
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 20 August 2021
Sec. Nutrition and Food Science Technology
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.715287
This article is part of the Research TopicSustainable Solutions in Food TechnologyView all 8 articles
Omega-3 (n-3) fatty acids (FA) play an essential role in human physiology and health. As a result, a variety of n-3 FA-fortified functional foods have become commercially available for human consumption. These fortified functional foods are created through various processes; however, nutri-priming, a potentially promising fortification approach, has not been utilized to develop plant-based n-3 fortified foods. We sought to determine whether nutri-priming is a viable option to enrich seeds and sprouts with n-3 FA. Additionally, we assessed whether n-3 FA nutri-priming would inhibit germination of the primed seeds. To address these goals, we nutri-primed brown flax in three priming solutions, control [0% fish oil (FO)], 10% FO and a 20% FO solution, and determined the FA content and profile of seeds and sprouts and germination percentage of primed seeds. n-3 FA nutri-priming with FO altered the FA profile in seeds and sprouts, with increases in the absolute content of 20:5 n-3, 22:6 n-3, 22:5 n3, 18:4 n-3, and 20:4 n-6. However, n-3 FA nutri-priming did not increase the absolute content of 18:2 n-6, 18:3 n-3, total saturated FA, total monounsaturated FA, total polyunsaturated FA, total n-6 FA, or total n-3 FA. Our results also showed that n-3 nutri-priming decreased the germination percentage of primed seeds, with 10 and 20% FO priming solution reducing germination by 4.3 and 6.2%, respectively. Collectively, n-3 nutri-priming modified the n-3 FA profile in flax; however, the process does not increase the total n-3 FA content and inhibits germination of primed seeds. Further research utilizing different seed types, oil types, and oil concentrations needs to be conducted to fully determine if n-3 nutri-priming is a commercially viable approach for n-3 fortification of seeds and sprouts.
Omega-3 (n-3) fatty acids (FA) are polyunsaturated FA (PUFA) that play an important role in human physiology and health (1). n-3 FA are key components of the cell membrane's phospholipid bilayer, which provide protection and structure for cells (1). Additionally, specific n-3 FA have critical physiological functions; eicosapentaenoic acid (EPA, 20:5 n-3) and docosahexaenoic acid (DHA, 22:6 n-3) are essential for brain development (1–3). Furthermore, the enrichment of the diets with n-3 FA has been shown to reduce the risk and death from coronary heart disease and cardiovascular diseases (4). Despite the importance of n-3 FA, the body cannot, or is inefficient at biosynthesizing n-3 FA, making diet the only meaningful method to obtain certain beneficial n-3 FA. For instance, alpha-linolenic acid (LNA, 18:3 n-3) is an essential n-3 FA that cannot be biosynthesized by humans and therefore must be obtained by diet. Additionally, DHA and EPA can be biosynthesized from ALA, but this process is inefficient, with 5% of ALA being converted to EPA and <1% into DHA (1–5). As a result of n-3 FA having a key role in human health, and diet being the primary source of certain n-3 FA, a wide variety of functional foods (i.e., foods that possess positive human health benefits in addition to basic nutrition) enriched with n-3 FA have become commercially available (5, 6), such as n-3 fortified milk (7, 8) and eggs (9–11).
There are numerous methods to create functional foods fortified with n-3 FA (5, 12). For example, n-3-fortified eggs are produced by supplementing chicken feed with n-3 FA, typically in the form of flax or linseeds (Linum usitatissimum L.), which contain a high content of LNA (9–11, 13, 14). As a result of the n-3 FA supplementation, the produced eggs contain up to 5 to 6 times more n-3 FA (primarily LNA) than conventional eggs (11, 15–17). However, methods to create plant-based functional foods fortified with long-chain n-3 FA, are currently limited (5, 18). This limitation leads to the exclusion of a growing group of the population who predominately follow a plant-based diet, such as vegans, vegetarians, pescatarian, and flexiterians from consuming certain functional foods fortified with long-chain n-3 FA (19–21). One method that may be used to develop plant-based n-3 FA fortified functional foods on a commercial scale is nutri-priming, the process of imbibing seeds in a nutrient-rich solution then redrying to their original weight (22–25). Nutri-priming ensures micronutrient availability to the seed and has been shown to improve germination, seedling vigor, resilience, root development, and productivity in multiple crops (22–25). Additionally, in some instances, such as in corn (Zea mays L.) (26), chickpea (Cicer arietinum L.) (27), and wheat (Triticum aestivum L.) (27, 28), zinc nutri-priming increased the zinc content of seeds and seedlings (22, 29). Yet, despite the benefits of this fortification approach, there are currently no known examples in the scientific literature of using this process to create n-3 fortified seeds and sprouts.
