
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 11 August 2021
Sec. Nutrition and Microbes
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.712327
This article is part of the Research TopicGut Microbial Response to Host Metabolic Phenotypes: Volume 1View all 24 articles
Background: The incidence of renal cell carcinoma (RCC) is increasing year by year. It is difficult to have complete treatment so far. Studies have shown that tryptophan metabolite Kynurenine (Kyn) affects cell proliferation, migration, apoptosis, adhesion, and differentiation. Our aim is to explore whether Kyn activates aromatic hydrocarbon receptor (AhR) to mediate RCC metastasis.
Methods: We collected RCC tissues and feces from RCC patients. 16S rRNA technology was performed to analyze the gut microbial composition of RCC patients. LC-MS/MS was used to analyze the gut microbial metabolites. The AhR was inhibited and treated with Kyn. Immunofluorescence was used to measure the degree of AhR activation. The migration and invasion ability of 786-O cells was tested by Transwell assay. Flow cytometry and cell cycle assay were utilized to observe the apoptosis and cycle of 786-O cells. CCK-8 assay was used to detect 786-O cells proliferation. qRT-PCR and Western blot were used to detect AhR and EMT-related genes expression level.
Results: AhR expression was up-regulated in RCC tissues. RCC gut microbiota was disordered. The proportion of Kyn was increased in RCC. After being treated with Kyn, the migration, invasion, and proliferation ability of 786-O cells were decreased. Furthermore, the expression of EMT-related protein E-cadherin decreased, and the expression of N-cadherin and Vimentin increased. The proportion of 786-O cells in the S phase increased. The apoptosis rate of 786-O cells was inhibited.
Conclusion: The tryptophan metabolite Kyn could activate AhR. Kyn could promote 786-O cells migration and invasion. Gut microbiota could activate AhR through its tryptophan metabolite Kyn to mediate RCC metastasis.
Renal cell carcinoma (RCC) is still an elusive cancer in lack of biomarkers. It was the eighth most common malignant tumor in the United States (1). In addition to the increase in newly diagnosed cases, RCC patients' prevalence and overall survival rate have also increased significantly (2). Studies have shown a link between the gut microbiota and metastatic RCC (mRCC). Evidence showed that RCC patients have a lower abundance of bifidobacteria, compared with healthy adults (3). However, it needs experimental verification about whether there is a connection between the gut microbiota and RCC needs to.
Many life activities are mediated by metabolites of gut microbiota. Tryptophan is an essential aromatic amino acid, and it is considered necessary in many metabolites between gut microbiota and the host (4, 5). Many tryptophan metabolites derived from abundant microbiota exhibit the activation potential of aromatic hydrocarbon receptor (AhR) (6). Some endogenous tryptophan metabolites are recognized as AhR ligands, including tryptamine (TRA), indole, 5-hydroxyindole-3-acetic acid (5-HIAA), Kynurenine (Kyn), kynurenic acid (KA), and xanthine acid (XA) (7, 8). Tryptophan metabolites as ligands can activate AhR signals in many diseases, such as inflammation, oxidative stress damage, cancer, aging-related diseases, cardiovascular disease (CVD), and chronic kidney disease (CKD) (4). We screened tryptophan metabolites to verify the regulatory relationship between tryptophan metabolites and AhR.
AhR is a cytoplasmic ligand-activated transcription factor involved in various cellular processes. It can mediate the toxicity (including carcinogenicity) of polycyclic aromatic hydrocarbons and induce many enzymes expression. It can participate in critical biological processes, such as signal transduction, cell differentiation, and cell apoptosis (9). Recent studies have shown that AhR is related to CVD, CKD, and RCC (10). Tryptophan catabolites can activate AhR to enhance tumor malignancy and inhibit anti-tumor immunity (11, 12). More studies revealed AhR can be activated by many endogenous ligands. Different ligands bind and activate AhR, which can translocate AhR to the nucleus and induce a series of genes expression (8).
Epithelial-mesenchymal transition (EMT) is a process in which epithelial cells lose their polarized structure and gain the migration and invasion ability. It is believed to be the cause of cancer metastasis (13). EMT biomarkers such as Vimentin, N-cadherin, and MMP9 are overexpressed in cancer and are involved in promoting cancer cells metastasis (14). Many studies have shown that AhR activity leads to loss of cell contact inhibition and changes in extracellular matrix remodeling (15). This study intends to explore the internal relationship between metastatic RCC and the gut microbiota and its metabolism (tryptophan metabolism) and verify whether the tryptophan metabolite Kyn promotes EMT and RCC pathological process by activating AhR.
The clinical characteristics of all subjects were presented in Table 1. To study whether AhR expression in RCC was abnormal, we used qRT-PCR and Western blot to detect AhR expression. Compared with the Control tissues group, the AhR mRNA expression in the RCC tissues group was significantly increased (Figure 1A). AhR protein expression was increased in both the cytoplasm and the nucleus (Figure 1B). It showed that AhR expression was abnormally increased in RCC. RCC was often accompanied by EMT conversion (16). We next detected the expression of E-cadherin, N-cadherin, and Vimentin related to EMT. Compared with the Control tissues group, E-cadherin expression in the RCC tissues group was inhibited, but N-cadherin and Vimentin expressions were significantly up-regulated (Figures 1C,D). It showed that EMT accompanied the RCC patients, and AhR expression was abnormal.
