
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 07 October 2021
Sec. Clinical Nutrition
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.729510
This article is part of the Research Topic Nutritional Assessment Tools for Identification and Monitoring of Malnutrition in Patients with Chronic Disease - Volume 1 View all 13 articles
 Mojgan Behrad Nasab1
Mojgan Behrad Nasab1 Mohammad Esmail Akbari2*
Mohammad Esmail Akbari2* Samira Rastgoo3
Samira Rastgoo3 Somayeh Gholami4
Somayeh Gholami4 Azadeh Hajipour5
Azadeh Hajipour5 Nazanin Majidi6
Nazanin Majidi6 Maryam Gholamalizadeh7
Maryam Gholamalizadeh7 Samaneh Mirzaei Dahka8
Samaneh Mirzaei Dahka8 Saied Doaei9*
Saied Doaei9* Mark O. Goodarzi10
Mark O. Goodarzi10Background: Nutrient imbalance can frequently occur in patients with indications for parenteral nutrition (PN) after gastrointestinal surgery. This study aimed to compare the recommendations of a surgeon to those of a dietitian in the field of parenteral nutrition.
Methods: This study was performed on 256 patients undergoing gastrointestinal surgery who received PN, which included 120 patients who received PN based on recommendations of the surgeons and 136 patients who were referred to receive PN under the supervision of a dietitian in Razi Hospital in Rasht, Iran. Data on PN and clinical outcomes of the patients were collected.
Results: Patients under the supervision of dietitians received higher vitamin B complex and lipids and lower vitamin A and vitamin E than the surgeon-supervised patients (all P < 0.001). In the group receiving PN under the supervision of a surgeon, the level of blood glucose (207 vs. 182, P < 0.01), sodium (138 vs. 136, P = 0.01), potassium (3.97 vs. 3.53, P < 0.01), and white blood cell count (9.83 vs. 9.28, P < 0.01) increased significantly at the end of the PN compared to baseline. In the group receiving PN under the supervision of a dietician, the level of serum Cr (1.23 vs. 1.32, P = 0.04), Mg (2.07 vs. 1.84, P < 0.01), and pH (7.45 vs. 7.5, P = 0.03) significantly improved after receiving parenteral nutrition compared to baseline.
Conclusion: The amounts of nutrients recommended for PN by the surgeon and dietitian were different. Implementation of dietitian recommendations in critically ill patients under PN can improve patients' clinical parameters.
Parenteral Nutrition (PN) is applied as a method of nutrition therapy for ICU patients when bowel failure prevents adequate oral or enteral nutrition (1). After the patient is admitted to the ICU and the patient does not tolerate enteral nutrition for more than 2–3 days, it is recommended to start PN as an alternative or supplemental diet therapy (2). By preventing malnutrition and reducing stress, PN has positive effects on critical care, especially of people older than 50 years of age (3). On average, ~34,000 patients in the United States receive PN each year. (4). Parenteral nutrition can lead to a moderate increase in pre-albumin, which is one of the markers of survival in critically ill patients (5, 6). However, PN is an expensive nutritional support and may have serious side effects if not properly administered (1). As a result, it is important to provide nutritional recommendations based on standard guidelines in order to minimize nutritional complications. The American Society for Parenteral and Enteral Nutrition (ASPEN) and the European Society for Clinical Nutrition and Metabolism (ESPEN) formed special groups to encourage proper use of PN to promote benefits and reduce risks (1). PN standardization was developed by ESPEN and ASPEN to increase patient safety and clinical suitability (7).
Nutrient imbalance can frequently occur in patients after gastrointestinal surgery with indications for PN (8). Energy-protein deficiency is a common clinical problem in critically ill patients. On the other hand, the risk of overfeeding in parenteral nutrition is greater than that of enteral nutrition (2), and there is a risk of circulatory infection with increasing calorie supply in parenteral nutrition (9) The prevalence of carbohydrate metabolism disorders in critically ill patients is very high, which complicates insulin therapy and the amount of metabolic control achieved (10). High levels of blood glucose in patients with PN can lead to increased mortality (11). Parenteral nutrition can also exacerbate liver and biliary disorders (12).
