
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 20 August 2021
Sec. Clinical Nutrition
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.700132
This article is part of the Research Topic Sarcopenia, Frailty and Nutrition in Liver Diseases View all 14 articles
 Yue Yin1†
Yue Yin1† Yiling Li2†
Yiling Li2† Lichun Shao3†
Lichun Shao3† Shanshan Yuan4†
Shanshan Yuan4† Bang Liu5
Bang Liu5 Su Lin6
Su Lin6 Yida Yang7
Yida Yang7 Shanhong Tang8
Shanhong Tang8 Fanping Meng9
Fanping Meng9 Yunhai Wu10
Yunhai Wu10 Yu Chen11
Yu Chen11 Bimin Li12
Bimin Li12 Qiang Zhu13
Qiang Zhu13 Xingshun Qi1*
Xingshun Qi1*Objective: At present, the association of body mass index (BMI) with the prognosis of liver cirrhosis is controversial. Our retrospective study aimed to evaluate the impact of BMI on the outcome of liver cirrhosis.
Methods: In the first part, long-term death was evaluated in 436 patients with cirrhosis and without malignancy from our prospectively established single-center database. In the second part, in-hospital death was evaluated in 379 patients with cirrhosis and with acute gastrointestinal bleeding (AGIB) from our retrospective multicenter study. BMI was calculated and categorized as underweight (BMI <18.5 kg/m2), normal weight (18.5 ≤ BMI < 23.0 kg/m2), and overweight/obese (BMI ≥ 23.0 kg/m2).
Results: In the first part, Kaplan–Meier curve analyses demonstrated a significantly higher cumulative survival rate in the overweight/obese group than the normal weight group (p = 0.047). Cox regression analyses demonstrated that overweight/obesity was significantly associated with decreased long-term mortality compared with the normal weight group [hazard ratio (HR) = 0.635; 95% CI: 0.405–0.998; p = 0.049] but not an independent predictor after adjusting for age, gender, and Child–Pugh score (HR = 0.758; 95%CI: 0.479–1.199; p = 0.236). In the second part, Kaplan–Meier curve analyses demonstrated no significant difference in the cumulative survival rate between the overweight/obese and the normal weight groups (p = 0.094). Cox regression analyses also demonstrated that overweight/obesity was not significantly associated with in-hospital mortality compared with normal weight group (HR = 0.349; 95%CI: 0.096-1.269; p = 0.110). In both of the two parts, the Kaplan–Meier curve analyses demonstrated no significant difference in the cumulative survival rate between underweight and normal weight groups.
Conclusion: Overweight/obesity is modestly associated with long-term survival in patients with cirrhosis but not an independent prognostic predictor. There is little effect of overweight/obesity on the short-term survival of patients with cirrhosis and with AGIB.
Overweight/obesity, which is defined as excessive body fat accumulation, is a common public health problem (1). The global age-standardized prevalence of obesity defined by high body mass index (BMI) is increased from 3.2 to 10.8% in men and from 6.4 to 14.9% in women between 1975 and 2014 (2). Overweight/obesity is considered a risk factor for liver diseases (3, 4). Increased adipose tissues lead to triglyceride deposition in the liver, produce various transduction signals that alter lipid and glucose metabolisms, and then cause insulin resistance and increased release of free fatty acids, which are the causes of hepatic steatosis (5). Hepatic steatosis can contribute to lipid peroxidation and hepatic stellate cell activation, which further induce cellular injury and inflammation and accelerate the progression of liver fibrosis and cirrhosis (6, 7). However, the impact of BMI on outcomes in liver cirrhosis is still controversial. Some studies demonstrated that obesity was an independent risk factor for cirrhosis-related death or hospitalization (8). Other studies supported that the patients with cirrhosis and with obesity had a lower mortality than those without (9). Considering the controversy of the existing evidence, this study aimed to examine the effect of BMI on the prognosis of patients with liver cirrhosis.
This retrospective study was carried out following the rules of the 1975 Declaration of Helsinki and approved by the Medical Ethical Committee of the General Hospital of Northern Theater Command with an approval number of Y (2021) 023. It was divided into two major parts. In both of the two parts, if the patients are lacking height and weight, they were excluded from the current study.
In the first part, we retrospectively selected patients with cirrhosis and without malignancy from our prospectively established database (10). Eligible patients should be consecutively admitted to our department and underwent an endoscopy and contrast-enhanced CT or MRI scans between December 2014 and December 2020. They were regularly followed via telephone or through outpatient visits and/or by reviewing medical records until February 2021. Death and the patients with liver transplantation during follow-up were recorded. Liver transplantation-free survival was the major endpoint of the first part. Patients who underwent liver transplantation were followed until the time point when the liver transplantation was performed.