Therefore, the purpose of this study was to determine whether nutri-priming is a viable option for n-3 fortification for seeds and sprouts. A potential limitation to n-3 FA nutri-priming may be the detrimental effects of n-3 FA on seed germination. Oils, such as crude oil and sunflower oil, have been shown to inhibit the germination and growth of various crops (30–32), likely because the oil forms a hydrophobic film on the seed and its roots, thus, preventing water and gas exchange (32). Consequently, the n-3 FA nutri-priming process may reduce germination and may not be a suitable option for creating n-3 fortified sprouts. The second aim of this study was therefore to determine whether n-3 nutri-priming inhibits germination of primed seeds.
To address these aims, we nutri-primed brown flax in three priming solutions, control (100% deionized water, no fish oil (FO) addition), 10% FO (90% deionized water plus 10% FO), and a 20% FO (80% deionized water plus 20% FO) solution, and determined the FA content and profile in seeds and sprouts as well as their germination percentage. We used flax and FO because they are commonly used to produce n-3 fortified functional foods (33–35) because they contain a high content of total n-3 FA (Σn-3 FA), and, in comparison to FO, a different profile of n-3 FA. Flax is enriched in LNA while FO is enriched with long-chain n-3 FA such as EPA and DHA (5). The utilization of these two sources of n-3 FA should create a functional food with enhanced n-3 FA content and profile that appeals to a wide range of consumers.
“Brown Flax” (Linum usitatissimum) from King's Agriseeds Inc. (Lancaster, PA) was used for the experiment. Eighteen replicates of 1 (±0.05) g of flax seeds (~180 seeds) were primed with either a control, 10%, or a 20% FO-water solution following the procedure described in Holub and Nagpurkar (36). The nutri-priming solution was created by mixing the respective ratio of FO derived from anchovy (Omega-3 Fish Oil EE - 40% EPA and 20% DHA, Jedwards International Inc. Braintree, MA) and deionized water (e.g., 1 mL of FO and 4 mL of deionized water for the 20% FO treatment) and vortexing for 10 minutes at 2,500 RPM. Seeds were then primed with the nutri-priming solution using 50 mL conical tubes on an orbital shaker set at 220 RPM for 5 hours. The constant movement by the orbital shaker prevented the separation of the oil and water and ensured that the seeds were in continual contact with the FO. Subsequently, seeds were removed and thoroughly rinsed with deionized water.
After seeds were rinsed, they were left to dry for at least 24 hours at room temperature (22°C). Subsequently, half of the replicates (n = 9) were placed in petri dishes (Fisherbrand™ Polystyrene, 100 mm, Pittsburg, PA) lined with three filter papers (Cytiva Whatman™ Qualitative Filter Paper: Grade 1, 90 mm, Maidstone, UK) to undergo sprouting. The remaining 9 replicates were used to quantify the FA content and profile in flax seeds. Filter papers were initially saturated with deionized water and then re-watered ad libitum for 10 days. Every 24 hours for 10 days, seeds were scored for germination. A seed was considered germinated if the radicle length reached 3 mm or if cotyledons fully emerged. Once a seed germinated, it was removed from the petri dish and stored at −20°C until further analysis. At the end of 10 days, the total number of germinated seeds and the total number of seeds that failed to germinate were calculated. Additionally, germination percentages were calculated by dividing the number of germinated seeds by the total number of seeds present in the petri dish.
Fatty acid analysis and calculations of flax seeds and sprouts were conducted as described in Goosen et al. (37) with the exception of using 150 mg of dried sample instead of 500 mg.
Absolute FA measures were analyzed using a two-way ANOVA, and multiple comparisons were made using a Tukey HSD test. The two factors used in the analysis were priming solution (control, 10, 20%) and plant stage (seed or sprout). Differences were considered significant with an adjusted P < 0.05. Furthermore, a principal component analysis (PCA) was conducted on the absolute FA data (i.e., mg/g) using the package “ggfortify” to identify FA that were important in explaining the variability between treatment groups (38). Germination data were analyzed using a general linear model with a beta-regression distribution with the “betareg” function from the R “betareg” package, and multiple comparisons were made using a Tukey HSD test (39). Differences were considered significant with an adjusted P < 0.05. All figures were created using the “ggplot2” package in R (40), and all statistical analyses were performed in R version 4.0.2 (41).
Absolute FA content and profile in seeds and sprouts was influenced by the nutri-priming solution, with an increase of fish-derived FA in the n-3 nutri-primed treatment groups (Table 1). PCA revealed that absolute FA content and profile of nutri-primed seeds and sprouts with 10 and 20% FO addition were similar, with apparent clustering away from the control (Figure 1). The FA driving clustering differences were primarily EPA, DHA, docosapentaenoic acid (DPA, 22:5 n-3), stearidonic acid (SDA, 18:4 n-3), and arachidonic acid (AA, 20:4 n-6) (Supplemental Figure 1). When comparing these FA independently, an increase of absolute content of EPA, DHA, DPA, SDA, and AA was seen in seeds and sprouts for both 10% and 20% FO groups when compared to the control group (Table 1, Figure 2). Furthermore, a dose-response relationship was observed between % FO and EPA, DHA, DPA, and AA (Table 1). As FO percentage increased in the nutri-priming solution, so did the absolute content of EPA, DHA, DPA, and AA; with increases of 34.6, 36.6, 45.5, and 25.8% in seeds, respectively, and 38.8, 37.9, 50, and 33.3% in sprouts, respectively. The content of SDA in seeds remained relatively consistent while the amount of SDA in sprouts increased by 66.7% between treatment groups. Despite increases in fish-derived FA, the absolute content of LA, LNA, total FA (ΣFA), total saturated FA (ΣSFA), total monounsaturated FA (ΣMUFA), ΣPUFA, total n-6 FA (Σn-6 FA), and Σn-3 FA did not increase as a result of n-3 FA nutri-priming (Table 1).
