- 1Department of Neurology, People's Hospital of Ningxia Hui Autonomous Region, Yinchuan, China
- 2Department of Pulmonary and Critical Care Medicine, Xi'an No.3 Hospital, Xi'an, China
Purpose: The role of allium vegetables or garlic supplements on reducing cancer risk was inconsistent between laboratory study findings and related epidemiologic studies.
Methods: Studies assessing the effect of allium vegetables and garlic supplement consumption on cancer risk were included in our meta-analysis. We used fixed- or random-effects models to pool effect measures to evaluate the highest and lowest consumption. A dose-response regression analysis was used to assess the association between allium vegetables, garlic supplements, and cancer risk.
Results: In a pooled analysis of 22 studies with 25 reports on allium vegetables, a high consumption of allium vegetables showed no significant association with cancer risk (relative risk [RR] = 0.97, 95% confidence interval [CI] 0.92–1.03) in a fixed-effects model. Similarly, garlic supplements were not found to be significantly associated with an increased risk of cancer (RR = 0.97, 95% CI 0.84–1.12) in a random-effects model involving a pooled analysis of 10 studies with 11 reports. Consumption of allium vegetables did not significantly correspond with cancer risk (P for nonlinearity = 0.958, P for linearity = 0.907).
Conclusion: In this meta-analysis, we found no evidence that higher consumption of allium vegetables or garlic supplements reduced the risk of cancer; however, this finding requires further validation.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/#recordDetails, identifier: CRD42021246947.
Introduction
The burden of cancer incidence and mortality is rapidly increasing worldwide. According to estimates from the World Health Organization (WHO), there were 19.3 million new cancer cases and almost 10.0 million cancer deaths in 2020 (https://gco.iarc.fr/today/home). Over the past decade, the search for dietary factors with effects on cancer prevention have led to the rapid expansion of the field of dietary patterns and cancer. Studies of individual nutrients and phytochemicals have revealed associations between certain dietary factors and cancer risk (1). Allium vegetables, mainly garlic, onion, and leeks, contained a high level of organosulfur compounds and flavonoids. Evidence from laboratory studies indicates that allium vegetables have anticancer effects through a variety of pathways (2–5). Therefore, allium vegetable consumption has been regarded as a potential dietary strategy for preventing pan-cancer. However, clinical findings examining the effects of allium vegetables on cancer risk reduction have been inconsistent. Previous systemic reviews and meta-analyses have shown that high consumption of allium vegetables might have a protective effect against cancer (6, 7). However, other studies have found no significant association between allium vegetable consumption and risk of cancer (7, 8).
Garlic supplements, as the most widely used type of allium vegetable-related supplements, were evaluated in the majority of previous meta-analyses. However, some studies have reported that garlic supplements were associated significantly with an increased risk of cancer growth (8, 9). Recently, an additional trial (10) with long-term follow-up assessed the effects of garlic supplements on cancer risk but did not detect any beneficial the benefits of garlic supplements on gastric cancer incidence.
Moreover, most previously published meta-analyses have focused on gastrointestinal cancers, like colorectal and stomach cancer (8, 11). However, to the best of our knowledge, no meta-analysis has comprehensively evaluated the role of allium vegetables or garlic supplements in total cancer prevention. Due to this conflicting evidence, as well as the limitations of previous reviews and the availability of new findings, the systematic review and meta-analysis was conducted to evaluate the effects of allium vegetables and garlic supplements on pan-cancer risk.
Methods
Protocol and Guidance
This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (12). The protocol for this review was registered with PROSPERO (CRD42021246947).
Inclusion and Exclusion Criteria
After preliminary searches, we decided to include prospective studies, and took the pooled analysis of retrospective studies as our next work. Thus, we only considered trials to be eligible if they involved prospective study designs, including cohort, case-cohort, or intervention studies; if they investigated the relationship between allium vegetables or garlic supplements and malignancy risk; and if they provided or allowed for the calculation of relative risks (RR) with 95% confidence intervals (CIs).
We excluded studies if they were designed as nonhuman studies, case reports, or case series or had a retrospective study design, such as a case-control study; if they were editorials, or letters without the analyses of primary data; or, if they did not provide sufficient data; if they did not use an analytic epidemiologic design.
Data Sources and Searches
One of the authors (Q.F. Zhang) performed the search of several databases: Medline, Embase, and Web of Science from inception to May 1, 2021. The following combined search terms were used: MeSH headings and keywords relating to “neoplasms,” the MeSH heading “allium,” and keywords related to the allium vegetables and garlic supplements. Ongoing or unpublished eligible studies were also searched through ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP). To maximize the identification of relevant studies, the reference lists of eligible studies were also reviewed. If multiple studies remained with the same or partially overlapping populations, we extracted the data with the largest sample size or the longest follow-up duration. If duplicate studies offered results related to different outcomes, we included them all in the pooled analysis of specific outcomes. An English-language restriction was also included in our search strategy.
Two researchers (Q. F. Zhang and Q. Zhao) independently screened all titles and abstracts of studies meeting the inclusion criteria. They then obtained full-text versions and performed further screening if studies were deemed eligible. We resolved any disagreements by consensus.
Data Extraction and Quality Assessment
Two independent researchers (Q.F. Zhang and Y. Shen) used a standard form to extract data from the included studies, including first author, publication year, study design, geographic region, sample size (number of cases and total participants), duration of follow-up, demographics of participants, tumor site, exposure, and effect sizes with 95% CIs of highest vs. lowest consumption category. We extracted effect sizes that represented the greatest degree of control for potential confounders. If person-years were not presented, they were using the number of subjects multiplied by the mean duration of follow-up. When a study reported an outcome of interest but without estimates, we tried to contact the author for related data. We resolved any disagreements by consensus.
Two researchers (Y. Shen and F.P. Zhao) independently assessed the quality of included studies by the Newcastle-Ottawa Scale (NOS; http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). Publication bias was assessed qualitatively by visual estimation of funnel plots and quantitatively by calculation of Egger's test (13). We considered P-value less than to represent the possibility of small-study effects.
Data Synthesis and Analysis
The primary outcome for this meta-analysis was the incidence of cancer. We considered the RRs and 95% CI as the effect sizes for all studies. The association between allium vegetables/garlic supplements and cancer risk was quantified by pooling the RRs for the highest vs. lowest consumption category using random-effects or fixed-effects models according to the extent of between-study heterogeneity. The extent of heterogeneity was quantified by the Q test (14) and I2 score (15). If significant heterogeneity was not observed (I2 < 50%), we used a fixed-effects model to pool outcomes; otherwise, a random-effects model was used (I2 ≥ 50%).