Surgeons, internists, critical care medicine specialists, pharmacists, and dietitians are responsible for providing nutritional recommendations for ICU patients with PN. Dietitians may apply different nutritional recommendations compared with the other specialists based on different training and responsibilities. Applying the advice of dietitians to assess nutritional requirements and determine the amount of nutritional supplements needed can be effective in improving the health status of critically ill patients. However, some surgeons prefer to order nutritional recommendations directly and not all patients with PN indications are referred to a dietitian. No study has been done to compare the nutritional recommendations of the dietitians with surgeons and their effects on patients. So, the aim of this study was to compare the biochemical and pathological parameters and parenteral nutrition of ICU patients under supervision of surgeons or a dietician.
This retrospective study was performed on 256 patients with Gastroenterologic disease undergoing gastrointestinal surgery with intestinal failure and indication for TPN, which included 120 patients who received PN based on recommendations of the surgeons and 136 patients who received PN under the supervision of a single dietitian in the years 2019 and 2020 in Razi Hospital in Rasht, Iran. The sample size was estimated based on a previous similar study (13). Inclusion criteria were indication for receiving PN, age between 50 and 80 years, and consent to participate in the study. The exclusion criteria were lack of access to sufficient information on the amount of PN received and receiving enteral or oral nutrition along with PN.
Age, sex, weight, height, BMI, duration of hospitalization, medical history including chronic diseases (i.e., diabetes, chronic kidney diseases, hyperlipidemia, and hypertension), diagnosed disease, pathological indices, the Acute Physiologic Assessment and Chronic Health Evaluation II (APACHE II), Glasgow coma score (GCS), and blood glucose (BG), sodium (Na), potassium (K), urea nitrogen (BUN), creatinine (Cr), white blood cell count (WBC), magnesium (Mg), albumin (Alb), calcium (Ca), and pH were extracted from the medical records before and after TPN. Information related to the nutritional recommendations in the field of TPN, including the amount and percentage of dextrose, amino acids, lipids, vitamins, and minerals were collected from ICU sheets after PN was finished. SMOFlipid® (Fresenius Kabi, United states) was used as the lipid source, which is a composite parenteral nutrition (PN) lipid, comprised of soybean oil (30%), medium-chain triglycerides (MTCs, 30%), olive oil (25%), and fish oil (15%). The mean essential fatty acid content of SMOFlipid is 35 mg/mL (range of 28 to 50 mg/mL) linoleic acid (omega-6) and 4.5 mg/mL (range of 3–7 mg/mL) α-linolenic acid (omega-3) (14). The amount of macro-nutrients administration was determined according to the patient's weight. The amount of micro-nutrients prescribed was vitamin A 50,000 IU/d, vitamin E 100 IU/d, vitamin C 500 mg/d, vitamin B complex containing vitamin B1 10 mg/d, vitamin B2 4 mg/d, vitamin B3 40 mg/d, vitamin B5 6 mg/d, and vitamin B6 4 mg/d.
The two groups receiving parenteral nutrition under the supervision of surgeons or a dietitian were compared in terms of demographic and pathological indicators using independent T-test and Chi-square methods. Also, the amounts of macronutrients and micronutrients received in the two groups were compared by independent T-test. The two groups were compared regarding the number of patients who received the nutrients using Chi-square and Fisher's exact test. The values of clinical and biochemical parameters before and after PN in each group were compared by paired t-test. All analyzes were performed using SPSS software version 21 and the significance level was considered as P >0.05.
Written consent forms were obtained from all participants or their first-degree relatives. This study was approved by the ethics committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran.) code IR.SBMU.CRC.REC.1398.015).
No significant difference was found in terms of sex (males: 46.7 vs. 50.0%), age (62 ± 12 vs. 67 ± 18 years), weight (73 ± 6 vs. 75 ± 7 kg), height (165 ± 6 vs. 168 ± 6 cm), duration of hospitalization (27 ± 31 vs. 22 ± 8 days), and BMI (27 ± 2.7 vs. 26 ± 3 kg/m2) between dietitian-supervised and surgeon supervised groups (Table 1). In addition, no significant difference was seen between the two groups in terms of history of hypertension (50 vs. 47%), hyperlipidemia (23.5 vs. 13%), ischemic heart disease (8.8 vs. 6.7%), and hemoglobin level (9 ± 1 vs. 9 ± 1) (Table 1).
