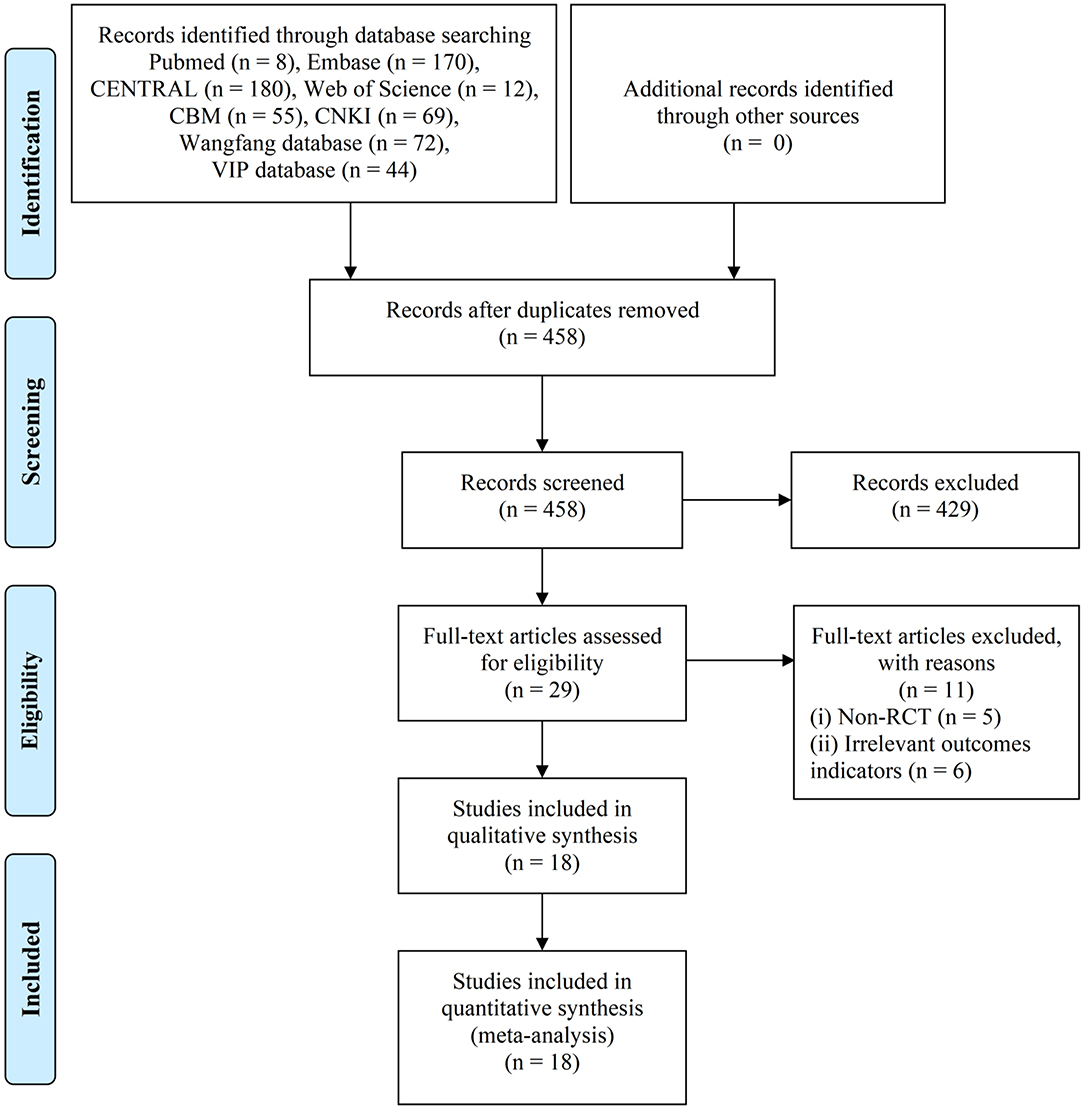
Figure 1. PRISMA flow diagram of the literature search process. PRISMA, the Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Eighteen RCTs (20–23, 25–38) were included in this study, involving 1,210 patients with 618 cases in the experimental group and 592 cases in the control group. Furthermore, a total of four RCTs (20, 34, 36, 37) adopted BJOEI + FOLFOX4, and four RCTs (28, 29, 31, 38) employed BJOEI + XELOX. Due to the diverse combination therapy of BJOEI, subgroup analysis was considered. Additional details are summarized in Table 1.