Dose-Response Meta-Analysis
To precisely assess the effect of allium vegetable consumption on cancer risk, a random-effects dose-response meta-analysis was performed, as described previously (16). Moreover, we used the restricted cubic splines with four knots at fixed percentiles (5%, 35%, 65%, and 95%) of the distribution to assess the potential curve-linear relationship between allium vegetable consumption and cancer risk (17). For each study, we used the mean or median level of each category consumption for assigning to each corresponding RR. If data were not available, we assigned the midpoint of the upper and lower boundaries of each category as the average consumption.
Meta-Regression, Subgroup, and Sensitivity Analyses
Meta-regression and subgroup analyses were performed to identify potential sources of heterogeneity. We stratified included studies into different subgroups according to the number of cases, sex, location, follow-up period, allium vegetable species, and tumor sites. We conducted further sensitivity analyses by excluding one study per iteration. Because the probability of false-negative results is rather high after the adjustment of multiple testing, we did not adjust p values in the present meta-analysis.
Results
Eligible Studies and Study Characteristics
The search and selection procedures of literature are presented in Supplementary Figure 1. Finally, twenty-two studies on allium vegetables involving 13,677 patients and 10 studies on garlic supplements involving 6,555 patients were included in this meta-analysis. The main characteristics of included studies are summarized in Table 1.
The assessment of risk of bias and quality showed that all included studies had NOS scores of ≥7, indicting a lower risk of bias and better quality.
Highest vs. Lowest Consumption Category
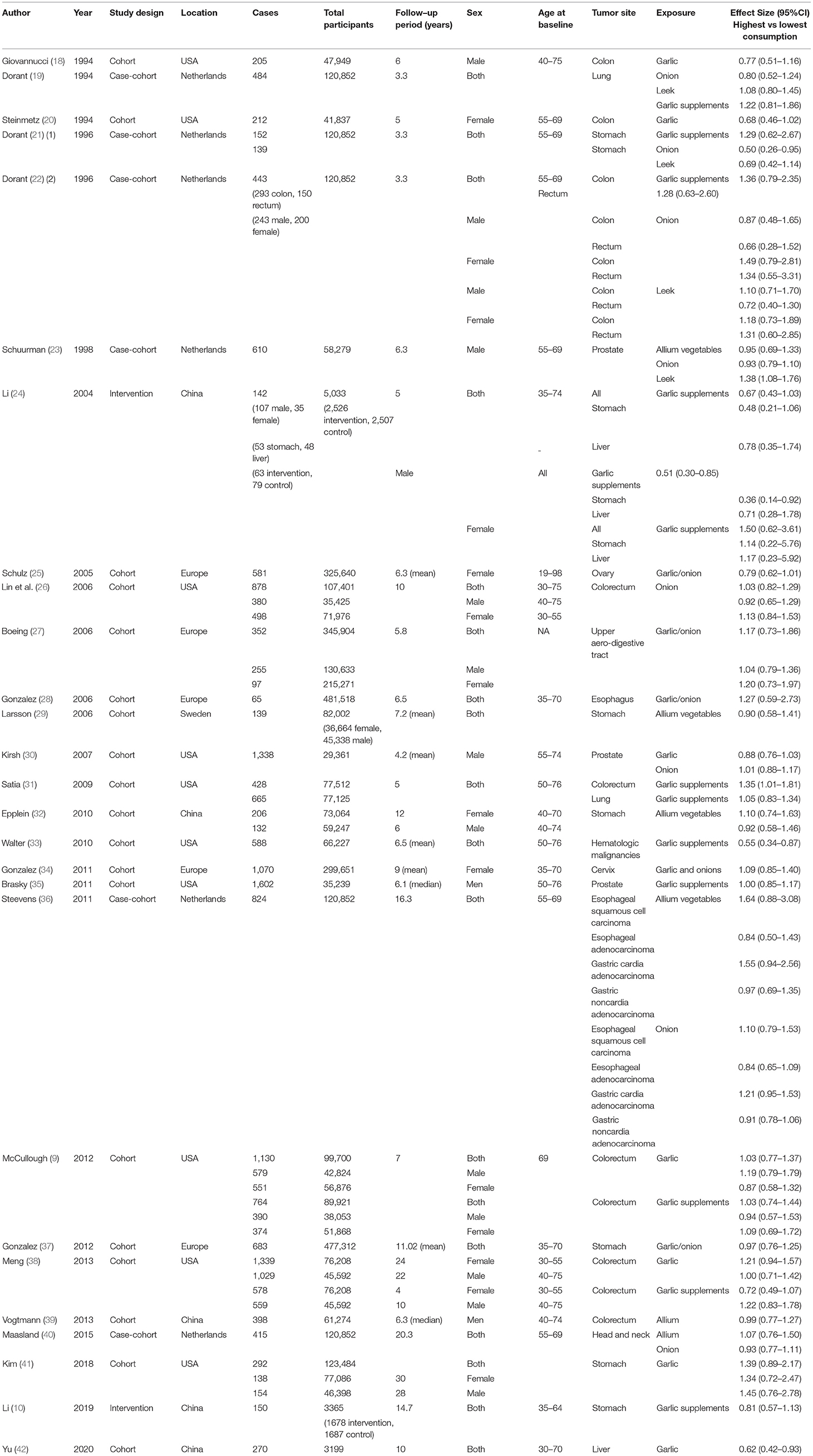
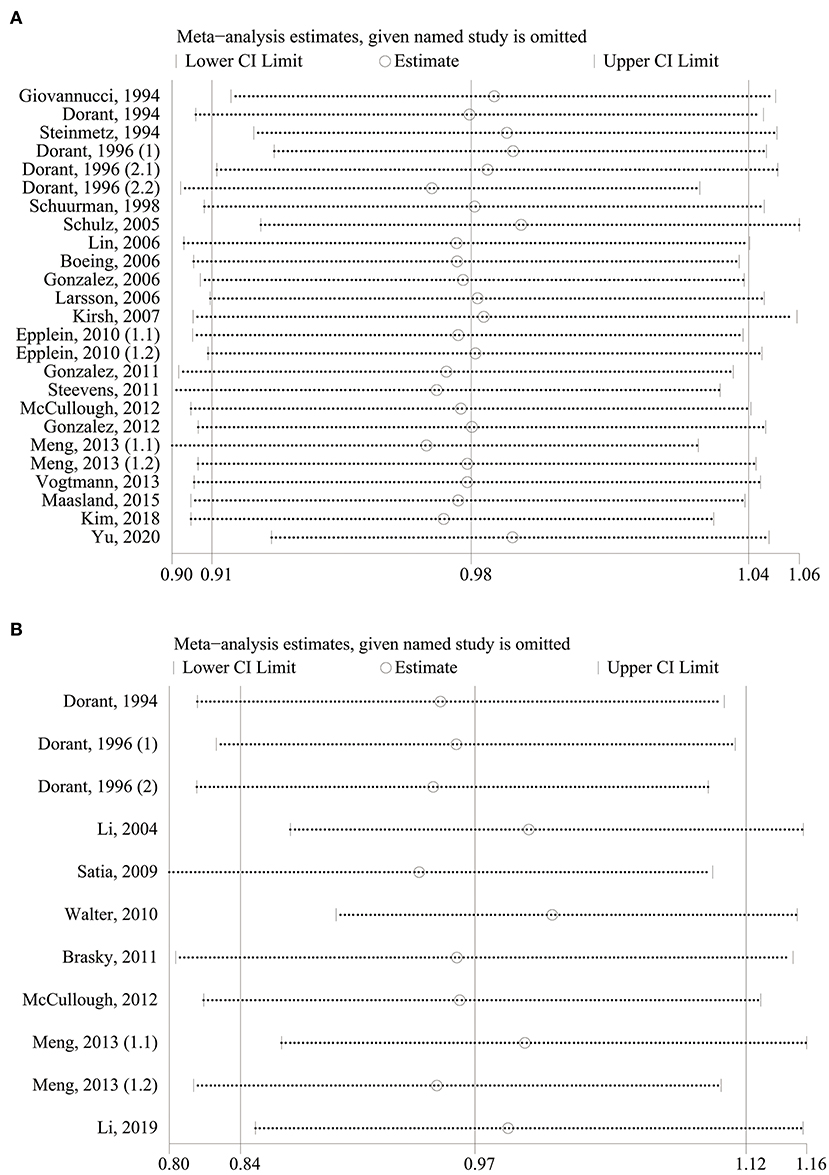
As shown in Figure 1, in the pooled analysis of the 22 studies (9, 18–23, 25–30, 32, 34, 36–42) with 25 reports on allium vegetable consumption, no significant association was observed between a high consumption of allium vegetables and cancer risk (RR, 0.97; 95% CI, 0.92–1.03; P = 0.356; I2 = 22.0%; Ph = 0.161). Garlic supplements were also not found to be significantly associated with an increased risk of cancer (RR, 0.97; 95% CI, 0.84–1.12; P = 0.705; I2 =50.5%; Ph = 0.027) in a random-effects model involving a pooled analysis of 10 studies (9, 10, 19, 21, 22, 24, 31, 33, 35, 38) with 11 reports (Figure 2).
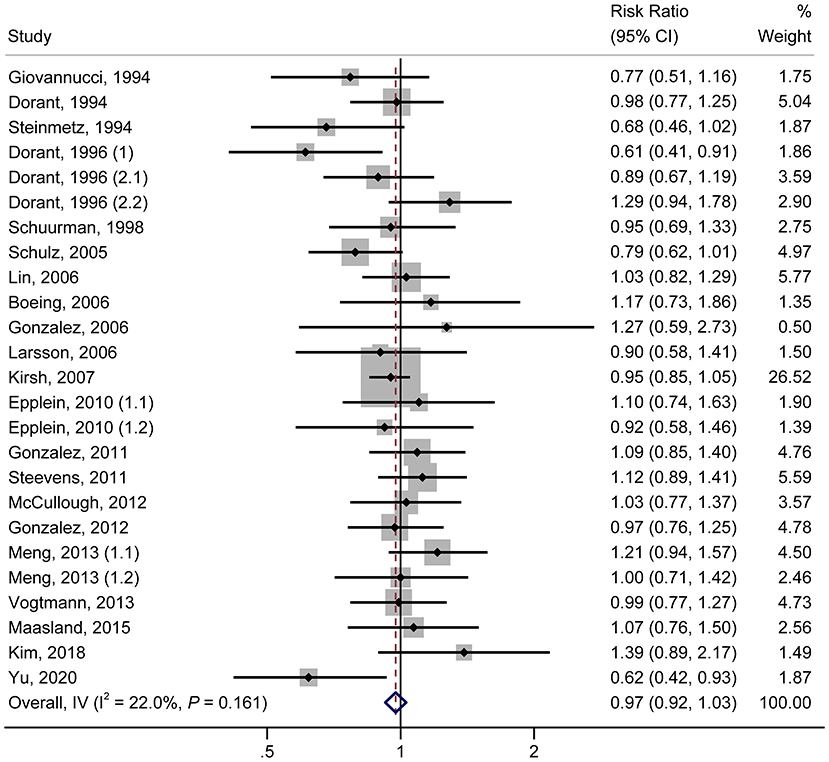
Figure 1. Forest plot of the association between allium vegetable consumption and cancer risk (highest vs. lowest category of allium vegetable consumption).
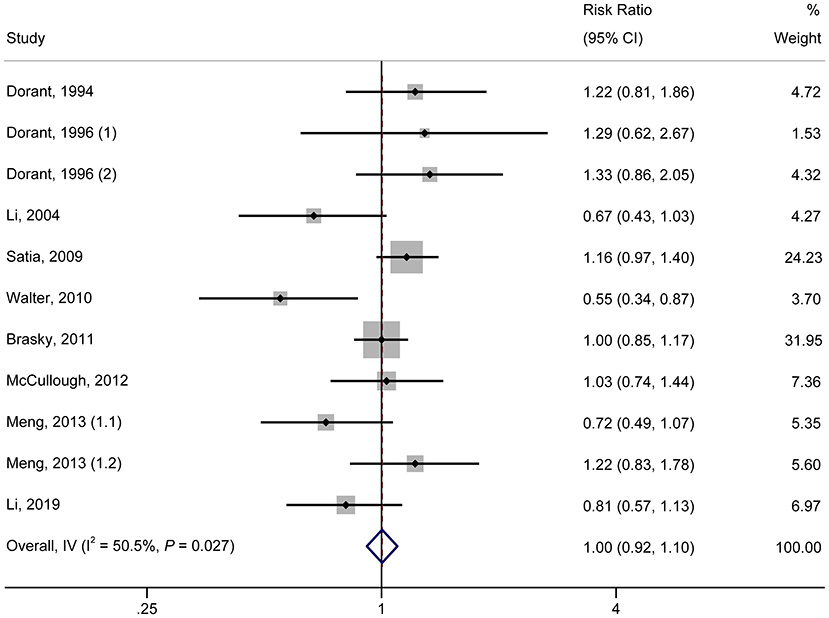
Figure 2. Forest plot of the association between garlic supplement consumption and cancer risk (highest vs. lowest category of supplement consumption).
