
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr. , 14 October 2021
Sec. Nutritional Epidemiology
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.709846
Background: The association between Parkinson's disease (PD) risk and alcohol intake is a controversial topic.
Objectives: To systematically assess the association between PD risk and alcohol intake.
Methods: PubMed and Embase databases were searched for eligible studies with prospective design on PD risk and alcohol intake. A meta-analysis with a random-effects model and dose-response analysis was performed. Relative risk ratios (RRs) with 95% CIs were calculated.
Results: Eleven prospective studies were included. Overall, a higher intake of alcohol was inversely associated with PD risk (RR: 0.81, 95% CI: 0.70–0.95, I2 = 73.7%). Significant differences existed between the specific types of alcoholic beverages and geographic area. Specifically, a significant association existed for beer (RR: 0.78, 95% CI: 0.65–0.94, I2 = 0.0%) and studies conducted in Asia (RR: 0.66, 95% CI: 0.55–0.80, I2 = 37.3%). Dose-response analysis indicated a nonlinear relationship between PD risk and alcohol exposure. No evidence for publication bias was detected.
Conclusions: In summary, our meta-analysis suggests that alcohol consumption was associated with a decreased risk of PD, with a nearly U-shaped association. Future studies are warranted to clarify the question of a specific type of alcoholic beverage-dependent association, geographic area effect, and possible threshold effects regarding both the adverse and beneficial effects of alcohol.
Parkinson's disease (PD) characterized by three cardinal motor impairments: bradykinesia, rigidity, and tremor, is the second most common neurodegenerative disorder after Alzheimer's disease (1, 2). The incidence of PD increases with age and ~1% of the population > 60 years is affected by PD (2). Progress in understanding the etiology of PD is limited. Based on epidemiological studies, the broadest consistent evidence suggests that an inverse association between PD risk and cigarette smoking exists (3). Other risk factors, demonstrated with some consistency, include less physical activity, fewer doses of coffee intake, pesticides/insecticides, and increased intake of dairy products (4).
Alcohol consumption is another important lifestyle exposure following cigarette smoking and coffee consumption and is associated with multiple health outcomes, namely, cancers, neuropsychiatric disorders, and cardiovascular diseases (5, 6). Some evidence has shown that light to moderate alcohol intake has a decreased risk, while heavy intake carries an increased risk of disease or death (5, 6).
The brain is a key target of alcohol action. Prolonged and excessive alcohol consumption contributes to elevated oxidative stress, neuroimmune response, glutamate excitotoxicity, and their interplay effects that lead to permanent neuronal damage via mitochondrial lipid peroxidation and DNA or protein damage (7, 8). Thus, a potential association between alcohol intake and PD risk is hypothesized. The first study investigation was published in 1980 but did not yield significant findings (9). Since then, several studies have addressed the issue and both positive and negative results have been reported (10–30). Given the limitations of retrospective studies and single study power in previous studies, no strong evidence of an association exists. The aim of this systematic review and meta-analysis, therefore, was to quantitatively evaluate the effect of alcohol consumption on the risk of PD by summarizing the evidence from prospective studies using a dose-response approach.
Our study was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (31).
We identified all studies in the PubMed and EMBASE databases (up to August 21, 2021), and reference lists of relevant original papers, meta-analyses, and review articles. The following search terms were used: “{Parkinson[Title/Abstract] AND (liquor[Title/Abstract] OR ethanol[Title/Abstract] OR spirits[Title/Abstract] OR beer[Title/Abstract] OR wine[Title/Abstract] OR alcohol[Title/Abstract])}”. There were no publication years or language restrictions, but the search was limited to human studies.
We included prospective cohort studies or case-control nested in prospective cohort studies that reported an association between alcohol consumption and PD risk and provided adjusted relative risks (RRs) and 95% CIs, or other data to estimate the association and risk. If multiple articles were based on the same case series, only the study with the longest follow-up period or largest sample size was included.
The last name of the first author, publication year, country, sample size, identification of PD, exposure variables of interest (wine, spirits/hard liquor, beer, and/or total alcohol), assessment of alcohol exposure, the adjusted RRs with corresponding 95% CIs in the multivariable analysis, and potential confounders considered were extracted from each eligible study.
