- 1Department of Food Science and Nutrition, Zhejiang University, Hangzhou, China
- 2School of Forestry, Northeast Forestry University, Harbin, China
Recently, owing to well-controlled release, enhanced distribution and increased permeability, nanocarriers used for alternative drug and food-delivery strategies have received increasingly attentions. Nanocarriers have attracted a large amount of interest as potential carriers of various bioactive molecules for multiple applications. Drug and food-based delivery via polymeric-based nanocarriers and lipid-based nanocarriers has been widely investigated. Nanocarriers, especially liposomes, are more and more widely used in the area of novel nano-pharmaceutical or food-based design. Herein, we aimed to discuss the recent advancement of different surface-engineered nanocarriers type, along with cutting-edge applications for food and nanomedicine and highlight the alternative of phytochemical as nanocarrier. Additionally, safety concern of nanocarriers was also highlighted.
Introduction
There have been major breakthroughs in the field of nanotechnology, especially in the fields of materials science, electronics, photonics, supramolecular assembly, drug delivery, agriculture and food industries. In particular, the application of nanotechnology in medicine, usually termed nanomedicine, has provided a key impetus for the development of various types of drug-carrying nanomaterials (1, 2), such as liposomes, micelles, polymeric nanoparticles, albumin-based formulations (3). Typical nanomaterials possess several common characteristics: high surface-to-volume ratio, enhanced electrical conductivity, superparamagnetic behavior, spectral shift of optical absorption, and unique fluorescence properties. Nanomedicine is being taken as a promising way in drug delivery and diagnostics. To date, due to improved penetration and delivery of drugs into specific regions of skin, nanocarriers taken as alternative drug-delivery strategies have gained increasingly interest. A large number of studies have focused on nanocarriers as effective diagnostic or therapeutic tools for serious diseases, such as cancer, infectious or neurodegenerative diseases (4, 5). Besides, nanocarriers could not only improve the solubility of hydrophobic nutraceuticals more efficiently, but also they have almost no effect on the appearance of final food products, such as, drinks and beverages (6). Research has highlighted the complexity of the physicochemical and biochemical processes involved in the bioavailability of biocomponents, such as release, absorption, distribution, metabolism, and excretion, which contribute to increase the bioavailability of bioactive compounds used in food fortification (7).
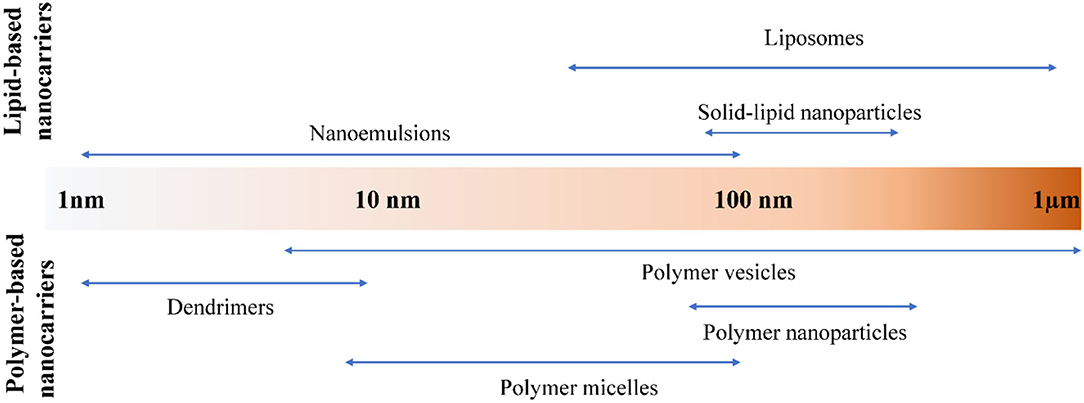
In general, nanocarriers are colloidal in size with diameters ranging from 1 to 1,000 nm (8). The nano-range size of medical nanocarrier is generally 1–100 nm. According to the different materials used to construct the carrier, it can be roughly divided into two types: lipid-based nanocarrier and polymer-based or dendritic nanocarrier. Among them, lipid-based nanocarriers can be divided into liposomes and solid-lipid nanoparticles. Nanocarriers based on polymers and dendritic branches are classified into dendritic branches, polymer micelles, polymer vesicles, nanoparticles and nano-colloids (8). Their particle size range is shown in Figure 1. In this review, we will have a brief overview of nanoencapsulation systems applicable to nanomedicine and food ingredients. And then, application of these nanocarriers for drug usage and different food bioactive components will be covered.
Lipid-Based Nanocarriers
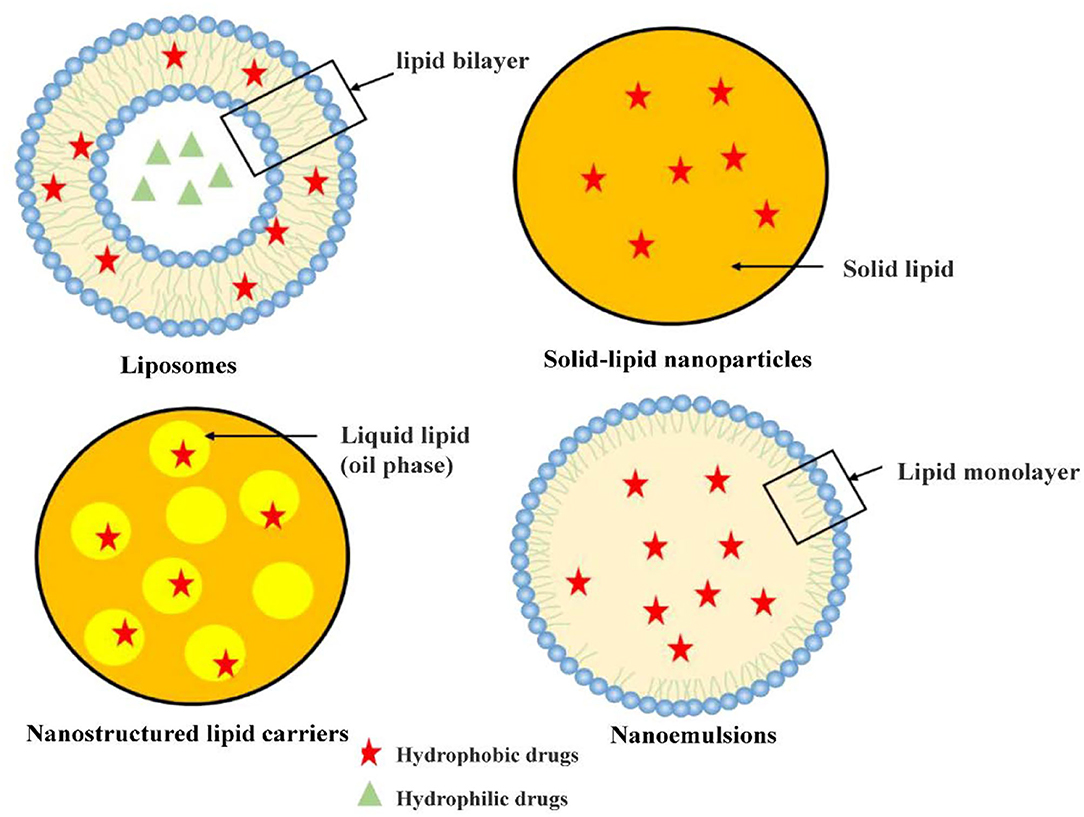
Lipid-based nanocarriers (LNs) are generally non-spherical in shape, which is either determined by the electrostatic interaction between polar/ionogenic phospholipid head and the solvent or due to non-polar lipid hydrocarbon moieties present in the solvent (9, 10). The unique physicochemical properties of LNs which in the form of liposomes or solid core lipid nanoparticles with excellent biocompatibility makes them candidates as carriers for drugs and food application. These LNs made of uniform lipid bilayers or solid cores can entrap various cytotoxic drugs. Hydrophilic drug will be trapped in the aqueous region, while the lipophilic drug will be captured in the lipid leaflets. Additionally, LNs carry drugs safely to the destination-tumor site, release it in a gradual manner, and are then degraded (9). Recently, research on lipid-based nanomaterials is blooming and main categories, including liposomes, solid lipid nanoparticles (SLNs), and nanostructured lipid carriers (NLCs) and nanoemulsions, have been receiving great attention in current research and clinical trials.
Liposomes
Liposomes, the first phospholipid vesicle system developed in the 1960s (11), are composed of phospholipid bilayer similar to the plasma membrane of human cells. Therefore, liposomes have good biocompatibility and can promote drug diffusion across the plasma membrane (12). Liposomes can be designed as self-assembled vesicles comprising one or multiple concentric lipid bilayers that enclose an aqueous core (13). Liposomes, ranged from 20 nm to more than 1 μm, have the typical structure encapsulate with a hydrophobic bilayer and a hydrophilic core. Therefore, liposomes can hold and stabilize hydrophilic drugs in the hydrophilic core and encapsulate lipophilic drugs in lipid bilayer. Therefore, liposomes have good ability to carry both hydrophilic and hydrophobic drugs in the aqueous lumen and lipid bilayer, respectively, which contributing to the versatility of liposomes. In addition, liposome system also has the advantages of easy modification and targeting potential, which could be constructed with the surface modified with appropriated molecules (or ligands) to actively bind a target molecule of certain cells, system, or tissue (14). Since Doxil® was approved by the FDA in 1995 as the first long-circulating liposome for cancer treatment, many chemicals have also been reported to be able to encapsulate liposomes. However, due to the limited bilayer space of liposomes, it is difficult to achieve high drug loading of hydrophobic drugs. It is necessary to strike a delicate balance between high drug loading and particle size distribution and stability of liposomes. To better improve clinical translation, further research is needed for targeted drug delivery by nano-carriers to reduce toxicity, enhance permeability and retention effects, and minimize the shielding effect of protein corona (15). Therefore, it is critical to optimize the composition, preparation methods and properties of the lipid bilayer.