Dietitian-supervised patients had a higher burden of chronic diseases (79.4 vs. 53.3%, p = 0.025), diabetes (61.8 vs. 23.3%, P = 0.002), chronic kidney disease (CKD) (20 vs. 0%, P = 0.03), and levels of BG (209 ± 31 vs. 182 ± 28 mg/dl, P = 0.001), Na (141 ± 5 vs. 135 ± 3 mEq/L, P < 0.001), K (4 ± 0.4 vs. 3 ± 0.3 mEq/L, P < 0.001), BUN (47 ± 14 vs. 38 ± 10 mg/dl, P = 0.004), and Cr (1.32 ± 0.2 vs. 1.11 ± 0.1 mg/dl, P < 0.001) compared to the surgeon-supervised patients. Surgeon-supervised patients had higher APACHE II score (21 ± 1 vs. 14 ± 2, P < 0.001) and GCS (15 vs. 8 ± 0.5, P < 0.001) compared to the dietitian-supervised patients.
Regarding the percentages of the patients who received different nutrients and met the recommended amounts, the results showed that the number of patients receiving lipid (P < 0.001) and vitamin B complex was significantly higher in dietitian-supervised group, while the number of patients receiving vitamin A and vitamin E was significantly higher in surgeon-supervised group (Table 2). Regarding nutritional recommendations, the number of days that each patient received lipids (5.59 ± 1.13 vs. 2 ± 2.55 days, P < 0.001) and vitamin B complex (8.1 ± 2.8 vs. 0 days, P = 0.001) was higher in the dietitian-supervised group compared to the surgeon-supervised group (Table 2).
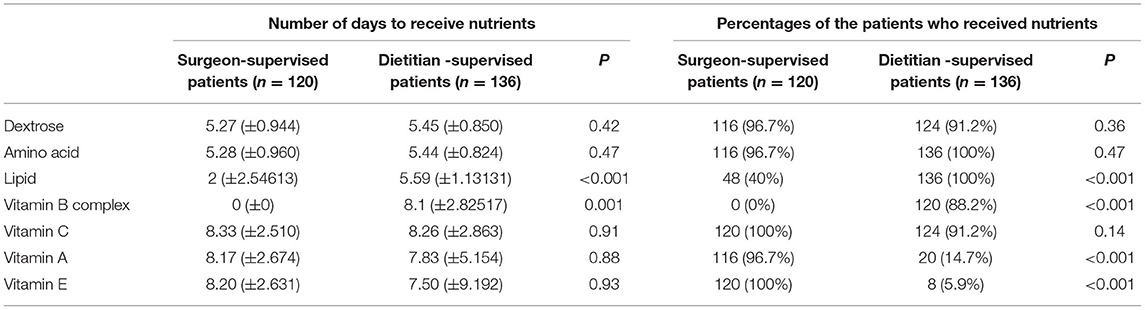
Table 2. Average number of days to receive nutrients and the percentages of the patients who received nutrients among patients under surgeon and dietitian recommendations.
In the group receiving parenteral nutrition under the supervision of a surgeon, the level of BG (207 ± 35 vs. 182 ± 28, P < 0.01), sodium (138 ± 3 vs. 136 ± 3 mg/dl, P = 0.01), potassium (3.97 ± 0.4 vs. 3.53 ± 0.4, P < 0.01), and white blood cell count (9.83 ± 2.5 vs. 9.28 ± 2.4 109/L, P < 0.01) increased significantly at the end of the parenteral nutrition period compared to baseline. In the group receiving parenteral nutrition under the supervision of a dietician, the level of serum Cr (1.23 ± 0.2 vs. 1.32 ± 0.2 mg/dl, P = 0.04), Mg (2.07 ± 0.2 vs. 1.84 ± 0.2 mg/dl, P < 0.01), and pH (7.45 vs. 7.5, P = 0.03) significantly improved after receiving parenteral nutrition compared to baseline. Serum urea, albumin and calcium levels after parenteral nutrition in the two groups were not significantly different from the baseline levels (Table 3).