In the second part, we retrospectively selected patients with cirrhosis and with acute gastrointestinal bleeding (AGIB) who received terlipressin and/or somatostatin/octreotide from our multicenter study (registration number: NCT03846180), which has been further updated after some publications (11–14). Notably, in this part, the eligible patients were consecutively admitted to 13 centers from 8 provinces or municipalities in China between January 2010 and December 2018, and patients who underwent transjugular intrahepatic portosystemic shunt, splenectomy, surgical shunt, or liver transplantation were excluded from the study. In-hospital death was the major endpoint of the second part.
Liver cirrhosis was diagnosed based on clinical manifestations, laboratory tests, radiological examinations, and/or histological data. AGIB was defined as hematemesis, melena, and/or hematochezia within 5 days before admission (15).
BMI was calculated by dividing weight in kilograms by the square of height in meters (16). According to the WHO classification for Asian populations, all patients were categorized as underweight (BMI <18.5 kg/m2), normal weight (18.5 ≤ BMI < 23.0 kg/m2), and overweight/obese (BMI ≥ 23.0 kg/m2) (17).
First, continuous variables were described as mean ± SD and median (range), and categorical variables were described as frequency (percentage). The difference was compared using Mann–Whitney U test and Chi-squared test or Fisher's exact test. Second, survival probability curves were calculated by the Kaplan–Meier curve analyses and compared by the log-rank test. Third, univariate Cox regression analyses were performed to explore the association of BMI with mortality, and multivariable Cox regression analyses were performed by adjusting for age, gender, and Child–Pugh scores to identify whether BMI was an independent predictor of death. Hazard ratios (HRs) with 95% CIs were calculated. Fourth, time-dependent receiver operating characteristic (T-ROC) curve analyses were used to evaluate the performance of BMI for predicting death, and area under the curve (AUC) and concordance index (C-index) were calculated. A two-tailed p < 0.05 was considered statistically significant. All statistical analyses were performed by using SPSS version 26.0 (IBM Corp, Armonk, New York, USA) and R version 4.0.3 with packages survival, survminer, and timeROC (R Foundation for Statistical Computing, Vienna, Austria).
Overall, 436 of 527 patients with cirrhosis registered in our prospective database had BMI data at their admissions and were included in the present study. Among them, 30 (6.9%) were underweight and 234 (53.7%) overweight/obese. The median BMI was 17.34 (range: 14.82–18.42 kg/m2) in underweight group, 21.02 (range: 18.52–22.95 kg/m2) in normal weight group, and 25.47 (range: 23.01–37.37 kg/m2) in overweight/obese group. During a median follow-up period of 2.28 (range: 0.03–5.59 years), 1 patient was lost to follow-up, 6 underwent liver transplantation, and 85 died. Among them, 62 (72.9%) patients died of liver diseases, 14 (16.5%) non-liver diseases, and 9 (10.6%) unknown causes. The mortality was 30% (9/30) in underweight group, 23.3% (40/172) in normal weight group, and 15.4% (36/234) in overweight/obese group (Figure 1).
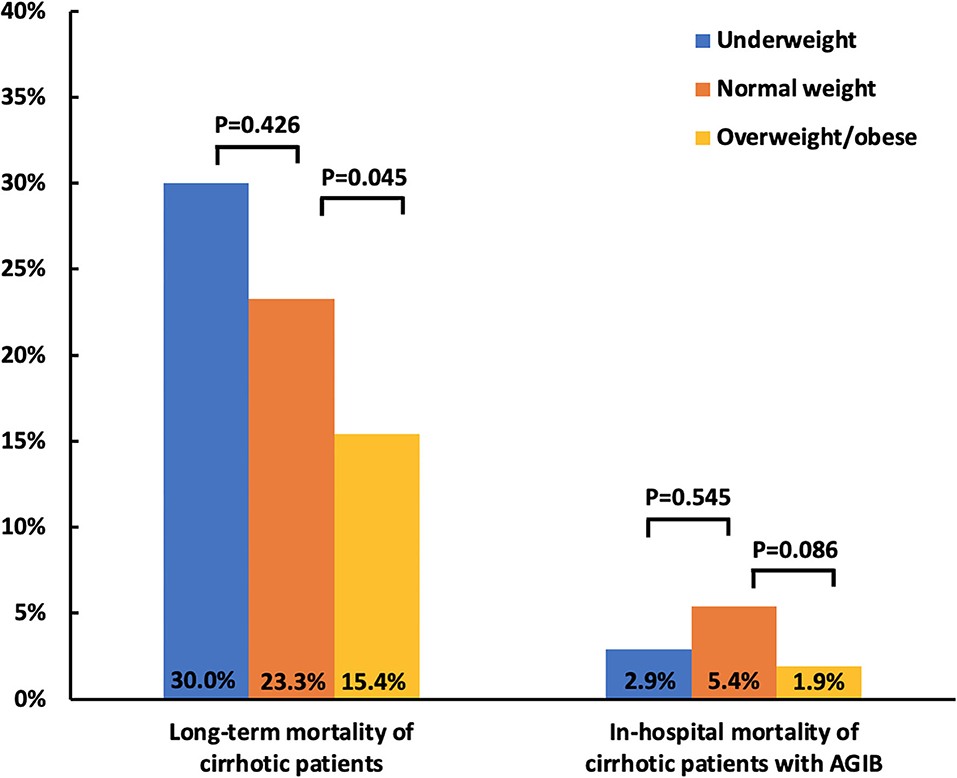
Figure 1. Bar charts showing the association of BMI with mortality in patients with cirrhosis. BMI, body mass index; AGIB, acute gastrointestinal bleeding.
