
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 26 May 2021
Sec. Nutrition and Microbes
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.685540
This article is part of the Research TopicRemodeling Composition and Function of Microbiome by Dietary Strategies - Functional Foods PerspectiveView all 8 articles
Weaning piglets experienced the transformation from breast milk to solid feed and present the proliferation of pathogens, the presence of diarrhea, poor growth performance and even death. Plant extracts and probiotics have certain potential in improving animal growth performance, antioxidant capacity and immune function. The purpose of this study was to explore the effects of dietary yucca schidigera extract (YSE) and oral Candida utilis (CU) on growth performance and intestinal health weaned piglets. According to a 2 × 2 factorial design with the main factors being CU (orally administered 1 mL of 0.85% saline with or without CU; fed basal diet with or without 120 mg/kg YSE), forty 28 d healthy weaned piglets were randomly allocated into four groups of 10 barrows each: (1) piglets fed basal diet and orally administered 1 mL of 0.85% saline (CON); (2) piglets fed basal diet and orally administered 1 mL 1 × 109 cfu/mL C. utilis in 0.85% saline (CU); (3) piglets fed the basal diet containing YSE (120 mg/kg) and orally administered 1 mL of 0.85% saline (YSE); (4) Piglets fed the basal diet containing 120 mg/kg YSE and 1 mL 1 × 109 cfu/mL C. utilis in 0.85% saline (YSE+CU). This study lasted 28 days and evaluated the effects of dietary YSE and oral CU on growth performance, immunity, antioxidant function, ileal morphology, and intestinal microflora in weaned piglets. Dietary YSE increased ADG, the spleen and lymph node indexes, serum GLU, BUN, T-SOD, T-AOC, CAT concentrations, ileal villus height and villus height/crypt depth, jejunal occludin, and β-definsin-2 concentrations and ileal occludin concentration in weaned piglets (P < 0.05); decreased the diarrhea rate and mortality, rectal pH and urine pH, the BUN and MDA concentrations, crypt depth (P < 0.05); improved the diversity of cecal microflora. Orally CU increased ADG, and ADFI, the T-SOD, T-AOC, and CAT activity, ileal villus height, villus height/crypt depth, jejunum occludin, and β-definsin-2 concentrations (P < 0.05); reduced the diarrhea rate and mortality, urine pH, the BUN and MDA concentrations, crypt depth (P < 0.05); improved the diversity of cecal microflora. Dietary YSE and orally CU increased the T-SOD, T-AOC, and CAT activity, villus height/crypt depth, jejunal occludin concentration; reduced the diarrhea rate of weaned piglets by 28%, gastric pH, ileal pH, cecal pH and urine pH, MDA, crypt depth; improved the diversity of cecal microflora. YSE and CU could improve the growth performance, reduce the diarrhea rate, improve intestinal health, and increase the diversity and abundance of cecal microflora in weaned piglets and expected to be used as antibiotics alternative feed additives in the production of weaned piglets.
The transformation of weaned piglets from breast milk to solid feed will cause intestinal flora disorder, and then lead to diarrhea, growth performance decline, and even death of piglets, and finally bring huge economic losses to animal husbandry. Therefore, the use of active ingredients in weaned piglets can protect the stability of gastrointestinal microorganisms, prevent or slow down diarrhea, improve growth performance and promote the development of antibiotics alternative feed additives. Many studies have pointed out that plants and their extracts play an important role in promoting animal growth, enhancing immunity and maintaining animal health (1–4). YSE is a natural plant extract, which is generally recognized as safe (GRAS), so it can be used in food, cosmetics, pharmaceutical and feed industry. Studies have confirmed that YSE can improve animal feed intake, feed conversion efficiency, growth rate and maintain animal health (5, 6), mainly because YSE has a significant effect on anti-inflammation, antibacterial and enhancing immunity in livestock and poultry (7, 8). Probiotics have attracted wide attention because of their enhancement of animal immunity and effective defense against the invasion of pathogenic bacteria. CU is a kind of forage yeast, which is proved to be a kind of microorganism rich in cell protein, which can improve the balance of intestinal microecology and is beneficial to the growth of the host (9). However, the research on YSE and CU in weaned piglets is insufficient, which limits their application in production. This paper explores the effects of YSE and CU on growth performance and intestinal health and their probiotic mechanism and provided an experimental basis for the application of YSE and CU.
YSE with an active ingredient content of 60% was purchased from Xi'an Lutian Biotechnology Co., Ltd. (Xi'an, China). CU was purchased from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (No. DSM 2361).
YPD culture medium comprised 2% (w/v) glucose, 1% (w/v) yeast powder, 2% (w/v) peptone, 2% (w/v) agar, and was adjusted to pH 6.0 and sterilized for 15 min at 115°C.
