
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 20 January 2022
Sec. Clinical Nutrition
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.781647
This article is part of the Research Topic Clinical Nutrition and Oncologic Outcomes - Volume 1 View all 28 articles
 Weipu Mao1,2†
Weipu Mao1,2† Keyi Wang1†
Keyi Wang1† Yuan Wu3†
Yuan Wu3† Jinliang Ni4†
Jinliang Ni4† Houliang Zhang4†
Houliang Zhang4† Yidi Wang4†
Yidi Wang4† Zonglin Wu1
Zonglin Wu1 Ruiji Liu2
Ruiji Liu2 Jiang Geng4*
Jiang Geng4* Shuqiu Chen2*
Shuqiu Chen2* Ming Chen2*
Ming Chen2*Background: We conducted a multi-institutional clinical study to assess the prognostic value of the advanced lung cancer inflammatory index (ALI) and modified ALI (mALI) in patients with renal cell carcinoma (RCC).
Methods: We collected 440 patients who underwent laparoscopic nephrectomy at three centers from 2014 to 2019. ALI was defined as body mass index (BMI) × serum albumin (ALB)/neutrophil-to-lymphocyte ratio (NLR) and mALI as L3 muscle index × ALB/NLR. Kaplan-Meier curves, receiver operating characteristic (ROC) curves and Cox survival analysis were used to assess the effect of ALI and mALI on overall survival (OS). In addition, we performed 1:1 propensity score matching (PSM) for the high mALI and low mALI groups to further explore the impact of mALI on survival in RCC patients.
Results: The optimal cut-off values for ALI and mALI were 40.6 and 83.0, respectively. Based on the cut-off values, we divided the patients into high ALI and low ALI groups, high mALI and low mALI groups. ALI and mALI were significantly associated with the AJCC stage, Fuhrman grade, T stage, and M stage. Low ALI (p = 0.002) or low mALI (p < 0.001) was associated with poorer prognosis. ROC curves showed that mALI was a better predictor of OS than ALI. Multivariate Cox regression analysis showed that low mALI (aHR = 2.22; 95% CI 1.19–4.13, p = 0.012) was an independent risk factor for OS in RCC patients who underwent nephrectomy, while ALI (aHR = 1.40; 95% CI 0.73–2.66, p = 0.309) was not significantly associated. Furthermore, after PSM analysis, we found that mALI remained an independent risk factor for OS (aHR = 2.88; 95% CI 1.33–6.26, p = 0.007) in patients with RCC.
Conclusions: For RCC patients undergoing laparoscopic nephrectomy, low ALI and low mALI were associated with poor prognosis, and preoperative mALI can be used as a potential independent prognostic indicator for RCC patients.
Renal cancer, also known as renal cell carcinoma (RCC), is a malignant tumor originating from the urinary tubular epithelium of the renal parenchyma, and its incidence accounts for 2.2% of adult malignancies worldwide (1). Approximately 25–30% of RCC patients have developed locally advanced or metastatic lesions at the time of initial diagnosis (2, 3). For patients with locally advanced and metastatic renal cancer, although targeted drug therapy has achieved certain efficacy and more clinical trials of drugs are ongoing, the overall prognosis is still poor (4, 5). The preferred treatment for early stage non-metastatic RCC remains radical nephrectomy or partial nephrectomy. Early recurrence or metastasis is still found in 20–30% of patients treated with surgery at follow-up (6). Therefore, the search for better prognostic predictors can be of great help in developing individualized follow-up and treatment plans.
A growing number of studies have confirmed the importance of systemic inflammatory response, local immune response and nutritional status in the progression of malignancy and patient prognosis (7–9). Several blood indicators, including neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and lymphocyte-to-monocyte ratio (LMR), have been shown correlate with the prognosis of patients with RCC (10, 11). In addition, indicators reflecting nutritional status, such as serum albumin (ALB), hemoglobin and sarcopenia have been identified as postoperative prognostic factors in patients with RCC (12, 13). Jafri et al. (14) developed the advanced lung cancer inflammation index (ALI) to assess the degree of systemic nutrition and inflammation in patients with metastatic non-small cell lung cancer (NSCLC). The ALI combines body mass index (BMI, kg/m2), serum ALB (g/dL) and NLR and is defined as BMI × ALB/NLR. In addition, Kim et al. (15) replaced BMI with L3 muscle index (cm2/m2) to construct a modified ALI (mALI) score and found that low mALI was an independent prognostic risk factor for shorter overall survival (OS).
In this study, we aimed to assess the prognostic value of ALI and mALI on OS in patients undergoing laparoscopic nephrectomy in a multicenter clinical study.