Dose-Response Meta-Analysis
A random-effects, dose-response meta-analysis was performed to precisely assess the relationship between allium vegetable consumption and risk of cancer. A total of 14 studies (9, 19, 21–23, 26, 29, 30, 32, 38–42) with 20 reports were included. We did not find a curvilinear association between allium vegetable consumption and risk of cancer (P = 0.958 for nonlinearity; P = 0.907 for linearity) (Figure 3).
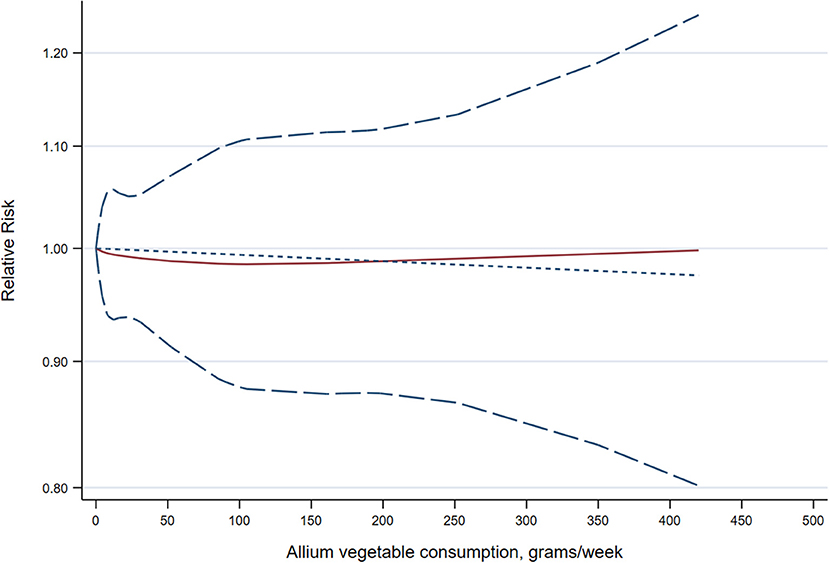
Figure 3. Dose-response meta-analysis of the association between allium vegetable consumption and cancer risk. The solid line represents the linear relationship, and the short-dashed line represents the nonlinear relationship, with the long-dashed lines representing the 95% confidence intervals for the spline model.
Subgroup Analysis
In an analysis of individual allium vegetables, garlic intake was investigated in seven studies (9, 18, 20, 30, 38, 41, 42) with eight reports, which showed no association with cancer risk (RR, 0.93; 95% CI, 0.79–1.09). An analysis of eight studies (19, 21–23, 26, 30, 36, 40) with nine reports related to onion consumption showed that a high amount of onion consumption was not associated with cancer risk (RR, 0.96; 95% CI, 0.90–1.03). Moreover, four studies (19, 21–23) with five reports investigated the consumption of leeks and risk of cancer, with the pooled analysis showing no association between leek consumption and cancer risk (RR, 1.08; 95% CI, 0.88–1.34) (Table 2). Another subgroup analysis based on tumor site showed that allium vegetable consumption was not significantly associated with the risk of cancer in the digestive tract (RR, 1.01; 95% CI, 0.94–1.09) or the non-digestive tract (RR, 0.94; 95% CI, 0.87–1.01).
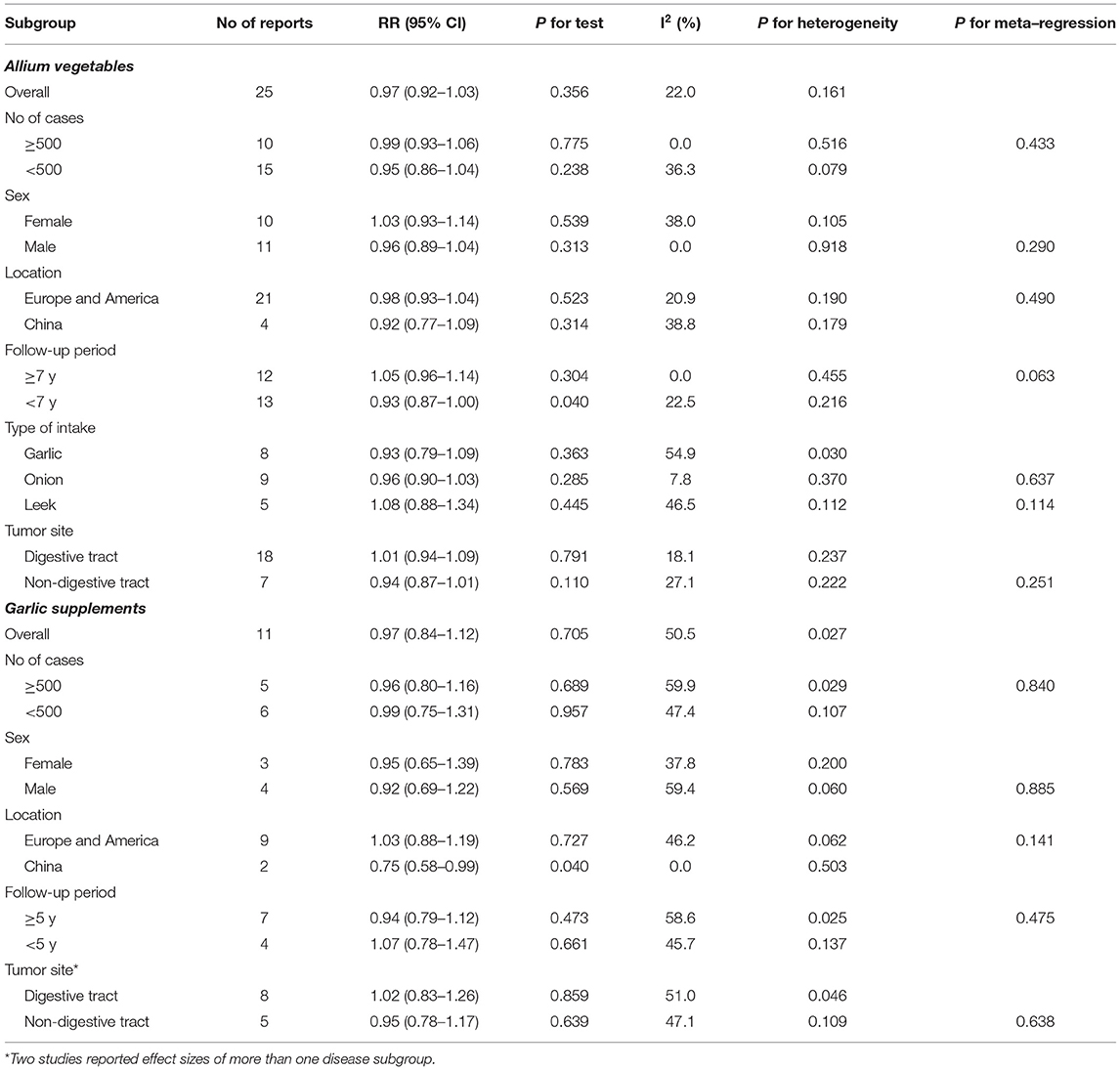
Table 2. Subgroup analysis of the association between allium vegetable and supplement consumption and cancer risk.