As shown in Figure 2, in terms of random sequence generation, six RCTs (20, 22, 23, 28, 29, 38) were considered to have a low bias risk by applying a random number table or random envelope. Three RCTs (30, 31, 36) were marked as “high risk” because they divided patients according to hospitalization period, ID, and postoperative chemotherapy, respectively. The other nine RCTs (21, 25–27, 32–35, 37) did not describe the specific randomized method and were evaluated as “uncertain risk.” None of the trials reported the methods of allocation concealment and blinding procedures, which indicated that there were unclear bias risks.
In total, 17 RCTs (20, 22, 23, 25–38) with 1,170 patients presented ORR data. To explore the potential effect differences in ORR, we conducted a subgroup analysis according to the different combination therapies of BJOEI, namely, BJOEI + FOLFOX4, BJOEI + XELOX, and BJOEI + other chemotherapeutics. As shown in Figure 3, the results demonstrated that compared with the control group, the experimental group of patients with GC exhibited a significantly improved ORR (RR = 1.52, 95% CI: 1.36–1.69, Z = 7.35, P < 0.00001). Furthermore, subgroup analysis showed that there were statistically significant differences in ORR between the BJOEI intervention and control groups in patients who received BJOEI combined with FOLFOX4 (RR = 1.55, 95% CI: 1.26–1.90, Z = 4.15, P < 0.0001), XELOX (RR = 1.53, 95% CI: 1.24–1.88, Z = 4.01, P < 0.0001), and other chemotherapeutics (RR = 1.48, 95% CI: 1.25–1.76, Z = 4.56, P < 0.00001).
In total, 17 RCTs (10, 20, 22, 23, 25–33, 35–38) recorded CBR data. We conducted a subgroup analysis according to the different combination therapies of BJOEI, namely, BJOEI + FOLFOX4, BJOEI + XELOX, and BJOEI + other chemotherapeutics. As shown in Figure 4, the results demonstrated that, compared with the control group, the experimental group of patients with GC exhibited significantly improved CBR (RR = 1.17, 95% CI: 1.11–1.23, Z = 5.70, P < 0.00001). Subgroup analysis showed that there were statistically significant differences in CBR between the BJOEI intervention and control groups in patients who received BJOEI combined with FOLFOX4 (RR = 1.11, 95% CI: 1.01–1.22, Z = 2.06, P = 0.04), XELOX (RR = 1.25, 95% CI: 1.11–1.41, Z = 3.76, P = 0.0002), and other chemotherapeutics (RR = 1.16, 95% CI: 1.08–1.25, Z = 3.88, P = 0.0001).
As shown in Figure 5, 11 RCTs (20, 25, 26, 28–30, 32, 34–36, 38) reported the performance status data of the BJOEI and control groups with a slight heterogeneity (P = 0.27, I2 = 18% <50%). A meta-analysis demonstrated that the BJOEI group experienced ~72% superiority in terms of this outcome compared with the control group, and the difference was statistically significant (RR = 1.72, 95% CI: 1.46–2.01, Z = 6.62, P < 0.00001).