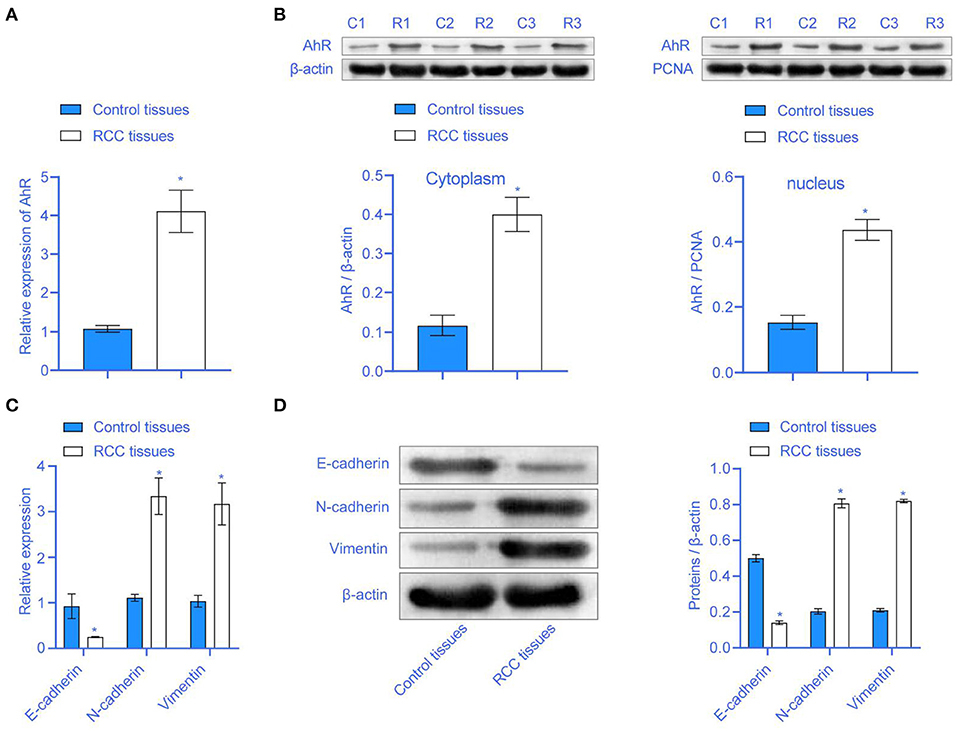
Figure 1. The expression of AhR was higher in RCC. (A) The mRNA expression of AhR was higher in the RCC tissues group. (B) The expression of AhR protein in the cytoplasm and nucleus. (C,D) E-cadherin, N-cadherin, and Vimentin were abnormally expressed in RCC tissues group * compared with Control tissues group, P < 0.05.
Next, we aim to explore whether RCC affected the gut microbiota of RCC patients. PCA analysis showed that the microbial community similarity of the clinical samples of the Control tissues group and the RCC tissues group was low (Figure 2A). Anosim analysis further helped obtain an R-value of 0.266, showing that the difference between groups was more significant than the difference within groups (Figure 2B). The OUT Venn diagram showed that the Control tissues group had 155 unique OUT numbers, the RCC tissues group had 819 unique OUT numbers, and the two groups had 134 OUT numbers in total (Figure 2C). Chao1, Shannon, and Simpson's indexes showed the difference between the two groups was significant (Figure 2D) (P < 0.05). The Rank-abundance curve indicated that the RCC tissues group curve had a larger range on the horizontal axis, and the species richness was higher (Figure 2E). The abundance bar graph showed a significant difference in species composition between the Control tissues group and RCC tissue group (Figure 2F). The heat map showed that compared with the Control tissues group, the abundance of Bacteroides and Akkermansia was increased significantly in RCC tissues group, while the abundance of Blautia, Bifidobacterium, and Megamonas was decreased significantly (Figure 2G). As shown above, the gut microbiota of RCC patients was imbalanced.
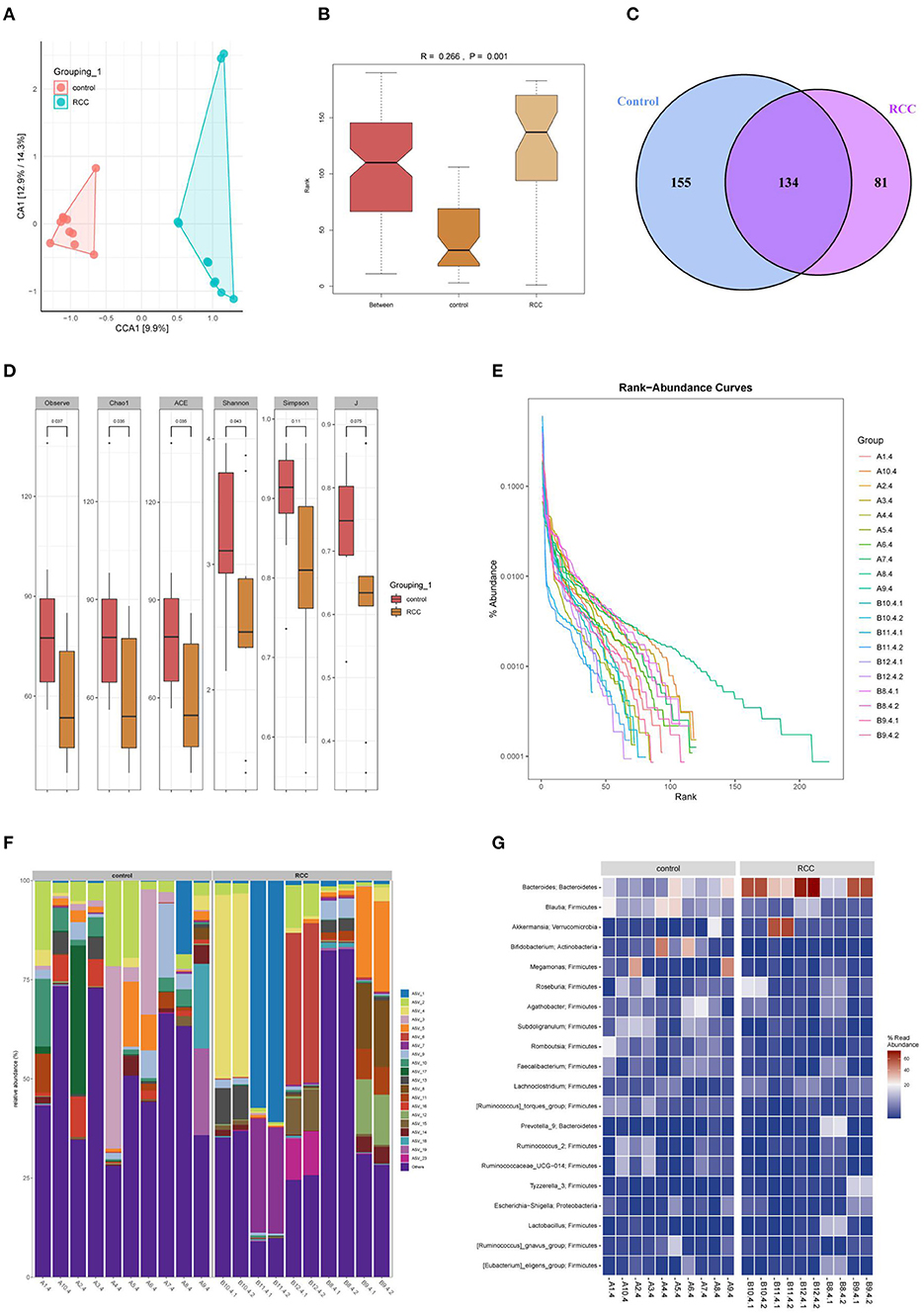
Figure 2. Gut microbiota imbalance caused by RCC. (A) The similarity between the Control tissues and RCC tissues groups. (B) The difference between the two groups. (C) OUT Venn diagram. (D) Box chart. (E) Rank-abundance curve. (F) Histogram of the distribution of phylum species. (G) Heat map of phylum-species distribution.