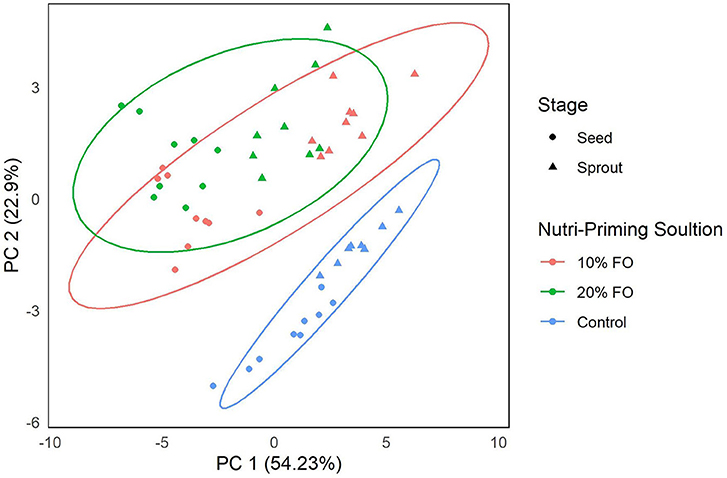
Figure 1. Principal component analysis of FA content (mg per g of sample) of flax seeds and sprouts by nutri-priming solution. Samples are color-coded by nutri-priming treatment (control, 10% fish oil (FO), and 20% FO) and shapes signify plant sage (seed or sprout).
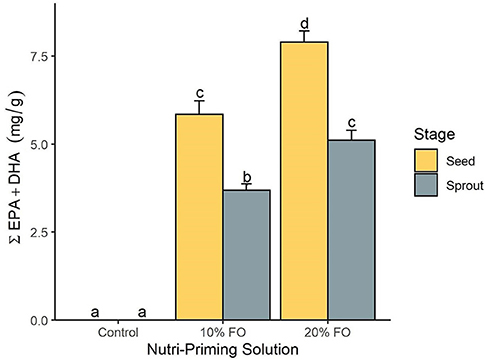
Figure 2. The total content of EPA and DHA (mg per g of sample) by nutri-priming solution and plant stage. Values are expressed as mean and standard error. Means without a common letter differ (P < 0.05).
Plant stage (i.e., seed and sprout) influenced the absolute FA content, with sprouts having a consistently lower FA content than their seed counterparts in all treatment groups (Table 1, Figure 2). For instance, in the 20% FO treatment group, EPA, DHA, DPA, AA, SDA, ALA, and LA decreased by 35.3, 35.5, 43.8, 28.2, 33.3, 13, and 9.1%, respectively. Similar losses were observed in the control and 10% FO group (Table 1).
Germination percentage was affected by the nutri-priming solution (P < 0.001, Figure 3). As the inclusion of FO% increased in the nutri-priming solution, germination percentage decreased (Figure 3). The highest inclusion rate (i.e., 20% FO) resulted in the lowest germination percentage with 87.7% (±0.6), followed by FO 10% with 89.5% (±0.7), and then the control group with 93.5% (±0.3) (Figure 3).
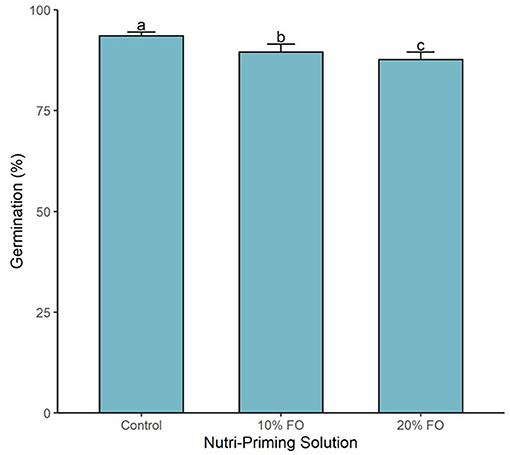
Figure 3. Germination percentage of sprouts by nutri-priming solution. Values are expressed as mean and standard error. Means without a common letter differ (P < 0.05).