Compared with normal weight group, underweight group had significantly lower proportions of hepatitis C virus (HCV) (0 vs. 11.6%; p = 0.034) and international normalized ratio (INR) (1.31 ± 0.40 vs. 1.34 ± 0.26; p = 0.026) and higher platelet count (PLT) (163.10 ± 137.51 vs. 97.65 ± 66.90; p = 0.004) (Table 1). The Kaplan–Meier curve analyses demonstrated no significant difference in the cumulative survival rate between normal weight and underweight groups (p = 0.190) (Figure 2A). Univariate Cox regression analyses also demonstrated that underweight was not significantly associated with long-term mortality (HR = 1.617; 95%CI: 0.783–3.338; p = 0.194) (Supplementary Table 1).
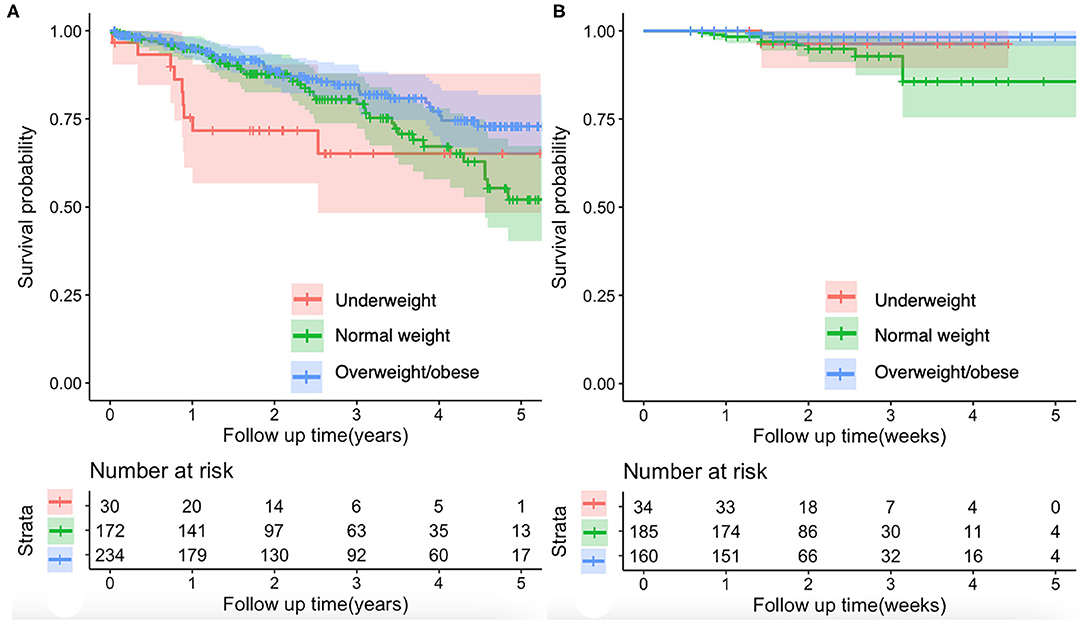
Figure 2. Kaplan–Meier curves showing the effect of BMI on the mortality of patients with cirrhosis. (A) Long-term mortality of patients with cirrhosis. There was no significant difference in the cumulative survival rate between normal weight and underweight groups (log-rank test, p = 0.190). The overweight/obese group had a significantly higher cumulative survival rate than the normal weight group (log-rank test, p = 0.047). (B) In-hospital mortality of patients with cirrhosis and with AGIB. There was no significant difference in the cumulative survival rate between normal weight and underweight groups (log-rank test, p = 0.491) or between normal weight and overweight/obese groups (log-rank test, p = 0.094). AGIB, acute gastrointestinal bleeding.