In this multi-institutional study, we collected 590 patients with RCC who underwent partial or radical nephrectomy between January 2014 and December 2019 at the Department of Urology, Zhongda Hospital Southeast University, the Department of Urology, Shanghai Tenth People's Hospital, and the Department of Urology, Shidong Hospital. All patients were operated by the most experienced urologists in that hospital. Exclusion criteria were as follows: patients combined with other malignancies; patients received other anticancer treatments before surgery; patients lacked complete medical records or were lost to follow-up; patients lacked preoperative laboratory test data. We excluded 150 patients, resulting in 440 patients included in the final study. The methodology of this study followed the criteria in the Declaration of Helsinki (revised in 2013) and received ethical approval from the Ethics Committee of Zhongda Hospital Southeast University (ZDKYSB077) and Ethics Committee of Shanghai Tenth People's Hospital of Tongji University (SHSY-IEC-BG/02.04/04.0-81602469).
Clinicopathological features, laboratory test data, and imaging results for all patients were available from the electronic medical record. Laboratory test data were measured 2 days prior to surgery or closest to the time of surgery, and laboratory data included serum ALB (g/dL), neutrophils, and lymphocytes. The L3 muscle index (cm2/m2) in the imaging results was determined based on our previous study (13). In addition, we included gender, age, BMI [weight (kg)/height2 (m2)], cardiovascular disease, diabetes, hypertension, smoking, type of surgery, laterality, AJCC stage, T stage, N stage, M stage, and Fuhrman grade. OS was calculated from the date of surgical treatment to the date of last follow-up or death.
Categorical variables were expressed as numbers and percentages and analyzed with chi-square tests. As previously described, ALI = BMI × ALB/NLR, and mALI = L3 muscle index × ALB/NLR. Optimal cut-off values for ALI and mALI were determined using X-tile software (version 3.6.1). Kaplan-Meier curves were used to assess the effect of ALI and mALI on OS. Receiver operating characteristic (ROC) curves were used to compare the effect of ALI and mALI's predictive ability on OS and was calculated using the area under the curve (AUC). Univariate and multivariate Cox regression were used to assess the relationship between ALI, mALI and OS, and the associated adjusted hazard ratio (aHR) and 95% confidence interval (CI) were calculated. In multivariate Cox regression analysis, three models were constructed to further assess the relationship between ALI, mALI and OS. Base model: adjusted for age, gender, BMI, hypertension, cardiovascular diseases, diabetes and smoking; core model: base model variables plus surgery type and laterality; extended model: core model variables plus AJCC stage, T stage, N stage, M stage and Fuhrman grade.
Based on the optimal cut-off value of mALI determined by the X-tile software, we divided the patients into a high mALI group (n = 216) and a low mALI group (n = 214). Considering the differences in some variables between the two groups, we used the “Matching” package in R software to perform 1:1 propensity score matching (PSM) for the high mALI and low mALI groups, adjusting for gender, age, BMI, cardiovascular disease, diabetes, hypertension, smoking, surgery type, laterality, AJCC stage, T stage, N stage, M stage, and Fuhrman grade to further explore the effect of mALI on OS in patients with RCC. Statistical analyses were performed using SPSS software (version 26.0), Graphpad Prism (version 8.3.0), and R software (version 3.6.2). P value < 0.05 was considered statistically significant.
According to the X-tile software, the optimal cut-off values for ALI and mALI were 40.6 and 83.0, respectively (Figure 1). Based on the cut-off values, we divided the patients into high ALI and low ALI groups, and high mALI and low mALI groups. The clinicopathological characteristics of all patients were shown in Table 1. Chi-square testing showed that ALI was associated with BMI, hypertension, surgery type, AJCC stage, T stage, M stage and Fuhrman grade, whereas mALI was statistically associated with BMI, surgery type, AJCC stage, T stage, M stage and Fuhrman grade. A higher proportion of patients with BMI ≥ 25kg/m2, underwent partial nephrectomy, AJCC I/II stage, T1/T2 stage, M0 stage, and Fuhrman I/II grade were in the high ALI and high mALI groups compared with the low ALI and low mALI groups. In addition, we found that higher T stage, M stage, AJCC stage and Fuhrman grade were associated with lower ALI and lower mALI (Figure 2).