The association between allium vegetable consumption and cancer risk were similarly nonsignificant in other subgroup analyses based on the number of cases, sex, and location. However, a stratified analysis based on the duration of follow-up period indicated that a high amount of allium vegetable consumption was marginally associated with a decreased risk of cancer in patients with a follow-up period of less than 7 years (RR, 0.93; 95% CI, 0.87–1.00) (Table 2).
Moreover, in the subgroup analysis of garlic supplements, the consumption of garlic supplements was not associated with cancer risk according to the number of cases, sex, follow-up period, or tumor site. Stratified by study location, however, garlic supplement consumption was marginally associated with a decreased cancer risk in Chinese patients (RR, 0.75; 95% CI, 0.58–0.99); however, only two reports were pooled in this subgroup.
Sensitivity Analysis
As shown in Figure 4, in our sensitivity analysis, the specific RRs from 25 reports for allium vegetable consumption ranged from a low of 0.96 (95% CI, 0.91–1.02; I2 = 17.6%; Ph = 0.219) after omitting the report by Meng, 2013 (1.1) (38) to a high of 0.99 (95% CI, 0.93–1.04; I2 = 17.2%; Ph = 0.225) after omitting the report by Schulz, 2005 (25), but they were, in general, similar. The 11 report-specific RRs for garlic supplement intake ranged from a low of 0.96 (95% CI, 0.86–1.06; I2 = 47.2%; Ph = 0.048) after omitting the report by Satia, 2009 (31) to a high of 1.03 (95% CI, 0.94-1.13; I2 = 34.2%; Ph = 0.134) after omitting the report by Walter, 2010 (33), which were again similar. The results of sensitivity analysis showed that there was no significant variation in the pooled RR after excluding any one of the studies, proving the stability of the overall results.
Meta-Regressions
Our univariate meta-regression analysis found no statistically significant differences, revealing that the analyzed stratification factors were not a source of heterogeneity. However, we did find that the cancer risk was significantly higher in studies with allium vegetable consumption and a longer duration of follow-up (P for interaction = 0.017; Supplementary Figures 2, 3).
Publication Bias
No publication bias was observed in this meta-analysis. No asymmetry were found through a funnel plot analysis. Moreover, Egger's test (P = 0.910 for allium vegetables; P = 0.426 for garlic supplements) detected no significant small-study effects (Supplementary Figures 4, 5).
Discussion
Principal Findings
To our knowledge, the current meta-analysis was the first to assess the effects of allium vegetable and supplement consumption on pan-cancer risk. In our findings, there was no significant effect of higher consumption of allium vegetables on cancer risk. Furthermore, results from a dose-response meta-analysis revealed no nonlinear or linear relationship between allium vegetable consumption and cancer risk. Meanwhile, a subgroup analysis in patients with a follow-up duration of less than 7 years showed that a higher amount of allium vegetable consumption was marginally related to a decreased cancer risk. Our meta-regression analysis also revealed that the follow-up period might be a potential source of heterogeneity. Neither garlic and onion, nor leek consumption were associated with cancer risk. We also found that garlic supplements were not significantly associated with cancer risk. Our findings do not support the conclusion that allium vegetable or supplement intake are protective factors against cancer. Our comprehensive cancer risk assessment based on the consumption of allium vegetables or supplements enables a general understanding of the association between allium vegetable and supplement consumption and cancer risk.
Comparison With Other Studies
Many previous meta-analyses have focused on the association between allium vegetable consumption and risk of gastrointestinal cancers, including colorectal cancer (8, 43) and stomach cancer (6, 44) with inconsistent findings. In 2014, two meta-analyses involving observational studies found a higher consumption of allium vegetables was not associated with colorectal cancer risk (8, 43). Similar nonsignificant results was found in a meta-analysis assessing the effects of allium vegetables on esophageal cancer (7). However, consumption of a large amount of allium vegetables was found to reduce the risk of stomach cancer based on data mainly derived from case-control studies (6, 44). In our pooled analysis of only prospective studies, we found that higher allium vegetable consumption did not reduce the risk of cancer. A subgroup analysis also showed consistently nonsignificant results for different tumor sites, including the digestive tract and non-digestive tract. Though our study did suggest that higher allium vegetable consumption may have a protective role against cancer risk in patients with a follow-up duration of less than 7 years, the statistical difference was marginal for this finding. Our meta-regression analysis also found that cancer risk was significantly higher in studies with allium vegetable consumption in patients with a longer duration of follow-up. The follow-up period may therefore explain the heterogeneity in the pooled RR. We hypothesize that a longer follow-up period may have led to the development of cancer in more patients. Overall, allium vegetable consumption was not associated with cancer risk in the current meta-analysis.
Findings related to garlic, the mostly widely analyzed individual allium vegetable item, and its role in cancer risk has varied across studies and cancer types. A meta-analysis of 18 studies in 2018 revealed that high garlic consumption was associated with a reduced gastric cancer risk (11). Furthermore, its protective effect has been proven for upper aerodigestive tract cancer (7), esophageal cancer (7), and prostate cancer (45). However, conclusions from other meta-analyses did not support a protective role of garlic consumption in head and neck cancer (7), colorectal cancer (46). It is worth noting that previous meta-analyses always combined garlic and garlic supplements, however, garlic supplements have been proven to be significantly associated with an increased risk of certain cancer types, including colorectal cancer (8). In the current meta-analysis, we conducted a subgroup analysis exploring the role of garlic consumption on cancer prevention without garlic supplements, finding no association between higher garlic consumption and reduced cancer risk after pooling the results of eight reports, including five on colorectal cancer (9, 18–20, 38) (1,2), one on stomach cancer [Kim, 2018 (41)], one on prostate cancer [Kirsh, 2007 (30)], and one on liver cancer[Yu, 2020 (42)]. It is worth exploring whether the addition of retrospective studies, such as case-control studies, would have led to different results. However, these pooled results did not support a protective role of garlic in cancer prevention.
