- 1School of Life Sciences, Jilin University, Changchun, China
- 2Key Laboratory for Molecular Enzymology and Engineering, Jilin University, Ministry of Education, Changchun, China
- 3Key Laboratory for Evolution of Past Life and Environment in Northeast Asia, Jilin University, Ministry of Education, Changchun, China
Background/Aim: Essential oils of sunflower receptacles (SEOs) have antibacterial and antioxidant potential. However, the differences of biological activities from the different varieties of sunflowers have not been studied till now. The purpose of this study was to compare the differences of chemical compounds, antioxidant activities, and inhibitory activities against xanthine oxidase (XO) of SEOs from the three varieties of sunflowers including LD5009, SH363, and S606.
Methods: SEOs were extracted by using the optimal extraction conditions selected by response surface methodology (RSM). Chemical compounds of SEOs were identified from the three varieties of sunflowers by gas chromatography-mass spectrometry (GC-MS). Antioxidant activities of SEOs were detected by 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and iron ion reduction ability. Inhibitory activities of SEOs against XO were measured by using UV spectrophotometer. XO inhibitors were selected from the main chemical compounds of SEOs by the high-throughput selections and molecular simulation docking.
Results: The extraction yields of SEOs from LD5009, SH363, and S606 were 0.176, 0.319, and 0.580%, respectively. A total of 101 chemical compounds of SEOs were identified from the three varieties of sunflowers. In addition, the results of inhibitory activities against XO showed that SEOs can reduce uric acid significantly. Eupatoriochromene may be the most important chemical compounds of SEOs for reducing uric acid. The results of antioxidant activities and inhibitory activities against XO showed that SEOs of LD5009 had the strongest antioxidant and XO inhibitory activities. The Pearson correlation coefficient (r > 0.95) showed that γ-terpinene, (E)-citral, and L-Bornyl acetate were highly correlated with the antioxidant activities and XO inhibitory ability.
Conclusion: SEOs had antioxidant activities and XO inhibitory ability. It would provide more scientific information for utilization and selection of varieties of sunflowers, which would increase the food quality of sunflowers and incomes of farmers.
Introduction
Sunflower (Helianthus annuus L.) belongs to the family Compositae (Asteraceae), which originated in the south of America and spread to China in the seventeenth century (1); several varieties of sunflowers have been used as traditional medicines by Native Americans (2). Now, sunflowers are mainly planted in China (3), which are distributed widely in the north of China such as Inner Mongolia province, Jilin province, Liaoning province, Heilongjiang province, and Shanxi province (4). Among of them, Jilin province was the main producing area of sunflower (5). LD5009, SH363, and S606 are high yield sunflower varieties that are planted in Baicheng city of Jilin province. These three varieties have different commercial applications. LD5009 and SH363 are usually used for eating seeds, while S606 is used for pressing oil from seeds. It has been confirmed that the sunflower receptacles in Baicheng city of Jilin province had the biological functions of reducing uric acid and gout symptoms (6), which implied that sunflower receptacles in Baicheng city of Jilin province can be used as functional foods for treating hyperuricemia and gout. However, which variety of sunflowers had the higher biological activities is still unclear. So, it is difficult to select and distinguish the materials in food factory, which related to food quality and standard. So, it is very important to better understand the different biological activities from the different varieties of sunflowers.
Sunflower receptacles were usually used as pig feeds (7). Sometimes, they were discarded, which damaged the natural environment (8). So, it is very important to develop more applications of sunflower receptacles. Sunflower receptacles had many medicinal functions such as antigout (6), anti-inflammatory (9), antioxidant, and antimicrobial activities (10). In our previous research, it was proved that essential oils of sunflower receptacles (SEOs) had antioxidant and antimicrobial activities (10). The enzymatic hydrolysis products of sunflower receptacles adjusted the intestinal microorganisms and relieved the hyperuricemia by inhibiting the key proteins such as xanthine oxidase (XO), adenosine deaminase, and uric acid transporter 1 (11).
Sunflower oils are rich in unsaturated fatty acids such as oleic and linoleic acids (ω-6), which were considered that essential oils are good for human health (12, 13). Essential oils can be obtained by hydrodistillation (14). However, essential oils were difficult to be extracted with low extraction yield in many plants (15). Many factors of extraction would affect the extraction yields of essential oils such as liquid–solid ratio (16) and distillation time (17). Response surface methodology (RSM) can be used to select the optimal extraction conditions by evaluating the multiple parameters with less numbers of experiments (18). In previous studies, RSM was used to optimize the extraction conditions of essential oils (19) and flavonoids (20) successfully.
Different varieties of plants have different biological activities, which may be due to the different contents of chemical components. Varieties of pitaya fruits with different color peels had different antioxidant activities, which were because of the different contents of alkaloids and polyphenols (21). Different varieties of Polygonum multiflorum had different anti-inflammatory and antioxidant activities, which was because of the different contents of ellagic acid and quercetin (22). However, the biological activities and chemical compounds of SEOs from different varieties of sunflowers have not been reported till now.
The purpose of this study was to compare the differences of the chemical compounds, antioxidant activities, and inhibitory activities of SEOs against XO from varieties of sunflowers in Baicheng city of Jilin province in China. In this study, chemical compounds of SEOs were detected by gas chromatography-mass spectrometry (GC-MS). Antioxidant activities of SEOs were investigated by 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and iron ion reduction ability. Inhibitory activities of SEOs against XO were determined by both the experiments and molecular simulation docking. This study would provide more scientific information for using sunflower receptacles, which would reduce the waste of sunflower receptacles and increase the incomes of farmers.
Materials and Methods
Plant Materials
Three varieties of sunflowers were planted in Baicheng city of Jilin province in China (123°12′45″E, 44°52′23″N), which were planted in spring and harvested in autumn. Plant species were authenticated by Professor Shu-Wen Guan, School of Life Science, Jilin University. The samples of different varieties of sunflowers including LD5009, SH363, and S606 were collected in November 2020. The sunflower receptacles from the three varieties of sunflowers were air dried to remove water. The sunflower receptacles were powdered by grinder and passed through a 20-mesh standard sieve.
Chemical Reagents
Tetracycline hydrochloride, miconazole nitrate, DPPH, and (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) were purchased from the Source Leaf Biology Corporation Ltd. (Shanghai, China). Pentadecane, potassium persulfate (K2S2O8), ABTS, XO, and xanthine were purchased from the Dalian Meilun Biotechnology Corporation Ltd. (Dalian, China). The Iron Ion Reduction Capacity Kit was purchased from the Congyi Biology Corporation Ltd. (Shanghai, China). All the chemical reagents were of analytical grade.
