- Department of Nephrology, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu, China
Aims: We aimed to assess the association between dietary inflammation index (DII) with parathyroid hormone (PTH) and hyperparathyroidism (HP) in adults with/without chronic kidney disease (CKD).
Methods: Data were obtained from the 2003–2006 National Health and Nutrition Examination Survey (NHANES). The participants who were <18 years old, pregnant, or missing the data of DII, PTH, and CKD were excluded. DII was calculated based on a 24-h dietary recall interview for each participant. Weighted multivariable regression analysis and subgroup analysis were conducted to estimate the independent relationship between DII with PTH and the HP in the population with CKD/non-CKD.
Results: A total of 7,679 participants were included with the median DII of −0.24 (−2.20 to 1.80) and a mean PTH level of 43.42 ± 23.21 pg/ml. The average PTH was 45.53 ± 26.63 pg/ml for the participants in the highest tertile group compared with 41.42 ± 19.74 pg/ml in the lowest tertile group (P < 0.0001). The rate of HP was 11.15% overall, while the rate in the highest DII tertile was 13.28 and 8.60% in the lowest DII tertile (P < 0.0001). The participants with CKD tended to have higher PTH levels compared with their counterparts (61.23 ± 45.62 vs. 41.80 ± 19.16 pg/ml, P < 0.0001). A positive association between DII scores and PTH was observed (β = 0.46, 95% CI: 0.25, 0.66, P ≤ 0.0001), and higher DII was associated with an increased risk of HP (OR = 1.05, 95% CI: 1.02, 1.08, P = 0.0023). The results from subgroup analysis indicated that this association was similar in the participants with different renal function, gender, age, BMI, hypertension, and diabetes statuses and could also be appropriate for the population with CKD.
Conclusions: Higher consumption of a pro-inflammatory diet appeared to cause a higher PTH level and an increased risk of HP. Anti-inflammatory dietary management may be beneficial to reduce the risk of HP both in the population with and without CKD.
Introduction
Parathyroid hormone (PTH) is a single-stranded peptide hormone, containing 84 amino acids, which are synthesized and secreted by the chief cells of the parathyroid gland, with the main function of increasing the serum Ca2+ and decreasing the serum phosphorus levels (1). The secretion of PTH is also mainly regulated by th zvbe concentration of serum Ca2+ and phosphorus (2, 3). Serum Ca2+ regulates PTH secretion through the interaction with calcium-sensitive receptors (CASR) on the surface of parathyroid cells (4, 5). Serum phosphorus enhances the stability of PTH mRNA and stimulates the proliferation of parathyroid cells to increase the secretion of PTH both directly and indirectly (3, 6). Bone and kidney are the main target organs of PTH (7–9). For a variety of pathological reasons, the parathyroid glands can secrete excessive PTH and cause hyperparathyroidism (HP), which can be classified as primary, secondary, and tertiary (10). HP appeared to be associated with an increased risk of poor clinical outcomes and death, which is often observed in patients with chronic kidney disease (CKD) (11).
Chronic kidney disease refers to a chronic clinical condition of renal, structural, and functional disorders and is characterized by a higher inflammation status (12). Recent studies have revealed the global prevalence of CKD to be about 10%, as well as the increasing disease burdens (13–15). In patients with CKD, abnormal regulation in calcium, phosphorus, vitamin D, and PTH is accompanied by a decline in renal function, which could lead to secondary HP, which is associated with the increased risk of fracture, cardiovascular disease, and death (16–18). Thus, the management of the PTH level in patients with CKD is of great significance.
The association between inflammation and PTH remains unclear. Several animal and human studies indicated that the PTH level may be associated with inflammation (19–21). Previous studies observed a decreased inflammation status after parathyroidectomy in patients with HP (22, 23). However, the inflammation level after parathyroidectomy varies widely among studies; both increased (24) and even no-change results (25) have been reported before. Chen et al. (19) found that inflammatory markers, including C-reactive protein (CRP), red cell distribution width (RDW), and platelet-to-lymphocyte ratio (PLR) levels increased with increasing serum PTH concentration in the US adults, indicating a positive relationship between inflammation and PTH. In in vitro studies, several inflammatory cytokines, such as interleukin-8 (IL-8), tumor necrosis factor-α (TNF-α), etc., have been proved to enhance PTH synthesis and secretion through nuclear factor-κB (NF-κB) and affect CASR transcription (26–28). It could be inferred that the consumption of an inflammatory diet may also have an impact on the PTH. Dietary inflammatory index (DII) was a literature-derived and population-based scoring system designed to evaluate the inflammatory potential of diets (29). A positive value for DII corresponded to an pro-inflammatory diet, and a negative value for DII corresponded to an anti-inflammatory diet. The higher scores suggested a more pro-inflammatory effect, and the more negative scores suggested a more anti-inflammatory effect. In fact, previous studies indicated a significant association between DII and the risk of various cancers (30, 31), obesity (32), cardiovascular disease (33), sarcopenia (34), and so on. However, the association between the dietary inflammatory potential and PTH has not been reported before.
In this study, we aimed to assess the impact of DII on PTH and HP, using data from the National Health and Nutrition Examination Survey (NHANES). We hypothesized that the increased consumption of pro-inflammatory diet would be associated with higher PTH levels and increased risk of HP. In addition, with regard to the fact that patients with CKD may have more elevated PTH levels than the population with non-CKD, we further examined this association in subgroups stratified by renal function.
Materials and Methods
Study Population
In this study, we obtained data from the NHANES. NHANES is a program of studies administered by the National Center for Health Statistics (NCHS), part of the U.S. Centers for Disease Control and Prevention (CDC), aimed to assess the health and nutrition status of the U.S. population through interviews, physical examinations, and laboratory tests. The NHANES is conducted on a 2-year cycle, and the data are still being updated. Because this study adopted a stratified multistage probability sampling method, the included samples had good representativeness (35). All NHANES data are publicly available at www.cdc.gov/nchs/nhanes/.