In addition to garlic, other individual allium vegetable items have also been investigated for their effects on cancer risk. For example, onions have been demonstrated to be a protective factor against gastric cancer (6), esophageal cancer (7), and laryngeal cancer (7), but not colorectal (43) or prostate (45) cancers. As with garlic, the current meta-analysis showed no protective effects against cancer for onions or leeks.
Garlic supplements contains various mixture that must be prepared at a proper temperature, which have been demonstrated to be associated with toxicity (47). Accumulating evidence indicates important diversity between garlic supplements and raw/cooked garlic (48). Allicin, as the major biologically active element of garlic, is not present in garlic supplement (49). A previous meta-analysis that included five studies with 11 reports on the effects of garlic supplements showed that use of these supplements was significantly associated with an increased risk of colorectal cancer (8); however, another analysis comprising 10 reports demonstrated no significant association (46). In 2019, a blinded randomized placebo-controlled trial enrolled 3,365 residents in a region at high risk for gastric cancer in China (10). In a report of its long-term results, this study revealed that garlic supplements yielded a statistically significant decrease in gastric cancer mortality but a statistically insignificant decrease in incidence. By including the results of those recently published studies, the current meta-analysis provides an independent estimate of the effects of garlic supplements on cancer risk, showing that these supplements play neither a protective nor a harmful role in cancer. Notably, however, our pooled analysis of two reports from China showed inconsistent results, revealing that garlic supplement may decrease the risk of cancer incidence by 25%. However, we speculate that the small sample size with insufficient power generated less stable results.
Although many systematic reviews have studied the association between allium vegetable consumption and cancer risk, some issues still exist and need to be resolved, especially related to the discrepancies in their findings. First, retrospectively designed studies, such as case-control studies, have been enrolled in some previous meta-analyses. In general, case-control studies are more prone to recall and selection biases and are not entirely controlled for confounders, including those known to be protective factors, which may bias the results (8). Second, dietary factors are generally considered to be mainly related to gastrointestinal cancers, although laboratory evidence indicates that some ingredients in allium vegetables may also exert anticancer effects on other cancer types besides colorectal or stomach cancer (2, 50–52). More epidemiologic studies, especially prospective studies, are required to further verify the role of allium vegetables in non-digestive tract cancers. Third, supplements provide the possibility of conducting randomized controlled trials for assessing the effects of allium vegetable on cancer. However, it remains unclear whether specific ingredients in allium vegetables play the major anticancer role or if supplements play the same role as raw allium vegetables. Both of these factors make the results of previously pooled analyses unreliable. To avoid the influence of these factors, the current meta-analysis included prospectively designed studies and separately pooled allium vegetables and supplements. We believe our findings offer more stable and comprehensive evidence about the role of allium vegetables.
Strengths and Limitations
This present meta-analysis had several strengths. Because only prospective studies were included, our findings are unlikely to be influenced by recall or selection bias. Also, unlike most previous studies that assessed only specific cancer types, the associations between allium vegetables and multiple cancer types were examined in our study. Additionally, garlic supplements were analyzed separately from allium vegetables due to differences in their properties and effects. Moreover, our results based on pooled estimates were consistent in stratified analyses across various scenarios, indicating the robustness of our findings. Furthermore, we quantified the association between consumption of allium vegetables and cancer risks by performing linear and non-linear dose-response analyses. Finally, our subgroup, publication bias, and sensitivity analyses demonstrated the reliability and stability of the results.
However, some limitations existed the current meta-analysis. First, the measurements of dose level of allium vegetables or garlic supplements (daily, weekly, monthly, or gram, serving, clove) varied across the included studies, making it impossible to accurately compare equivalent consumption. Also, the included studies only reported use or nonuse, leading to the impossibility of assessing the dose-response relationship between garlic supplements and cancer risk. Second, although we pooled effect sizes adjusting for most established risk factors, the causes of cancer incidence are complicated and sometimes unclear, which may have introduced confounders that could have influenced our findings. Third, many laboratory studies have demonstrated the role of garlic components in inhibiting tumor growth and progression, suggesting that they might also play a role in prolonging the survival of cancer patients. However, whether allium vegetables or supplements could reduce cancer mobility was not analyzed in the current study, limiting the implications of our findings. Last, though prospective studies are less likely to be influenced by recall or selection bias, well-conducted retrospective studies provide less-biased evidence. The addition of retrospective studies in the pooled analysis was believed to provide more detailed information and strength the reliability of the findings, which was also our next work.
Implications
Cancer incidence and mortality is a major burden in most countries worldwide. Currently, not only treatment but also prevention of cancer has been of great concern to prolong lifespan of population. Considering the anticancer effects of certain active compounds present in allium vegetables, such as organosulfur, researchers have shown an increasingly interest in the ability of these substances to prevent cancer. Our findings suggest that neither allium vegetables or supplements have clinically relevant effects on cancer prevention; therefore, there is little evidence to support the idea that allium vegetables or supplements can reduce cancer in incidence. Additional large, randomized controlled trials are needed to confirm these results.