The above experiments indicated that RCC could lead to gut microbiota disturbance. We detected tryptophan metabolites. First, we uploaded the metabolic data on the online website (https://www.metaboanalyst.ca/MetaboAnalyst/ModuleView.xhtml) to get the heat map (Figure 3A). The heat map showed our nine tryptophan metabolites (L-Kynurenine, Tryptamine, Indole, 3-Methylindole, Indoxyl Sulfate potassium, salt, Indole-3-acetic acid, 3-Indolepropionic acid, and 3-Indoleacrylicacid Kynurenic acid) in different groups were different. Percentage of the distribution ratio of tryptophan metabolites in the Control tissues group and the RCC tissues group indicated meaningful differences (Figure 3B). Then, original data were concluded. 3-Indoleacrylicacid, Indoxyl Sulfate potassium salt, and 3-Methylindole were significantly reduced (Figure 3C). The Spearman's rank correlation was performed to analyze the correlation between the top 20 gut microbiota and nine tryptophan metabolites. The results showed that L-Kynurenine was negatively correlated with Agathobacter. Tryptamine was negatively correlated with Escherichia-Shigella. Indole was positively correlated with Tyzzerella_3. 3-Methylindole was positively correlated with Romboutsia, Bifidobacterium, and [Ruminococcus]_torques_group. Indoxyl Sulfate potassium salt was positively correlated with Subdoligranulum and [Ruminococcus]_torques_groups. Indole-3-acetic acid was positively correlated with Romboutsia, Blautia, Bifidobacterium, and [Ruminococcus]_torques_group. Indole-3-acetic acid was negatively correlated with Bacteroides and Akkermansia. 3-Indolepropionic acid was negatively correlated with Roseburia, Prevotella_9, and Megamonas. 3-Indoleacrylicacid was positively correlated with Blautia. 3-Indoleacrylicacid was negatively correlated with Akkermansia. Kynurenic acid was negatively correlated with Prevotella_9 and Akkermansia. The above results indicated that gut microbiota imbalance in RCC patients might lead to tryptophan metabolites disorders (Figure 3D). Next, we analyzed the correlation between the tryptophan metabolites and AhR, E-cadherin, N-cadherin, and Vimentin. The results revealed AhR was significantly negatively correlated with L-Kynurenine. E-cadherin was significantly positively correlated with 3-Indolepropionic acid (Figure 3E). The above indicated that the disturbance of tryptophan metabolites from gut microbiota was related to the abnormal expression of EMT and AhR in RCC.
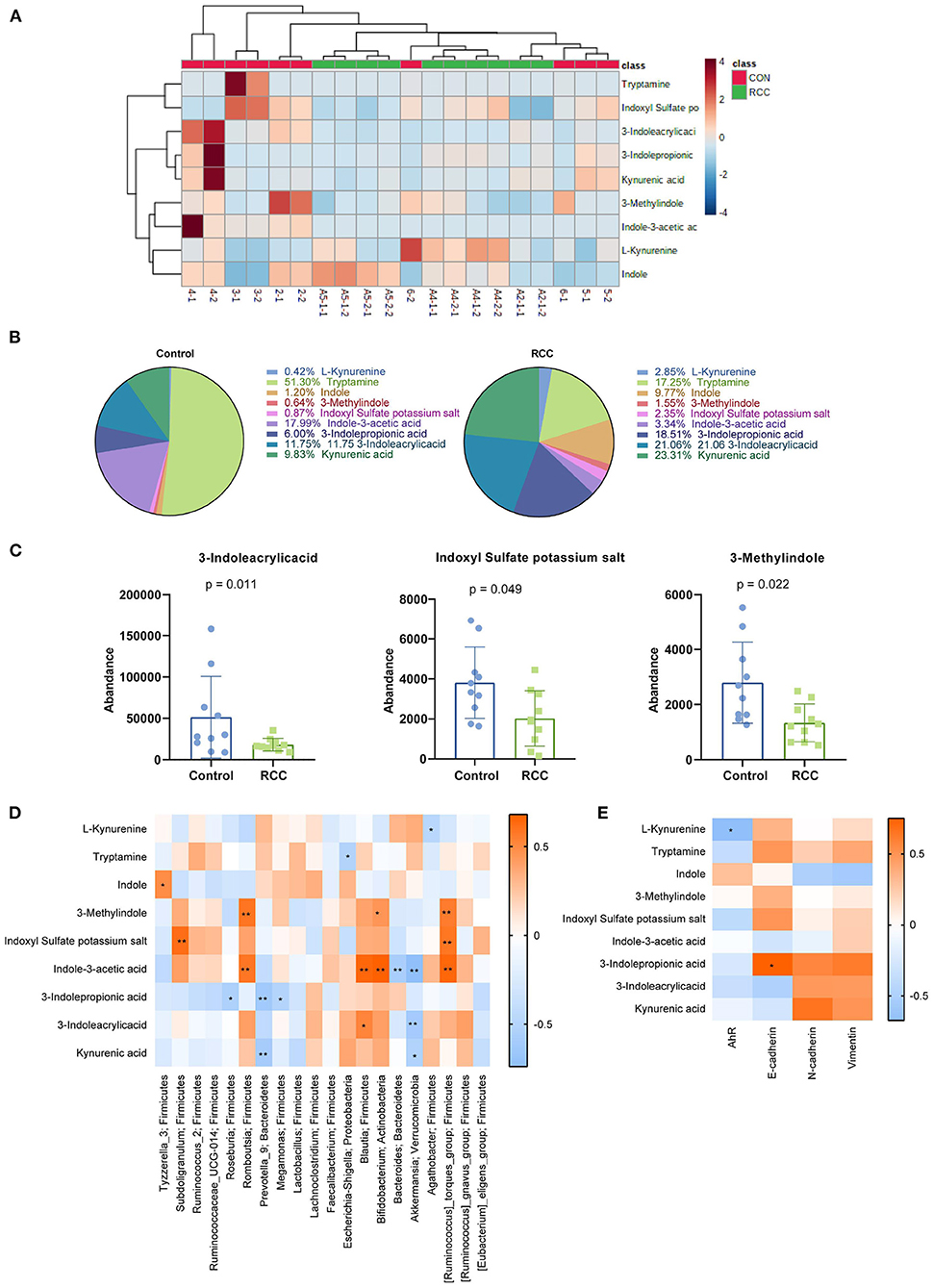
Figure 3. The content of Kyn was high in RCC. (A) Heat map of metabolites (2-1, 2-2, 3-1, 3-2, 4-1, 4-2.5-1, 5-2, 6-1, and 6-2 belong to control group, A2-1-1, A2-1-2, A4-1-1, A4-1-2, A4-2-1, A4-2-2, A5-1-1, A5-1-2, A5-2-1, and A5-2-2 belong to RCC group). (B) Percentage of nine tryptophan metabolites. (C) Three metabolites with reduced content in RCC. (D) The relationship between 9 tryptophan metabolites and the top 20 gut microbiota. (E) The correlation between the tryptophan metabolites and AhR, E-cadherin, N-cadherin, and Vimentin.