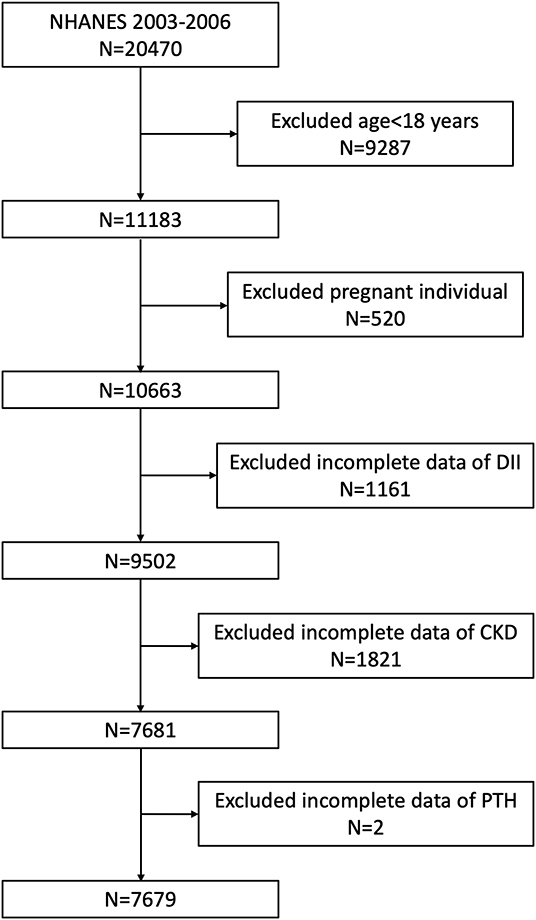
This study was based on the data from two 2-year NHANES surveys from 2003 to 2006. A total of 20,470 participants were enrolled at first; after the exclusion of individuals aged <18 years old (n = 9,287), who were pregnant (n = 520), missing the dietary data relating to DII (n = 1,161), missing the data of diagnosing CKD (n = 1,821), and missing the data of PTH (n = 2), 7,679 participants were included in our final analysis (Figure 1).
The NCHS Ethics Review Board granted the human subject approval for the conduction of NHANES, and written informed consent was obtained from all the participants.
Exposure and Outcome Definitions
Dietary inflammation index was designed as an exposure variable. The dietary data in NHANES were obtained by a 24-h dietary recall interview at the mobile examination center (MEC) (36), which have been validated by the Nutrition Methodology Working Group before (37). The data of 24-h dietary recall interviews were used to calculate the DII scores for each individual, and it could quantify the inflammatory potential of diets. A higher positive DII score indicated a pro-inflammatory diet, and a lower negative DII score indicated an anti-inflammatory diet (29). A total of 27 food parameters were available in NHANES and were used for the calculation of DII, including anti-inflammatory food parameters (alcohol, β-carotene, fibers, folic acid, magnesium, zinc, selenium, vitamin A, vitamin B-6, vitamin C, vitamin E, monounsaturated fatty acid, niacin, riboflavin, n-3 fatty acid, n-6 fatty acid, polyunsaturated fatty acid, caffeine, and thiamin), and pro-inflammatory food parameters (cholesterol, carbohydrates, energy, fats, iron, vitamin B-12, protein, and saturated fat). Studies have shown that the predictive ability was not affected when <30 dietary parameters were used to calculate DII scores (38–40). DII was analyzed as a continuous variable, and the participants were divided into tertiles from the total sample for further analysis.
The PTH level and HP were designed as outcome variables. The Elecsys 1010 analyzer (Roche Diagnostics) was used to determine the serum intact PTH level. The Elecsys 1010 analyzer was a fully automatic run-oriented analyzer system for the determination of immunological tests, using the ECL/Origen electrochemiluminescent process. All components and reagents for routine analysis were integrated into the analyzer. PTH was measured on the Elecsys 1010, using a sandwich principle. There was no difference in the equipment, lab method, or lab site between the combined two survey cycles in our analysis. HP was defined as PTH > 65 pg/ml according to previous studies (1, 41). The detailed process of measuring PTH was available at www.cdc.gov/nchs/nhanes/.
Other Study Variables
Baseline variables in this study included age (years), gender, race, educational level, systolic blood pressure (mmHg), diastolic blood pressure (mmHg), body mass index (kg/m2), serum glucose (mg/dl), serum phosphorus (mg/dl), serum iron (ug/dl), serum CRP (mg/dl), serum 25 (OH) D (nmol/L), serum calcium (mg/dl), urinary creatinine (mg/dl), urinary albumin (mg/L), urinary creatinine (mg/dl), eGFR (ml/min/1.73 m2), parathyroid hormone (pg/ml), hypertension, diabetes, albuminuria, low eGFR, and CKD. All detailed measurement processes of study variables were publicly available at www.cdc.gov/nchs/nhanes/.
We calculated urinary albumin: creatinine ratio (ACR) and defined albuminuria as ACR > 30 mg/g. The data about gender, age, and serum creatinine were used to calculate the estimated glomerular filtration rate (eGFR, ml/min/1.73 m2) according to the CKD Epidemiology Collaboration equation for each participant (42), and eGFR lower than 60 ml/min/1.73 m2 was used to define low eGFR. We defined CKD as the presence of either albuminuria or low eGFR according to Kidney Disease: Improving Global Outcomes 2012 recommendations (12).
Statistical Analysis
All statistical analyses were conducted according to CDC guidelines (43). All estimates were calculated, accounting for NHANES sample weight. Continuous variables were presented as mean with SD or a median with an interquartile range, and categorical variables were presented as frequency or percentage. Either weighted Student's t-test (for continuous variables) or weighted chi-square test (for categorical variables) were conducted to calculate the differences in different DII groups (tertiles). To examine the association between DII and PTH levels, weighted multivariable linear regression explored PTH as a continuous variable, and weighted multivariable logistic regression for HP was used as a categorical variable in three different models. In model 1, no covariates were adjusted. Model 2 was adjusted for gender, age, and race. Model 3 was adjusted for gender, age, race, education level, serum glucose, serum phosphorus, serum iron, serum calcium, serum CRP serum, serum 25 (OH) D, systolic blood pressure, diastolic blood pressure, body mass index, hypertension, diabetes, chronic kidney disease, protein intake, calcium intake, and phosphorus intake. To further explore the association between DII with PTH and HP in different population settings, the subgroup analysis was performed by stratified weighted multivariate regression analysis. In addition, it has been well-recognized that patients with CKD often have elevated parathyroid hormone levels; the CKD status was treated as a prespecified potential effect modifier. An interaction term was added to test the heterogeneity of associations between the subgroups. P < 0.05 was considered statistically significant. All analysis was performed using Empower software (www.empowerstats.com; X&Y solutions, Inc., Boston MA) and R version 3.4.3 (http://www.R-project.org, The R Foundation).