Conclusions
Overall, the current meta-analysis found that higher consumption of allium vegetables or garlic supplements were not associated with a decrease in cancer risk. Randomized controlled trials or well-designed cohort studies are warranted to confirm these findings.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
Material preparation, data collection, and analysis were performed by QifZ, QinZ, YS, FZ, and YZ. The first draft of the manuscript was written by QifZ and QinZ. All authors commented on previous versions of the manuscript, read and approved the final manuscript, and contributed to the study conception and design.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.746944/full#supplementary-material
References
1. Steck SE, Murphy EA. Dietary patterns and cancer risk. Nat Rev Cancer. (2020) 20:125–38. doi: 10.1038/s41568-019-0227-4
2. Chu Q, Lee DT, Tsao SW, Wang X, Wong YC. S-allylcysteine, a water-soluble garlic derivative, suppresses the growth of a human androgen-independent prostate cancer xenograft, CWR22R, under in vivo conditions. BJU Int. (2007) 99:925–32. doi: 10.1111/j.1464-410X.2006.06639.x
3. Xiao J, Xing F, Liu Y, Lv Y, Wang X, Ling MT, et al. Garlic-derived compound S-allylmercaptocysteine inhibits hepatocarcinogenesis through targeting LRP6/Wnt pathway. Acta pharmaceutica Sinica B. (2018) 8:575–86. doi: 10.1016/j.apsb.2017.10.003
4. Katsuki T, Hirata K, Ishikawa H, Matsuura N, Sumi S, Itoh H. Aged garlic extract has chemopreventative effects on 1,2-dimethylhydrazine-induced colon tumors in rats. J Nutr. (2006) 136:847S–51S. doi: 10.1093/jn/136.3.847S
5. Tung YC, Tsai ML, Kuo FL, Lai CS, Badmaev V, Ho CT, et al. Se-Methyl-L-selenocysteine Induces Apoptosis via Endoplasmic Reticulum Stress and the Death Receptor Pathway in Human Colon Adenocarcinoma COLO 205 Cells. J Agric Food Chem. (2015) 63:5008–16. doi: 10.1021/acs.jafc.5b01779
6. Turati F, Pelucchi C, Guercio V, La Vecchia C, Galeone C. Allium vegetable intake and gastric cancer: a case-control study and meta-analysis. Mol Nutr Food Res. (2015) 59:171–9. doi: 10.1002/mnfr.201400496
7. Guercio V, Turati F, La Vecchia C, Galeone C, Tavani A. Allium vegetables and upper aerodigestive tract cancers: a meta-analysis of observational studies. Mol Nutr Food Res. (2016) 60:212–22. doi: 10.1002/mnfr.201500587
8. Zhu B, Zou L, Qi L, Zhong R, Miao X. Allium vegetables and garlic supplements do not reduce risk of colorectal cancer, based on meta-analysis of prospective studies. Clin Gastroenterol Hepatol. (2014) 12:1991–2001 e1–4. doi: 10.1016/j.cgh.2014.03.019
9. McCullough ML, Jacobs EJ, Shah R, Campbell PT, Gapstur SM. Garlic consumption and colorectal cancer risk in the CPS-II Nutrition Cohort. Cancer Causes Control: CCC. (2012) 23:1643–51. doi: 10.1007/s10552-012-0042-7
10. Li WQ, Zhang JY, Ma JL Li ZX, Zhang L, Zhang Y, et al. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: follow-up of a randomized intervention trial. BMJ. (2019) 366:l5016. doi: 10.1136/bmj.l5016
11. Li Z, Ying X, Shan F, Ji J. The association of garlic with Helicobacter pylori infection and gastric cancer risk: A systematic review and meta-analysis. Helicobacter. (2018) 23:e12532. doi: 10.1111/hel.12532
12. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
13. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
14. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
15. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
16. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. (1992) 135:1301–9. doi: 10.1093/oxfordjournals.aje.a116237
17. Harrell FE. Jr, Lee KL, Pollock BG Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. (1988) 80:1198–202. doi: 10.1093/jnci/80.15.1198
18. Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res. (1994) 54:2390–7.
19. Dorant E, van den Brandt PA, Goldbohm RA. A prospective cohort study on Allium vegetable consumption, garlic supplement use, and the risk of lung carcinoma in The Netherlands. Cancer Res. (1994)54:6148–53.
20. Steinmetz KA, Kushi LH, Bostick RM, Folsom AR, Potter JD. Vegetables, fruit, and colon cancer in the Iowa Women's Health Study. Am J Epidemiol. (1994) 139:1–15. doi: 10.1093/oxfordjournals.aje.a116921
21. Dorant E, van den Brandt PA, Goldbohm RA, Sturmans F. Consumption of onions and a reduced risk of stomach carcinoma. Gastroenterology. (1996) 110:12–20. doi: 10.1053/gast.1996.v110.pm8536847
22. Dorant E, van den Brandt PA, Goldbohm RA. A prospective cohort study on the relationship between onion and leek consumption, garlic supplement use and the risk of colorectal carcinoma in The Netherlands. Carcinogenesis. (1996) 17:477–84. doi: 10.1093/carcin/17.3.477
23. Schuurman AG, Goldbohm RA, Dorant E, van den Brandt PA. Vegetable and fruit consumption and prostate cancer risk: a cohort study in The Netherlands. Cancer Epidemiol Biomarkers Prev. (1998) 7:673–80.
24. Li H, Li HQ, Wang Y, Xu HX, Fan WT, Wang ML, et al. An intervention study to prevent gastric cancer by micro-selenium and large dose of allitridum. Chin Med J. (2004) 117:1155–60.
25. Schulz M, Lahmann PH, Boeing H, Hoffmann K, Allen N, Key TJ, et al. Fruit and vegetable consumption and risk of epithelial ovarian cancer: the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. (2005) 14:2531–5. doi: 10.1158/1055-9965.EPI-05-0159
26. Lin J, Zhang SM, Wu K, Willett WC, Fuchs CS, Giovannucci E. Flavonoid intake and colorectal cancer risk in men and women. Am J Epidemiol. (2006) 164:644–51. doi: 10.1093/aje/kwj296
27. Boeing H, Dietrich T, Hoffmann K, Pischon T, Ferrari P, Lahmann PH, et al. Intake of fruits and vegetables and risk of cancer of the upper aero-digestive tract: the prospective EPIC-study. Cancer Causes Control: CCC. (2006) 17:957–69. doi: 10.1007/s10552-006-0036-4
28. Gonzalez CA, Pera G, Agudo A, Bueno-de-Mesquita HB, Ceroti M, Boeing H, et al. Fruit and vegetable intake and the risk of stomach and oesophagus adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST). Int J Cancer. (2006) 118:2559–66. doi: 10.1002/ijc.21678