The activities or contents of glucose (GLU), total cholesterol (T-CHO), Aspartate aminotransferase (AST), Alanine transaminase (ALT), blood urea nitrogen (BUN), total superoxide dismutase (T-SOD), total antioxidant capacity (T-AOC), malondialdehyde (MDA), catalase (CAT), acetylcholinesterase (A-CHE) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Power Fecal DNA Isolation Kit was purchased from Germany Qiagen reagent company (Dusseldorf, Germany).
The rabbit-anti rat β-definsin-2 (ab178728, 1:1000) and the rabbit-anti rat occludin (ab31721; 1:1000) were purchased from Abcam (Cambridge, MA, USA). The rabbit-anti rat β-actin (5125S, 1:1000) was purchased from CST (Danvers, USA). The F(ab)2 of goat-anti rabbit Ig was purchased from Fantibody (FAB127288, 1: 2500).
The experiment was established as a 2 × 2 factorial design with the main factors being C. utilis (orally administered 1 mL of 0.85% saline with or without C. utilis; fed basal diet with or without 120 mg/kg YSE). Forty 28-day-old weaned piglets (Rongchang × Landrace × Large white) with similar parity and body weight (7.51 ± 0.54 kg) were randomly divided into 4 treatments of 10 barrows each: (1) piglets fed basal diet and orally administered 1 mL of 0.85% saline (CON); (2) piglets fed basal diet and orally administered 1 mL 1 × 109 cfu/mL C. utilis in 0.85% saline (CU); (3) piglets fed the basal diet containing YSE (120 mg/kg) and orally administered 1 mL of 0.85% saline (YSE); (4) Piglets fed the basal diet containing 120 mg/kg YSE and 1 mL 1 × 109 cfu/mL C. utilis in 0.85% saline (YSE+CU).
The ingredients and the compositions of the basal diet are formulated according to the NRC (2012) recommendations (Table 1). Piglets were fed at 08:00, 12:00, and 18:00, and kept in 4 mechanically ventilated and temperature-controlled (30 ± 1.2°C) 30 m2 room. Each piglet was kept in individual pen (1.5 m length × 0.5 m width × 0.8 m height). Food and water were provided ad libitum. All experimental procedures were approved by the License of Experimental Animals (SYXK 2014-0002) of the Animal Experimentation Ethics Committee of Southwest University, Chongqing, China.
The experimental period lasted for 28 days. Feed intake and incidence of diarrhea in pigs were recorded daily during the entire experimental period. The pigs were weighed on day 0 and day 28 prior to the morning feed.
Prior to the morning feed on day 29, five piglets were selected from each group and a 10 mL blood sample was collected. The blood sample was undisturbed for 60 min and centrifuged at 3,500 g for 10 min at 4°C to harvest the serum. Serum was stored at −20°C for biochemical analysis and enzyme linked immunosorbent assay (ELISA). After blood sampling, the five pigs with a similar average weight selected from each group were anesthetized with an intravenous injection of sodium pentobarbital (50 mg/kg Basal body weight) and bled by exsanguination. Cardiac, liver, thymus, kidney, spleen, pancreas, mesenteric lymph nodes were sampled and weighed. Then a 2–3 cm of jejunal and ileal tissue was excised from the midpoint of the jejunum, gently rinsed using cold saline, and placed into 10% formalin solution before hematoxylin and eosin (H&E) staining. The jejunal and ileal mucosa were rinsed by cold saline, and the mucosa was scraped gently by a scalpel blade and immediately frozen in liquid N2 and stored at −80°C for western blot analysis. The digesta contents of stomach, jejunum, ileum, colon, cecum, rectum and urine were collected for pH measure and he digesta contents of colon were collected for 16S rDNA sequencing.
The digesta contents of stomach, jejunum, ileum, colon, cecum, rectum and urine were measured by pH meter (METTLER TOLEDO, S220, Switzerland).
The presence of GLU, T-CHO, AST, ALT, BUN, T-AOC, T-SOD, CAT, MDA, and A-CHE in serum were determined using colorimetric methods with a reagent kit according to the manufacturer's instructions (Nanjin Jianchen Institute of Bioengineering, Nanjing, Jiangshu, China).
The morphology of the ileum was analyzed by H&E staining as reported by Wang et al. (10) Sliced samples were viewed under an optical microscope (Carl Zeiss Inc., Oberkochen, Bayern, Germany). Each sample photographed Five pictures and five fields in each picture were used to analyze villus height and crypt depth using image analysis software (Intronic GmbH & Co., Rothenstein, Berlin, Germany).
