- 1Faculty of Health and Medical Sciences, University of Surrey, Guildford, United Kingdom
- 2Pharmacognosy and Phytotherapy, University College London (UCL) School of Pharmacy, London, United Kingdom
- 3Graduate Institute of Integrated Medicine, College of Chinese Medicine, Chinese Medicine Research Center, China Medical University, Taichung, Taiwan
- 4Beyond Alcohol Ltd., London, United Kingdom
Myrcene (β-myrcene) is an abundant monoterpene which occurs as a major constituent in many plant species, including hops and cannabis. It is a popular flavouring and aroma agent (food additive) used in the manufacture of food and beverages. This review aims to report on the occurrence, biological and toxicological profile of β-myrcene. The main reported biological properties of β-myrcene—anxiolytic, antioxidant, anti-ageing, anti-inflammatory, analgesic properties—are discussed, with the mechanisms of activity. Here we also discuss recent data regarding the safety of β-myrcene. Overall, β-myrcene has shown promising health benefits in many animal studies. However, studies conducted in humans is lacking. In the future, there is potential for the formulation and production of non-alcoholic beers, functional foods and drinks, and cannabis extracts (low in THC) rich in β-myrcene.
Introduction
Myrcene (7-methyl-3-methylene-1,6-octadiene) is a popular food additive used as a flavouring agent in the manufacture of food and beverages (1). It is further used in consumer products, such as cosmetics, soaps, and detergents. In addition to its use in a variety of consumable products, β-Myrcene is used as a starting material for commercially important scents and flavours such as menthol, nerol, geraniol, and linalool (2). β-Myrcene has a high production volume of 58,076 kg for Europe and 1,188 kg for the USA (3).
β-Myrcene is a pleasant-smelling, olefinic, acyclic unsubstituted monoterpene which occurs naturally in a large number of plant species (4–6), especially in the essential oils of plants such as hops, cannabis, lemongrass, verbena and bay (4, 7), as well as in citrus fruits and citrus juices (8).
In brewing, β-myrcene is one of the most potent aromatic flavour components of hop essential oils and in all analysed hop varieties is considered the most odour-active volatile (9, 10). Myrcene largely determines the “green hop aroma” in beer and is a primary substance in dry hopped beers (11), with a “herbaceous, resinous, green, balsamic, fresh hop” like odour (12, 13). It is also the major constituent of hop essential oil and can constitute as much as 70% of the essential oil by volume (14).
In addition to the flavour of hops, β-myrcene contributes significantly to cannabis aromas, and may function analogously to the endocannabinoid system. β-Myrcene characteristically gives cannabis strains a mildly sweet flavour profile and provides scent notes that are spicy, earthy and musky (15). Cannabis strains which contain high concentrations of myrcene (>0.5% myrcene), are likely to induce sedative qualities (“couch-lock effect”), which are classically attributed to Cannabis indica Lam (a synonym of C. sativa L.) strains (16). On the other hand, strains low in β-myrcene (<0.5%) are likely to induce a more energic “high” (17). β-Myrcene may also have a role in assisting cannabinoids to be absorbed across the blood-brain barrier, increasing transport into the brain and enhancing psychoactive responses; however, there is limited robust data supporting this claim (18).
β-Myrcene reported biological activities include analgesic (19), sedative (20), antidiabetic (21), antioxidant (22), anti-inflammatory (23), antibacterial (24), and anticancer effects (25).
Despite the therapeutic benefits observed, β-myrcene has come under scientific scrutiny due to an alleged risk as a potential human carcinogen. The uncertainty of the safety of myrcene, stems from studies conducted by the National Toxicology Program, USA (NTP) which has showed an increased incidence of kidney and liver neoplasms in rodents (26). In 2018, the FDA took regulatory action to no longer permit the use of β-myrcene, as a food additive based on legal action taken against the FDA under the Delaney Clause (A federal health statute which prohibits FDA approval of any food additive which caused cancer in humans or animals). Importantly, the FDA confirmed that there was no safety concern for β-myrcene to public health under the conditions of its intended use. Several other regulatory and scientific expert bodies have since argued that β-myrcene is safe under conditions of intended use as a flavouring substance (27) and it must be noted that countless permitted food products continue to naturally contain significant levels of β- myrcene.
The wide application of β-myrcene in industry and for domestic use coupled with safety concerns, has raised the interest to critically review its biochemical and pharmacological properties. Thus, the aim of this review is to explore and assess some of the important biological activities of β-myrcene and to assess its suitability for commercial use in food industry and phytotherapy.
Approach and Methods
This review paper collected the literature published on the phytochemistry, pharmacodynamics, pharmacology, pharmacokinetics, health benefits and occurrence of myrcene in different plant species and toxicity. Relevant information on myrcene was gathered from worldwide accepted scientific search engines and databases, including Web of Science, PubMed, Elsevier, Wiley Online Library, ResearchGate and Google Scholar. The key words used for the searches were “myrcene,” combined with “monoterpene,” “phytochemistry,” “pharmacokinetics,” “pharmacology”; specialist pharmacological terms included,” “anxiolytic,” “sedative” “antioxidant” “skin” “anti-inflammatory” “pain,” “analgesic,” and “toxicology.” Most of the cited information in this article were from peer-reviewed journals directly published in English. No time period limitation was considered. In addition, reference lists of identified publications were hand searched to identify other studies potentially eligible for inclusion. Species names were cross-checked in MPNS (https://mpns.science.kew.org/).
Physical and Chemical Properties of β-Myrcene
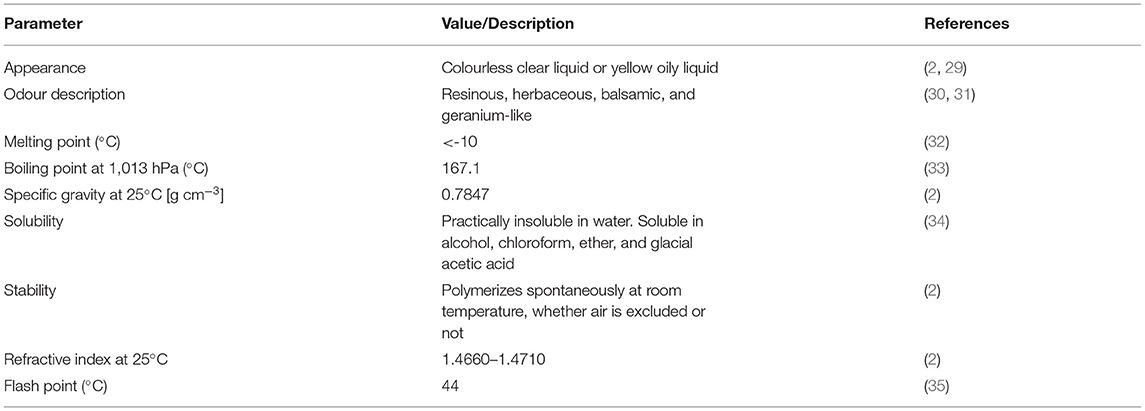
Monoterpenes are a class of terpenes; consisting of two isoprene units (5 carbon bases each) (28). Myrcene (C10H16, molecular weight 136.23 g/mol) (29) is classified as an acyclic monoterpene, with properties listed in Table 1.
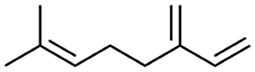
Myrcene exists in two isomeric forms, namely β-myrcene (7-methyl-3-methylene-1,6-octadiene) and α-myrcene (2-methyl-6-methylene-1,7-octadiene). The most common is the naturally occurring isomer β-myrcene, which contains an isopropylidene group and is often denoted as “myrcene” in the literature. The other is α-myrcene, which exists in the isopropenyl form (2). In β-myrcene there are three carbon-carbon double bonds (two of them being conjugated) and a gem-dimethyl terminal (Figure 1) (36).
Biosynthesis Production of β-Myrcene
Monoterpenes are produced in plants by the stereospecific condensation of two isomeric five-carbon units: isopentenyl diphosphate (IDP) and dimethylallyl diphosphate (DMADP) (37). Geranyl diphosphate (GPP) is thus formed via condensation of IPP with DMADP, by GPP synthase (GPPS) (38, 39). Geranyl diphosphate (GPP) then undergoes hydrolysis catalysed by a prenolpyrophosphatase to form geraniol. Consequently, β-myrcene is produced by the dehydration and isomerization of geraniol (37).
Industrial Synthesis of Myrcene
β-Pinene is an important starting material for the synthesis of β- myrcene. Up until the 1950s, β-pinene was extracted from pine or spruce resins by tapping trees Nowadays, β-pinene can be isolated from the waste streams of paper mills, by distillation and desulphurisation from crude sulphate turpentine (CST). The proportion of β-pinene in CST, depends largely on the age, season, geographical location, and variety of the tree (40).
The most common method to produce myrcene industrially is through the pyrolysis of β-pinene (2). In the future, engineered microbial platforms may provide alternative means for sustainable and environmentally friendly large-scale production of myrcene (41–43).
