
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Nutr., 25 November 2021
Sec. Clinical Nutrition
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.780205
This article is part of the Research TopicDyslipidemia, Obesity and Coronavirus Disease 2019 (Covid-19)View all 10 articles
Background: Carnitine, a potential substitute or supplementation for dexamethasone, might protect against COVID-19 based on its molecular functions. However, the correlation between carnitine and COVID-19 has not been explored yet, and whether there exists causation is unknown.
Methods: A two-sample Mendelian randomization (MR) analysis was conducted to explore the causal relationship between carnitine level and COVID-19. Significant single nucleotide polymorphisms from genome-wide association study on carnitine (N = 7,824) were utilized as exposure instruments, and summary statistics of the susceptibility (N = 1,467,264), severity (N = 714,592) and hospitalization (N = 1,887,658) of COVID-19 were utilized as the outcome. The causal relationship was evaluated by multiplicative random effects inverse variance weighted (IVW) method, and further verified by another three MR methods including MR Egger, weighted median, and weighted mode, as well as extensive sensitivity analyses.
Results: Genetically determined one standard deviation increase in carnitine amount was associated with lower susceptibility (OR: 0.38, 95% CI: 0.19–0.74, P: 4.77E−03) of COVID-19. Carnitine amount was also associated with lower severity and hospitalization of COVID-19 using another three MR methods, though the association was not significant using the IVW method but showed the same direction of effect. The results were robust under all sensitivity analyses.
Conclusions: A genetic predisposition to high carnitine levels might reduce the susceptibility and severity of COVID-19. These results provide better understandings on the role of carnitine in the COVID-19 pathogenesis, and facilitate novel therapeutic targets for COVID-19 in future clinical trials.
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread across the world and led to substantial morbidity and mortality (1). Global efforts have been invested in treatment options concerning the pandemic, but no effective therapy has been found so far. Although a number of drugs have been put in clinical trials, evidence from a large living systematic review and network meta-analysis suggested that only glucocorticoids, such as dexamethasone, probably reduce mortality and mechanical ventilation in patients with severe COVID-19 (2). Coincidentally, L-carnitine, a potentially promising substitute for dexamethasone, has been found to mimic some of the biological activities of glucocorticoids, especially immunomodulation, while with less side effect (3). L-Carnitine was shown to have antiviral and anti-inflammatory effects in HCV and HIV infections (4, 5). Meanwhile, L-carnitine is an essential nutrient with a major role in cellular energy production (6), while abnormal lipid metabolism is a common symptom of COIVD-19 (7). Furthermore, earlier investigations have suggested that L-carnitine might be a supportive and therapeutic option to prevent the harm caused by COVID-19 based on its molecular functions (8, 9). However, the correlation between carnitine and COVID-19 has not been explored yet, and whether there exists causation is still unknown.
Here, to evaluate the correlation between carnitine level in the body and risk of COVID-19, we employed the two-sample Mendelian randomization (MR) approach to explore the causal role of carnitine on COVID-19 (10, 11). As a result, we found that higher carnitine level was causally associated with decreased susceptibility and severity of COVID-19.
We obtained summary statistics for carnitine from a previous genome-wide association study (GWAS) on human metabolic traits (12). This study analyzed the genetic influences on human blood metabolites using the GWAS method in 7,824 European-ancestry participants. The study design like the collection of samples, quality control procedures and imputation methods have been described in the original publication. Single nucleotide polymorphisms (SNP) that passed the generally accepted genome-wide significance threshold (P < 5E−08) for carnitine were chosen as instrument variants. None of the significant SNPs for carnitine was suggestively associated with body mass index (BMI) searched in GWAS Catalog (13) (P < 1E−05). Then we clumped instrument variables based on 1,000 Genomes Project linkage disequilibrium (LD) structure, and kept index SNPs (R2 < 0.001 with any other associated SNP within 10 Mb) with the lowest P-value. The final summary information for each instrument was shown in Supplementary Tables 1–3.