Solid-Lipid Nanoparticles (SLN)
Solid-lipid nanoparticles (SLN) were developed in the 1990s in order to combine the advantages of polymer nanocarriers, such as strong drug loading capacity, controllable drug delivery, good biocompatibility of lipid emulsions and improvement of drug bioavailability (16, 17). SLN can be prepared by a variety of technologies including heat or cold homogenization, which is easy to scale up production, has good preparation repeatability and does not require toxic organic solvents in the preparation process (16, 18). The main feature of SLN is that it contains lipids that remain solid at room temperature. Biocompatible substances such as triglycerides, fatty acids, steroids and biowaxes are often used to prepare SLN systems. Due to their small sizes and large surface area, SLN are suitable to be covered with functionalized ligands moieties, antibody and other functional group (19). SLNs can be orally administered as aqueous dispersions or in the dosage forms of capsules, tablets, and pellets (20). Among the different types of nanocarriers, SLN are at the forefront of the potential application in oral drug delivery systems (21). SLN have many advantages like easy manufacturing, the stability of pharmaceuticals, increased drug content, effective release of drug and high long-term stability. Additionally In terms of drug delivery, SLN system can efficiently encapsulate antitumor drugs and other substances with poor water-solubility due to its high lipid content (22).
Nanostructured Lipid Carriers (NLCs)
Nanostructured lipid carriers (NLCs), also known as lipid-based formulations, have been broadly studied as drug delivery system. They have a solid matrix at room temperature and are considered superior to many other traditional lipid-based nanocarriers such as nanoemulsions, liposomes and SLNs due to their enhanced physical stability, improved drug loading capacity, and biocompatibility (23). To enhance biocompatibility and superior formulation properties, researchers modified SLN by replacing a fraction of solid lipids with liquid lipids to obtain nanostructured lipid carrier (NLC). Unlike SLN, the lipid matrix of NLC consisted of mixture of solid and liquid lipids with controlled levels that possess improved capacity of bioactive retention along with controlled release attributes (24). Compared to other lipid-based formulations, NLCs are biocompatible systems distinguished by a rigid morphology which contributes to unique properties (25). The wide variety of lipids used in topical lipid nanoparticulate formulations may be classified as fatty acids, waxes, steroids, partial glycerides, and triglycerides (26). Generally, NLC can be divided into three types: one is to use structurally different lipids to construct NLC; the other is to use amorphous lipids to construct NLC; the most common NLC system is composed of a mixture of solid lipids and liquid lipids (27, 28). Compared with solid lipids, drugs have a higher solubility in oil, thus common NLC has a stronger encapsulation ability for drugs than SLN (17). However, NLC has the disadvantage of difficult surface functionalization (29).
Nanoemulsions
Nanoemulsions, referred to as dispersed systems with ≤100 nm droplets, are gaining importance in healthcare and cosmetics sectors as a result of the unique properties of nanosized droplets, such as high surface area (30). The small-sized droplet with a high surface area makes nanoemulsions important in many industries (31). High and low energy methods are used to prepare nanoemulsions, such as high-pressure homogenization, ultrasonication, phase inversion temperature and emulsion inversion point, bubble bursting method (32). Based on the components, Nanoemulsions can be categorized in three types, that is oil in water (O/W) type, water in oil (W/O) type, and bi-continuous/multiple emulsion where micro domains of oil and water phases are inter-dispersed (W/O/W and O/W/O) (33–36). Additionally, based on surface charge over the nano-droplets, nanoemulsions are categorized as neutral, anionic and cationic nanoemulsions (35). Digestible oils such as soybean oil, sesame oil, cottonseed oil or safflower oil are usually used to dissolve lipophiles (37). Compared to other nanocarriers, nanoemulsions are easy to prepare and do not necessarily require organic solvents/cosolvents, especially carriers prepared from edible oil with a low risk of toxicity. Based on the above advantages, nanoemulsions have been used to wrap paclitaxel, polyenetaxel, curcumin, retinoic acid and other anti-tumor drugs (38–42), and a large number of studies have reported using nanoemulsions to package essential oils, nutrients and other substances (43, 44).
Additionally, nanoemulsions have potential application in the food industry for the delivery of nutraceuticals, coloring and flavoring agents, and antimicrobials. The nanoemulsion formulations of active ingredients can be used for developing biodegradable coating and packaging films to enhance the quality, functional properties, nutritional value, and shelf life of foods. Considering nanoemulsions are susceptible to destabilization, essential oils (EOs) incorporated in nanoemulsions could be helpful to improved stability of nanoemulsion for high long-term stability food products (45). Nanoemulsion based edible nanocoatings containing flavor and coloring ingredients, antioxidants, enzymes, antimicrobials, and antibrowning agents can be used to coat foods such as meats, dairy products such as cheese, fresh produce, and fresh cuts including fruit and vegetables and confectionaries to improve their shelf life (45). Totally speaking, the different classification of nanocarriers based on lipids were listed below (Figure 2).
Anocarriers Based on Polymers Branches
In general, polymers are made up of multiple linear and branched co-polymers or cross-linked polymer networks. The physical and chemical changes in the polymer in response to external factors like pH and temperature provide them with distinctive features (10). Polymer-based nanocarriers have tree-like structures and consist of hyperbranched polymers, dendrigrafts, dendrons and dendrimers. Each of these four classes reflects the structural features of these complex macromolecular architectures (46).
Polymer Nanoparticles
Polymer nanoparticles, which were invented in the 1970s, are more stable than liposomes and their preparation methods are easy to reproduce. In addition, the controlled release properties of polymer nanoparticles are easier to control and surface modification is convenient (47). In general, polymer nanoparticles can be divided into two categories: nanospheres and nano microcapsules. Nanospheres are “matrix type,” in which drugs are dispersed throughout the matrix, while microcapsules are “repository type,” in which drugs are present in a core surrounded by a polymer shell. In recent years, biodegradable nanoparticles have shown great potential as drug carriers due to their good biocompatibility.
However, the encapsulation rate of polymer nanoparticles is low, and due to the high molecular weight of polymer, polymer nanoparticles can easily induce immune response. To overcome these shortcomings, a new carrier system emerges out which is known as lipid polymer hybrid nanoparticles (LPNs). Clearly, the polymer controls the drug release and the lipid increases the loading efficiency as well as permeation. Thus, LPNs have good potential to enhance physical stability and biocompatibility (48). Zhang et al. (49) constructed a polymer-lipid hybrid nanoparticle in which polyetaxel is encapsulated in the polymer core, which is encapsulated in a monolayer of lipids. Compared with pure polymer nanoparticles (37%), this system has a higher encapsulation rate (59%). In addition, the lipid-controlled release barrier on the surface of the system inhibits the burst release effect, and it takes 20 h for the mixed nanoparticles to release 50% of the drug, compared to only 7 h for the pure polymer nanoparticles. LPNs system has been explored for the oral delivery of several thrombolytic agents. Previous reports demonstrated that chitosan and lipid nanoparticles increased the oral bioavailability of heparin (50). Khan (51) reported that cisplatin loaded lipid-polymer hybrid nanoparticles was an effective delivery system for controlled drug delivery at tumor sites, having promise as a platform for controlled delivery of cisplatin in cancer therapy.
Polymer Micelles
Polymer micelles are a class of nano-colloids, which can be formed by self-assembly of amphiphilic block copolymers in aqueous solution (52). In polymer micelles, hydrophobic drugs are encapsulated in the hydrophobic core, while the hydrophilic shell plays a role in maintaining particle stability, which makes it suitable for intravenous injection (53). It usually presents a core-shell structure, and its particle size is generally distributed in the range of 5–100 nm (54). Unlike nanoparticles, polymer micelles are typically characterized by critical micellar concentration (CMC). Therefore, when the concentration is diluted below the CMC, the micelles disassemble into free unimers (55). Nonetheless, polymer micelles mainly have low CMC values, which render them relatively insensitive to dilution, thus leading to enhanced circulation times. According to research results, polymer micelles have been able to successfully deliver a variety of hydrophobic drugs and DNA and can be functionalized by many different ligands (56, 57). In addition, compared to non-polymer-stabilized micelles, polymer-stabilized micelles can reduce rapid drug clearance via strengthening of the micellar structure and increase in the available drug amount in plasma, thus broadening pharmaceutical applications of micelles (58).