To further explore the effect of tryptophan metabolites on RCC, we inhibited AhR. We treated 786-O cells with different concentrations of Kyn. The immunofluorescence results showed that Kyn could activate AhR in 786-O cells (Figure 4A). Cell viability assay showed that 786-O cells viability was increased in Low-Kyn and High-Kyn groups compared with the Control group. Compared with the AhR antagonist group, the 786-O cells viability in the Low-Kyn + AhR antagonist and High-Kyn + AhR antagonist groups was increased (Figure 4B). Then, we checked the cell cycle and cell apoptosis. Kyn was added to treat 786-O cells, and the results suggested that the cells number arrested in the G1/G2 phase decreased, and the cells number in the S phase increased. The AhR of 786-O cells was inhibited. Then Kyn was added to treat 786-O cells. The cells number arrested in the G1/G2 phase was also significantly reduced, and cells number in the S phase was increased substantially (Figure 4C). It indicated that Kyn could regulate the normal life cycle of 786-O cells. Flow cytometry was used to measure the 786-O cells apoptosis (Figure 4D). The results suggested that Kyn could effectively inhibit 786-O cells apoptosis. In summary, Kyn could activate AhR to inhibit 786-O cells apoptosis.
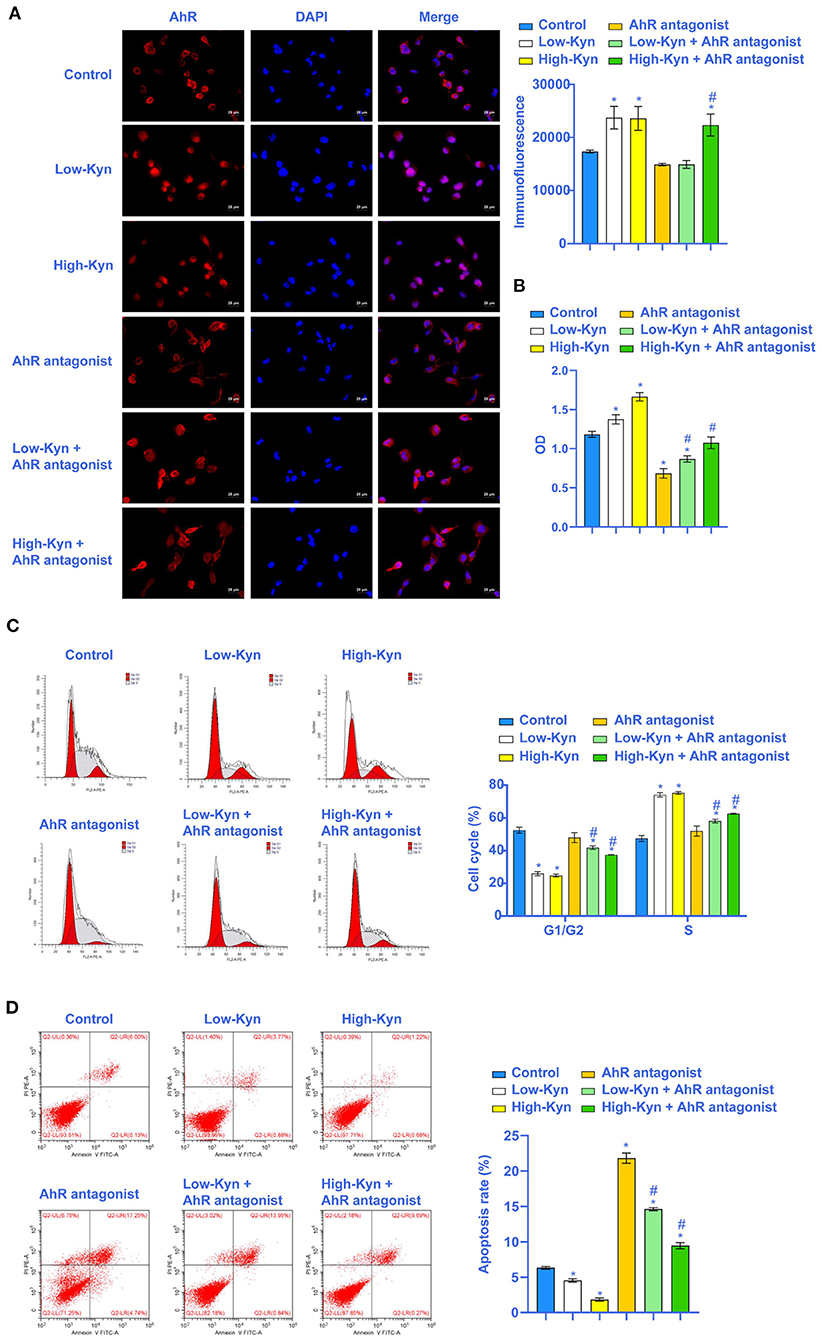
Figure 4. Kyn could inhibit 786-O cells apoptosis. (A) AhR was activated by Kyn. (B) CCK-8 assay was used to measure the cell vitality. (C) Kyn could stabilize the 786-O cells cycle (×200, Scale bar = 50μm). (D) Flow cytometry was used to measure the apoptosis rate of 786-O cells. * Compared with the Control group, P < 0.05. # Compared with the AhR antagonist group, P < 0.05.