Analysis, Extraction, and Quantification of β-Myrcene
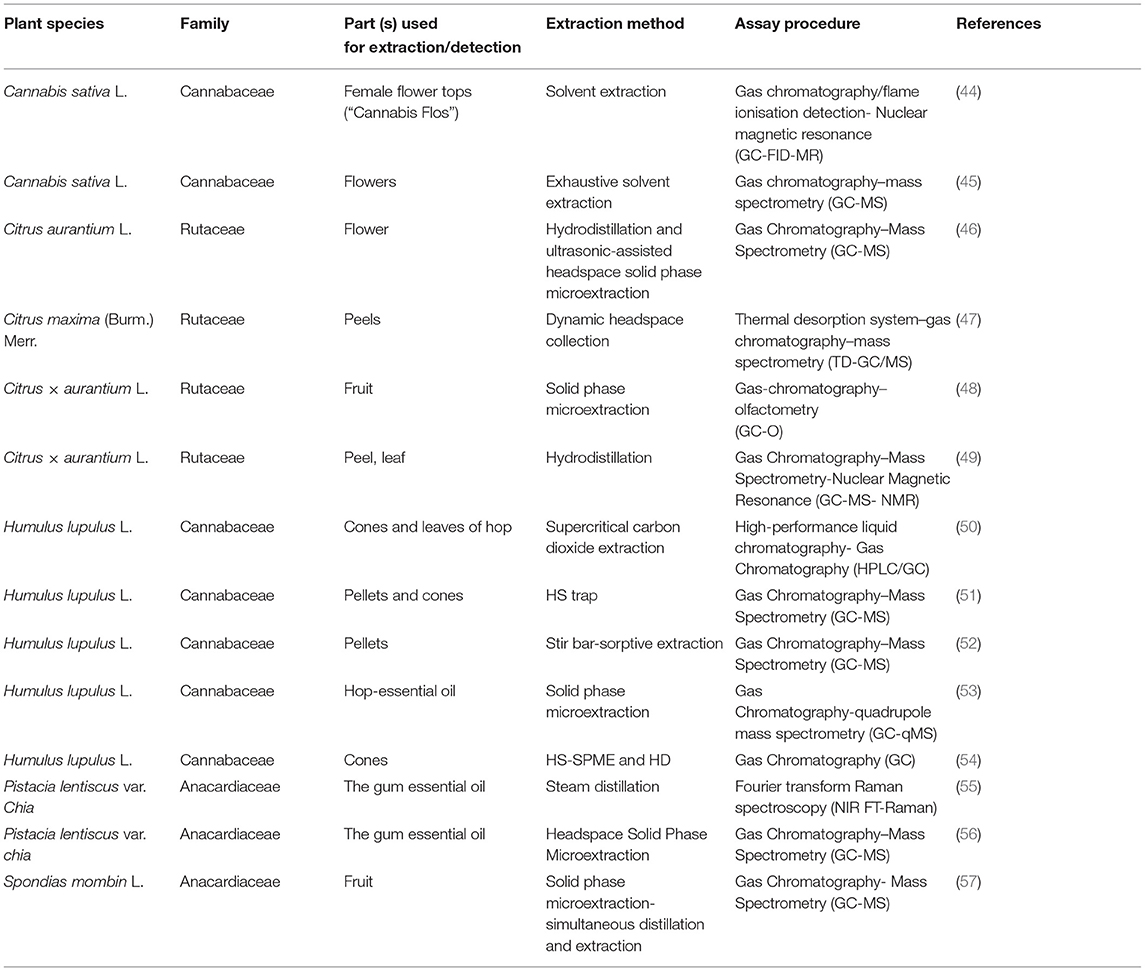
β-Myrcene is considered an important intermediate compound which can be derivatized to produce numerous end products, such as, citronellol, citronellal, geraniol, nerol and linalool (2). Several methods of determining β-myrcene content have been published and developed to achieve more simple, rapid and efficient quantitative analytical methods (Table 2) (55, 58). Naturally, β-myrcene exists as complex mixtures with other monoterpenes. Thus, it would be difficult to isolate β-myrcene in large quantities from these complex mixtures. Therefore, there is a need for an efficient and economical method for separating β-myrcene from other terpenes. Hydro-distillation and solvent extraction were used extensively followed by Gas Chromatography (GC) coupled with Mass Spectrometry (MS) or Flame ionisation detector (FID) for quantification (58, 59). The drawbacks of these techniques were mainly the tendency of losing essential volatile compounds (including β-myrcene) during the process of solvent evaporation leading to decrease the yield and altering its odour characteristics (59, 60). Also, these methods require a lengthy process from 3 to 4 h of distillation allowing possibility of changing the chemical composition of analyte or risk of indefinable loss (51, 61).
In contrast, extraction efficiency and flexibility of supercritical fluid CO2 extraction are much better than the earlier methods, since there is a greater control in temperature and pressure parameters (50). The yield of extracts obtained by supercritical CO2 extraction is highly dependent on the temperature and pressure applied during the extraction process. Higher recovery percentage of β-myrcene was obtained at lower temperatures and pressures of supercritical fluid (62). Also, at 40°C and 20.0 MPa, β- myrcene, L-caryophyllene and α-humulene were detected at higher concentrations allowing for their importance in beverage industry as flavouring and aroma additives (63). The demand for using environmentally clean extraction techniques and the need for faster, more powerful, and cheaper analytical procedures is therefore driving the industrial production into methods like supercritical fluid CO2 extraction.
All these techniques had noticeable disadvantages such as excessive use of solvents, longer operation time and/ or large volumes of sample (60, 62). Also, they require the use of highly sophisticated, uneconomical devices with a limited lifetime (53). Furthermore, these techniques are quite complex in operation and are labour intensive, thus, they are not fully efficient when a routine analysis of a large number of samples is needed.
Solid phase microextraction (SPME) has emerged as an alternative to traditional techniques (53). It has been successfully employed to determine the volatile composition of hop and also in different food products (54, 64). This technique has its own characteristics, such as efficient extraction procedures, short analysis of time, economical with low production cost, and high selectivity and sensitivity when coupled with suitable detection modes. Moreover, by using SPME, all the steps of extraction technique can be introduced within a single process without any interruption, resulting in a high sample processing (53).
SPME can also be used with (GC) and (MS) analysis, to provide a full metabolomic profile of the intended plant (53, 65, 66). Hops of the Saaz variety have been studied by Gonçalves et al. using SPME, allowing for profiling the terpenoid metabolomic pattern found in the essential oil. β-Myrcene was dominated by 53.0% of the total volatile fraction at 40°C. The disadvantages of SPME are that the extraction conditions, age of fibre, and matrix could affect the amount of sample absorbed on the fibre. Controlling these factors can produce a highly efficient and reproducible method for extraction and analysis of β-myrcene. Fourier transform Raman Spectroscopy has also been used to determine the percentage of β-myrcene in mastic gum oil based on band intensity measurements (55). The method is extremely rapid, simple and non-destructive for the sample.
There are still some limitations in obtaining a pure extract of β-myrcene allowing for accurate analysis and quantification. Thus, proper selection of extraction techniques and pre- determined sample parameters are necessary for efficient analysis of β-myrcene.
Pharmacokinetics of β-Myrcene
Most previously published data on the absorption, distribution, metabolism and excretion of β-myrcene have been conducted in experimental animals, such as, rabbits and rats (26). In a pharmacokinetic study, blood levels as high as 14.1 ± 3.0 μg/mL β-myrcene (peak value) were detected 60 min after oral administration of 1.0 g/kg bw β-myrcene to female rats (67). β-Myrcene showed a pattern of elimination mostly by urine with an elimination half-life of 285 min, however no studies examined the possibility of biliary excretion (3, 67). β-Myrcene was mainly distributed in adipose tissue and in several major organs, including the liver, brain, kidneys, and gonads. β-Myrcene is bioavailable in human plasma within 30 min after consumption of a single dose. The high degree of bioavailability in plasma is a crucial step towards its useful usage in the food and beverage industry. Furthermore, β-myrcene reaches the plasma unaltered, with a peak concentration between 2 and 4 h (68). This could partially explain its health benefits and applications on human health. More research is needed to characterise the kinetics of β-myrcene in human metabolism.
Urinary excretion of conjugates of two diols (10-hydroxylinalool and 7-methyl-3-methylene-oct-6-ene-1,2-diol), and two hydroxy acids (10-carboxylinalool and 2-hydroxy-7-methyl-3-methylene-oct-6-enoic acid) was observed in male rabbits administered β-myrcene by gavage (69, 70). Diols were formed due to an oxidation reaction occurred at the 3,10-double bond through a 3,10-epoxide intermediate. The metabolites were isolated by using rat-liver microsomal cytochrome P450 enzymes and confirmed by undergoing enzymatic degradation by β-Glucuronidase/Arylsulfatase.
In rats, several metabolites were isolated from the urine after oral administration of β-myrcene, such as, 10-hydroxylinalool, 1-hydroxymethyl-4-isopropenyl cyclohexanol, 7-methyl-3-methylene-oct-6-ene-1,2-diol, 10-carboxylinalool, 2-hydroxy-7-methyl-3-methylene-oct-6-enoic acid (5). Their formation involved a sequence of oxidation reactions of the terminal double bonds by microsomal cytochrome P450 2B and epoxidation of the 1,2- and 3,10-epoxide intermediates, with subsequent hydrolysis to diols. This CYP-catalysed reaction was inhibited by several non-specific inhibitors of cytochrome P450 preventing from conversion of β-myrcene into 10-hydroxylinalool.
Synergistic Effects of β-Myrcene With Other Active Natural Products
Studying the synergistic effect of monoterpenes, is important for determining the qualities present in different plant varieties. One study suggested that monoterpenes, including, β-myrcene found within a plant with other terpenes, may generate synergistic interactions (71). For example, β-myrcene can contribute to the overall flavour of beer due to its synergistic effect with other hop essential oils, such as, linalool. Other volatile compounds such as limonene, 3-carene and caryophyllene have been found to have an important role in the aroma and flavour of beverages and food products (72). There is also possibility of synergy between these compounds and β-myrcene, particularly if they share similar structure and notes (73, 74).
β-Myrcene contained within the cannabis plant may potentiate the innate anti-nociceptive properties of cannabinoids by lowering resistance across the blood brain barrier (BBB) and improving permeability, leading to an increase in transportation of cannabinoids into the brain (18). Additionally, the effect of β-myrcene as peripheral and central analgesics could be mediated to boost endocannabinoid derived central actions, when other terpenes are synergistically interacted with it (75). The terpenes were suggested to regulate the affinity of THC for CB1 receptor, which contributes to the improved analgesic effects of the cannabis plant (76, 77). Thus, one may be able to see a higher level of effects, rather than using an isolated component itself.
β-Myrcene found within the cannabis plant possesses anti-inflammatory, analgesic and sedative activities, which is additional to the effects of classical phytocannabinoids and may generate synergistic interactions (77, 78). β-Myrcene may act in synergy with tetrahydrocannabinol and other cannabinoids, such as CBD to enhance cannabis activity and eventually increase its psychoactive potential (77). However, a recent study suggested that cannabis-derived terpenoids functional effects were not detected, either alone or when combined with Δ9-tetrahydrocannabinol and cannabidiol (79). Thus, their ability to produce entourage activity by direct effects at cannabinoid receptors cannot be fully determined. The study concluded that none of the tested terpenes present in the cannabis plant (β-myrcene, pinene, caryophyllene and limonene) has a direct interaction with CB1 or CB2 receptors. Also, there were unaltered modifications to the activity of Δ9-tetrahydrocannabinol and cannabidiol. This study is in agreement with a previous study conducted by Santiago and his colleague, in which they rule out the direct activation of CB1 or CB2 or modulating the signalling of the phytocannabinoid agonist Δ9-tetrahydrocannabinol by β-myrcene in cannabis plant (80). However, both studies cannot rule out the existence of an entourage effect of β-myrcene as they only examined cannabinoids signalling through one pathway. There are possibility of entourage effects emerging through the impact of terpenoids on other pathways of endocannabinoid system or through non-cannabinoid receptor mechanisms that are important for the behavioural effect of Cannabis strains (81, 82).
Purity of Commercially Available Myrcene
β-Myrcene is available commercially in an untreated technical grade (purity, 75%). High-purity β-myrcene (purity, >90%) is extracted using rectification (2). Impurities in β-myrcene are mainly other monoterpenes including: β-pinene, limonene, dl-limonene and psi-limonene. Dipeptine from a cyclization reaction and isomers and dimers of β-myrcene have also been observed (2).
Polymerization inhibitors are chemical compounds added to high purity β-myrcene to prevent their auto-polymerisation and to prolong its shelf-life (26). If β-myrcene is stored at 3°C, there is no loss by polymerisation for up to 12 months without an inhibitor. Most commercial food products containing myrcene, have polymerisation inhibitors such as α-tocopherol (2).