About 100 mg jejunal and ileal mucosa was homogenized in 1 mL RIPA buffer [50 mM Tris-base, 1.0 mM ethylene diamine tetraacetic acid (EDTA), 150 mM NaCl, 0.1% sodium dodecyl sulfate (SDS), 1% Tritox-100, 1% sodium deoxycholate, and 1 mM phenylmethylsulfonyl fluoride (PMSF)] and separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were transferred to a polyvinylidene fluoride (PVDF) membrane by the semi-dry transfer method. The PVDF membranes were blocked in a blocking buffer overnight at 4°C, then incubated in blocking buffer with rabbit-anti rat β-definsin-2, β-actin, occludin and incubated in blocking buffer with F(ab)2 of goat-anti rabbit Ig labeled with horseradish peroxidase and with diluted in phosphate buffered saline solution. The PVDF membrane was soaked in a chemiluminescent liquid (Millipore, Massachusetts, USA). Pictures were photographed using a Chemiluminescence Imaging System (Bio-Rad).
The total DNA of cecum contents was extracted with the MOBIO Power Fecal DNA Isolation Kit and sent to Chengdu Luoning Biological Technology Co for sequencing and analysis of microbial 16S rDNA fragments. The specific primers with Barcode were synthesized to amplify the 16S rDNA V4 region of the sample using the primers 515F (5′-GTGCC AGCMG CCGCG GTAA-3′) and 806R (5′-GGACT ACHV GGGTW TCTAAT-3′). Three replicates were performed for each sample, and the recovered products of PCR were detected and quantified with Qubit 2.0. The corresponding proportion was mixed according to the sequencing quantity requirements of each sample. the library was constructed by Illumina's TruSeq DNA PCR-Free Sample Prep Kit. Illumina's MiSeq Reagent Kit v2 was used for MiSeq sequencing. PE reads obtained from Miseq sequencing were spliced with FLASH (https://ccb.jhu.edu/software/FLASH/). Data filtering was completed after the removal of low-quality bases and contaminated sequences of joints. The samples were then analyzed with UPARSE (11).
The average daily feed intake, average daily weight gain and feed conversion rate (FCR), diarrhea incidence and organ index were calculated according to the following formula: Average daily gain (ADG) (g/d) = (final weight-initial weight) (g)/days (d) (Equation 1); Average daily feed intake (ADFI) (g/d) = total feed intake (g)/days (d) (Equation 2); Feed/Gain = average daily weight gain (g)/average daily food intake (g) (Equation 3); Diarrhea incidence (%) = the number of pigs with diarrhea/(the number of pigs × test days) × 100% (Equation 4); The incidence of diarrhea (%) = the number of diarrhea × days/(number of piglets × days of trial) × 100% (Equation 5); Organ index (g/kg) = organ wet weight (g)/pig live weight (kg) (Equation 6).
Data were analyzed by two-way with CU (2 levels) and YSE (2 levels) analysis of variance using the GLM procedure (SAS Institute 222 Inc.; Cary, NC, USA). The values presented in the tables represent means and pooled SEMs. The Student-Neuman-Keuls test was performed to identify differences among groups. Significance was set at P < 0.05.
The effects of oral YSE and CU on the growth performance of weaned piglets were shown in Table 2. Piglets dietary administered YSE increased the final weight and ADG (P < 0.05), and tended to reduce the Feed/Gain (P = 0.087), and had no significant effect on ADFI (P> 0.05). Piglets orally administered CU increased final weight, ADG, and ADFI (P < 0.05), didn't affected Feed/Gain (P > 0.05). Piglets orally administered CU or dietary administered YES decreased the diarrhea rate and mortality. Piglets orally administered CU and dietary administered YSE reduced the diarrhea rate as much as 28%. There was no the interaction effect on final weight, ADG, and ADFI between YSE and CU (P > 0.05), and there was a significant trend in the interaction effect on the Feed/Gain (P = 0.057).
As shown in Table 3, piglets dietary administered YSE increased the spleen index and lymph node index (P < 0.05), had a tend to increase the pancreatic index (P = 0.07), had no significant effect on cardiac, liver, thymus, kidney, pancreas indexes (P > 0.05). Piglets orally administered CU tended to increase spleen index (P = 0.05) and lymph node index (P = 0.08) and had no significant effect on cardiac, liver, thymus, kidney, pancreas indexes (P > 0.05). There were significant interaction effects on spleen index and lymph node index between YSE and CU (P < 0.05), a significant trend on liver index (P < 0.05), and no interaction effect on cardiac, liver, thymus, kidney and pancreas indexes (P> 0.05).