Compared with normal weight group, overweight/obese group had significantly higher proportions of hepatitis B virus (HBV) (44.0 vs. 34.3%; p = 0.048) and alcohol abuse (49.1 vs. 35.5%; p = 0.006) (Table 1) and lower mortality (15.4 vs. 23.3%; p = 0.045) (Figure 1). The Kaplan-Meier curve analyses demonstrated that overweight/obese group had a significantly higher cumulative survival rate than normal weight group (p = 0.047) (Figure 2A). Univariate Cox regression analyses demonstrated that overweight/obesity was significantly associated with decreased long-term mortality (HR = 0.635; 95%CI: 0.405–0.998; p = 0.049). After adjusting for age, gender, and Child–Pugh score, overweight/obesity was not an independent predictor of decreased long-term mortality (HR = 0.758; 95%CI: 0.479–1.199; p = 0.236) (Supplementary Table 1).
Time-dependent receiver operating characteristic analyses of BMI for predicting long-term mortality of patients with cirrhosis are shown in Figure 3A. The AUCs at 1-, 2-, and 3-year during follow up were 0.613 (95%CI: 0.487–0.740), 0.566 (95%CI: 0.472–0.659), and 0.585 (95%CI: 0.495–0.675), respectively. C-index was 0.568 (95% CI: 0.497–0.639).
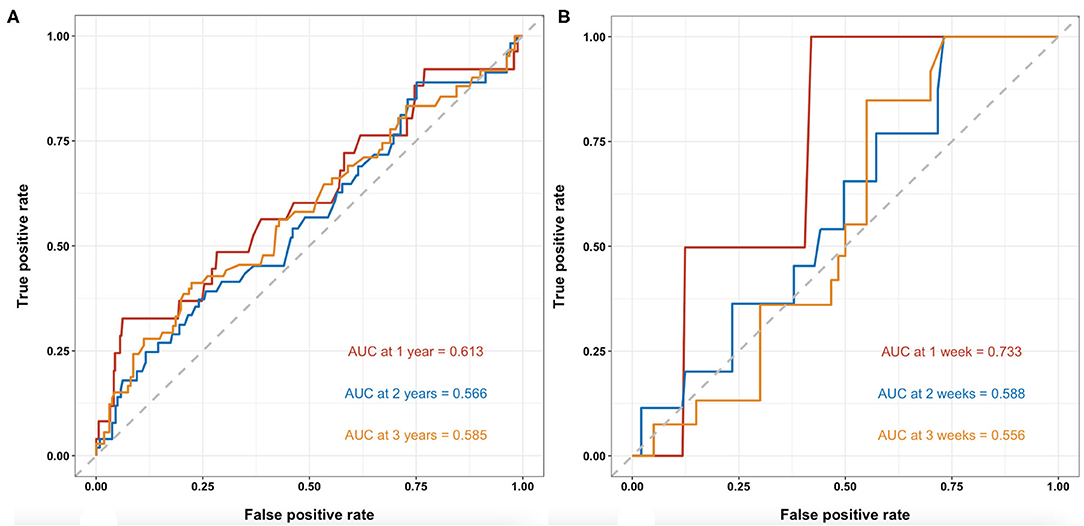
Figure 3. T-ROC curves of BMI for the predictability of mortality in patients with cirrhosis. (A). Long-term mortality of patients with cirrhosis at 1-, 2-, and 3-year during follow-up. The AUCs were 0.613 (95%CI: 0.487–0.740), 0.566 (95%CI: 0.472–0.659), and 0.585 (95%CI: 0.495–0.675), respectively. (B) In-hospital mortality of patients with cirrhosis with AGIB at 1-, 2-, and 3-week during hospitalization. The AUCs were 0.733 (95%CI: 0.527–0.938), 0.588 (95%CI: 0.422–0.755), and 0.556 (95%CI: 0.410–0.702), respectively. T-ROC, time-dependent receiver operating characteristic; BMI, body mass index; AUC, area under the curve.
Overall, 379 of 1,582 patients with cirrhosis and with AGIB recorded in our multicenter study had BMI data at their admissions and were included in the present study. Among them, 34 (9.0%) were underweight and 160 (42.2%) overweight/obese. The median BMI was 17.70 (range: 16.33–18.49 kg/m2)in underweight group, 21.09 (range: 18.56–22.99 kg/m2) in normal weight group, and 25.15 (range: 23.03–43.94 kg/m2) in overweight/obese group. During a median hospitalization period of 13 (range: 4–48) days, 14 patients died. Among them 11 (78.6%) patients died of liver diseases and 3 (21.4%) died of non-liver diseases. In-hospital mortality was 2.9% (1/34) in underweight group, 5.4% (10/185) in normal weight group, and 1.9% (3/160) in overweight/obese group (Figure 1).