Extraction of Essential Oils
RSM for Optimal Extraction Conditions
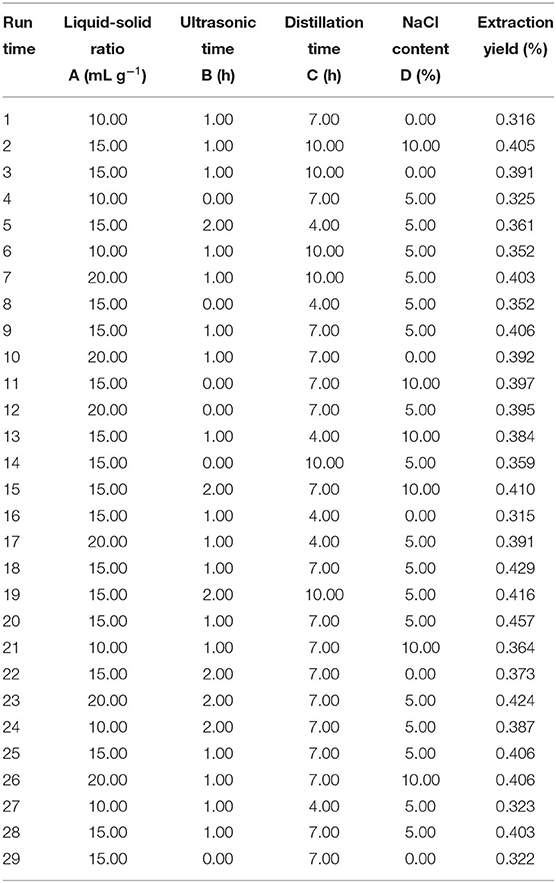
RSM was widely used to extract the essential oils from plants (23). Therefore, RSM was used to optimize the extraction conditions of SEOs (16). Based on the single-factor experiments (24), the experiments with four factors and three levels were designed by the Box–Behnken (Stat-Ease Incorporation, Minneapolis, Minnesota, USA). The four factors included liquid–solid ratio (A), ultrasonic time (B), distillation time (C), and the concentration of sodium chloride (NaCl) (D). The ranges of independent variables and the levels are shown in Table 1. The test variable range was converted from −1 to 1.
Extraction of SEOs
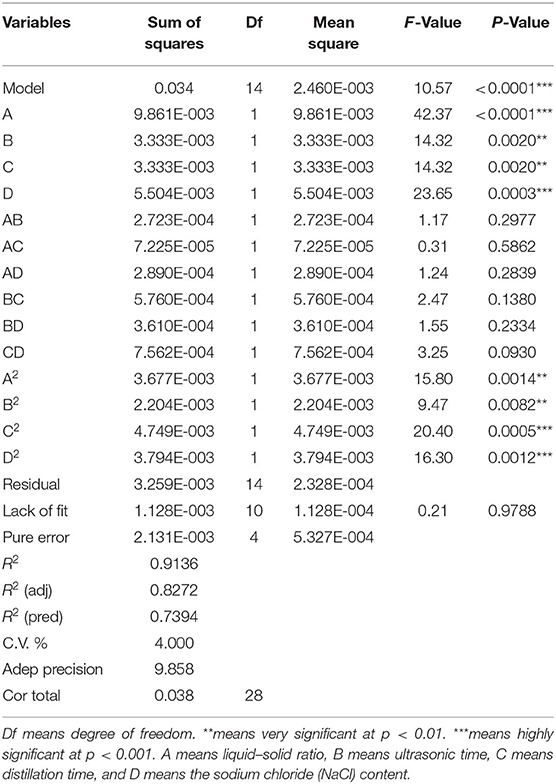
SEOs were extracted from the three varieties of sunflowers (LD5009, SH363, and S606). According to the model and the verification test, the optimal extraction conditions of SEOs were determined by RSM as follows: the liquid–solid ratio was 17.5:1 (ml/g). The ultrasonic time was 1.5 h. The distillation time was 8 h. The NaCl content was 6%. Based on the above optimal extraction conditions by RSM, the conditions of SEOs extraction were finally determined as follows: 1,750 ml 6% NaCl was added to 100 g powders of sunflower receptacles with ultrasonic time for 1.5 h. SEOs were extracted by hydrodistillation in the Clevenger-type apparatus with distillation time for 8 h. Sodium sulfate (Na2SO4) was used to remove water from SEOs. The extractions of SEOs were repeated 30 times to provide enough SEOs for the analysis of biological activities. SEOs were stored at 2–4°C for later use.
Chemical Compounds of SEOs
Gas chromatography-mass spectrometry was widely used to identify and quantify the chemical compounds of essential oils (24). Chemical compounds of SEOs were analyzed by GC-MS with the Agilent (5975C, Agilent Technologies Corporation Ltd., Santa Clara, California, USA). SEOs of 10 μl were diluted with 10 μl n-hexane. The separation was achieved by using the HP-INNOWax Capillary Column (30 × 0.25 mm id, 0.25 μm film thickness) (Agilent Technologies, Santa Clara, California, USA). Helium was used as the carrier gas at a flow rate of 1 ml/min. Injector and detector temperatures were set at 250 and 280°C, respectively. The oven was maintained at 60°C for 3 min, 240°C for 5 min, and held at 240°C for 15 min. Electronic ion mode was set at 70 eV. Mass spectra were recorded in the range of m/z 50–550 amu and the ion source temperature was 230°C.
Retention indices (RIs) of the separated compounds on the HP-INNOWax Capillary Column were determined on the basis of homologous series of n-alkanes (C9-C30). The chemical compounds of SEOs were identified on the basis of comparison of their RIs. Mass spectra with published data and computer matching with the National Institute of Standards and Technology (NIST, 15.0). The library provided computer analysis of GC-MS system (25). The relative proportions of SEOs constituents were expressed as percentages obtained by peak area normalization. All the relative response factors were set as 1.
Determination of Antioxidant Activities
Antioxidant activities of SEOs were detected by ABTS, DPPH, and the iron ion reduction. It was because that a single antioxidant method cannot accurately evaluate the antioxidant activities of SEOs.
Determination of ABTS Radical Scavenging Activity
ABTS radical scavenging activity was determined by the modified protocol from Kang and Song (26). ABTS working solution was prepared with 2.6 mM K2S2O8 and 7.4 mM ABTS, which was incubated at room temperature in the dark for 12 h and diluted with 40–45 times ethanol. We added 0.5 ml SEOs to 2 ml ABTS working solution and incubated at room temperature in the dark for 6 min. The absorbance was measured at 734 nm by a spectrophotometer (SP-752, Shanghai Instrument Corporation, Ltd., Shanghai, China). Trolox was used as a positive control.
The ABTS scavenging rate was determined by the following formula:
where, A0 was the absorbance of the negative control without SEOs and A1 was the absorbance of the test sample with SEOs.
Determination of DPPH Free-Radical Scavenging Activity
DPPH free-radical scavenging activity was determined according to the modified protocol from Das et al. (27). Ethanol and DPPH were mixed to prepare 0.08 mM DPPH solution, which was stored in the dark. Here, 1 ml sample and 3 ml DPPH solution were mixed and kept at room temperature for 30 min in the dark. The absorption value was measured at 517 nm. Anhydrous ethanol and Trolox were used as negative control and positive control, respectively.
The DPPH radical scavenging capacity was determined by the following formula:
where, A0 was the absorbance of the negative control without SEOs and A1 was the absorbance of the test sample with SEOs.
Determination of Iron Ion Reduction Ability
The Iron Ion Reduction Capacity Kit (A003-96T Iron Ion Reduction Ability Test Kit, Congyi Biology Corporation, Ltd., Shanghai, China) was used to determine the iron ion reducing ability of SEOs (10). Antioxidant substances can convert ferric iron of potassium ferricyanide into ferrous ions to form Prussian blue, which has a maximum absorption peak at 700 nm. The greater absorbance value, the better antioxidant activities of the SEOs.
Inhibitory Activities Against XO
Determination of Inhibitory Activities of SEOs Against XO
Xanthine oxidase reacted with xanthine to produce uric acid and the content of uric acid was measured by UV spectrophotometer at 290 nm to evaluate the inhibitory activities of SEOs against XO (28, 29). The experiment of inhibitory activities of SEOs against XO was modified according to the method of Bou-Salah et al. (30).
