- 1Department of Epidemiology and Health Statistics, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, China
- 2Clinical Epidemiology Unit, Qilu Hospital of Shandong University, Jinan, China
- 3Clinical Research Center, Qilu Hospital of Shandong University, Jinan, China
- 4State Key Laboratory of Genetic Engineering, Collaborative Innovation Center for Genetics and Development, School of Life Sciences, Fudan University, Shanghai, China
- 5Fudan University Taizhou Institute of Health Sciences, Taizhou, China
- 6Human Phenome Institute, Fudan University, Shanghai, China
- 7Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden
- 8Department of Epidemiology and Health Statistics & Key Laboratory of Ministry of Education for Gastrointestinal Cancer, Fujian Medical University, Fuzhou, China
Background: We aimed to explore the relationship between lifestyle factors, cancer family history, and gastric cancer risk.
Methods: We examined the association between lifestyle factors, cancer family history, and gastric cancer risk based on a population-based case-control study in Taixing, China, with 870 cases and 1928 controls. A lifestyle score was constructed considering body shape, smoking, alcohol drinking, tooth brushing habit, and food storage method. Unconditional logistic regression models were used to calculate odd ratios (ORs) and 95% confidence intervals (CIs).
Results: Compared with participants with a lifestyle score of 0, subjects with a lifestyle score of 1 (OR 0.59, 95%CI 0.43–0.83), 2 (OR 0.42, 95%CI 0.30–0.59), 3 (OR 0.29, 95%CI 0.20–0.41), 4 (OR 0.20, 95%CI 0.13–0.32), or 5 (OR 0.10, 95%CI 0.04–0.22) had a lower risk of gastric cancer (P for trend < 0.001). Overall, 34% of gastric cancer cases (95%CI 27–41%) can be attributed to non-compliance with ≥3 healthy lifestyle. Family history of early-onset cancer is closely related to the occurrence of gastric cancer, with an OR ranging from 1.77 to 3.27. Regardless of family history, a good lifestyle is associated with a reduced risk of gastric cancer, with an OR value between 0.38 and 0.70.
Conclusions: The early-onset cancer family history is closely related to the occurrence of gastric cancer and a good lifestyle is associated with a reduced risk of gastric cancer regardless of family history. Our results provide a basis for identifying and providing behavior guidance of high-risk groups of gastric cancer.
Introduction
Gastric cancer (GC) was the fifth most common cancer in the world in 2020 (1), and more than 40% of new cases and deaths from GC occurred in China (2). Family history is closely related to disease and is widely used to identify high-risk groups of many diseases, including GC (3–5). However, not all people with a family history of malignancy will get GC, which suggests that environmental and lifestyle factors play an important role in the occurrence of GC.
A number of large-scale cohort studies and meta-analyses have examined the relationship between smoking (6), alcohol drinking (7), poor oral hygiene habits (8), BMI and body composition (9, 10), diet (11–15), physical activity (16–18) and the risk of GC. The observed reduced GC risk associated with refrigerator usage also suggests that food storage method may be one of the GC-risk-related lifestyle factors (19). The reality that these lifestyle factors often appear together makes it important to explore their combined effects on GC risk. We assumed that people with healthier lifestyle have a lower GC risk than those with no or fewer healthy lifestyle factors, and the beneficial effects increase with the number of healthy lifestyle factors. Among people with different types of family history of malignancy, exploring the relationship between combined lifestyle factors and GC risk will provide information to develop the behavioral guidance for GC high-risk groups.
However, there were few studies on the relationship between combined lifestyle factors and the risk of GC (20–22). In a prospective cohort study, Jin et al. found that participants with a better lifestyle had a lower risk of GC. Compared with participants with high genetic risk and poor lifestyle, participants with high genetic risk and good lifestyle have a lower risk of GC (21). Family history reflects the common genetic background and common environmental exposure, so the genetic risk represented by the polygenic risk score does not fully represent the family history. Therefore, we still need to explore the relationship between a variety of lifestyle factors and the risk of GC among people with different types of family history of malignancy.
Taixing is one of the areas with high incidence of GC in China. Since 2010, a population-based case-control study aiming at exploring the etiology of upper gastrointestinal cancers has been carried out in Taixing, China. Based on the data collected in this project, we explored the relationship between combined lifestyle factors, family history of cancer, and GC risk, in order to provide bases for the prevention and control of GC.
Materials and Methods
Study Design and Setting
We have previously reported the study design and participant recruitment process in detail (23). In short, we conducted a population-based case-control study in Taixing from October 2010 to September 2013. In order to collect the newly diagnosed GC cases, we recruited cases from the endoscopy units in the four largest local hospitals. Potential missing cases were additionally identified by comparing our case list with the records of the local Cancer Registry. The GC cases included in our study were approximately 75% of the estimated new GC cases during the study period and were a good representative of the local GC patients. The potential controls were randomly selected from the local Population Registry, which provides the basic information of people living in Taixing. Trained staff used a structured electronic questionnaire to conduct face-to-face interviews with participants in the hospitals (for cases) or community (for controls and for cases identified from the local Cancer Registry). In order to reduce potential recall bias, all GC cases were interviewed before they knew their diagnoses.