Results
Baseline Characteristics of Participants
The weighted demographic characteristics and other covariates of included individuals were shown in Table 1. A total of 7,679 participants were included in this study, of whom 48.34% were males and 51.66% were females, with an average age of 46.73 ± 18.80 years old. Median DII was −0.24 (−2.20 to 1.80), and the ranges of DII for tertiles 1–3 were −6.60 to −1.50, −1.50 to 1.08, and 1.08 to 6.50, respectively. Among different tertiles of DII, gender, race, education level, systolic blood pressure, diastolic blood pressure, serum iron, serum CRP, serum 25 (OH) D, urinary creatinine, serum creatinine, parathyroid hormone, and HP, whether having hypertension, diabetes, low eGFR and CKD, were significantly different, while no significant difference was observed in BMI, serum glucose, serum phosphorus, serum calcium, urinary albumin, eGFR, and whether having albuminuria between different tertiles. Mean PTH was 43.42 ± 23.21 pg/ml, and the average PTH was 45.53 ± 26.63 pg/ml for the participants in the highest tertile group compared with 41.42 ± 19.74 pg/ml in the lowest tertile group (P < 0.0001). The rate of HP was 11.15% overall, while the rate in the highest DII tertile was 13.28 and 8.60% in the lowest DII tertile (P < 0.0001).
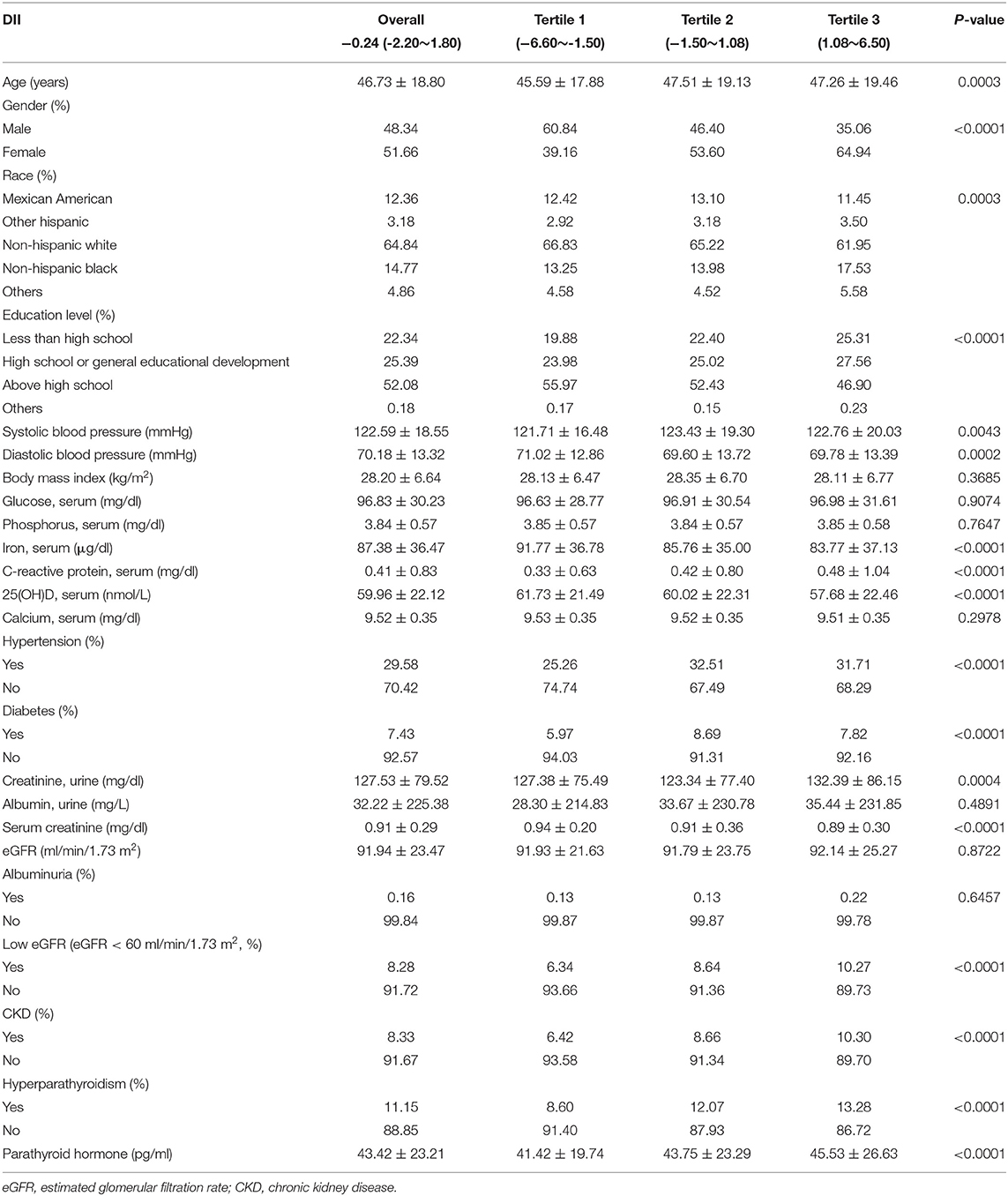
Table 1. Weighted baseline characteristics of the participants according to different dietary inflammatory indexes (DIIs).
As for the PTH levels of the participants based on different renal conditions, the participants with albuminuria, low eGFR, and CKD tended to have higher PTH levels compared with their counterparts. In the CKD group, mean PTH was 68.40 ± 55.29 pg/ml for the highest tertile group while 51.12 ± 32.84 pg/ml for the lowest tertile group (P = 0.0002). Mean PTH was 68.45 ± 55.36 pg/ml for the highest tertile and 51.42 ± 32.91 pg/ml for the lowest tertile in the low eGFR group (P = 0.0003). In the albuminuria group, mean PTH was 131.86 ± 94.31 pg/ml for the highest tertile group while 95.06 ± 110.67 pg/ml for the lowest tertile group; however, there was no significant difference (P = 0.7248). Mean eGFR of the patients with CKD was 48.96 ± 10.64 ml/min/1.73 m2, and 1.78% of them were <15 ml/min/1.73 m2. We also calculated the mean PTH level according to different stages of CKD. The PTH level in the participants with CKD, stages 1 to 5, was 40.50 ± 18.45, 43.58 ± 19.97, 56.01 ± 32.16, 122.30 ± 81.21, and 239.12 ± 136.89 pg/ml. The participants tended to show a higher PTH level with the CKD progression (Table 2).