As shown in Table 4, piglets dietary administered YSE reduced rectal pH and urine pH (P < 0.05), and tended to decrease colon pH (P = 0.09), and had no significant effect on jejunum pH, ileum pH, and cecal pH (P > 0.05). Piglets orally administered CU reduced urine pH (P < 0.05) and had no significant effect on stomach pH, jejunum pH, ileum pH, colon pH, cecal pH, and rectal pH (P > 0.05). There were interaction effects on gastric pH, ileal pH, cecal pH, and urine pH between CU and YSE (P < 0.05), and there were no the interaction effects on jejunum pH, colon pH and cecal pH (P > 0.05).
As shown in Table 5, dietary supplementation of YSE increased serum GLU and BUN concentrations, serum T-SOD, T-AOC, CAT activity (P < 0.05), and decreased serum BUN and MDA concentrations (P < 0.05), and had no effect on serum T-CHO concentration, serum AST, ALT and A-CHE activity (P > 0.05). Piglets orally administered CU decreased serum MDA concentration (P < 0.05), increased serum T-SOD, T-AOC and CAT activity (P < 0.05), and had no significant effect on serum GLU, T-CHO concentration, serum AST, ALT, A-CHE activity (P < 0.05). Piglets dietary administered YSE and oral administered CU had significant interaction effects on serum T-SOD, T-AOC, and CAT activity and serum MDA concentration (P < 0.05), and had a decrease trend on serum BUN concentration (P = 0.06), no the interaction effect on serum GLU and T-CHO concentration, and serum T-SOD, AST, ALT and A-CHE activity (P > 0.05).
As shown in Figure 1, the structure of ileal villi of piglets in the control group was incomplete, there was shedding linearity, and the villi were sparsely arranged. Intestinal morphology and structure were not fully recovered, but the development of ileal villus structure of the three experimental groups was better than that of the control group, and the mucosal development was improved in varying degrees.
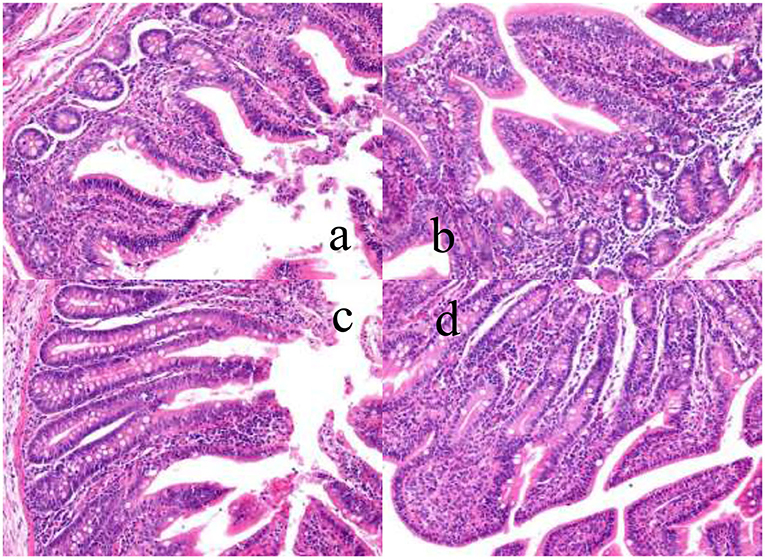
Figure 1. The effects of dietary YSE and oral CU on villus height and crypt depth of ileum in weaned pigs ( ×400). (a) represents piglets fed basal diet and orally administered 1 mL of 0.85% saline (CON), (b) represents piglets fed basal diet and orally administered 1 mL 1 × 109 cfu/mL C. utilis in 0.85% saline (CU), (c) represents piglets fed the basal diet containing YSE (120 mg/kg) and orally administered 1 mL of 0.85% saline (YSE), (d) represents Piglets fed the basal diet containing 120 mg/kg YSE and 1 mL 1 × 109 cfu/mL C. utilis in 0.85% saline (YSE+CU).
As shown in Table 6, dietary supplementation of YSE increased ileal villus height, villus height/crypt depth (P < 0.05), and decreased crypt depth (P < 0.05). Piglets orally administered CU increased ileal villus height and villus height/crypt depth (P < 0.05), tended to increase villus height (P <0.10), and decreased crypt depth (P < 0.05). There were significant interaction effects on between YSE and CU was significant in crypt depth, villus height/crypt depth (P <0.01), and trended to increase villus height (P = 0.08).
Figure 2 and Table 7 showed that dietary supplementation of YSE increased jejunal occludin, β-definsin-2 and ileal occludin concentration (P < 0.05), but had no significant effect on ileal β-definsin-2 levels (P > 0.05). Oral administration of CU increased the levels of occludin and β-definsin-2 in jejunum (P < 0.05), and had no significant effect on the concentration of occludin and β-definsin-2 in ileal mucosa (P > 0.05). There was the interaction effect on the concentration of occludin in jejunal mucosa between YSE and CU (P < 0.05), but not at the level of β-definsin-2 in jejunal and ileal mucosa (P > 0.05).