Sixteen RCTs referred to this outcome. The main ADRs were neutropenia (3 RCTs) (28, 29, 34), leukopenia (10 RCTs) (26, 29, 33, 35–38), thrombocytopenia (7 RCTs) (20, 22, 26, 29, 33, 36, 37), nausea and vomiting (10 RCTs) (20, 22, 23, 26, 31, 33, 34, 36–38), diarrhea (8 RCTs) (20, 22, 26, 30, 34, 36–38), liver damage (9 RCTs) (20, 21, 23, 26, 31, 32, 35, 37, 38), renal damage (3 RCTs) (20, 31, 37), alopecia (3 RCTs) (20, 21, 37), hand-foot syndrome (6 RCTs) (23, 26, 28, 29, 33, 38), stomatitis (2 RCTs) (26, 33), anemia (3 RCTs) (26, 29, 33), and peripheral sensory nerve toxicity (5 RCTs) (26, 28, 31, 34, 38). Meta-analysis showed that there was a statistically significant difference between the two groups (RR = 0.72, 95% CI: 0.66–0.78, Z = 7.60, P < 0.00001). Compared with the control group, the BJOEI group exhibited fewer of the following ADRs: neutropenia (RR = 0.44, 95% CI: 0.27–0.74, Z = 3.10, P = 0.002), leukopenia (RR = 0.68, 95% CI: 0.58–0.79, Z = 4.91, P < 0.00001), nausea and vomiting (RR = 0.79, 95% CI: 0.65–0.95, Z = 2.46, P = 0.01), diarrhea (RR = 0.70, 95% CI: 0.52–0.94, Z = 2.40, P = 0.02), liver damage (RR = 0.49, 95% CI: 0.30–0.81, Z = 2.81, P =0.005), hand-foot syndrome (RR = 0.73, 95% CI: 0.54–1.00, Z = 1.99, P = 0.05), and peripheral sensory nerve toxicity (RR = 0.69, 95% CI: 0.51–0.93, Z = 2.42, P = 0.02). However, no statistically significant differences were detected in the occurrence of thrombocytopenia, renal damage, alopecia, stomatitis, and anemia. The results of ADR were shown in Figure 6.
According to the Cochrane Handbook for Systematic Reviews of Interventions (39), I2 values between 0 and 40% indicated that heterogeneity might not be important. Therefore, we eliminated the included studies with I2 ≥ 40% one by one and then conducted a meta-analysis. The results showed that in the CBR of BJOEI + XELOX, after excluding Zhang et al. (38), the heterogeneity was decreased from 59 to 32% (P = 0.0002; Figure 7). After excluding Ma et al. (31), the heterogeneity was decreased from 59 to 0% (P = 0.04; Figure 8). The data suggested that Zhang et al. (38) and Ma et al. (31) were the main reasons for the heterogeneity in the CBR of BJOEI + XELOX. In terms of ADRs, after excluding Ma et al. (31), the heterogeneity of nausea and vomiting decreased was from 47 to 36% (P = 0.14; Figure 9), and the heterogeneity of peripheral sensory nerve toxicity was decreased from 41 to 0% (P = 0.57; Figure 10). In addition, after deleting Zhang et al. (38), the heterogeneity of peripheral sensory nerve toxicity was decreased from 41% to 1% (P = 0.005; Figure 11). These findings suggest that Zhang et al. (38) and Ma et al. (31) might explain the heterogeneity in ORR and ADRs.
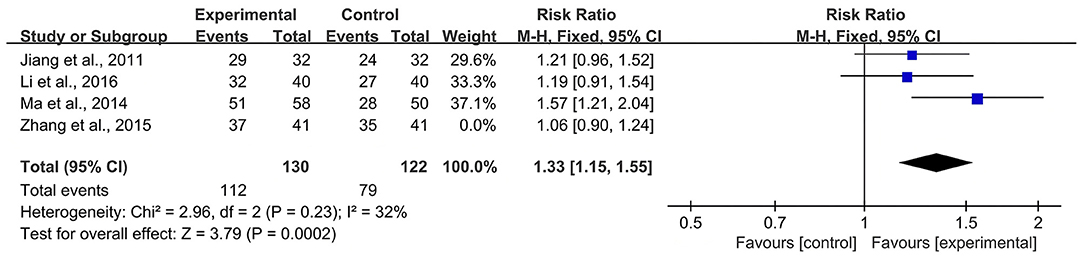
Figure 7. Forest plot of sensitivity analysis of CBR with BJOEI combined with XELOX treatment vs. pure XELOX treatment (a). CBR, clinical benefit rate; BJOEI, Brucea javanica oil emulsion injection.