Polymer Vesicles
Polymer vesicles (or polymersomes) are also similar to “storehouse” systems, but have different properties from polymer nano-microcapsules. Polymer vesicle membranes are composed of special amphiphilic block copolymers, biomimetic analogs of natural phospholipids, ranging in size from tens of nanometers to tens of microns, and can be well customized to the desired size (59). The hydrophobic segments of copolymers gather together to reduce contact with water, while the hydrophilic groups are distributed on the outer side of the membrane, thus forming a typical bilayer membrane structure similar to that of liposomes (60). Therefore, similar to liposomes, polymer vesicles can encapsulate hydrophobic substances in the membrane, while sub-encapsulating hydrophilic groups in the water-based core which are more complicated than polymer micelles with a simple core–shell nanostructure. Recently, polymer vesicles have attracted increasing attention due to their low permeability, superior stability and toughness (61). Polymer vesicles evolved from a simple “hollow sphere” to “smart” nanocarrier which were responsive to external stimuli (62), and presented significant advances for promising applications in biomedical field, including drug delivery, gene therapy, magnetic resonance imaging, theranostics over the past decades (59).
Dendrimers
Dendrimers are highly branched polymer molecules consisting of a core and branches connected around the core, typically between 5 and 20 nm (<100 nm) in size (63). Due to the particularity of dendritic molecules, dendritic molecules, as nanocarriers, have the advantages of high stability, easy size control and easy surface functionalization (64) Dendrites have been reported to improve the solubility of refractory anticancer drugs through ionic interactions, hydrogen bonding and hydrophobic interactions. In addition, dendritic branches have multiple sites for binding multiple drugs or targeting groups (65). Compared to liposomes, dendrimers present some advantages. dendrimers can be covalently bound to drugs, which are stable (63). However, once liposomes are systems composed of several components, drugs cannot be covalently attached to their outer surface and are difficult to stabilize (66). The aforementioned different type of polymer-based nanocarriers were bellowed (Figure 3).
Application of Nanocarrier in the Delivery of Pharmaceuticals
Nanoparticle drug delivery systems have been used in the clinic since the early 1990's. The past decade has witnessed tremendous advances in the field of nanoparticle engineering technologies. Recently, nanotechnology-based drug delivery systems such as nanoparticles (67), polymer micelles (68, 69), polymer vesicles (70), dendrimer (71), liposomes (72), SLN (73), NLC (74) and nanoemulsion (75) have been demonstrated to be excellent platforms for drug delivery to address the oral delivery challenges. Among them, liposomes most commonly was used in nanoformulation for drug delivery of a wide range (76). Liposomes have been widely used to develop drug delivery agents with clinical potential due to their biocompatibility, ease of modification and ability to reduce drug side effects. Because liposomes can simultaneously contain hydrophobic substances in bilayer and hydrophilic substances in core, and cationic liposomes can bind therapeutic genes, liposomes based on the combination of two drugs, drug and metal, and drug and therapeutic genes have been reported (77). Additionally, liposomes have also been explored as “chemical containers,” designed to serve as nanoscale reaction vessels for remotely controlled reactions (78). It has been demonstrated that liposome-based nanocarrier has effective therapeutics on anti-cancers, anti-hyperlipidemics, anti-inflammatories, antimalarials, anti-parasitics (79). Despite significant advances in medical science and technology, cancer remains a disease with limited treatment approaches. The combination of anti-tumor drugs based on liposome can improve the anticancer effect, anti-proliferation activity, pro-apoptotic activity and cytotoxicity of the drugs. Meanwhile, the preparation can reduce the systemic toxicity of drugs. In addition, the co-delivery of chemotherapy drugs using liposomes can destroy drug resistance in cancer cells (77). It has been reported that encapsulation of etoposide with positively charged monolsomes can enhance its antitumor activity and reduce etoposide effects (such as bone marrow suppression) (80).
Generally speaking, the driving force of nanocarrier in tumor tissues was based on enhanced permeability and retention (EPR) mechanism (81, 82). Utilizing the passive mechanisms of EPR still remains a critical design parameter of nanocarrier delivery to tumors, however, inefficient drug release and uptake by the endosomes can limit their therapeutic efficacy (83). Correspondingly, it does not necessarily ensure delivery of the cargo within the tumor cell via EPR mechanism facilitates (84). Hence, directing nanocarrier surface-modified with ligand motifs specific to such receptors provides a promising way to exploit the receptor-mediated active endocytosis mechanisms to achieve intracellular delivery of the nanocarrier. This mechanism of “active targeting” has been investigated for various receptors. Recently, converging lines of evidence suggest that cell surface receptor, long non-coding RNAs (lncRNAs) and cytokines such as integrins, folate, growth factors, which are known to express on disease cells and their microenvironments, were explored for developing counter marker functionalized drug carrier to recognize the target disease cells (74, 85, 86). Especially in antitumor treatment, cell surface receptor or tumor necrosis factor such as epidermal growth factor receptor (EGFR) (87, 88) and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) (89) has been successfully designed as a target or mediator of ligand-nanoparticle or ligand-drug conjugates for active drug delivery.
Application of Lipid-Based Vehicles for Nanoencapsulation of Food Industry
Currently, in the preparation of safe food, with superior nutritional and sensory characteristics, and multiple health benefits, nanofoods based on the application of nanotechnologies was newly proposed. Hence, for delivering functional ingredients and nutraceuticals into body cells and protect them against detrimental environmental and food process factors, food bioactive ingredients, including phenolic compounds, antioxidants, natural food colorants, antimicrobial agents, essential oils, minerals, flavors, essential fatty acids, and vitamins, could be placed within nanocarriers such as nanoemulsions, NLCs, SLNs, micro-emulsions and nano-liposomes so that a controlled/targeted release could be achieved (90–94). Commonly, food lipids used to encapsulate nutrients and functional components have been proven to be effective in protecting, controlling the release, and enhancing the action of bioactive compounds (95). Especially, the stability and absorption of polyunsaturated fatty acids, which are easily affected by heat and light, can be improved by wrapping them with liposomes. Nanoencapsulation of essential oils offers numerous advantages such as ease of handling, stability, protection against oxidation, enhanced distribution, solubility, controlled release, with less or no adverse effect on organoleptic properties of applicable food items with enhanced bioavailability (96). Bai et al. (97) prepared coix seed oil liposomes, which improved the stability of coix seed oil, improved the solubility of coix seed oil in water and significantly promoted its absorption in intestine. When using liposomes to wrap antioxidant components, liposomes can not only improve the resistance of antioxidant to adverse environment, but also enhance its antioxidant effect to a certain extent. According to previous reports, resveratrol liposomes show better ROS quenching ability than resveratrol, and can exert better antioxidant activity in cells (98). Apart from the attribute of saving bioactive food ingredients from environmental damage, it is also believed that one of the ways to disguise their displeasure attributes is through nanoencapsulation, especially active ingredients with undesirable flavor or properties that cannot be applied directly to food. Even though oleuropein from olive has numerous benefits, but it cannot be used for food fortification because of its presence in enzymatic browning reactions, bitter taste and irregularly absorbed in the gastrointestinal tract due to antioxidant activity. It has been demonstrated that adding olive leaf extract-loaded nanostructured lipid carriers powder as the source rich of oleuropein to sauce, could improve the undesirable appearance, color, odor, and flavor of the extract powder (polyphenols such as oleuropein) and also enjoy high durability of antioxidant properties of the polyphenols existing in the nanocarrier (99). It also reported that lipid-based nanocarriers stabilized by hydrophobically modified starch or sucrose stearate for the delivery of lutein could be appropriate for hydrophobic bioactive compounds in nutraceutical beverages (100).
Besides, lipid-based nanocarrier can also be used for solubilization of adjuvant or flavoring agent in food industry and improve its processing sensitivity. Lee et al. (101) has demonstrated the influences of carrier lipids (medium-chain triglyceride, coconut, olive, and soybean oil, and cocoa butter) on the physical stability of β-caryophellene-loaded lipid nanocarriers when they were exposed to various food processing conditions including thermal, freeze-thaw, and salt treatments, and revealed that the β-caryophellene-loaded lipid nanocarriers with medium-chain triglyceride oil could greatly benefit from application to various food processing.
