- 1Department of Emergency Medicine and National Clinical Research Center for Geriatrics, Research Laboratory of Emergency Medicine, Disaster Medicine Center, West China Hospital, Sichuan University, Chengdu, China
- 2West China School of Nursing, West China Hospital, Sichuan University, Chengdu, China
- 3Department of Cardiology, West China Hospital, Sichuan University, Chengdu, China
- 4Chinese Evidence-Based Medicine Center, West China Hospital, Sichuan University, Chengdu, China
- 5Health Management Center, West China Hospital, Sichuan University, Chengdu, China
- 6Department of General Practice and National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu, China
Objective: The relationship between combined healthy lifestyle and cardiovascular (CV) events in diabetes is unclear. We aim to investigate the association between a healthy lifestyle score (HLS) and the risk of mortality and CV events in diabetes.
Methods: We examined the associations of six lifestyle factors scores (including healthy diet, moderate alcohol and regular coffee intakes, never smoking, physical activity, and normal weight) with diabetes in the Atherosclerosis Risk in Communities (ARIC) study of 3,804 participants with diabetes from the United States at baseline. Primary outcomes included all-cause mortality, CV mortality, and composite CV events (heart failure hospitalizations, myocardial infarction, fatal coronary heart disease, and stroke).
Results: Among these diabetic participants, 1,881 (49.4%), 683 (18.0%), and 1,600 (42.0%) cases of all-cause mortality, CV mortality, and CV events were documented, respectively, during the 26 years of follow-up. Further, the prevalence of these adverse events became lower with the increase of HLS (all P < 0.001). In the risk-factors adjusted Cox regression model, compared to participants with HLS of 0, participants with HLS of 2 had significant lower risk of all-cause mortality (HR = 0.811, 95% CI: 0.687–0.957, P = 0.013), CV mortality (HR = 0.744, 95% CI: 0.576–0.962, P = 0.024), and CV events (HR = 0.789, 95% CI: 0.661–0.943, P = 0.009). The association of HLS with CV events was stronger for women than men (P for interaction <0.05).
Conclusion: Adherence to a healthy lifestyle was associated with a lower risk of CV events and mortality in diabetics. Our findings suggest that the promotion of a healthy lifestyle would help reduce the increasing healthcare burden of diabetes.
Clinical Trial Registration: https://clinicaltrials.gov, Identifier: NCT00005131.
Introduction
Diabetes affects more than 400 million people globally (~8.4% of the world's population). This number may rise dramatically to 693 million (9.9%) by 2045 with an aging population and the prevalence of unhealthy lifestyles worldwide (1). The cardiovascular (CV) system is the primary system affected by diabetes, and despite major therapeutic advances that have improved outcomes over the past 2 decades, cardiovascular disease (CVD) remains the leading cause of morbidity and mortality in patients with diabetes (1, 2). Compared to the general population, the risk for CVD in diabetes is two to five times higher, independent of other risk factors (3). The global burden of diabetes is exacerbated by the fact that a large proportion of patients develop CVD, which affects their life expectancy and accounts for the majority of their healthcare costs (4, 5).
Although some studies indicate that pharmacologic management is considerably effective in improving CV events, studies of pharmacologic treatment in secondary prevention show contradictory results in its ability to reduce CV events; furthermore, it is costly and may have unpleasant side effects (6). In contrast, adherence to a healthy lifestyle has become a mainstream approach to lower CV burden through primary and secondary prevention emphasized by guidelines (2, 6, 7). In epidemiological studies, ~72.6% of incident diabetes were attributable to a combination of CV risk factors over a period of about a decade (8). This includes an adherence to multiple healthy lifestyle factors including lower weight, healthy dietary patterns, being physically active, not smoking, and moderate alcohol consumption. All of these have been associated with up to a 90% reduction of incidences of diabetes (9, 10), nearly an 80% reduction of coronary heart disease (CHD), a 67.9% reduction in ischemic heart disease, and a 50% reduction in ischemic strokes (11–13). In addition, non-adherence to healthy lifestyles contributes to 60.7% of all-cause mortality, and 71.7% of for CV mortality in general population (14).
Common pathways associated with a healthy lifestyle may play a protective role in reducing risks of CV events, such as anti-inflammatory action, inhibition of platelet activity, improved insulin resistance, and enhanced antioxidant activity (7, 15, 16). Adherence to a healthy lifestyle, which alleviates comprehensive CV risk factors, is more likely to improve CV events in patients with diabetes. However, previous studies largely focused on the association between adherence to healthy lifestyle and total mortality (17–19), and little is known if cardiac protective effects persist throughout the course of diabetes. Therefore, there is a need for well-controlled studies of the potential effects of lifestyle interventions to better understand how a healthy lifestyle can be implemented in clinical practice for diabetic individuals.
Thus, this study aimed to examine the associations of a combination of modifiable, healthy lifestyle factors with the risks of all-cause mortality and CV events among individuals with type 1 or type 2 diabetes in a large, long-term follow-up cohort study.
Methods
Study Population
This study was based on the Atherosclerosis Risk in Communities (ARIC) study. ARIC data are available through The National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center. ARIC is a large prospective study covering four communities (Minneapolis, MN; Forsyth County, North Carolina; Washington County, Maryland; and Jackson, MS) in the United States. At baseline (1987–1989), 15,792 community residents aged between 45 and 64 participated in this study. Follow-up was conducted every 3 years to update medical history, lifestyle, CV events and other health-related events. The institutional review committee at each site approved the study, and participants provided informed consent.
In this study, data from 1987 were used as the baseline for ARIC, including 4,464 participants with type 1 or type 2 diabetes at baseline. Diabetes is defined as a blood glucose concentration ≥ 126 mg/dL, a non-fasting blood glucose concentration ≥ 200 mg/dL, self-reported history of diabetes, or use of diabetes drugs in the past 2 weeks. Thus, type 1 and type 2 diabetes cannot be distinguished according to the definition in ARIC study. We excluded participants without a complete assessment of a healthy lifestyle (N = 117), patients with diagnosed with a myocardial infarction, stroke, or malignant tumor (N = 264; a diagnosis of these diseases might cause changes in lifestyle habits; in addition, they contributed significantly to mortality), patients with implausible caloric intakes (≤ 500 kcal/day or >5,000 kcal/day, N = 66), those who were neither white nor black (N = 24), and participants without covariates (N = 184). The final participant count was 3,804.