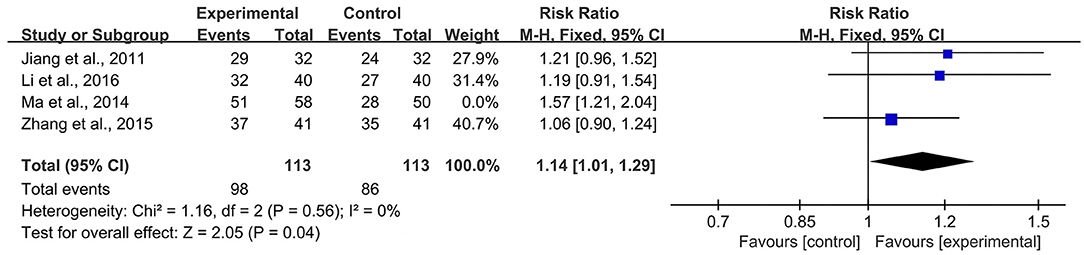
Figure 8. Forest plot of sensitivity analysis of CBR with BJOEI combined with XELOX treatment vs. pure XELOX treatment (b). CBR, clinical benefit rate; BJOEI, Brucea javanica oil emulsion injection.

Figure 9. Forest plot of sensitivity analysis of ADRs of nausea and vomiting. ADRs, adverse drug reactions.
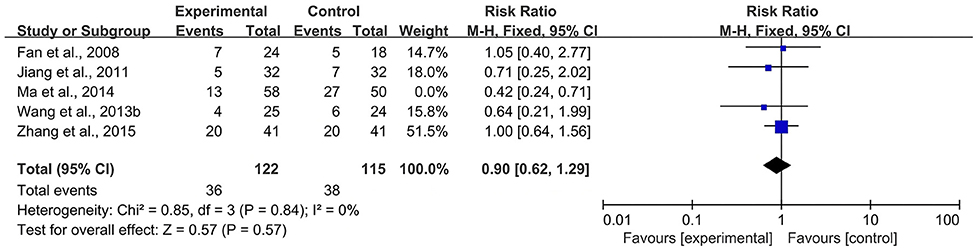
Figure 10. Forest plot of sensitivity analysis of ADRs of peripheral sensory nerve toxicity (a), ADRs, adverse drug reactions.
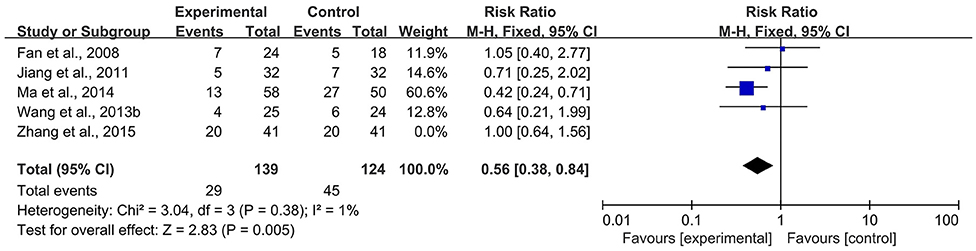
Figure 11. Forest plot of sensitivity analysis of ADRs of peripheral sensory nerve toxicity (b). ADRs, adverse drug reactions.
A funnel plot of publication bias for ORR is displayed in Figure 12, which indicates that there was no evidence of significant publication bias.
Based on the GRADE criteria, the ORR, CBR, performance status, and ADRs were all assessed as low-quality evidence, owing to the existence of clinical heterogeneity and low participant numbers in most studies (Tables 2, 3).
Despite advances in disease screening and modern technology, GC remains one of the most common malignant tumors. Its metastasis, morbidity, and mortality rates are all on the rise, while the cure, radical resection, and 5-year postoperative survival rates of patients with advanced GC are low (40). In recent years, TCM has made great progress in anti-tumor therapy, and the manufacturing technologies of Chinese medicine compounds, Chinese patent medicine, Chinese medicine extract, and Chinese medicine monomers have developed more rapidly. BJOEI is a Chinese patent medicine that is widely used in the treatment of various cancers, such as lung (41) and several gastrointestinal cancers (42, 43). Previous studies have shown that its antitumor effects might be related to the following mechanisms: 1) inhibition of DNA synthesis in tumor cells (44, 45); 2) induction of tumor cell apoptosis and differentiation (46–48); 3) anti-angiogenesis (49); and 4) reversion of drug resistance (50).