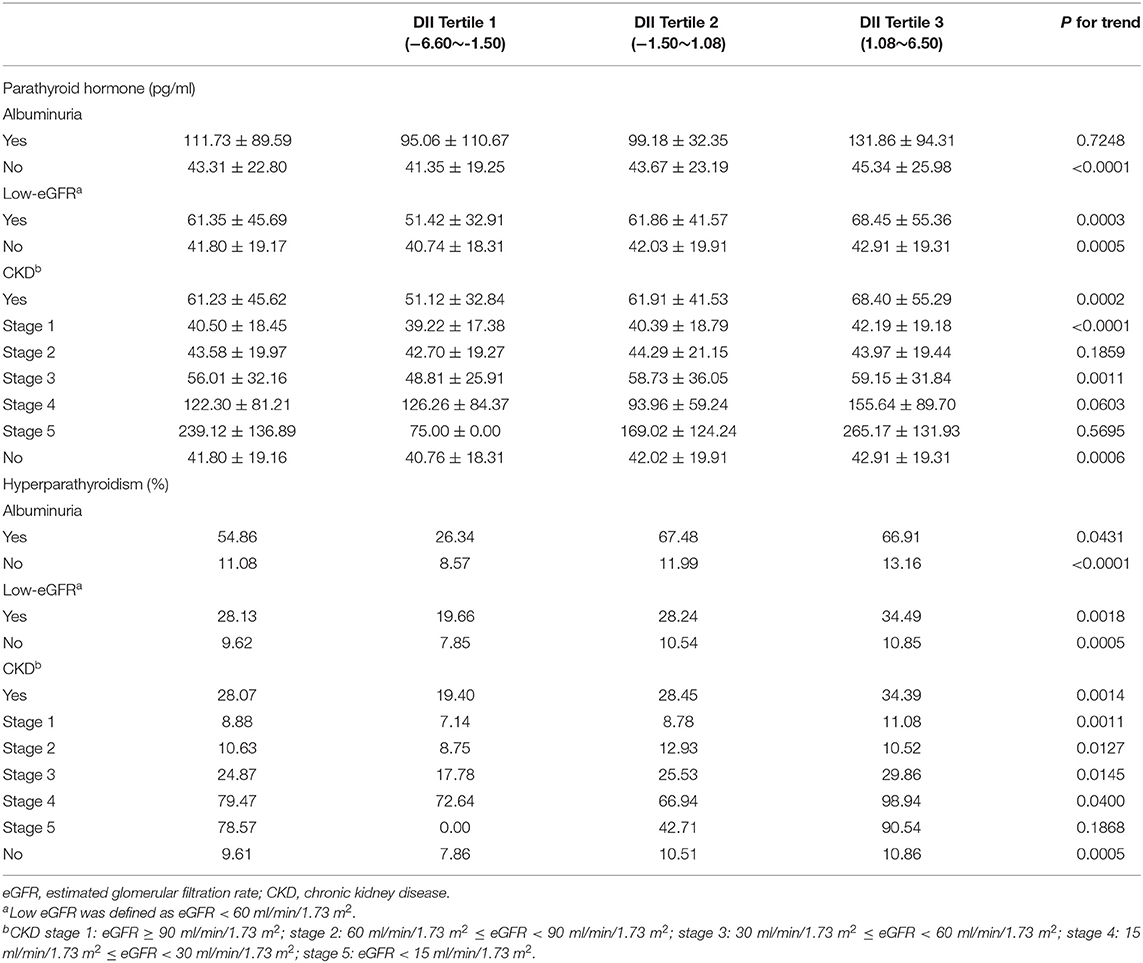
Table 2. Parathyroid hormone (PTH) and the prevalence of hyperparathyroidism (HP) based on different renal population settings, weighted.
For the prevalence of HP, participants with reduced renal function tended to have higher rates of HP. In the population with CKD, 28.07% of the participants had HP, while it was 9.61% for those without CKD. Similar results could be observed in the albuminuria and low-eGFR population as well (Albuminuria: 54.86 vs. 11.08%, low eGFR: 28.13 vs. 9.62%) (Table 2).
Higher DII Was Associated With Higher PTH Level and Higher Risk of HP
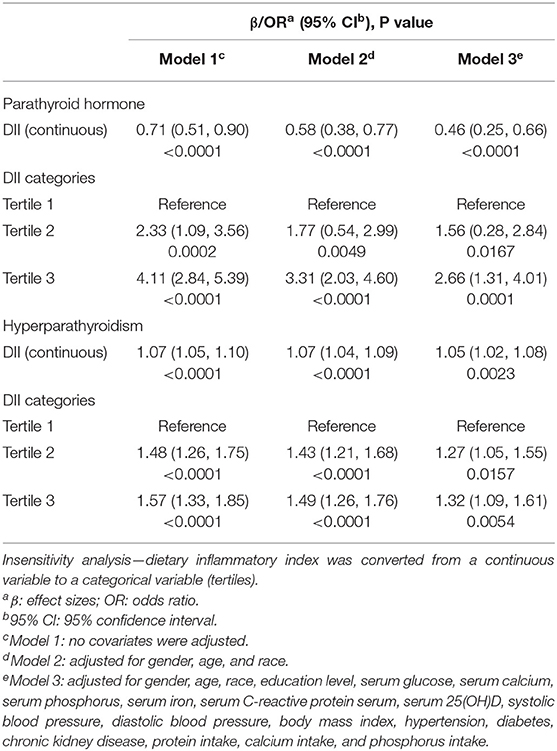
Weighted multivariable regression analysis was conducted to estimate the association of DII with the PTH level and HP in three different models (Table 3). The results revealed a positive association between DII scores and PTH with statistical significance (Model 1, β = 0.71, 95% CI: 0.51, 0.90, P < 0.0001; Model 2, β = 0.58, 95% CI: 0.38, 0.77, P < 0.0001; Model 3, β = 0.46, 95% CI: 0.25, 0.66, P ≤ 0.0001). According to the results of the fully adjusted model (Model 3), each unit of the increased DII score was associated with a PTH increase by.46 pg/ml, suggesting that the higher DII scores were associated with a higher PTH level. This association remained statistically significant after DII was grouped as tertiles. The fully adjusted effect size (reference to Tertile 1) was 1.56 (95% CI: 0.28, 2.84, P = 0.0167) for Tertile 2 and 2.66 (95% CI: 1.31, 4.01, P = 0.0001) for Tertile 3.
In terms of HP, we also observed that increased DII was associated with a higher risk of HP (Model 1, OR = 1.07, 95% CI: 1.05, 1.10, P < 0.0001; Model 2, OR = 1.07, 95% CI: 1.04, 1.09, P < 0.0001; Model 3, OR = 1.05, 95% CI: 1.02, 1.08, P = 0.0023). In Model 3, which adjusted for all covariates, the results indicated that each unit of increased DII score was associated with a 5% increase of risk of HP. In sensitivity analysis, the adjusted OR (reference to Tertile 1) was 1.27 (95% CI: 1.05, 1.55, P = 0.0157) for Tertile 2 and 1.32 (95% CI: 1.09, 1.61, P = 0.0054) for Tertile 3, suggesting a stable positive relationship between increased DII and higher risk of HP with statistical significance.