Summary statistics of COVID-19 susceptibility and severity for the selected instrument variables were obtained from the COVID-19 Host Genetics Initiative (14) (https://www.covid19hg.org/, Release 5). GWAS on COVID-19 susceptibility involved 42,557 patients with COVID-19 and 1,424,707 population controls, while GWAS on COVID-19 severity involved 5,582 very severe respiratory confirmed patients with COVID-19 and 709,010 population controls. Meanwhile, we also analyzed GWAS on COVID-19 hospitalization involving 9,986 hospitalized patients with COVID-19 and 1,877,672 population controls, since hospitalized patients with COVID-19 were mostly with severe symptoms. Harmonization was undertaken to rule out strand mismatches and ensure alignment of SNP effect sizes. The final summary information was shown in Supplementary Tables 1–3.
The current study only utilized publicly available summarized results from published genome-wide association studies. No individual-level data were involved.
We hypothesized that carnitine level as a protective factor could causally decrease the risk of COVID-19, and the following assumptions were satisfied: the genetic variants used as instrumental variables are associated with carnitine level; the genetic variants are not associated with any confounders; the genetic variants are associated with COVID-19 through carnitine level (namely horizontal pleiotropy should not be present).
To comprehensively evaluate the causative effect of carnitine on COVID-19, we performed the two-sample MR analysis using the random effects inverse variance weighted (IVW) method, which is most widely used in MR studies and could provide robust causal estimates under the absence of directional pleiotropy. Meanwhile, we verified the results using another three MR methods, namely Mendelian randomization Egger regression, weighted median and weighted mode. To evaluate potential violations of the model assumptions in the MR analysis, we further conducted comprehensive sensitivity analyses. Cochran's Q test was computed to check heterogeneity across the individual causal effects. Mendelian Randomization Pleiotropy RESidual Sum and Outlier (MR-PRESSO) analysis was conducted to detect outlier instrument (15). MR-Egger regression was performed to evaluate the directional pleiotropy of instruments (16). To evaluate the strength of each instrument variable, we computed the F-statistic of each SNP as described by a previous study (17). Leave-one-out analysis was conducted with the inverse variance weighted method to assess the influence of individual variants on the observed association. And reverse causal inference was conducted to explore whether COVID-19 susceptibility and severity have a causal impact on carnitine level with Steiger analysis (18). The statistical power calculated at http://cnsgenomics.com/shiny/mRnd/ is sufficient (1.00) assuming the true causal OR of carnitine on COVID-19 is 0.8, given the involved sample size and the significance level α as 0.05 (19). The main statistical analyses were conducted using the R package TwoSampleMR 0.5.5 (20).
We analyzed the role of carnitine on COVID-19 using the two-sample MR approach. Results showed that each one standard deviation (1-SD) increase in carnitine amount was associated with a lower risk of COVID-19 (OR: 0.38, 95% CI: 0.19~0.74, P: 4.77E−03), and the results were verified using the weighed median and weighted mode methods (Figure 1A). Meanwhile, higher carnitine amount was associated with lower severity and hospitalization of COVID-19 using three MR methods, though such association was not significant using the IVW method (Figures 1B,C). The funnel plot displays symmetric pattern of effect size variation around the point estimate (Figures 1D–F).