In this study, we searched as many RCTs as we could and conducted a meta-analysis to evaluate the treatment efficacy and safety of BJOEI in patients with GC. All available data from the collected trials were applied without intentional selection. The results showed that BJOEI combined with chemotherapy was superior to single chemotherapy in improving ORR, CBR, and performance status. Considering that the different patient regimens might lead to high outcome heterogeneity, to obtain a more convincing conclusion, we conducted a subgroup analysis according to chemotherapeutic regimens. The results showed that for each BJOEI + FOLFOX4 and BJOEI + XELOX sub-group, the ORR and CBR were significantly improved by the addition of the BJOEI intervention. Furthermore, we have paid special attention to neutropenia, leukopenia, nausea and vomiting, diarrhea, liver damage, hand-foot syndrome, and peripheral sensory nerve toxicity, which are common symptoms of chemotherapy-associated ADRs. The meta-analysis showed that the BJOEI group had fewer symptoms related to the above ADRs. However, more RCTs are needed to further demonstrate the positive effect of BJOEI in ameliorating chemotherapy-associated toxicities.
Although we strictly conducted this meta-analysis according to the review procedure released by the Cochrane Collaboration, this study has several limitations. First, the duration of the intervention is an important factor in the evaluation of efficacy. The observation time of the included studies was mainly concentrated at 12 and 6 W, and the longest was 24 W (in only one RCT). Furthermore, the long-term effects of BJOEI in the treatment of GC remain unknown. Moreover, high-quality original studies were scarce in this study. The problems in most RCTs included unexplained randomization methods, insufficient attention to allocation concealment, low utilization rate of blinding, and unreported lost follow-up cases. Finally, recent advances have renewed the hope that immune and targeted agents can be leveraged to improve patient survival (51, 52). Although chemotherapy is still the backbone of therapy against GC, studies should also investigate the efficacy of BJOEI combined with immunotherapy or targeted therapy.
Due to the limitations associated with the poor quality of pooled studies, it is difficult to draw a definitive conclusion. Nevertheless, our study suggests the positive effect of BJOEI in facilitating the management of ORR, CBR, performance status, and ADRs in patients with GC. More prospectively designed, large-sample, and multicenter RCTs are expected to offer persuasive evidence to demonstrate the efficacy and safety of BJOEI.
JL designed this study. XW and HW performed the online database search. LC, JW, TL, and SL contributed to the data collection, extraction, and analysis. XW, HW, and LC prepared the original draft and finished the revision of the manuscript. All authors have read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
3. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. (2020) 396:635–48. doi: 10.1016/S0140-6736(20)31288-5
4. Yamada Y, Boku N, Mizusawa J, Iwasa S, Kadowaki S, Nakayama N. Docetaxel plus cisplatin and S-1 versus cisplatin and S-1 in patients with advanced gastric cancer (JCOG1013): an open-label, phase 3, randomised controlled trial. Lancet Gastroenterol Hepatol. (2019) 4:501–10. doi: 10.1016/S2468-1253(19)30083-4
5. Wang QW, Zhang XT, Lu M, Shen L. Impact of duration of adjuvant chemotherapy in radically resected patients with T4bN1-3M0/TxN3bM0 gastric cancer. World J Gastrointest Oncol. (2018) 10:31–9. doi: 10.4251/wjgo.v10.i1.31
6. Digklia A, Wagner AD. Advanced gastric cancer: current treatment landscape and future perspectives. World J Gastroenterol. (2016) 22:2403–14. doi: 10.3748/wjg.v22.i8.2403
7. Wang Q, Liu X, Chen C, Chen J, Xu B, Chen L. A predictive signature for oxaliplatin and 5-fluorouracil based chemotherapy in locally advanced gastric cancer. Transl Oncol. (2021) 14:100901. doi: 10.1016/j.tranon.2020.100901