Participants
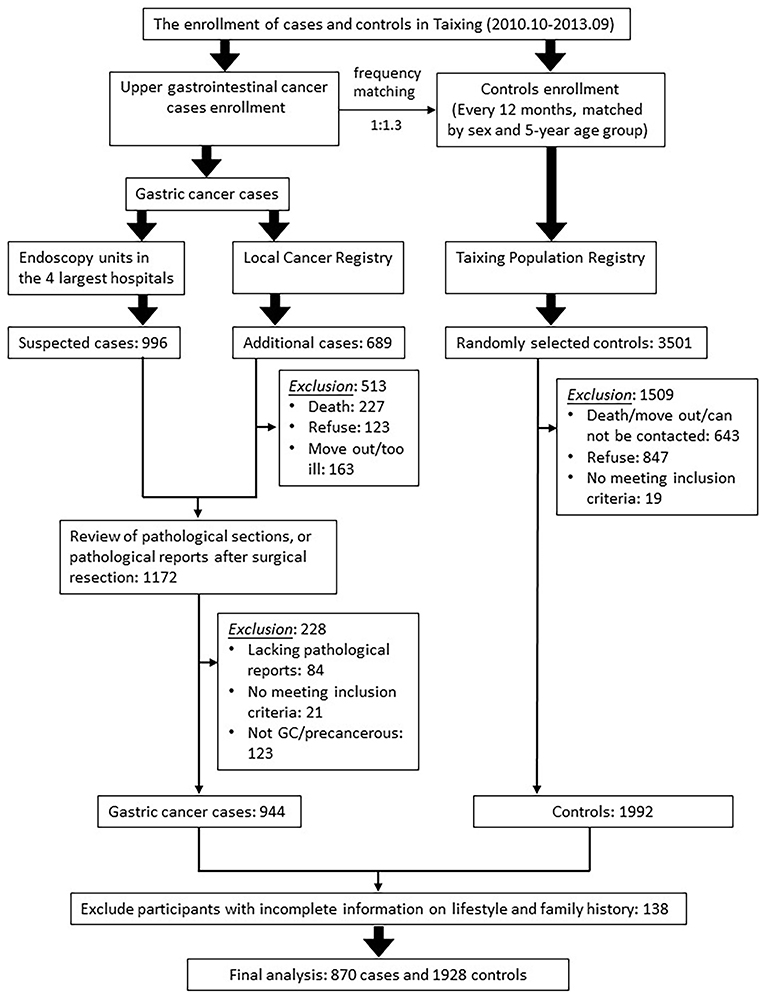
The process of inclusion and exclusion of cases and controls is shown in Figure 1. The basic inclusion criteria for participants were aged 40–85 and lived in Taixing for more than 5 years before the date of diagnosis or interview. The additional inclusion criteria for cases were: first, they were confirmed by pathological diagnosis or endoscopy; second, they have been independently verified by pathologists. The additional inclusion criteria for controls were: first, they were not diagnosed as GC; second, they were randomly selected by the frequency-matched method by gender and the 5-year age group. Based on the response rate of the controls in pilot study (response rate: 75%), we selected controls for the upper gastrointestinal cancer cases (esophageal cancer and GC) at a ratio of 1.3:1. Because the gender and age distribution of patients with esophageal cancer and GC were similar, all qualified controls were included in this analysis. We excluded those participants who were uncooperative or unable to participate in the investigation due to various reasons, such as mental illness. After excluding 138 subjects with missing lifestyle information and cancer family history information, this study finally included 870 GC cases and 1928 controls.
Data Collection
The structured electronic questionnaire includes information on demographics (age, sex, marriage, education, family size), family wealth score, smoking and drinking status, body shape, tooth brushing, food storage method, and family history of malignancy. We defined smoking and drinking as smoking at least one cigarette every 1–3 days for 6 months and consuming alcoholic beverages at least once a week for 6 months, respectively (24). The participants were divided into never smoking/drinking and current smoker/drinker or former smoker/drinker according to their smoking or drinking history. Due to the low prevalence of obesity in Taixing city, we revised the Stunkard's Figure Rating Scale (Supplementary Figure S1), which shows the different body shapes of males and females from extremely thin (body shape 1) to extremely fat (body shape 7 for males and body shape 9 for females). The trained staff showed and introduced the revised Stunkard Graphic Rating Scale briefly, and then asked the participants to choose the closest body type 10 years before the interview. Healthy body shapes were defined as body shape 3 and body shape 4 based on previous studies (23, 25). Participants were asked how many times a day they brushed their teeth and were divided into ≥1/day and ≥2/day groups according to the frequency of tooth brushing. We collected information on food storage method by asking participants to choose the option that was closest to their food storage method 10 years before interview from six options. The six options were using airtight box inside the refrigerator, open box inside the refrigerator, airtight box outside the refrigerator, open box outside the refrigerator, plastic bag, or cloth wrap. We defined using open boxes outside the refrigerator, plastic bag, and cloth wrap as a bad food storage method, and using airtight boxes inside the refrigerator, open boxes inside the refrigerator, and airtight boxes outside the refrigerator as a good food storage method. For family history of cancer, the information we collected includes the number of siblings and children, and the cancer status of their parents, siblings, and children. For those relatives who had cancer, we further collected the information about cancer type and the age of diagnosis. A positive family history of a certain type of cancer is defined as having at least one first-degree relative suffering from the cancer. A positive family history of early onset cancer was defined as having at least one first-degree relative who was diagnosed with the cancer at or before the age of 45 (26). The status of H. pylori infection was obtained by quantitative detection of H. pylori immunoglobulin G antibody using immunoblotting assay (H. pylori IgG Antibody Detection Kit; Syno Gene Digital Technology, Taizhou, China).
Lifestyle Score
After reviewing the literatures, we identified some changeable lifestyle factors related to GC risk, such as smoking, alcohol drinking, oral hygiene habits, food storage method, BMI and body composition, diet, and physical activity. Because the food storage method reflected the carcinogens that might be produced by the food (such as nitrites), and physical activity variables have not been collected in our study, the lifestyle factors finally included in our analysis are smoking, alcohol drinking, toothbrushing, food storage method, and body shape. One point was assigned to participants for the following low risk lifestyle factors: appropriate body type (shape 3/4), never smoking, never drinking, brushing teeth twice a day or more, and good food storage method. We summed the points for the five lifestyle factors to get a healthy lifestyle score which ranges from 0 (least healthy) to 5 (most healthy). Because the median lifestyle score of the participants was 2, we divided the study subjects into two groups (≤2 vs. ≥3) according to their lifestyle scores.