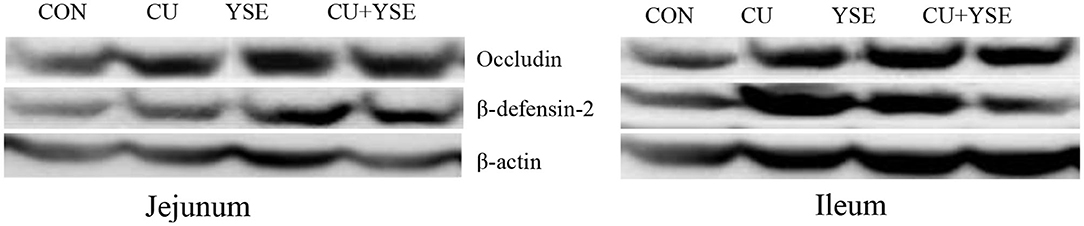
Figure 2. The effect of dietary YSE and oral CU on level of intestinal mucosa β-definsin-2 and occludin concentrations. Piglets fed basal diet and orally administered 1 mL of 0.85% saline (CON); piglets fed basal diet and orally administered 1 mL 1 × 109 cfu/mL C. utilis in 0.85% saline (CU); piglets fed the basal diet containing YSE (120 mg/kg) and orally administered 1 mL of 0.85% saline (YSE); Piglets fed the basal diet containing 120 mg/kg YSE and 1 mL 1 × 109 cfu/mL C. utilis in 0.85% saline (YSE+CU).
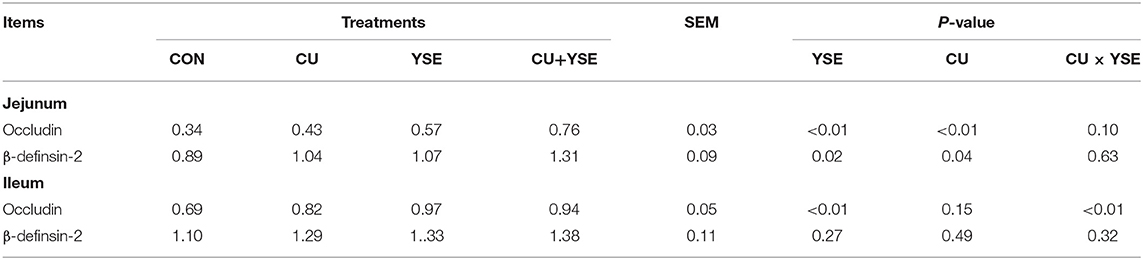
Table 7. Effects of dietary YSE and oral CU on level of intestinal mucosa β-definsin-2 and occludin concentration.
A total of 688,961 sequences were obtained from 12 samples in 4 groups by Miseq sequencing. After filtering some low abundance sequence, 566,122 sequences with 308 bp the average length were clustered into 3,498 OTU. These bacteria were divided into 21 phyla, 38 classes, 72 orders, 116 families, 294 genera, and 305 species. A Venn diagram was used to explore similarities and differences in microbial communities among groups, showing that the intestinal microbial communities in the caecum contents of piglets in the four groups had 959 common OTUs, accounting for 84.53% (Figure 3A). There were 244 specific OTUs in the control group, accounting for 0.87%, 246 specific OTU in the CU group, 230 specific OTUs in the YSE group, 319 specific OTUs in CU+YSE group.