We sought to determine whether n-3 nutri-priming is a viable option for the fortification of flax seeds and sprouts. Additionally, we assessed whether n-3 nutri-priming inhibits germination of nutri-primed seeds. We found that n-3 nutri-priming with FO influenced FA content and profile in seeds and sprouts (Table 1). Specifically, we observed an increase in the absolute content of EPA, DHA, DPA, SDA, and AA due to FO nutri-priming (Figure 2, Table 1). Additionally, as the percent of FO increased in the nutri-priming solution, so did the absolute content of EPA, DHA, DPA, and AA in both the seeds and sprouts (Figure 2, Table 1). This increase was expected, as flax does not contain EPA, SDA, DHA, DPA, and AA, while FO contains a moderate to high amount of each of these FA (5, 33, 34, 42, 43). In contrast, n-3 FA nutri-priming did not increase the absolute content of LA, LNA, ΣFA, ΣSFA, ΣMUFA, ΣPUFA, and Σn-6 FA and Σn-3 FA (Table 1). The primary reason for this is that flax is naturally high in Σn-3 FA, primarily LNA. We found that flax comprised of approximately 290 mg LNA per gram sample, which accounts for 56% of the total FA content (Supplemental Table 1). This finding is consistent with other studies that demonstrated flax to contain 39.9% to 60.4% of LNA (5, 33, 34). Because of flax having a high amount of LNA, the moderate increase in other n-3 FA, such as EPA, DHA, DPA, and SDA, did not significantly increase the overall content of total n-3 FA in seeds or sprouts. Potentially, n-3 FA nutri-priming with a higher percentage of FO or using other seeds with a low to moderate total n-3 FA content, such as mung bean (44), sunflower (45, 46), sesame (46), or lentil (45), may result in an increased content of ΣPUFA and Σn-3 FA.
The second objective of our study was to assess whether n-3 FA nutri-priming inhibits germination. We found that n-3 FA nutri-priming decreased the germination percentage of primed flax seeds. The decrease in germination percentage, while significant, is modest when compared to studies that assessed the effect of the presence of soil oils on crop germination (30–32). For instance, sunflower oil decreased wheat germination by 20% (32), and crude oil decreased corn germination by 37.5 to 93.8% (30). Further research utilizing different seed types is required to determine whether the reduction in germination percentage is a flax-specific or a general trend associated with n-3 FA nutri-priming.
Our results also indicate that n-3 nutri-primed seeds and sprouts can be used as a functional food to increase EPA and DHA in diets. The American Heart Association recommends consuming two fatty fish servings per week, which amounts to approximately 250 mg of EPA and DHA per day (47). Similarly, the World Health Organization (48), Food and Agriculture Organization (48), the Dietary Guidelines for Americans (49), and the European Food Safety Authority (50) recommend that adults consume 250 mg of EPA and DHA per day. To reach the international dietary recommended amount of EPA and DHA, one would need to consume 31.6, 48.9, 42.8, or 67.6 g of the 20% FO-treated seeds, 20% FO-treated sprouts, 10% FO-treated seeds, and 10% FO-treated sprouts, respectively. For the 20% FO-treated seed treatment group, which contained the highest combined EPA and DHA content (Table 1), the consumption of 31.6 grams of flax seed exceeds the Flax Council of Canada recommendation of consuming 8–16 g of flax per day and the daily recommended amount (1.1–1.6 grams per day) of LNA by Dietary Guidelines for Americans (49, 51). However, the consumption of more than 16 g of flax is safe and may be beneficial for human health (52). Cunnane et al. (53) concluded that consuming 50 g of flax per day was palatable, safe, and beneficial to human health by increasing n-3 FA in blood plasma and erythrocytes and reducing postprandial glucose response. Additionally, the consumption of 30 g of milled flaxseed every day for 6 months decreased systolic and diastolic blood pressure in patients with peripheral arterial disease (54), while the daily consumption of 40 g of milled flax seeds reduced cholesterol levels (51). Lastly, no clinical trial has reported toxicity due to dietary supplementation of flax (52). Therefore, the daily consumption of 30 to 50 g of n-3 nutri-primed flax seeds and sprouts is most likely a feasible and safe amount of flax to consume.
Other potential drawbacks including areas of future studies for n-3 nutri-priming are the sustainability and acceptability of FO, the cost-effectiveness of this approach, the commercial application of this process, and the effects of n-3 nutri-priming on the sensory and nutritional components of flax seeds and sprouts. The sustainability of FO has been called into question due to the rapid decline of fish stocks from overfishing and climate change (55–57). A typical FO supplement contains 1,000 mg of FO, which translates to 300 mg of EPA and DHA (58, 59). n-3 nutri-priming with 20% FO solution would utilize at least 25 times more FO to deliver the same amount of EPA and DHA as a typical FO supplement. Therefore, the results of this study indicate that n-3 nutri-priming with FO may be less cost-effective at increasing EPA and DHA in diets than typical FO supplements. Another major problem is that FO is animal-based which makes it unappealing to some consumers, like vegetarians and vegans, and even to some omnivores due to its fishy taste and odor (12, 33, 43, 60). Therefore, the effect of n-3 nutri-priming on the sensory components of flax seeds and sprouts needs to be thoroughly evaluated through sensory evaluation studies to gauge consumer acceptability. To alleviate these potential drawbacks, n-3 nutri-priming with alternative plant-based sustainable oils, such as echium oil (Echium plantagineum), may be a possible solution (61). Echium oil is a neutral, plant-based, and sustainable source of n-3 FA, primarily SDA (13–14% of total FA) (61, 62). While echium oil does not contain EPA or DHA, it has a high amount of SDA (an intermediate in the biosynthetic conversion of LNA to EPA), which the body can readily convert to EPA (43, 62). Most importantly, echium oil and specifically SDA show similar health benefits as FO, EPA, and DHA (43, 63–66). Furthermore, additional studies are required to understand the long-term functional stability of n-3 FA fortified seeds and sprouts and to determine if there are any effects on other nutritional components such as protein, carbohydrate, fiber, and antinutrient content. The long-term stability of the n-3 FA primed seeds and sprouts could be a major concern as n-3 PUFA are highly prone to oxidative degradation (67). Incorporating an antioxidant, such as vitamin E, into the n-3 FA nutri-priming process may mitigate this concern and extend the shelf life of n-3 FA fortified seeds and sprouts.