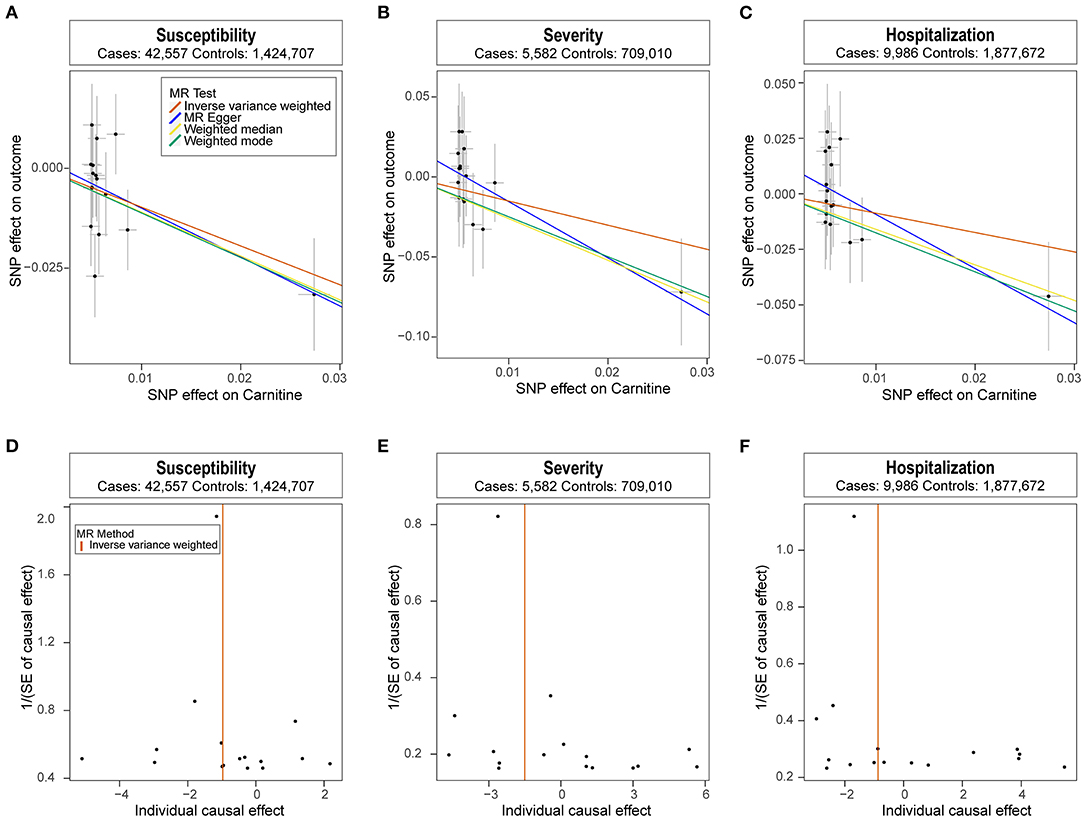
Figure 1. Mendelian randomization analysis results. (A-C) Scatter plot of single nucleotide polymorphism (SNP) potential effects on carnitine level vs. COVID-19. The 95% CI for the effect size on COVID-19 is shown as vertical lines, while the 95% CI for the effect size on carnitine level is shown as horizontal lines. The slope of fitted lines represents the estimated Mendelian randomization (MR) effect per method. (D-F) Forest plot showing results from the Mendelian randomization analysis to evaluate potential causal associations between carnitine and COVID-19. Estimates are per 1 standard deviation (SD) increase in the trait.
Next, we performed extensive sensitivity analyses to validate the causal association between carnitine and COVID-19. No heterogeneity of effects was detected using Cochran's Q test (Table 1). The F statistics of all the instrument variables were above 10 (ranging from 29 to 293), indicating the absence of weakness in the selected instruments. The intercept of MR-Egger is not significantly deviated from zero, suggesting no apparent horizontal pleiotropy (Table 1). Directionality examination by Steiger analysis did not suggest a violation of the causality either. The MR-PRESSO analysis detected no potential instrumental outlier at the nominal significance level of 0.05. The leave-one-out results suggest that the causal effect was not driven by single instrumental variable (Supplementary Figures 1, 2).
Recent studies have proposed the potential role of carnitine as therapy options for COVID-19, but whether there is any correlation between them has not been explored yet. Meanwhile, unmeasured confounding factors in clinical studies can potentially bias the association evidence, as is a common criticism inherent to observational studies. Here, based on results from comprehensive two-sample MR analyses, we demonstrated that higher carnitine level was causally associated with decreased susceptibility and severity of COVID-19.