Statistical Analysis
Pearson chi-squared test and Wilcoxon rank-sum test were used to evaluate the difference of distribution of the demographic, lifestyle factors and family history of cancer between case and control groups. The family wealth score was calculated based on the ownership of household appliances by the multiple correspondence analysis and classified according to the quintiles among controls (27). We used unconditional logistic regression models to calculate odds ratios (ORs) and 95% confidence intervals (CIs) to evaluate the association between family history of cancer, lifestyle factors and the risk of GC. We adjusted age (continuous) and sex in model 1 and further adjusted education (illiteracy/primary school/primary high school or above), marriage (unmarried/married/divorced or widowed), family size (<=1/2–3/>3) and family wealth score (Q1–Q5) and H. pylori in model 2. In Model 3, we further adjusted the GC family history, smoking, drinking, toothbrushing, food storage method, and body shape, where appropriate. In addition, we also calculated the adjusted population attributable fractions (PAF) and 95% CIs to estimate the proportion of cases attributable to lifestyle factors and lack of adherence to healthy lifestyle. PAFs were estimated based on the method proposed by Bruzzi (28). This method assumes that the cases and controls are random samples from the study population, and the exposure and confounding information are unbiased. In a case-control study, the number of cases is x and the number of controls is n−x. For exposure k, there are two levels of exposure and no exposure, and the number of cases exposed to k is x1. After adjusting for other confounding factors, the effect of k is OR. The formula for PAF is:
which is equivalent to the formula proposed by Miettinen (29).
The 95% CIs were estimated by the bootstrap method. We sampled 1000 bootstrap samples using replacement sampling, and estimated the PAF of each bootstrap sample. The 2.5th and 97.5th percentiles of PAFs of the bootstrap samples form a good approximation of the 95% confidence interval (30). Sensitivity analysis was conducted by excluding cases from the local Cancer Registry. All analyses were performed using Stata software (version 16.0). Two-sided P-values less than 0.05 were considered statistically significant.
Result
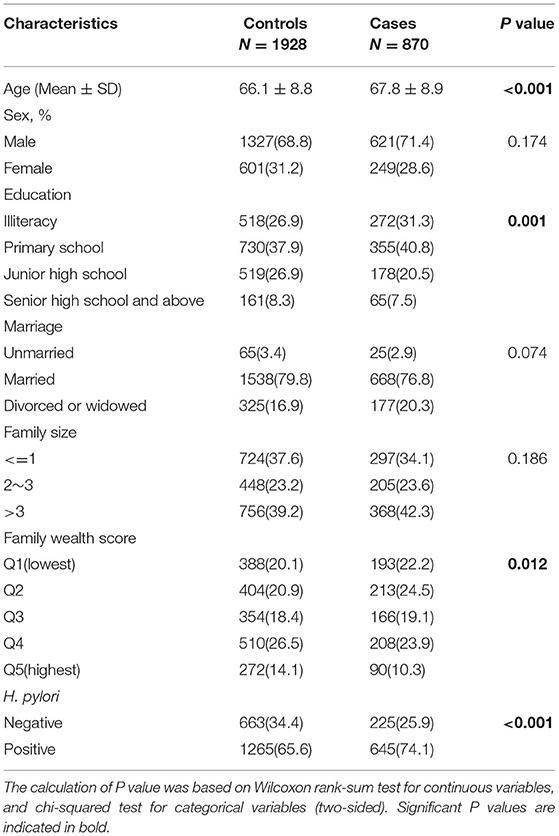
Table 1 presents the basic information of cases and controls. The mean age of cases was slightly higher than that of controls, with 67.8 years for GC cases and 66.1 years for controls. Compared with controls, cases had lower education level, lower family wealth score, and were more likely to have H. pylori infection. There were no significant differences between cases and controls in terms of sex, marital status and family size.