Definition of the Healthy Lifestyle Score
The healthy lifestyle score (HLS) was calculated based on six factors: healthy diet, moderate alcohol, coffee consumption, physical activity, normal body weight, and being a non-smoker. Each health lifestyle factor added a score of 1 to the HLS, thus HLS ranged from 0 to 6. In ARIC, participants' dietary intake was assessed by the 66 food item-frequency semi-quantitative Food Frequency questionnaire (FFQ) by Willett et al. (20). Participants were asked about the frequency of specified portions of 66 foods, and their daily nutrient intake was calculated by multiplying the nutrient content of a specific part of each food by its daily intake frequency, and then adding up all foods. Dietary quality was assessed using the Mediterranean diet scale of Trichopoulou et al. (21). The original score was based on the intake of 9 items, and possible scores on the Mediterranean diet scores ranged from 0 to 9. This diet is rich in vegetables, fruits, extra virgin olive oil, nuts, legumes and whole grains, while containing low to medium levels of animal products. It has been identified as a high-quality diet for the prevention of diabetes (22). We removed the alcohol score from the Mediterranean Diet since alcohol intake was measured as an independent component of HLS. We defined a healthy diet as the highest two quintiles of Mediterranean diet scores which was scored 1, whereas the lowest three quintiles scored 0, as in previous methods (9, 18, 23, 24). The mean (SD) of Mediterranean diet score for total population was 4.0 (1.8), and mean (SD) was 5.7 (0.9) for the top two quintiles of Mediterranean diet score. Guidelines in the United States define moderate alcohol intakes as consuming 5–15 g of alcohol per day for women and 5–30 g per day for men (25). Moderate alcohol intakes scored 1 point, whereas intakes outside of this range scored 0. Coffee consumption was defined as drinking ≥ 2 servings per day and scored 1 point, while drinking <2 servings per day scored 0. According to previous reports, general and diabetic individuals with a regular intake of ≥ two cups per day has a lower risk of CV and all-cause mortality (26, 27). In Visit 1, 3, and 5, participants answered questions about participation in up to four exercise activities, and how often they participated, by the Baecke Questionnaire (28). A score of 1 point was assigned to those performing healthy physical activity, defined as physical activity of ≥ 150 min per week of vigorous intensity or ≥ 300 min of moderate intensity (≥15 MET-hour/week), while 0 point was assigned for those who did not accomplish enough exercise (29). Trained staff measured the participants' weight and height during follow-up, which was used to calculate body mass index (BMI). Normal body weight was defined as a BMI from 18.5 to 24.9 kg/m2 according to the WHO standard and scored 1 point, whereas the range of BMI <18.5 or ≥ 25.0 kg/m2 was scored 0 (30). Smoking status was assessed according to smoking history, never smoked was scored 1 point, while past smoking or present smoking was scored 0.
Because lifestyle factors may be affected by the risk of death over a long period of time, in order to obtain an accurate assessment of a healthy lifestyle, detailed and repeated measurements of healthy lifestyle factors were applied to calculate the HLS. The Mediterranean Diet score, alcohol and coffee consumption, and physical activities were calculated by the average of 3-year repeated measurements. To minimize reverse causality, we applied the lifelong maximum BMI.
Definition of Outcomes
Our primary results contained CV events, CV disease mortality, and all-cause mortality. CV events and CV deaths were determined by annual telephone calls with participants or agents, active monitoring of local discharge and death records, and the National Death Index. CV events were defined as myocardial infarction, fatal coronary heart disease, stroke, or hospitalized heart failure (31). Stroke was defined as the discharge diagnostic code [International Classification of Diseases ninth revision (ICD-9), code 430-438]. The incident stroke was defined as the first known or probable stroke incident among participants with a history of stroke without a doctor's diagnosis at the baseline (32). Heart failure was recorded according to discharge diagnostic code (ICD-9, code 428). CV disease mortality was defined as deaths with an ICD-9 code of 390 to 459. All-cause mortality was defined as death from any cause.
Covariate Assessment
At baseline, participants' socio-demographic information (age, sex, race, income, and education), health behaviors (smoking, alcohol consumption, physical activity, dietary intake), and medicine use (antihypertensive or diabetic drug use) were collected through a standard self-reporting questionnaire. The concentration of total cholesterol was determined by using an enzymatic method. The trained technicians measured the blood pressure of the subjects three times, then took the average values of the second and third times. We defined hypertension as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or using antihypertensive drugs in the past 2 weeks. The concentration of blood glucose was determined by improved hexokinase or glucose-6-phosphate dehydrogenase method.
Statistical Analysis
Categorical variables are reported as frequencies and percentages and were compared using a chi-squared test. Continuous variables were reported as the mean ± standard deviation and compared using an analysis of variance.
The Cox proportional hazards regression model was used to assess the relationship of HLS with the time to the all-cause mortality, CV mortality, and CV events for individuals with diabetes. The group with an HLS of 0 was set as reference group in the Cox regression model. To further determine if these relationships were independent of risk factors, the model was adjusted according to demographic variables (age, sex, race, education) (< high school, high school, or >high school), annual household income (<16,000; 16,000–35,000; >35,000 US$), heart rate, systolic blood pressure, total cholesterol, high density lipoprotein cholesterol, low density lipoprotein cholesterol, triglycerides, creatinine, blood glucose, and total caloric intake. In addition, after adjusting for confounding factors, the Cox regression analysis was performed to evaluate the association between HLS and all-cause mortality in different subgroups of age, sex, and race, as well as their interactions. In order to explain the different results in the subgroup of sex, the correlation of BMI with adjusted hazard ratios (HRs) for all-cause mortality was analyzed using linear splines with five evenly spaced knots in men and women.
A two-tailed P < 0.05 was considered significant for all tests. All statistical analyses were performed using SPSS version 26.0 (IBM Corp, Armonk, NY, USA), and R software 3.5.0 (Vienna, Austria).