Subgroup Analysis
We conducted the subgroup analysis stratified by low eGFR, CKD, gender, age, BMI, hypertension, and diabetes to further explore the association of DII with the PTH level and HP in different population settings by stratified weighted multivariate regression analysis and tested the interactions (Table 4). Regarding the correlation between DII scores and the PTH level, the test for interaction was significant for low eGFR (P for interaction = 0.0004) and CKD P (for interaction = 0.0003), indicating significant dependence on renal function. However, the positive association was statistically significant both in subgroups stratified by low eGFR and in subgroups stratified by CKD (all P for trend < 0.05). In subgroups stratified by gender, age, BMI, hypertension, and diabetes, the positive association between DII and PTH was still significant (P for trend <0.05) and P for interaction >0.05, suggesting that the correlation between DII scores and the PTH level was similar in the population with different gender, age, BMI, hypertension status, and diabetes status.
As for the association between DII scores and HP, results of subgroup analysis stratified by CKD or no CKD demonstrated that DII was positively associated with a higher risk of HP (OR = 1.07, P for trend = 0.0023) in the CKD subgroup, and a similar result (OR = 1.04, P for trend = 0.0121) was observed in the non-CKD group. In addition, an interaction test was performed to evaluate if there was any significant dependence of the effect modifier (CKD) on the association. P for interaction >0.05 means no significant dependence, indicating that the magnitude of the association was the same for the population with/without CKD (P for interaction = 0.4942). Similarly, we did not find any significant dependence on low eGFR, gender, age, BMI, hypertension status, and diabetes status (all P for interaction > 0.05). These results indicated that the positive association between DII scores and HP was similar in the population with different renal function conditions (including CKD and low eGFR), gender, age, BMI, hypertension status, and diabetes status and could also be appropriate for the participants with reduced renal function.
Discussion
In this cross-sectional study with 7,679 adults, a significant positive association of DII with the PTH level and HP was observed, indicating that higher consumption of pro-inflammatory diet may contribute to the higher PTH level and an increased risk of HP. The association between the exposure variable and outcome variables was still stable after adjustment for covariates. Subgroup analysis stratified by the renal condition (low eGFR and CKD) and other variables showed that this positive association was not affected, suggesting that this association could be appropriate for the population with different renal function conditions, gender, age, BMI, hypertension status, and diabetes status.
To our knowledge, this is the first study that assesses the association between the dietary inflammatory potential with the PTH level and the risk of HP. Patel et al. (44) investigated whether dietary calcium intake will influence the serum PTH concentrations in apparently healthy Indian adolescents and found that subjects with higher calcium intake had lower PTH. Cheung et al. (45) suggested that low dietary magnesium intake could alter the vitamin D-PTH relationship in adults who were overweight or obese. In another study assessing the effect of the dietary approaches to stop hypertension (DASH) diet, which is rich in fiber and low-fat dairy and is useful for lowering blood pressure on the PTH level, no significant effect was observed (46). Additionally, the cause-and-effect relationship between PTH and inflammation still remains controversial. Cheng et al. (19) assessed the association between the PTH level and inflammatory markers among the U.S. adults and reported that higher inflammatory markers were associated with a higher PTH level. Similar results have been reported before in other studies (22, 47–49). Another study observed upregulation of inflammatory genes in the adipose tissue from primary patients with HP when compared with the healthy controls (19). However, conflicting results were found for the effects of inflammatory markers on patients with HP after parathyroidectomy: both increased (24, 50, 51) and decreased (22) and even unchanged levels of inflammatory markers have been reported (52). The inconsistent results indicated an unclear cause-and-effect association between PTH and inflammation. The results of this study indicated that higher pro-inflammatory dietary intake was positively associated with PTH and an increased risk of HP; in another way, a higher inflammatory level may be associated with a higher PTH level and a higher risk of HP. The association was similar in the population with different renal function conditions, gender, age, BMI, hypertension status, and diabetes status according to the subgroup analysis.
Regarding the positive association between DII scores and the PTH level, we observed significant dependence on low eGFR (P for interaction = 0.0004) and CKD (P for interaction = 0.0003), indicating that renal function may be affected by this association. Serious disorders of calcium and phosphorus metabolism were commonly observed in patients with CKD; we speculated that the effect of the pro-inflammatory diet on PTH may be interfered by the metabolism disorder (53). The interplay between Renin-Angiotensin-Aldosterone System (RAAS) and PTH may also influence this association (54). What is more, patients with end-stage CKD were treated with hemodialysis usually, and the management of hemodialysis can affect the calcium and phosphorus metabolic status (55, 56). However, the data of hemodialysis in NHANES were missing, so we could not exclude the influence of different dialysis modes or conduct further analysis. Additionally, it was worth noting that this positive association was still statistically significant both in subgroups stratified by low eGFR and CKD (all P-value <0.05), suggesting that, although, renal function may affect this association, it was similar in the population with/without low eGFR and CKD.
The exact mechanism of this positive association of DII with PTH and HP remains unclear. A possible explanation to support the results might be the effect of diet on pro-inflammatory markers, such as IL-8, TNF-α, etc., Previous studies have demonstrated that inflammation was closely associated with the activation of the NF-κB pathway (57–59). NF-κB could serve as a direct regulator of genes related to cell proliferation, such as cyclin D1, p21, p27, and p53, thus, involved in cell cycles (60, 61). It also plays a key role in angiogenesis and tumor growth by promoting antiapoptotic mechanisms and the expression of inflammatory cytokines; in turn, these cytokines also contribute to its activation (62, 63). NF-κB could play a role in PTH regulation as well. Mao et al. (26) found that local NF-κB activation could promote the synthesis and secretion of PTH and mediated the transcriptional activation of PTH directly. We hypothesized that the elevated inflammatory level may lead to the activation of NF-κB; thus, similar regulation by NF-κB may underlie the development of parathyroid hyperplasia and enhanced PTH synthesis and secretion. Angeletti et al. (27) evaluated the effects of IL-8 on the PTH level by incubation of parathyroid cells with recombinant IL-8 in vitro and found that IL-8 increased both PTH secretion and PTH mRNA expression. A previous study also reported that TNF-α could affect CASR transcription via NF-κB in human renal tubular cells (64). A significant down-expression of CASR mRNA, thus, upsetting the Ca2+ set point and enhancing the sensitivity of parathyroid glands to Ca2+ concentration may be another potential explanation of our results.