Heterogeneity was measured using the I2 test (32). The random-effects model, incorporating the heterogeneity across studies, was adopted to combine the risk estimates (33). Subgroup analysis was performed according to gender, geographic area, and type of alcohol consumption. Sensitivity analysis was performed by excluding one study at a time. Another sensitivity analysis restricted to those studies that provided risk estimates adjusted for smoking and coffee intake was also performed. Potential publication bias was assessed by Begg's funnel plots and Egger's test (34).
In this study, grams were used as the measure for the assessment of alcohol exposure and we defined one drink as 13 g of ethanol (35), except when the dose was well-defined in the original study. We first performed a comparison of the highest vs. lowest level of alcohol exposure. A dose-response analysis with a one-stage robust error meta-regression (REMR) model (36) was adopted. Using the REMR model, only the study-specific adjusted RRs with 95% CIs for at least two levels of alcohol consumption were reported. When the median level of exposure range of alcohol exposure was unavailable, the midpoint of each category of alcohol consumption was used. When the upper exposure categories were open-ended, we assumed that the width was the same interval as the adjacent category. All analyses were performed using STATA software (version 15.0, STATA Corp., College Station, TX, USA).
Figure 1 shows the flowchart. The initial search of the PubMed and Embase databases identified 1,403 studies and 453 duplicate records were excluded. After examining the title and abstract, 881 studies were also removed, and thus, 69 studies remained for assessment of the full text. Sixty of 69 studies were further excluded for the following reasons: retrospective case-control studies, comment letters without original data reported, review, conference abstracts, case report, meta-analysis, reports of the same study population, and no data available regarding alcohol consumption and PD risk. The manual literature search identified two studies (22, 24). Thus, 11 eligible studies were included (20–30). We only included the data from external control subjects for the Swedish Twin Registry study in which two control groups were reported (23).
Table 1 presents the basic characteristics of the included studies. There were three case-control studies nested within prospective cohorts (20, 22, 23) and eight prospective cohort studies (21, 24–30). Four studies were conducted in the United States (20, 21, 25, 26), two in Asia (24, 28), and five in Europe (22, 23, 27, 29, 30). Ascertainment of PD was identified by reviewing death certificates, hospital discharge summaries, and other types of medical records. All of the included studies had data on total alcohol intake, while only six studies had information on specific types of alcohol consumption (20, 21, 25–27, 29, 30). Alcohol exposure was measured using a mailed or self-administered questionnaire, or an in-person interview questionnaire. The reference category of alcohol exposure was nondrinkers in seven studies (20–23, 25–28) and light consumers (0–4.9 g/day) in another three studies (24, 29, 30).
Figure 2 shows the study-specific adjusted RRs with 95% CIs and pooled results. The overall analysis suggested that the highest category of alcohol consumption was associated with a decreased risk of PD (RR: 0.81, 95% CI: 0.70–0.95). Substantial heterogeneity was identified (I2 = 73.7%).
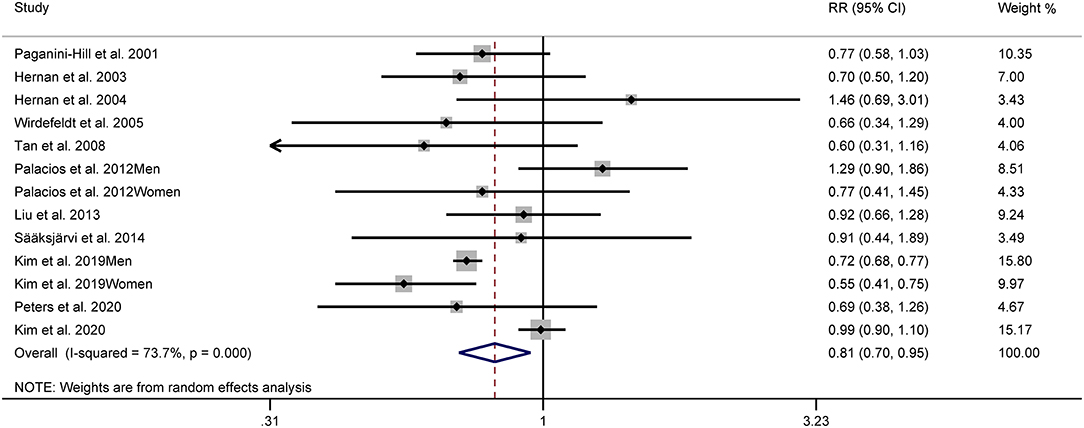
Figure 2. Forest plots for the relationship between Parkinson's disease (PD) risk and alcohol consumption.