Results
Baseline Characteristics
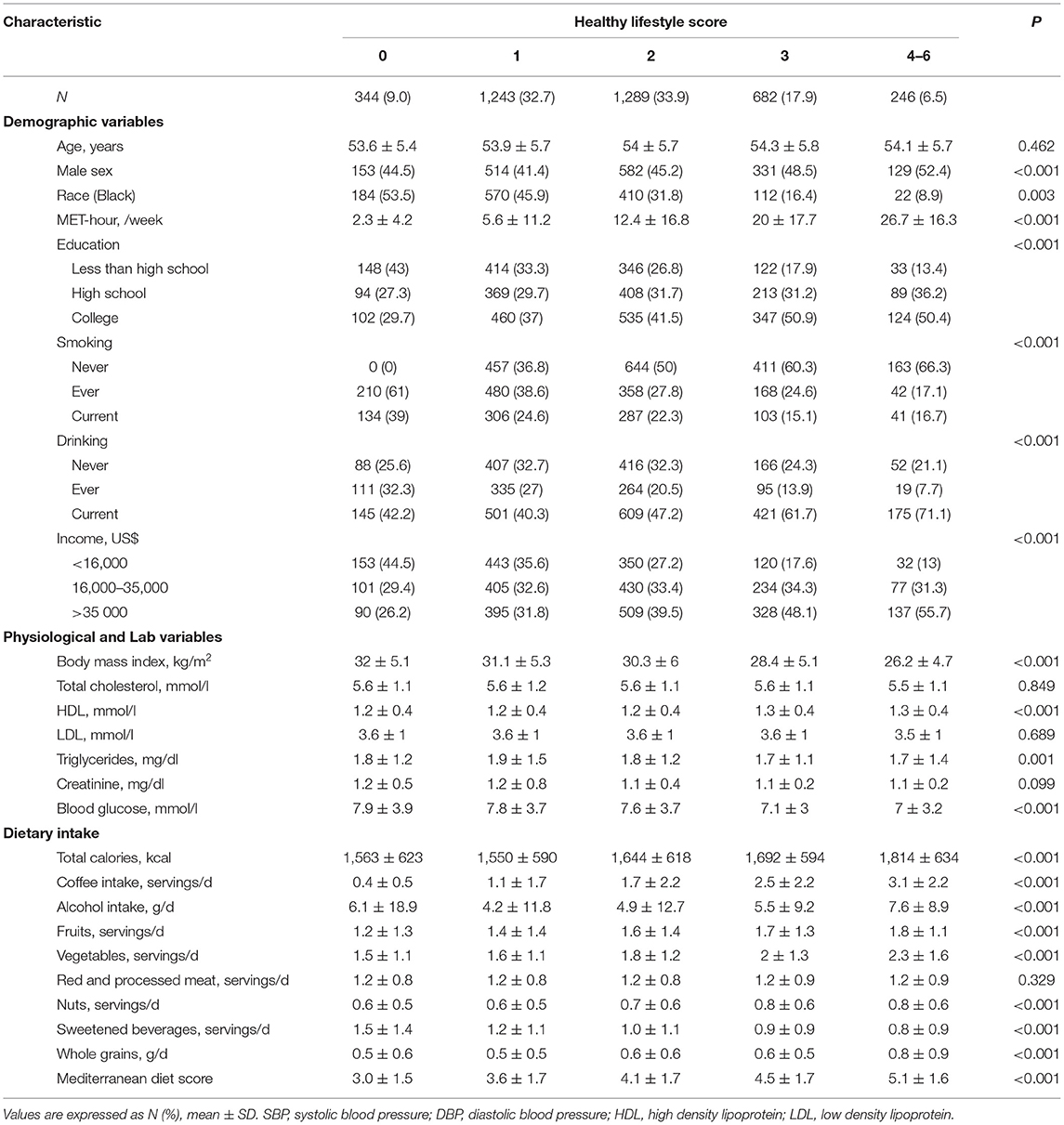
There were 3,804 participants with diabetes included in our prospective analyses. The diabetics with HLS of 1, 2, 3, 4, 5, and 6 were as follows, respectively: 344 (9.0%), 1,243 (32.7%), 1,289 (33.9%), 682 (17.9%), 210 (5.5%), 33 (0.9%), and 3 (0.1%). As the proportion of diabetics in the population with HLS of 5 or 6 is too low to analyze, the population was divided into five groups with an HLS of 0, 1, 2, 3, and 4–6, respectively. Baseline (1987–1989) characteristics are described and compared in Table 1. Participants with a higher HLS were more likely to be male and Caucasian, have lower levels of biomarkers for CV disease, have more healthy behaviors such as physical activity and a healthy diet (i.e., more vegetables, fruits, nuts, and whole grains, less beverages, and higher Mediterranean diet score); moderate drinking and coffee consumption, keeping fit, and never smoking (P < 0.001 for all).
Healthy Lifestyle Pattern and Adverse Outcome
During the 26 years of follow-up, we documented 1,881 (49.4%), 683 (18.0%), and 1,600 (42.0%) cases of all-cause mortality, CV mortality, and CV events among diabetic participants. Participants with a higher HLS had a lower incidence of these adverse outcomes (P < 0.001, Figure 1). In risk-factors adjusted in the Cox regression model (Table 2), compared to participants without any healthy lifestyle factors, participants with only 2 healthy lifestyle factors had a significantly lower risk of all-cause mortality (HR = 0.811, 95% CI: 0.687–0.957, P = 0.013), CV mortality (HR = 0.744, 95% CI: 0.576–0.962, P = 0.024), and CV events (HR = 0.789, 95% CI: 0.661–0.943, P = 0.009); while participants with an HLS ≥ 4 demonstrated a significant lower HR of all-cause mortality (0.593, 95% CI: 0.389–0.905, P = 0.015), CV mortality (0.593, 95% CI: 0.389–0.905, P = 0.015), and CV events (0.677, 95% CI: 0.511–0.896, P = 0.006) than an HLS of 0.
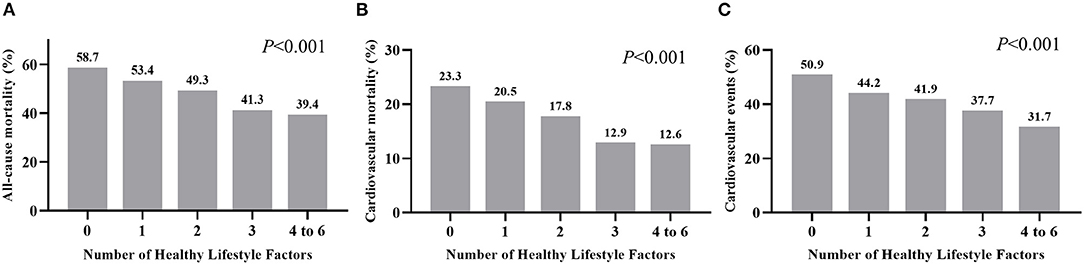
Figure 1. Incidence of all-cause mortality (A), cardiovascular mortality (B), and cardiovascular events (C) grouped by number of healthy factors for diabetic individuals.