Natural Occurrences of β-Myrcene
β-Myrcene is a component of the hydrocarbon fraction of many essential oils (83). It occurs naturally in over 200 plants and is present in the emissions of many trees in different parts of the world (26). Exposure to β-myrcene from natural food sources, is estimated to be 16,500 times more than from its synthetic use as a flavour substance (8).
The concentrations of β-myrcene in essential oils varies considerably between different plant species and varieties as well as plant parts (botanical drugs) (84). It can be found in significant quantities in the essential oils of hops and cannabis. Supplementary Tables 1–3 supply an overview of the relative concentrations of β-myrcene in essential oils and food products. The highest content of β-myrcene was found in Hops (Maximum: 10 g/kg dry weight) (3). The final concentration of β-myrcene in beer (0.4–80 μg/L) is much lower than in hops (52, 58). This is possibly due to dilution, variable extraction methods and it being destroyed by heating processes (58).
Quantification of β-myrcene from C. sativa extract has been studied in different varieties of C. sativa (85, 86). A more comprehensive description can be found in a recent study by Ibrahim et al. (45), which examined three varieties of C. sativa (45). One variety has high THC content (HP), the other one with high CBD content (HD), and the last was an intermediate variety containing both THC and CBD at a significant level. β-Myrcene content was higher in the intermediate variety than the other two varieties (0.87–1.32 mg/g). In the HD variety, β-myrcene content was 0.54–0.68 mg/mL compared to 0.19– 0.72 mg/g in HP varieties. Thus, the observations from this study may help in differentiation and the selection of specific variety of C. sativa based on their β-myrcene content.
Variations also exist between different geographical areas, season of harvesting, part of the plant and agronomical factors in different essential oils (22, 87). GC/MS analysis has shown that differences in β-myrcene concentrations exist between the different life cycles of a plant (vegetative and flowering) (84). Additionally, distillation periods and extraction methods can influence the yield of β-myrcene (88, 89).
Important Pharmacological and Biological Effects of β-Myrcene
In the following we discuss some of the most salient pharmacological and biological effects (Supplementary Table 4).
Central Nervous System Effects and Neurobehavioral Activity
Myrcene is well-known for its anxiolytic and sedative effects, which are both desired therapeutic actions, but may also pose some risks. Sedating agents can create drowsiness and impair motor coordination, and this can be assessed using the “rota-rod test” in animal studies measuring the length of time a rodent can balance on a rotating horizontal rod. A relatively high dosage of 200 mg/kg (1,468 μmol/kg) body wt myrcene, led to a 48% decrease in the time of permanence on the bar in the rota-rod test. The same dose of myrcene prolonged barbiturate sleep time 2.6 times. This was more intense in the presence of citral (20). Similarly, a single oral dose of β-myrcene, prolonged potentiated pentobarbital sleeping time, when administered 60 min before the barbiturate, possibly by inhibiting the barbiturates metabolism via cytochrome P450 (CYP) (78).
The main essential oil obtained from Cannabis sativa L. (Cannabaceae; hemp) (myrcene content: 22.9%), demonstrated measurable effects on the autonomic nervous system in healthy human subjects (n = 5). Inhalation of cannabis essential oil for 5 min improved nerve activity and was shown to relive stress and anxiety (Sweet almond oil was used as a control). The subjects generally felt more relaxed, energetic, calm, and an elevated mood, five min post inhalation. The study also used an electroencephalogram (EEG) to measure brain activity and results showed that there was an increase in theta (4–8 Hz) and alpha (8–13 Hz) brain wave activity in the posterior brain region, which is comparable to the EEG waves of individuals undergoing meditation (90).
Myrcene has also been shown to function as an anticonvulsant agent. Myrcene, obtained from Lippia alba (Mill.) N.E.Br. ex Britton and P.Wilson increased the latency of pentylenetetrazol-induced (PTZ) convulsions and increased the percentage of survival in female Swiss mice (91). Similarly, essential oils from Cinnamosma madagascariensis Danguy (8.9% myrcene) was evaluated in vivo for its anticonvulsant effects in Wistar rats that underwent induced seizures using pentylenetetrazole (PTZ). The study demonstrated the antiepileptic potential, by attenuating convulsions with moderate sedative effects. The possible mechanism of action was linked to glutamatergic and GABAergic neurotransmission (92). On the other hand, Da-Silva et al. (93) were unable to demonstrate the protective role of myrcene against PTZ-induced seizures. They were also unable to demonstrate benzodiazepine-like anxiolytic activity and the anti-depressive and antipsychotic effects of myrcene (93).
β-Myrcene may have significant clinical potential in adjuvant therapies, both as a pure compound and as a part of extract preparations. Popular anxiolytic essential oils are rich in other terpenoid alcohols, such as linalool, geraniol and citronellol, which might work synergistically with β-myrcene. Due to limited studies in human participants, small sample sizes, short duration of β-myrcene application, limited diverse administration of β-myrcene, the potential beneficial effect of β-myrcene on neurological disorders need further and more rigorous assessment.
Antioxidant Activity
Antioxidant agents are accountable for the prevention of ageing and degenerative diseases such as atherosclerosis, cardiovascular diseases, cancer, diabetes and neurological illnesses (94). They also have an important role in inhibiting lipid oxidation within food products. In recent decades, there has been growing interest in the use of naturally occurring antioxidants in food preservation (22).
Selected monoterpenes have been studied for their potential antioxidant capacities, which can be attributed by the presence of conjugated double bonds that create chain breaking antioxidant activity. Chemical antioxidant assays like the DPPH assay (22, 95), are excluded here since they are of no pharmacological relevance. There is no evidence for therapeutic benefits on the basis of such chemical assays (96).
In vivo myrcene (97–100) demonstrate some relevant antioxidant effects. Ciftci et al. treated female Sprague-Dawley rats exposed to the environmental contaminant 2,3,7,8-tetracholorodibenzo-p-dioxin (TCDD) with myrcene [with a high dose of up to 200 mg/kg bw per day (1,468 μmol/kg) for 30 or 60 days]. These rats had a decrease in hepatic lipid peroxidation via activation of antioxidant and radical scavenger properties (97). Myrcene, again at a high dose of 200 mg/kg (1,468 μmol/kg) played a neuroprotective role in cerebral ischemia/reperfusion injury in C57Bl/J6 mice. In addition, myrcene increased glutathione along with other antioxidant enzymes such as glutathione peroxidase (GPx) and superoxide dismutase, thereby preventing oxidative damage and protecting brain tissue (99).The effects of orally administered β-myrcene (7.5 mg/kg bw; 55 μmol/kg bw) against ethanol-induced gastric ulcers in male Wistar rats is mediated through antioxidant effects via increased levels of GPx, glutathione reductase (GR) and total glutathione in gastric tissue (98). Importantly, future studies investigating antioxidant activity of β-myrcene, need to use appropriate dosage levels that have therapeutic effects in humans. This requires a comprehensive investigation into the recommended dosage of β-myrcene in humans. Additionally, most studies in the literature utilise chemical assays to detect antioxidant activity of β-myrcene. Future studies should use pharmacologically relevant in vivo or cell-based models to measure antioxidant activity.
Anti-ageing Activity
As myrcene is an effective antioxidant compound, it may play a protective role against UVB-induced human skin photo-ageing. UVB exposure is associated with an overproduction of reactive oxygen species (ROS), which is a primary factor in oxidative skin damage (101). Abnormal production of ROS, activates numerous cell surface cytokines, growth factor receptors and mitogen activated protein kinases (MAPKs) (102). Exposure to UV radiation has also been shown to activate matrix metalloproteinases (MMPs), leading to the atrophy of collagen and elastin fibres (103). To date, only one study has investigated the role of β-myrcene and anti-ageing. Myrcene ameliorated skin ageing via decreased production of ROS, MMP-1, MMP-3, interleukin-6 (IL-6) and increased transforming growth factor type 1 (TGF-1) and type I procollagen secretions in UVB-irradiated human dermal fibroblasts. β-Myrcene treatment also downregulated phosphorylation of MAPK-related signalling molecules. Thus, myrcene may have a vital role against age-associated skin oxidative damage in skin care products (104).
Anti-inflammatory Activity
In vitro β-myrcene is a powerful anti-inflammatory agent. Its ability to lessen inflammation occurs via prostaglandin E-2 (PGE-2) (105). In a study by Souza et al. (106) myrcene was shown to be effective in inhibiting the inflammatory response induced by lipopolysaccharide, including cell migration (leucocytes, neutrophils, mononuclear macrophages and eosinophils) and production of nitric oxide in mouse models of pleurisy. A significant inhibition of γ-interferon and interleukin (IL)-4 was also observed (106). Male Wistar rats with isoproterenol induced heart failure showed signs of cardiac function abnormalities. On the other hand, rats who were pre-treated with myrcene were protected from cardiac failure (p < 0.001) and inflammatory signals were abrogated. β-Myrcene was involved in suppressing fibrotic markers such as matrix metalloproteinases (MMP-2 and MMP-9) and regulating the expression of inducible nitric oxide synthase (iNOS), Transforming growth factor beta (TGF-β) and miRNA (profibrotic agents) with potential future benefits in treating cardiac failure (107).
In an in vitro cartilage degradation model of osteoarthritis, myrcene (25–50 μg/mL; 183.5–367 μmol/kg) showed anti-inflammatory and anticatabolic effects on human chondrocytes. Cartilage degradation and osteoarthritis progression was slowed down. Myrcene decreased interleukin IL-1β-induced nuclear factor-κB (NF-κB) and jun terminal kinase (JNK). It further decreased ERK1/2, p38 activation and the expression of inflammatory iNOS. Myrcene decreased catabolic responses (matrix metalloprotease MMP1 and MMP13), whilst increasing the expression of anticatabolic genes (tissue inhibitor of metalloproteases TIMP1 and TIMP3). Additionally, myrcene decreased the expression of non-cartilage specific collagen I induced by IL-1β, thus promoting the maintenance of the differentiated chondrocyte phenotype (23).
The anti-inflammatory activity of β-myrcene may not only be credited to its antioxidant potential, but also with its interaction with signal pathway cascades involving cytokines and transcription factors. Thus, plant oils rich in β-myrcene could serve as an option help to alleviate anti-inflammatory diseases and their symptoms.
Antinociceptive Activity
β-Myrcene has shown central and peripheral analgesic effects (108). Intraperitoneal administration of β-myrcene (10 mg/kg; 73 μmol/kg) provided antinociception in mice who underwent tests of acute pain (19). This effect was antagonised centrally by previous administration of naloxone (opioid antagonist) and yohimbine (α2 adrenergic antagonist), implying the role of the opioid and noradrenergic systems. The results imply that the antinociceptive effect is mediated by the release of endogenous opioids through the a2-adrenoreceptors (19). In addition, the peripheral sites were antagonised by inhibitors of nitric oxide synthesis (109).