Application of Polymer-Based Nano-Carriers in Food Processing
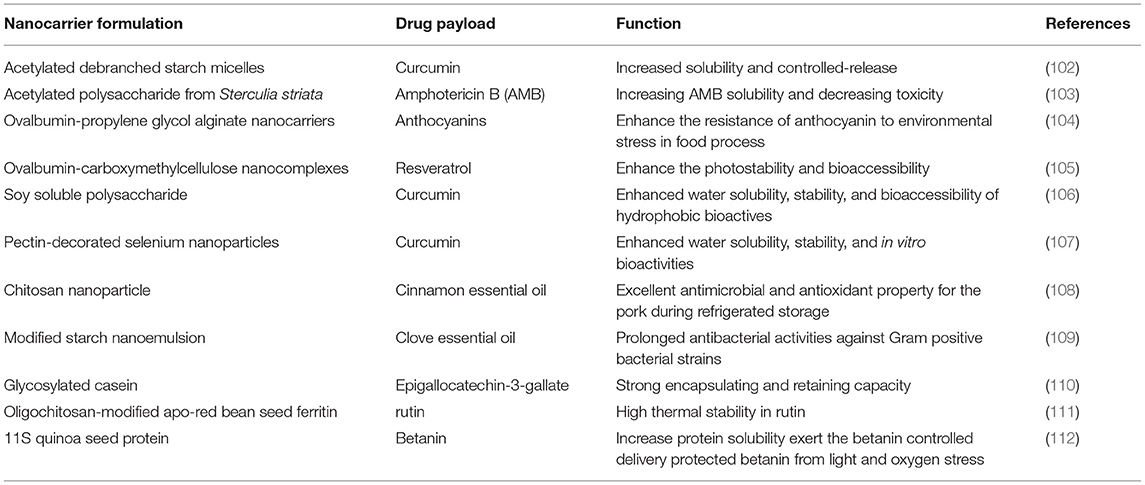
Due to its excellent encapsulation performance, sustained release ability, as well as efficient biocompatibility and biodegradability, polymer-based nanocarriers are also widely used for embedding food functional factors, nutrients and additives (listed in Table 1). Generally speaking, there are two type of polymer material, including natural and synthetic polymer nanoparticles, were used as polymer-based nanocarrier. Compared with synthetic polymers such as polylactide–polyglycolide copolymers, polyacrylates and polycaprolactones (PCL), polylactic acid (PLA), poly (lactic-co-glycolic acid) (PLGA) which are often used in nanoparticle synthesis (113–115), natural polymers polysaccharides are more concerned by food researchers because of their good safety. However, in most cases, polysaccharides need to be modified to have the desired nanocervative function. Modified-polysaccharides-based wall materials mainly include modified chitosan, modified starch and other materials. As for chitosan concern, it is mucoadhesive and soluble only at acidic pH. Hence, chemical modification of chitosan is being carried out to enhance its mucoadhesive properties. Chitosan derivatives like trimethyl chitosan (TMC), thiolated chitosan, chitosan-ethylenediaminetetraacetic acid, etc. have showed improved solubility and mucoadhesive properties (116). It is reported that the modified chitosan wall material has good solubility and film forming properties. Peng et al. (117) synthesized water-soluble methoxy poly (ethylene glycol)-graft-chitosan and applied it to prepare nano-microcapsules encapsulated in seaweed oil. The results showed that such modified chitosan nano-microcapsules had a high encapsulation rate on seaweed oil and could avoid the effects of ultraviolet light and heat on the quality of seaweed oil. Rusli et al. (118) successfully embedded fish oil vulnerable to oxidative damage by taking the product of maillard reaction between protein and carbohydrate as the wall material of nano-microcapsules. In particular, the wall material itself has some antioxidant activity, which can better protect fish oil composition (119). Notably, natural plant- and microbial-derived polysaccharides, such as soy soluble polysaccharide (106), modified pectin (107), can also be used as nanocarriers.
Additionally, some type of food-derived proteins with special structures can be used as nanocarriers (120). Protein nanocages, usually assembled from multiple subunits in a high symmetry, are three-dimensional shell-like containers, which possess an intrinsic homogeneous chamber that is segregated from the bulk environment by protein walls (121). Structurally, natural nanocages include ferritin, heat shock protein, DNA-binding protein, and encapsuling (121–123) with a modifiable exterior surface. Previous research demonstrated that applications of chitosan and transglutaminase in protein modification will improve ferritin functionalization as a nanocarrier for food bioactive molecules (111). It has been reported that betanin loaded 11S quinoa seed protein nanovehicle could exert the betanin controlled delivery for pharmaceutical and nutraceutical food products (112). Additionally, meat-, milk-, egg-, and silk-based protein, as well as plant-based proteins, such as zein proteins, soy proteins, pea proteins have been reported as possible carriers for bioactive compounds (120, 124). It has been demonstrated that casein and glycosylated casein display strong encapsulating and retaining capacity to epigallocatechin-3-gallate (EGCG), effectively protect EGCG from degradation in alkaline pH and displayed a slow and sustained release in intestinal fluid. Therefore, glycosylated casein could be used as a promising and effective nanocarrier for EGCG (110).
The application of polymer-based nanocapsule to plant essential oil is a hot spot in the field of food science. Nano microcapsules containing essential oil can play a role in food flavoring, anti-corrosion and anti-oxidation. The essential oil in traditional spices is attached to the carrier of wood fiber, which is difficult to be fully utilized. However, the use of nano-microcapsules to wrap the essential oil in spices can reduce volatilization loss, inhibit quality deterioration and facilitate storage and transportation (125). Bai et al. (126) used chitosan/polycaprolactone nanoparticles as wall materials to prepare tea tree essential oil microcapsules, which showed higher protection against volatilization and exhibits broader anti-bacterial spectrum. Moreover, polymer-coated essential oils are good for keeping fresh food. Wu et al. (127) prepared nano microcapsules of citrus essential oil using chitosan and sodium tripolyphosphate as wall materials, and studied its film-forming ability and fresh-keeping function for fish. The results showed that citrus essential oil nanocapsules could significantly prolong the shelf life of marine fish and have a broad prospect of application in the field of marine product preservation. Hu et al. (108) reported that chitosan nanoparticles loaded with cinnamon essential oil exhibited the excellent antimicrobial and antioxidant property for the pork during refrigerated storage. In addition, polymer nanocarriers solve the problems of limited application of hydrophobic food functional components in aqueous food, such as poor water solubility, poor stability and low bioavailability. Liu et al. (102) reported that acetylated debranched starch micelles could be designed as a promising nanocarrier for controlled-release of curcumin in simulated digestive fluid.
Safety of Nanocarriers
Although a large number of research reports that lipid-based and polymer-based nanocarriers are expected to be applied to the pharmaceuticals and food industry, however, safety should not be underestimated. It is hypothesized that nanocarriers having properties such as nano-size, high surface to volume ratio, and infuse efficiently from the intestinal barrier to circulation may cause toxicity since they differ from bulk material (128). Nanoparticles may exert toxic effects such as oxidative stress, inflammation and DNA damage in animals, after entering the human body, contacting with cells and producing toxic effects (129, 130). Recently, increasing evidences have demonstrated that the induction of autophagy by nanoparticles can also cause nanotoxicity (131, 132). Additionally, concern about penetration of biopersistent solid nanoparticles into the skin which could lead to damage to viable epidermal cells or accumulation in secondary organs following biodistribution was also in the spotlight (133, 134). Fortunately, there are increasing reports on the safety evaluation of nanocarriers. A growing body of evidence suggests that certain bioactive substance-loaded nanocarriers are efficient and safe formulations that can be used in food or medicine. In assessing the gel properties, pharmacokinetics, morphology, anticancer activity and immunohistopathology after thermosensitive irinotecan-encapsulated solid lipid nanoparticles (SLN) rectal administration to tumor-bearing mice, it was easily administered to the anus, gelling rapidly and strongly after rectal administration, inducing no damage to the rat rectum and no body weight loss in tumor-bearing mice (135). Futhermore, mutiple nanocarrier, including bio-nanocapsule-liposome nanocarrier (136), poly{(benzyl-L-aspartate)-co-[N-(3-aminopropyl) imidazole-L-aspartamide]}-poly(ethylene glycol) (137), Polysaccharide-based nanocarriers (138), (139), Poly(aspartic acid-graft-imidazole)-poly(ethylene glycol) [P(Asp-g-Im)-PEG] (140), amphiphilic copolymer of poly(epsilon-caprolactone)-b-poly(ethylene glycol)-b- poly(epsilon-caprolactone) (141), were demonstrated to be safety in vivo in treatment cancer, inflammatory bowel disease. Additionally, some material was demonstrated to be safety such as starch-based nanoparticles (139). While a vast array of nanocarrier is under development, many of which are undergoing advanced clinical trials. However, relatively few have achieved full translation to clinical practice. This slow uptake may be due, in part, to the need for a rigorous demonstration of safety in these new nanotechnologies.
For food safety as regards, although the safety (or the safety dose) of most food materials has been established, the safety of food obtained through nano processing needs to be further explored. It has been demonstrated that ingestion biodegradable polymers chitosan-sodium alginate-oleic acid based nano-carrier loaded with lutein (LNCs) with dose of 10 mg/kg body weight was demonstrated to revealed no mortality with no morphological and clinical changes in rats (128). However, there are few safety-evaluation studies on nanocarriers used in food under strict scrutiny. Therefore, the application of effective nanocarriers in the real food industry still needs a large space for safety evaluation.
Conclusion and Future Prospect
Owing to marked progress in the understanding of nanoformulation discoveries and petentional applications, substantial efforts done for successful management of lipid-based or polymer-based nanocarrier with least adverse effects as medicine or as an protectant, enhancer or improver for bioactive ingredient in the food industry. In terms of nanomedicine, mutiple researches have focused on targeted delivery of anticancer drugs. Therefore, the wider use of nanocarriers in medicine should be covered. Moreover, nanocarrier used in nanomedicine may transform into possibly higher toxicity and immunogenicity as well, thus increasing manufacturing cost and unexpected risks. Hence, designing new nanoformulations require sufficient proofs to demonstrate that nanoformulation by certain nanocarrier is clinically more active, adequately stable and cost-effective. Considering food application respect, low-cost, efficient, safety and edible nanocarrier formulations has a broad application prospect in food preservation, preservative, functional ingredient enhancement and flavor ingredient sustained release, which is also the urgent demand in food industry. Therefore, effective, safe and non-toxic nanomaterials for food use require multiple experimental data and proofs. To conclude, nanocarriers have broad prospects in food and medicine, and should receive more attention, especially for safe phytochemical.
Author Contributions
HL and QC conceived the review topics. HL wrote the initial draft. SZ revised the manuscript. JW and QC revised and supervised the entire work. All authors read and approved the submitted version.