Compared with normal weight group, underweight group had significantly lower proportion of men (47.1 vs. 75.1%; p = 0.001) and higher PLT (113.32 ± 81.32 vs. 86.69 ± 65.13; p = 0.004) (Table 2). The Kaplan–Meier curve analyses demonstrated no significant difference in the cumulative survival rate between normal weight and underweight groups (p = 0.491) (Figure 2B). Univariate Cox regression analyses also demonstrated that underweight was not significantly associated with in-hospital mortality (HR = 0.494; 95%CI: 0.063–3.866; p = 0.502) (Supplementary Table 1).
Compared with normal weight group, overweight/obese group had significantly lower alanine aminotransferase (ALT) (60.49 ± 141.16 vs. 67.77 ± 238.96; p = 0.039) and higher serum creatinine (SCr) (75.36 ± 27.03 vs. 70.05 ± 22.73; p = 0.044) (Table 2). The Kaplan–Meier curve analyses demonstrated no significant difference in the cumulative survival rate between normal weight and overweight/obese groups (p = 0.094) (Figure 2B). Univariate Cox regression analyses also demonstrated that overweight/obesity was not significantly associated with in-hospital mortality (HR = 0.349; 95%CI: 0.096–1.269; p = 0.110) (Supplementary Table 1).
Time-dependent receiver operating characteristic analyses of BMI for predicting in-hospital mortality of patients with cirrhosis and with AGIB are shown in Figure 3B. The AUCs at 1-, 2-, and 3-week during hospitalizations were 0.733 (95%CI: 0.527–0.938), 0.588 (95%CI: 0.422–0.755), and 0.556 (95%CI: 0.410–0.702), respectively. C-index was 0.610 (95% CI: 0.477–0.742).
The first objective of the present work was to explore the relationship of BMI with the long-term prognosis of patients with cirrhosis. We found that overweight/obesity was inversely associated with long-term mortality of patients with liver cirrhosis. This finding supported the “obesity paradox” that overweight/obese patients could have superior survival. It was first proposed by Fleischmann et al. (18) in patients undergoing hemodialysis (18) and further validated in subjects with chronic diseases, such as cardiovascular diseases, hypertension, and diabetes (19–22).
The pathophysiology of the “obesity paradox” remains to be elucidated, and there are some underlying explanations (Supplementary Figure 1). First, fat storage in overweight/obese patients may protect the balance of muscle protein catabolism in chronic wasting diseases (23). Body protein is crucial for survival because it can maintain cell function and support cell architecture (24). Muscle protein metabolism is preserved in patients with obesity and with chronic cardiac failure, indicating better outcomes but increased in patients without obesity (25). Similarly, sarcopenia, which is mainly caused by increased muscle protein metabolism (26, 27), is associated with lower BMI in patients with liver cirrhosis (28), and further leads to higher mortality (29). Second, adipose tissue, which has been recognized as an endocrine organ, can secrete diverse adipokines (30, 31). Adiponectin, an anti-inflammatory adipokine, can inhibit the proliferation and activation of hepatic stellate cells, which produce extracellular matrix proteins in the case of liver injury and promote the occurrence of liver fibrosis (32). Leptin, another adipokine, can prevent ectopic lipid accumulation in non-adipose tissues, augment immune response, and improve bacterial clearance and survival in animal models (33, 34). Both of which are increased in overweight/obese patients with liver cirrhosis, probably improving the outcomes of the patients (35). Third, patients with cirrhosis and with hepatic edema have systemic vasodilation and underfilled arteries, decreasing effective circulatory blood volume and activating cardiac sympathetic nervous system (SNS) and renin-angiotensin-aldosterone system (RAAS) which can stimulate sodium and water retention. Prolonged sodium and water retention will cause hyponatremia and pulmonary edema and increase cardiac afterload (36, 37). Overweight/obesity can alleviate the activities of cardiac SNS and RAAS, thereby inhibiting hyperdynamic circulation and conferring survival benefits (38). Fourth, overweight/obese patients are more likely to receive medical interventions, including antihypertensive drugs for decreasing systolic blood pressure, which can make short-term hemodynamic status more stable (39, 40), and statins for treating hyperlipidemia (41), which can have a favorable impact on outcomes of cirrhosis and portal hypertension (42).