The above experimental results indicated that Kyn could inhibit the 786-O cells apoptosis. Next, we tested whether Kyn could affect the invasion and EMT process of 786-O cells. Compared with the Control group, 786-O cells migration and invasion ability in the Low-Kyn and High-Kyn groups was increased. Compared with the AhR antagonist group, 786-O cells migration and invasion ability in the Low-Kyn + AhR antagonist and High-Kyn + AhR antagonist groups were increased. It suggested that Kyn could promote the 786-O cells migration and invasion (Figures 5A,B). Finally, qRT-PCR and Western blot were used to detect the expression of E-cadherin, N-cadherin, and Vimentin related to EMT genes. Kyn was added to treat 786-O cells. The E-cadherin expression in 786-O cells was inhibited, but N-cadherin and Vimentin expressions were significantly up-regulated (Figure 5C). In short, Kyn could activate AhR to promote the migration, invasion, and EMT process of 786-O cells.
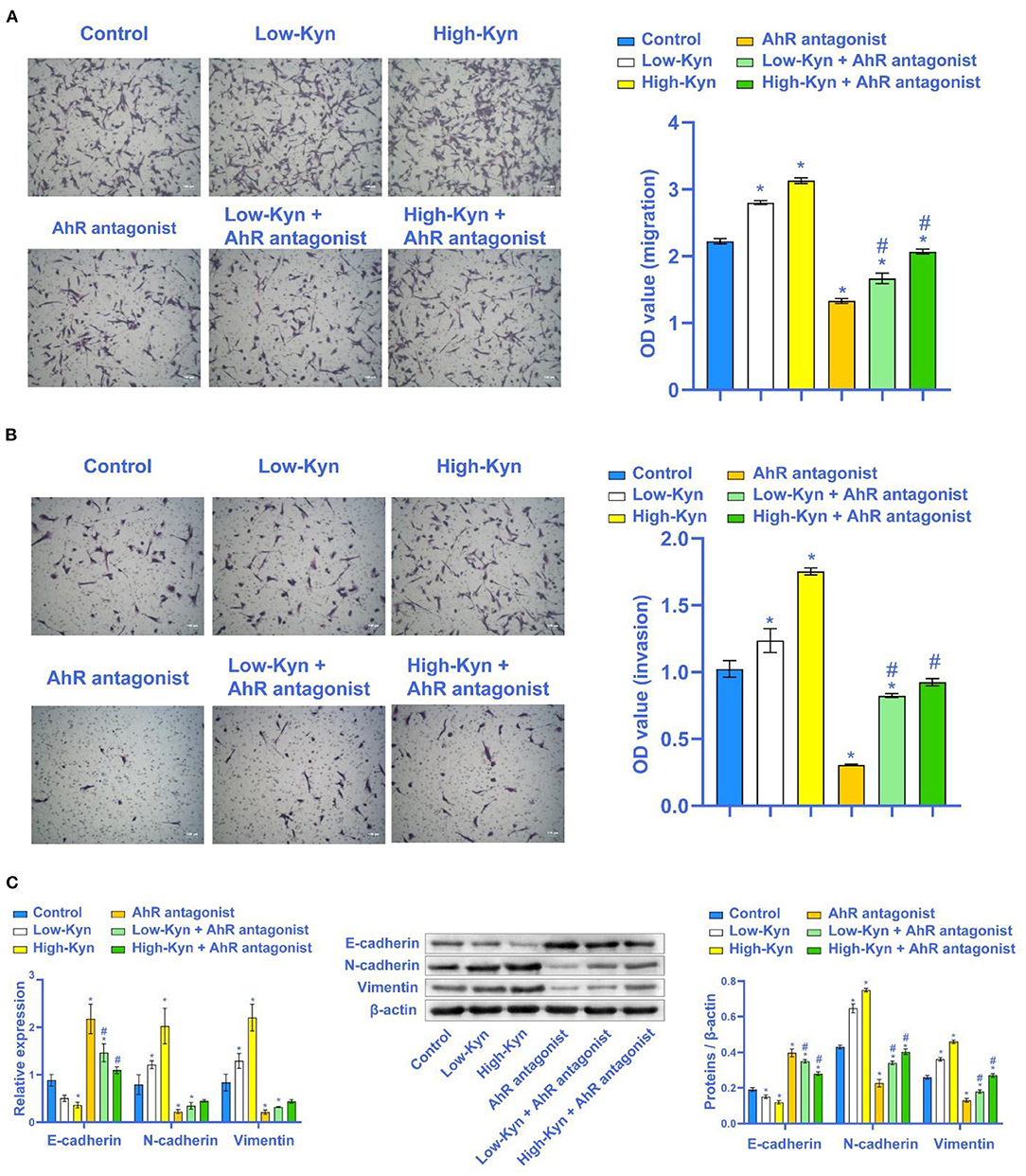
Figure 5. Kyn could promote the migration and invasion of 786-O cells. (A) and (B) Transwell was used to detect the migration and invasion ability of 786-O cells (×100, Scale bar = 100μm). (C) E-cadherin was inhibited by Kyn. N-cadherin and Vimentin were activated by Kyn. * Compared with the Control group, P < 0.05. # Compared with the AhR antagonist group, P < 0.05.
We collected 10 RCC tissues samples and Control tissues (at least 5 cm from the tumor tissues) from the Xiangya hospital central south university. We also collected patient feces. These samples came from RCC patients after surgery. None of these patients received chemotherapy or radiotherapy before surgery. Patients with infectious diseases, autoimmune diseases, or multiple primary cancers were excluded. We also collected 10 normal human feces. Before the sample collection, all subjects did not receive antibiotics or similar drugs. The research was approved by the Ethics Association and related hospitals.