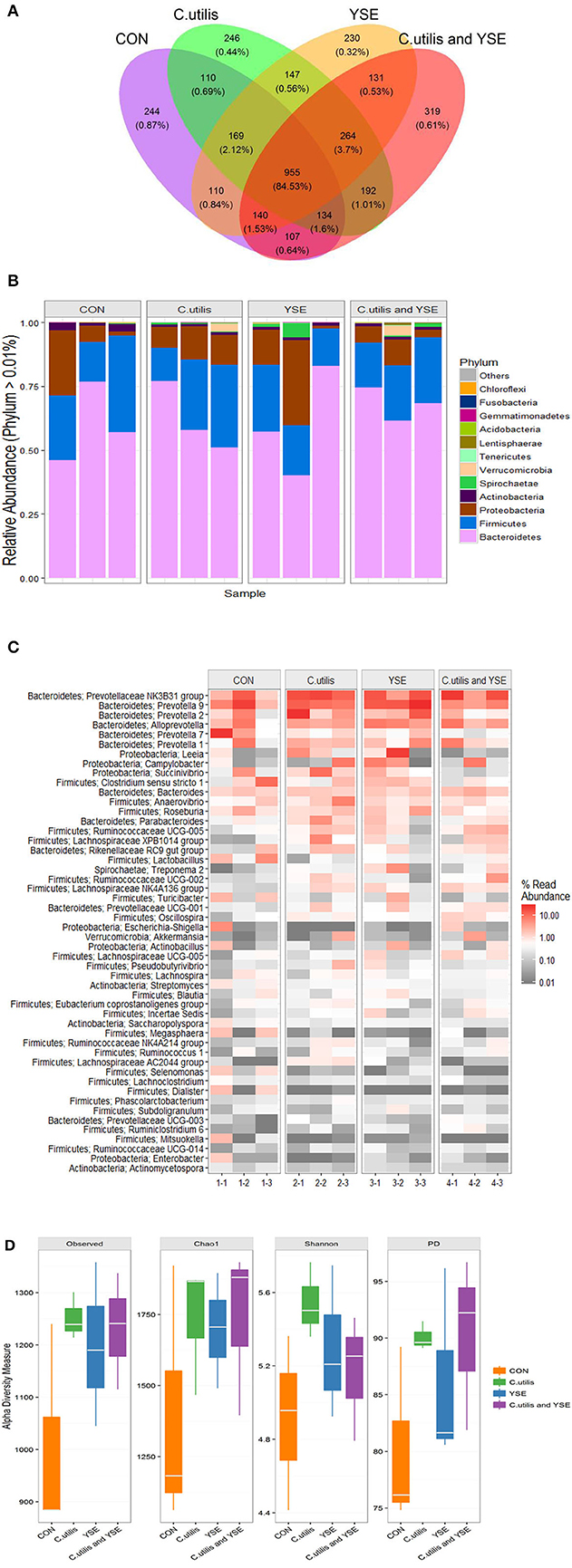
Figure 3. Effects of dietary YSE and oral CU on cecal microflora of weaned piglet. (A) The Ven pot; (B) phylum barplot; (C) genus heatmap; (D) Alpha diversity analysis; piglets fed basal diet and orally administered 1 mL of 0.85% saline (CON); piglets fed basal diet and orally administered 1 mL 1 × 109 cfu/mL C. utilis in 0.85% saline (C. utilis); piglets fed the basal diet containing YSE (120 mg/kg) and orally administered 1 mL of 0.85% saline (YSE); Piglets fed the basal diet containing 120 mg/kg YSE and 1 mL 1 × 109 cfu/mL C. utilis in 0.85% saline (C. utilis and YSE).
Table 8 and Figure 3B showed dietary supplementation of YSE decreased the relative abundance of acidobacteria in cecum of weaned piglets (P < 0.05), the relative abundance of Spirochaetae had an increasing trend (P = 0.072). However, it had no significant effect on the relative abundance of Bacteroides, Firmicutes, Proteobacteria, Actinobacteria, Verrucomicrobia, Tenericutes, and Lentisphaerae (P > 0.05). Oral administration of CU decreased the relative abundance of Proteobacteria and Actinobacteria (P < 0.05), and increased the relative abundance of Verrucomicrobia microbacteria, but had no effects on the relative abundance of Bacteroides, Firmicutes, Spirochaetae, Tenericutes, Lentisphaerae, and Acidobacteria (P > 0.05). There was no an interaction effect on the relative abundance of Proteus bacteria between YSE and CU, and decreased the relative abundance of Proteus bacteria (P < 0.05). YSE and CU had certain effects on the structure of cecal microflora of weaned piglets (P < 0.05).
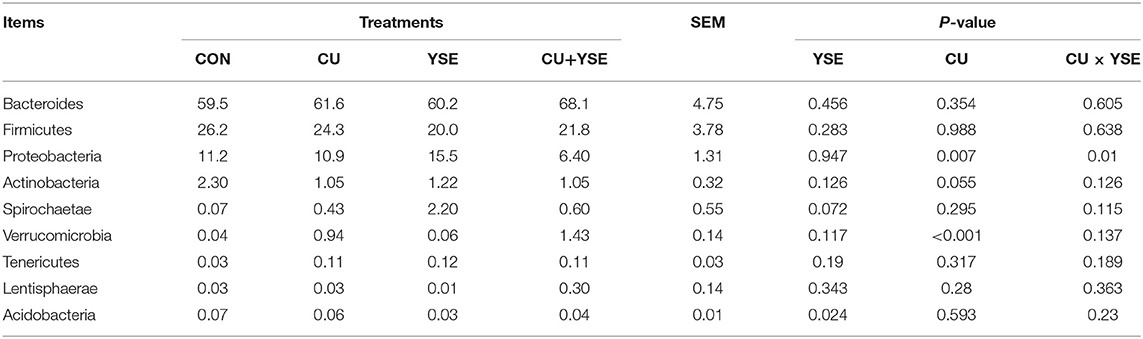
Table 8. Effects of dietary YSE and oral CU on the relative abundance of caecum microflora at phylum level.