29. Larsson SC, Bergkvist L, Wolk A. Fruit and vegetable consumption and incidence of gastric cancer: a prospective study. Cancer Epidemiol Biomarkers Prev. (2006) 15:1998–2001. doi: 10.1158/1055-9965.EPI-06-0402
30. Kirsh VA, Peters U, Mayne ST, Subar AF, Chatterjee N, Johnson CC, et al. Prospective study of fruit and vegetable intake and risk of prostate cancer. J Natl Cancer Inst. (2007) 99:1200–9. doi: 10.1093/jnci/djm065
31. Satia JA, Littman A, Slatore CG, Galanko JA, White E. Associations of herbal and specialty supplements with lung and colorectal cancer risk in the VITamins and Lifestyle study. Cancer Epidemiol Biomarkers Prev. (2009) 18:1419–28. doi: 10.1158/1055-9965.EPI-09-0038
32. Epplein M, Shu XO, Xiang YB, Chow WH, Yang G, Li HL, et al. Fruit and vegetable consumption and risk of distal gastric cancer in the Shanghai Women's and Men's Health studies. Am J Epidemiol. (2010) 172:397–406. doi: 10.1093/aje/kwq144
33. Walter RB, Brasky TM, Milano F, White E. Vitamin, mineral, and specialty supplements and risk of hematologic malignancies in the prospective VITamins And Lifestyle (VITAL) study. Cancer Epidemiol Biomarkers Prev. (2011) 20:2298–308. doi: 10.1158/1055-9965.EPI-11-0494
34. Gonzalez CA, Travier N, Lujan-Barroso L, Castellsague X, Bosch FX, Roura E, et al. Dietary factors and in situ and invasive cervical cancer risk in the European prospective investigation into cancer and nutrition study. Int J Cancer. (2011) 129:449–59. doi: 10.1002/ijc.25679
35. Brasky TM, Kristal AR, Navarro SL, Lampe JW, Peters U, Patterson RE, et al. Specialty supplements and prostate cancer risk in the VITamins and Lifestyle (VITAL) cohort. Nutr Cancer. (2011) 63:573–82. doi: 10.1080/01635581.2011.553022
36. Steevens J, Schouten LJ, Goldbohm RA, van den Brandt PA. Vegetables and fruits consumption and risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Int J Cancer. (2011) 129:2681–93. doi: 10.1002/ijc.25928
37. Gonzalez CA, Lujan-Barroso L, Bueno-de-Mesquita HB, Jenab M, Duell EJ, Agudo A, et al. Fruit and vegetable intake and the risk of gastric adenocarcinoma: a reanalysis of the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) study after a longer follow-up. Int J Cancer. (2012) 131:2910–9. doi: 10.1002/ijc.27565
38. Meng S, Zhang X, Giovannucci EL, Ma J, Fuchs CS, Cho E. No association between garlic intake and risk of colorectal cancer. Cancer Epidemiol. (2013) 37:152–5. doi: 10.1016/j.canep.2012.11.002
39. Vogtmann E, Xiang YB Li HL, Levitan EB, Yang G, Waterbor JW, et al. Fruit and vegetable intake and the risk of colorectal cancer: results from the Shanghai Men's Health Study. Cancer Causes Control: CCC. (2013) 24:1935–45. doi: 10.1007/s10552-013-0268-z
40. Maasland DH, van den Brandt PA, Kremer B, Goldbohm RA, Schouten LJ. Consumption of vegetables and fruits and risk of subtypes of head-neck cancer in the Netherlands Cohort Study. Int J Cancer. (2015) 136:E396–409. doi: 10.1002/ijc.29219
41. Kim H, Keum N, Giovannucci EL, Fuchs CS, Bao Y. Garlic intake and gastric cancer risk: Results from two large prospective US cohort studies. Int J Cancer. (2018) 143:1047–53. doi: 10.1002/ijc.31396
42. Yu CX, Chen YS, Ge ZJ, Zhang YH, Xu X, Tian T, et al. Dietary habits and risk of hepatocellular carcinoma among hepatitis B surface antigen carriers: A prospective cohort study in China. J Dig Dis. (2020) 21:406–15. doi: 10.1111/1751-2980.12878
43. Turati F, Guercio V, Pelucchi C, La Vecchia C, Galeone C. Colorectal cancer and adenomatous polyps in relation to allium vegetables intake: a meta-analysis of observational studies. Mol Nutr Food Res. (2014) 58:1907–14. doi: 10.1002/mnfr.201400169
44. Zhou Y, Zhuang W, Hu W, Liu GJ, Wu TX, Wu XT. Consumption of large amounts of Allium vegetables reduces risk for gastric cancer in a meta-analysis. Gastroenterology. (2011) 141:80–9. doi: 10.1053/j.gastro.2011.03.057
45. Zhou XF, Ding ZS, Liu NB. Allium vegetables and risk of prostate cancer: evidence from 132,192 subjects. Asian Pac J Cancer Prev. (2013) 14:4131–4. doi: 10.7314/APJCP.2013.14.7.4131
46. Chiavarini M, Minelli L, Fabiani R. Garlic consumption and colorectal cancer risk in man: a systematic review and meta-analysis. Public Health Nutr. (2016) 19:308–17. doi: 10.1017/S1368980015001263
47. Rahman K, Lowe GM. Garlic and cardiovascular disease: a critical review. J Nutr. (2006) 136:736S–40S. doi: 10.1093/jn/136.3.736S
48. Fleischauer AT, Arab L. Garlic and cancer: a critical review of the epidemiologic literature. J Nutr. (2001) 131:1032S–40S. doi: 10.1093/jn/131.3.1032S
49. Lawson LD, Wang ZJ. Low allicin release from garlic supplements: a major problem due to the sensitivities of alliinase activity. J Agric Food Chem. (2001) 49:2592–9. doi: 10.1021/jf001287m
50. Tang FY, Chiang EP, Chung JG, Lee HZ, Hsu CY. S-allylcysteine modulates the expression of E-cadherin and inhibits the malignant progression of human oral cancer. J Nutr Biochem. (2009) 20:1013–20. doi: 10.1016/j.jnutbio.2008.09.007
51. Xu YS, Feng JG, Zhang D, Zhang B, Luo M, Su D, et al. S-allylcysteine, a garlic derivative, suppresses proliferation and induces apoptosis in human ovarian cancer cells in vitro. Acta Pharmacol Sin. (2014) 35:267–74. doi: 10.1038/aps.2013.176
Keywords: cancer risk, supplement, epidemiology, meta-analysis, allium vegetable
Citation: Zhang Q, Zhao Q, Shen Y, Zhao F and Zhu Y (2022) Allium Vegetables, Garlic Supplements, and Risk of Cancer: A Systematic Review and Meta-Analysis. Front. Nutr. 8:746944. doi: 10.3389/fnut.2021.746944
Received: 20 August 2021; Accepted: 21 December 2021;
Published: 23 March 2022.
Edited by:
Catherine Frances Hughes, Ulster University, United KingdomReviewed by:
Shivendra Vikram Singh, St. Jude Children's Research Hospital, United StatesIsao Oze, Aichi Cancer Center, Japan
Copyright © 2022 Zhang, Zhao, Shen, Zhao and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Zhu, emh1eWFuMjAyMUB5ZWFoLm5ldA==
 Qifan Zhang1
Qifan Zhang1 Yan Zhu
Yan Zhu