Human RCC 786-O cells were cultured in a T25 cell culture flask (690175, Greiner Bio-One Vilvoorde, Belgium) supplemented with 100 U/ml penicillin. The cells were cultured in a 5% CO2 medium containing 10% fetal bovine serum (FBS) (Gibco). The medium was replaced with a serum-free medium. 786-O cells were inoculated into a six-well culture plate and incubated for at least 12. The cells were grouped as the Control tissues group (786-O cells), the Low-Kyn group (786-O cells were cultured in 0.2 mmol/L Kyn for 24 h), the High-Kyn group (786-O cells were cultured in 2 mmol/L Kyn for 24 h), the AhR antagonist group (786-O cells were pretreated with DMF (3′, 4′-dimethoxyflavone) for 12 h), the Low-Kyn + AhR antagonist group (786-O cells were pretreated with DMF and cultured at 0.2 mmol/L Kyn), and the High-Kyn + AhR antagonist group (786-O cells were pretreated with DMF and cultured under two mmol/L Kyn) (17).
We de-joined and filtered the raw data with low-quality. The representative sequence of each out was annotated. We have separated and filtered the low-quality raw data. We have annotated the representative sequence of each OTU. The random sampling method was adopted, and the OTU analysis was performed with the number of effective sequences drawn. The alpha diversity indexes were calculated. A dilution curve was constructed. We obtained the R-value by analyzing the distance matrix between samples. Finally, the composition and abundance of the gut microbiota were identified.
Fecal samples from all subjects were centrifuged at 1,150 g and 4°C for 10 min. Then the supernatant was further divided into 100 μl, put into the labeled tube and stored at −80°C before preparing for metabonomics analysis. Each bisection sample was separated from 300 μl cold acetonitrile, mixed and then swirled for 30 s before analysis. The mixture was deproteinized by centrifugation at 4°C (21,130 g, 30 min), and 1 μl of supernatant was injected into UPLC. The ion source parameters and scanning parameters were optimized.
About 0.02 g tissues in Trizol were put into a 1.5 ml centrifuge tube, and 1 ml of Trizol was added to the homogenizer to grind and homogenize thoroughly. About 500 μl of the cells in Trizol were placed in a 1.5 ml centrifuge tube. Fluorescence quantitative PCR (quantstudio1, Thermo, USA) was performed on a fluorescence quantitative PCR machine. The reaction conditions were pre-denaturation at 95°C for 10 min, denaturation at 94°C for 15 s, and annealing at 60°C for 30 s, a total of 40 cycles. The internal reference primer was β-actin, and the primer sequence was shown in Table 2.
We cut 0.025 g tissues, and then washed the tissues with ice-cold PBS. We added 300 μl RIPA lysate and ground the tissues repeatedly in the biological sample homogenizer until no tissue masses were visible. For primary antibodies, we used rabbit anti-AhR (1:1,000, #83200, CST), rabbit anti-E-cadherin (1:5,000, 20874-1-AP, Proteintech), mouse anti-N-cadherin (1:6,000, 66219-1-Ig, Proteintech), rabbit anti-Vimentin (1:5,000, ab92547, Abcam), rabbit anti-PCNA (1:5,000, 10205-2-AP, Proteintech) and mouse anti-β-actin (1:5,000, 60008-1-Ig, Proteintech). We rinsed the membrane three times with TBST for 10 min each time. Then we incubated secondary antibodies HRP-conjugated Affinipure Goat Anti-Mouse IgG (H + L) (1:5,000, SA00001-1, Proteintech) and HRP-conjugated Affinipure Goat Anti-Rabbit IgG (H+L) (1:6,000, SA00001-2, Proteintech). The film was immersed in SuperECL Plus (K-12045-D50, Advansta, USA) for luminescence development.
We used the CCK-8 kit (NU679, Dojindo Molecular Technologies, Inc., Japan) to analyze the cell viability. The cells were taken in a logarithmic growth phase and were digested with trypsin. At the density of 5 × 103 cells/well, the cells were inoculated into a 96-well plate with 100 μl/well. Ten microliter CCK8 solution of complete culture medium was co-cultured with cells in each well. The absorbance (OD) value at 450 nm was analyzed by a Bio-Tek microplate reader after incubation with 5 % CO2 at 37°C for 4 h.
We diluted Matrigel with 100 μl cold, serum-free DMEM medium per well. Then we placed 500 μl complete medium containing 10% FBS in the lower chamber. The cells were digested with trypsin to form a single cell. We put the upper chamber into a new hole with PBS. We used 0.1% crystal violet for 5 min.
Cells were collected with trypsin digestion solution (c0201, Beyotime, China) without EDTA. We collected about 2 × 105 cells. Five hundred microliter of binding buffer was added to the cell suspension. We added 5 μl annexin V-FITC (kga108, keygen, China) and mixed well. Finally, we added 5 μl Propidium Iodide (PI) (mb2920, Meilunbio, China) into the mixture and mixed them well. Flow cytometry was used to observe the changes within 1 h.
The slices were cleaned 2~3 times with PBS. Then the slices were fixed with 4% paraformaldehyde for 30 min. The primary antibody AhR was dripped onto the slices at 4°C overnight. The second antibody was incubated by dropping 50–100 μl anti-rabbit IgG labeled fluorescent antibody. Then the slices were incubated at 37°C for 90 min. Finally, the slices were washed with PBS 3 times, 5 min each. DAPI solution was used to stain the nucleus at 37°C for 10 min. Then PBS was washed 3 times, 5 min each. The slices were placed under a fluorescent microscope for observation.
We took out the fixed sample. One microliter of pre-cooled PBS was added to the sample for cell suspension. We added 150 μl PI working solution into the cell solution and stained it at 4°C for 30 min. PI was excited by 488 nm argon ion laser and received by 630 nm pass filter. 1 × 104 cells were collected by FSC/SSC scatterplot. Adhesion cells and fragments were excluded by gating technique. The percentage of cell cycle on PI fluorescence histogram was analyzed.
All experimental data were analyzed by GraphPad 8.0 software. Measurement data were expressed as mean ± standard deviation. The unpaired T-test was used between the two groups conforming to the normal distribution. One-way analysis of variance (ANOVA) was used for comparison between multiple groups. P < 0.05 was considered statistically significant.
This study found that RCC has a certain internal relationship with gut microbiota and tryptophan metabolite. Through cell experiments, we found that Kyn may promote EMT by activating AhR, further promote RCC cells migration and invasion and inhibit RCC cells apoptosis.
The gut microbiota is closely related to cancer (18). We found that the composition of gut microbiota in RCC patients was changed significantly. Bacteroides and Akkermansia were increased significantly, while Blautia, Bifidobacterium, and Megamonas were decreased significantly. Recent studies have shown that gut microbiota composition affects the success of immune checkpoint blocking therapy for RCC (19). Clinical studies have shown that patients treated with vascular endothelial growth factor tyrosine kinase inhibitor (VEGF-TKIs) combined with mRCC have higher Bacteroides and lower Prevotella (20). These results indicated that the gut microbiota composition changes were related to the occurrence and development of RCC.