Table 9 and Figure 3C showed that the relative abundance of bacteria at the cecal microflora level of weaned piglets. Dietary supplementation of YSE increased the relative abundance of Bacteroides in cecum of weaned piglets (P < 0.05), and decreased the relative abundance of Clostridiaceae, Veillonellaceae, Erysipelotrichaceae, Acidaminococcaceae, Streptococcaceae, Campylobacteraceae, and Streptomycetaceae in weaned piglets (P < 0.05). It had no significant effect on the relative abundance of bacteria in Prevotellaceae, Porphyromonadaceae, Rikenellaceae, Cytophagaceae, Ruminococcaceae, Lactobacillaceae, and so on (P > 0.05). Oral administration of CU increased the relative abundance of Bacteroidales, Lachnospiraceae, and Ruminococcaceae (P < 0.05), and significantly decreased the relative abundance of Clostridiaceae, Streptococcaceae, Enterobacteriaceae, and Streptomycetaceae (P < 0.05). There no significant effect on the relative abundance of bacteria in Prevotellaceae, Porphyromonadaceae, Rikenellaceae, and Cytophagaceae (P > 0.05). YSE and CU had significant interaction effects on the relative abundance of Bacteroidales, Lachnospiraceae, Erysipelotrichaceae, Streptococcaceae, Campylobacteraceae, Enterobacteriaceae, Succinivibrionaceae, and Streptomycetaceae (P < 0.05).
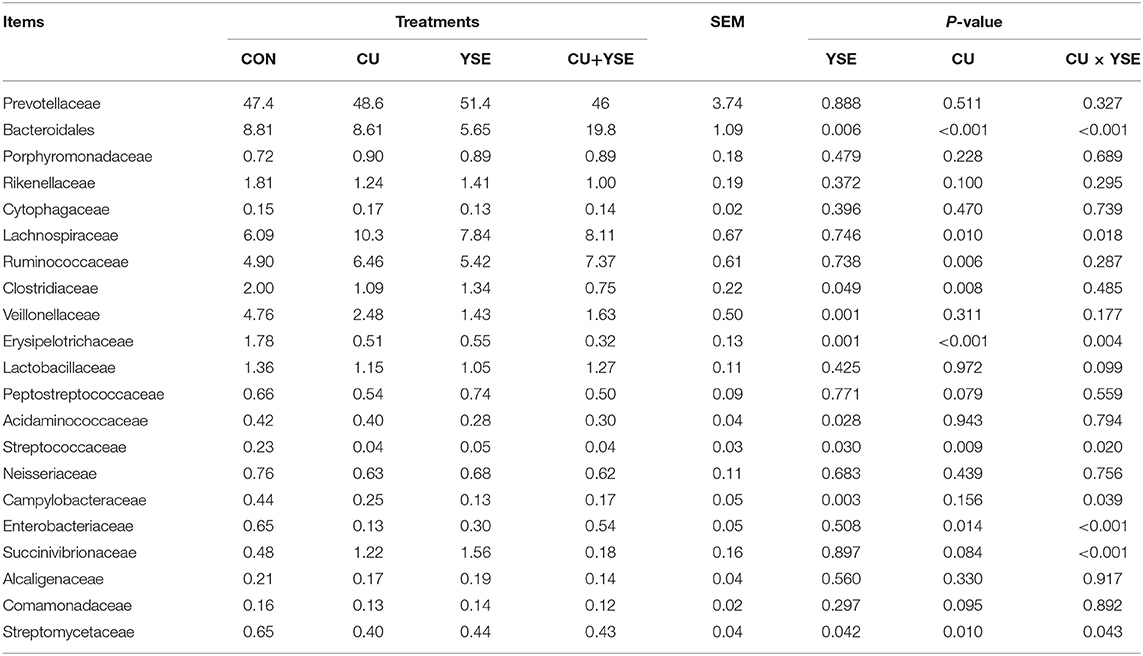
Table 9. Effects of dietary YSE and oral CU on the relative abundance of caecum microflora at family level.
Table 10 and Figure 3D showed that compared with the control group, the Observed species, Chao1 and Fisher indexes of YSE group, CU group and mixed group were significantly higher than those of the control group (P < 0.05). The Shannon index in the YSE group and CU group was significantly higher than that in the control group (P < 0.05), but there was no significant difference between the mixed group and the control group (P > 0.05). There was no significant difference in PD index between CU group and mixed group (P > 0.05), but it was significantly higher than that in YSE group and control group (P < 0.05), and that in YSE group was significantly higher than that in control group (P < 0.05).