Carnitine occurs in two forms known as D-carnitine and L-carnitine, and only L-carnitine is biologically active in the body (21). L-carnitine is an essential carrier for long-chain fatty acids from the cytosol through the inner mitochondrial membrane into the matrix, where β-oxidation takes place (22). Although no studies have investigated the correlation between carnitine and COVID-19, previous research on carnitine might provide some insights from molecular aspects of why L-carnitine might protect against COVID-19. L-carnitine plays an important role in fatty acid metabolism and could act as an adjuvant agent in the improvement of dyslipidemia (23). L-carnitine was previously found to increase high-density lipoprotein and lower triglyceride, total cholesterol and low-density cholesterol (23), while dyslipidemia has been shown to be associated with the risk and severity of COVID-19 repeatedly (7, 24). In addition, as an effective antioxidant, L-carnitine was involved in modulating the mechanisms of the immune system and the nervous system (8), and could inhibit the expression of inflammatory factors (25, 26). Antioxidants supplementation has been recommended in therapeutic strategies against COVID-19 (27), since antioxidant therapy could improve oxygenation rates, glutathione levels and strengthen the immune response (28). Meanwhile, anti-inflammatory drugs were suggested to potentially inhibit a key enzyme in the replication and transcription of SARS-CoV-2 (29), and anti-inflammatory agents have also been proposed as potential therapies for COVID-19 due to their prevention of cardiovascular events. Furthermore, L-carnitine plays a critical role in energy production, as it transports long-chain fatty acids into the mitochondria so they can be oxidized to produce energy (30). More energy for the immune system means more immune cells can be produced to protect against infection from virus. Lastly, current evidence showed that severely ill patients with COVID-19 tend to have a high concentration of pro-inflammatory cytokines, such as IL-6, compared to those who are moderately ill, and the high level of cytokines also indicates a poor prognosis in COVID-19 (31). L-carnitine could suppress the production of pro-inflammatory cytokines by preventing the hyperosmolarity-induced oxidative stress (32), and thus might help prevent patients with COVID-19 from cytokine storm. Taken together, so many biological functions make L-carnitine a potential therapeutic option to protect against COVID-19. Unfortunately, no clinical or epidemiological studies have investigated the correlation between them. Here, using the MR approach, we clarified the protective role of carnitine on COVID-19 susceptibility and severity from a genetic perspective. Future clinical or functional studies could put importance to this, and further explore the role of carnitine in protecting against COVID-19.
Based on the Mendelian randomization results obtained from large-scale GWAS summary data, we demonstrated that carnitine might have a protective role on COVID-19, and carnitine might be a therapy which is worth further exploration in clinical trials. These results help better understand the role of carnitine on COVID-19, and will facilitate therapeutic drugs in future clinical trials.
The strength of our work is the Mendelian randomization method which could avoid confounding factors. And comprehensive sensitivity analyses guaranteed the reliability of the association. Meanwhile, current results have very important clinical implications. Up till now, global efforts were still invested in finding effective drugs for COVID-19. Our findings provided some guidance and new direction for future clinical trials. There were also some limitations to the current study. Though we identified a protective role of carnitine against COVID-19, the biological mechanism of this protection was still unclear. Further clinical and functional studies were necessary to clarify the effect of carnitine on COVID-19.
Summary statistics of carnitine could be found in http://mips.helmholtz-muenchen.de/proj/GWAS/gwas/index.php (ID: M15500). Summary statistics of COVID-19 could be downloaded from the COVID-19 Host Genetics Initiative (https://www.covid19hg.org/, release 5). The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Code or algorithm used to generate results in this study are available from the corresponding authors on reasonable request.
CL and HS conceived the study. CL performed the statistical analyses and prepared the drafted manuscript. RO, QW, and HS contributed to writing and editing of the manuscript. All authors reviewed and approved the final manuscript.