Tryptophan catabolism has become a critical metabolic regulation factor for tumor progression (21). Different cancers prognosis showed that an essential indicator of tryptophan metabolism is serum KTR. When the serum KTR increased, tryptophan was metabolizing through indoleamine 2,3-dioxygenase 1 (IDO1) or tryptophan 2,3 -Dioxygenase (TDO) through the Kyn pathway. At the same time, other researchers have reported higher KTR in the serum of patients with advanced RCC or resistance to immune checkpoint inhibitor (22, 23). The composition of the gut microbiota determines several tryptophan metabolites because they are catabolism products of gut microbiota. These tryptophan-derived microbial catabolites are important signaling molecules in the host and the microorganisms (24, 25). Targeted metabolomics studies have shown that RCC patients have elevated Kyn pathway metabolites (26). Our experiment revealed that the distribution of tryptophan metabolites in gut microbiota changed significantly in RCC. Through correlation analysis, it was found that the tryptophan metabolites were correlated considerably with Agathobacter, Escherichia-Shigella, Romboutsia, and Akkermansia, etc. Studies have shown that microorganisms mediate gut Trp metabolism changes and participate in the occurrence of cancer (27). This was consistent with our research. Through correlation analysis, we also found that tryptophan metabolites of gut microbiota were significantly correlated with AhR and E-cadherin expressions in RCC. These results indicated that the Kyn metabolic pathway of the gut microbiota might be involved in the pathogenetic progress of RCC. This experiment assessed that the Kyn pathway was operable and could be used as a therapeutic target for RCC.
Kyn is the main product of the tryptophan metabolic pathway catalyzed by TDO2 and IDO in tumor cells. Kyn proved that AhR could be activated. AhR in an autocrine/paracrine manner can inhibit the anti-tumor immune response and promote tumor cell survival and movement (28). AhR was identified as a ligand-activated transcription factor of the basic helix-loop-helix (bHLH) Per-Arnt-Sim (PAS) family, and it played an important role in a wide range of physiological and pathological conditions (29–31). AhR participates in the induction of Slug expression, and this process inhibits E-cadherin expression. MMPs expression is also a target of the AhR pathway. Odibenzo-p-dioxin (TCDD) exposure up-regulated the expression and activity of MMP9 in a variety of malignant tumors, including melanoma cells, urothelial cancer cells, prostate cancer cells, and gastric cancer cells (32). AhR is involved in the induction of EMT by PCBs in HCC cells (33). In this study, we aimed to study the influence of AhR on the progress of EMT in RCC. Our results showed that AhR was highly expressed in RCC. In addition, Kyn could promote 786-O cells migration and invasion and inhibit 786-O cells apoptosis by activating AhR.
Fecal microorganisms and metabolites are often affected by diet (34). In this study, the subjects were not given a standardized diet like other studies (3) but kept the original eating habits, which is the limitation of this study. However, non-invasive research can be overcome by expanding the sample size. In addition, the effect of tryptophan metabolites of gut microbiota on the AhR activation pathway of the host itself was complex, which may require more evidence to prove its specific regulatory mechanism. Based on these factors, we will expand the sample size in future research and explore the influence of tryptophan metabolites of gut microbiota on RCC in combination with clinical and animal experiments.
In conclusion, through the research of this subject, we have verified that the RCC gut microbiota metabolism is disordered, and the Kyn metabolism is increased. In vitro experiments further confirmed that Kyn could promote 786-O cells migration and invasion and the progress of EMT and inhibit 786-O cells apoptosis by activating AhR. Our design clarified that the gut microbiota could activate AhR through its tryptophan metabolism to mediate the metastasis of RCC.
The original contributions presented in the study are publicly available. This data can be found here: https://www.ncbi.nlm.nih.gov/sra/PRJNA735071.
All experiments were performed according to the guidelines set by the Medical Ethics Committee of Xiangya Hospital of Central South University (202105187), Changsha, Hunan, China. Written informed consent was obtained from all participates. The patients/participants provided their written informed consent to participate in this study.
GD, XC, and YH designed the study, performed the research, analyzed data, and wrote the paper. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of Hunan (2020JJ5895).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siegel RL, Miller KD. Cancer Statistics, 2017. CA Cancer J Clin. (2017) 67:7–30. doi: 10.3322/caac.21387
2. Adashek JJ, Salgia MM, Posadas EM, Figlin RA, Gong J. Role of biomarkers in prediction of response to therapeutics in metastatic renal-cell carcinoma. Clin Genitourin Cancer. (2019) 17:e454–60. doi: 10.1016/j.clgc.2019.01.004
3. Dizman N, Hsu J, Bergerot PG, Gillece JD, Folkerts M, Reining L, et al. Randomized trial assessing impact of probiotic supplementation on gut microbiome and clinical outcome from targeted therapy in metastatic renal cell carcinoma. Cancer Med. (2021) 10:79–86. doi: 10.1002/cam4.3569
4. Liu J-R, Miao H. Gut microbiota-derived tryptophan metabolism mediates renal fibrosis by aryl hydrocarbon receptor signaling activation. Cell Mol Life Sci. (2021) 78:909–22. doi: 10.1007/s00018-020-03645-1
5. Trott JF, Kim J. Inhibiting tryptophan metabolism enhances interferon therapy in kidney cancer. Oncotarget. (2016) 7:66540–57. doi: 10.18632/oncotarget.11658