8. Sexton RE, Al HM, Diab M, Azmi AS. Gastric cancer: a comprehensive review of current and future treatment strategies. Cancer Metastasis Rev. (2020) 39:1179–203. doi: 10.1007/s10555-020-09925-3
9. Li J, Sun GZ, Lin HS, Pei YX, Qi X, An C, et al. The herb medicine formula “Yang Wei Kang Liu” improves the survival of late stage gastric cancer patients and induces the apoptosis of human gastric cancer cell line through Fas/Fas ligand and Bax/Bcl-2 pathways. Int Immunopharmacol. (2008) 8:1196–206. doi: 10.1016/j.intimp.2008.04.007
10. Wang XM, Zhu GH, Yang HY, Gao RK, Wu Z, Zhang Y, et al. An investigation of the antigastric cancer effect in tumor microenvironment of radix rhei et rhizome: a network pharmacology study. Evidence-Based Complement Alternative Med. (2021) 2021:9. doi: 10.1155/2021/9913952
11. Feng Y, Wu Z, Li J. Effect of fuzheng jiedu prescription combined with 5-Fu against postoperative recurrence and metastasis of gastric cancer in mice: an exploration based on endoplasmic reticulum stress. Chin J Experi Tradit Med Formulae. (2021) 27:75–83. doi: 10.13422/j.cnki.syfjx.20211699
12. Yoon BK, Lim ZY, Jeon WY, Cho NJ, Kim JH, Jackman JA. Medicinal activities and nanomedicine delivery strategies for Brucea javanica oil and its molecular components. Molecules. (2020) 25:25414. doi: 10.3390/molecules25225414
13. Zhou JP, Yang HX. Meta-analysis on efficacy and safety of Brucea javanica oil emulsion injection combined with chemotherapy for patients with advanced gastric carcinoma. China J Chin Mater Med. (2016) 41:326–32. doi: 10.4268/cjcmm20160226
14. Wang K, Chen Q, Shao Y, Yin S, Liu C, Liu Y, et al. Anticancer activities of TCM and their active components against tumor metastasis. Biomed Pharmacother. (2021) 133:111044. doi: 10.1016/j.biopha.2020.111044
15. Zhao N, Li YH, Wu XK, Wang GY, Cai DY, Han FJ. [Effect of Brucea javanica fruit oil emulsion combined cisplatin on the growth inhibition of transplanted tumor in human ovarian cancer SKOV3 nude mice: an experimental study]. Zhongguo Zhong Xi Yi Jie He Za Zhi. (2015) 35:57–62. doi: 10.7661/CJIM.2015.01.0057
16. Qiu ZH, Zhang WW, Zhang HH, Jiao GH. Brucea javanica oil emulsion improves the effect of radiotherapy on esophageal cancer cells by inhibiting cyclin D1-CDK4/6 axis. World J Gastroenterol. (2019) 25:2463–72. doi: 10.3748/wjg.v25.i20.2463
17. Wu JR, Liu SY, Zhu JL, Zhang D, Wang KH. Efficacy of Brucea javanica oil emulsion injection combined with the chemotherapy for treating gastric cancer: a systematic review and meta-analysis. Evid Based Complement Alternat Med. (2018) 2018:6350782. doi: 10.1155/2018/6350782
18. Yang XP, Huang YL, Shen HP, Liu Y, Yuan Y. Systematic review of yadanzi oil grease injection combined with chemotherapy for gastric cancer. Chin J Experi Traditional Med Formulae. (2016) 22:208–12. doi: 10.13422/j.cnki.syfjx.2016040208
19. Wei HB. The effect of Brucea javanica oil grease injection plus chemotherapy in the treatment of patients with gastric cancer. Gansu College Traditinal Chin Med. (2014) 2014:42.
20. Cui LH. Analysis of curative effect of oleum fructus bruceae and chemotherapy in treatment of advanced carcinoma of stomach. China Foreign Med Treatment. (2017) 36:150–2. doi: 10.16662/j.cnki.1674-0742.2017.17.150
21. Tan BL, Zhang JR. Clinical efficacy of Brucea javanica oil injection in the treatment of stage III gastric carcinoma and the effects on the immunological function of the organism. World Chin Med. (2017) 12:2093–5. doi: 10.3969/j.issn.1673-7202.2017.09.027
22. Tong GD, Hu RB. Brucea javanica oil emulsion combined with SOX chemotherapy in treatment of stage IIIb-IV gastric cancer. Int Med Health Guidance News. (2019) 21:3584–7. doi: 10.3760/cma.j.issn.1007-1245.2019.21.024
23. You J, He FG, Wang Z, Yuan MQ, Zhu H. Effects of Brucea javanica oil combined with chemotherapy of weekly TX regimen in treating patients with advanced gastric cancer. Chin J Pharmacoepidemiol. (2018) 27:310–3.
24. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
25. Deng YF, Wang CB, Liu YM. Clinical efficacy of Brucea javanica oil interventional chemotherapy in the treatment of advanced cardiac cancer. J Pract Oncol. (2009) 24:603–4. doi: 10.13267/j.cnki.syzlzz.2009.06.016
26. Fan XQ, Zhou XQ, Li XY, Zhou RG, Tang XY, Pan DJ. Study of Brucea fruit water in oil emulsion combine with mFOLFOX regimen on old patients with advanced gastric cancer. Modern J Integrated Traditional Chin Western Med. (2008) 27:4229–30.
27. Gao J. Avanica oil joint MF /CF clinical observation of advanced gastric cancer chemotherapy. Med J Chin People's Health. (2011) 23:1108–9. doi: 10.3969/j.issn.1672-0369.2011.09.031
28. Jiang BG, Wang ZX, Wang LH. Clinical observation of XELOX regimen combined with Brucea javanica oil emulsion in the treatment of 32 patients with advanced gastric cancer. Guiding J Traditional Chin Med Pharmacy. (2011) 17:60–1. doi: 10.13862/j.cnki.cn43-1446/r.2011.11.053
29. Li YM, Li DF, Wang YQ. Clinical observation of capecitabine + oxaliplatin combined with Brucea javanica oil in the treatment of advanced gastric cancer. Chin J Clin Res. (2016) 29:374–6. doi: 10.13429/j.cnki.cjcr.2016.03.027
30. Liu HJ, Xu H, Ning SQ. Treatment of advanced gastric cancer with DX chemotherapy and bruceolic oil emulsion. Practical J Clin Med. (2010) 7:76–7.
31. Ma YB, Ge R, Wang C, Ma XM. Effect of postoperative application of Brucea javanica oil injection adjuvant chemotherapy in treating gastric cancer. Chin J Experi Tradit Med Formulae. (2014) 20:178–80. doi: 10.13422/j.cnki.syfjx.2014180178
32. Wang ZG, Liu LM, Sun LL. Observation on the therapeutic effect of Brucea javanica oil emulsion combined with euphoridine on senile advanced gastric cancer. China Modern Med. (2009) 16:243–4.
33. Wang JY, Ma W, Jing WJ, Qi BH, Ma J, Yang XJ, et al. Efficacy of Brucea javanica oil combined with chemotherapy in treatment of advanced gastric cancer. J Shaanxi University Chin Med. (2013) 36:49–50. doi: 10.13424/j.cnki.jsctcm.2013.02.049
34. Wang YT, Yang LP. Clinical observation of Brucea javanica oil emulsion injection combined with oxaliplatin 5-fluorouracil leucovorin calcium regimen in the treatment of advanced gastric cancer. J Pract Med Techn. (2013) 20:427–8.
35. Wang P. Efficacy of chemotherapy, radiotherapy and Brucea javanica oil emulsion injection in the treatment of advanced gastric cancer. J Pract Oncol. (2004) 1:78–9.
36. Wang J. Clinical observation of adjuvant chemotherapy with Brucea javanica oil emulsion in the treatment of advanced gastric cancer. J Emerg Traditional Chin Med. (2013) 22:1005–6.