We evaluated the efficacy of n-3 nutri-priming of flax seeds and sprouts to increase n-3 FA and determined if this process inhibited germination. We demonstrated that FO nutri-priming of flax modified the FA profile of flax seeds and sprouts with the inclusion of beneficial FA, specifically EPA, DHA, DPA, SDA, and AA. Nutri-priming, however, did not increase the total content of n-3 FA of flax. This was because the modest increase in FO-derived n-3 FA, such as EPA, DHA, DPA, and SDA did not offset the naturally large amount of LNA present in flax. Additionally, our results also demonstrate that nutri-priming decreases germination. Therefore, n-3 nutri-priming does not seem to be a viable option for n-3 fortification of flax seeds or sprouts. However, further research utilizing other seed types, oil types, and oil concentrations is required to fully determine whether nutri-priming is indeed a viable commercial method for creating plant-based n-3 fortified functional foods.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
EM conducted the study and wrote the manuscript. JK and HD designed the study, edited, and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
This research was supported by UVM Ventures (Vermont Dept. of Economic Development #07120-19-17) and in part by Botanical Intelligence LLC.
This research was partially funded through Botanical Intelligence LLC, the current patent holder of Method of fortifying seeds with an essential fatty acid, fortified seed and food product (WO 2005/065468 A1). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Erika Bueno and Allison Unger for their help with the study and manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.715287/full#supplementary-material
FO, fish oil; LA, linoleic acid; LNA, alpha-linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid; SDA, stearidonic acid; AA, arachidonic acid; FA, fatty acid; MUFA, monounsaturated fatty acids; n-3 FA, n-3 fatty acids; n-6 FA, n-6 fatty acids; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids.
1. Institute of Medicine Food and Nutrition Board. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: National Academies Press (2005). doi: 10.17226/10490
2. Coates PM, Blackman MR, Cragg GM, Levine M, Moss J, White JD. Encyclopedia of Dietary Supplements. Coates PM, Paul MC, Blackman M, Blackman MR, editors. London, and New York, NY: CRC Press (2004). doi: 10.1201/b13959
3. Turpeinen APM. Functional Foods. Second ed. Saarela M, Woodhead Publishing (2011). doi: 10.1016/B978-1-84569-690-0.50028-X
4. Hu Y, Hu FB, Manson JE. Marine omega-3 supplementation and cardiovascular disease: an updated meta-analysis of 13 randomized controlled trials involving 127 477 participants. J Am Heart Assoc. (2019) 8:e013543. doi: 10.1161/JAHA.119.013543
5. Ganesan B, Brothersen C, McMahon DJ. Fortification of foods with omega-3 polyunsaturated fatty acids. Crit Rev Food Sci Nutr. (2014) 54:98–114. doi: 10.1080/10408398.2011.578221
6. Siró I, Kápolna E, Kápolna B, Lugasi A. Functional food. Product development, marketing and consumer acceptance—A review. Appetite. (2008) 51:456–67. doi: 10.1016/j.appet.2008.05.060
7. Unger AL, Bourne DE, Walsh H, Kraft J. Fatty acid content of retail cow's milk in the northeastern United States—what's in it for the consumer? J Agric Food Chem. (2020) 68:4268–76. doi: 10.1021/acs.jafc.9b07390
8. Marsanasco M, Calabró V, Piotrkowski B, Chiaramoni NS. del V. Alonso S Fortification of chocolate milk with omega-3, omega-6, and vitamins E and C by using liposomes. Eur J Lipid Sci Technol. (2016) 118:1271–81. doi: 10.1002/ejlt.201400663
9. Lemahieu C, Bruneel C, Ryckebosch E, Muylaert K, Buyse J, Foubert I. Impact of different omega-3 polyunsaturated fatty acid (n-3 PUFA) sources (flaxseed, Isochrysis galbana, fish oil and DHA Gold) on n-3 LC-PUFA enrichment (efficiency) in the egg yolk. J Funct Foods. (2015) 19:821–7. doi: 10.1016/j.jff.2015.04.021
10. Ehr IJ, Persia ME, Bobeck EA. Comparative omega-3 fatty acid enrichment of egg yolks from first-cycle laying hens fed flaxseed oil or ground flaxseed. Poult Sci. (2017) 96:1791–9. doi: 10.3382/ps/pew462