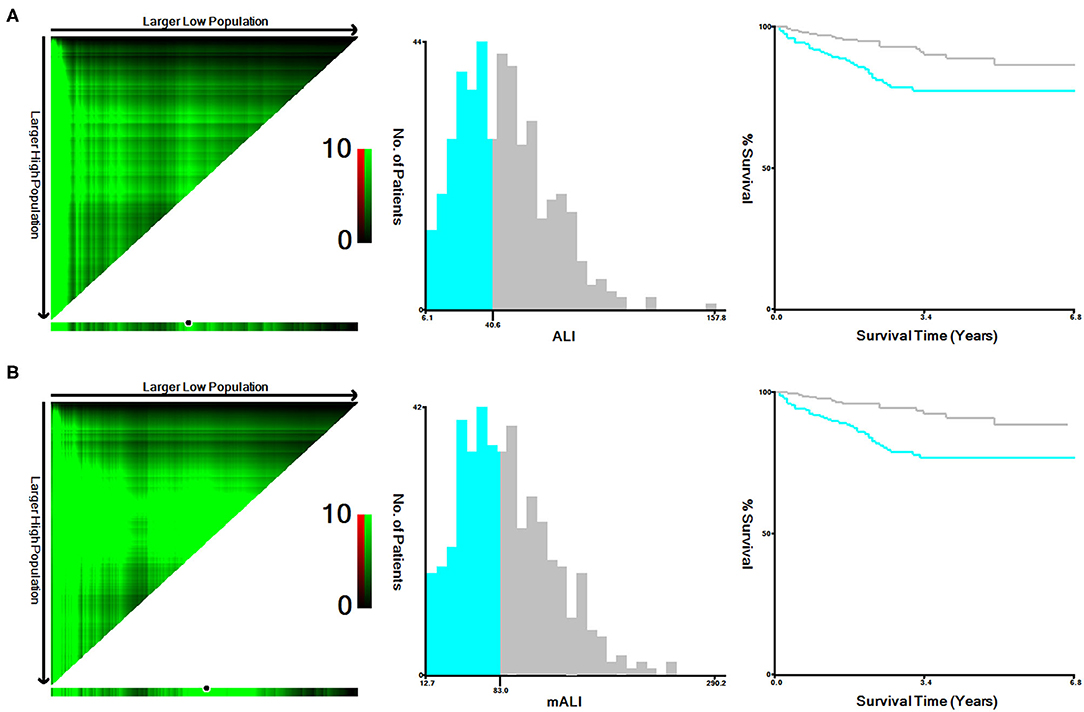
Figure 1. X-tile stratified analysis of ALI and mALI. (A) ALI; (B) mALI. ALI, advanced lung cancer inflammation index; mALI, modified advanced lung cancer inflammation index.
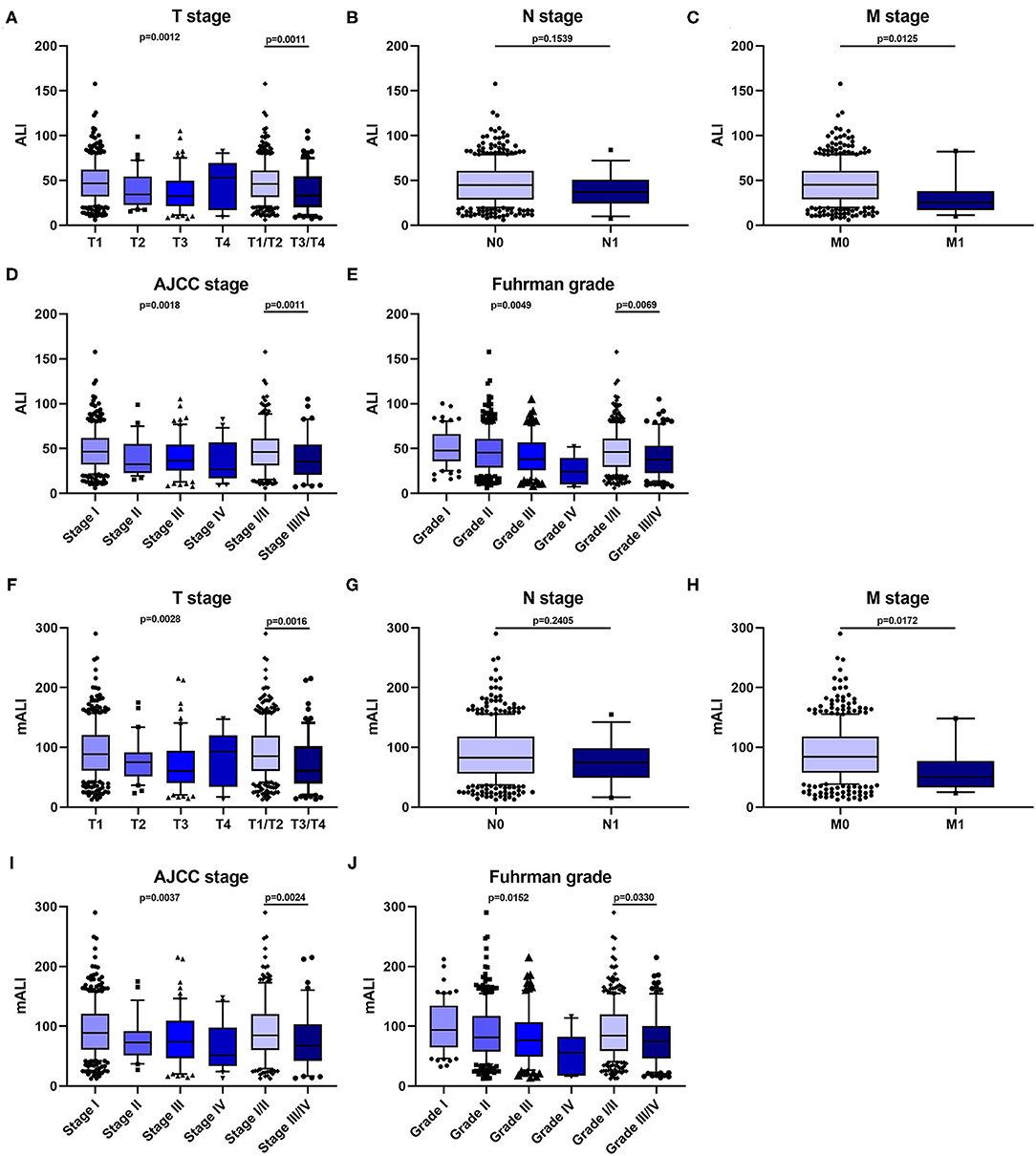
Figure 2. Relationship between patients' clinical characteristics and ALI, mALI. (A–E) Levels of ALI in different T-stage (A), N-stage (B), M-stage (C), AJCC stage (D), and Fuhrman grade (E). (F–J) Levels of ALI in different T-stage (F), N-stage (G), M-stage (H), AJCC stage (I), and Fuhrman grade (J). ALI, advanced lung cancer inflammation index; mALI, modified advanced lung cancer inflammation index.
We performed survival analysis for the high ALI and low ALI groups, as well as for the high mALI and low mALI groups. Kaplan-Meier curves showed that low ALI (p = 0.002) and low mALI (p < 0.001) were associated with worse prognosis (Figure 3). Subsequently, we used ROC curves to assess the predictive ability