Significant differences existed based on stratified analyses by geographic area. The pooled RRs were 0.66 (95% CI: 0.55–0.80, I2 = 37.3%), 0.88 (95% CI: 0.71–1.10, I2 = 37.3%), and 0.98 (95% CI: 0.89–1.08, I2 = 0.0%) for Asian, American, and European studies, respectively. No significant association between alcohol consumption and PD risk was demonstrated when a stratified analysis was based on sex. The pooled RRs were 0.81 (95% CI: 0.61–1.08, I2 = 61.3%) in male studies and 0.79 (95% CI: 0.57–1.08, I2 = 64.0%) in female studies. Considering subgroups of specific types of alcoholic beverages, the pooled results showed a RR of 0.78 (95% CI: 0.65–0.94, I2 = 0.0%) for beer, 0.98 (95% CI: 0.77–1.24, I2 = 58.0%) for liquor, and 0.95 (95% CI: 0.85–1.08, I2 = 2.5%) for wine.
Sensitivity analyses suggested that our results were not stable. Exclusion of the national program of 6,795,816 Koreans aged ≥ 40 years (24) did not yield a significant association (RR: 0.90, 95% CI: 0.79–1.03). Furthermore, heterogeneity was markedly decreased (I2 = 24.1%).
Another sensitivity analysis was restricted to those studies that provided risk estimates adjusted for smoking and coffee (20, 21, 23–27, 29). The pooled RR was 0.84 (95% CI: 0.72–0.98, I2 = 8.1%).
As suggested by Begg's funnel plots (Figure 3) and Egger's linear regression test (p = 0.697), no evidence of publication bias was found.
According to the criteria of the REMR model, 10 studies were included in the dose-response analysis (20–23, 25–30). A nonlinear association (p = 0.0018) between alcohol consumption and the risk of PD was confirmed (Figure 4). The strongest inverse association was observed for consumption of 26–35 g/day and no further reduction in risk of PD with increasing consumption of alcohol over this mount was identified.
In this comprehensive meta-analysis of 11 prospective studies, we found a slightly decreased risk of PD in those who consume alcohol. Dose-response analysis provided evidence for a significant, nonlinear, and inverse association between PD risk and alcohol consumption.
Interestingly, previous evidence hinted that there may be significant differences in the risk of PD associated with specific types of alcoholic beverages (20, 21, 25, 26, 29, 30). Two studies showed that beer consumption was associated with a decreased risk of PD, but wine or liquor with an increased risk of PD, although some results were not statistically significant (21, 26). In another four studies, there was no difference between beer, wine, and liquor, with a non-significant inverse (20, 29, 30) or positive (26) relationship reported. After pooling these results, we found that a decreased PD risk was only associated with beer consumption, but not liquor and wine. This indicated that other ingredients except ethanol may confer a beverage-specific relationship. Beer has a much lower ethanol content, but higher antioxidants levels, such as folic acid, niacin, purine, and other phenolics, which were thought to mediate the neuroprotective effects of alcohol (7, 37). Moreover, beer beverage intake is associated with high urate concentration, which has been shown to be neuroprotective effects in animal models (7, 38). There is also limited epidemiological evidence that uric acid (UA) prevents the development of PD. In a Netherlands cohort of approximately 5,000 participants (age ≥ 55 years; the average follow-up, 9.4 years), De Lau et al. (39) found that a higher concentration of UA was independently associated with a lower risk of PD, with significant evidence for a dose-exposure relationship (39). Another US cohort of 15,792 participants aged 45–64 years with nearly 20 years of follow-up also supported the previous finding that UA may be a protective factor against PD, especially in men (40). Taken together, it is thought that beer consumption may be associated with a deceased PD risk; however, this issue warrants further verification in future studies.