11. Khan SA, Khan A, Khan SA, Beg MA, Ali A, Damanhouri G. Comparative study of fatty-acid composition of table eggs from the Jeddah food market and effect of value addition in omega-3 bio-fortified eggs. Saudi J Biol Sci. (2017) 24:929–35. doi: 10.1016/j.sjbs.2015.11.001
12. Taneja A, Singh H. Challenges for the delivery of long-chain n-3 fatty acids in functional foods. Annu Rev Food Sci Technol. (2012) 3:105–23. doi: 10.1146/annurev-food-022811-101130
13. Palmquist DL. Omega-3 fatty acids in metabolism, health, and nutrition and for modified animal product foods. Prof Anim Sci. (2009) 25:207–49. doi: 10.15232/S1080-7446(15)30713-0
14. Bourre JM, Galea F. An important source of omega-3 fatty acids, vitamins D and E, carotenoids, iodine and selenium: a new natural multi-enriched egg. J Nutr Health Aging. (2006) 10:371–6. Available online at: http://www.ncbi.nlm.nih.gov/pubmed/17066208
15. Morris DH. The novel egg –Opportunities for flax in omega-3 production. Flax Counc Can. (2003) 8:2008. Available online at: http://www.flaxcouncil.ca/english/pdf/novelegg.pdf
16. Nain S, Renema RA, Korver DR, Zuidhof MJ. Characterization of the n-3 polyunsaturated fatty acid enrichment in laying hens fed an extruded flax enrichment source. Poult Sci. (2012) 91:1720–32. doi: 10.3382/ps.2011-02048
17. Rajasekaran A, Kalaivani M. Designer foods and their benefits: a review. J Food Sci Technol. (2013) 50:1–16. doi: 10.1007/s13197-012-0726-8
18. Bhowmick MK. Seed priming: a low-cost technology for resource-poor farmers in improving pulse productivity. In: Rakshit A, Singh HB, editors. Advances in Seed Priming. Singapore: Springer Singapore (2018). p. 187–208. doi: 10.1007/978-981-13-0032-5_11
19. Saari UA, Herstatt C, Tiwari R, Dedehayir O, Saku JM. The vegan trend and the microfoundations of institutional change: a commentary on food producers' sustainable innovation journeys in Europe. Trends Food Sci Technol. (2021) 107:161–7. doi: 10.1016/j.tifs.2020.10.003
20. Leitzmann C. Vegetarian nutrition: past, present, future. Am J Clin Nutr. (2014) 100:496S−502S. doi: 10.3945/ajcn.113.071365
21. Perry BD, Grace DC. How growing complexity of consumer choices and drivers of consumption behaviour affect demand for animal source foods. Ecohealth. (2015) 12:703–12. doi: 10.1007/s10393-015-1091-7
22. Farooq M, Usman M, Nadeem F, Rehman HU, Wahid A, Basra SMA, et al. Seed priming in field crops: potential benefits, adoption and challenges. Crop Pasture Sci. (2019) 70:731. doi: 10.1071/CP18604
23. Hussain HA, Hussain S, Anjum SA, Hussain S. Priming and Pretreatment of Seeds and Seedlings. Hasanuzzaman M, Fotopoulos V, editors. Singapore: Springer Singapore (2019). doi: 10.1007/978-981-13-8625-1
24. Singh H, Jassal RK, Kang JS, Sandhu SS, Kang H, Grewal K. Seed priming techniques in field crops -a review. Agric Rev. (2015) 36:251–64. doi: 10.18805/ag.v36i4.6662
25. Lutts S, Benincasa P, Wojtyla L, Kubala S, Pace R, Lechowska K, et al. Seed priming: new comprehensive approaches for an old empirical technique. In: New Challenges in Seed Biology - Basic and Translational Research Driving Seed Technology. (2016). p. 1–46. doi: 10.5772/64420
26. Harris D, Rashid A, Miraj G, Arif M, Shah H. ‘On-farm' seed priming with zinc sulphate solution—a cost-effective way to increase the maize yields of resource-poor farmers. F Crop Res. (2007) 102:119–27. doi: 10.1016/j.fcr.2007.03.005
27. Harris D, Rashid A, Miraj G, Arif M, Yunas M. ‘On-farm' seed priming with zinc in chickpea and wheat in Pakistan. Plant Soil. (2008) 306:3–10. doi: 10.1007/s11104-007-9465-4
28. Rehman A, Farooq M, Naveed M, Nawaz A, Shahzad B. Seed priming of Zn with endophytic bacteria improves the productivity and grain biofortification of bread wheat. Eur J Agron. (2018) 94:98–107. doi: 10.1016/j.eja.2018.01.017
29. Marques E, Darby HM, Kraft J. Benefits and limitations of non-transgenic micronutrient biofortification approaches. Agronomy. (2021) 11:464. doi: 10.3390/agronomy11030464