Lemongrass essential oil (15–20% β-myrcene) presented strong analgesic effects similar to peripheral-acting opioids, when assessed under different experimental models of pain in rats. Unlike morphine, no tolerance was observed after 5 days of repeated dosing in rats (105).
β-Myrcene may play a significant role in treating pain through interaction with transient receptor potential cation channel subfamily V member 1 (TRPV1) channels (110) involved in peripheral nociception (detection of noxious heat and pain) (111). On the other hand, a more recent study was unable to confirm the result of terpenoids being able to activate TRPV1 channels, suggesting that additional molecular targets must be explored (112).
The Safety of Myrcene
In the following we discuss the safety of β-myrcene (Supplementary Table 5).
Adverse Skin Reactions
In a European multicentre study, of 1,511 consecutive dermatitis patients, only one patient reacted adversely to 3% β-oxidised myrcene (containing 30% of β -myrcene) (113), this indicates that myrcene is hypoallergenic on the skin and is safe for topical use. Undiluted β-myrcene was moderately irritating to rabbit skin (114); but was neither irritating nor sensitising after being tested at 4% (n = 25) (115). β-Myrcene (5%) was sensitising to two of eleven patients sensitive to tea tree oil (116).
Acute Toxicity
In mice and rats, the acute oral toxicity of β-myrcene was low, with an approximate lethal dose (ALD) of >5.06 g/kg bw (37,143 μmol/kg bw) and 11.39 g/kg bw (83,609 μmol/kg bw), respectively. Administration of β-myrcene via intraperitoneal injection had a lower ALD in mice and rats (2.25 g/kg bw and 5.06 g/kg bw, respectively; 16,516 μmol/kg bw and 37,143 μmol/kg bw, respectively), which is likely due to drug- induced chemical peritonitis (117). The acute oral LD50 in rats and the acute dermal LD50 in rabbits were reported to exceed 5 g/kg body weight (36,703 μmol/kg bw), following oral administration and dermal application (114).
Subacute and Sub-chronic Toxicity
In a 14 week gavage study (Good Laboratory Practise compliant), male and female F344/N rats and B6C3F1 mice (10/group) were given doses of β-myrcene at 0.25, 0.5, 1, 2, or 4 g/kg bw (1,835, 3,670, 7,341, 14,681, 29,362 μmol/kg bw) for 5 days per week (human equivalent daily dose range: 17.5–280 g) (26). All 4 g/kg (29,362 μmol/kg) mice and rats died within 2 weeks, with other deaths observed in groups administered >0.5 g/kg (>3,670 μmol/kg). At the end of the 14 weeks, renal tubule necrosis significantly increased in rats of each dosage groups (not tested in mice). In rats with a dosage >1 g/kg (>7,341 μmol/kg), increased chronic inflammation, inflammation of the forestomach, mesenteric lymph node atrophy and olfactory epithelium degeneration was observed (26).
In a 90 day toxicity study utilising groups of male and female Sprague Dawley rats (10/sex and group), at the request of the EFSA, β-myrcene was administered in a diet containing 0, 700, 2,100, or 4,200 ppm of β-myrcene daily designed to provide targeted doses of 50, 150, or 300 mg/kg bw/day (367, 1,101, 2,202 μmol/kg bw/day) (118). No effects on mortalities, clinical signs of toxicity, haematology and clinical chemistry parameters and organ weights in the presence of β-myrcene within the diet was reported. Furthermore, the histopathological findings observed were not related to ingestion of β-myrcene and were either incidental or spontaneous. The oral NOEL for both sexes of rats, was the highest dose tests: 115 and 136 mg/kg bw/day (844 and 998 μmol/kg bw/day) for males and females (118). It should be noted that this calculated NOEL, is several orders of magnitude greater than human exposures from β-myrcene (119).
Reproductive Toxicology
In a 3 month Gavage Study of β-myrcene, no effects on the weight of reproductive organs, sperm count or oestrous cyclicity was observed in doses of up to 2 g/kg (14,681 μmol/kg) in rats and up to 1 g/kg (7,341 μmol/kg) in mice (26). Administration of high doses of β-myrcene (1,200 mg/kg bw/day; 8,809 μmol/kg bw/day) on days 6–15 of pregnancy, was found to induce embryofoetal toxicity in pregnant Wistar rats. High doses decreased maternal body weight gain, increased the incidence of foetal skeletal malformations, lowered the number of visible implantation sites and the number of live foetuses. Additionally, foetal weights were lower, in comparison to the control group. The no-observable-adverse-effect level (NOAEL) of oral administration of β-myrcene for maternal and offspring toxicity was 500 mg/kg bw/day (3,670 μmol/kg bw/day) (67).
Two addition studies of β-myrcene on reproductive and development toxicity, have also indicated the adverse effects of β-myrcene on birth weight, peri and post-natal mortality, as well as foetal developmental abnormalities in Wistar rats (120, 121). The NOELS for fertility and general reproductive performance has been estimated as 250 mg/kg bw (1,835 μmol/kg bw) (120) and 300 mg/kg bw (2,202 μmol/kg bw) (121). No data on the reproductive or developmental toxicity of β-myrcene in humans is currently available.
Mutagenicity and Genotoxicity
Myrcene inhibited cyclophosphamide induced sister-chromatid exchanges in Chinese hamster V79 cells and cultured hepatic tumour cells (122). Myrcene had no genotoxic potential in mammalian cells in vitro and did not induce chromosome aberrations or sister-chromatid exchange. β-Myrcene reduced CP-induced sister-chromatid exchanges in human lymphocytes in a dose dependent manner. Also, it did not influence the genotoxicity of methane sulfonate and benzo[a]pyrene (123). Additionally, β-myrcene (doses ranging from 100 to 1,000 μg/mL; 734 to 7,341 μmol/kg) reduced the cytotoxic and mutagenic effects of CP in V79 Chinese hamster cells, when tested with rat liver S9. The authors suggested that myrcene had the ability to inhibit cytochrome P-450 isoenzymes which activates compounds with mutagenic and carcinogenic properties (123).
No evidence of chromosomal aberrations in bone marrow cells of rats administered β-myrcene (0.1, 0.5, or 1.0 g/kg bw; 734, 3,670, 7,341 μmol/kg bw) was observed. Although there was no evidence of myrcene-induced clastogenicity, there was a dose-dependent increase in the mitotic index of bone marrow cells at 24 h (124). Additionally, there was no increase in the frequency of micronucleated normochromatic erythrocytes, a biomarker of both acute and cumulative chromosomal damage, in B6C3F1 mice treated with β-myrcene (0.25 to 2 g/kg; 1,835 to 14,681 μmol/kg) by gavage for 3 months (26).
β-Myrcene expressed antimutagenic activities against aflatoxin B1 (AFB1) in Salmonella typhimurium (TA100). Doses of 1.5 and 3.0% of β-Myrcene, showed inhibitory actions of 65 and 73%, respectively when tested with 1.0 μg/plate AFB1 in the presence of exogenous metabolic activation (rat liver S9) using TA100 and the pre-incubation method (125). The NTP (2010) and (126), concluded that β-myrcene was not mutagenetic based on the negative Ames test using Salmonella strains (TA97, TA98, TA100, and TA1535) with and without metabolic activation. It was also negative in the Escherichia coli test system (strain WP2 uvrApKM101) with and without metabolic activation (S9 fraction from Aroclor 1254-induced rat or hamster liver), and in an in vivo micronucleus assay in B6C3F1 mice (26).
β-Myrcene inhibited the activity of pentoxyresorufin-O-depenthylase (PROD), a selective marker for mono-oxygenase CYP2B1, necessary for the activation of genotoxins in rats (127). β-Myrcene also demonstrated protective effects against t-butyl hydroperoxide induced genotoxicity in human B lymphoid NC-NC cells, which was predominantly mediated by their radical scavenging activity (128).
Carcinogenicity/Anti-carcinogenic Activity
Multiple studies have demonstrated that β-myrcene exposure had anticarcinogenic potential in in vitro models. Administration of β-myrcene suppressed the in vitro formation of N-Nitrosodimethylamine (NDMA), a potent carcinogen, by 88% (129). In MCF-7 cells, β-myrcene (IC50, 291 μM) inhibited the breast cancer cells growth in vitro, but was slightly toxic to normal Chang liver cells (IC50, 9.5 mM; 9,500 μmol/kg) (130). Additional studies have also implicated the cytotoxic effect of β-myrcene against a broad range of cancer cells, such as MCF-7 breast carcinoma, HT-29 colon adenocarcinoma (131), P388 leukaemia cells (132) and other tumour cell lines (25, 133, 134).
On the other hand, in a model of 7,12-dimethylbenz[a]anthracene (DMBA)-induced mammary carcinogenesis, β-myrcene did not exhibit significant chemopreventive activities. β-myrcene did not reduce the total number of mammary tumours or the median tumour latency period in a group of female Sprague-Dawley rats (age, 6 weeks) fed diets containing β-myrcene (purity, 94.3%) (135).
In the 2010 NTP study, a gavage study in groups of male and female F344/N rats and B6C3F1 mice was conducted over 2 years. Rats and mice were given doses of β-myrcene (0, 250, 500 or 100 mg/kg/day; 0, 1,835, 3,670, 734 μmol/kg/day) in corn oil for 5 days per week (26). The β-myrcene provided had a purity of 90% and contained other impurities, such as ψ-limonene, (±)-limonene and isomers and dimers of β-myrcene. The presence of other components could render the carcinogenic results attributed to myrcene in the NTP study as potentially invalid. The doses given to the rodents had a strength five orders of magnitude greater than the exposure to food flavouring additives containing β-myrcene, which is normally found in a human population (27).
The results of the NTP study showed that there was an increased incidence of renal tubule adenoma or carcinoma in treated male rats (not for female rats, or mice). Furthermore, there was clear evidence of a dose-dependent increase in hepatocellular adenoma, hepatocellular carcinoma, and hepatoblastoma in male B6C3F1 mice (26). Spontaneous tumours were also observed in the vehicle control group, this is of no surprise as the male B6C3F1 mouse is known for having a high background incidence of hepatocellular tumours and may not be of relevance to humans (27).
Regulatory Positions
The U.S. FDA conducted a safety review of β-myrcene, following the submission of a Food Additive Petition (FAP 5A3810) by a coalition of NGOs (136). The petition requested the removal of the use of synthetic β-myrcene, as previously approved in the food additive regulations (21 CFR 172.515) (8, 137). The petition was based on the results of carcinogenicity studies undertaken in mice and rats treated with β-myrcene, from the National Toxicology Program (NTP) (26).