Age, gender, and body function may interact with the relationship between BMI and prognosis (43, 44). Accordingly, this study adjusted some potential confounding factors, including age, gender, and Child–Pugh score, in multivariable Cox regression analysis. By comparison, the previous study by Karagozian et al. (9) selected patients with cirrhosis from the National Inpatient Sample database, which was lacking laboratory data, such as hepatic function (9). Thus, these results should be more reliable. This study found that BMI was not an independent risk factor of decreased long-term mortality, indicating that the prognostic impact of BMI might not be as strong as Child–Pugh score. This finding can be explained by the hypothesis of “reverse causation” that overweight/obesity may not be a cause for a better outcome, but a consequence (45–47). Another explanation is that BMI is convenient but unable to comprehensively measure body composition (2, 48), such as muscle and subcutaneous and visceral adipose tissue and their specific distributions in the body (49). Besides, body weight may be masked by fluid retention resulting from ascites in patients with cirrhosis, inaccurately or falsely evaluating the prognostic impact of BMI.
This study demonstrated that BMI was not significantly associated with in-hospital outcomes of patients with cirrhosis and with AGIB, which is consistent with the previous study regarding the association of obesity with in-hospital mortality of patients with non-variceal gastrointestinal bleeding (50). This may be explained by the complexity of evaluating the outcomes of acute injuries, which should not be attributed to the effect of body weight alone. Fatal injuries brought by decompensated events are far beyond the potential benefits of overweight/obesity.
There were some limitations in this study. First, we did not obtain the dynamic changes of BMI during follow-up. Second, we did not have an external validation cohort to verify the present findings. Third, BMI data were missing in a proportion of our AGIB patients, probably producing a selection bias. Fourth, the interventions used during the hospitalization and follow-up, which might be beneficial for the outcomes, were not available. Fifth, we did not evaluate the specific values of muscle mass or the distribution of adipose tissue due to the absence of dual-energy X-ray absorptiometry, waist circumference, and waist to hip ratio.
In conclusion, there is a modest association of overweight/obesity with decreased long-term mortality of patients with cirrhosis. However, BMI cannot act as an independent prognostic predictor of liver cirrhosis. In the future, prospective large-scale studies should be attempted to combine BMI with other indicators involved in measuring muscle mass and adipose tissue to more precisely predict the clinical outcomes of liver cirrhosis.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Medical Ethical Committee of the General Hospital of Northern Theater Command. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
XQ: conceptualization and supervision. YYi and XQ: methodology, formal analysis, visualization, and writing—original draft. YL, LS, SY, BL, SL, YYa, ST, FM, YW, YC, BL, QZ, and XQ: resource. YYi, YL, LS, SY, BL, SL, YYa, ST, FM, YW, YC, BL, QZ, and XQ: data curation and writing—review and editing. All authors have made an intellectual contribution to the manuscript and approved the submission.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank the participants of the study, including Han Deng, Ran Wang, Xiangbo Xu, Zhaohui Bai, Qianqian Li, Kexin Zheng, Le Wang, Fangfang Yi, Yanyan Wu, Li Luo, and Mengyuan Peng of our study team, for their efforts in setting up and updating our single-center prospectively established database.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.700132/full#supplementary-material
Supplementary Figure 1. Schematic diagram showing the underlying explanations of obesity paradox. SNS, sympathetic nervous system; RAAS, renin-angiotensin-aldosterone system.
BMI, body mass index; AGIB, acute gastrointestinal bleeding; HR, hazard ratio; T-ROC, time-dependent receiver operating characteristic; AUC, area under the curve; C-index, concordance index; HCV, hepatitis C virus; INR, international normalized ratio; PLT, platelet count; HBV, hepatitis B virus; ALT, alanine aminotransferase; SCr, serum creatinine; SNS, sympathetic nervous system; RAAS, renin-angiotensin-aldosterone system.
1. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. (2000) 894:i–xii, 1–253.
2. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. (2016) 387:1377–96. doi: 10.1016/S0140-6736(16)30054-X
3. Hourigan LF, Macdonald GA, Purdie D, Whitehall VH, Shorthouse C, Clouston A, et al. Fibrosis in chronic hepatitis C correlates significantly with body mass index and steatosis. Hepatology. (1999) 29:1215–9. doi: 10.1002/hep.510290401
4. Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology. (1997) 25:108–11. doi: 10.1002/hep.510250120
5. Adler M, Schaffner F. Fatty liver hepatitis and cirrhosis in obese patients. Am J Med. (1979) 67:811–6. doi: 10.1016/0002-9343(79)90740-X
6. Berzigotti A, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Morillas R, et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology. (2011) 54:555–61. doi: 10.1002/hep.24418
7. Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. (1999) 30:1356–62. doi: 10.1002/hep.510300604
8. Ioannou GN, Weiss NS, Kowdley KV, Dominitz JA. Is obesity a risk factor for cirrhosis-related death or hospitalization? A population-based cohort study. Gastroenterology. (2003) 125:1053–9. doi: 10.1016/S0016-5085(03)01200-9
9. Karagozian R, Bhardwaj G, Wakefield DB, Baffy G. Obesity paradox in advanced liver disease: obesity is associated with lower mortality in hospitalized patients with cirrhosis. Liver Int. (2016) 36:1450–6. doi: 10.1111/liv.13137
10. Zheng K, Guo X, Yi F, Wang L, Mancuso A, Qi X. No association between ischemic stroke and portal vein thrombosis in liver cirrhosis. Biomed Res Int. (2020) 2020:8172673. doi: 10.1155/2020/8172673
11. Xu X, Liu B, Lin S, Li B, Wu Y, Li Y, et al. Terlipressin may decrease in-hospital mortality of cirrhotic patients with acute gastrointestinal bleeding and renal dysfunction: a retrospective multicenter observational study. Adv Ther. (2020) 37:4396–413. doi: 10.1007/s12325-020-01466-z
12. Xu X, Lin S, Yang Y, Chen Y, Liu B, Li B, et al. Development of hyponatremia after terlipressin in cirrhotic patients with acute gastrointestinal bleeding: a retrospective multicenter observational study. Expert Opin Drug Saf. (2020) 19:641–7. doi: 10.1080/14740338.2020.1734558
13. Bai Z, Li B, Lin S, Liu B, Li Y, Zhu Q, et al. Development and validation of CAGIB score for evaluating the prognosis of cirrhosis with acute gastrointestinal bleeding: a retrospective multicenter study. Adv Ther. (2019) 36:3211–20. doi: 10.1007/s12325-019-01083-5
14. Li Q, Wu Y, Zhu Q, Meng F, Lin S, Liu B, et al. External validation of Liaoning score for predicting esophageal varices in liver cirrhosis: a Chinese multicenter cross-sectional study. Ann Transl Med. (2019) 7:755. doi: 10.21037/atm.2019.11.78
15. Li Y, Han B, Li H, Song T, Bao W, Wang R, et al. Effect of admission time on the outcomes of liver cirrhosis with acute upper gastrointestinal bleeding: regular hours versus off-hours admission. Can J Gastroenterol Hepatol. (2018) 2018:3541365. doi: 10.1155/2018/3541365
16. Rosenbaum M, Leibel RL, Hirsch J. Obesity. N Engl J Med. (1997) 337:396–407. doi: 10.1056/NEJM199708073370606
17. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. (2004) 363:157–63. doi: 10.1016/S0140-6736(03)15268-3
18. Fleischmann E, Teal N, Dudley J, May W, Bower JD, Salahudeen AK. Influence of excess weight on mortality and hospital stay in 1346 hemodialysis patients. Kidney Int. (1999) 55:1560–7. doi: 10.1046/j.1523-1755.1999.00389.x
19. Costanzo P, Cleland JG, Pellicori P, Clark AL, Hepburn D, Kilpatrick ES, et al. The obesity paradox in type 2 diabetes mellitus: relationship of body mass index to prognosis: a cohort study. Ann Intern Med. (2015) 162:610–8. doi: 10.7326/M14-1551
20. Zamora E, Lupón J, de Antonio M, Urrutia A, Coll R, Díez C, et al. The obesity paradox in heart failure: is etiology a key factor? Int J Cardiol. (2013) 166:601–5. doi: 10.1016/j.ijcard.2011.11.022
21. Lechi A. The obesity paradox: is it really a paradox? Hypertension. Eat Weight Disord. (2017) 22:43–8. doi: 10.1007/s40519-016-0330-4
22. Czapla M, Juárez-Vela R, Łokieć K, Karniej P. The association between nutritional status and in-hospital mortality among patients with heart failure-a result of the retrospective nutritional status heart study 2 (NSHS2). Nutrients. (2021) 13:1669. doi: 10.3390/nu13051669
23. Braun N, Gomes F, Schütz P. “The obesity paradox” in disease–is the protective effect of obesity true? Swiss Med Wkly. (2015) 145:w14265. doi: 10.4414/smw.2015.14265
24. Liu Z, Barrett EJ. Human protein metabolism: its measurement and regulation. Am J Physiol Endocrinol Metab. (2002) 283:E1105–12. doi: 10.1152/ajpendo.00337.2002