In the present study, for the first time, the performance of dietitians was compared to surgeons in parenteral feeding of patients after gastrointestinal surgery. The results indicated that patients with worsening conditions were referred to a dietitian. Moreover, patients under the supervision of dietitians received higher vitamin B complex and lipids than the group under the supervision of surgeons. In the surgeon-supervised group, the patients received higher amounts of vitamin A and vitamin E than the dietitian-supervised patients. In the group receiving parenteral nutrition under the supervision of a surgeon, the level of BG, sodium, potassium, and white blood cells count increased significantly at the end of the PN compared to baseline. In the group receiving PN under the supervision of a dietician, the level of serum Cr, Mg, and pH significantly improved after receiving parenteral nutrition compared to baseline.
Providing parenteral nutrition can be vital for patients with intestinal failure, but achieving the desired amount and balance is a complicated issue and many factors such as age, degree of inflammation, number of failing organs, comorbidities, estimated length of stay, gastrointestinal function, fluids and electrolytes, and BG control must be considered in parenteral nutrition planning. Patients admitted to the ICU should receive PN within 24–48 h if they are unable to tolerate enteral nutrition.
Tignanelli et al. reported that mortality was lower in patients with nutritional counseling and that malnutrition should be prevented in order to prevent adverse consequences. Malnutrition increases the risk of disease, adverse surgical outcomes, length of stay in the hospital, and cost burden. Disease-induced stress in ICU patients may accelerate the development of malnutrition.
Patients receiving nutritional care from dietitians were reported to reach the target dietary intake faster and their clinical outcomes were improved (15). Vankrunkelsven et al. examined parenteral administration of micronutrients including phosphate, magnesium, iron and B-complex vitamins including vitamins B12, B1, and folic acid and concluded that nutrient deficiency may be related to the degree of inflammation (16).
In the study by Heyland et al., most ICU patients did not receive adequate nutritional support, especially early in their illness, and their energy and protein requirements were not correctly estimated (17).
We found that patients receiving parenteral nutrition under the supervision of a dietitian received similar dextrose compared with the patients under the supervision of a surgeon. Several previous reports indicated that receiving dextrose parenterally during the first week in the ICU leads to fewer secondary infections, less weakness, rapid recovery, and reduced patient mortality (18–21). However, hyperglycemia is an independent risk factor for short-term infection in patients undergoing surgery (14, 15). The risk of hyperglycemia as a part of the endocrine metabolic response to stress is present in almost all patients in the ICU. If the requirement for intravenous dextrose in patients is not specifically assessed and determined, it may increase the risk of hyperglycemia. In the present study, the blood sugar level of patients under the supervision of a surgeon increased significantly after receiving intravenous nutrition.
In the present study, patients receiving parenteral nutrition under the supervision of a dietitian received more lipid than patients under the supervision of a surgeon. Lipids should be considered as an integral part of PN to provide energy and ensure a supply of essential fatty acids. Providing essential fatty acids in PN using standard lipid emulsions can lead to additional clinical benefits such as reductions in both infection rate and length of hospital stay (22, 23). Various mixtures of lipid emulsions, including soybean oil, medium chain triglycerides, olive oil, and omega-3 rich fish oil, are widely available for parenteral nutrition. Omega-3 fatty acids can have beneficial immune-regulating and anti-inflammatory effects in a wide range of patients undergoing surgery (23). The addition of EPA and DHA to lipid emulsions may improve cell membrane function and inflammation, and reducing the length of stay of critically ill patients in the ICU (23). Pradelli et al. in a systematic review reported that omega-3 fatty acid-containing PN was associated with clinically significant improvement in patient outcomes (24). In the present study, SMOFlipid was used as the lipid source of TPN, which is rich in omega-3 fatty acids and higher lipid intake in the group under the supervision of a dietitian was associated with improved serum creatinine and pH levels and no increase in BG levels. However, high intake of unsaturated fatty acids in critically ill patients may be associated with side effects such as disturbed liver function or altered balances of antioxidants (25) and should be recommended according to the patient's requirements.
The results of this study indicated that dietitians may be better able to assess the nutritional requirements of critically ill patients and significantly help to improve the biochemical and pathological parameters of these patients. In line with the present study, evidence suggests that dietitians are key members of the ICU care team who can help improve patient outcomes (26). Severely ill patients who had sufficient nutritional intake were less likely to develop pneumonia, pulmonary insufficiency, gastrointestinal bleeding, or the need for mechanical ventilation (27).