of ALI and mALI for OS. We found that mALI had a better ability to predict OS than ALI (Supplementary Figure 1). In addition, univariate Cox regression analysis showed that low ALI and low mALI were associated with poorer OS (Table 2). Multivariate Cox regression analysis showed that mALI was consistently an independent risk factor for OS, whether in the basic model (low mALI vs. high mALI: aHR = 3.09; 95% CI 1.68–5.69, p < 0.001), core model (low mALI vs. high mALI: aHR = 2.20; 95% CI 1.17–4.14, p = 0.014) or extended model (low mALI vs. high mALI: aHR = 2.22; 95% CI 1.19–4.13, p = 0.012), while ALI was statistically significant only in the basic model (Table 2).
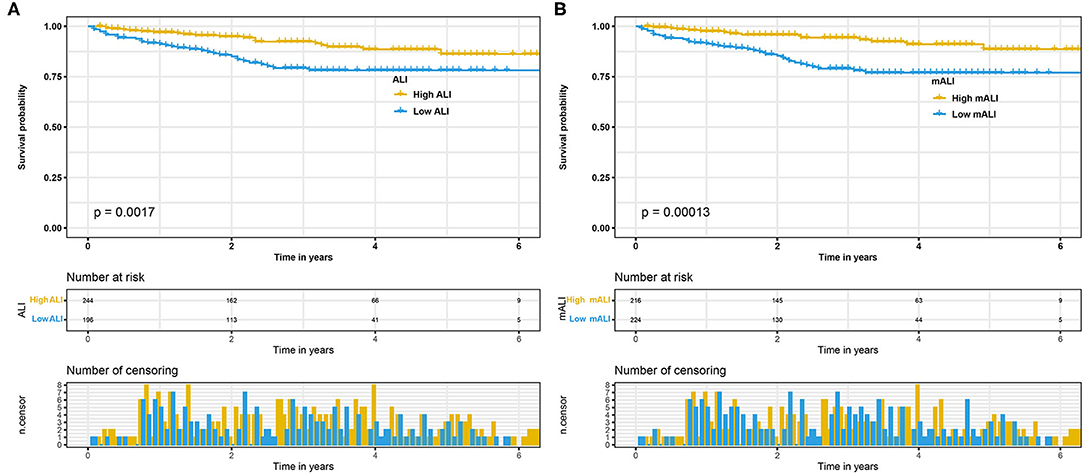
Figure 3. Kaplan-Meier curves for OS stratified by ALI and mALI before PSM. (A) ALI OS; (B) mALI OS. OS, overall survival; ALI, advanced lung cancer inflammation index; mALI, modified advanced lung cancer inflammation index; PSM, propensity score matching.