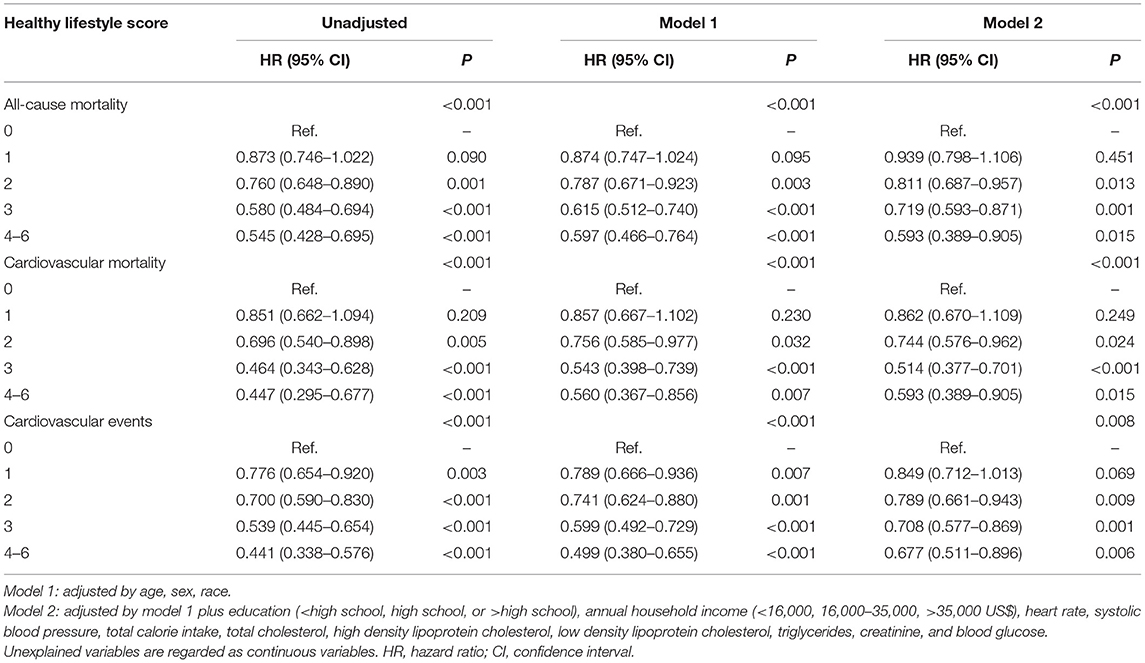
Table 2. Adjusted hazard ratios for the association of healthy lifestyle score with all-cause mortality, cardiovascular mortality, and cardiovascular events.
Subgroup Analysis
Figure 2 shows subgroup analyses stratified by age, sex, and race. As is evident, there was no interaction for age and race, with similar risk of all-cause mortality (Figure 2), CV mortality (Supplementary Figure 1), and CV events (Supplementary Figure 2) between younger (<51 years) vs. older adults (≥51 years) and white vs. black individuals. However, the risk of all three outcomes was affected by sex (P < 0.05 for interaction), where the association between higher HLS and risk reduction was significant for women.
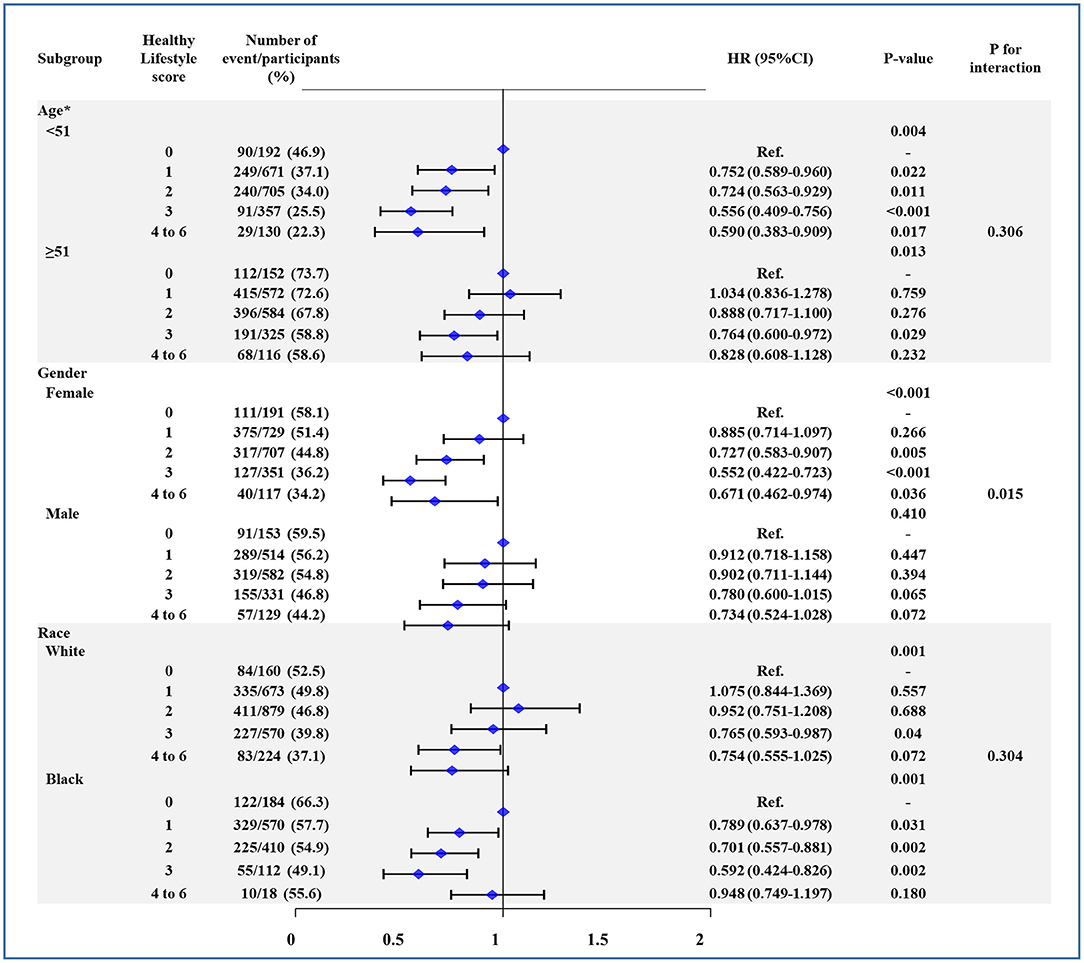
Figure 2. The association between healthy lifestyle score (HLS) and all-cause mortality in subgroup of age, sex, and race. Age was divided by median. Models were adjusted by age, sex, center-race, education (< high school, high school, or >high school), annual household income (<16,000; 16,000–35,000; >35,000 US$), heart rate, systolic blood pressure, total caloric intake, total cholesterol, high density lipoprotein cholesterol, low density lipoprotein cholesterol, triglycerides, creatinine, and blood glucose. CI, confidence interval. HR, hazard ratio.
Factors Analysis Between Men and Women
In order to explore the differences between men and women and the association between HLS and adverse events, we analyzed the factors of six binary variables of HLS. As Table 3 shows, in risk-factors adjusted Cox regression model, six binary variables of HLS were all significantly associated with all-cause mortality for women. Meanwhile, only a healthy diet, physical activity, and never smoking had a significantly protective effect against all-cause mortality (Table 3). Moreover, the associations of normal body weight with all-cause mortality were stronger for women than men (P = 0.002 for interaction). Given the unexpected result that men with normal weight (WHO standard: BMI is 18.5–25 kg/m2) did not have a lower risk of all-cause mortality, we conducted a linear spline between the association of BMI and the adjusted HR for all-cause mortality (Figure 3). There was a U-shape and a linear association between BMI and HR of all-cause mortality in men and women, respectively. For men, participants with the lowest risk of all-cause death were overweight (WHO standard: BMI is 25–30 kg/m2).