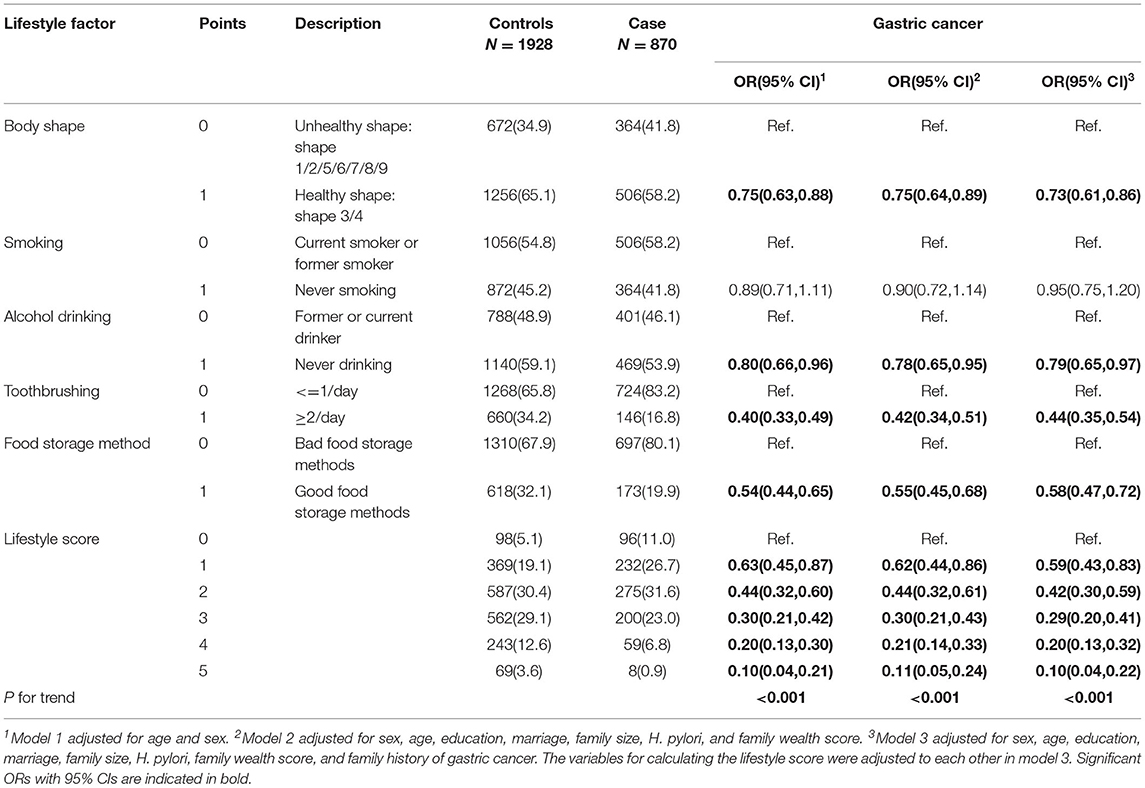
Table 2 shows the association between lifestyle factors, lifestyle score and the risk of GC. We observed that most individual lifestyle factors were associated with a reduced GC risk in the multivariate analysis: appropriate body type (OR 0.73, 95%CI 0.61–0.86), never drinking (OR 0.79, 95%CI 0.65–0.97), brushing teeth twice a day or more (OR 0.44, 95%CI 0.35–0.54), and good food preservation methods (OR 0.58, 95%CI 0.47–0.72). A good lifestyle was associated with a reduced GC risk in a dose-response pattern (P for trend < 0.001). Participants with five good lifestyle factors had only one-tenth GC risk (95%CI 0.04–0.22) compared with participants without any good lifestyle factor. However, we did not observe an association between smoking and GC risk. We further explored the relationship between food storage method and the risk of GC and found that storing food in airtight containers and low temperatures were associated with reduced GC risks, with ORs of 0.53 (95%CI 0.42–0.67) and 0.70 (95%CI 0.56–0.87), respectively (Supplementary Table S1). We also found that more than 95% of females did not smoke, and more than 70% of males smoked in both cases and controls (Supplementary Table S2).
Table 3 shows the PAFs according to individual and combined lifestyle factors and the risk of GC. We calculated PAFs after converting OR to > 1. The estimated PAFs attributable to non-adherence to healthy lifestyle factors were 12% (95%CI 6–16%) for body shape, 10% (95%CI 1–16%) for alcohol drinking, 47% (95%CI 38–54%) for tooth brushing, and 34% (95%CI 22–43%) for food storage method. In combination, 34% (95%CI 27–41%) of GC cases were attributable to non-adherence to healthy lifestyles (<=2 healthy lifestyle factors).
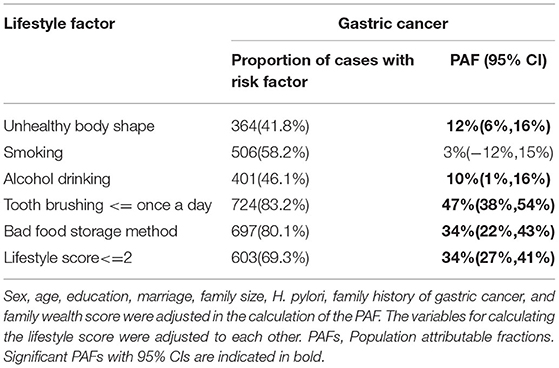
Table 3. PAFs according to individual and combined lifestyle factors and the risk of gastric cancer.
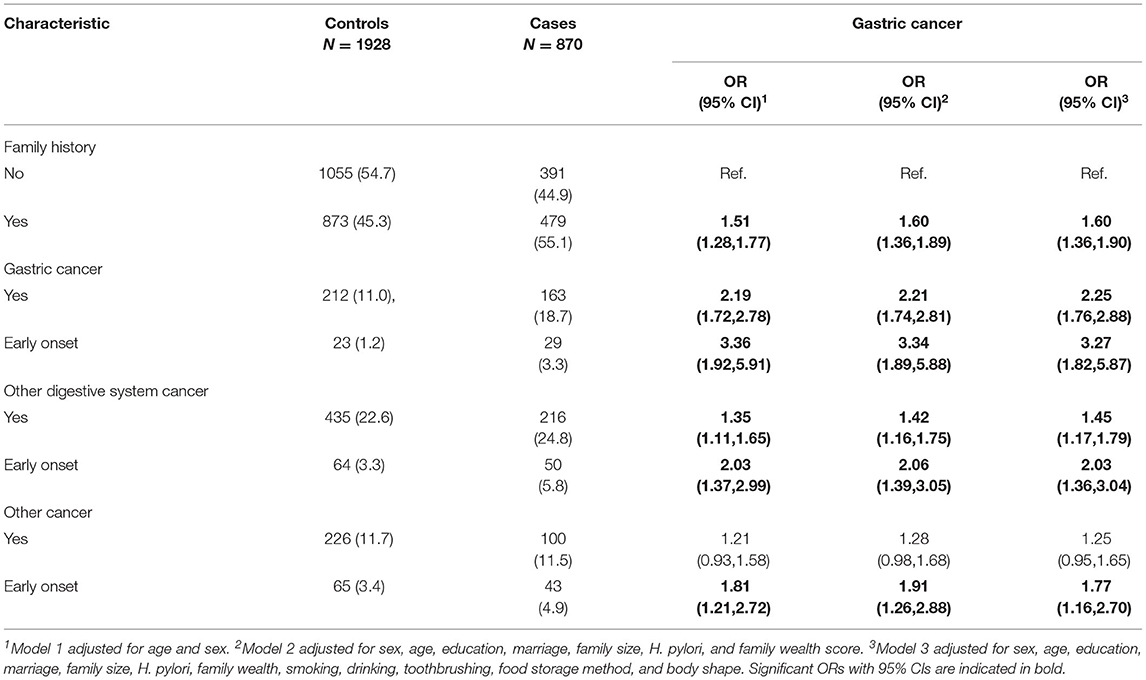
The relationship between family history of cancer and the risk of GC is showed in Table 4. Compared with people with no family history of cancer, people with family history of GC and other digestive system cancers showed an increased risk of GC, with OR of 2.25 (95%CI 1.76–2.88) and 1.45 (95%CI 1.17–1.79) respectively. People with family history of early-onset GC and early-onset other digestive system cancers had even higher risk of GC, with OR of 3.27 (95%CI 1.82–5.87) and 2.03 (95%CI 1.36–3.04) respectively. We did not observe a relationship between GC risk and family history of non-digestive cancers, but we observed that people with an early-onset family history of non-digestive system cancers had an increased risk of GC, with OR of 1.77 (95%CI 1.16–2.70).