The intake of vitamins A and E in the group under the supervision of the surgeon was higher and the intake of B vitamins was lower than the group under the supervision of the dietitian. Because of the risk of toxicity, fat soluble vitamins such as vitamin E and vitamin A should not be prescribed at high doses without proven deficiency. It was reported that patients with renal failure may be at risk for symptomatic vitamin A toxicity if given PN with standard retinol supplementation. However, vitamin E may reduce the length of mechanical ventilation in ICU patients (28, 29). On the other hand, critical illness in adults is characterized by absolute or relative thiamine depletion, which is associated with an almost 50% increase in mortality. Vitamin B1 is likely to be used in high-risk patients to prevent Wernicke's encephalopathy and heart failure. Moreover, administration of vitamin B1 may be used as adjunctive therapy in septic shock (30–33).
However, this study was limited to the ICU patients undergoing surgery, which makes it difficult to generalize the results to other patients. Moreover, the dietitian-supervised patients were significantly different compared with surgeon-supervised patients in terms of history of chronic diseases and pathological and biochemical parameters which may influence nutritional recommendations as well as the biochemical changes observed after TPN. In addition, individual dietary requirements of the patients were not assessed. Future longitudinal studies are needed to confirm these results and to investigate the effects of dietitian and surgeon PN recommendations on health outcomes of the patients.
The results indicated that patients with worsening conditions were referred to a dietitian. Moreover, patients under the supervision of dietitians received higher vitamin B complex and lipids than the surgeon-supervised patients. In the surgeon-supervised group, the patients received higher amounts of vitamin A and vitamin E than the dietitian-supervised patient. Biochemical changes suggestive of better outcomes were observed in the dietician-supervised group. Future longitudinal studies are needed to investigate the effects of dietitian and surgeon PN recommendations on health outcomes of the patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Data may be made available upon request.
The written consent forms were obtained from all participants or their first-degree relatives. This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (code IR.SBMU.CRC.REC.1398.015). The patients/participants provided their written informed consent to participate in this study.
SD, MB, MA, MG, SR, SG, AH, and NM designed the study, involved in the data collection, analysis, and drafting of the manuscript. SMD, MOG, and SD were involved in the design of the study, analysis of the data, and critically reviewed the manuscript. All authors read and approved the final manuscript.
Funding for this study was provided by Shahid Beheshti University of Medical Sciences, Tehran Iran (code 16415) and Guilan University of Medical Sciences, Rasht, Iran (code 578).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This study was conducted at Shahid Beheshti University of Medical Sciences, Tehran, Iran and Guilan University of Medical Sciences, Rasht, Iran. We acknowledge the staff of the mentioned centers for their kind cooperation.
1. Worthington P, Balint J, Bechtold M, Bingham A, Chan LN, Durfee S, et al. When is parenteral nutrition appropriate? J Parent Enteral Nutr. (2017) 41:324–77. doi: 10.1177/0148607117695251