Funding
This research was funded by the Science and Technology Project of Yunnan province (No. 202002AA1000056-3).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen H, Gu Z, An H, Chen C, Chen J, Cui R, et al. Precise nanomedicine for intelligent therapy of cancer. Sci China Chem. (2018) 61:1503–52. doi: 10.1007/s11426-018-9397-5
2. Germain M, Caputo F, Metcalfe S, Tosi G, Spring K, Aslund AKO, et al. Delivering the power of nanomedicine to patients today. J Control Release. (2020) 326:164–71. doi: 10.1016/j.jconrel.2020.07.007
3. Bordat A, Boissenot T, Nicolas J, Tsapis N. Thermoresponsive polymer nanocarriers for biomedical applications. Adv Drug Deliv Rev. (2019) 138:167–92. doi: 10.1016/j.addr.2018.10.005
4. Simon J, Christmann S, Mailänder V, Wurm FR, Landfester K. Protein corona mediated stealth properties of biocompatible carbohydrate-based nanocarriers. Isr J Chem. (2018) 58:1363–72. doi: 10.1002/ijch.201800166
5. Roberts TC, Langer R, Wood MJA. Advances in oligonucleotide drug delivery. Nat Rev Drug Discovery. (2020) 19:673–94. doi: 10.1038/s41573-020-0075-7
6. McClements DJ, Jafari SM. Improving emulsion formation, stability and performance using mixed emulsifiers: a review. Adv Colloid Interface Sci. (2018) 251:55–79. doi: 10.1016/j.cis.2017.12.001
7. Dima C, Assadpour E, Dima S, Jafari SM. Nutraceutical nanodelivery; an insight into the bioaccessibility/bioavailability of different bioactive compounds loaded within nanocarriers. Crit Rev Food Sci Nutr. (2020) 2020:1–35. doi: 10.1080/10408398.2020.1792409
8. Nicolas J, Mura S, Brambilla D, Mackiewicz N, Couvreur P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem Soc Rev. (2013) 42:1147–235. doi: 10.1039/C2CS35265F
9. Wacker M. Nanocarriers for intravenous injection—the long hard road to the market. Int J Pharm. (2013) 457:50–62. doi: 10.1016/j.ijpharm.2013.08.079
10. Jahangir MA, Taleuzzaman M, Kala C, Gilani SJ. Advancements in polymer and lipid-based nanotherapeutics for cancer drug targeting. Curr Pharm Des. (2020) 26:5119–27. doi: 10.2174/1381612826999200820173253
11. Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. (2013) 65:36–48. doi: 10.1016/j.addr.2012.09.037
12. Guimarães D, Cavaco-Paulo A, Nogueira E. Design of liposomes as drug delivery system for therapeutic applications. Int J Pharm. (2021) 601:120571. doi: 10.1016/j.ijpharm.2021.120571
13. Raemdonck K, Braeckmans K, Demeester J, De Smedt SC. Merging the best of both worlds: hybrid lipid-enveloped matrix nanocomposites in drug delivery. Chem Soc Rev. (2014) 43:444–72. doi: 10.1039/C3CS60299K
14. Lima PHC, Butera AP, Cabeça LF, Ribeiro-Viana RM. Liposome surface modification by phospholipid chemical reactions. Chem Phys Lipids. (2021) 237:105084. doi: 10.1016/j.chemphyslip.2021.105084
15. Cheng Z, Li M, Dey R, Chen Y. Nanomaterials for cancer therapy: current progress and perspectives. J Hematol Oncol. (2021) 14:85. doi: 10.1186/s13045-021-01096-0
16. Muller RH, Mader K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art. Eur J Pharm Biopharm. (2000) 50:161–77. doi: 10.1016/S0939-6411(00)00087-4
17. Yuan H, Wang L-L, Du Y-Z, You J, Hu F-Q, Zeng S. Preparation and characteristics of nanostructured lipid carriers for control-releasing progesterone by melt-emulsification. Colloids Surf B Biointerf. (2007) 60:174–9. doi: 10.1016/j.colsurfb.2007.06.011
18. Saupe A., Rades T. (2006). “Solid lipid nanoparticles,” in Nanocarrier Technologies (Berlin: Springer), 41–50.
19. Rostami E, Kashanian S, Azandaryani AH, Faramarzi H, Dolatabadi JEN, Omidfar K. Drug targeting using solid lipid nanoparticles. Chem Phys Lipids. (2014) 181:56–61. doi: 10.1016/j.chemphyslip.2014.03.006
20. Ezzati Nazhad Dolatabadi J, Valizadeh H, Hamishehkar H. Solid lipid nanoparticles as efficient drug and gene delivery systems: recent breakthroughs. (2015) 5:151–9. doi: 10.15171/apb.2015.022
21. Kamble MS, Vaidya KK, Bhosale AV, Chaudhari PD. Solid lipid nanoparticles and nanostructured lipid carriers. Int J Pharm Chem Biol Sci. (2012) 2:681–91.
22. Wong HL, Bendayan R, Rauth AM, Li Y, Wu XY. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv Drug Deliv Rev. (2007) 59:491–504. doi: 10.1016/j.addr.2007.04.008
23. Haider M, Abdin SM, Kamal L, Orive G. Nanostructured lipid carriers for delivery of chemotherapeutics: a review. Pharmaceutics. (2020) 12:288. doi: 10.3390/pharmaceutics12030288
24. Akhavan S, Assadpour E, Katouzian I, Jafari SM. Lipid nano scale cargos for the protection and delivery of food bioactive ingredients and nutraceuticals. Trends Food Sci Technol. (2018) 74:132–46. doi: 10.1016/j.tifs.2018.02.001
25. Muller RH, Shegokar R, Keck C. 20 years of lipid nanoparticles (SLN and NLC): present state of development and industrial applications. Curr Drug Discov. (2011). 8:207–27. doi: 10.2174/157016311796799062
26. Souto EB, Baldim I, Oliveira WP, Rao RH, Yadav N, Gama FM, et al. SLN and NLC for topical, dermal, and transdermal drug delivery. Expert Opin Drug Deliv. (2020) 17:357–77. doi: 10.1080/17425247.2020.1727883
27. Müller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. (2002) 54:S131–55. doi: 10.1016/S0169-409X(02)00118-7
28. Müller R, Petersen R, Hommoss A, Pardeike J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv Drug Deliv Rev. (2007) 59:522–30. doi: 10.1016/j.addr.2007.04.012
29. Puri A, Loomis K, Smith B, Lee J-H, Yavlovich A, Heldman E, et al. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Critic Rev Therap Drug Carrier Syst. (2009) 26:6. doi: 10.1615/CritRevTherDrugCarrierSyst.v26.i6.10
30. Ashaolu TJ. Nanoemulsions for health, food, and cosmetics: a review. Environ Chem Lett. (2021) 19:3381–95. doi: 10.1007/s10311-021-01216-9
31. Azmi NA, Elgharbawy AAM, Motlagh SR, Samsudin N, Salleh HM. Nanoemulsions: factory for food, pharmaceutical and cosmetics. Processes. (2019) 7:pr7090617. doi: 10.3390/pr7090617
32. Gupta A, Eral HB, Hatton TA, Doyle PS. Nanoemulsions: formation, properties and applications. Soft Matter. (2016) 12:2826–41. doi: 10.1039/C5SM02958A
33. Sigward E, Mignet N, Rat P, Dutot M, Muhamed S, Guigner JM, et al. Formulation and cytotoxicity evaluation of new self-emulsifying multiple W/O/W nanoemulsions. Int J Nanomed. (2013) 8:611–25. doi: 10.2147/IJN.S35661
34. Singh Y, Meher JG, Raval K, Khan FA, Chaurasia M, Jain NK, et al. Nanoemulsion: concepts, development and applications in drug delivery. J Controll Release. (2017) 252:28–49. doi: 10.1016/j.jconrel.2017.03.008
35. Rai VK, Mishra N, Yadav KS, Yadav NP. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: formulation development, stability issues, basic considerations and applications. J Control Release. (2018) 270:203–25. doi: 10.1016/j.jconrel.2017.11.049
36. Zhang MW, Corona PT, Ruocco N, Alvarez D, de Molina PM, Mitragotri S, et al. Controlling complex nanoemulsion morphology using asymmetric cosurfactants for the preparation of polymer nanocapsules. Langmuir. (2018) 34:978–90. doi: 10.1021/acs.langmuir.7b02843
37. Constantinides PP, Tustian A, Kessler DR. Tocol emulsions for drug solubilization and parenteral delivery. Adv Drug Deliv Rev. (2004) 56:1243–55. doi: 10.1016/j.addr.2003.12.005
38. Ganta S, Amiji M. Coadministration of paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol Pharm. (2009) 6:928–39. doi: 10.1021/mp800240j
39. Chinsriwongkul A, Opanasopit P, Ngawhirunpat T, Rojanarata T, Sila-On W, Ruktanonchai U. Oleic acid enhances all-trans retinoic acid loading in nano-lipid emulsions. PDA J Pharm Sci Technol. (2010) 64:113–23.