This study was supported by the funding of the National Natural Science Foundation of China (81871000 and 81901294), the China Postdoctoral Science Foundation (2019M653424), the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYJC18038), the National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (Grant No. Z20192006), and the Science Foundation of Chengdu Science and Technology Bureau (2019-YF05-00307-SN).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.780205/full#supplementary-material
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; MR, Mendelian randomization; GWAS, genome-wide association study; SNP, single nucleotide polymorphism; LD, linkage disequilibrium; SD, standard deviation; IVW, inverse variance weighted.
1. Li H, Liu SM, Yu XH, Tang SL, Tang CK. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int J Antimicrob Agents. (2020) 55:105951. doi: 10.1016/j.ijantimicag.2020.105951
2. Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. (2020) 370:m2980. doi: 10.1136/bmj.m2980
3. Liu Y, Van Der Leij FR. Long-term effects of neonatal treatment with dexamethasone, L-carnitine, and combinations thereof in rats. Pediatr Res. (2011) 69:148–53. doi: 10.1203/PDR.0b013e318205178b
4. Tsukuda Y, Suda G, Tsunematsu S, Ito J, Sato F, Terashita K, et al. Anti-adipogenic and antiviral effects of l-carnitine on hepatitis C virus infection. J Med Virol. (2017) 89:857–66. doi: 10.1002/jmv.24692
5. Moretti S, Alesse E, Di Marzio L, Zazzeroni F, Ruggeri B, Marcellini S, et al. Effect of L-carnitine on human immunodeficiency virus-1 infection-associated apoptosis: a pilot study. Blood. (1998) 91:3817–24. doi: 10.1182/blood.V91.10.3817
6. Vasiljevski ER, Summers MA, Little DG, Schindeler A. Lipid storage myopathies: Current treatments and future directions. Prog Lipid Res. (2018) 72:1–17. doi: 10.1016/j.plipres.2018.08.001
7. Sorokin AV, Karathanasis SK, Yang ZH, Freeman L, Kotani K, Remaley AT. COVID-19-Associated dyslipidemia: implications for mechanism of impaired resolution and novel therapeutic approaches. FASEB J. (2020). doi: 10.1096/fj.202001451
8. Budhwar S, Sethi K, Chakraborty M. A rapid advice guideline for the prevention of novel coronavirus through nutritional intervention. Curr Nutr Rep. (2020) 9:119–28. doi: 10.1007/s13668-020-00325-1
9. Fakhrolmobasheri M, Khanahmad H, Kahlani MJ, Shiravi AA, Shahrokh SG, Zeinalian M. L-Carnitine Can Extinguish the COVID19 Fire: A Review on Molecular Aspects. Zenodo (2020). Available online at: https://zenodo.org/record/3740145 (accessed April 4, 2020).
10. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. (2008) 27:1133–63. doi: 10.1002/sim.3034
11. Bandres-Ciga S, Noyce AJ, Traynor BJ. Mendelian randomization—a journey from obscurity to center stage with a few potholes along the way. JAMA Neurol. (2020) 77:7–8. doi: 10.1001/jamaneurol.2019.3419
12. Shin SY, Fauman EB, Petersen AK, Krumsiek J, Santos R, Huang J, et al. An atlas of genetic influences on human blood metabolites. Nat Genet. (2014) 46:543–50. doi: 10.1038/ng.2982
13. Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C, et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. (2019) 47:D1005–d12. doi: 10.1093/nar/gky1120
14. COVID-19 Host Genetics Initiative. The COVID-19 Host Genetics Initiative, a global initiative to elucidate the role of host genetic factors in susceptibility and severity of the SARS-CoV-2 virus pandemic. Eur J Hum Genet. (2020) 28:715–8. doi: 10.1038/s41431-020-0636-6
15. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
16. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. (2017) 32:377–89. doi: 10.1007/s10654-017-0255-x
17. Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol. (2016) 40:597–608. doi: 10.1002/gepi.21998
18. Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. (2017) 13:e1007081. doi: 10.1371/journal.pgen.1007081
19. Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. (2013) 42:1497–501. doi: 10.1093/ije/dyt179
20. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife. (2018) 7:e34408. doi: 10.7554/eLife.34408
21. Rebouche CJ. Kinetics, pharmacokinetics, and regulation of L-carnitine and acetyl-L-carnitine metabolism. Ann NY Acad Sci. (2004) 1033:30–41. doi: 10.1196/annals.1320.003
22. Hoppel C. The role of carnitine in normal and altered fatty acid metabolism. Am J Kidney Dis. (2003) 41(4 Suppl 4):S4–12. doi: 10.1016/S0272-6386(03)00112-4
23. Askarpour M, Hadi A, Symonds ME, Miraghajani M, Omid S, Sheikhi A, et al. Efficacy of l-carnitine supplementation for management of blood lipids: a systematic review and dose-response meta-analysis of randomized controlled trials. NMCD. (2019) 29:1151–67. doi: 10.1016/j.numecd.2019.07.012
24. Wei X, Zeng W, Su J, Wan H, Yu X, Cao X, et al. Hypolipidemia is associated with the severity of COVID-19. J Clin Lipidol. (2020) 14:297–304. doi: 10.1016/j.jacl.2020.04.008
25. Lee BJ, Lin JS, Lin YC, Lin PT. Effects of L-carnitine supplementation on oxidative stress and antioxidant enzymes activities in patients with coronary artery disease: a randomized, placebo-controlled trial. Nutr J. (2014) 13:79. doi: 10.1186/1475-2891-13-79
26. Wang S, Xu J, Zheng J, Zhang X, Shao J, Zhao L, et al. Anti-Inflammatory and antioxidant effects of acetyl-L-carnitine on atherosclerotic rats. Med Sci Monit Int Med J Exp Clin Res. (2020) 26:e920250. doi: 10.12659/MSM.920250
27. Derouiche S. Oxidative stress associated with SARS-Cov-2 (COVID-19) Increases the severity of the lung disease—a systematic review. J Infect Dis Epidemiol. (2020) 6:121. doi: 10.23937/2474-3658/1510121
28. Soto ME, Guarner-Lans V, Soria-Castro E, Manzano Pech L, Pérez-Torres I. Is antioxidant therapy a useful complementary measure for Covid-19 treatment? an algorithm for its application. Medicina. (2020) 56:386. doi: 10.3390/medicina56080386
29. Gimeno A, Mestres-Truyol J, Ojeda-Montes MJ, Macip G, Saldivar-Espinoza B, Cereto-Massagué A, et al. Prediction of novel inhibitors of the main protease (M-pro) of SARS-CoV-2 through consensus docking and drug reposition. Int J Mol Sci. (2020) 21:3793. doi: 10.3390/ijms21113793
30. Pietrzak I, Opala G. The role of carnitine in human lipid metabolism. Wiadomosci lekarskie. (1998) 51:71–5.
31. Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine Storm in COVID-19: The current evidence and treatment strategies. Front Immunol. (2020) 11:1708. doi: 10.3389/fimmu.2020.01708
32. Hua X, Deng RZ, Zhang ZD, Su ZT, De-Quan L, Pflugfelder SC. L-Carnitine suppresses the production of pro-inflammatory cytoiknes by preventing the hyperosmolarity-induced oxidative stress in human corneal epithelial cells. Invest Ophthalmol Vis Sci. (2014) 55:3058. doi: 10.3109/02713683.2014.957776
Keywords: carnitine, COVID-19, protective, Mendelian randomization (MR), causation
Citation: Li C, Ou R, Wei Q and Shang H (2021) Carnitine and COVID-19 Susceptibility and Severity: A Mendelian Randomization Study. Front. Nutr. 8:780205. doi: 10.3389/fnut.2021.780205
Received: 20 September 2021; Accepted: 28 October 2021;
Published: 25 November 2021.
Edited by:
Andree Kurniawan, University of Pelita Harapan, IndonesiaReviewed by:
Chandrayani Simanjorang, State Polytechnic of Nusa Utara, IndonesiaCopyright © 2021 Li, Ou, Wei and Shang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huifang Shang, aGZzaGFuZzIwMDJAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.