Our stratified analyses based on gender indicated that no significant association existed in women or men. Further analysis by geographic area revealed a significant association for Asia, but not the United States or Europe. The significance of geographic area disparities is unclear. One possible explanation is that a chance result was obtained in the US or European group as the limited number of included studies were included. An alternative explanation was that the potential residual confounding effect could not be excluded in the Asia group. The Korean study revealed a strong effect (RR range, 0.55–0.77) (28), but the risk estimates were not adjusted for caffeine intake, an important risk factor for PD. Finally, different lifestyles and genetic backgrounds may account for part of the difference.
In the dose-response analysis, a nearly U-shaped association between PD risk and alcohol consumption was identified, with the strongest inverse association observed for consumption of 26–35 g/day and no further reduction in risk of PD with increasing consumption of alcohol over this amount. Similarly, a 2019 systematic review of meta-analyses covering 34 risk factors concluded that light or moderate alcohol consumption was associated with a decreased risk of Alzheimer's disease (41). Regarding the cognitive decline, there was some evidence that the relationship with alcohol consumption was thought to be U- and J-shaped (8). All of these findings were supported by current biological mechanisms of alcohol conferring neurodegenerative disorders risk, with a protective or harmful effect reported in several reviews (7, 8, 42). A wide range of dose-risk estimates was identified in our study; however, additional studies are needed to provide a precise estimate.
Several meta-analyses of the relationship between alcohol consumption and PD risk have been published (35, 43–45). In a meta-analysis of 13 case-control studies published until September 2004, Ishihara and Bryne reported that ever alcohol drinkers were associated with a decreased risk of PD compared with nondrinkers (RR: 0.81, 95% CI: 0.70–0.92) (43). In 2012, another meta-analysis of 22 case-control and two cohort studies assessed alcohol exposure as “drinking” vs. “nondrinking” and the result (RR: 0.90, 95% CI: 0.84–0.96) (44) were in agreement with Ishihara and Bryne (43). Two years later, a meta-analysis of 24 case-control studies and eight prospective studies showed that alcohol consumption was inversely associated with PD risk (35). Furthermore, the dose-response curve showed a significant negative, linear association (35). In 2019, based on 26 case-control and five prospective studies, Jiménez-Jiménez et al. (45) estimated crude and diagnostic odds ratios with 95% CIs to measure the association between PD risk and alcohol consumption. An inverse association between PD risk and alcohol consumption was only supported by evidence of retrospective case-control studies but not prospective ones (45). Noticeable, in previous meta-analyses, the vast majority of included studies were retrospective studies that were always subjected to recall and selective biases. Additionally, we performed a comparison of the highest vs. lowest categories of alcohol exposure rather than “ever” or “never,” which is thought to be inadequate to assess the complexity of alcohol (46). Thus, our meta-analysis of 11 prospective studies may provide a relatively precise estimation. Specially, a nonlinear relationship between PD risk and alcohol exposure was observed.
The main strengths of this meta-analysis included a large number of prospective studies with large sample sizes, estimation of total and specific types of alcoholic beverages, and dose-response analysis with the one-stage method. Also, some potential limitations need to be addressed. First, significant heterogeneity was identified. As indicated by the results of sensitivity and subgroup analyses, confounding factors may have accounted for, in part, heterogeneity. Second, because of the nature of observational design, a residual confounding effect could not be neglected. Third, we noted that the potential measurement bias of alcohol exposure may twist the true relationship between alcohol consumption and PD risk. For example, alcohol exposure was only measured in the past 12 months before baseline, but not lifetime (26), and thus, “former drinkers” may become “never drinkers” at the baseline examination and the relationship between alcohol exposure and PD risk may have been distorted. A further study providing a more detailed exposure, namely, “former drinkers” and “current drinkers” would help us fully understand the relationship between alcohol exposure and PD. Fourth, our results were not stable. When the Korean study (28) was removed from the sensitivity analyses, there was no significant association between PD risk and alcohol intake. Finally, potential publication bias is always a concern in meta-analyses, although little evidence for publication bias was identified by Begg's funnel plots and Egger's linear regression test.