Inhibitory activity of SEOs against XO was determined by the following formula:
A is the absorbance of the solution with 0.5 ml XO, 3.5 ml phosphate-buffered saline (PBS) (70 mM, pH = 7.5), and 1 ml xanthine. B is the absorbance of the solution with 4 ml PBS (70 mM, pH = 7.5) and 1 ml xanthine. C is the absorbance of the solution with 0.5 ml XO and 0.5 ml essential oils (different density), 1 ml xanthine, and 3 ml PBS (70 mM, pH = 7.5). D is the absorbance of the solution with 3.5 ml PBS (70 mM, pH = 7.5), 0.5 ml SEOs with different densities, and 1 ml xanthine.
Molecular Docking
High-throughput virtualization was used to screen the protease inhibitors. Chemical compounds with similar structures to allopurinol were screened from 101 chemical compounds of SEOs. Open Babel was used to process the ligand structure data file (SDF) structure information in batches and convert them into three-dimensional (3D) program database (PDB) files, which were suitable for molecular docking (31). The PDB structure of XO was obtained from the PDB database (PDBID: 3 nvw).
The AutoDock Vina Software (Scripps Research Institute, San Diego, California, USA) was used for molecular docking between the target inhibitor and XO (32), which was downloaded from the website (http://vina.scrip.edu). The results were analyzed by the Discovery Studio version 3.5 (33). The Lamarckian genetic algorithm (GA) was used to calculate the possible conformations of protein receptors and inhibitors (34).
Statistical Analysis
All the experiments were conducted with the three replications. The one-way ANOVA and the mean comparisons were performed on antioxidant data by using the program Statistical Package for the Social Sciences (SPSS) version 20.0 (IBM Corporation, New York, USA). The Duncan's multiple range tests were used to calculate the mean values. Differences between mean values at p < 0.05 were considered as statistically significant. The chemical compounds, antioxidant activities, and XO inhibitory activity of SEOs were analyzed by the Pearson's correlation coefficient with R programming language (R-4.1.0 for Windows 32/64 bit).
Results and Discussion
Extraction of SEOs
RSM for Optimal Extraction Conditions of SEOs
The extraction yields of SEOs with different conditions are shown in Table 2. A second-order polynomial equation was used to express the extraction yields of SEOs as a function of the coding variables. The empirical equation was used to calculate and predict the extraction yield of sunflower receptacles. It was given as follows:
where, A: liquid–solid ratio, B: ultrasonic time, C: distillation time, and D: NaCl content.
The ANOVA is shown in Table 3. The model was highly significant (p < 0.001), indicating that the equation was sufficient to predict production under any combination of variable values. A, D, and C2 were very important model items because the p < 0.001. B, C, A2, B2, and D2 were very important model terms because the p < 0.01. However, AB, AC, AD, BC, BD, and CD were not very important model terms because the p-value was bigger than 0.05.
The correlation coefficient between the experimental data and the model was very high (R2 = 0.9136), indicating that the model cannot explain 8.64% of the total changes. After adjusting the R2 (pred) of 0.7394 and R2 (adj) of 0.8272, the value of the coefficient R2 (adj) was within a reasonable range (35).
In this model, the p-value of the liquid–solid ratio (A) was <0.001, indicating that the liquid–solid ratio had the most influence on the extraction yield of SEOs. According to the coefficients of the regression equation, the results showed that the liquid–solid ratio (A) had the greatest effect on the extraction yield of SEOs. The concentration of NaCl (D) had the greater influence on the extraction yield of SEOs. Ultrasonic time (B) and distillation time (C) had the great influence factors on the extraction yield of SEOs. So, the order of the influence factors was as follows: A > D > B > C.
The advantage of RSM was that the interaction of multiple factors can be considered at the same time on the experimental results (36). The response surface and contour lines of the interaction of A (liquid–solid ratio), B (ultrasonic time), C (distillation time), and D (NaCl content) are shown in Figure 1. The interactions among factors had a significant impact on the extraction yields of SEOs (Figure 2). The contour plots of various factors can reflect the interaction influence on the response value. Distillation time and NaCl content had the greatest influence on the extraction yields of SEOs. The contour plots of ultrasonic time and distillation time, NaCl content and ultrasonic time, and ultrasonic time and liquid–solid ratio indicated that the interactions were obvious. The results showed that the extraction yield had an upward trend. Adding an appropriate amount of inorganic salt during the extraction process could increase the extraction yield of essential oil from lavender (37).
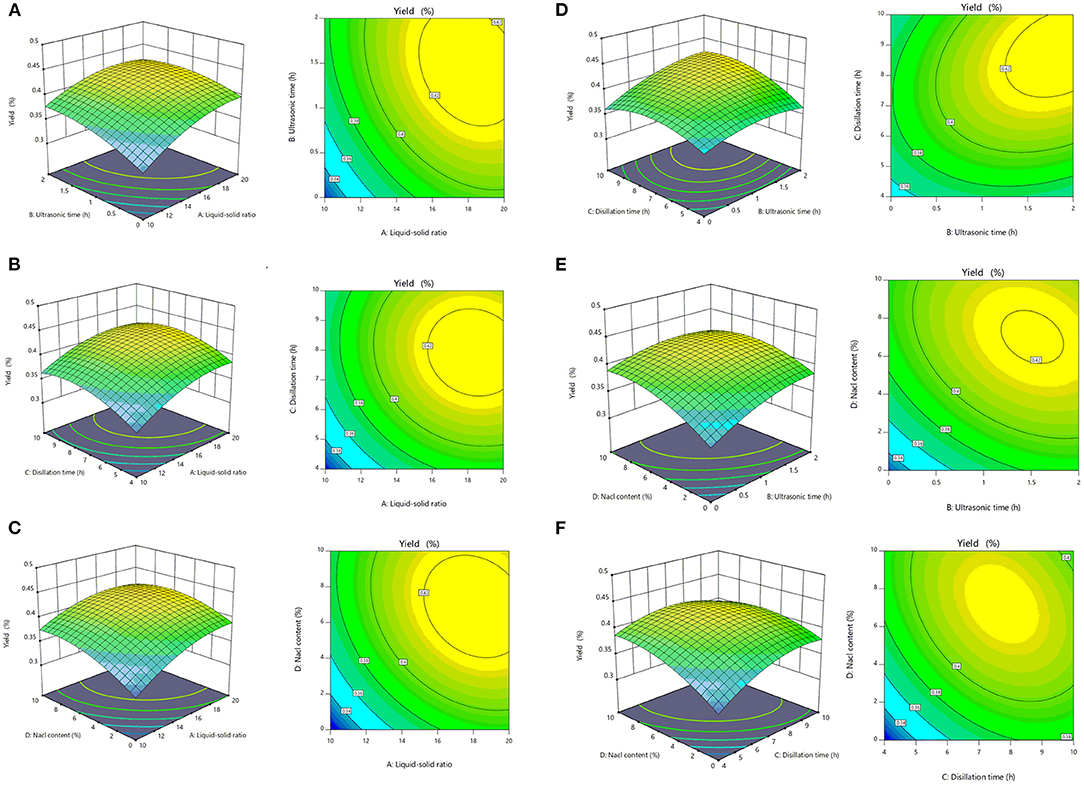
Figure 2. The mutual effects of the response surface plots and contour plots for the extraction yields of essential oils of sunflower receptacles (SEOs). (A) Ultrasound time and liquid–solid ratio, (B) distillation time and liquid–solid ratio, (C) sodium chloride (NaCl) content and liquid–solid ratio, (D) distillation time and ultrasonic time, (E) NaCl content and ultrasonic time, (F) NaCl content and distillation time.