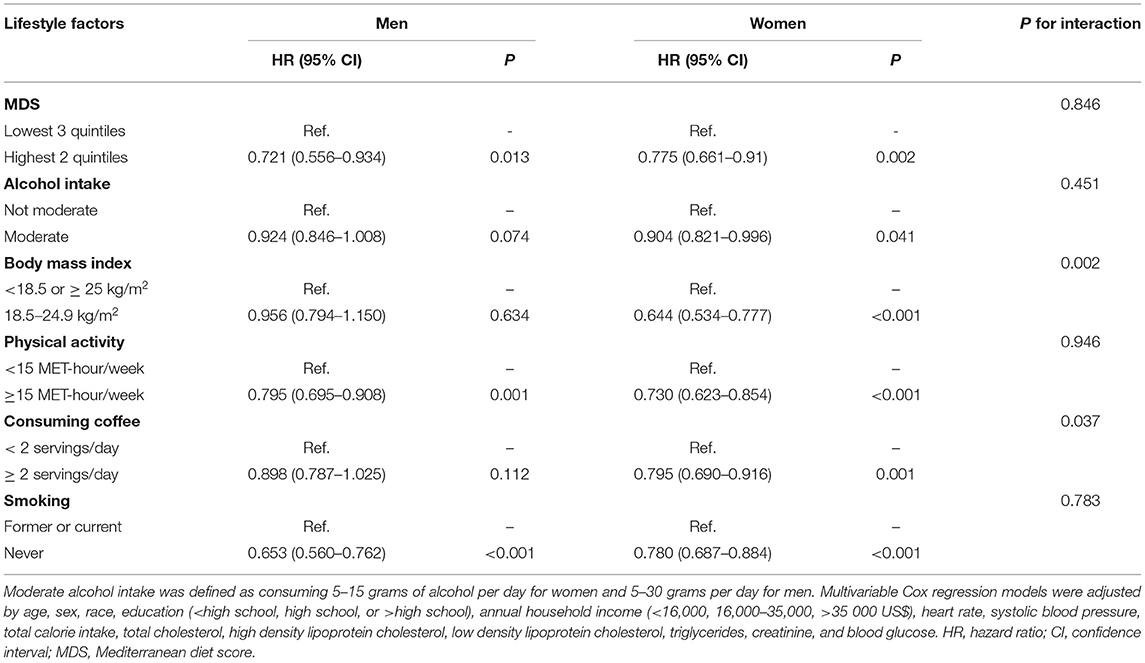
Table 3. Multivariable Cox regression model for association of healthy lifestyle factors and all-cause mortality for man and woman.
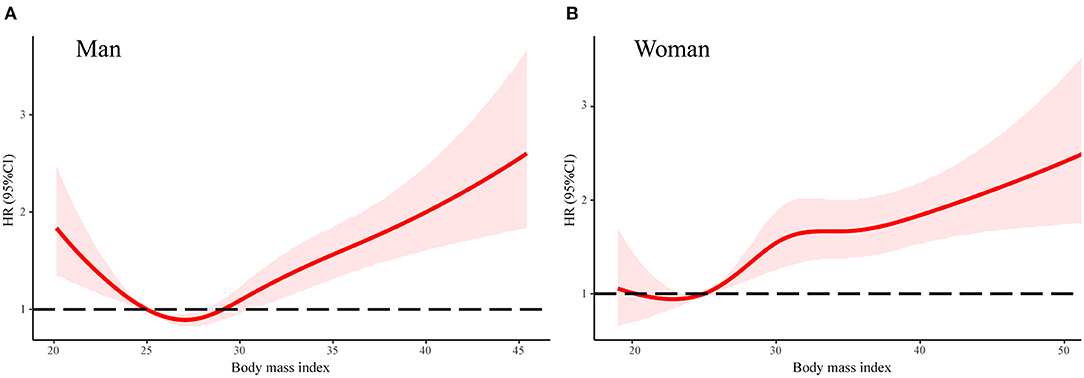
Figure 3. The association between body mass index and all-cause mortality in linear splines curve for men (A) and women (B). Models were adjusted by age, sex, center-race, education (< high school, high school, or >high school), annual household income (<16,000; 16,000–35,000; >35,000 US$), heart rate, systolic blood pressure, total caloric intake, total cholesterol, high density lipoprotein cholesterol, low density lipoprotein cholesterol, triglycerides, creatinine, and blood glucose. CI, confidence interval. HR, hazard ratio.
Discussion
In this community-based cohort study, a healthy lifestyle—including a healthy diet (reflected by the highest two quintiles of the Mediterranean diet scores), never smoking, moderate alcohol intake (5–15 g for men; 5–30 g for women), regular coffee consumption (≥2 cups/d), physical activity (≥15 MET-hour/week), and normal body weight (BMI: 18.5–24.9 kg/m2)—was associated with a lower risk of all-cause mortality, CV mortality, and CV events among diabetic participants. This association also suggests that participants with a higher HLS had a gradually decreased (81 to 59%) risk of adverse outcomes. Therefore, assuming causal relations, the risk of these three adverse outcomes might be reduced by one fifth to two fifths when all diabetic participants have an HLS ≥ 2.
In the subgroup analysis, we found that an association between HLS and adverse events of diabetic patients were consistent with age and race (Caucasian vs. African Americans), but this association was stronger in women than in men. Analyzing the difference in the distribution of single lifestyle factors might explain this phenomenon. As shown, in risk-factors adjusted in the Cox regression model, all six healthy lifestyle factors are significantly associated with all-cause mortality in women, while only healthy diet, physical activity, and never smoking had significant protective effects against mortality in men. Moreover, it appears as though men cannot benefit by having a normal body weight. According to our analysis of linear splines, there was a non-linear (U-shape) association between BMI and risk of all-cause mortality in men. Like several studies have shown, the lowest risk group for men with diabetes mellitus was the overweight group (33–35). In fact, there is growing evidence that overweight patients with CVD survive longer than their normal weight counterparts, an effect called the “obesity paradox” (36). Inconsistently, some studies did not support the obesity paradox for diabetic individuals (37, 38). Moreover, according to previous studies, women with a low-risk lifestyle had a lower risk of cancer, CV disease, diabetes, and mortality than men; however, the reasons for this disparity are not totally clear (23, 39–41).