In conclusion, our meta-analysis suggested that alcohol consumption was associated with a decreased risk of PD (a nearly U-shaped association). Due to the above-listed limitations, the need for caution regarding our findings is warranted. The specific type of alcoholic beverage-dependent association, geographic area effect, and possible alcohol threshold for adverse and beneficial effects remain to be clarified in future studies.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
CS and HT designed the research and wrote the first draft. CS and XW performed the second literature search. CS, HT, XW, and PW extracted and confirmed the data. CS performed the statistical analysis. CS, HT, XW, PW, NW, and JH reviewed and rewrote the study. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Gonzalez-Hunt CP, Sanders LH. DNA damage and repair in Parkinson's disease: recent advances and new opportunities. J Neurosci Res. (2021) 99:180–9. doi: 10.1002/jnr.24592
2. Tysnes OB, Storstein A. Epidemiology of Parkinson's disease. J Neural Transm. (2017) 124:901–5. doi: 10.1007/s00702-017-1686-y
3. Gallo V, Vineis P, Cancellieri M, Chiodini P, Barker RA, Brayne C, et al. Exploring causality of the association between smoking and Parkinson's disease. Int J Epidemiol. (2019) 48:912–25. doi: 10.1093/ije/dyy230
4. Kyrozis A, Ghika A, Stathopoulos P, Vassilopoulos D, Trichopoulos D, Trichopoulou A. Dietary and lifestyle variables in relation to incidence of Parkinson's disease in Greece. Eur J Epidemiol. (2013) 28:67–77. doi: 10.1007/s10654-012-9760-0
5. Gmel G, Hasan OSM, Imtiaz S, Popova S, Probst C, Roerecke M, et al. The relationship between different dimensions of alcohol use and the burden of disease-an update. Addiction. (2017) 112:968–1001. doi: 10.1111/add.13757
6. Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. (2011) 342:d671. doi: 10.1136/bmj.d671
7. Kamal H, Tan GC, Ibrahim SF, Shaikh MF, Mohamed IN, Mohamed RMP, et al. Alcohol use disorder, neurodegeneration, Alzheimer's and Parkinson's disease: interplay between oxidative stress, neuroimmune response and excitotoxicity. Front Cell Neurosci. (2020) 14:282. doi: 10.3389/fncel.2020.00282
8. Peng B, Yang Q, B Joshi R, Liu Y, Akbar M, Song BJ, et al. Role of alcohol drinking in Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis. Int J Mol Sci. (2020) 21:2316. doi: 10.3390/ijms21072316
9. Baumann RJ, Jameson HD, McKean HE, Haack DG, Weisberg LM. Cigarette smoking and Parkinson disease: 1. Comparison of cases with matched neighbors. Neurology. (1980) 30:839–43. doi: 10.1212/WNL.30.8.839
10. Wang WZ, Fang XH, Cheng XM, Jiang DH, Lin ZJ. A case-control study on the environmental risk factors of Parkinson's disease in Tianjin, China. Neuroepidemiology. (1993) 12:209–18. doi: 10.1159/000110319
11. Hellenbrand W, Seidler A, Boeing H, Robra BP, Vieregge P, Nischan P, et al. Diet and Parkinson's disease. I: A possible role for the past intake of specific foods and food groups. Results from a self-administered food-frequency questionnaire in a case-control study. Neurology. (1996) 47:636–43. doi: 10.1212/WNL.47.3.636
12. Liou HH, Tsai MC, Chen CJ, Jeng JS, Chang YC, Chen SY, et al. Environmental risk factors and Parkinson's disease: a case-control study in Taiwan. Neurology. (1997) 48:1583–8. doi: 10.1212/WNL.48.6.1583
13. Benedetti MD, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, et al. Smoking, alcohol, and coffee consumption preceding Parkinson's disease: a case-control study. Neurology. (2000) 55:1350–8. doi: 10.1212/WNL.55.9.1350