The FDA stated that β-myrcene did not demonstrate genotoxic potential and was unlikely to induce tumours in humans at its current exposure level as a food flavouring chemical (8). Although the FDA stated that β-myrcene did not pose a risk to the public health under conditions of intended use, it was removed from the food additive regulations under the Delaney Clause of the Federal Food, Drug, and Cosmetic Act in October 2019. This clause requires the Food and Drug Administration (FDA) to ban food additives which are found to cause or induce cancer in humans or animals as indicated by testing (137).
The EFSA stated that there are no safety concern relating to β-myrcene, including no evidence of a potential genotoxic or mutagenic activity (138). Additionally, the Expert Panel of the Flavour and Extract Manufacturers Association (FEMA) most recently evaluated the safety of 54 citrus derived natural flavour complexes. The FEMA panel confirmed that β-myrcene is “generally recognised as safe (GRAS)” (139). According to the Joint FAO/WHO Expert Committee on Food Additives (JEFCA), β-myrcene is safe to use as a flavour ingredient and is not of concern at its current estimated intake (140). More recently, the RIFM stated that is safe to use for fragrances, and that there is no evidence for genotoxic, skin sensitising, phototoxic/photoallergenic effects (141).
Conclusion
β-Myrcene is an abundant compound which occurs naturally as a major constituent in many plant species. Despite it is being common practise to attribute the biological properties of an essential oil to β-myrcene, as with all herbal preparations, the role of a range of metabolites and the specific composition of an essential oil is a crucial parameter to be taken into consideration in such an assessment (142).
The many significant biological properties of β-myrcene coupled with its non-allergic, non-toxic and antimutagenic activities offers the possibility of incorporating this natural product into medical or cosmetic products. Botanicals, such as raw hops are rich in β-myrcene, and play an important role in enriching the aroma of beer (26). There is a growing trend for alcohol free functional drinks in the beverage sector, since health consciousness is rising (143). The use of non-alcoholic functional beverages could offer the interesting health-related properties of β-myrcene e.g., relaxing, stress reducing and sleep enhancing, without the negative effects of ethanol on the liver and other organs. Companies such as Three Spirit Drinks (Beyond Alcohol Ltd.), are currently promoting the use of β-myrcene in their functional non-alcoholic beverage formulations as well as developing novel methods to create further β-myrcene rich products. The suggested anxiolytic effects of myrcene may also lead to the development of more functional non-alcoholic hop based products that are able to provide the perception of relaxation without the harmful effects of alcohol. Additionally, subsequent to the international relaxation of marijuana prohibition legislation, breeding work has already resulted on Cannabis chemotypes producing 97% of monoterpenoid content as β-myrcene (77). Such a preparation may lead to novel approaches in treatment of numerous clinical conditions.
The significant biological role of β-myrcene in plant essential oils may be limited due to the existing chemical variability. Fluctuations of the chemical composition occur due to harvesting time, climate, age of plant, plant parts, and extraction methods used. There is also possibility that β-myrcene found within plant essential oils act synergistically with other components within the essential oil to enhance health benefits. Therefore, potential synergism and antagonism should be further studied.
At present the FDA no longer permits the use of pure β-myrcene as a flavouring agent due to a legal challenge based on the Delaney Clause. Currently, no data is available that correlates the therapeutic use of pure β-myrcene with health benefits in human participants. Most of the studies on health benefits of β-myrcene in this review were in animal models or cell culture. Few studies conducted in humans (n = 2) were found and these included humans inhaling plant essential oil extracts containing <25% of β-myrcene. Thus, more robust, randomised, controlled clinical trials/intervention studies are needed using pure β-myrcene preparations to evaluate and replicate its beneficial effects in humans.
The dosages applied in the NTP study were five-six orders of magnitude greater than human exposure and there are also doubts of the purity of the β-myrcene used, thus casting serious doubts on the relevance to humans. The NTP concluded that the renal tumours in the low dose group of male F344 rats, were possibly due to α2u-globulin nephropathy. This is not applicable to humans, as the protein α2u-globulin responsible for this effect in rodents is not present in humans. In contrast, the susceptibility of different strains of rats to renal carcinogenicity varies widely (27), with Bastaki et al., finding a lack of renal toxicity to β-myrcene when using Sprague-Dawley rats. In addition, the B6C3F1 male mice included in the NTP study are recognised for having a high and variable background incidence of hepatocellular tumours; on this basis the EFSA has rejected its relevance for human health (144). Furthermore, based on this review, numerous studies have indicated the anti-mutagenic and anti-metastatic effects of β-myrcene on several different cancer cell lines (25, 145), highlighting the positive effects of β-myrcene on cancer prevention.
Overall, the evidence reviewed here points to β-myrcene being safe if consumed at a level, as it is common for food use (estimated daily intakes for β-myrcene is 1.23 μg/kg bw/day for a 60 kg person). More in-depth studies of β-myrcene toxicity in human target-organs and the establishment of protective exposure limits are needed to enhance the safe and effective use of β-myrcene.
Many questions remain yet to be answered, and not just to clarify the mechanisms of activity β-myrcene exerts in the human body, but also with regards to adopting consistent methodological criteria for future clinical research. Importantly, it needs to be evaluated to what extent β-myrcene achieves and maintains concentrations required for affecting neuronal activity in the brain. Additionally, comparisons of the intensity and durability of β-myrcene with conventional medicine needs to be investigated. Furthermore, sufficient knowledge of the efficient extraction and analysis methods of β-myrcene would help in maximising β-myrcene extraction whilst retaining its organoleptic qualities. Overall, a wide range of interesting biological activities and biochemical modifications in healthy subjects are likely to emerge from future research on β-myrcene.
Author Contributions
SS, GS, DL, and MH were responsible for the study conception. SS, FQ, and GS drafted the manuscript. SS, FQ, GS, DL, and MH provided data and sense checked data analysis and critically reviewed the manuscript. All authors contributed to and approved the final version of the manuscript.
Conflict of Interest
GS and DL were employed by the company Beyond Alcohol Ltd (trading as Three Spirit Drinks). GS and DL are employed by Beyond Alcohol Ltd., who use plants containing β-myrcene in their products. This study has arisen out of an assessment of the compound's safety initially planned as a collaborative project between Beyond Alcohol Ltd., and UCL, which, however, was not possible due to the pandemic of 2020. SS, FQ, and MH have been advising Beyond Alcohol Ltd., on the pharmacology and safety of β-myrcene.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.699666/full#supplementary-material
References
1. Leung AY. Encyclopedia of Common Natural Ingredients Used in Food, Drugs and Cosmetics. New York, NY: John Wiley & Sons (1980).
2. Behr A, Johnen L. Myrcene as a natural base chemical in sustainable chemistry: a critical review. ChemSusChem. (2009) 2:1072–95. doi: 10.1002/cssc.200900186
4. Guenther E, Althausen D. β-myrcene. In: Guenther E, editor. The Essential Oils. The Constituents of Essential Oils. New York, NY: D. Van Nostrand Company (1949). p. 8–10.
5. Madhava Madyastha K, Srivatsan V. Metabolism of β-myrcene in vivo and in vitro: its effects on rat-liver microsomal enzymes. Xenobiotica. (1987) 17:539–49. doi: 10.3109/00498258709043961
6. Cesta MF, Hard GC, Boyce JT, Ryan MJ, Chan PC, Sills RC. Complex histopathologic response in rat kidney to oral β-myrcene: an unusual dose-related nephrosis and low-dose alpha2u-globulin nephropathy. Toxicol Pathol. (2013) 41:1068–77. doi: 10.1177/0192623313482057
7. Simonsen JL, Owen LN. The Terpenes: The Simpler Acyclic and Monocyclic Terpenes and Their Derivatives. Cambridge: University Press (1953).
9. Briggs DE, Boulton CA, Brookes PA, Stevens R. 8 - The chemistry of hop constituents. In: Brewing. Woodhead Publishing (2004). p. 255–305.
10. Buglass AJ, Caven-Quantrill DJ. 16 - Applications of natural ingredients in alcoholic drinks. In: Baines D, Seal R, editors. Natural Food Additives, Ingredients and Flavourings. Oxford: Woodhead Publishing (2012). p. 358–416.
11. Haslbeck K, Bub S, Schoenberger C, Zarnkow M, Jacob F, Coelhan M. On the fate of β-myrcene during fermentation – the role of stripping and uptake of hop oil components by Brewer's yeast in dry-Hopped wort and beer. BrewingScience. (2017) 70:159–69. doi: 10.23763/BRSC17-16HASLBECK
12. Howard GA, Slater CA. Evaluation of Hops VII. Composition of the essential oil of Hops. J Inst Brewing. (1957) 63:491–506. doi: 10.1002/j.2050-0416.1957.tb06290.x
13. Dresel M, Praet T, Opstaele F, Van Holle A, Naudts D, De Keukeleire D, et al. Comparison of the analytical profiles of volatiles in single-hopped worts and beers as a function of the hop variety. BrewingScience. (2015) 68:8–28.
14. Wolfe PH. A study of factors affecting the extraction of flavor when dry hopping beer (dissertation). Oregon State University (2012).
15. Louis BWS editor. Cannabinoids and the entourage effect. In: Cannabis: A Clinician's Guide. CRC Press (2018).
16. Piomelli DR, Ethan B. The Cannabis sativa versus Cannabis indica debate: an interview with Ethan Russo, MD. Cannabis Cannabinoid Res. (2016) 1:44–6. doi: 10.1089/can.2015.29003.ebr
17. Hanuš LO, Hod Y. Terpenes/terpenoids in cannabis: are they important? Med Cannabis Cannabinoids. (2020) 3:25–60. doi: 10.1159/000509733
18. Hartsel JA, Eades J, Hickory B, Makriyannis A. Chapter 53 - Cannabis sativa and Hemp. In: R. C. Gupta, editor. Nutraceuticals. Boston, MA: Academic Press (2016). p. 735–54.
19. Rao VS, Menezes AM, Viana GS. Effect of myrcene on nociception in mice. J Pharm Pharmacol. (1990) 42:877–8. doi: 10.1111/j.2042-7158.1990.tb07046.x
20. Gurgel do Vale T, Couto Furtado E, Santos JG, Viana GSB. Central effects of citral, myrcene and limonene, constituents of essential oil chemotypes from Lippia alba (Mill.) N.E. Brown Phytomed. (2002) 9:709–14. doi: 10.1078/094471102321621304
21. Al-Omari SM. The Effect of Thujone and Myrcene on Diabetes Mellitus in Albino Rats. Faculty of Graduate Studies University of Jordan (2007).
22. Ojeda-Sana AM, van Baren CM, Elechosa MA, Juárez MA, Moreno S. New insights into antibacterial and antioxidant activities of rosemary essential oils and their main components. Food Control. (2013) 31:189–95. doi: 10.1016/j.foodcont.2012.09.022
23. Rufino AT, Ribeiro M, Sousa C, Judas F, Salgueiro L, Cavaleiro C, et al. Evaluation of the anti-inflammatory, anti-catabolic and pro-anabolic effects of E-caryophyllene, myrcene and limonene in a cell model of osteoarthritis. Eur J Pharmacol. (2015) 750:141–50. doi: 10.1016/j.ejphar.2015.01.018
24. Inoue Y, Shiraishi A, Hada T, Hamashima H, Shimada J. The antibacterial effects of myrcene on Staphylococcus aureus and its role in the essential oil of the tea tree (Melaleuca alternifolia). Nat Med. (2004) 58:10–14.