It is well-established that a healthy lifestyle related to a lower risk of CVD and mortality in most healthy people (24, 42). Regarding associations between lifestyle and health outcomes among diabetic patients, previous studies largely focused on total mortality (17–19). In the current study, we addressed a major limitation in previous studies by the average of 3-year repeated measurements of dietary and lifestyle factors to capture potential changes of lifestyle practices. Moreover, we evaluated the associations of an overall 6-factor healthy lifestyle (different from previous 4 or 5-factor healthy lifestyle and usually did not include coffee consuming) and CV events (including myocardial infarction, fatal coronary heart disease, stroke, or hospitalized heart failure) and CV mortality, in addition to all-cause mortality. Our findings were in line with an observational study which included 867 newly diagnosed diabetic patients, in which a greater number of healthy behavior changes within the 1st year of diagnosis were associated with a lower risk of CV outcomes (43).
Substantial evidence supports the benefits of lifestyle modification for the prevention of all-cause mortality and CV events. Moderate alcohol consumption, never or quitting smoking, physical activity, regular consumption of coffee, staying fit, and following a Mediterranean Diet have been reported to reduce the risk of diabetes and improve CV health in patients with diabetes (22, 26, 44–46). All of these healthy lifestyle factors were related to insulin resistance (47–52), which is a key pathophysiological mechanism for increasing the morbidity and mortality of diabetes. Further, the modified lifestyle factors have been shown to effectively alter the lipid profile and, thus, could alleviate pathways related to CV disease (53–56).
Since the 1980's—especially since 2013—the American Heart Association, the American College of Cardiology, and the European Society of Cardiology have launched clinical practice guidelines on lifestyle management to prevent CV disease and diabetes, as well as improving CV health (57). This study provides additional evidence that adherence to a comprehensive healthy lifestyle could alleviate the risk of CVD and mortality for people with diabetes. Thus, HLS may serve as a useful and simple tool to screen for high risk of CV events and mortality in primary prevention. Through risk stratification, diabetic individual lifestyle intervention and risk management could be further adopted by healthcare professionals.
Limitations
There are some limitations which should be mentioned. First, although this study came from the ARIC with a large sample size that has been regularly updated for over 30 years, these results should be further confirmed by other prospective cohorts and randomized controlled studies. Second, only 1% of diabetic participants had an HLS of 5 to 6 (this is significantly lower than the general population in other studies) (11, 23); thus, analysis for these populations was limited. Third, the detailed and repeated measurements of lifestyle factors in this study enabled us to take dynamic changes over time into account, but it was also limited by its reliance on self-reported lifestyle factors. Therefore, measurement errors are inevitable. Fourth, some potential confounding factors, such as medication, health insurance, diabetic complications, and quality of treatment, may not have been adequately adjusted. Fifth, top two quintiles of healthy diet score were usually defined as healthy diet for general or diabetic population in previous studies (9, 18, 23, 24), but there is no evidence that any diet score greater than a certain point can be classified as healthy diet for diabetic individuals. Finally, the cardiac metabolic risk of type 1 diabetes is different from that of type 2 diabetes, but they are indistinguishable according to the definition of diabetes in ARIC study.
Conclusion
In this cohort, adherence to an overall healthy lifestyle was associated with a lower risk of CV events and mortality in diabetic individuals. Given the increasing prevalence of diabetes and continuous high risk of adverse outcomes, our findings suggest that the promotion of a healthy lifestyle would help reduce the gradually increasing healthcare burdens via non-drug interventions. Future research should measure a more accurate range of healthy lifestyle factors for diabetic individuals, such as alcohol consumption and BMI.
Data Availability Statement
ARIC data are available through the NIH NHLBI-sponsored Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) at https://biolincc.nhlbi.nih.gov.
Ethics Statement
The studies involving human participants were reviewed and approved by The institutional review committee at each site approved the study. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
DL, YJ, and RZ designed the research. DL, YJ, QW, and YML analyzed the data. DL and YJ wrote the first draft of the manuscript. JY, YL, FL, XL, ZZ, and ZW reviewed the manuscript and provided critical scientific input. RZ holds the primary responsibility for the final content of the manuscript. All the authors have approved the final draft of the manuscript.
Funding
This work was supported financially by grants from the Sichuan Science and Technology Program (Nos. 2020YFS0154, 2020YFSY0014), 1·3·5 Project for Disciplines of Excellence-Clinical Research Incubation Project, Sichuan University West China Hospital (Nos. 2018HXFH001, 2018HXFH027, and 2020HXFH050), Sichuan University West China Nursing Discipline Development Special Fund Project (HXHL20046, HXHL19023), Chengdu Science and Technology Bureau (2019-YF05-00322-SN), National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (Z20191009).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the staff and participants of the ARIC study, and BioLINCC for their important contributions.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.698608/full#supplementary-material
Supplementary Figure 1. The association between healthy lifestyle score (HLS) and cardiovascular mortality in subgroup of age, sex, and race. The detailed description is the same as Figure 1.
Supplementary Figure 2. The association between healthy lifestyle score (HLS) and cardiovascular events in subgroup of age, sex, and race. The detailed description is the same as Figure 2.
Abbreviations
ARIC, Atherosclerosis Risk in Communities; BMI, body mass index; CVD, cardiovascular disease; FFQ, Food Frequency questionnaire; HLS, healthy lifestyle score.