30. Ogboghodo IA, Iruaga EK, Osemwota IO, Chokor JU. An assessment of the effects of crude oil pollution on soil properties, germination and growth of maize (zea mays) using two crude types – forcados light and escravos light. Environ Monit Assess. (2004) 96:143–52. doi: 10.1023/B:EMAS.0000031723.62736.24
31. Zhu Y, Song Z, Chen J, Li C, Xiao B, Wang Q. Effects of oil-contaminated soil on the seed germination and seedling growth of selected crops. In: World Automation Congress. Puerto Vallarta, Mexico: IEEE (2012) p. 1–4. Available online at: https://ieeexplore.ieee.org/document/6321312
32. Polonskiy VI, Polonskaya DE. Possible mechanisms of multidirectional oil impacts on seed germination. Russ Agric Sci. (2015) 41:120–2. doi: 10.3103/S1068367415020202
33. Goyal A, Sharma V, Upadhyay N, Gill S, Sihag M. Flax and flaxseed oil: an ancient medicine; modern functional food. J Food Sci Technol. (2014) 51:1633–53. doi: 10.1007/s13197-013-1247-9
34. Morris D. Description and composition of flax. In: Flax—A health and nutrition primer. (2007) p. 9–21. Available online at: http://www.flaxcouncil.ca/english/pdf/FlxPrmr_4ed_Chpt1.pdf%5Cnhttp://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Description+and+Composition+of+Flax#0
35. Ruxton CHS, Reed SC, Simpson MJA, Millington KJ. The health benefits of omega-3 polyunsaturated fatty acids: a review of the evidence. J Hum Nutr Diet. (2004) 17:449–59. doi: 10.1111/j.1365-277X.2004.00552.x
36. Holub BJ, Nagpurkar A. Method of fortifying seeds with an essential fatty acid, fortified seed and food product. United States patent US 7,416,752 (2008). p. 1–23.
37. Goossen CP, Bosworth SC, Darby HM, Kraft J. Microwave pretreatment allows accurate fatty acid analysis of small fresh weight (100 g) dried alfalfa, ryegrass, and winter rye samples. Anim Feed Sci Technol. (2018) 239:74–84. doi: 10.1016/j.anifeedsci.2018.02.014
38. Tang Y, Horikoshi M. ggfortify: unified interface to visualize statistical result of popular R packages. R J. (2016) 8:474 Available online at: https://journal.r-project.org/
39. Cribari-Neto F, Zeileis A. Beta regression in R. J Stat Softw. (2010) 34:1–24. doi: 10.18637/jss.v034.i02
40. Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York, NY: Springer-Verlag (2016). Available online at: https://ggplot2.tidyverse.org
41. R Core Team. R: A Language and Environment for Statistical Computing. Vienna (2020). Available online at: https://www.r-project.org/
42. Durmuş M. Fish oil for human health: omega-3 fatty acid profiles of marine seafood species. Food Sci Technol. (2019) 39:454–61. doi: 10.1590/fst.21318
43. Whelan J. Dietary stearidonic acid is a long chain (n-3) polyunsaturated fatty acid with potential health benefits. J Nutr. (2009) 139:5–10. doi: 10.3945/jn.108.094268
44. Abdel-Rahman E-SA, El-Fishawy FA, El-Geddawy MA, Kurz T, El-Rify MN. The changes in the lipid composition of mung bean seeds as affected by processing methods. Int J Food Eng. (2007) 3:1–10. doi: 10.2202/1556-3758.1186
45. Marton M, Mandoki Z, Csapo J. Evaluation of biological value of sprouts I. Fat content, fatty acid composition. Acta Univ Sapientiae Aliment. (2010) 3:53–65.
46. Li D, Hu X. Fatty acid content of commonly available nuts and seeds. In: Nuts and Seeds in Health and Disease Prevention. Elsevier (2011) p. 35–42. doi: 10.1016/B978-0-12-375688-6.10004-0
47. American Heart Association. The American Heart Association Diet and Lifestyle Recommendations. (2018) p. 1–5. Available online at: https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/aha-diet-and-lifestyle-recommendations
48. Joint FAO/WHO Expert Consultation on Fats and Fatty Acids in Human Nutrition. Interim summary of conclusions and dietary recommendations on total fat and fatty acids. In: From the Joint FAO/WHO Expert Consultation on Fats and Fatty Acids in Human Nutrition. Geneva (2008). Available online at: http://www.who.int/nutrition/topics/FFA_summary_rec_conclusion.pdf
49. U.S. Department of Health and Human Services and U.S. Department of Agriculture. Dietary Guidelines for Americans, 2020-2025. 9th ed. (2020). Available online at: DietaryGuidelines.gov.