25. Bai X, Tang J. Myrcene exhibits antitumor activity against lung cancer cells by inducing oxidative stress and apoptosis mechanisms. Nat Prod Commun. (2020) 15:1–7. doi: 10.1177/1934578X20961189
26. NTP. NTP Technical Report on the Toxicology and Carcinogenesis Studies of β-Myrcene (CAS No. 123-35-3) in F344/N Rats and B6C3F1 Mice (Gavage Studies). Research Triangle Park, NC, National Institutes of Health (2010).
27. Felter SP, Llewelyn C, Navarro L, Zhang X. How the 62-year old Delaney Clause continues to thwart science: case study of the flavor substance β-myrcene. Regul Toxicol Pharmacol. (2020) 115:104708. doi: 10.1016/j.yrtph.2020.104708
28. Aldred EM, Buck C, Vall K. Chapter 22 – terpenes. In: Aldred EM, Buck C, Vall K, editors. Pharmacology. Edinburgh: Churchill Livingstone (2009). p. 167–74.
29. NCBI. PubChem Compound Summary for CID 31253, Myrcene. (2020). Available online at: https://pubchem.ncbi.nlm.nih.gov/compound/Myrcene (accesse July 15, 2020).
30. Masanetz C, Grosch W. Key odorants of parsley leaves (Petroselinum crispum [Mill.] Nym. ssp. crispum) by Odour-activity values. Flavour Fragran J. (1998) 13:115–24. doi: 10.1002/(SICI)1099-1026(199803/04)13:2%3C115::AID-FFJ706%3E3.0.CO;2-6
31. Steinhaus M, Schieberle P. Comparison of the most odor-active compounds in fresh and dried hop cones (Humulus lupulus L. Variety Spalter Select) based on GC?Olfactometry and odor dilution techniques. J Agric Food Chem. (2000) 48:1776–83. doi: 10.1021/jf990514l
32. Chemicals Inspection and Testing Institute. Bioaccumulation Data of Existing Chemicals Based on the CSCL Japan. Japan Chemical Industry Ecology-Toxicology and Information Center (1992).
33. Veith SR, Pratsinis SE, Perren M. Aroma retention in sol?gel-made silica particles. J Agric Food Chem. (2004) 52:5964–71. doi: 10.1021/jf049356a
34. Merck Index. The Merck index: An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck & Co., Inc (2013).
35. Sigma-Aldrich. [online] (2020). Available online at: https://www.sigmaaldrich.com/catalog/product/aldrich/m100005?lang=en®ion=GB (accessed July 13, 2020).
36. Narushima H, Omori T, Minoda Y. Microbial oxidation of β-myrcene. In: Moo-Young M, Vezina C, Singh K, editors. Fermentation Products. London: Elsevier (1981). p. 525–31.
37. Eggersdorfer M. Terpenes. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH (2000). p. 33–34.
38. Dudareva N, Negre F, Nagegowda DA, Orlova I. Plant volatiles: recent advances and future perspectives. Crit Rev Plant Sci. (2006) 25:417–40. doi: 10.1080/07352680600899973
39. Nagegowda DA. Plant volatile terpenoid metabolism: biosynthetic genes, transcriptional regulation and subcellular compartmentation. FEBS Lett. (2010) 584:2965–73. doi: 10.1016/j.febslet.2010.05.045
40. Erman MB, Kane BJ. Chemistry around pinene and pinane: a facile synthesis of cyclobutanes and oxatricyclo-derivative of pinane from CIS-and Trans-pinanols. Chem Biodivers. (2008) 5:910–9. doi: 10.1002/cbdv.200890104
41. Reiling KK, Yoshikuni Y, Martin VJ, Newman J, Bohlmann J, Keasling JD. Mono and diterpene production in Escherichia coli. Biotechnol Bioeng. (2004) 87:200–12. doi: 10.1002/bit.20128
42. Kirby J, Keasling JD. Metabolic engineering of microorganisms for isoprenoid production. Nat Prod Rep. (2008) 25:656–61. doi: 10.1039/b802939c
43. Kim EM, Eom JH, Um Y, Kim Y, Woo HM. Microbial synthesis of myrcene by metabolically engineered Escherichia coli. J Agric Food Chem. (2015) 63:4606–12. doi: 10.1021/acs.jafc.5b01334
44. Romano LL, Hazekamp A. Cannabis oil: chemical evaluation of an upcoming cannabis- based medicine. Cannabinoids. (2013) 1:1–11.
45. Ibrahim EA, Wang M, Radwan MM, Wanas AS, Majumdar CG, Avula B, et al. Analysis of terpenes in Cannabis sativa L. Using GC/MS: method development, validation, and application. Planta Med. (2019) 85:431–8. doi: 10.1055/a-0828-8387
46. Rahimi A, Hashemi P, Talei G, Borzuei M, Ghiasvand A. Comparative analyses of the volatile components of Citrus aurantium L. flowers using ultrasonic-assisted headspace SPME and hydrodistillation Combined with GC-MS and evaluation of their antimicrobial activities. Anal Bioanal Chem Res. (2014) 1:83–91.
47. Shao Q, Liu H, Zhang A, Wan Y, Hu R, Li M. Analysis of volatile components extracted from the peels of four different Chinese pomelos using TDS-GC-MS. J Sci Food Agric. (2014) 94:3248–54. doi: 10.1002/jsfa.6677
48. Miyazaki T, Plotto A, Baldwin EA, Reyes-De-Corcuera JI, Gmitter FG Jr. Aroma characterization of tangerine hybrids by gas-chromatography-olfactometry and sensory evaluation. J Sci Food Agric. (2012) 92:727–35. doi: 10.1002/jsfa.4663
49. Lota M, De Rocca Serra D, Tomi F, Casanova J. Chemical variability of peel and leaf essential oils of 15 species of mandarins. Biochem Syst Ecol. (2001) 29:77–104. doi: 10.1016/S0305-1978(00)00029-6
50. Kiujkeleire DD, David F, Hauhebaert K, Sandra P. Automated reporting on the quality of hops and hop products. J Inst Brewing. (1998) 104:75–82. doi: 10.1002/j.2050-0416.1998.tb00978.x
51. Aberl A, Coelhan M. Determination of volatile compounds in different hop varieties by headspace-trap GC/MS–in comparison with conventional hop essential oil analysis. J Agric Food Chem. (2012) 60:2785–92. doi: 10.1021/jf205002p
52. Kishimoto T, Wanikawa A, Kagami N, Kawatsura K. Analysis of Hop-derived terpenoids in beer and evaluation of their behavior using the stir bar–sorptive extraction method with GC-MS. J Agric Food Chem. (2005) 53:4701–7. doi: 10.1021/jf050072f
53. Gonçalves J, Figueira J, Rodrigues F, Camara JS. Headspace solid-phase microextraction combined with mass spectrometry as a powerful analytical tool for profiling the terpenoid metabolomic pattern of hop-essential oil derived from Saaz variety. J Sep Sci. (2012) 35:2282–96. doi: 10.1002/jssc.201200244
54. Vázquez-Araújo L, Rodríguez-Solana R, Cortés-Diéguez SM, Domínguez JM. Use of hydrodistillation and headspace solid-phase microextraction to characterize the volatile composition of different hop cultivars. J Sci Food Agric. (2013) 93:2568–74. doi: 10.1002/jsfa.6078
55. Daferera D, Pappas C, Tarantilis P, Polissiou M. Quantitative analysis of α-pinene and β-myrcene in mastic gum oil using FT-Raman spectroscopy. Food Chem. (2002) 77:511–15. doi: 10.1016/S0308-8146(01)00382-X
56. Zachariadis GA, Langioli AV. Headspace solid phase microextraction for terpenes and volatile compounds determination in mastic gum extracts, mastic oil and human urine by GC- MS. Anal Lett. (2012) 45:993–1003. doi: 10.1080/00032719.2012.670787
57. Ceva-Antunes PMN, Bizzo HR, Alves SM, Antunes OAC. Analysis of volatile compounds of taperebá (Spondias mombin L.) and cajá (Spondias mombin L.) by simultaneous distillation and extraction (SDE) and solid phase microextraction (SPME). J Agric Food Chem. (2003) 51:1387–92. doi: 10.1021/jf025873m
58. Okaru AO, Lachenmeier DW. The food and beverage occurrence of furfuryl alcohol and myrcene-two emerging potential human carcinogens? Toxics. (2017) 5:9. doi: 10.3390/toxics5010009
59. Eyres G, Dufour JP. Hop essential oil: analysis, chemical composition and odor characteristics. In: Preedy V, editor. Beer in Health and Disease Prevention. Amsterdam: Elsevier (2009). p. 239–54.