40. Li X, Du L, Wang C, Liu Y, Mei X, Jin Y. Highly efficient and lowly toxic docetaxel nanoemulsions for intravenous injection to animals. Die Pharm. (2011) 66:479–83. doi: 10.1691/ph.2011.1015
41. Ahmed K, Li Y, McClements DJ, Xiao H. Nanoemulsion-and emulsion-based delivery systems for curcumin: encapsulation and release properties. Food Chem. (2012) 132:799–807. doi: 10.1016/j.foodchem.2011.11.039
42. Gaoe H, Pang Z, Pan S, Cao S, Yang Z, Chen C, et al. Anti-glioma effect and safety of docetaxel-loaded nanoemulsion. Arch Pharm Res. (2012) 35:333–41. doi: 10.1007/s12272-012-0214-8
43. McClements DJ. Nanoscale nutrient delivery systems for food applications: improving bioactive dispersibility, stability, and bioavailability. J Food Sci. (2015) 80:N1602–11. doi: 10.1111/1750-3841.12919
44. Donsi F, Ferrari G. Essential oil nanoemulsions as antimicrobial agents in food. J Biotechnol. (2016) 233:106–20. doi: 10.1016/j.jbiotec.2016.07.005
45. Guerra-Rosas MI, Morales-Castro J, Ochoa-Martínez LA, Salvia-Trujillo L, Martín-Belloso O. Long-term stability of food-grade nanoemulsions from high methoxyl pectin containing essential oils. Food Hydrocoll. (2016) 52:438–46. doi: 10.1016/j.foodhyd.2015.07.017
46. Atav R. 8—Dendritic molecules and their use in water repellency treatments of textile materials (2018). In: J. Williams (editor). Waterproof and Water Repellent Textiles and Clothing. Woodhead Publishing, 191–214.
47. Patel T, Zhou J, Piepmeier JM, Saltzman WM. Polymeric nanoparticles for drug delivery to the central nervous system. Adv Drug Deliv Rev. (2012) 64:701–5. doi: 10.1016/j.addr.2011.12.006
48. Hallan SS, Kaur P, Kaur V, Mishra N, Vaidya B. Lipid polymer hybrid as emerging tool in nanocarriers for oral drug delivery. Artif Cells Nanomed Biotechnol. (2016) 44:334–49. doi: 10.3109/21691401.2014.951721
49. Zhang L, Chan JM, Gu FX, Rhee J-W, Wang AZ, Radovic-Moreno AF, et al. Self-assembled lipid– polymer hybrid nanoparticles: a robust drug delivery platform. ACS Nano. (2008) 2:1696–702. doi: 10.1021/nn800275r
50. Hallan SS, Nidhi KV, Jain V, Mishra N. Development and characterization of polymer lipid hybrid nanoparticles for oral delivery of LMWH. Artif Cells Nanomed Biotechnol. (2017) 45:1631–1639. doi: 10.1080/21691401.2016.1276920
51. Khan MM, Madni A, Torchilin V, Filipczak N, Pan J, Tahir N, et al. Lipid-chitosan hybrid nanoparticles for controlled delivery of cisplatin. Drug Deliv. (2019) 26:765–72. doi: 10.1080/10717544.2019.1642420
52. Cabral H, Miyata K, Osada K, Kataoka K. Block copolymer micelles in nanomedicine applications. Chem Rev. (2018) 118:6844–92. doi: 10.1021/acs.chemrev.8b00199
53. Adams ML, Lavasanifar A, Kwon GS. Amphiphilic block copolymers for drug delivery. J Pharm Sci. (2003) 92:1343–55. doi: 10.1002/jps.10397
54. Oerlemans C, Bult W, Bos M, Storm G, Nijsen JFW, Hennink WE. Polymeric micelles in anticancer therapy: targeting, imaging and triggered release. Pharm Res. (2010) 27:2569–89. doi: 10.1007/s11095-010-0233-4
55. Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. (2007) 24:1. doi: 10.1007/s11095-006-9132-0
56. Gothwal A, Khan I, Gupta U. Polymeric micelles: recent advancements in the delivery of anticancer drugs. Pharm Res. (2016) 33:18–39. doi: 10.1007/s11095-015-1784-1
57. Raval N, Maheshwari R, Shukla H, Kalia K, Torchilin VP, Tekade RK. Multifunctional polymeric micellar nanomedicine in the diagnosis and treatment of cancer. Mater Sci Eng C-Mater Biol Appl. (2021) 126:112186. doi: 10.1016/j.msec.2021.112186
58. Wen S-N, Chu C-H, Wang Y-C, Huang H-Y, Wang Y-J, Lin J-Y, et al. Polymer-stabilized micelles reduce the drug rapid clearance. J Nanomater. (2018) 2018:5818592. doi: 10.1155/2018/5818592
59. Zhu Y, Yang B, Chen S, Du J. Polymer vesicles: mechanism, preparation, application, and responsive behavior. Prog Polym Sci. (2017) 64:1–22. doi: 10.1016/j.progpolymsci.2015.05.001
60. Discher BM, Won Y-Y, Ege DS, Lee JC, Bates FS, Discher DE, et al. Polymersomes: tough vesicles made from diblock copolymers. Science. (1999) 284:1143–6. doi: 10.1126/science.284.5417.1143
61. Jiang W, Zhou Y, Reviews DYJCS. Hyperbranched polymer vesicles: from self-assembly, characterization, mechanisms, and properties to applications. Chem Rev Soc. (2015) 44:3874–89. doi: 10.1039/C4CS00274A
62. Liu D, Sun H, Xiao Y, Chen S, Cornel EJ, Zhu Y, et al. Design principles, synthesis and biomedical applications of polymer vesicles with inhomogeneous membranes. J Controll Release. (2020) 326:365–86. doi: 10.1016/j.jconrel.2020.07.018
63. Dias AP, da Silva Santos S, da Silva JV, Parise-Filho R, Igne Ferreira E, Seoud OE, et al. Dendrimers in the context of nanomedicine. Int J Pharm. (2020) 573:118814. doi: 10.1016/j.ijpharm.2019.118814
64. Mousa SA, Chidlowsky E, Bawarski WE, Bharali DJ. Emerging nanopharmaceuticals (2017). In: Nanomedicine in Cancer. Stanford: Pan Stanford, 125–154.
65. Khandare JJ, Jayant S, Singh A, Chandna P, Wang Y, Vorsa N, et al. Dendrimer versus linear conjugate: influence of polymeric architecture on the delivery and anticancer effect of paclitaxel. Bioconjug Chem. (2006) 17:1464–72. doi: 10.1021/bc060240p
66. Lee CC, MacKay JA, Fréchet JMJ, Szoka FC. Designing dendrimers for biological applications. Nat Biotechnol. (2005) 23:1517–26. doi: 10.1038/nbt1171
67. Liu BY, Wu C, He XY, Zhuo RX, Cheng SX. Multi-drug loaded vitamin E-TPGS nanoparticles for synergistic drug delivery to overcome drug resistance in tumor treatment. Sci Bull. (2016) 61:552–60. doi: 10.1007/s11434-016-1039-5
68. Sheikhpour M, Barani L, Kasaeian A. Biomimetics in drug delivery systems: a critical review. J Controlled Release. (2017) 253:97–109. doi: 10.1016/j.jconrel.2017.03.026
69. Liu Y, Yang GZ, Baby T, Tengjisi, Chen D, Weitz DA, et al. (2020). Stable polymer nanoparticles with exceptionally high drug loading by sequential nanoprecipitation. Angew. Chem. (2020) 59:4720–28. doi: 10.1002/anie.201913539
70. Jia L, Wang R, Fan YN. Encapsulation and release of drug nanoparticles in functional polymeric vesicles. Soft Matter. (2020) 16:3088–95. doi: 10.1039/D0SM00069H
71. Wei T, Chen C, Liu J, Liu C, Posocco P, Liu XX, et al. Anticancer drug nanomicelles formed by self-assembling amphiphilic dendrimer to combat cancer drug resistance. Proc Natl Acad Sci USA. (2015) 112:2978–83. doi: 10.1073/pnas.1418494112
72. Li T, Cipolla D, Rades T, Boyd BJ. Drug nanocrystallisation within liposomes. J Controll Release. (2018) 288:96–110. doi: 10.1016/j.jconrel.2018.09.001
73. Mirchandani Y, Patravale VB, Brijesh S. Solid lipid nanoparticles for hydrophilic drugs. J Controll Release. (2021) 335:457–64. doi: 10.1016/j.jconrel.2021.05.032
74. Majumder J, Taratula O, Minko T. Nanocarrier-based systems for targeted and site specific therapeutic delivery. Adv Drug Deliv Rev. (2019) 144:57–77. doi: 10.1016/j.addr.2019.07.010
75. Hormann K, Zimmer A. Drug delivery and drug targeting with parenteral lipid nanoemulsions—a review. J Controll Release. (2016) 223:85–98. doi: 10.1016/j.jconrel.2015.12.016
76. Afzal M, Ameeduzzafar AKS, Alruwaili NK, Al-Abassi FA, Al-Malki AA, et al. Nanomedicine in treatment of breast cancer? A challenge to conventional therapy. Sem Cancer Biol. (2021) 69:279–92. doi: 10.1016/j.semcancer.2019.12.016
77. Zununi Vahed S, Salehi R, Davaran S, Sharifi S. Liposome-based drug co-delivery systems in cancer cells. Mater Sci Eng C Mater Biol Appl. (2017) 71:1327–41. doi: 10.1016/j.msec.2016.11.073
78. Al-Jamal WT, Kostarelos K. Liposomes: from a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine. Acc Chem Res. (2011) 44:1094–104. doi: 10.1021/ar200105p