Considering the effect of other confounding variables, we performed a 1:1 PSM analysis for the high mALI and low mALI groups and adjusted for the 14 variables of gender, age, BMI, cardiovascular disease, diabetes, hypertension, smoking, surgery type, laterality, AJCC stage, T stage, N stage, M stage, and Fuhrman grade (Figure 4). After the PSM analysis, 154 patients were included in the high mALI and low mALI groups, respectively. Clinicopathological characteristics of 308 patients after PSM were shown in Supplementary Table 1. We performed survival analysis in 308 patients and Kaplan-Meier curves still showed that low mALI (p = 0.034) was associated with a poorer prognosis (Figure 5). Univariate and multivariate Cox regression analyses showed that low mALI was associated with a higher risk and that low mALI was associated with a 188% higher risk compared to high mALI in the extended model (aHR = 2.88; 95% CI 1.33–6.26, p = 0.007) (Table 2).
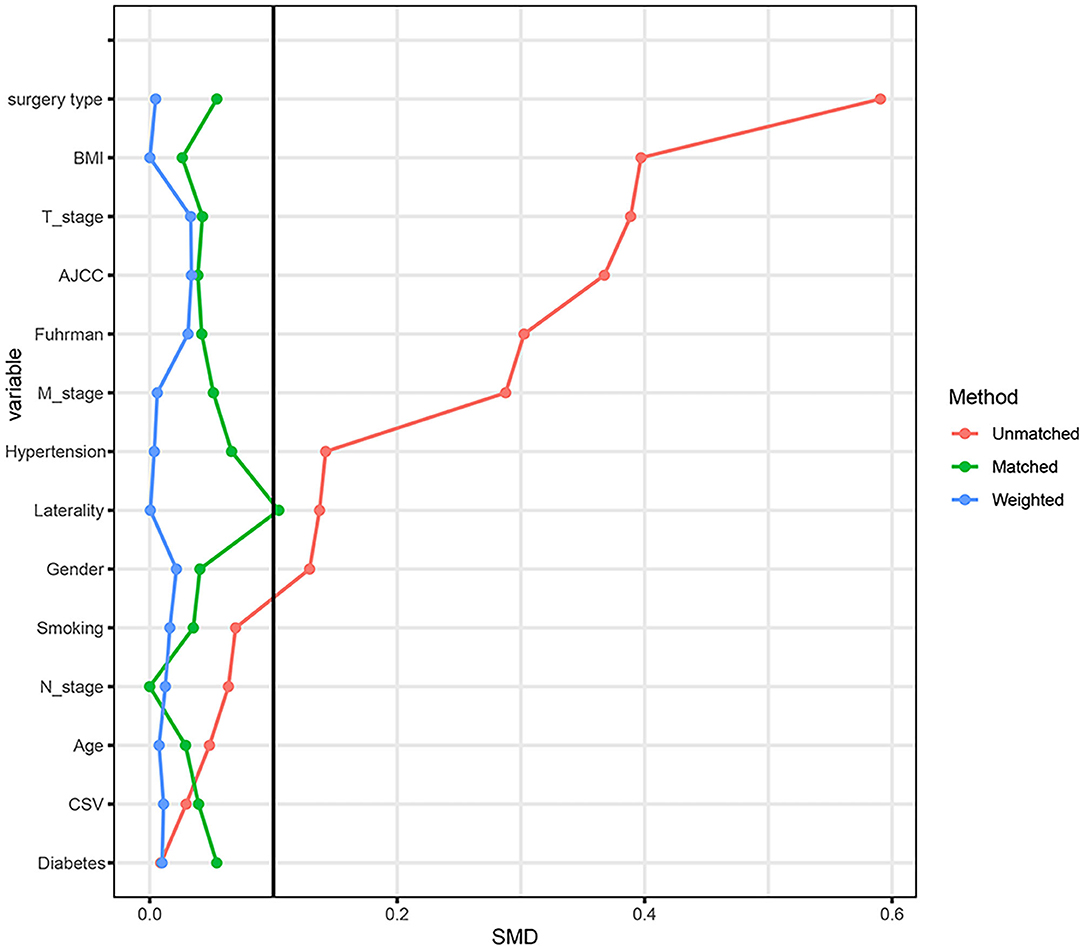
Figure 4. The standardized mean difference (SMD) results of different variables after PSM. PSM, propensity score matching.

Figure 5. Kaplan-Meier curves for OS stratified by mALI after PSM. OS, overall survival; mALI, modified advanced lung cancer inflammation index; PSM, propensity score matching.