2. Oshima T, Hiesmayr M, Pichard C. Parenteral nutrition in the ICU setting: need for a shift in utilization. Curr Opin Clin Nutr Metab Care. (2016) 19:144–50. doi: 10.1097/MCO.0000000000000257
3. Wischmeyer PE, Hasselmann M, Kummerlen C, Kozar R, Kutsogiannis DJ, Karvellas CJ, et al. A randomized trial of supplemental parenteral nutrition in underweight and overweight critically ill patients: the TOP-UP pilot trial. Crit Care. (2017) 21:1–14. doi: 10.1186/s13054-017-1736-8
4. Lappas BM, Patel D, Kumpf V, Adams DW, Seidner DL. Parenteral nutrition: indications, access, and complications. Gastroenterol Clin. (2018) 47:39–59. doi: 10.1016/j.gtc.2017.10.001
5. Anderson J, Peterson K, Bourne D, Boundy E. Effectiveness of intradialytic parenteral nutrition in treating protein-energy wasting in hemodialysis: a rapid systematic review. J Renal Nutr. (2019) 29:361–9. doi: 10.1053/j.jrn.2018.11.009
6. Marsen TA, Beer J, Mann H. group GI-T. Intradialytic parenteral nutrition in maintenance hemodialysis patients suffering from protein-energy wasting Results of a multicenter, open, prospective, randomized trial. Clin Nutr. (2017) 36:107–17. doi: 10.1016/j.clnu.2015.11.016
7. Directors ABo, Standardization TFoPN, Kochevar M, Guenter P, Holcombe B, Malone A, et al. ASPEN statement on parenteral nutrition standardization. J Parent Enteral Nutr. (2007) 31:441–8. doi: 10.1177/0148607107031005441
8. Möller-Loswick AC, Zachrisson H, Bennegård K, Sandström R, Lundholm K. Insufficient effect of total parenteral nutrition to improve protein balance in peripheral tissues of surgical patients. J Parent Enteral Nutr. (1991) 15:669–75. doi: 10.1177/0148607191015006669
9. Dissanaike S, Shelton M, Warner K, O'Keefe GE. The risk for bloodstream infections is associated with increased parenteral caloric intake in patients receiving parenteral nutrition. Crit Care. (2007) 11:1–9. doi: 10.1186/cc6167
10. Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. J Parent Enteral Nutr. (2014) 38:196–204. doi: 10.1177/0148607113502674
11. Drincic AT, Knezevich JT, Akkireddy P. Nutrition and hyperglycemia management in the inpatient setting (meals on demand, parenteral, or enteral nutrition). Curr Diab Rep. (2017) 17:1–12. doi: 10.1007/s11892-017-0882-3
12. Wu G, Jiang Y, Zhu X, Jin D, Han Y, Han J, et al. Prevalence and risk factors for complications in adult patients with short bowel syndrome receiving long-term home parenteral nutrition. Asia Pac J Clin Nutr. (2017) 26:591–7. doi: 10.6133/apjcn.082016.08
13. Wu S-C, Chen T-A, Tsai I-J, Wang Y-C, Cheng H-T, Tzeng C-W, et al. Lipid-free parenteral nutrition is associated with an increased risk of hepatic dysfunction in surgical critically ill patients: a retrospective observational study. Healthcare. (2021) 9:1096. doi: 10.3390/healthcare9091096
14. Mirtallo J, Johnson D. Task force for the revision of safe practices for parenteral nutrition. Safe practices for parenteral nutrition [published corrrection appears in JPEN J Parenter Enteral Nutr. 2006; 30: 177]. J Parenter Enteral Nutr. (2004) 28:S39–70. doi: 10.1177/0148607104028006s39
15. Tignanelli CJ, Sheetz KH, Petersen A, Park PK, Napolitano LM, Cooke CR, et al. Utilization of Intensive Care Unit Nutrition Consultation Is Associated With Reduced Mortality. J Parenter Enteral Nutr. (2020) 44:213–9. doi: 10.1002/jpen.1534
16. Vankrunkelsven W, Gunst J, Amrein K, Bear DE, Berger MM, Christopher KB, et al. Monitoring and parenteral administration of micronutrients, phosphate and magnesium in critically ill patients: the VITA-TRACE survey. Clin Nutrition. (2020).