50. EFSA Panel on Dietetic Products N and A (NDA). Scientific opinion on the tolerable upper intake level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). EFSA J. (2012) 10:2815. doi: 10.2903/j.efsa.2012.2815
51. The Flax Council of Canada. Flax a healthy food. p. 3–6. Available online at: https://flaxcouncil.ca/resources/nutrition/general-nutrition-information/flax-a-healthy-food/ (accessed April 26, 2021).
52. Parikh M, Maddaford TG, Austria JA, Aliani M, Netticadan T, Pierce GN. Dietary flaxseed as a strategy for improving human health. Nutrients. (2019) 11:1171. doi: 10.3390/nu11051171
53. Cunnane SC, Ganguli S, Menard C, Liede AC, Hamadeh MJ, Chen Z-Y, et al. High α-linolenic acid flaxseed (Linum usitatissimum): some nutritional properties in humans. Br J Nutr. (1993) 69:443–53. doi: 10.1079/BJN19930046
54. Rodriguez-Leyva D, Weighell W, Edel AL, LaVallee R, Dibrov E, Pinneker R, et al. Potent antihypertensive action of dietary flaxseed in hypertensive patients. Hypertension. (2013) 62:1081–9. doi: 10.1161/HYPERTENSIONAHA.113.02094
55. Jenkins DJA, Sievenpiper JL, Pauly D, Sumaila UR, Kendall CWC, Mowat FM. Are dietary recommendations for the use of fish oils sustainable? Can Med Assoc J. (2009) 180:633–7. doi: 10.1503/cmaj.081274
56. McClanahan T, Allison EH, Cinner JE. Managing fisheries for human and food security. Fish Fish. (2015) 16:78–103. doi: 10.1111/faf.12045
57. Gordon TAC, Harding HR, Clever FK, Davidson IK, Davison W, Montgomery DW, et al. Fishes in a changing world: learning from the past to promote sustainability of fish populations. J Fish Biol. (2018) 92:804–27. doi: 10.1111/jfb.13546
58. National Institute of Health. Omega-3 fatty acids - fact sheet. (2020). Available online at: https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional/#en30 (accessed April 26, 2021).
59. Albert BB, Derraik JGB, Cameron-Smith D, Hofman PL, Tumanov S, Villas-Boas SG, et al. Fish oil supplements in New Zealand are highly oxidised and do not meet label content of n-3 PUFA. Sci Rep. (2015) 5:7928. doi: 10.1038/srep07928
60. Hernandez EM. Issues in fortification and analysis of omega-3 fatty acids in foods. Lipid Technol. (2014) 26:103–6. doi: 10.1002/lite.201400004
61. Kitessa S, Abeywardena M, Wijesundera C, Nichols P. DHA-containing oilseed: a timely solution for the sustainability issues surrounding fish oil sources of the health-benefitting long-chain omega-3 oils. Nutrients. (2014) 6:2035–58. doi: 10.3390/nu6052035
62. MIR M. Echium oil: A valuable source of n-3 and n-6 fatty acids. Oléagineux, Corps gras, Lipides. (2008) 15:252–6. doi: 10.1051/ocl.2008.0203
63. Botelho PB, Mariano KDR, Rogero MM, de Castro IA. Effect of Echium oil compared with marine oils on lipid profile and inhibition of hepatic steatosis in LDLr knockout mice. Lipids Health Dis. (2013) 12:38. doi: 10.1186/1476-511X-12-38
64. Kuhnt K, Fuhrmann C, Köhler M, Kiehntopf M, Jahreis G. Dietary echium oil increases long-chain n−3 pufas, including docosapentaenoic acid, in blood fractions and alters biochemical markers for cardiovascular disease independently of age, sex, and metabolic syndrome. J Nutr. (2014) 144:447–60. doi: 10.3945/jn.113.180802
65. Kavanagh K, Flynn DM, Jenkins KA, Wilson MD, Chilton FH. Stearidonic and γ-linolenic acids in echium oil improves glucose disposal in insulin resistant monkeys. Prostaglandins, Leukot Essent Fat Acids. (2013) 89:39–45. doi: 10.1016/j.plefa.2013.04.003
66. Surette ME. Dietary omega-3 PUFA and health: Stearidonic acid-containing seed oils as effective and sustainable alternatives to traditional marine oils. Mol Nutr Food Res. (2013) 57:748–59. doi: 10.1002/mnfr.201200706
Keywords: agronomic, functional food, biofortification, eicosapentaenoic acid, docosahexaenoic acid, alpha-linolenic acid
Citation: Marques E, Darby H and Kraft J (2021) Omega-3 Fatty Acid Fortification of Flax Through Nutri-Priming. Front. Nutr. 8:715287. doi: 10.3389/fnut.2021.715287
Received: 26 May 2021; Accepted: 08 July 2021;
Published: 20 August 2021.
Edited by:
Jooyeoun Jung, University of Nebraska-Lincoln, United StatesReviewed by:
Anand Zanwar, Bharati Vidyapeeth Deemed University, IndiaCopyright © 2021 Marques, Darby and Kraft. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jana Kraft, SmFuYS5LcmFmdEB1dm0uZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.