60. Tyśkiewicz K, Gieysztor R, Konkol M, Szałas J, Rój E. Essential oils from Humulus lupulus scCO2 extract by hydrodistillation and microwave-assisted hydrodistillation. Molecules. (2018) 23:2866. doi: 10.3390/molecules23112866
61. Eri S, Khoo BK, Lech J, Hartman TG. Direct thermal desorption– gas chromatography and gas chromatography– mass spectrometry profiling of hop (Humulus lupulus L.) essential oils in support of varietal characterization. J Agric Food Chem. (2000) 48:1140–9. doi: 10.1021/jf9911850
62. Ligor M, Stankevičius M, Wenda-Piesik A, Obelevičius K, RagaŽinskiene O, Stanius Z, et al. Comparative gas chromatographic–mass spectrometric evaluation of hop (Humulus lupulus L.) essential oils and extracts obtained using different sample preparation methods. Food Anal Methods. (2014) 7:1433–42. doi: 10.1007/s12161-013-9767-5
63. Langezaal CR, Chandra A, Katsiotis ST, Scheffer JJ, De Haan AB. Analysis of supercritical carbon dioxide extracts from cones and leaves of a Humulus lupulus L cultivar. J Sci Food Agric. (1990) 53:455–63. doi: 10.1002/jsfa.2740530404
64. Plutowska B, Wardencki W. Headspace solid-phase microextraction and gas chromatography–olfactometry analysis of raw spirits of different organoleptic quality. Flavour Fragr J. (2009) 24:177–85. doi: 10.1002/ffj.1930
65. Czerwiński J, Zygmunt B, Namieśnik J. Head-space solid phase microextraction for the GC-MS analysis of terpenoids in herb based formulations. Fresenius' J Anal Chem. (1996) 356:80–83. doi: 10.1007/s0021663560080
66. Lachenmeier K, Musshoff F, Madea B, Lachenmeier DW. Application of experimental design to optimise solid-phase microextraction of orange juice flavour. Electron J Environ Agric Food Chem. (2006) 5:1380–8. doi: 10.5281/zenodo.438134
67. Delgado IF, Carvalho RR, De Almeida Nogueira ACM, Mattos AP, Figueiredo LH, Oliveira SHP, et al. Study on embryo-foetotoxicity of β-myrcene in the rat. Food Chem Toxicol. (1993) 31:31–5. doi: 10.1016/0278-6915(93)90175-X
68. Papada E, Gioxari A, Amerikanou C, Galanis N, Kaliora AC. An absorption and plasma kinetics study of monoterpenes present in Mastiha oil in humans. Foods. (2020) 9:1019. doi: 10.3390/foods9081019
69. Ishida T, Asakawa Y, Takemoto T, Aratani T. Terpenoids biotransformation in mammals III: biotransformation of α-pinene, β-pinene, pinane, 3-carene, carane, myrcene, and p-cymene in rabbits. J Pharm Sci. (1981) 70:406–415. doi: 10.1002/jps.2600700417
70. Ishida T. Biotransformation of terpenoids by mammals, microorganisms, plant-cultured cells. Chem Biodivers. (2005) 2:569–90. doi: 10.1002/cbdv.200590038
71. Štěrba K, Cejka P, Culík J, Jurková M, Krofta K., Pavlovič M, et al. Determination of linalool in different hop varieties using a new method based on fluidized-bed extraction with gas chromatographic-mass spectrometric detection. J Am Soc Brewing Chem. (2015) 73:151–8. doi: 10.1094/ASBCJ-2015-0406-01
73. Zhu J, Xiao Z. Characterization of the major odor-active compounds in dry jujube cultivars by application of gas chromatography–olfactometry and odor activity value. J Agric Food Chem. (2018) 66:7722–34. doi: 10.1021/acs.jafc.8b01366
74. Niu Y, Wang P, Xiao Q, Xiao Z, Mao H, Zhang J. Characterization of odor-active volatiles and odor contribution based on binary interaction effects in mango and vodka cocktail. Molecules. (2020) 25:1083. doi: 10.3390/molecules25051083
76. McPartland JM, Russo EB. Cannabis and cannabis extracts: greater than the sum of their parts? J Cannabis Ther. (2001) 1:103–32. doi: 10.1300/J175v01n03_08
77. Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. (2011) 163:1344–64. doi: 10.1111/j.1476-5381.2011.01238.x
78. Freitas JC, Presgrave OA, Fingola FF, Menezes MA, Paumgartten FJ. Effect of beta-myrcene on pentobarbital sleeping time. Braz J Med Biol Res. (1993) 26:519–23.
79. Finlay DB, Sircombe KJ, Nimick M, Jones C, Glass M. Terpenoids from cannabis do not mediate an entourage effect by acting at cannabinoid receptors. Front Pharmacol. (2020) 11:359. doi: 10.3389/fphar.2020.00359
80. Santiago M, Sachdev S, Arnold JC, McGregor IS, Connor M. Absence of entourage: terpenoids commonly found in Cannabis sativa do not modulate the functional activity of Δ9-THC at human CB1 and CB2 receptors. Cannabis Cannabinoid Res. (2019) 4:165–76. doi: 10.1089/can.2019.0016
81. Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. (2004) 431:312–6. doi: 10.1038/nature02913
82. Ibsen MS, Connor M, Glass M. Cannabinoid CB1 and CB2 receptor signaling and bias. Cannabis Cannabinoid Res. (2017) 2:48–60. doi: 10.1089/can.2016.0037
83. Krings U, Berger RG. Terpene bioconversion–how does its future look? Nat Prod Commun. (2010) 5:1507–22. doi: 10.1177/1934578X1000500927
84. Gauvin A, Smadja J. Essential oil composition of four Psiadia species from Reunion Island: a chemotaxonomic study. Biochem Syst Ecol. (2005) 33:705–14. doi: 10.1016/j.bse.2004.12.013
85. Casano S, Grassi G, Martini V, Michelozzi M. Variations in terpene profiles of different strains of Cannabis sativa L. Acta Hortic. (2011) 925:115–21. doi: 10.17660/ActaHortic.2011.925.15
86. Giese M, Lewis M, Giese L, Smith K. Method for the analysis of cannabinoids and terpenes in cannabis. J AOAC Int. (2015) 98:1503–22. doi: 10.5740/jaoacint.15-116
87. Ojala A, Huopalahti R, NykÄNen A, Kallio H. Variation of Angelica archangelica subsp. archangelica (Apiaceae) in northern Fennoscandia: 5. Variation in composition of essential oil. Ann Bot Fenn. (1986) 23:325–32.
88. Sørensen JM, Katsiotis ST. Parameters influencing the yield and composition of the essential oil from cretan vitex agnus-castus fruits. Planta Med. (2000) 66:245–50. doi: 10.1055/s-2000-10685
89. Morsy NFS. A short extraction time of high quality hydrodistilled cardamom (Elettaria cardamomum L. Maton) essential oil using ultrasound as a pretreatment. Indust Crops Prod. (2015) 65:287–92. doi: 10.1016/j.indcrop.2014.12.012
90. Gulluni N, Re T, Loiacono I, Lanzo G, Gori L, Macchi C, et al. Cannabis essential oil: a preliminary study for the evaluation of the brain effects. Evid Based Complement Altern Med. (2018). 2018:1709182. doi: 10.1155/2018/1709182
91. Viana GS, do Vale TG, Silva CM, Matos FJ. Anticonvulsant activity of essential oils and active principles from chemotypes of Lippia alba (Mill.) N.E. Brown. Biol Pharm Bull. (2000) 23:1314–7. doi: 10.1248/bpb.23.1314
92. Rakotosaona R, Randrianarivo E, Rasoanaivo P, Nicoletti M, Benelli G, Maggi F. Effect of the leaf essential oil from Cinnamosma madagascariensis Danguy on pentylenetetrazol-induced seizure in rats. Chem Biodivers. (2017) 14:e1700256. doi: 10.1002/cbdv.201700256
93. da-Silva VA, de-Freitas JC, Mattos AP, Paiva-Gouvea W, Presgrave OA, Fingola FF, et al. Neurobehavioral study of the effect of beta-myrcene on rodents. Braz J Med Biol Res. (1991) 24:827–31.
94. Gutteridge JM. Free radicals in disease processes: a compilation of cause and consequence. Free Radic Res Commun. (1993) 19:141–58. doi: 10.3109/10715769309111598
95. Wojtunik KA, Ciesla LM, Waksmundzka-Hajnos M. Model studies on the antioxidant activity of common terpenoid constituents of essential oils by means of the 2,2-diphenyl-1-picrylhydrazyl method. J Agric Food Chem. (2014) 62:9088–94. doi: 10.1021/jf502857s
96. Heinrich M, Appendino G, Efferth T, Fürst R, Izzo AA, Kayser O, et al. Best practice in research – overcoming common challenges in phytopharmacological research. J Ethnopharmacol. (2020) 246:112230. doi: 10.1016/j.jep.2019.112230
97. Ciftci O, Ozdemir I, Tanyildizi S, Yildiz S, Oguzturk H. Antioxidative effects of curcumin, β-myrcene and 1,8-cineole against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced oxidative stress in rats liver. Toxicol Ind Health. (2011) 27:447–453. doi: 10.1177/0748233710388452
98. Bonamin F, Moraes TM, Dos RC, Kushima H, Faria FM, Silva MA, et al. The effect of a minor constituent of essential oil from Citrus aurantium: the role of β-myrcene in preventing peptic ulcer disease. Chem Biol Interact. (2014) 212:11–19. doi: 10.1016/j.cbi.2014.01.009
99. Ciftci O, Oztanir MN, Cetin A. Neuroprotective effects of β-myrcene following global cerebral ischemia/reperfusion-mediated oxidative and neuronal damage in a C57BL/J6 mouse. Neurochem Res. (2014) 39:1717–23. doi: 10.1007/s11064-014-1365-4
100. Hoseini SM, Yousefi M, Hoseinifar SH, Van Doan H. Antioxidant, enzymatic and hematological responses of common carp (Cyprinus carpio) fed with myrcene- or menthol-supplemented diets and exposed to ambient ammonia. Aquaculture. (2019) 506:246–55. doi: 10.1016/j.aquaculture.2019.03.048
101. Hwang E, Park SY, Lee HJ, Lee TY, Sun ZW, Yi TH. Gallic acid regulates skin photoaging in UVB-exposed fibroblast and hairless mice. Phytother Res. (2014) 28:1778–88. doi: 10.1002/ptr.5198
102. Zhang M, Hwang E, Lin P, Gao W, Ngo HTT Yi .-H. Prunella vulgaris L. exerts a protective effect against extrinsic aging through Nf-κB, MAPKs, AP-1, and TGF-β/smad signaling pathways in UVB-aged normal human dermal fibroblasts. Rejuven Res. (2018) 21:313–22. doi: 10.1089/rej.2017.1971
103. Brennan M, Bhatti H, Nerusu KC, Bhagavathula N, Kang S, Fisher GJ, et al. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem Photobiol. (2003) 78:43–8.2. doi: 10.1562/0031-8655(2003)078<0043:MMITMC>2.0.CO;2
104. Hwang E, Ngo HTT, Park B, Seo SA, Yang JE, Yi TH. Myrcene, an aromatic volatile compound, ameliorates human skin extrinsic aging via regulation of MMPs production. Am J Chin Med. (2017) 45:1113–24. doi: 10.1142/S0192415X17500604
105. Lorenzetti BB, Souza GE, Sarti SJ, Santos Filho D, Ferreira SH. Myrcene mimics the peripheral analgesic activity of lemongrass tea. J Ethnopharmacol. (1991) 34:43–8. doi: 10.1016/0378-8741(91)90187-I
106. Souza M, Siani AC, Ramos M, Menezes-de-Lima O, Henriques M. Evaluation of anti-inflammatory activity of essential oils from two Asteraceae species. Pharmazie. (2003) 58:582–6.
107. Tian J, Zhang R, Weng Y, Qin Q, Zhang X, Liu A, et al. Myrcene enhances the cardioprotective effect through matrix remodelling in an experimental model of heart failure. Arch Med Sci. (2020) 16:1–12. doi: 10.5114/aoms.2020.95875
108. Paula-Freire LI, Molska GR, Andersen ML, Carlini EL. Ocimum gratissimum essential oil and its isolated compounds (Eugenol and Myrcene) reduce neuropathic pain in mice. Planta Med. (2016) 82:211–6. doi: 10.1055/s-0035-1558165
109. Duarte ID, dos Santos IR, Lorenzetti BB, Ferreira SH. Analgesia by direct antagonism of nociceptor sensitization involves the arginine-nitric oxide-cGMP pathway. Eur J Pharmacol. (1992) 217:225–7. doi: 10.1016/0014-2999(92)90881-4
110. Jansen C, Shimoda LMN, Kawakami JK, Ang L, Bacani AJ, Baker JD, et al. Myrcene and terpene regulation of TRPV1. Channels. (2019) 13:344–66. doi: 10.1080/19336950.2019.1654347
111. Philippaert K, Vennekens R. Transient Receptor Potential (TRP) cation channels in diabetes. In: Szallasi A, editor. TRP Channels as Therapeutic Targets: From Basic Science to Clinical Use. Elsevier Science (2015). p. 343–63.