25. Aquilani R, La Rovere MT, Febo O, Boschi F, Iadarola P, Corbellini D, et al. Preserved muscle protein metabolism in obese patients with chronic heart failure. Int J Cardiol. (2012) 160:102–8. doi: 10.1016/j.ijcard.2011.03.032
26. Anand AC. Nutrition and muscle in cirrhosis. J Clin Exp Hepatol. (2017) 7:340–57. doi: 10.1016/j.jceh.2017.11.001
27. Sinclair M, Gow PJ, Grossmann M, Angus PW. Review article: sarcopenia in cirrhosis–aetiology, implications and potential therapeutic interventions. Aliment Pharmacol Ther. (2016) 43:765–77. doi: 10.1111/apt.13549
28. Hanai T, Shiraki M, Nishimura K, Ohnishi S, Imai K, Suetsugu A, et al. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition. (2015) 31:193–9. doi: 10.1016/j.nut.2014.07.005
29. Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis. (2012) 16:95–131. doi: 10.1016/j.cld.2011.12.009
30. Ronti T, Lupattelli G, Mannarino E. The endocrine function of adipose tissue: an update. Clin Endocrinol. (2006) 64:355–65. doi: 10.1111/j.1365-2265.2006.02474.x
31. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. (2011) 11:85–97. doi: 10.1038/nri2921
32. Buechler C, Haberl EM, Rein-Fischboeck L, Aslanidis C. Adipokines in liver cirrhosis. Int J Mol Sci. (2017) 18:1392. doi: 10.3390/ijms18071392
33. Tsochatzis E, Papatheodoridis GV, Archimandritis AJ. The evolving role of leptin and adiponectin in chronic liver diseases. Am J Gastroenterol. (2006) 101:2629–40. doi: 10.1111/j.1572-0241.2006.00848.x
34. Hsu A, Aronoff DM, Phipps J, Goel D, Mancuso P. Leptin improves pulmonary bacterial clearance and survival in ob/ob mice during pneumococcal pneumonia. Clin Exp Immunol. (2007) 150:332–9. doi: 10.1111/j.1365-2249.2007.03491.x
35. Marra F, Aleffi S, Bertolani C, Petrai I, Vizzutti F. Adipokines and liver fibrosis. Eur Rev Med Pharmacol Sci. (2005) 9:279–84.
36. Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. (1999) 341:577–85. doi: 10.1056/NEJM199908193410806
37. John S, Thuluvath PJ. Hyponatremia in cirrhosis: pathophysiology and management. World J Gastroenterol. (2015) 21:3197–205. doi: 10.3748/wjg.v21.i11.3197
38. Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. (1997) 96:3423–9. doi: 10.1161/01.CIR.96.10.3423
39. Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. (2001) 38:789–95. doi: 10.1016/S0735-1097(01)01448-6
40. Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J. (2008) 156:13–22. doi: 10.1016/j.ahj.2008.02.014
41. Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff. (2009) 28:w822–31. doi: 10.1377/hlthaff.28.5.w822
42. Bosch J, Gracia-Sancho J, Abraldes JG. Cirrhosis as new indication for statins. Gut. (2020) 69:953–62. doi: 10.1136/gutjnl-2019-318237
43. Leavey SF, McCullough K, Hecking E, Goodkin D, Port FK, Young EW. Body mass index and mortality in 'healthier' as compared with 'sicker' haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. (2001) 16:2386–94. doi: 10.1093/ndt/16.12.2386
44. Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med. (1998) 338:1–7. doi: 10.1056/NEJM199801013380101
45. Machado MV, Cortez-Pinto H. Obesity paradox in cirrhosis: is it real or just an illusion? Liver Int. (2016) 36:1412–4. doi: 10.1111/liv.13154
46. Tobias DK, Hu FB. Does being overweight really reduce mortality? Obesity. (2013). 21:1746–9. doi: 10.1002/oby.20602
47. Banack HR, Kaufman JS. The obesity paradox: understanding the effect of obesity on mortality among individuals with cardiovascular disease. Prev Med. (2014) 62:96–102. doi: 10.1016/j.ypmed.2014.02.003
48. Bhaskaran K, Dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3·6 million adults in the UK. Lancet Diabetes Endocrinol. (2018) 6:944–53. doi: 10.1016/S2213-8587(18)30288-2
49. Nishikawa H, Osaki Y. Liver cirrhosis: evaluation, nutritional status, and prognosis. Mediators Inflamm. (2015) 2015:872152. doi: 10.1155/2015/872152
Keywords: body mass index, liver cirrhosis, obesity, prognosis, outcome
Citation: Yin Y, Li Y, Shao L, Yuan S, Liu B, Lin S, Yang Y, Tang S, Meng F, Wu Y, Chen Y, Li B, Zhu Q and Qi X (2021) Effect of Body Mass Index on the Prognosis of Liver Cirrhosis. Front. Nutr. 8:700132. doi: 10.3389/fnut.2021.700132
Received: 25 April 2021; Accepted: 23 July 2021;
Published: 20 August 2021.
Edited by:
Liana Gheorghe, Fundeni Clinical Institute, RomaniaReviewed by:
Shalimar, All India Institute of Medical Sciences, IndiaCopyright © 2021 Yin, Li, Shao, Yuan, Liu, Lin, Yang, Tang, Meng, Wu, Chen, Li, Zhu and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingshun Qi, eGluZ3NodW5xaUAxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.