Our study is the first study assessing the prognostic value of ALI and mALI in RCC. In this multi-institutional retrospective study, we included clinical data from 440 patients who underwent nephrectomy and used Kaplan-Meier curves, ROC curves, and univariate and multivariate Cox regression analyses to explore the correlation between ALI, mALI, and OS. We found that low ALI and low mALI were correlated with poor prognosis, mALI was an independent risk factor for OS, and mALI was a better predictor of OS in RCC patients than ALI. In addition, we further performed 1:1 PSM on patients in the low mALI and high mALI groups and found that mALI was still an independent risk factor for OS.
L3 muscle index is a common indicator of sarcopenia. Sarcopenia is an age-related syndrome and is considered an emerging indicator that can reflect nutritional status (16). Sarcopenia is defined as an age-related syndrome of reduced skeletal muscle mass, decreased muscle strength and/or decreased physical performance (17). Currently, sarcopenia can be assessed by measuring the L3 lumbar skeletal muscle index (SMI) (18). In recent years, there were increasing evidences that oncology patients often had comorbid sarcopenia. The prevalence of sarcopenia ranged from 20 to 70% in different tumors (19). Studies have shown that as SMI decreases, oncology patients have a poorer prognosis and an increased risk of complications (20). Our previous studies found that sarcopenia was a risk factor for survival time in patients with bladder cancer and RCC (13, 21). Sarcopenia played an important role in the treatment prognosis of oncology patients, and nutritional, exercise and pharmacological interventions for patients with sarcopenia could reduce the occurrence of post-treatment complications and improve the prognosis of patients with oncology (22).
ALB is a product synthesized by the liver and is an important component of human serum protein, which has an important role in the transport and synthesis of substances in the organism. Serum ALB is a common marker used to assess the nutritional status of patients, and low serum ALB level indicates that the patient is malnourished (23). In addition to being an indicator of nutritional status, serum ALB may also be associated with mechanisms of inflammatory response (24). Studies have demonstrated that preoperative low serum ALB levels may be considered as a marker of systemic inflammation and a poor prognostic indicator of survival outcome in cancer patients (25, 26).
Many studies have shown that the development of malignant tumors is closely related to the tumor microenvironment (27). Inflammatory cells, such as neutrophils, lymphocytes, and monocytes, are important components of the tumor microenvironment, and their mediated inflammatory responses can promote tumor cell proliferation, invasion, metastasis, and immune escape (7, 28). The combination of multiple inflammatory cells, such as NLR, PLR and LMR, has been shown to correlate with the prognosis of various cancers (29, 30). NLR is an evaluation indicator reflecting the systemic inflammatory response and is one of the earliest and most classical inflammatory indicators found. The literature reports that preoperative NLR levels are significantly associated with postoperative tumor survival in a variety of solid tumors (31).
In order to better assess patient prognosis, Jafri et al. (14) developed an index (ALI) that could reflect the degree of systemic nutrition and inflammation in patients based on three indicators: BMI, ALB and NLR, and found that low ALI was a poor prognostic indicator for patients with advanced NSCLC. In addition, subsequent studies have shown that low ALI can be used to assess the prognosis of various malignancies, such as small cell lung cancer (SCLC), colorectal cancer and pancreatic carcinoma (32, 33). Considering that BMI cannot directly measure body fat and skeletal muscle content, Kim et al. (15) replaced BMI with L3 muscle index to construct a modified ALI (mALI) score and found that low mALI was an independent prognostic risk factor for SCLC patients OS shortening. In the present study, we compared the predictive ability of ALI and mALI for OS, and found that mALI better predicted OS in RCC patients and that mALI was an independent risk factor for OS.
Despite the positive results obtained in this study, there are several limitations to this study. First, although this study was a three-institution multicenter study, it was still a retrospective study and required an expanded sample for prospective studies. Second, we did not assess patients' quality of life or postoperative nutritional status. Final, we did not include other treatments in the study, which may also have an impact on prognosis.
In general, we found that both ALI and mALI were associated with poor prognosis in patients with RCC, but mALI was a better predictor of OS than ALI, and mALI was an independent prognostic factor for OS in patients with RCC undergoing laparoscopic nephrectomy.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Zhongda Hospital Southeast University (ZDKYSB077) and Ethics Committee of Shanghai Tenth People's Hospital of Tongji University (SHSY-IEC-BG/02.04/04.0-81602469). The patients/participants provided their written informed consent to participate in this study.
WM, JG, SC, and MC: conception and design. JG, SC, and MC: administrative support. WM, KW, YW, and JN: Provision of study materials or patients and manuscript writing. WM, HZ, YW, ZW, and RL: collection and assembly of data. WM and KW: data analysis and interpretation. All authors contributed to the article, final approval of manuscript, and approved the submitted version.