79. Uner M, Damgali S, Ozdemir S, Celik B. Therapeutic potential of drug delivery by means of lipid nanoparticles: reality or illusion? Curr Pharm Des. (2017) 23:6573–91. doi: 10.2174/1381612823666171122110638
80. Harfouche R, Basu S, Soni S, Hentschel DM, Mashelkar RA, Sengupta S. Nanoparticle-mediated targeting of phosphatidylinositol-3-kinase signaling inhibits angiogenesis. Angiogenesis. (2009) 12:325. doi: 10.1007/s10456-009-9154-4
81. Li YP, Xiao K, Zhu W, Deng WB, Lam KS. Stimuli-responsive cross-linked micelles for on-demand drug delivery against cancers. Adv Drug Deliv Rev. (2014) 66:58–73. doi: 10.1016/j.addr.2013.09.008
82. Shakeran Z, Keyhanfar M, Varshosaz J, Sutherland DS. Biodegradable nanocarriers based on chitosan-modified mesoporous silica nanoparticles for delivery of methotrexate for application in breast cancer treatment. Mater Sci Eng C Mater Biol Appl. (2021) 118. doi: 10.1016/j.msec.2020.111526
83. Bilal M, Qindeel M, Raza A, Mehmood S, Rahdar A. Stimuli-responsive nanoliposomes as prospective nanocarriers for targeted drug delivery. J Drug Deliv Sci Technol. (2021) 66:102916. doi: 10.1016/j.jddst.2021.102916
84. Master AM, Sen Gupta A. EGF receptor-targeted nanocarriers for enhanced cancer treatment. Nanomedicine. (2012) 7:1895–906. doi: 10.2217/nnm.12.160
85. Yu B, Tai HC, Xue WM, Lee LJ, Lee RJ. Receptor-targeted nanocarriers for therapeutic delivery to cancer. Mol Membr Biol. (2010) 27:286–98. doi: 10.3109/09687688.2010.521200
86. Zuo XL, Chen ZQ, Gao W, Zhang Y, Wang JG, Wang JF, et al. M6A-mediated upregulation of LINC00958 increases lipogenesis and acts as a nanotherapeutic target in hepatocellular carcinoma. J Hematol Oncol. (2020) 13:5. doi: 10.1186/s13045-019-0839-x
87. Chang M-H, Pai C-L, Chen Y-C, Yu H-P, Hsu C-Y, Lai P-S. Enhanced antitumor effects of epidermal growth factor receptor targetable cetuximab-conjugated polymeric micelles for photodynamic therapy. Nanomaterials. (2018) 8:121. doi: 10.3390/nano8020121
88. Habban Akhter M, Sateesh Madhav N, Ahmad J. Epidermal growth factor receptor based active targeting: a paradigm shift towards advance tumor therapy. Artif Cells Nanomed Biotechnol. (2018) 46:1188–98. doi: 10.1080/21691401.2018.1481863
89. Vaughan HJ, Zamboni CG, Radant NP, Bhardwaj P, Revai Lechtich E, Hassan LF, et al. Poly(beta-amino ester) nanoparticles enable tumor-specific TRAIL secretion and a bystander effect to treat liver cancer. Mol Therapy Oncol. (2021) 21:377–88. doi: 10.1016/j.omto.2021.04.004
90. Park SJ, Garcia CV, Shin GH, Kim JT. Development of nanostructured lipid carriers for the encapsulation and controlled release of vitamin D3. Food Chem. (2017) 225:213–9. doi: 10.1016/j.foodchem.2017.01.015
91. Assadpour E, Mahdi Jafari S. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit Rev Food Sci Nutr. (2019) 59:3129–51. doi: 10.1080/10408398.2018.1484687
92. Hsu CY, Wang PW, Alalaiwe A, Lin ZC, Fang JY. Use of lipid nanocarriers to improve oral delivery of vitamins. Nutrients. (2019) 11:68. doi: 10.3390/nu11010068
93. Feng SM, Sun YX, Wang P, Sun PL, Ritzoulis C, Shao P. Co-encapsulation of resveratrol and epigallocatechin gallate in low methoxyl pectin-coated liposomes with great stability in orange juice. Int J Food Sci Technol. (2020) 55:1872–80. doi: 10.1111/ijfs.14323
94. Souri J, Almasi H, Hamishehkar H, Amjadi S. Sodium caseinate-coated and β-cyclodextrin/vitamin E inclusion complex-loaded nanoliposomes: a novel stabilized nanocarrier. LWT. (2021) 151:112174. doi: 10.1016/j.lwt.2021.112174
95. Caddeo C, Pucci L, Gabriele M, Carbone C, Fernàndez-Busquets X, Valenti D, et al. Stability, biocompatibility and antioxidant activity of PEG-modified liposomes containing resveratrol. Int J Pharm. (2018) 538:40–7. doi: 10.1016/j.ijpharm.2017.12.047
96. Prakash B, Kujur A, Yadav A, Kumar A, Singh PP, Dubey NK. Nanoencapsulation: an efficient technology to boost the antimicrobial potential of plant essential oils in food system. Food Control. (2018) 89:1–11. doi: 10.1016/j.foodcont.2018.01.018
97. Bai C, Peng H, Xiong H, Liu Y, Zhao L, Xiao X. Carboxymethylchitosan-coated proliposomes containing coix seed oil: characterisation, stability and in vitro release evaluation. Food Chem. (2011) 129:1695–702. doi: 10.1016/j.foodchem.2011.06.033
98. Vanaja K, Wahl M, Bukarica L, Heinle H. Liposomes as carriers of the lipid soluble antioxidant resveratrol: evaluation of amelioration of oxidative stress by additional antioxidant vitamin. Life Sci. (2013) 93:917–23. doi: 10.1016/j.lfs.2013.10.019
99. Soleimanifard M, Sadeghi Mahoonak A, Sepahvand A, Heydari R, Farhadi S. Spanish olive leaf extract-loaded nanostructured lipid carriers: production and physicochemical characterization by Zetasizer, FT-IR, DTA/TGA, FE-SEM and XRD. J Food Process Preserv. (2019) 43:e13994. doi: 10.1111/jfpp.13994
100. Sedaghat Doost A, Afghari N, Abbasi H, Nikbakht Nasrabadi M, Dewettinck K, Van der Meeren P. Nano-lipid carriers stabilized by hydrophobically modified starch or sucrose stearate for the delivery of lutein as a nutraceutical beverage model. Colloids Surf A Physicochem Eng Aspects. (2020) 605:125349. doi: 10.1016/j.colsurfa.2020.125349
101. Lee MH, Lee IY, Chun YG, Kim B-K. Formulation and characterization of β-caryophellene-loaded lipid nanocarriers with different carrier lipids for food processing applications. LWT. (2021) 149:111805. doi: 10.1016/j.lwt.2021.111805
102. Liu Q, Li F, Ji N, Dai L, Xiong L, Sun Q. Acetylated debranched starch micelles as a promising nanocarrier for curcumin. Food Hydrocoll. (2021) 111:106253. doi: 10.1016/j.foodhyd.2020.106253
103. Sombra FM, Richter AR, de Araújo AR, de Oliveira Silva Ribeiro F, Souza Mendes J, et al. Development of amphotericin B-loaded propionate Sterculia striata polysaccharide nanocarrier. Int J Biol Macromol. (2020) 146:1133–41. doi: 10.1016/j.ijbiomac.2019.10.053
104. Zhang X, Zeng Q, Liu Y, Cai Z. Enhancing the resistance of anthocyanins to environmental stress by constructing ovalbumin-propylene glycol alginate nanocarriers with novel configurations. Food Hydrocoll. (2021) 118:106668. doi: 10.1016/j.foodhyd.2021.106668
105. Xiong W, Ren C, Li J, Li B. Enhancing the photostability and bioaccessibility of resveratrol using ovalbumin-carboxymethylcellulose nanocomplexes and nanoparticles. Food and Function. (2018) 9:3788–97. doi: 10.1039/C8FO00300A
106. Chen F-P, Ou S-Y, Chen Z, Tang C-H. Soy soluble polysaccharide as a nanocarrier for curcumin. J Agric Food Chem. (2017) 65:1707–14. doi: 10.1021/acs.jafc.6b05087
107. Wu Y, Liu H, Li ZH, Huang DY, Nong LZ, Ning ZX, et al. Pectin-decorated selenium nanoparticles as a nanocarrier of curcumin to achieve enhanced physicochemical and biological properties. IET Nanobiotechnol. (2019) 13:880–6. doi: 10.1049/iet-nbt.2019.0144
108. Hu J, Wang X, Xiao Z, Bi W. Effect of chitosan nanoparticles loaded with cinnamon essential oil on the quality of chilled pork. LWT Food Sci Technol. (2015) 63:519–26. doi: 10.1016/j.lwt.2015.03.049
109. Anwer MK, Jamil S, Ibnouf EO, Shakeel F. Enhanced antibacterial effects of clove essential oil by nanoemulsion. J Oleo Sci. (2014) 63:347–54. doi: 10.5650/jos.ess13213
110. Xue J, Tan C, Zhang X, Feng B, Xia S. Fabrication of epigallocatechin-3-gallate nanocarrier based on glycosylated casein: stability and interaction mechanism. J Agric Food Chem. (2014) 62:4677–84. doi: 10.1021/jf405157x