37. Wu CY, Zhang YC, Dai GX, Wang LJ, Ye JK. Clinical observation of bruceolic oil emulsion combined with FOLFOX4 in treating advanced carcinoma of stomach. Med Pharm J Chin People Liberation Army. (2012) 24:29–31. doi: 10.3969/j.issn.2095-140X.2012.08.009
38. Zhang XM, Shao YP, Xue H. Clinical observation of Brucea javanica oil injection combined with oxaliplatin and xeloda in the treatment of elderly patients with advanced gastric cancer. China Pharmacy. (2015) 26:3769–71. doi: 10.6039/j.issn.1001-0408.2015.27.09
39. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
40. Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci. (2020) 21:4012. doi: 10.3390/ijms21114012
41. Lu YY, Huang XE, Cao J, Xu X, Wu XY, Liu J, et al. Phase II study on Javanica oil emulsion injection (Yadanzi(R)) combined with chemotherapy in treating patients with advanced lung adenocarcinoma. Asian Pac J Cancer Prev. (2013) 14:4791–4. doi: 10.7314/APJCP.2013.14.8.4791
42. Liu J, Huang XE, Tian GY, Cao J, Lu YY, Wu XY, et al. Phase II study on safety and efficacy of Yadanzi(R) (Javanica oil emulsion injection) combined with chemotherapy for patients with gastric cancer. Asian Pac J Cancer Prev. (2013) 14:2009–12. doi: 10.7314/APJCP.2013.14.3.2009
43. Wang J, Ye HB, Dong Y. Effects of javanica oil emulsion injection combined with radiotherapy versus radiotherapy alone on the efficacy and safety in patients with esophageal cancer: a pooled analysis of 1269 cases. J BUON. (2017) 22:985–95.
44. Liu Y, Cao Y, Li CG, Kong WW, Sui X. Effects of Brucea javanica oil emulsion combined with thermotherapy on proliferation and p-Akt expression in human bladder cancer BIU-87 cells. Clin J Med Office. (2013) 41:1035–8. doi: 10.3969/j.issn.1671-3826.2013.1.17
45. Gu KL. Experimental analysis of the effect of Brucea javanica oil emulsion on bladder cancer. Biotech World. (2016) 4:187–8.
46. Lai Y, Chen ZJ. Research progress on anti-leukemia effect and mechanism of toxic Chinese herbs. Chin Traditional Patent Med. (2016) 38:875–9. doi: 10.3969/j.issn.1001-1528.2016.04.034
47. Lau ST, Lin ZX, Zhao M, Leung PS. Brucea javanica fruit induces cytotoxicity and apoptosis in pancreatic adenocarcinoma cell lines. Phytother. Res. (2008) 22:477–86. doi: 10.1002/ptr.2344
48. Zhang H, Yang JY, Zhou F, Wang LH, Zhang W, Sha S, et al. Seed oil of Brucea javanica induces apoptotic death of acute myeloid leukemia cells via both the death receptors and the mitochondrial-related pathways. Evid Based Complement Alternat Med. (2011) 2011:965016. doi: 10.1155/2011/965016
49. Jiang B, Xu DH, Xu X, Lv QH. Effect of Brucea javanica oil on apoptosis and vascular endothelial growth factor secretion of A549 cells. Chin Pharm J. (2009) 44:1387–91.
50. Ding YX, Qu J, Zhang JJ, Lv XY. Reversal effect of Brucea javanica oil emulsion combined siRNA-ERCC1 on human lung adenocarcinoma A549 / DDP Cells. Chin J Experi Traditional Med Formulae. (2012) 18:235–9. doi: 10.13422/j.cnki.syfjx.2012.20.095
51. Patel TH, Cecchini M. Targeted therapies in advanced gastric cancer. Curr Treat Options Oncol. (2020) 21:70. doi: 10.1007/s11864-020-00774-4
Keywords: Brucea javanica oil emulsion injection, gastric cancer, efficacy, safety, meta-analysis
Citation: Wang X, Wang H, Cao L, Wu J, Lu T, Li S and Li J (2021) Efficacy and Safety of Brucea javanica Oil Emulsion Injection in the Treatment of Gastric Cancer: A Systematic Review and Meta-Analysis. Front. Nutr. 8:784164. doi: 10.3389/fnut.2021.784164
Received: 27 September 2021; Accepted: 05 November 2021;
Published: 09 December 2021.
Edited by:
Abdur Rauf, University of Swabi, PakistanReviewed by:
Nasiara Karim, University of Malakand, PakistanCopyright © 2021 Wang, Wang, Cao, Wu, Lu, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Li, cWZtMjAyMGppZWxpQHllYWgubmV0
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.