According to the model and the verification test, the optimal extraction conditions of SEOs were determined as follows: the liquid–solid ratio was 17.5:1 (ml/g). The ultrasonic time was 1.5 h. The distillation time was 8 h. The NaCl content was 6%.
Analysis of the Extraction Yields of SEOs From Different Varieties of Sunflowers
The results showed that the extraction yields of SEOs from different varieties of sunflowers were much different (Table 4). The extraction yields of SEOs from LD5009, SHE363, and S606 were 0.176, 0.319, and 0.580%, respectively.
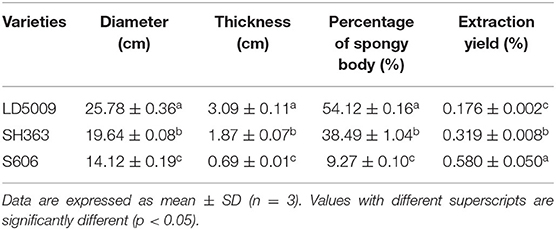
Table 4. The morphological characters and the extraction yields of SEOs from different varieties of sunflowers.
The extraction yield of SEOs of S606 was significantly higher than that of LD5009 and SH363, which may be related to the content of SEOs in different varieties of sunflowers. Sunflower receptacles were mainly composed of outer surrounding cells and inner spongy bodies. The morphological characters of sunflower receptacles from different varieties of sunflowers were much different (Figure 1; Table 4). The order of the sunflower receptacles size from big to small was as follows: LD5009 > SH363 > S606. The thickness and percentage of the spongy bodies of S606 were significantly lower than that of LD5009 and SH363. The results showed that the sunflower receptacles of S606 with smaller spongy bodies had much more SEOs. The sunflower receptacles of LD5009 with bigger spongy bodies had a little amount of SEOs. So, the results suggested that SEOs were not in the inner spongy bodies of sunflower receptacles, but may be in the outer surrounding cells of sunflower receptacles.
Chemical Compounds of SEOs
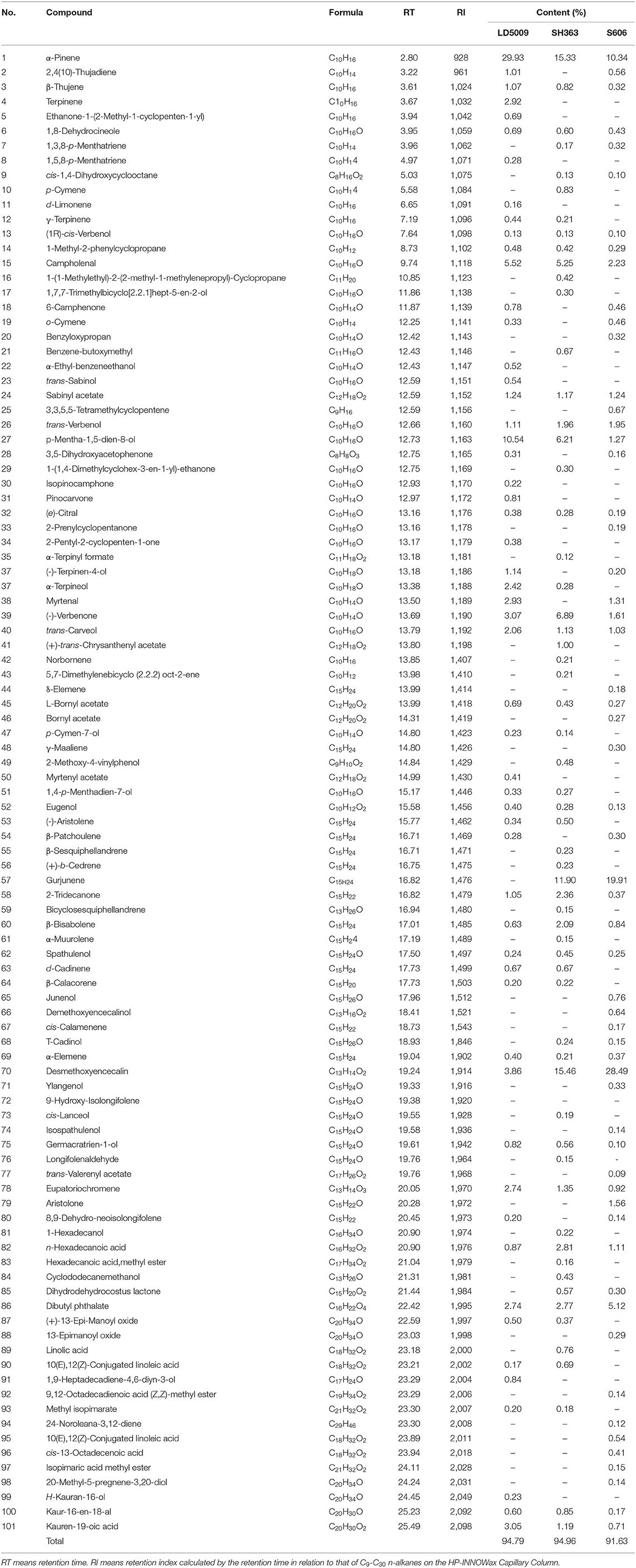
A total of 101 chemical compounds of SEOs were identified by GC-MS (Table 5; Figure 3). A total of 55 chemical compounds of SEOs were identified from LD5009 of which monoterpenoids accounted for 35.31%. Oxygenated monoterpenes accounted for 41.54%, and sesquiterpenes accounted for 4.46%. In previous study, the sunflower essential oils of “chianti,” “mammoth,” and wild-growing H. strumosus in north Alabama were dominated by monoterpenes, in particular α-pinene (50.65, 48.91, and 58.65%, respectively), sabinene (6.81, 17.01, and 1.91%, respectively), β-pinene (5.79, 3.27, and 4.52%, respectively), and limonene (7.2, 7.1, and 3.8%, respectively) (2). In this study, the main chemical compounds of SEOs from LD5009 were α-pinene (29.93%), p-mentha-1,5-dien-8-ol (10.54%), α-campholenal (5.52%), desmethoxyencecalin (3.86%), verbenone (3.07%), kauren-19-oic acid (3.05%), terpinene (2.92%), and α-terpineol (2.42%). A total of 61 chemical compounds of SEOs from SH363 were identified by GC-MS, accounting for 94.96% of its total contents. Among them, monoterpenoids accounted for 18.50%, oxygenated monoterpenes accounted for 33.76%, and sesquiterpenes accounted for 38.57%. The main chemical compounds of SEOs from SH363 were desmethoxyencecalin (15.46%), α-pinene (15.33%), gurjunene (11.90%), p-mentha-1,5-dien-8-ol (6.21%), campholenal (5.25%), and verbenone (6.89%). A total of 58 chemical compounds were identified from S606 by GC-MS, accounting for 91.63% of its total contents, volatile monoterpenoids accounted for 12.18%, oxygen-containing monoterpenes accounted for 13.54%, and sesquiterpenes accounted for 62.05%. The main chemical compounds of SEOs from S606 were desmethoxyencecalin (28.49%), gurjunene (19.91%), α-pinene (10.34%), dibutyl phthalate (5.12%), campholenal (2.23%), trans-verbenol (1.95%), and verbenone (1.61%). It is concluded that monoterpenes are the major compounds of essential oils in sunflower receptacles, leaves, and flowers (38).