112. Heblinski M, Santiago M, Fletcher C, Stuart J, Connor M, McGregor IS. Terpenoids commonly found in Cannabis sativa do not modulate the actions of phytocannabinoids or endocannabinoids on TRPA1 and TRPV1 channels. Cannabis Cannabinoid Res. (2020) 4:305–17. doi: 10.1089/can.2019.0099
113. Matura M, Sköld M, Börje A, Andersen KE, Bruze M, Frosch P, et al. Selected oxidized fragrance terpenes are common contact allergens. Contact Dermatitis. (2005) 52:320–8. doi: 10.1111/j.0105-1873.2005.00605.x
114. Moreno OM. Acute Oral Toxicity Studies of Myrcene in Mice, Rats and Rabbits. Unpublished report to the Research Institute of Fragrance Materials (1972).
115. Kligman A. Report to RIFM (Unpublished report). Woodcliff Lake, NJ: Research Institute for Fragrance Materials, Inc. (1972).
116. Hausen BM, Reichling J, Harkenthal M. Degradation products of monoterpenes are the sensitizing agents in tea tree oil. Am J Contact Dermat. (1999) 10:68–77. doi: 10.1016/S1046-199X(99)90002-7
117. Paumgartten FJR, Delgado IF, Alves EN, Nogueira ACMDA, Presgrave RDF, Neubert D. Single dose toxicity study of beta-myrcene, a natural analgesic substance. Braz J Med Biol Res. (1990) 23:873–7.
118. Bastaki M, Aubanel M, Bauter M, Cachet T, Demyttenaere J, Diop MM, et al. Absence of renal adverse effects from β-myrcene dietary administration in OECD guideline-compliant subchronic toxicity study. Food Chem Toxicol. (2018) 120:222–9. doi: 10.1016/j.fct.2018.07.004
119. Felter SP, Boobis AR, Botham PA, Brousse A, Greim H, Hollnagel HM, et al. Hazard identification, classification, and risk assessment of carcinogens: too much or too little? - Report of an ECETOC workshop. Crit Rev Toxicol. (2020) 50:72–95. doi: 10.1080/10408444.2020.1727843
120. Delgado IF, de Almeida Nogueira ACM, Souza CAM, Costa AMN, Figueiredo LH, Mattos AP, et al. Peri- and postnatal developmental toxicity of β-myrcene in the rat. Food Chem Toxicol. (1993) 31:623–8. doi: 10.1016/0278-6915(93)90044-Y
121. Paumgartten FJ, De-Carvalho RR, Souza CA, Madi K, Chahoud I. Study of the effects of beta-myrcene on rat fertility and general reproductive performance. Braz J Med Biol Res. (1998) 31:955–65. doi: 10.1590/S0100-879X1998000700012
122. Röscheisen C, Zamith H, Paumgartten FJ, Speit G. Influence of β-myrcene on sister-chromatid exchanges induced by mutagens in V79 and HTC cells. Mut Res Lett. (1991) 264:43–9. doi: 10.1016/0165-7992(91)90044-5
123. Kauderer B, Zamith H, Paumgartten FJR, Speit G, Holden HE. Evaluation of the mutagenicity of β-myrcene in mammalian cells in vitro. Environ Mol Mutagen. (1991) 18:28–34. doi: 10.1002/em.2850180106
124. Zamith HP, Vidal MN, Speit G, Paumgartten FJ. Absence of genotoxic activity of beta-myrcene in the in vivo cytogenetic bone marrow assay. Braz J Med Biol Res. (1993) 26:93–8.
125. Kim, J.-O., Kim, Y.-S., Lee, J.-H. N., Rhee, S.-H., Moon, S.-H., et al. Antimutagenic effect of the major volatile compounds identified from mugwort (Artemisia asictica nakai) leaves. J Korean Soc Food Nutr. (1992) 21:308–13.
126. Gomes-Carneiro MR, Viana ME, Felzenszwalb I, Paumgartten FJ. Evaluation of beta-myrcene, alpha-terpinene and (+)- and (-)-alpha-pinene in the Salmonella/microsome assay. Food Chem Toxicol. (2005) 43:247–52. doi: 10.1016/j.fct.2004.09.011
127. De-Oliveira AC, Ribeiro-Pinto LF, Paumgartten JR. In vitro inhibition of CYP2B1 monooxygenase by beta-myrcene and other monoterpenoid compounds. Toxicol Lett. (1997) 92:39–46. doi: 10.1016/S0378-4274(97)00034-9
128. Mitić-Culafić D, Žegura B, Nikolić B, Vuković-Gačić B, KneŽević-Vukčević J, Filipič M. Protective effect of linalool, myrcene and eucalyptol against t-butyl hydroperoxide induced genotoxicity in bacteria and cultured human cells. Food Chem Toxicol. (2009) 47:260–6. doi: 10.1016/j.fct.2008.11.015
129. Sawamura M, Sun SH, Ozaki K, Ishikawa J, Ukeda H. Inhibitory effects of citrus essential oils and their components on the formation of N-nitrosodimethylamine. J Agric Food Chem. (1999) 47:4868–72. doi: 10.1021/jf9903206
130. Chaouki W, Leger DY, Liagre B, Beneytout JL, Hmamouchi M. Citral inhibits cell proliferation and induces apoptosis and cell cycle arrest in MCF-7 cells. Fundam Clin Pharmacol. (2009) 23:549–56. doi: 10.1111/j.1472-8206.2009.00738.x
131. Saleh MMH, Hashem FA, Glombitza KW. Cytotoxicity and in vitro effects on human cancer cell lines of volatiles of Apium graveolens var. filicum. Pharm Pharmacol Lett. (1998) 8:97–9.
132. Okamura K, Iwakami S, Matsunaga T. Biological activity of monoterpenes from trees. Toyama-Ken Yakuji Kenkyusho Nenpo. (1993) 20:95–101.
133. Silva SL, Figueiredo PM, Yano T. Cytotoxic evaluation of essential oil from Zanthoxylum rhoifolium Lam. leaves. Acta Amazonica. (2007) 37:281–6. doi: 10.1590/S0044-59672007000200015
134. Ferraz RP, Bomfim DS, Carvalho NC, Soares MB, da Silva TB, Machado WJ, et al. Cytotoxic effect of leaf essential oil of Lippia gracilis Schauer (Verbenaceae). Phytomedicine. (2013) 20:615–21. doi: 10.1016/j.phymed.2013.01.015
135. Russin WA, Hoesly JD, Elson CE, Tanner MA, Gould MN. Inhibition of rat mammary carcinogenesis by monoterpenoids. Carcinogenesis. (1989) 10:2161–4. doi: 10.1093/carcin/10.11.2161
136. Hallagan JB, Hall RL, Drake J. The GRAS provision - the FEMA GRAS program and the safety and regulation of flavors in the United States. Food Chem Toxicol. (2020) 138:111236. doi: 10.1016/j.fct.2020.111236
137. Mog SR, Zang YJ. Safety assessment of food additives: case example with myrcene, a synthetic flavoring agent. Toxicol Pathol. (2019) 47:1035–37. doi: 10.1177/0192623319879634
138. EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids. Scientific Opinion on Flavouring Group Evaluation 78, Revision 2 (FGE.78Rev2): consideration of aliphatic and alicyclic and aromatic hydrocarbons evaluated by JECFA (63rd meeting) structurally related to aliphatic hydrocarbons evaluated by EFSA in FGE.25Rev3. EFSA J. (2015) 13:4067. doi: 10.2903/j.efsa.2015.4067
139. Cohen SM, Eisenbrand G, Fukushima S, Gooderham NJ, Guengerich FP, Hecht SS, et al. FEMA GRAS assessment of natural flavor complexes: citrus-derived flavoring ingredients. Food Chem Toxicol. (2019) 124:192–218. doi: 10.1016/j.fct.2018.11.052
140. Joint FAO/WHO Expert Committee on Food Additives. Safety Evaluation of Certain Food Additives/Prepared by the Sixty-Third Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JEFCA) (2006).
141. Api AM, Belmonte F, Belsito D, Biserta S, Botelho D, Bruze M, et al. RIFM fragrance ingredient safety assessment, myrcene, CAS Registry Number 123-35-3. Food Chem Toxicol. (2020) 144:111631. doi: 10.1016/j.fct.2020.111631
142. Heinrich M, Barnes J, Prieto-Garcia J, Gibbons S, Williamson EM. Fundamentals of Pharmacognosy and Phytotherapy. Edinburgh; London: Elsevier Health Sciences (2018).
143. Hagemann M, Schmidt-Cotta V, Marchioni E, Braun S. Chance for dry-hopped non-alcoholic beverages? Part. 2: health properties and target consumers. BrewingScience. (2017) 70:188–23. doi: 10.23763/BrSc17-10Hagemann
144. EFSA. Scientific Opinion on Flavouring Group Evaluation 78, Revision 1 (FGE. 78Rev1): consideration of aliphatic and alicyclic and aromatic hydrocarbons evaluated by JECFA (63rd meeting) structurally related to aliphatic and aromatic hydrocarbons evaluated by EFSA in FGE. 25Rev2. EFSA J. (2011) 9:2178. doi: 10.2903/j.efsa.2011.2178
Keywords: myrcene, hop, toxicology, phytochemistry, biological activities, plant biotechnology, non-alcoholic beer
Citation: Surendran S, Qassadi F, Surendran G, Lilley D and Heinrich M (2021) Myrcene—What Are the Potential Health Benefits of This Flavouring and Aroma Agent? Front. Nutr. 8:699666. doi: 10.3389/fnut.2021.699666
Received: 23 April 2021; Accepted: 09 June 2021;
Published: 19 July 2021.
Edited by:
Fátima Martel, Universidade Do Porto, PortugalReviewed by:
Isabel O'Neill Gaivão, University of Trás-os-Montes and Alto Douro, PortugalDamião Pergentino De Sousa, Federal University of Paraíba, Brazil
Copyright © 2021 Surendran, Qassadi, Surendran, Lilley and Heinrich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shelini Surendran, cy5zdXJlbmRyYW5Ac3VycmV5LmFjLnVr; Geyan Surendran, Z2V5YW5AdGhyZWVzcGlyaXRkcmlua3MuY29t
 Shelini Surendran
Shelini Surendran Fatimah Qassadi
Fatimah Qassadi Geyan Surendran4*
Geyan Surendran4* Michael Heinrich
Michael Heinrich