One of the strengths of our study was that it was based on nationwide, population-based sampling survey data, and sample weights were adopted, which make the study much more representative. It is noteworthy that we performed subgroup analysis stratified by different renal functions and found that this association was similar in different population settings and could also be appropriate for the participants with CKD. However, the limitations of this study cannot be ignored. First, due to the cross-sectional study design, we cannot make a causal inference. Second, the food intake data were based on a 24-h dietary recall; a recall bias was inevitable, and the daily variability of food intake cannot be reflected. With regard to DII scores, a total of 45 food parameters were included according to the design, but only 27 food parameters were available in the NHANES data. Although, previous studies reported that the predictive ability was not affected when <30 dietary parameters were used with DII calculating (38–40), the impact on accuracy cannot be ignored. Third, HP was defined as intact PTH > 65 pg/ml in our analysis. However, optimal PTH levels in CKD stages 3–5 remain unknown (65). Although most of the participants with CKD in this study were in stage 1 and stage 2, we also used this HP definition in patients with CKD stages 3–5; thus, it may affect the accuracy. In addition, some potential confounders, such as the hemodialysis condition (the patients with non-dialysis dependent or hemodialysis CKD, a hemodialysis type, etc.,), drugs use, and fibroblast growth factor 23 (FGF23), may influence this association, but these confounders were not available in NHANES data (56, 66–68). Another limitation was that PTH was only assayed at a single time point; thus, no repeat measurements of PTH were conducted.
Conclusion
In this cross-sectional study with 7,679 adults, a significant positive association between DII with PTH and HP was observed, indicating that higher consumption of pro-inflammatory potential correlates positively with the higher PTH level and an increased risk of HP. This association exists both in the populations with CKD and with no CKD. Our finding suggests that anti-inflammatory dietary management may reduce the risk of HP. Further research and clinical settings are still needed to validate their potential application.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: www.cdc.gov/nchs/nhanes/.
Ethics Statement
The studies involving human participants were reviewed and approved by the NCHS Ethic Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
ZQ: data analysis, software, and writing of the original draft. QY: formal analysis and software. RL: methodology and writing of the original draft. BS: conceptualization, funding acquisition, writing, reviewing, and editing. All the authors approved the final version.
Funding
This work was supported by the State Key Research Programme of China (Grant nos. 2016YFC1103004 and 2016YFC1103003), the National Natural Science Foundation of China (Grant no. 82000702), the Science and Technology Achievement Transformation Fund of West China Hospital of Sichuan University (Grant no. CGZH19006), National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (Grant no. Z2018B10), and Med+ Biomaterial Institute of West China Hospital/West China School of Medicine of Sichuan University (Grant no. ZYME20001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the assistance of Yingfei Xu in data processing.
References
1. Raposo L, Martins S, Ferreira D, Guimarães JT, Santos AC. Vitamin D, parathyroid hormone and metabolic syndrome – the PORMETS study. BMC Endocr Disord. (2017) 17:71. doi: 10.1186/s12902-017-0221-3
2. Bilezikian JP, Cusano NE, Khan AA, Liu J-M, Marcocci C, Bandeira F. Primary hyperparathyroidism. Nat Rev Dis Primers. (2016) 2:16033. doi: 10.1038/nrdp.2016.33
3. Naveh-Many T, Rahamimov R, Livni N, Silver J. Parathyroid cell proliferation in normal and chronic renal failure rats. The effects of calcium, phosphate, vitamin D. J Clin Invest. (1995) 96:1786–93. doi: 10.1172/JCI118224
4. Brown EM. The pathophysiology of primary hyperparathyroidism. J Bone Miner Res. (2002) 17(Suppl. 2):N24–9. doi: 10.1359/jbmr.2002.17.11.2087
5. Paolo RG, Selene P. Does angiotensin II regulate parathyroid hormone secretion or not? Clini Endocrinol. (2018) 89:568–9. doi: 10.1111/cen.13798
6. Moallem E, Kilav R, Silver J, Naveh-Many T. RNA-protein binding and post-transcriptional regulation of parathyroid hormone gene expression by calcium and phosphate. J Biol Chem. (1998) 273:5253–9. doi: 10.1074/jbc.273.9.5253
7. Sitges-Serra A, Bergenfelz A. Clinical update: sporadic primary hyperparathyroidism. Lancet. (2007) 370:468–70. doi: 10.1016/S0140-6736(07)61213-6
8. Silverberg SJ, Clarke BL, Munro P, Francisco B, Stephanie B, Cusano NE, et al. Current issues in the presentation of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab. (2014) 99:3580–94. doi: 10.1210/jc.2014-1415
9. Smit MA, Van K, Joke V, Charlotte VN, SnjeŽana K. Clinical guidelines and PTH measurement: does assay generation matter? Endocr Rev. (2019) 40:1468–80. doi: 10.1210/er.2018-00220
10. Shindo M, Lee JA, Lubitz CC, Mccoy KL, Orloff LA, Tufano RP, et al. The changing landscape of primary, secondary, and tertiary hyperparathyroidism: highlights from the American College of Surgeons Panel, “what's new for the surgeon caring for patients with hyperparathyroidism”. J Am Coll Surg. (2016) 222:1240–50. doi: 10.1016/j.jamcollsurg.2016.02.024
11. Kritchevsky SB, Tooze JA, Neiberg RH, Schwartz GG, Hausman DB, Johnson MA, et al. 25-Hydroxyvitamin D, parathyroid hormone, and mortality in black and white older adults: The health ABC study. J Clin Endocr Metab. (2012) 97:4156–65. doi: 10.1210/jc.2012-1551
12. Chapter 1: definition classification of CKD. Kidney Int Suppl. (2013) 3:19–62. doi: 10.1038/kisup.2012.64
13. Hill NR, Fatoba ST, Oke JL, Hirst JA, O'Callaghan C, Lasserson DS, et al. Global prevalence of chronic kidney disease – a systematic review and meta-analysis. PLoS ONE. (2016) 11:e0158765. doi: 10.1371/journal.pone.0158765
14. Ackland P. Prevalence, detection, evaluation, and management of chronic kidney disease. BMJ. (2014) 348:f7688. doi: 10.1136/bmj.f7688
15. Jager KJ, Fraser SDS. The ascending rank of chronic kidney disease in the global burden of disease study. Nephrol Dial Transplant. (2017) 32:ii121–28. doi: 10.1093/ndt/gfw330
16. Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, therapeutic options. Clin J Am Soc Nephrol. (2011) 6:913–21. doi: 10.2215/CJN.06040710
17. Block GA, Martin KJ, de Francisco ALM, Turner SA, Avram MM, Suranyi MG, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. (2004) 350:1516–25. doi: 10.1056/NEJMoa031633
18. Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. (2001) 22:477–501. doi: 10.1210/edrv.22.4.0437
19. Cheng S-P, Liu C-L, Liu T-P, Hsu Y-C, Lee J-J. Association between parathyroid hormone levels and inflammatory markers among US adults. Mediators Inflamm. (2014) 2014:709024. doi: 10.1155/2014/709024
20. Christensen MHE, Dankel SN, Nordbo Y, Varhaug JE, Almas B, Lien EA, et al. Primary hyperparathyroidism influences the expression of inflammatory and metabolic genes in adipose tissue. PLoS ONE. (2011) 6:e20481 doi: 10.1371/journal.pone.0020481
21. Lutfioglu M, Sakallioglu U, Sakallioglu EE, Diraman E, Ciftci G, Tutkun F. Dietary-induced hyperparathyroidism affects serum and gingival proinflammatory cytokine levels in rats. J Periodontol. (2010) 81:150–7. doi: 10.1902/jop.2009.090353
22. Grey A, Mitnick MA, Shapses S, Ellison A, Gundberg C, Insogna K. Circulating levels of interleukin-6 and tumor necrosis factor-alpha are elevated in primary hyperparathyroidism and correlate with markers of bone resorption–a clinical research center study. J Clin Endocrinol Metab. (1996) 81:3450–4. doi: 10.1210/jcem.81.10.8855783
23. Alakus H, Goksu M. Does parathyroidectomy affect the neutrophil/lymphocyte ratio, a systemic inflammatory marker? Cureus. (2021) 13:7. doi: 10.7759/cureus.13708
24. Farahnak P, Lrfars G, Sten-Linder M, Nilsson IL. Mild primary hyperparathyroidism: vitamin d deficiency and cardiovascular risk markers. J Clin Endocrinol Metabol. (2011) 96:2112–8. doi: 10.1210/jc.2011-0238
25. Bollerslev J, Rosen T, Mollerup CL, Nordenström J, Baranowski M, Franco C, et al. Effect of surgery on cardiovascular risk factors in mild primary hyperparathyroidism. J Clin Endocrinol Metab. (2009) 94:2255–61.