References
1. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. (2020) 41:255–323 doi: 10.1093/eurheartj/ehz486
2. Das SR, Everett BM, Birtcher KK, Brown JM, Januzzi JL, Kalyani RR, et al. 2020 Expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American college of cardiology solution set oversight committee. J Am Coll Cardiol. (2020) 76:1117–45 doi: 10.1016/j.jacc.2020.05.037
3. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. (2010) 375:2215–22. doi: 10.1016/S0140-6736(10)60484-9
4. Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the US in 2002. Diabetes Care. (2003) 26:917–32. doi: 10.2337/diacare.26.3.917
5. Paterson AD, Rutledge BN, Cleary PA, Lachin JM, Crow RS. The effect of intensive diabetes treatment on resting heart rate in type 1 diabetes: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care. (2007) 30:2107–12. doi: 10.2337/dc06-1441
6. Napoli R, Formoso G, Piro S, Targher G, Consoli A, Purrello F. Management of type 2 diabetes for prevention of cardiovascular disease. An expert opinion of the Italian Diabetes Society. Nutr Metab Cardiovasc Dis. (2020) 30:1926–36. doi: 10.1016/j.numecd.2020.07.012
7. Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. (2014) 63:2960–84. doi: 10.1016/j.jacc.2013.11.003
8. Lv J, Yu C, Guo Y, Bian Z, Yang L, Chen Y, et al. Adherence to a healthy lifestyle and the risk of type 2 diabetes in Chinese adults. Int J Epidemiol. (2017) 46:1410–20. doi: 10.1093/ije/dyx074
9. Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. (2001) 345:790–7. doi: 10.1056/NEJMoa010492
10. Mozaffarian D, Kamineni A, Carnethon M, Djoussé L, Mukamal KJ, Siscovick D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: the cardiovascular health study. Arch Intern Med. (2009) 169:798–807. doi: 10.1001/archinternmed.2009.21
11. Lv J, Yu C, Guo Y, Bian Z, Yang L, Chen Y, et al. Adherence to healthy lifestyle and cardiovascular diseases in the Chinese population. J Am Coll Cardiol. (2017) 69:1116–25. doi: 10.1016/j.jacc.2016.11.076
12. Chiuve SE, Rexrode KM, Spiegelman D, Logroscino G, Manson JE, Rimm EB. Primary prevention of stroke by healthy lifestyle. Circulation. (2008) 118:947–54. doi: 10.1161/CIRCULATIONAHA.108.781062
13. Chomistek AK, Chiuve SE, Eliassen AH, Mukamal KJ, Willett WC, Rimm EB. Healthy lifestyle in the primordial prevention of cardiovascular disease among young women. J Am Coll Cardiol. (2015) 65:43–51. doi: 10.1016/j.jacc.2014.10.024
14. Li Y, Pan A, Wang DD, Liu X, Dhana K, Franco OH, et al. Impact of healthy lifestyle factors on life expectancies in the US population. Circulation. (2018) 138:345–55. doi: 10.1161/CIRCULATIONAHA.117.032047
15. Vetter C, Scheer F. A healthy lifestyle - reducing T2DM risk in shift workers? Nat Rev Endocrinol. (2019) 15:194–6. doi: 10.1038/s41574-019-0164-z
16. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart association task force on clinical practice guidelines. Circulation. (2019) 140:e563–95. doi: 10.1161/CIR.0000000000000677
17. Nöthlings U, Ford ES, Kröger J, Boeing H. Lifestyle factors and mortality among adults with diabetes: findings from the European - prospective investigation into cancer and nutrition potsdam study*. J Diabetes. (2010) 2:112–7. doi: 10.1111/j.1753-0407.2010.00069.x
18. Patel YR, Gadiraju TV, Gaziano JM, Djoussé L. Adherence to healthy lifestyle factors and risk of death in men with diabetes mellitus: The Physicians' Health Study. Clin Nutr. (2018) 37:139–43. doi: 10.1016/j.clnu.2016.11.003
19. Lin CC, Li CI, Liu CS, Lin WY, Fuh MM, Yang SY, et al. Impact of lifestyle-related factors on all-cause and cause-specific mortality in patients with type 2 diabetes: the Taichung Diabetes Study. Diabetes Care. (2012) 35:105–12. doi: 10.2337/dc11-0930
20. Willett WC, Reynolds RD, Cottrell-Hoehner S, Sampson L, Browne ML. Validation of a semi-quantitative food frequency questionnaire: comparison with a 1-year diet record. J Am Diet Assoc. (1987) 87:43–7.
21. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. (2003) 348:2599–608. doi: 10.1056/NEJMoa025039
22. Becerra-Tomás N, Blanco Mejía S, Viguiliouk E, Khan T, Kendall CWC, Kahleova H, et al. Mediterranean diet, cardiovascular disease and mortality in diabetes: A systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Crit Rev Food Sci Nutr. (2020) 60:1207–27. doi: 10.1080/10408398.2019.1565281
23. Li Y, Schoufour J, Wang DD, Dhana K, Pan A, Liu X, et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: prospective cohort study. BMJ. (2020) 368:l6669. doi: 10.1136/bmj.l6669
24. van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ. (2008) 337:a1440. doi: 10.1136/bmj.a1440
25. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. (2012) 142:1009–18. doi: 10.3945/jn.111.157222
26. Komorita Y, Iwase M, Fujii H, Ohkuma T, Ide H, Jodai-Kitamura T, et al. Additive effects of green tea and coffee on all-cause mortality in patients with type 2 diabetes mellitus: the Fukuoka Diabetes Registry. BMJ Open Diabetes Res Care. (2020) 8:1252. doi: 10.1136/bmjdrc-2020-001252
27. O'Keefe JH, Bhatti SK, Patil HR, DiNicolantonio JJ, Lucan SC, Lavie CJ. Effects of habitual coffee consumption on cardiometabolic disease, cardiovascular health, and all-cause mortality. J Am Coll Cardiol. (2013) 62:1043–51. doi: 10.1016/j.jacc.2013.06.035
28. Richardson MT, Ainsworth BE, Wu HC, Jacobs DR Jr, Leon AS. Ability of the Atherosclerosis Risk in Communities (ARIC)/Baecke Questionnaire to assess leisure-time physical activity. Int J Epidemiol. (1995) 24:685–93. doi: 10.1093/ije/24.4.685
29. WHO Guidelines Approved by the Guidelines Review Committee. WHO Guidelines on Physical Activity and Sedentary Behaviour. Geneva: World Health Organization © World Health Organization 2020. (2020).
30. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. (2004) 363:157–63. doi: 10.1016/S0140-6736(03)15268-3
31. Hussain A, Sun W, Deswal A, de Lemos JA, McEvoy JW, Hoogeveen RC, et al. Association of NT-ProBNP, blood pressure, and cardiovascular events: The ARIC study. J Am Coll Cardiol. (2021) 77:559–71. doi: 10.1016/j.jacc.2020.11.063
32. Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. (1999) 30:736–43. doi: 10.1161/01.STR.30.4.736
33. Zaccardi F, Dhalwani NN, Papamargaritis D, Webb DR, Murphy GJ, Davies MJ, et al. Nonlinear association of BMI with all-cause and cardiovascular mortality in type 2 diabetes mellitus: a systematic review and meta-analysis of 414,587 participants in prospective studies. Diabetologia. (2017) 60:240–8. doi: 10.1007/s00125-016-4162-6
34. Costanzo P, Cleland JG, Pellicori P, Clark AL, Hepburn D, Kilpatrick ES, et al. The obesity paradox in type 2 diabetes mellitus: relationship of body mass index to prognosis: a cohort study. Ann Intern Med. (2015) 162:610–8. doi: 10.7326/M14-1551
35. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. (2013) 309:71–82. doi: 10.1001/jama.2012.113905
36. Morse SA, Gulati R, Reisin E. The obesity paradox and cardiovascular disease. Curr Hypertens Rep. (2010) 12:120–6. doi: 10.1007/s11906-010-0099-1
37. Carnethon MR, De Chavez PJ, Biggs ML, Lewis CE, Pankow JS, Bertoni AG, et al. Association of weight status with mortality in adults with incident diabetes. JAMA. (2012) 308:581–90. doi: 10.1001/jama.2012.9282
38. Sasaki A, Horiuchi N, Hasegawa K, Uehara M. Mortality and causes of death in type 2 diabetic patients. A long-term follow-up study in Osaka District, Japan. Diabetes Res Clin Pract. (1989) 7:33–40. doi: 10.1016/0168-8227(89)90042-9
39. O'Doherty MG, Cairns K, O'Neill V, Lamrock F, Jørgensen T, Brenner H, et al. Effect of major lifestyle risk factors, independent and jointly, on life expectancy with and without cardiovascular disease: results from the Consortium on Health and Ageing Network of Cohorts in Europe and the United States (CHANCES). Eur J Epidemiol. (2016) 31:455–68. doi: 10.1007/s10654-015-0112-8
40. Stenholm S, Head J, Kivimäki M, Kawachi I, Aalto V, Zins M, et al. Smoking, physical inactivity and obesity as predictors of healthy and disease-free life expectancy between ages 50 and 75: a multicohort study. Int J Epidemiol. (2016) 45:1260–70. doi: 10.1093/ije/dyw126
41. Nyberg ST, Batty GD, Pentti J, Virtanen M, Alfredsson L, Fransson EI, et al. Obesity and loss of disease-free years owing to major non-communicable diseases: a multicohort study. Lancet Public Health. (2018) 3:e490–7. doi: 10.1016/S2468-2667(18)30139-7
42. Loef M, Walach H. The combined effects of healthy lifestyle behaviors on all cause mortality: a systematic review and meta-analysis. Prev Med. (2012) 55:163–70. doi: 10.1016/j.ypmed.2012.06.017
43. Long GH, Cooper AJ, Wareham NJ, Griffin SJ, Simmons RK. Healthy behavior change and cardiovascular outcomes in newly diagnosed type 2 diabetic patients: a cohort analysis of the ADDITION-Cambridge study. Diabetes Care. (2014) 37:1712–20. doi: 10.2337/dc13-1731
44. Pan A, Wang Y, Talaei M, Hu FB. Relation of smoking with total mortality and cardiovascular events among patients with diabetes mellitus: a meta-analysis and systematic review. Circulation. (2015) 132:1795–804. doi: 10.1161/CIRCULATIONAHA.115.017926
45. Polsky S, Akturk HK. Alcohol consumption, diabetes risk, and cardiovascular disease within diabetes. Curr Diab Rep. (2017) 17:136. doi: 10.1007/s11892-017-0950-8
46. Hu G, Jousilahti P, Barengo NC, Qiao Q, Lakka TA, Tuomilehto J. Physical activity, cardiovascular risk factors, and mortality among Finnish adults with diabetes. Diabetes Care. (2005) 28:799–805. doi: 10.2337/diacare.28.4.799
47. Barazzoni R, Gortan Cappellari G, Ragni M, Nisoli E. Insulin resistance in obesity: an overview of fundamental alterations. Eat Weight Disord. (2018) 23:149–57. doi: 10.1007/s40519-018-0481-6
48. Haj Mouhamed D, Ezzaher A, Neffati F, Douki W, Gaha L, Najjar MF. Effect of cigarette smoking on insulin resistance risk. Ann Cardiol Angeiol. (2016) 65:21–5. doi: 10.1016/j.ancard.2014.12.001
49. Jacobs S, Boushey CJ, Franke AA, Shvetsov YB, Monroe KR, Haiman CA, et al. A priori-defined diet quality indices, biomarkers and risk for type 2 diabetes in five ethnic groups: the Multiethnic Cohort. Br J Nutr. (2017) 118:312–20. doi: 10.1017/S0007114517002033
50. Zhang D, Liu X, Liu Y, Sun X, Wang B, Ren Y, et al. Leisure-time physical activity and incident metabolic syndrome: a systematic review and dose-response meta-analysis of cohort studies. Metabolism. (2017) 75:36–44. doi: 10.1016/j.metabol.2017.08.001
51. Schrieks IC, Heil AL, Hendriks HF, Mukamal KJ, Beulens JW. The effect of alcohol consumption on insulin sensitivity and glycemic status: a systematic review and meta-analysis of intervention studies. Diabetes Care. (2015) 38:723–32. doi: 10.2337/dc14-1556
52. Loureiro LMR, Reis CEG, da Costa THM. Effects of Coffee Components on Muscle Glycogen Recovery: A Systematic Review. Int J Sport Nutr Exerc Metab. (2018) 28:284–93. doi: 10.1123/ijsnem.2017-0342
53. Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ. (1999) 319:1523–8. doi: 10.1136/bmj.319.7224.1523
54. Silva RC, Diniz Mde F, Alvim S, Vidigal PG, Fedeli LM, Barreto SM. Physical activity and lipid profile in the ELSA- Brasil Study. Arq Bras Cardiol. (2016) 107:10–9. doi: 10.5935/abc.20160091
55. Willett W, Hennekens CH, Castelli W, Rosner B, Evans D, Taylor J, et al. Effects of cigarette smoking on fasting triglyceride, total cholesterol, and HDL-cholesterol in women. Am Heart J. (1983) 105:417–21. doi: 10.1016/0002-8703(83)90358-7
56. Cai L, Ma D, Zhang Y, Liu Z, Wang P. The effect of coffee consumption on serum lipids: a meta-analysis of randomized controlled trials. Eur J Clin Nutr. (2012) 66:872–7. doi: 10.1038/ejcn.2012.68
57. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. (2019) 140:e596–646. doi: 10.1161/CIR.0000000000000678
Keywords: diabetes, healthy lifestyle, mortality, cardiovascular events and mortality, cohort study
Citation: Li D, Jia Y, Yu J, Liu Y, Li F, Liu Y, Wu Q, Liao X, Zeng Z, Wan Z and Zeng R (2021) Adherence to a Healthy Lifestyle and the Risk of All-Cause Mortality and Cardiovascular Events in Individuals With Diabetes: The ARIC Study. Front. Nutr. 8:698608. doi: 10.3389/fnut.2021.698608
Received: 21 April 2021; Accepted: 09 June 2021;
Published: 05 July 2021.
Edited by:
Anne Marie Minihane, University of East Anglia, United KingdomReviewed by:
Todd Hagobian, California Polytechnic State University, United StatesMichelle Weech, University of Reading, United Kingdom
Copyright © 2021 Li, Jia, Yu, Liu, Li, Liu, Wu, Liao, Zeng, Wan and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Zeng, emVuZ3J1aV8wNTI0QDEyNi5jb20=
 Dongze Li
Dongze Li Yu Jia1
Yu Jia1 Xiaoyang Liao
Xiaoyang Liao