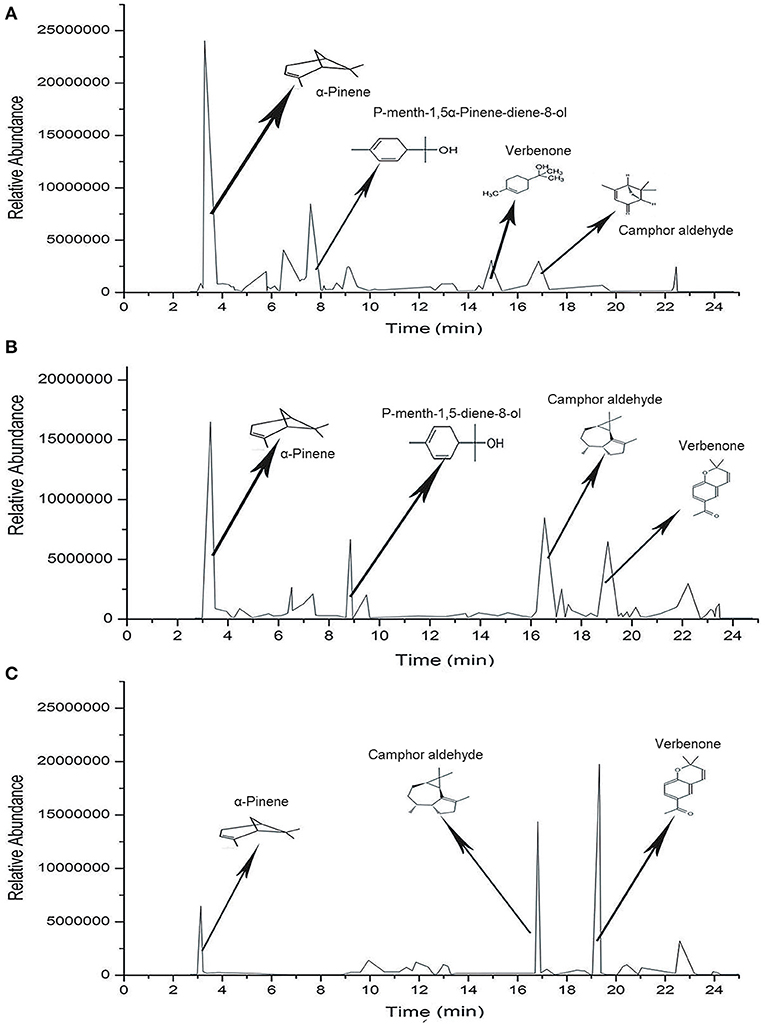
Figure 3. Chromatograms of the chemical compounds of SEOs from different varieties of sunflowers. (A) Chromatogram of chemical compounds of SEOs from LD5009. (B) Chromatogram of chemical compounds of SEOs from SH363. (C) Chromatogram of chemical compounds of SEOs from S606.
Analysis of Antioxidant Activities
All the SEOs from the three varieties of sunflowers had antioxidant activities (Figure 4). However, the antioxidant activities of SEOs from LD5009, SH363, and S606 were different. The antioxidant activities of SEOs were listed as followers: LD5009 > SH363 > S606. The antioxidant activities of SEOs in the three varieties of sunflowers were increased with high SEOs concentration, which indicated that the antioxidant activities of SEOs were highly dependent on the concentrations of SEOs.
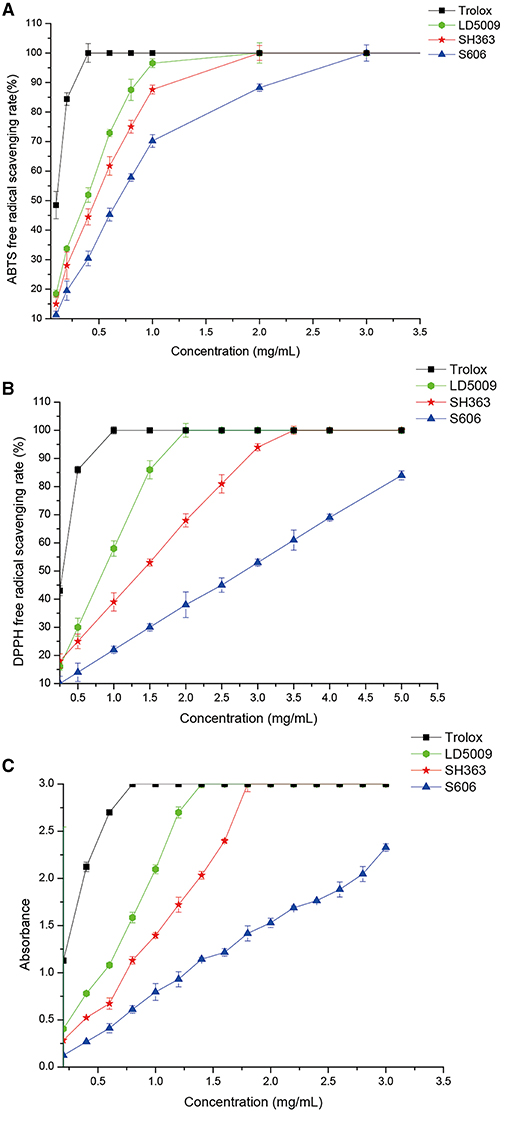
Figure 4. Antioxidant activities of SEOs from the different varieties of sunflowers. (A) ABTS free-radical scavenging rate of SEOs from different varieties of sunflowers. (B) DPPH free radical scavenging rate of SEOs from different varieties of sunflowers. (C) Iron ion reduction ability of SEOs from different varieties of sunflowers.
Previous studies proved that monoterpenoids (carveol) in essential oils had antioxidant activities (39, 40). Eugenol, γ-terpinene, and α-terpinolene had good antioxidant activities. However, α-pinene had very low antioxidant activity, while terpinene and α-terpinene have no antioxidant activity (40). Carveol, eugenol, γ-terpinene, α-pinene, and α-terpinolene were all belonged to monoterpenoids (Table 5). The contents of monoterpenoids of SEOs from LD5009 and SH363 were much higher than that of SEOs from S606. Antioxidant activities of SEOs from LD5009 and SH363 were higher than that of S606. So, antioxidant activities of SEOs related with the content of monoterpenoids.
Inhibitory Activity of SEOs Against XO
Determination of XO Inhibitory Rate
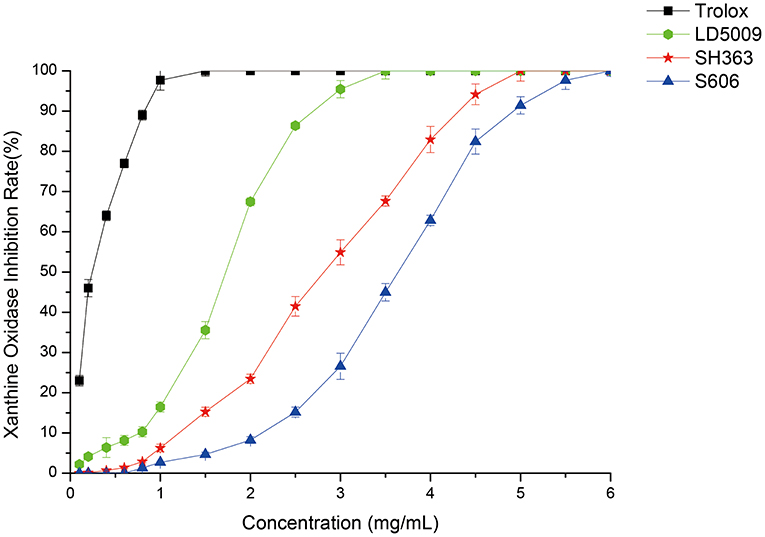
As shown in Figure 5, SEOs had a significant inhibitory effect on XO. SEOs of different sunflower varieties had different inhibitory abilities against XO. The inhibitory activities of SEOs against XO were ordered as follows: LD5009 > SH363 > S606. Previous studies showed that gout was caused by hyperuricemia. Uric acid was produced by the reaction of XO with xanthine (41). XO inhibitors may inhibit the activity of XO and reduce the content of uric acid in the blood, thereby avoid the occurrence of hyperuricemia (42). SEOs had a good inhibitory activities against XO. SEOs had the functions for reducing uric acid and inhibiting the production of gout.