We conducted a stratified analysis for the association between lifestyle and the risk of GC. We found that participants with more healthy lifestyle factors (lifestyle score≥3) had a lower risk of GC irrespective of family history of GC (no family history of cancer: OR 0.42, 95%CI 0.32–0.57; with family history of GC: OR 0.54, 95%CI 0.33–0.91; with family history of other digestive system cancers: OR 0.70, 95%CI 0.47–1.06; with family history of non-digestive cancers: OR 0.38, 95%CI 0.19–0.76, respectively) (Table 5). Formal test for heterogeneity of results across strata also revealed no significant interaction between family history of cancer and lifestyle factors (P for interaction: 0.396).
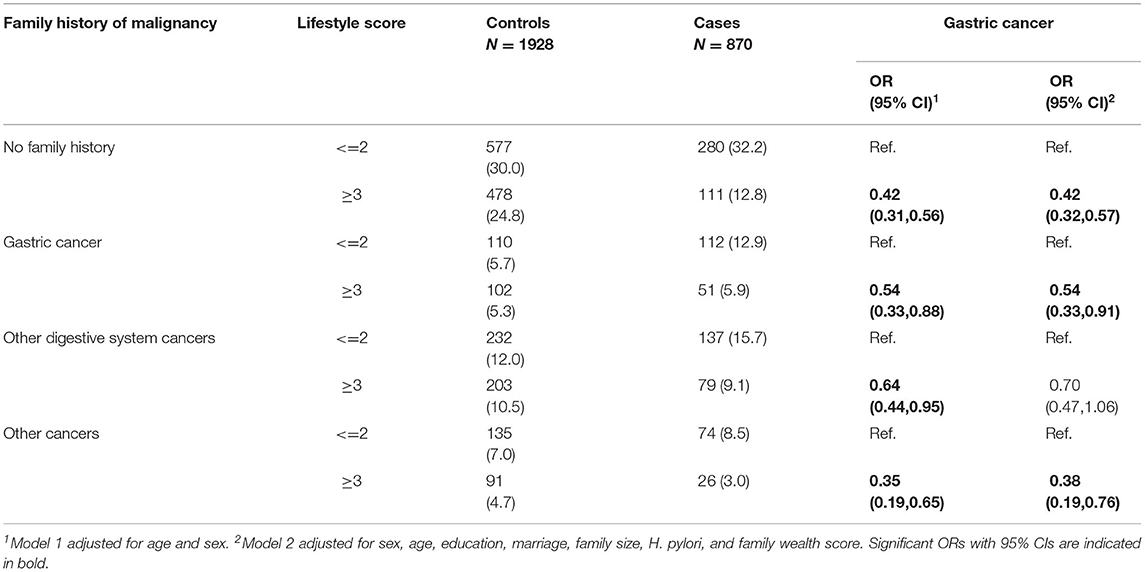
Table 5. Association between lifestyle score and the risk of gastric cancer stratified by family history of malignancy.
We also conducted a sensitivity analysis by excluding cases identified only from the local Cancer Registry, and the results showed no substantial changes (Supplementary Tables S3–S5).
Discussion
Based on a population-based case-control study conducted in Taixing, China, we explored the association between family history of cancer, combined lifestyle factors, and the risk of GC. We found that a family history of cancer was associated with an increased GC risk. The magnitude of the association was strongest for the family history of early-onset GC, followed by the family history of GC, the family history of early-onset other digestive system cancers, the family history of early-onset non-digestive system cancers, and the family history of other digestive system cancers. Regardless of the family history of cancer, a good lifestyle is associated with a lower GC risk. Our findings provide a solid foundation to guide the prevention and control of GC.
We found that a family history of GC was significantly associated with an increased risk of GC, which was consistent with the conclusions of previous studies (31, 32). In addition, we also found that family history of other digestive system cancers and family history of early-onset other cancers were associated with an increased risk of GC. Family history reflects common environmental factors, common lifestyle, and common genetic background. One important risk factor associated with GC and affecting each other among family members is H. pylori infection. The H. pylori infection rate of family members of GC patients is higher than that of the general population, and the precancerous histological changes of the gastric mucosa are more serious in these people (33, 34). In patients with H. pylori infection and with a family history of GC, H. pylori eradication therapy can reduce the risk of GC (35). Other risk factors can also explain part of the association between family history and increased risk of GC. Previous studies have also found an association between genetic background factors such as IL-17 polymorphisms (36) and cell proliferation-related genetic polymorphisms (37) and an increased GC risk.
Most of the interviewees in our study have a relatively low education level, and the memory of weight may not be as accurate as the memory of body shape. Body shape not only reflects the weight information, but also reflects the information of body composition (23). In order to reduce recall bias and incorporate body composition information into the analysis, our study used Stunkard body shape as a component of lifestyle variables (9, 38). Consistent with previous studies, our study also found that a suitable body size is associated with a lower GC risk (10, 39). Inappropriate body shapes include overweight and underweight, which are associated with high and low BMI respectively. High BMI is associated with an increased risk of gastroesophageal reflux, which is a risk factor for gastric cardia cancer (40, 41). In addition, overweight may increase the incidence of cancer, including GC, through insulin resistance, abnormalities of the IGF-I system and signaling, and other pathways (42). Some previous studies also showed that low BMI may be associated with an increased risk of GC (10, 23). Low BMI may be associated with malnutrition and low socioeconomic status, which have been linked with a higher risk of GC (41).