This study was supported by National Natural Science Foundation of China (No. 82070773), Natural Science Foundation of Jiangsu Province (No. BK20201271), Scientific Research Foundation of Graduate School of Southeast University (No. YBPY2173), Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. KYCX21_0156), Jiangsu Provincial Key Research and Development Program (No. BE2019751), Innovative Team of Jiangsu Provincial (No. 2017ZXKJQW07), and National Key Research and Development Program of China (No. SQ2017YFSF090096).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.781647/full#supplementary-material
Supplementary Figure 1. Comparison of area under ROC curves for ALI and mALI in predicting OS. ROC, receiver operating characteristic; OS, overall survival; ALI, advanced lung cancer inflammation index; mALI, modified advanced lung cancer inflammation index.
RCC, renal cell carcinoma; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; ALB, albumin; ALI, advanced lung cancer inflammatory index; mALI, modified advanced lung cancer inflammatory index; BMI, body mass index; OS, overall survival; ROC, receiver operating characteristic; AUC, area under the curve; aHR, adjusted hazard ratio; CI, confidence interval; PSM, propensity score matching; SMI, skeletal muscle index.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. (2015) 67:913–24. doi: 10.1016/j.eururo.2015.01.005
3. Mao W, Wang K, Xu B, Zhang H, Sun S, Hu Q, et al. ciRS-7 is a prognostic biomarker and potential gene therapy target for renal cell carcinoma. Mol Cancer. (2021) 20:142. doi: 10.1186/s12943-021-01443-2
4. Yong C, Stewart GD, Frezza C. Oncometabolites in renal cancer. Nat Rev Nephrol. (2020) 16:156–72. doi: 10.1038/s41581-019-0210-z
5. Bianchi M, Sun M, Jeldres C, Shariat SF, Trinh QD, Briganti A, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. (2012) 23:973–80. doi: 10.1093/annonc/mdr362
6. Tannir NM, Pal SK, Atkins MB. Second-line treatment landscape for renal cell carcinoma: a comprehensive review. Oncologist. (2018) 23:540–55. doi: 10.1634/theoncologist.2017-0534
7. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. (2014) 15:e493–503. doi: 10.1016/S1470-2045(14)70263-3
8. Souza Cunha M, Wiegert EVM, Calixto-Lima L, Oliveira LC. Relationship of nutritional status and inflammation with survival in patients with advanced cancer in palliative care. Nutrition. (2018) 51–52:98–103. doi: 10.1016/j.nut.2017.12.004
9. De Giorgi U, Procopio G, Giannarelli D, Sabbatini R, Bearz A, Buti S, et al. Association of systemic inflammation index and body mass index with survival in patients with renal cell cancer treated with nivolumab. Clin Cancer Res. (2019) 25:3839–46. doi: 10.1158/1078-0432.CCR-18-3661
10. Semeniuk-Wojtas A, Lubas A, Stec R, Syrylo T, Niemczyk S, Szczylik C. Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and c-reactive protein as new and simple prognostic factors in patients with metastatic renal cell cancer treated with tyrosine kinase inhibitors: a systemic review and meta-analysis. Clin Genitourin Cancer. (2018) 16:e685–93. doi: 10.1016/j.clgc.2018.01.010
11. Gu L, Ma X, Xie Y, Li H, Wang L, Chen L, et al. Pretreatment lymphocyte to monocyte ratio is an independent prognostic factor in metastatic clear cell renal cell carcinoma. Clin Genitourin Cancer. (2017) 15:e369–77. doi: 10.1016/j.clgc.2016.12.001
12. Motzer RJ, Bacik J, Schwartz LH, Reuter V, Russo P, Marion S, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. (2004) 22:454–63. doi: 10.1200/JCO.2004.06.132
13. Mao W, Wang K, Zhang H, Lu H, Sun S, Tian C, et al. Sarcopenia as a poor prognostic indicator for renal cell carcinoma patients undergoing nephrectomy in China: A multicenter study. Clin Transl Med. (2021) 11:e270. doi: 10.1002/ctm2.270
14. Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer. (2013) 13:158. doi: 10.1186/1471-2407-13-158
15. Kim EY, Kim N, Kim YS, Seo JY, Park I, Ahn HK, et al. Prognostic significance of modified advanced lung cancer inflammation index (ALI) in patients with small cell lung cancer_ comparison with original ALI. PLoS ONE. (2016) 11:e0164056. doi: 10.1371/journal.pone.0164056