Molecular Simulation Docking of SEOs and XO
Eupatoriochromene was screened to prove its inhibitory activities against XO from the chemical compounds of SEOs by high-throughput virtual screening and molecular simulation docking. The results of molecular simulation docking showed that eupatoriochromene can bind to XO very well (Figure 6; Supplementary Figure 1).
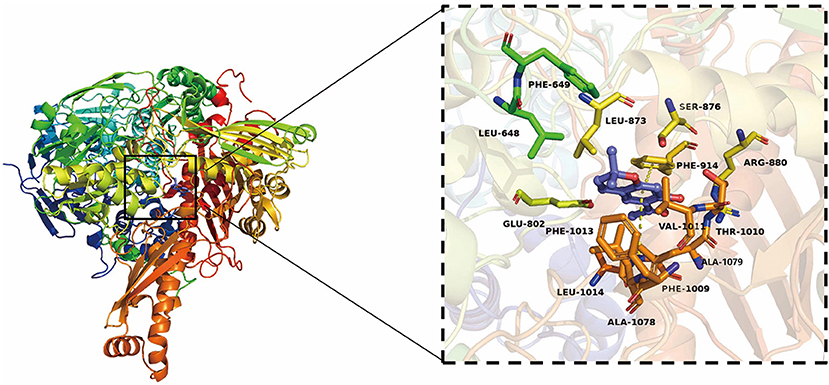
Figure 6. Three-dimensional (3D) structure of XO and the active residues of XO binding to eupatoriochromene.
The results of molecular simulation docking showed that the key active residues of XO combined to eupatoriochromene were Val1011, Phe1013, Leu648, Phe649, Leu1014, and Leu873, which formed Pi-alkyl in Figure 6. The benzene ring of Phe1009, Phe914, and eupatoriochromene formed Pi-Pi conjugated double bond. Ser876, Thr1010, Arg880, Ala1079, Ala1078, Glu802, and eupatoriochromene formed van der Waals forces. These weak intermolecular interactions played an important role in the interactions between eupatoriochromene and XO. Competitive inhibitors were inserted into the hydrophobic gap of XO to occupy its catalytic activity center, hindering the entry of the substrate and leading to its inactivation (43). Eupatoriochromene was a competitive inhibitor of xanthine in the system. Therefore, the main chemical compounds of SEOs may be eupatoriochromene, which had an inhibitory effect on XO.
The inhibitory abilities of SEOs on XO were listed as follows: LD5009 > SH363 > S606. The contents of eupatoriochromene in the three SEOs were 2.74% (LD5009), 1.35% (SH363), and 0.92% (S606). So, the inhibitory ability of SEOs against XO was possibly correlated with the content of eupatoriochromene in SEOs. The positive correlation phenomenon corresponded to the results of molecular simulation docking between XO and the chemical compounds of SEOs. It could be concluded that the inhibitory ability of SEOs effect on XO may be due to the main chemical components of SEOs such as eupatoriochromene.
Correlation Analysis Among the Chemical Compounds Antioxidant Activity and XO Inhibitory Activity
The Pearson correlation analysis is widely used in analysis of the correlation analysis between chemical compounds of SEOs and biological activities. The larger the Pearson coefficient (r), the greater the correlation between the chemical compounds and the biological activities (44). The results of correlation analysis showed that the chemical compounds of SEOs had the great correlation with antioxidant activities and XO inhibitory activity (Table 6; Supplementary Figure 2). The Pearson correlation coefficients of 12 chemical compounds of SEOs with antioxidant activities and XO inhibitory activity were r > 0.8, indicating that these chemical compounds of SEOs were highly positively correlated with antioxidant activities and XO inhibitory activity. The Pearson correlation coefficients of γ-terpinene, (E)-citral, and L-Bornyl acetate with both antioxidant activities and XO inhibition were r > 0.95, indicating that these chemical compounds of SEOs may play a major role in both the antioxidant activities and XO inhibitory activity. Some of the chemical compounds of SEOs were highly correlated only with the antioxidant activities (r > 0.95), while some of the chemical Compounds of SEOs were not highly correlated with XO inhibitory activity (r > 0.8), such as β-thujene, 1,8-dehydrocineole, 1-methyl-2-phenylcyclopropane, and p-mentha-1,5-dien-8-olandgermacratrien-1-ol. The results of molecular simulation docking showed that eupatoriochromene can bind to the active sites of XO and inhibit the activity of XO. Therefore, it can be concluded that eupatoriochromene might play an important role in the inhibition of XO activity.
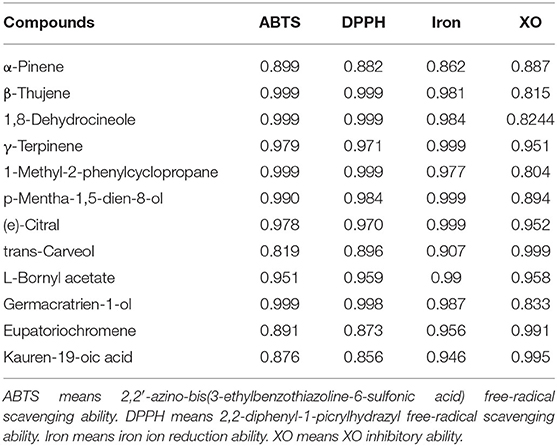
Table 6. The Pearson correlation coefficient (r) of the chemical compounds of SEOs with antioxidant activity and XO inhibition ability.
Application of SEOs
In this study, the results of antioxidant activities by ABTS, DPPH, and iron ion reduction ability showed that SEOs had antioxidant activities, implied that SEOs can be used as natural antioxidant to prevent food oxidation, which may keep food and fruit fresh for longer time. The antioxidant activities of SEOs from LD5009 were the highest among these three varieties of sunflower including LD5009, SH363, and S606. The seeds of LD5009 were always eaten directly as snacks, while the receptacles of LD5009 were discarded. It would provide a new application of sunflower receptacles in food sector that it can be developed to extract SEOs as natural antioxidant instead of throwing it away. SEOs had high XO inhibitory ability, implied that it can be used as healthcare food to reduce the uric acid for the patients with hyperuricemia (20), which would provide the evidence of its applications for hyperuricemia and gout (6).