Previous studies have shown that toothbrushing (43) and abstinence from alcohol can reduce the risk of GC (44), and our study supported this conclusion. Poor oral hygiene habits may lead to chronic inflammation, which is related to the occurrence of GC (43). Acetaldehyde, the metabolite of alcohol in the body, is internationally recognized as a Group 1 carcinogen to humans. In addition, alcohol also promotes the occurrence of cancer by changing the absorption and metabolism of carcinogens (45). Previous studies have shown that smoking is a risk factor for GC (41). However, no association between smoking and the GC risk was found in our study, which may be related to the homogeneous smoking habits by sex (more than 75% in males and less than 5% in females).
Our results showed that not putting food in low temperature or airtight containers may be associated with an increased risk of GC, with a high PAF of 34%. Food refrigeration and vacuum preservation can delay the spoilage process of food by preventing or inhibiting the growth of microorganisms (46). In addition, lower food storage temperature will also slow down the accumulation of carcinogens and preserve the beneficial substances in food. Studies have shown that for infant plant-based canned foods, after 24 h of refrigeration and storage at room temperature, the nitrate content increased by an average of 7 and 13%, and after 48 h of storage, the nitrate content increased by 15 and 29%, respectively (47). Higher temperatures will accelerate the aging process of fruits and vegetables and reduce the content of beneficial substances, such as carotenoids (48). These pieces of evidence strongly support our view.
In our study, compared with the people with the unhealthiest lifestyle (0 points), the risk of GC in the people with the healthiest lifestyle (5 points) was only one tenth, and this risk reduction was more eminent than that reported in previous studies (20–22, 49). The difference in the composition of lifestyle scores and the population may explain this observed heterogeneity. We found that a healthier lifestyle was associated with a lower risk of GC regardless of the family history of various cancer types, which was consistent with the finding of Jin and his colleagues (21). Compared with the polygenic risk score, which was used in Jin's study (21), family history was widely used in large-scale population screening programs for its convenience and cheapness. Our results will provide a solid foundation for the development of behavior guidance for GC high-risk groups in practical work.
There were some limitations in our study. First, all the information was collected through questionnaire interviews, and the recall bias was unavoidable. For majority of cases, we conducted interviews before cases knew their diagnoses, which might reduce the recall bias to a certain extent. In addition, we also conducted sensitivity analysis after excluding cases from the local Cancer Registry (interviews were conducted after they knew their diagnoses), and the results remained almost unchanged. This alleviated the concern of recall bias to some extent. Second, based on the case-control study design, the causal relationship between exposure and disease cannot be determined. Third, because the food storage method reflected the carcinogens that might be produced by the food (such as nitrites), and physical activity variables have not been collected in our study, our study did not include these two variables. Fourth, there may be potential biases in the selection of cases and controls. However, in our study, the response rates of both the case and the control were high, and there was no statistical difference in age and gender distribution between non-responders and responders, which can dispel this doubt to a certain extent.
In summary, based on this population-based case-control study conducted in Taixing, we confirmed the close relationship between the family history of cancer, especially the family history of early-onset cancer and the risk of GC. We also found that regardless of cancer family history, a good lifestyle was associated with reduced GC risk. The results of our study provide a solid foundation for the development of the behavior guidance for GC high-risk groups.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of School of Life Sciences, Fudan University, China (date: 19 February 2009), the Ethics Committee of Qilu Hospital of Shandong University, China (date: 8 March 2010), and the Stockholm Ethical Vetting Board, Sweden (2018/357-31). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Study supervision was performed by ML. The first draft of the manuscript was written by JM and YN. All authors contributed to the study conception and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, read, approved the final manuscript, and commented on previous versions of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (81573229, 81973116, and 82173591) and National Key Research and Development Program of China (2017YFC0907003). WY was also supported by a grant from the European Research Council (Consolidator Grant No.: 682663).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank personnel in the Fudan-Taizhou Institute of Health Sciences for their contributions to the recruitment of subjects. We would also like to thank the staff of the Taixing Center for Disease Control and Prevention for the assistance of sample collection. We also appreciate the support of these funds.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.774530/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Liu Q, Zeng X, Wang W, Huang R-L, Huang Y-J, Liu S, et al. Awareness of risk factors and warning symptoms and attitude towards gastric cancer screening among the general public in China: a cross-sectional study. BMJ Open. (2019) 9:e029638. doi: 10.1136/bmjopen-2019-029638