14. Tan XH, Wang SM, Xue NQ, Teng WT, Feng YQ. Study on the risk factors and its interaction on Parkinson disease. Zhonghua Liu Xing Bing Xue Za Zhi. (2004) 25:527–30. doi: 10.3760/j.issn:0254-6450.2004.06.017
15. Evans AH, Lawrence AD, Potts J, MacGregor L, Katzenschlager R, Shaw K, et al. Relationship between impulsive sensation seeking traits, smoking, alcohol and caffeine intake, and Parkinson's disease. J Neurol Neurosurg Psychiatry. (2006) 77:317–21. doi: 10.1136/jnnp.2005.065417
16. Fukushima W, Miyake Y, Tanaka K, Sasaki S, Kiyohara C, Tsuboi Y, et al. Fukuoka Kinki Parkinson's Disease Study Group. Alcohol drinking and risk of Parkinson's disease: a case-control study in Japan. BMC Neurol. (2010) 10:111. doi: 10.1186/1471-2377-10-111
17. Sipetic SB, Vlajinac HD, Maksimovic JM, Marinkovic JM, Dzoljic ED, Ratkov IS. Cigarette smoking, coffee intake and alcohol consumption preceding Parkinson's disease: a case-control study. Acta Neuropsychiatr. (2012) 24:109–14. doi: 10.1111/j.1601-5215.2011.00593.x
18. Kenborg L, Lassen CF, Ritz B, Andersen KK, Christensen J, Schernhammer ES, et al. Lifestyle, family history, and risk of idiopathic Parkinson disease: a large Danish case-control study. Am J Epidemiol. (2015) 181:808–16. doi: 10.1093/aje/kwu332
19. Grandinetti A, Morens DM, Reed D, MacEachern D. Prospective study of cigarette smoking and the risk of developing idiopathic Parkinson's disease. Am J Epidemiol. (1994) 139:1129–38. doi: 10.1093/oxfordjournals.aje.a116960
20. Paganini-Hill A. Risk factors for Parkinson's disease: the leisure world cohort study. Neuroepidemiology. (2001) 20:118–24. doi: 10.1159/000054770
21. Hernán MA, Chen H, Schwarzschild MA, Ascherio A. Alcohol consumption and the incidence of Parkinson's disease. Ann Neurol. (2003) 54:170–5. doi: 10.1002/ana.10611
22. Hernán MA, Logroscino G, Rodríguez LA. A prospective study of alcoholism and the risk of Parkinson's disease. J Neurol. (2004) 251:vII14–7. doi: 10.1007/s00415-004-1705-4
23. Wirdefeldt K, Gatz M, Pawitan Y, Pedersen NL. Risk and protective factors for Parkinson's disease: a study in Swedish twins. Ann Neurol. (2005) 57:27–33. doi: 10.1002/ana.20307
24. Tan LC, Koh WP, Yuan JM, Wang R, Au WL, Tan JH, et al. Differential effects of black versus green tea on risk of Parkinson's disease in the Singapore Chinese Health Study. Am J Epidemiol. (2008) 167:553–60. doi: 10.1093/aje/kwm338
25. Palacios N, Gao X, O'Reilly E, Schwarzschild M, McCullough ML, Mayo T, et al. Alcohol and risk of Parkinson's disease in a large, prospective cohort of men and women. Mov Disord. (2012) 27:980–7. doi: 10.1002/mds.25050
26. Liu R, Guo X, Park Y, Wang J, Huang X, Hollenbeck A, et al. Alcohol consumption, types of alcohol, and Parkinson's disease. PLoS ONE. (2013) 8:e66452. doi: 10.1371/journal.pone.0066452
27. Sääksjärvi K, Knekt P, Männistö S, Lyytinen J, Jääskeläinen T, Kanerva N, et al. Reduced risk of Parkinson's disease associated with lower body mass index and heavy leisure-time physical activity. Eur J Epidemiol. (2014) 29:285–92. doi: 10.1007/s10654-014-9887-2
28. Kim R, Yoo D, Jung YJ, Han K, Lee JY. Sex differences in smoking, alcohol consumption, and risk of Parkinson's disease: a nationwide cohort study. Parkinsonism Relat Disord. (2020) 71:60–5. doi: 10.1016/j.parkreldis.2019.12.006
29. Peters S, Gallo V, Vineis P, Middleton LT, Forsgren L, Sacerdote C, et al. Alcohol consumption and risk of Parkinson's disease: data from a large prospective European cohort. Mov Disord. (2020) 35:1258–63. doi: 10.1002/mds.28039