Conclusion
The optimal extraction conditions of SEOs were selected by RSM with liquid–solid ratio of 17.5:1 (ml/g), ultrasonic time of 1.5 h, distillation time of 8 h, and the concentration of NaCl of 6%. SEOs were extracted from three varieties of sunflowers (LD5009, SH363, and S606) by hydrodistillation in the Clevenger-type apparatus. The extraction yields of LD5009, SHE363, and S606 were 0.176, 0.319, and 0.580%, respectively. A total of 101 chemical compounds of SEOs were identified from the three varieties of sunflowers by GC-MS. The results of the antioxidant activities of SEOs showed that the antioxidant activities of SEOs from the three varieties of sunflowers were quite different (LD5009 > SH363 > S606). The analysis of the inhibitory activity of SEOs against XO and molecular simulation docking revealed that SEOs had strong inhibitory effect on XO, which may be related to eupatoriochromene of SEOs. The Pearson correlation coefficient (r > 0.95) showed that γ-terpinene, (E)-citral, and L-Bornyl acetate were highly correlated with antioxidant activities of SEOs and XO inhibitory ability. The results of the antioxidant activities and XO inhibitory activity both showed that SEOs have good antioxidant activities and XO inhibitory ability. This study would provide more scientific information for utilization of sunflower receptacles and good selection of different varieties of sunflowers, which would increase the incomes of farmers.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
LH, BG, W-WH, and W-NL conceived and designed the study. X-SL, MY, and Z-AQ performed the experiments. X-SL and LH analyzed the data. X-SL, LH, and Z-DD wrote the manuscript. All the authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 31870201), received research funding from the Key Laboratory for Evolution of Past Life and Environment in Northeast Asia by the Ministry of Education of China, and received funding from Jilin Teyi Food Technology Corporation Ltd.
Conflict of Interest
The authors declare that this study received funding from Jilin Teyi Food Technology Corporation Ltd. The funder had the following involvement in the study: design, sample collection and the decision to submit it for publication.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to Shu-Wen Guan, School of Life Sciences, Jilin University, for his help in the plant identification. We are grateful to Yi-Yan Lin for statistic analysis of morphological characters of sunflowers and modification of the reference format. We thank Jilin Teyi, Food Technology Corporation Ltd. for providing research funding and plant materials for this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.737157/full#supplementary-material
References
1. Yang J, Tang L, Guan YL, Sun WB. Genetic diversity of an alien invasive plant mexican sunflower (Tithonia diversifolia) in China. Weed Sci. (2012) 60:552–7. doi: 10.1614/WS-D-11-00175.1
2. Lawson SK, Sharp LG, Powers CN, McFeeters RL, Satyal P, Setzer WN. Essential oil compositions and antifungal activity of sunflower (Helianthus) species growing in North Alabama. Appl Sci. (2019) 9:3179. doi: 10.3390/app9153179
3. Smith BD. Eastern North America as an independent center of plant domestication. Proc Natl Acad Sci USA. (2006) 103:12223–8. doi: 10.1073/pnas.0604335103
4. Shi B, Zhao J. Recent progress on sunflower broomrape research in China. OCL. (2020) 27:30. doi: 10.1051/ocl/2020023
5. Liu J, Liu GS, Jan CC. Comparison of genetic diversity of the germplasm resources of confectionary sunflower (Helianthus annuus) in China based on RAPDs and AFLPs. Acta Bot Sin. (2003) 45:352–8.
6. Li L, Teng M, Liu Y, Qu Y, Zhang Y, Lin F, et al. Anti-Gouty arthritis and antihyperuricemia effects of sunflower (Helianthus annuus) head extract in gouty and hyperuricemia animal models. Biomed Res Int. (2017) 2017:5852076. doi: 10.1155/2017/5852076
7. Osman NS, Sapawe N, Sapuan MA, Fozi MFM, Azman M, Fazry AHZ, et al. Sunflower shell waste as an alternative animal feed. Mater Today Proc. (2018) 5:21905–10. doi: 10.1016/j.matpr.2018.07.049
8. Tan J, Hua X, Liu JR, Wang MM, Liu YX, Yang RJ, et al. Extraction of sunflower head pectin with superfine grinding pretreatment. Food Chem. (2020) 320:126631. doi: 10.1016/j.foodchem.2020.126631
9. Ukiya M, Akihisa T, Yasukawa K, Koike K, Takahashi A, Suzuki T, et al. Triterpene glycosides from the flower petals of sunflower (Helianthus annuus) and their anti-inflammatory activity. J Nat Prod. (2007) 70:813–6. doi: 10.1021/np078002l
10. Liu XS, Gao B, Li XL, Li WN, Qiao ZA, Han L. Chemical composition and antimicrobial and antioxidant activities of essential oil of sunflower (Helianthus annuus L.) receptacle. Molecules. (2020) 25:5244. doi: 10.3390/molecules25225244
11. Liu G, Chen XF, Lu X, Zhao JY, Li XL. Sunflower head enzymatic hydrolysate relives hyperuricemia by inhibiting crucial proteins (xanthine oxidase, adenosine deaminase, uric acid transporter1) and restoring gut microbiota in mice. J Funct Foods. (2020) 72:104055. doi: 10.1016/j.jff.2020.104055
12. Galúcio CS, Souza RA, Stahl MA, Sbaite P, Benites CI, Maciel MRW. Physicochemical characterization of monoacylglycerols from sunflower oil. Proc Food Sci. (2011) 1:1459–64. doi: 10.1016/j.profoo.2011.09.216
13. Aguirre MR, Velasco J, Ruiz-Méndez MV. Characterization of sunflower oils obtained separately by pressing and subsequent solvent extraction from a new line of seeds rich in phytosterols and conventional seeds. OCL. (2014) 21:D605. doi: 10.1051/ocl/2014033
14. Rubiolo P, Sgorbini B, Liberto E, Cordero C, Bicchi C. Essential oils and volatiles: sample preparation and analysis. A review. Flavour Fragr J. (2010) 25:282–90. doi: 10.1002/ffj.1984
15. Tongnuanchan P, Benjakul S. Essential oils: extraction, bioactivities, and their uses for food preservation. J Food Sci. (2014) 79:R1231–49. doi: 10.1111/1750-3841.12492
16. Liu K, Deng W, Hu W, Cao S, Zhong B, Chun J. Extraction of 'gannanzao' orange peel essential oil by response surface methodology and its effect on cancer cell proliferation and migration. Molecules. (2019) 24:499. doi: 10.3390/molecules24030499
17. Ferhat MA, Meklati BY, Smadja J, Chemat F. An improved microwave clevenger apparatus for distillation of essential oils from orange peel. J Chromatogr A. (2006) 1112:121–6. doi: 10.1016/j.chroma.2005.12.030
18. Myers RH, Montgomery DC, Anderson-Cook C. Response Surface Methodology: Process and Product Optimization Using Designed Experiments. Hoboken, NJ: John Wiley & Sons (2016).