3. Grogan RH. The importance of family history in the management of endocrine disease. Surg Clin North Am. (2019) 99:711–20. doi: 10.1016/j.suc.2019.04.016
4. Kastrinos F, Samadder NJ, Burt RW. Use of family history and genetic testing to determine risk of colorectal cancer. Gastroenterology. (2020) 158:389–403. doi: 10.1053/j.gastro.2019.11.029
5. Kim GH, Liang PS, Bang SJ, Hwang JH. Screening and surveillance for gastric cancer in the United States: Is it needed? Gastrointest Endosc. (2016) 84:18–28. doi: 10.1016/j.gie.2016.02.028
6. Kumar S, Metz DC, Ellenberg S, Kaplan DE, Goldberg DS. Risk factors and incidence of gastric cancer after detection of helicobacter pylori infection: a large cohort study. Gastroenterology. (2020) 158:527–36.e7. doi: 10.1053/j.gastro.2019.10.019
7. Deng W, Jin L, Zhuo H, Vasiliou V, Zhang Y. Alcohol consumption and risk of stomach cancer: a meta-analysis. Chem Biol Interact. (2021) 336:109365. doi: 10.1016/j.cbi.2021.109365
8. Ndegwa N, Ploner A, Liu ZW, Roosaar A, Axell T, Ye WM. Association between poor oral health and gastric cancer: a prospective cohort study. Int J Cancer. (2018) 143:2281–8. doi: 10.1002/ijc.31614
9. Liu AR, He QS, Wu WH, Du JL, Kuo ZC, Xia B, et al. Body composition and risk of gastric cancer: a population-based prospective cohort study. Cancer Med. (2021) 10:2164–74. doi: 10.1002/cam4.3808
10. Merry AHH, Schouten LJ, Goldbohm RA, van den Brandt PA. Body mass index, height and risk of adenocarcinoma of the oesophagus and gastric cardia: a prospective cohort study. Gut. (2007) 56:1503–11. doi: 10.1136/gut.2006.116665
11. Agudo A, Cayssials V, Bonet C, Tjønneland A, Overvad K, Boutron-Ruault MC, et al. Inflammatory potential of the diet and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Am J Clin Nutr. (2018) 107:607–16. doi: 10.1186/ISRCTN12136108
12. Fang X, Wei J, He X, An P, Wang H, Jiang L, et al. Landscape of dietary factors associated with risk of gastric cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. Eur J Cancer. (2015) 51:2820–32. doi: 10.1016/j.ejca.2015.09.010
13. Larsson SC, Bergkvist L, Wolk A. Fruit and vegetable consumption and incidence of gastric cancer: a prospective study. Cancer Epidemiol Biomarkers Prev. (2006) 15:1998–2001. doi: 10.1158/1055-9965.EPI-06-0402
14. Shimazu T, Wakai K, Tamakoshi A, Tsuji I, Tanaka K, Matsuo K, et al. Association of vegetable and fruit intake with gastric cancer risk among Japanese: a pooled analysis of four cohort studies. Ann Oncol. (2014) 25:1228–33. doi: 10.1093/annonc/mdu115
15. Bertuccio P, Alicandro G, Rota M, Pelucchi C, Bonzi R, Galeone C, et al. Citrus fruit intake and gastric cancer: The stomach cancer pooling (StoP) project consortium. Int J Cancer. (2019) 144:2936–44. doi: 10.1002/ijc.32046
16. Abioye AI, Odesanya MO, Abioye AI, Ibrahim NA. Physical activity and risk of gastric cancer: a meta-analysis of observational studies. Br J Sports Med. (2015) 49:224–9. doi: 10.1136/bjsports-2013-092778
17. Psaltopoulou T, Ntanasis-Stathopoulos I, Tzanninis IG, Kantzanou M, Georgiadou D, Sergentanis TN. Physical Activity and gastric cancer risk: a systematic review and meta-analysis. Clin J Sport Med. (2016) 26:445–64. doi: 10.1097/JSM.0000000000000316
18. Xie F, You Y, Huang J, Guan C, Chen Z, Fang M, et al. Association between physical activity and digestive-system cancer: An updated systematic review and meta-analysis. J Sport Health Sci. (2021) 10:4–13. doi: 10.1016/j.jshs.2020.09.009
19. Yan S, Gan Y, Song X, Chen Y, Liao N, Chen S, et al. Association between refrigerator use and the risk of gastric cancer: A systematic review and meta-analysis of observational studies. PLoS ONE. (2018) 13:e0203120. doi: 10.1371/journal.pone.0203120
20. Wang Z, Koh W-P, Jin A, Wang R, Yuan J-M. Composite protective lifestyle factors and risk of developing gastric adenocarcinoma: the Singapore Chinese Health Study. Br J Cancer. (2017) 116:679–87. doi: 10.1038/bjc.2017.7
21. Jin GF, Lv J, Yang M, Wang MY, Zhu M, Wang TOP, et al. Genetic risk, incident gastric cancer, and healthy lifestyle: A meta-analysis of genome-wide association studies and prospective cohort study. Lancet Oncol. (2020) 21:1378–86. doi: 10.1016/S1470-2045(20)30460-5
22. Buckland G, Travier N, Huerta JM, Bueno-de-Mesquita HB, Siersema PD, Skeie G, et al. Healthy lifestyle index and risk of gastric adenocarcinoma in the EPIC cohort study. Int J Cancer. (2015) 137:598–606. doi: 10.1002/ijc.29411
23. Yin X, Yang X, Zhang T, Yuan Z, Chen H, Jin L, et al. Changes of body mass index and body shape in relation to risk of gastric cancer: a population-based case-control study. J Cancer. (2021) 12:3089–97. doi: 10.7150/jca.56149
24. Yang X, Chen X, Zhuang M, Yuan Z, Nie S, Lu M, et al. Smoking and alcohol drinking in relation to the risk of esophageal squamous cell carcinoma: a population-based case-control study in China. Sci Rep. (2017) 7:17249. doi: 10.1038/s41598-017-17617-2
25. Yang X, Zhang T, Yin X, Yuan Z, Chen H, Plymoth A, et al. Adult height, body mass index change, and body shape change in relation to esophageal squamous cell carcinoma risk: a population-based case-control study in China. Cancer Med. (2019) 8:5769–78. doi: 10.1002/cam4.2444
26. Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci. (2020) 21:4012. doi: 10.3390/ijms21114012
27. Gao P, Yang X, Suo C, Yuan Z, Cheng H, Zhang Y, et al. Socioeconomic status is inversely associated with esophageal squamous cell carcinoma risk: Results from a population-based case-control study in China. Oncotarget. (2018) 9:6911–23. doi: 10.18632/oncotarget.24003
28. Bruzzi P, Green SB, Byar DP, Brinton LA, Schairer C. Estimating the population attributable risk for multiple risk-factors using case-control data. Am J Epidemiol. (1985) 122:904–14. doi: 10.1093/oxfordjournals.aje.a114174
29. Miettine.Os. Proportion of disease caused or prevented by a given exposure, trait or intervention. Am J Epidemiol. (1974) 99:325–32. doi: 10.1093/oxfordjournals.aje.a121617