26. Mao J, Wang M, Ni L, Gong W, Jiang X, Zhang Q, et al. Local NF-kappaB activation promotes parathyroid hormone synthesis and secretion in uremic patients. Endocrinology. (2021). doi: 10.1210/endocr/bqab084
27. Angeletti RH, D'Amico T, Ashok S, Russell J. The chemokine interleukin-8 regulates parathyroid secretion. J Bone Min Res. (1998) 13:1232–7. doi: 10.1359/jbmr.1998.13.8.1232
28. Hendy GN, Canaff L. Calcium-sensing receptor gene: regulation of expression. Front Physiol. (2016) 7:12. doi: 10.3389/fphys.2016.00394
29. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert J. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96. doi: 10.1017/S1368980013002115
30. Shivappa N, Godos J, Hebert JR, Wirth MD, Piuri G, Speciani AF, et al. Dietary inflammatory index and colorectal cancer risk-a meta-analysis. Nutrients. (2017) 9:17. doi: 10.3390/nu9091043
31. Zahedi H, Djalalinia S, Asayesh H, Mansourian M, Abdar ZE, Gorabi AM, et al. A higher dietary inflammatory index score is associated with a higher risk of incidence and mortality of cancer: a comprehensive systematic review and meta-analysis. Int J Preventive Med. (2020) 11:24. doi: 10.4103/ijpvm.IJPVM_332_18
32. Ruiz-Canela M, Zazpe I, Shivappa N, Hebert JR, Sanchez-Tainta A, Corella D, et al. Dietary inflammatory index and anthropometric measures of obesity in a population sample at high cardiovascular risk from the PREDIMED (PREvencion con DIeta MEDiterranea) trial. Br J Nutr. (2015) 113:984–95. doi: 10.1017/S0007114514004401
33. Ji M, Hong X, Chen M, Chen T, Zhang N. Dietary inflammatory index and cardiovascular risk and mortality: a meta-analysis of cohort studies. Medicine. (2020) 99:e20303. doi: 10.1097/MD.0000000000020303
34. Geng J, Deng L, Shi Q, Bian H, Su B. Dietary inflammatory potential and risk of sarcopenia: data from national health and nutrition examination surveys. Aging. (2020) 13:1913–28. doi: 10.18632/aging.202141
35. Zipf G, Chiappa M, Porter KS, Ostchega Y, Dostal J. National health and nutrition examination survey: plan and operations, 1999-2010. Vital Health Stat. (2013) 1:1–37.
36. Mazidi M, Kengne AP, Vatanparast H. Association of dietary patterns of American adults with bone mineral density and fracture. Public Health Nutr. (2018) 21:2417–23. doi: 10.1017/S1368980018000939
37. Plan and operation of the third National Health and Nutrition Examination Survey 1988-94. Vital Health Stat. (1994):1–407.
38. Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, et al. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr. (2014) 17:1825–33. doi: 10.1017/S1368980013002565
39. Park Y, Choi MK, Lee SS, Shivappa N, Han K, Steck SE, et al. Dietary inflammatory potential and risk of mortality in metabolically healthy and unhealthy phenotypes among overweight and obese adults. Clin Nutr. (2018) 38:682–8. doi: 10.1016/j.clnu.2018.04.002
40. Mohsen M, Nitin S, Wirth M, Hebert JR, Kengne AP. Greater dietary inflammatory index score is associated with higher likelihood of chronic kidney disease. Br J Nutr. (2018) 120:204–9. doi: 10.1017/S0007114518001071
41. Chen L, Pei JH, Kuang J. Moderators of the association between serum parathyroid hormone and metabolic syndrome in participants with elevated parathyroid hormone: NHANES 2003-2006. Horm Metab Res. (2020) 52:509–16. doi: 10.1055/a-1148-2546
42. Levey AS, Stevens LA, Schmid CH, Zhang YP, Castro AF, Feldman HI, et al. Chronic kidney dis epidemiology, a new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
43. Johnson CL, Paulose-Ram R, Ogden CL, Carroll M, Curtin LR. National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital Health Stat 2. (2013) 161:1–24.