19. Kaya DA, Ghica MV, Danila E, Ozturk S, Turkmen M, Albu Kaya MG, et al. Selection of optimal operating conditions for extraction of Myrtus communis L. Essential oil by the steam distillation method. Molecules. (2020) 25:2399. doi: 10.3390/molecules25102399
20. Qiao Z, Han L, Liu XS, Dai HN, Liu CM, Yan M, et al. Extraction, radical scavenging activities, and chemical composition identification of flavonoids from sunflower (Helianthus annuus L.) receptacles. Molecules. (2021) 26:403. doi: 10.3390/molecules26020403
21. Paśko P, Galanty A, Zagrodzki P, Ku YG, Luksirikul P, Weisz M, et al. Bioactivity and cytotoxicity of different species of pitaya fruits - a comparative study with advanced chemometric analysis. Food Biosci. (2021) 40:100888. doi: 10.1016/j.fbio.2021.100888
22. Wu YJ, Zhang ZF, Chen TB, Cheng CS, Zhang ZL, Zhou H, et al. Comparison of two Polygonum chinense varieties used in Chinese cool tea in terms of chemical profiles and antioxidant/anti-inflammatory activities. Food Chem. (2020) 310:125840. doi: 10.1016/j.foodchem.2019.125840
23. Yu HH, Song MW, Song YJ, Lee NK, Paik HD. Antibacterial effect of a mixed natural preservative against listeria monocytogenes on lettuce and raw pork loin. J Food Prot. (2019) 82:2001–6. doi: 10.4315/0362-028X.JFP-19-026
24. Lebanov L, Ghiasvand A, Paull B. Data handling and data analysis in metabolomic studies of essential oils using GC-MS. J Chromatogr A. (2021) 1640:461896 doi: 10.1016/j.chroma.2021.461896
25. Sparkman OD. Identification of essential oil components by gas chromatography/mass spectroscopy Robert P. Adams. J Am Soc Mass Spectrom. (1997) 8:671–2. doi: 10.1016/S1044-0305(97)00026-3
26. Kang JH, Song KB. Characterization of Job's tears (Coix lachryma-jobi L.) starch films incorporated with clove bud essential oil and their antioxidant effects on pork belly during storage. LWT Food Sci Technol. (2019) 111:711–8. doi: 10.1016/j.lwt.2019.05.102
27. Das S, Singh VK, Dwivedy AK, Chaudhari AK, Upadhyay N, Singh A, et al. Antimicrobial activity, antiaflatoxigenic potential and in situ efficacy of novel formulation comprising of Apium graveolens essential oil and its major component. Pestic Biochem Physiol. (2019) 160:102–11. doi: 10.1016/j.pestbp.2019.07.013
28. Tian Y, Lin L, Zhao M, Peng A, Zhao K. Xanthine oxidase inhibitory activity and antihyperuricemic effect of Moringa oleifera Lam. leaf hydrolysate rich in phenolics and peptides. J Ethnopharmacol. (2021) 270:113808. doi: 10.1016/j.jep.2021.113808
29. Nguyen MTT, Awale S, Tezuka Y, Le Tran Q, Watanabe H, Kadota S. Xanthine oxidase inhibitory activity of vietnamese medicinal plants. Biol Pharm Bull. (2004) 27:1414–21. doi: 10.1248/bpb.27.1414
30. Bou-Salah L, Benarous K, Linani A, Bombarda I, Yousfi M. In vitro and in silico inhibition studies of five essential oils on both enzymes human and boine xanthine oxidase. Indust Crops Prod. (2020) 143:111949. doi: 10.1016/j.indcrop.2019.111949
31. Xie XQS. Exploiting PubChem for virtual screening. Exp Opin Drug Discov. (2010) 5:1205–20. doi: 10.1517/17460441.2010.524924
32. Biesiada J, Porollo A, Velayutham P, Kouril M, Meller J. Survey of public domain software for docking simulations and virtual screening. Hum Genomics. (2011) 5:497–505. doi: 10.1186/1479-7364-5-5-497
33. Cao H, Hall J, Hille R. Substrate orientation and specificity in xanthine oxidase: crystal structures of the enzyme in complex with indole-3-acetaldehyde and guanine. Biochemistry. (2014) 53:533–41. doi: 10.1021/bi401465u
34. Pan Y, Lu Z, Li C, Qi R, Chang H, Han L, et al. Molecular dockings and molecular dynamics simulations reveal the potency of different inhibitors against xanthine oxidase. ACS Omega. (2021) 6:11639–49. doi: 10.1021/acsomega.1c00968
35. Ji YB, Dong F, Ma DB, Miao J, Jin LN, Liu ZF, et al. Optimizing the extraction of anti-tumor polysaccharides from the fruit of Capparis spionosa L. by response surface methodology. Molecules. (2012) 17:7323–35. doi: 10.3390/molecules17067323
36. Keshtegar B, Mert C, Kisi O. Comparison of four heuristic regression techniques in solar radiation modeling: kriging method vs RSM, MARS and M5 model tree. Renew Sust Energy Rev. (2018) 81:330–41. doi: 10.1016/j.rser.2017.07.054
37. Yu S, Xie X, Li S, Li W. Optimization of ultrasonic enhanced salt-containing hydrodistillation by response surface methodology. Chem Eng Technol. (2013) 36:801–9. doi: 10.1002/ceat.201200671
38. Ceccarini L, Macchia M, Flamini G, Cioni PL, Caponi C, Morelli I. Essential oil composition of Helianthus annuus L. leaves, and heads of two cultivated hybrids “Carlos” and “Florom 350”. Indust Crops Prod. (2004) 19:13–7. doi: 10.1016/S0926-6690(03)00076-1
39. Song Y, Wang LB, Bei Y, Qin DX, Ai LY, Ma QZ, et al. Carvacryl acetate, a semisynthetic monoterpenic ester obtained from essential oils, provides neuroprotection against cerebral ischemia reperfusion-induced oxidative stress injury via the Nrf2 signalling pathway. Food Funct. (2020) 11:1754–63. doi: 10.1039/C9FO02037C
40. Misharina TA, Terenina MB, Krikunova NI. Antioxidant Activity of essential oils. J Agric Food Chem. (2013) 2013:10835–47. doi: 10.1021/jf403496k
41. White WB, Saag KG, Becker MA, Borer JS, Gorelick PB, Whelton A, et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med. (2018) 378:1200–10. doi: 10.1056/NEJMoa1710895
42. Richette P, Doherty M, Pascual E, Barskova V, Becce F, Castaneda-Sanabria J, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheumat Dis. (2017) 76:29–42. doi: 10.1136/annrheumdis-2016-209707
43. Zafar H, Iqbal S, Javaid S, Khan KM, Choudhary MI. Xanthine oxidase inhibitory and molecular docking studies on pyrimidones. Med Chem. (2018) 14:524–35. doi: 10.2174/1573406413666171129224919
Keywords: sunflower (Helianthus annuus L.), essential oils, response surface methodology (RSM), GC-MS, antioxidant analysis, xanthine oxidase (XO)
Citation: Liu X-S, Gao B, Dong Z-D, Qiao Z-A, Yan M, Han W-W, Li W-N and Han L (2021) Chemical Compounds, Antioxidant Activities, and Inhibitory Activities Against Xanthine Oxidase of the Essential Oils From the Three Varieties of Sunflower (Helianthus annuus L.) Receptacles. Front. Nutr. 8:737157. doi: 10.3389/fnut.2021.737157
Received: 25 August 2021; Accepted: 04 October 2021;
Published: 19 November 2021.
Edited by:
Dejian Huang, National University of Singapore, SingaporeReviewed by:
Marco Iammarino, Istituto Zooprofilattico Sperimentale di Puglia e Basilicata (IZSPB), ItalyViduranga Y. Waisundara, Australian College of Business and Technology, Sri Lanka
Copyright © 2021 Liu, Gao, Dong, Qiao, Yan, Han, Li and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu Han, bHVoYW4mI3gwMDA0MDtqbHUuZWR1LmNu
†These authors have contributed equally to this work
 Xin-Sheng Liu1†
Xin-Sheng Liu1† Wei-Wei Han
Wei-Wei Han Wan-Nan Li
Wan-Nan Li Lu Han
Lu Han