30. Kim IY, Yang TO, Heath AK, Simpson RF, Reeves GK, Green J, et al. Alcohol intake and Parkinson's disease risk in the million women study. Mov Disord. (2020) 35:443–9. doi: 10.1002/mds.27933
31. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
32. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
33. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
34. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
35. Zhang D, Jiang H, Xie J. Alcohol intake and risk of Parkinson's disease: a meta-analysis of observational studies. Mov Disord. (2014) 29:819–22. doi: 10.1002/mds.25863
36. Xu C, Doi SAR. The robust error meta-regression method for dose-response meta-analysis. Int J Evid Based Healthc. (2018) 16:138–44. doi: 10.1097/XEB.0000000000000132
37. Sánchez-Muniz FJ, Macho-González A, Garcimartín A, Santos-López JA, Benedí J, Bastida S, et al. The nutritional components of beer and its relationship with neurodegeneration and Alzheimer's disease. Nutrients. (2019) 11:1558. doi: 10.3390/nu11071558
38. Huang TT, Hao DL, Wu BN, Mao LL, Zhang J. Uric acid demonstrates neuroprotective effect on Parkinson's disease mice through Nrf2-ARE signaling pathway. Biochem Biophys Res Commun. (2017) 493:1443–9. doi: 10.1016/j.bbrc.2017.10.004
39. de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum uric acid levels and the risk of Parkinson disease. Ann Neurol. (2005) 58:797–800. doi: 10.1002/ana.20663
40. Chen H, Mosley TH, Alonso A, Huang X. Plasma urate and Parkinson's disease in the atherosclerosis risk in communities (ARIC) study. Am J Epidemiol. (2009) 169:1064–9. doi: 10.1093/aje/kwp033
41. Anstey KJ, Ee N, Eramudugolla R, Jagger C, Peters R. A systematic review of meta-analyses that evaluate risk factors for dementia to evaluate the quantity, quality, and global representativeness of evidence. J Alzheimers Dis. (2019) 70:S165–86. doi: 10.3233/JAD-190181
42. Boulos C, Yaghi N, El Hayeck R, Heraoui GN, Fakhoury-Sayegh N. Nutritional risk factors, microbiota and Parkinson's disease: what is the current evidence? Nutrients. (2019) 11:1896. doi: 10.3390/nu11081896
43. Ishihara L, Brayne C. A systematic review of nutritional risk factors of Parkinson's disease. Nutr Res Rev. (2005) 18:259–82. doi: 10.1079/NRR2005108
44. Noyce AJ, Bestwick JP, Silveira-Moriyama L, Hawkes CH, Giovannoni G, Lees AJ, et al. Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann Neurol. (2012) 72:893–901. doi: 10.1002/ana.23687
45. Jiménez-Jiménez FJ, Alonso-Navarro H, García-Martín E, Agúndez JAG. Alcohol consumption and risk for Parkinson's disease: a systematic review and meta-analysis. J Neurol. (2019) 266:1821–1834. doi: 10.1007/s00415-018-9032-3
Keywords: Parkinson's disease, risk factors, alcohol, ethanol, meta-analysis
Citation: Shao C, Wang X, Wang P, Tang H, He J and Wu N (2021) Parkinson's Disease Risk and Alcohol Intake: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Front. Nutr. 8:709846. doi: 10.3389/fnut.2021.709846
Received: 14 May 2021; Accepted: 15 September 2021;
Published: 14 October 2021.
Edited by:
Claudio D'Addario, University of Teramo, ItalyReviewed by:
José María Huerta, Instituto de Salud Carlos III (ISCIII), SpainCopyright © 2021 Shao, Wang, Wang, Tang, He and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Tang, aHdlaXRhbmdAZ21haWwuY29t; Nan Wu, d3VuYW44ODFAdG1tdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.