17. Heyland DK, Schroter-Noppe D, Drover JW, Jain M, Keefe L, Dhaliwal R, et al. Nutrition support in the critical care setting: current practice in canadian ICUs–opportunities for improvement? J Parenter Enteral Nutr. (2003) 27:74–83. doi: 10.1177/014860710302700174
18. Berger MM, Reintam-Blaser A, Calder PC, Casaer M, Hiesmayr MJ, Mayer K, et al. Monitoring nutrition in the ICU. Clin Nutr. (2019) 38:584–93. doi: 10.1016/j.clnu.2018.07.009
19. Chow KW, Kelly DJ, Gupta R, Miller JD. Use of continuous glucose monitoring to assess parenteral nutrition–induced hyperglycemia in an adult patient with severe COVID-19. J Parenter Enteral Nutr. (2021) 45:208–11. doi: 10.1002/jpen.2032
20. Farrokhi F, Chandra P, Smiley D, Pasquel FJ, Peng L, Newton CA, et al. Glucose variability is an independent predictor of mortality in hospitalized patients treated with total parenteral nutrition. Endocr Pract. (2014) 20:41–5. doi: 10.4158/EP13131.OR
21. Ingels C, Vanhorebeek I, Van den Berghe G. Glucose homeostasis, nutrition and infections during critical illness. Clin Microbiol Infect. (2018) 24:10–5. doi: 10.1016/j.cmi.2016.12.033
22. Garnacho-Montero J, Ortiz-Leyba C, Jimenez-Jimenez F, Garcia-Garmendia J, Jiménez-Jiménez L, Garnacho-Montero M, et al. Clinical and metabolic effects of two lipid emulsions on the parenteral nutrition of septic patients. Nutrition. (2002) 18:134–8. doi: 10.1016/S0899-9007(01)00716-X
23. Mayer K, Klek S, García-de-Lorenzo A, Rosenthal MD, Li A, Evans DC, et al. Lipid use in hospitalized adults requiring parenteral nutrition. J Parenter Enteral Nutr. (2020) 44:S28–38. doi: 10.1002/jpen.1733
24. Pradelli L, Klek S, Mayer K, Alsaleh AJO, Rosenthal MD, Heller AR, et al. Omega-3 fatty acid-containing parenteral nutrition in ICU patients: systematic review with meta-analysis and cost-effectiveness analysis. Crit Care. (2020) 24:1–12. doi: 10.1186/s13054-020-03356-w
25. Wanten GJ. Parenteral lipid tolerance and adverse effects: fat chance for trouble? J Parenter Enteral Nutr. (2015) 39:33S−8S. doi: 10.1177/0148607115595973
26. Taylor B, Renfro A, Mehringer L. The role of the dietitian in the intensive care unit. Curr Opin Clin Nutr Metab Care. (2005) 8:211–6. doi: 10.1097/00075197-200503000-00017
27. Mulherin DW, Cogle SV. Updates in nutrition support for critically III adult patients. Hosp Pharm. (2017) 52:17–26. doi: 10.1310/hpj5201-17
28. Singer P, Berger MM, Van den Berghe G, Biolo G, Calder P, Forbes A, et al. ESPEN guidelines on parenteral nutrition: intensive care. Clin Nutr. (2009) 28:387–400. doi: 10.1016/j.clnu.2009.04.024
29. Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. (2019) 38:48–79. doi: 10.1016/j.clnu.2018.08.037
30. Collie JT, Greaves RF, Jones OA, Lam Q, Eastwood GM, Bellomo R. Vitamin B1 in critically ill patients: needs and challenges. Clin Chem Lab Med. (2017) 55:1652–68. doi: 10.1515/cclm-2017-0054
31. Fujii T, Luethi N, Young PJ, Frei DR, Eastwood GM, French CJ, et al. Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: the VITAMINS randomized clinical trial. JAMA. (2020) 323:423–31. doi: 10.1001/jama.2019.22176
32. Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. (2017) 151:1229–38. doi: 10.1016/j.chest.2016.11.036
33. Truwit JD, Hite RD, Morris PE, DeWilde C, Priday A, Fisher B, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA. (2019) 322:1261–70. doi: 10.1001/jama.2019.11825
Keywords: parenteral nutrition, dietitian, ICU patient, ICU, PN, total parenteral nutrition, surgeon, critically ill patients
Citation: Behrad Nasab M, Akbari ME, Rastgoo S, Gholami S, Hajipour A, Majidi N, Gholamalizadeh M, Mirzaei Dahka S, Doaei S and Goodarzi MO (2021) Comparison of Biochemical and Pathological Parameters and Parenteral Nutrition of ICU Patients Under Supervision of Dietitians and Surgeons. Front. Nutr. 8:729510. doi: 10.3389/fnut.2021.729510
Received: 23 June 2021; Accepted: 13 September 2021;
Published: 07 October 2021.
Edited by:
Lilia Castillo-Martinez, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), MexicoReviewed by:
José L. Villanueva-Juárez, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), MexicoCopyright © 2021 Behrad Nasab, Akbari, Rastgoo, Gholami, Hajipour, Majidi, Gholamalizadeh, Mirzaei Dahka, Doaei and Goodarzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saied Doaei, c2RvYWVlQHlhaG9vLmNvbQ==; Mohammad Esmail Akbari, cHJvZm1lYWtiYXJpQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.