6. Dong F, Hao F, Murray IA, Smith PB, Koo I, Tindall AM, et al. Intestinal microbiota-derived tryptophan metabolites are predictive of Ah receptor activity. Gut Microbes. (2020) 12:1–24. doi: 10.1080/19490976.2020.1788899
7. Dong F, Perdew GH. The aryl hydrocarbon receptor as a mediator of host-microbiota interplay. Gut Microbes. (2020) 12:1859812. doi: 10.1080/19490976.2020.1859812
8. Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, et al. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol. (2018) 8:13. doi: 10.3389/fcimb.2018.00013
9. Rothhammer V. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and CNS inflammation via the aryl hydrocarbon receptor. Nat Med. (2016) 22:586–97. doi: 10.1038/nm.4106
10. Zhao H, Lin Chen, Yang T, Feng Y-L, Vaziri ND, Liu B-L, et al. Aryl hydrocarbon receptor activation mediates kidney disease and renal cell carcinoma. J Transl Med. (2019) 17:302. doi: 10.1186/s12967-019-2054-5
11. Sadik A, Somarribas Patterson LF, Öztürk S, Mohapatra SR, Panitz V, Secker PF, et al. IL4I1 Is a metabolic immune checkpoint that activates the AHR and promotes tumor progression. Cell. (2020) 182:1252–70.e34. doi: 10.1016/j.cell.2020.07.038
12. Labadie BW, Bao R. Reimagining IDO pathway inhibition in cancer immunotherapy via downstream focus on the tryptophan-kynurenine-aryl hydrocarbon axis. Clin Cancer Res. (2019) 25:1462–71. doi: 10.1158/1078-0432.CCR-18-2882
13. Giannelli G. Role of epithelial to mesenchymal transition in hepatocellular carcinoma. J Hepatol. (2016) 64:798–808. doi: 10.1016/j.jhep.2016.05.007
14. Yang S, Liu Y. FOXP3 promotes tumor growth and metastasis by activating Wnt/β-catenin signaling pathway and EMT in non-small cell lung cancer. Mol Cancer. (2017) 16:124. doi: 10.1186/s12943-017-0700-1
15. Feng S. Role of aryl hydrocarbon receptor in cancer. Biochim Biophys Acta. (2013) 1836:197–210. doi: 10.1016/j.bbcan.2013.05.001
16. Liu H, Hu G. circPTCH1 promotes invasion and metastasis in renal cell carcinoma via regulating miR-485-5p/MMP14 axis. Theranostics. (2020) 10:10791–807. doi: 10.7150/thno.47239
17. Eisa NH, Reddy SV. Kynurenine promotes RANKL-induced osteoclastogenesis in vitro by activating the aryl hydrocarbon receptor pathway. Int J Mol Sci. (2020) 21:7931. doi: 10.3390/ijms21217931
18. Baffy G. Gut microbiota and cancer of the host: colliding interests. Adv Exp Med Biol. (2020) 1219:93–107. doi: 10.1007/978-3-030-34025-4_5
19. Derosa L, Routy B, Fidelle M, Iebba V, Alla L, Pasolli E, et al. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients. Eur Urol. (2020) 78:195–206. doi: 10.1016/j.eururo.2020.04.044
20. Pal SK, Li SM, Wu X, Qin H, Kortylewski M, Hsu J, et al. Stool bacteriomic profiling in patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor-tyrosine kinase inhibitors. Clin Cancer Res. (2015) 21:5286–93. doi: 10.1158/1078-0432.CCR-15-0724
21. Opitz CA, Somarribas Patterson LF, Mohapatra SR, Dewi DL, Sadik A, Platten M, et al. The therapeutic potential of targeting tryptophan catabolism in cancer. Br J Cancer. (2020) 122:30–44. doi: 10.1038/s41416-019-0664-6
22. Lucarelli G, Rutigliano M. Activation of the kynurenine pathway predicts poor outcome in patients with clear cell renal cell carcinoma. Urol Oncol. (2017) 35:461.e15–461.e27. doi: 10.1016/j.urolonc.2017.02.011
23. Li H, Bullock K, Gurjao C, Braun D, Shukla SA, Bossé D, et al. Metabolomic adaptations and correlates of survival to immune checkpoint blockade. Nat Commun. (2019) 10:4346. doi: 10.1038/s41467-019-12361-9
24. Hsu C-N, Tain YL. Developmental programming and reprogramming of hypertension and kidney disease: impact of tryptophan metabolism. Int J Mol Sci. (2020) 21:8705. doi: 10.3390/ijms21228705
25. Sumitomo M, Takahara K. Tryptophan 2,3-dioxygenase in tumor cells is associated with resistance to immunotherapy in renal cell carcinoma. Cancer Sci. (2021) 112:1038–47. doi: 10.1111/cas.14797
26. Kim K. Urine metabolomic analysis identifies potential biomarkers and pathogenic pathways in kidney cancer. OMICS. (2011) 15:293–303. doi: 10.1089/omi.2010.0094
27. Sun XZ, Zhao D-Y, Zhou Y-C, Wang Q-Q, Qin G, Yao S-K. Alteration of fecal tryptophan metabolism correlates with shifted microbiota and may be involved in pathogenesis of colorectal cancer. World J Gastroenterol. (2020) 26:7173–90. doi: 10.3748/wjg.v26.i45.7173
28. Opitz CA. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. (2011) 478:197–203. doi: 10.1038/nature10491
29. Zhou L. AHR Function in lymphocytes: emerging concepts. Trends Immunol. (2016) 37:17–31. doi: 10.1016/j.it.2015.11.007
30. Pilotte L. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc Natl Acad Sci USA. (2012) 109:2497–502. doi: 10.1073/pnas.1113873109
31. Song L, Guo L. Molecular mechanisms of 3,3'4,4',5-pentachlorobiphenyl-induced epithelial-mesenchymal transition in human hepatocellular carcinoma cells. Toxicol Appl Pharmacol. (2017) 322:75–88. doi: 10.1016/j.taap.2017.03.003
32. Li L, Wang T. TDO2 promotes the EMT of hepatocellular carcinoma through Kyn-AhR pathway. Front Oncol. (2020) 10:562823. doi: 10.3389/fonc.2020.562823
33. Venkateswaran N. MYC promotes tryptophan uptake and metabolism by the kynurenine pathway in colon cancer. Genes. (2019) 33:1236–51. doi: 10.1101/gad.327056.119
Keywords: rcc, AhR, KYN, EMT, gut microbiota
Citation: Dai G, Chen X and He Y (2021) The Gut Microbiota Activates AhR Through the Tryptophan Metabolite Kyn to Mediate Renal Cell Carcinoma Metastasis. Front. Nutr. 8:712327. doi: 10.3389/fnut.2021.712327
Received: 20 May 2021; Accepted: 20 July 2021;
Published: 11 August 2021.
Edited by:
Jie Yin, Hunan Agricultural University, ChinaReviewed by:
Shusong Wu, Hunan Agricultural University, ChinaCopyright © 2021 Dai, Chen and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yao He, aGV5YW8xOTg0QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.