111. Yang R, Zuo P, Zhang M, Meng D, Wang B, Zhen T. Transglutaminase induced oligochitosan glycosylation of ferritin as a novel nanocarrier for food bioactive molecules. Food Hydrocoll. (2019) 94:500–9. doi: 10.1016/j.foodhyd.2019.03.049
112. Martínez JH, Velázquez F, Burrieza HP, Martínez KD, Paula Domínguez Rubio A, dos Santos Ferreira C, et al. Betanin loaded nanocarriers based on quinoa seed 11S globulin. Impact on the protein structure and antioxidant activity. Food Hydrocolloids. (2019) 87:880–90. doi: 10.1016/j.foodhyd.2018.09.016
113. Ashour AE, Badran M, Kumar A, Hussain T, Alsarra IA, Yassin AEB. Physical PEGylation enhances the cytotoxicity of 5-fluorouracil-loaded PLGA and PCL nanoparticles. Int J Nanomed. (2019) 14:9259–73. doi: 10.2147/IJN.S223368
114. Chenthamara D, Subramaniam S, Ramakrishnan SG, Krishnaswamy S, Essa MM, Lin F-H, et al. Therapeutic efficacy of nanoparticles and routes of administration. Biomater Res. (2019) 23:20. doi: 10.1186/s40824-019-0166-x
115. Saha D, Kumar S, Ray D, Kohlbrecher J, Aswal VK. Role of physicochemical parameters associated with the hydrophobic vs. amphiphilic biodegradable polymer nanoparticles formation. J. Mol. Liquids. (2020) 318:113977. doi: 10.1016/j.molliq.2020.113977
116. Ways TM, Lau WM, Khutoryanskiy VV. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers. (2018) 10:10030267. doi: 10.3390/polym10030267
117. Peng H, Xiong H, Li J, Chen L, Zhao Q. Methoxy poly (ethylene glycol)-grafted-chitosan based microcapsules: synthesis, characterization and properties as a potential hydrophilic wall material for stabilization and controlled release of algal oil. J Food Eng. (2010) 101:113–9. doi: 10.1016/j.jfoodeng.2010.06.019
118. Rusli JK, Sanguansri L, Augustin MA. Stabilization of oils by microencapsulation with heated protein-glucose syrup mixtures. J Am Oil Chem Soc. (2006) 83:965–72. doi: 10.1007/s11746-006-5054-6
119. Islam MA, Park TE, Reesor E, Cherukula K, Hasan A, Firdous J, et al. Mucoadhesive chitosan derivatives as novel drug carriers. Curr Pharm Des. (2015) 21:4285–309. doi: 10.2174/1381612821666150901103819
120. Fathi M, Donsi F, McClements DJ. Protein-based delivery systems for the nanoencapsulation of food ingredients. Comprehens Rev Food Sci Food Saf. (2018) 17:920–36. doi: 10.1111/1541-4337.12360
121. Chen H, Tan X, Fu Y, Dai H, Wang H, Zhao G, et al. The development of natural and designed protein nanocages for encapsulation and delivery of active compounds. Food Hydrocoll. (2021) 121:107004. doi: 10.1016/j.foodhyd.2021.107004
122. Li H, Zang J, Tan X, Xia X, Wang Z, Du M. Purification ad characterizations of a nanocage ferritin GF1 from oyster (Crassostrea gigas). LWT. (2020) 127:109416. doi: 10.1016/j.lwt.2020.109416
123. Jones JA, Giessen TW. Advances in encapsulin nanocompartment biology and engineering. Biotechnol Bioeng. (2021) 118:491–505. doi: 10.1002/bit.27564
124. Yan Y, Hu J, Yao P. Effects of casein, ovalbumin, and dextran on the astringency of tea polyphenols determined by quartz crystal microbalance with dissipation. Langmuir. (2009) 25:397–402. doi: 10.1021/la8030123
125. Ni Z-J, Wang X, Shen Y, Thakur K, Han J, Zhang J-G, et al. Recent updates on the chemistry, bioactivities, mode of action, and industrial applications of plant essential oils. Trends Food Sci Technol. (2021) 110:78–89. doi: 10.1016/j.tifs.2021.01.070
126. Bai MY, Chang YT, Tsai JC, Wei DJ. Tea tree oil–containing chitosan/polycaprolactone electrospun nonwoven mats: a systematic study of its anti–bacterial properties in vitro. Int J Nanotechnol. 10:959. doi: 10.1504/IJNT.2013.058122
127. Wu C, Wang L, Hu Y, Chen S, Liu D, Ye X. Edible coating from citrus essential oil-loaded nanoemulsions: physicochemical characterization and preservation performance. RSC Adv. (2016) 6:20892–900. doi: 10.1039/C6RA00757K
128. Toragall V., Jayapala N., S., P, M., Vallikanan B. (2020). Biodegradable chitosan-sodium alginate-oleic acid nanocarrier promotes bioavailability and target delivery of lutein in rat model with no toxicity. Food Chem. 330:127195. doi: 10.1016/j.foodchem.2020.127195
129. Freyre-Fonseca V, Delgado-Buenrostro NL, Gutiérrez-Cirlos EB, Calderón-Torres CM, Cabellos-Avelar T, Sánchez-Pérez Y, et al. Titanium dioxide nanoparticles impair lung mitochondrial function. Toxicol Lett. (2011) 202:111–9. doi: 10.1016/j.toxlet.2011.01.025
130. Yu Y, Duan J, Yu Y, Li Y, Sun Z. Silica nanoparticle-induced blockage of autophagy leads to autophagic cell death in HepG2 cells. J Biomed Nanotechnol. (2017) 13:485–99. doi: 10.1166/jbn.2017.2351
131. Wu L, Zhang Y, Zhang C, Cui X, Zhai S, Liu Y, et al. Tuning cell autophagy by diversifying carbon nanotube surface chemistry. ACS Nano. (2014) 8:2087–99. doi: 10.1021/nn500376w
132. Feng SM, Wang LL, Shao P, Sun PL, Yang CS. A review on chemical and physical modifications of phytosterols and their influence on bioavailability and safety. Critic Rev Food Sci Nutr. (2021) 2021:1–20. doi: 10.1080/10408398.2021.1888692
133. Prow TW, Grice JE, Lin LL, Faye R, Butler M, Becker W, et al. Nanoparticles and microparticles for skin drug delivery. Adv Drug Deliv Rev. (2011) 63:470–91. doi: 10.1016/j.addr.2011.01.012
134. Roberts MS, Mohammed Y, Pastore MN, Namjoshi S, Yousef S, Alinaghi A, et al. Topical and cutaneous delivery using nanosystems. J Controll Release. (2017) 247:86–105. doi: 10.1016/j.jconrel.2016.12.022
135. Din FU, Choi JY, Kim DW, Mustapha O, Kim DS, Thapa RK, et al. Irinotecan-encapsulated double-reverse thermosensitive nanocarrier system for rectal administration. Drug Deliv. (2017) 24:502–10. doi: 10.1080/10717544.2016.1272651
136. Yoshimura K, Aoki H, Teruyama C, Iijima M, Tsutsumi H, Kuroda S, et al. A novel hybrid drug delivery system for treatment of aortic. Aneurysms. (2020) 21:5538. doi: 10.3390/ijms21155538
137. Sim T, Han SM, Lim C, Won WR, Lee ES, Youn YS, et al. A pH-sensitive polymer for cancer targeting prepared by one-step modulation of functional side groups. Macromol Res. (2019) 27:795–802. doi: 10.1007/s13233-019-7112-6
138. Cui M, Zhang M, Liu K. Colon-targeted drug delivery of polysaccharide-based nanocarriers for synergistic treatment of inflammatory bowel disease: a review. Carbohydr Polym. (2021) 272:118530. doi: 10.1016/j.carbpol.2021.118530
139. Yu M, Ji N, Wang Y, Dai L, Xiong L, Sun Q. Starch-based nanoparticles: stimuli responsiveness, toxicity, and interactions with food components. Compreh Rev Food Sci Food Saf. (2021) 20:1075–100. doi: 10.1111/1541-4337.12677
140. Sim T, Lim C, Hoang NH, Kim JE, Lee ES, Youn YS, et al. Synergistic photodynamic therapeutic effect of indole-3-acetic acid using a pH sensitive nano-carrier based on poly(aspartic acid-graft-imidazole)-poly(ethylene glycol). J Mater Chem B. (2017) 5:8498–505. doi: 10.1039/C7TB01651D
Keywords: lipid-based nanocarriers, polymer-based nanocarriers, phytochemical, nanoformulation, drug and food application
Citation: Lu H, Zhang S, Wang J and Chen Q (2021) A Review on Polymer and Lipid-Based Nanocarriers and Its Application to Nano-Pharmaceutical and Food-Based Systems. Front. Nutr. 8:783831. doi: 10.3389/fnut.2021.783831
Received: 27 September 2021; Accepted: 12 November 2021;
Published: 01 December 2021.
Edited by:
Mohammad Rezaul Islam Shishir, Shenzhen University, ChinaReviewed by:
Gulzar Ahmad Nayik, Govt Degree College, Shopian, IndiaMd Habban Akhter, DIT University, India
Copyright © 2021 Lu, Zhang, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qihe Chen, Y2hlbnFoQHpqdS5lZHUuY24=; Jinling Wang, d2FuZ2ppbmxpbmdAbmVmdS5lZHUuY24=
 Hongyun Lu
Hongyun Lu Shengliang Zhang1
Shengliang Zhang1 Jinling Wang
Jinling Wang Qihe Chen
Qihe Chen