16. Wilson D, Jackson T, Sapey E, Lord JM. Frailty and sarcopenia: the potential role of an aged immune system. Ageing Res Rev. (2017) 36:1–10. doi: 10.1016/j.arr.2017.01.006
17. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afz046
18. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. (2008) 9:629–35. doi: 10.1016/S1470-2045(08)70153-0
19. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc. (2014) 15:95–101. doi: 10.1016/j.jamda.2013.11.025
20. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. (2016) 57:58–67. doi: 10.1016/j.ejca.2015.12.030
21. Mao W, Ma B, Wang K, Wu J, Xu B, Geng J, et al. Sarcopenia predicts prognosis of bladder cancer patients after radical cystectomy: a study based on the Chinese population. Clin Transl Med. (2020) 10:e105. doi: 10.1002/ctm2.105
22. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. (2013) 31:1539–47. doi: 10.1200/JCO.2012.45.2722
23. McMillan DC, Watson WS, O'Gorman P, Preston T, Scott HR, McArdle CS. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer. (2001) 39:210–3. doi: 10.1207/S15327914nc392_8
24. Rodoman GV, Shalaeva TI, Dobretsov GE, Naumov EK, Obolenskii VN. [Serum albumin in systemic inflammatory reaction syndrome]. Anesteziol Reanimatol. (2006) 62–4. doi: 10.1016/j.jhep.2014.04.012
25. Ku JH, Kim M, Choi WS, Kwak C, Kim HH. Preoperative serum albumin as a prognostic factor in patients with upper urinary tract urothelial carcinoma. Int Braz J Urol. (2014) 40:753–62. doi: 10.1590/S1677-5538.IBJU.2014.06.06
26. Han S, Huang Y, Li Z, Hou H, Wu A. The prognostic role of preoperative serum albumin levels in glioblastoma patients. BMC Cancer. (2015) 15:108. doi: 10.1186/s12885-015-1125-0
27. Ji F, Liang Y, Fu SJ, Guo ZY, Shu M, Shen SL, et al. A novel and accurate predictor of survival for patients with hepatocellular carcinoma after surgical resection: the neutrophil to lymphocyte ratio (NLR) combined with the aspartate aminotransferase/platelet count ratio index (APRI). BMC Cancer. (2016) 16:137. doi: 10.1186/s12885-016-2189-1
28. Gu L, Ma X, Li H, Chen L, Xie Y, Zhao C, et al. Prognostic value of preoperative inflammatory response biomarkers in patients with sarcomatoid renal cell carcinoma and the establishment of a nomogram. Sci Rep. (2016) 6:23846. doi: 10.1038/srep23846
29. Dupre A, Malik HZ. Inflammation and cancer: what a surgical oncologist should know. Eur J Surg Oncol. (2018) 44:566–70. doi: 10.1016/j.ejso.2018.02.209
30. Demirdal T, Sen P. The significance of neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and lymphocyte-monocyte ratio in predicting peripheral arterial disease, peripheral neuropathy, osteomyelitis and amputation in diabetic foot infection. Diabetes Res Clin Pract. (2018) 144:118–25. doi: 10.1016/j.diabres.2018.08.009
31. Mei Z, Shi L, Wang B, Yang J, Xiao Z, Du P, et al. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: a systematic review and meta-analysis of 66 cohort studies. Cancer Treat Rev. (2017) 58:1–13. doi: 10.1016/j.ctrv.2017.05.005
32. Hua X, Chen J, Wu Y, Sha J, Han S, Zhu X. Prognostic role of the advanced lung cancer inflammation index in cancer patients: a meta-analysis. World J Surg Oncol. (2019) 17:177. doi: 10.1186/s12957-019-1725-2
Keywords: renal cell carcinoma, advanced lung cancer inflammatory index, modified advanced lung cancer inflammatory index, overall survival, biomarker
Citation: Mao W, Wang K, Wu Y, Ni J, Zhang H, Wang Y, Wu Z, Liu R, Geng J, Chen S and Chen M (2022) Prognostic Significance of Modified Advanced Lung Cancer Inflammation Index in Patients With Renal Cell Carcinoma Undergoing Laparoscopic Nephrectomy: A Multi-Institutional, Propensity Score Matching Cohort Study. Front. Nutr. 8:781647. doi: 10.3389/fnut.2021.781647
Received: 23 September 2021; Accepted: 24 December 2021;
Published: 20 January 2022.
Edited by:
Paula Ravasco, Santa Maria Hospital, PortugalReviewed by:
Bin Fu, The First Affiliated Hospital of Nanchang University, ChinaCopyright © 2022 Mao, Wang, Wu, Ni, Zhang, Wang, Wu, Liu, Geng, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Chen, bWluZ2NoZW5zZXVAMTI2LmNvbQ==; Shuqiu Chen, Y2hlbnNodXFpdXNldUAxNjMuY29t; Jiang Geng, Z2VuZ2ppYW5nc25Ac2luYS5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.