44. Patel P, Mughal MZ, Patel P, Yagnik B, Kajale N, Mandlik R, et al. Dietary calcium intake influences the relationship between serum 25-hydroxyvitamin D-3 (25OHD) concentration and parathyroid hormone (PTH) concentration. Arch Dis Childhood. (2016) 101:316–9. doi: 10.1136/archdischild-2015-308985
45. Cheung MM, DeLuccia R, Ramadoss RK, Aljahdali A, Volpe SL, Shewokis PA, et al. Low dietary magnesium intake alters vitamin D-parathyroid hormone relationship in adults who are overweight or obese. Nutr Res. (2019) 69:82–93. doi: 10.1016/j.nutres.2019.08.003
46. Hassoon, Michos ED, Miller ER III, Crisp Z, Appel LJ. Effects of different dietary interventions on calcitriol, parathyroid hormone, calcium, and phosphorus: results from the DASH trial. Nutrients. (2018) 10:367. doi: 10.3390/nu10030367
47. Guo C, Holland P, Jackson B, Hannon R, Rogers A, Harrison B, et al. Immediate changes in biochemical markers of bone turnover and circulating interleukin-6 after parathyroidectomy for primary hyperparathyroidism. Eur J Endocrinol. (2000) 142:451. doi: 10.1530/eje.0.1420451
48. Ogard CG, Engelmann MD, Kistorp C, Nielsen SL, Vestergaard H. Increased plasma N-terminal pro-B-type natriuretic peptide and markers of inflammation related to atherosclerosis in patients with primary hyperparathyroidism. Clin Endocrinol. (2005) 63:493–8. doi: 10.1111/j.1365-2265.2005.02363.x
49. Emam AA, Mousa SG, Ahmed KY, Al-Azab AA. Inflammatory biomarkers in patients with asymptomatic primary hyperparathyroidism. Med Princ Pract. (2011) 21:249–53. doi: 10.1159/000334588
50. Halabe A, Shohat B. Effect of parathyroid adenoma excision on interleukin-6 (IL-6) and IL-2 receptor levels. Metabolism. (2000) 49:192–4. doi: 10.1016/s0026-0495(00)91247-2
51. Almqvist EG, Bondeson AG, Bondeson L, Svensson J. Increased markers of inflammation and endothelial dysfunction in patients with mild primary hyperparathyroidism. Scand J Clin Lab Invest. (2011) 71:139–44. doi: 10.3109/00365513.2010.543694
52. Bollerslev J, Rosen T, Mollerup CL, Nordenstroem J, Baranowski M, Franco C, et al. Effect of surgery on cardiovascular risk factors in mild primary hyperparathyroidism. J Clin Endocrinol Metabol. (2009) 94:2255–61. doi: 10.1210/jc.2008-2742
53. Cannata-Andia JB, Martin-Carro B, Martin-Virgala J, Rodriguez-Carrio J, Bande-Fernandez JJ, Alonso-Montes C, et al. Chronic kidney disease-mineral and bone disorders: pathogenesis and management. Calcif Tissue Int. (2021) 108:410–22. doi: 10.1007/s00223-020-00777-1
54. Zheng MH, Li F, Xu F, Lin X, Yuan LQ. The interplay between the renin-angiotensin-aldosterone system and parathyroid hormone. Front Endocrinol. (2020) 11:539. doi: 10.3389/fendo.2020.00539
55. Cozzolino M, Ciceri P. Serum PTH levels in dialysis: better safe than sorry. Ther Adv Endocrinol Metabol. (2020) 11. doi: 10.1177/2042018820974172
56. Lotfollahi L, Ossareh S, Neyestani TR. Evaluation of 25-hydroxy vitamin D and 1,25-dihydroxy vitamin D levels in maintenance hemodialysis patients. Iran J Kidney Dis. (2021) 15:31–7.
57. Roshanravan N, Shabestari AN, Alamdari NM, Ostadrahimi A, Separham A, Parvizi R, et al. A novel inflammatory signaling pathway in patients with slow coronary flow: NF-kappaB/IL-1beta/nitric oxide. Cytokine. (2021) 143:155511. doi: 10.1016/j.cyto.2021.155511
58. Delhase M. IkappaB kinase and NF-kappaB signaling in response to pro-inflammatory cytokines. Methods Mol Biol. (2003) 225:7–17. doi: 10.1385/1-59259-374-7:7
59. Rahman A, Fazal F. Blocking NF-kappaB: an inflammatory issue. Proc Am Thorac Soc. (2011) 8:497–503. doi: 10.1513/pats.201101-009MW
60. Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-kappa B and I kappa B proteins: implications in cancer and inflammation. Trends Biochem.Sci. (2005) 30:43–52. doi: 10.1016/j.tibs.2004.11.009
61. Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-kappa B: its role in health and disease. J Mol Med. (2004) 82:434–48. doi: 10.1007/s00109-004-0555-y
62. Sakamoto K, Maeda S, Hikiba Y, Nakagawa H, Hayakawa Y, Shibata W, et al. Constitutive NF-kappa B Activation in colorectal carcinoma plays a key role in angiogenesis, promoting tumor growth. Clin Cancer Res. (2009) 15:2248–58. doi: 10.1158/1078-0432.CCR-08-1383
63. He J, Qin M, Chen Y, Hu Z, Ye L, Hui T. EZH2 promotes extracellular matrix degradation via nuclear factor-kappa B (NF-kappa B) and p38 signaling pathways in pulpitis. Inflammation. (2021). doi: 10.1007/s10753-021-01470-7
64. Canaff L, Hendy GN. NF-kappa B mediates transcriptional up-regulation of the human calcium-sensing receptor gene by pro-inflammatory cytokines. Faseb J. (2003) 17:A185.
65. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. (2017) 7:e1. doi: 10.1016/j.kisu.2017.10.001
66. Zeng SF, Querfeld U, Feger M, Haffner D, Hasan AA, Chu C, et al. Relationship between GFR, intact PTH, oxidized PTH, non-oxidized PTH as well as FGF23 in patients with CKD. Faseb J. (2020) 34:15269–81. doi: 10.1096/fj.202000596R
67. Imafuku T, Tanaka M, Tokunaga K, Miyamura S, Kato H, Tanaka S, et al. Effect of cinacalcet on the redox status of albumin in secondary hyperparathyroidism patients receiving hemodialysis. Biol Pharm Bull. (2020) 43:1583–90. doi: 10.1248/bpb.b20-00472
Keywords: dietary inflammatory index, parathyroid hormone, chronic kidney disease, hyperparathyroidism, National Health and Nutrition Examination Survery
Citation: Qin Z, Yang Q, Liao R and Su B (2021) The Association Between Dietary Inflammatory Index and Parathyroid Hormone in Adults With/Without Chronic Kidney Disease. Front. Nutr. 8:688369. doi: 10.3389/fnut.2021.688369
Received: 30 March 2021; Accepted: 01 June 2021;
Published: 25 June 2021.
Edited by:
Carla Maria Avesani, Karolinska Institutet, SwedenReviewed by:
Alisson Diego Machado, University of São Paulo, BrazilRosa Maria Affonso Moyses, University of São Paulo, Brazil
Copyright © 2021 Qin, Yang, Liao and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baihai Su, aW1zYmhAMTYzLmNvbQ==
 Zheng Qin
Zheng Qin Baihai Su
Baihai Su