30. D M. Bootstap 101: obtain robust confidence intervals for any statistic. Montreal, Canada: SAS Institute Inc (2004).
31. Song M, Camargo MC, Weinstein SJ, Best AF, Mannisto S, Albanes D, et al. Family history of cancer in first-degree relatives and risk of gastric cancer and its precursors in a Western population. Gastric Cancer. (2018) 21:729–37. doi: 10.1007/s10120-018-0807-0
32. Jiang XJ, Tseng CC, Bernstein L, Wu AH. Family history of cancer and gastroesophageal disorders and risk of esophageal and gastric adenocarcinomas: a case-control study. BMC Cancer. (2014) 14:60. doi: 10.1186/1471-2407-14-60
33. Brenner H, Bode G, Boeing H. Helicobacter pylori infection among offspring of patients with stomach cancer. Gastroenterology. (2000) 118:31–5. doi: 10.1016/S0016-5085(00)70411-2
34. Nam JH, Choi IJ, Cho SJ, Kim CG, Lee JY, Nam SY, et al. Helicobacter pylori infection and histological changes in siblings of young gastric cancer patients. J Gastroenterol Hepatol. (2011) 26:1157–63. doi: 10.1111/j.1440-1746.2011.06717.x
35. Choi IJ, Kim CG, Lee JY, Kim YI, Kook MC, Park B, et al. Family history of gastric cancer and helicobacter pylori treatment. N Engl J Med. (2020) 382:427–36. doi: 10.1056/NEJMoa1909666
36. Long ZW, Yu HM, Wang YN, Liu D, Chen YZ, Zhao YX, et al. Association of IL-17 polymorphisms with gastric cancer risk in Asian populations. World J Gastroenterol. (2015) 21:5707–18. doi: 10.3748/wjg.v21.i18.5707
37. Gao L, Nieters A, Brenner H. Cell proliferation-related genetic polymorphisms and gastric cancer risk: systematic review and meta-analysis. Eur J Hum Genet. (2009) 17:1658–67. doi: 10.1038/ejhg.2009.102
38. Stunkard AJ, Sorensen T, Schulsinger F. Use of the Danish Adoption Register for the study of obesity and thinness. Res Publ Assoc Res Nerv Ment Dis. (1983) 60:115–20.
39. Bae JM. Body mass index and risk of gastric cancer in Asian adults: A meta-epidemiological meta-analysis of population-based cohort studies. Cancer Res Treat. (2020) 52:369–73. doi: 10.4143/crt.2019.241
40. Nandurkar S, Locke GR, Fett S, Zinsmeister AR, Cameron AJ, Talley NJ. Relationship between body mass index, diet, exercise and gastro-oesophageal reflux symptoms in a community. Aliment Pharmacol Ther. (2004) 20:497–505. doi: 10.1111/j.1365-2036.2004.02156.x
41. Smyth EC, Nilsson M, Grabsch HI, van Grieken NCT, Lordick F. Gastric cancer. Lancet. (2020) 396:635–48. doi: 10.1016/S0140-6736(20)31288-5
42. Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metab-Clin Exp. (2019) 92:121–35. doi: 10.1016/j.metabol.2018.11.001
43. Wu H, Zhang J, Zhou B. Toothbrushing frequency and gastric and upper aerodigestive tract cancer risk: a meta-analysis. Eur J Clin Invest. (2021) 51:e13478. doi: 10.1111/eci.13478
44. Ma K, Baloch Z, He TT, Xia X. Alcohol consumption and gastric cancer risk: a meta-analysis. Med Sci Monit. (2017) 23:238–46. doi: 10.12659/MSM.899423
45. Na HK, Lee JY. Molecular basis of alcohol-related gastric and colon cancer. Int J Mol Sci. (2017) 18:1116. doi: 10.3390/ijms18061116
46. Gould GW. Methods for preservation and extension of shelf life. Int J Food Microbiol. (1996) 33:51–64. doi: 10.1016/0168-1605(96)01133-6
47. Tamme T, Reinik M, Roasto M, Meremäe K, Kiis A. Impact of food processing and storage conditions on nitrate content in canned vegetable-based infant foods. J Food Prot. (2009) 72:1764–8. doi: 10.4315/0362-028X-72.8.1764
48. Ngamwonglumlert L, Devahastin S, Chiewchan N, Raghavan V. Plant carotenoids evolution during cultivation, postharvest storage, and food processing: a review. Comprehen Rev Food Sci Food Safety. (2020) 19:1561–604. doi: 10.1111/1541-4337.12564
Keywords: family history, gastric cancer, case-control study, lifestyle factor, food storage
Citation: Man J, Ni Y, Yang X, Zhang T, Yuan Z, Chen H, Chen X, Lu M and Ye W (2021) Healthy Lifestyle Factors, Cancer Family History, and Gastric Cancer Risk: A Population-Based Case-Control Study in China. Front. Nutr. 8:774530. doi: 10.3389/fnut.2021.774530
Received: 12 September 2021; Accepted: 03 December 2021;
Published: 22 December 2021.
Edited by:
Wen-Qing Li, Peking University Cancer Hospital, ChinaCopyright © 2021 Man, Ni, Yang, Zhang, Yuan, Chen, Chen, Lu and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Lu, bHZtaW5nQHNkdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Jinyu Man1,2†
Jinyu Man1,2† Xiaorong Yang
Xiaorong Yang Hui Chen
Hui Chen Xingdong Chen
Xingdong Chen Ming Lu
Ming Lu Weimin Ye
Weimin Ye