Our results showed that CU had a positive effect on the growth performance of weaned piglets. Oral active yeast preparation for early weaned piglets can help to relieve diarrhea, promote piglet growth, improve survival rate and growth performance (12). Active yeast preparation can promote the growth performance and enhance the immune function of piglets, and the mechanism of action may be related to the main components of cell wall, β-glucan, phosphorylated oligosaccharides and intracellular active peptides (13, 14). In this study, we fed CU together with its fermentation medium to piglets. Nucleotides, amino acids, peptides and other flavor substances in yeast culture were important factors to improve the feed intake of piglets. Yeast culture could significantly increase the feed intake of piglets (15). Yeast culture could improve ADG and feed utilization of piglets (16–18). Yeast culture stimulated intestinal fermentation, increased the yield of volatile fatty acids and the products of bacterial fermentation, and provided some energy for pigs to improve nutrient utilization (19–21).
Dietary 120 mg/kg YSE and oral CU in weaned piglets was 27.9% lower than that of the control group. YSE saponins reduce the concentration of ammonia and provide a good environment for digestion and absorption of nutrients. It is also the result of antioxidant and anti-inflammatory effects of YSE polyphenols. Some studied showed that YSE reduced the concentration of ammonia in livestock barn or the content of ammonia nitrogen in feces, but had no significant effect on growth performance (22, 23). This may be caused by different feed formula, environment, adding amount, physiological stage of pigs and so on. The feed composition, especially the content of crude protein, may affect the effect of YSE (24). Dietary 120 mg/kg YSE in weaned piglets also decreased the ammonia emission and improved growth performance (23). The addition amount of YSE in pig diets was different in different physiological stages. Generally speaking, the addition amount of YSE was 50–200 mg/kg.
The organ index can reflect the functional status of animals to a certain extent (25). Our results showed that oral CU tended to promote the development of spleen and mesenteric lymph nodes, and dietary YSE in diet promoted the spleen, pancreas and mesenteric lymph nodes. The specific mechanism of CU in promoting immune function may be related to the composition of cell wall. A certain proportion of yeast culture increased the concentration of intestinal IFN-γ in weaned piglets (15). YSE increased the immune organ index at low dose (100 and 200 mg/kg), but decreased the immune organ index at high dose (26).
Our results showed that piglets dietary administered YSE reduced rectal pH and urine pH significantly, piglets orally administered CU reduced urine pH and piglets dietary administered YSE and orally administered CU decreased gastric pH, ileal pH, cecal pH and urine pH. Oral yeast preparation decreased the pH value of intestinal segment, especially hindgut segment (27–29). Probiotic yeast inhibited the growth of Escherichia coli, promote the proliferation of Bifidobacterium and Lactobacillus, and cause the increase of intestinal VFA content. Dietary YSE could reduce the pH value of rumen fluid of dairy cows (30).
Our study showed that dietary YSE decreased the content of plasma urea nitrogen, which was consistent with the results of previous studies (31). Dietary 750 mg/kg significantly increased the content of ALT in animals, but high doses of YSE may have side effects on animals (32). In this study, dietary 120 mg / kg of YES did not affect the content of ALT, which indicated that this addition was safe in weaned piglets. Our study results showed that the extract of CU could significantly improve the antioxidant capacity and the contents of plasma glucose, urea nitrogen and total cholesterol in weaned piglets. Dietary YSE significantly decreased the level of serum glucose in diabetic rats (33). The hypoglycemic function of YSE is related to its saponins (34–36). In this study, dietary supplementation of YSE decreased plasma MDA concentration and increased plasma SOD concentration, which may be related to the scavenging effect of YSE on superoxide free radicals or preventing the formation of superoxides or peroxides (37). Our study showed that CU increased T-SOD activity, CAT activity, TmurAOC, and decreased MDA content, indicating that CU may have more potential in improving animal antioxidant capacity, which may be related to the ability of CU to synthesize glutathione (38, 39). Among all the experimental groups, CU and YSE synergistically improved the antioxidant capacity of weaned piglets, and the mixed group improved the antioxidant capacity of weaned piglets most obviously.
Villus height, crypt depth and villus height / crypt depth are important indexes to evaluate the absorption function of small intestine. Our results showed that oral CU and diet supplemented with YSE promoted the development of ileal mucosa, and they synergistically increased villus height / crypt depth. Under the physiological state, the villous epithelial cells fall off normally. The exfoliated cells migrate, differentiate and produce mature villus cells from the base of the crypt to the villus end (40). The depth of crypt becomes shallower, the maturation rate of epithelial cells increases, and the absorptive capacity of villi increases. Qi et al. (41) found that dietary YSE in piglets to reduce the concentration of ammonia nitrogen in the contents of duodenum, jejunum, ileum, cecum and colon, improve the intestinal villus structure of piglets, increase the villus width of jejunum, increase the villus length and width of jejunum and the ratio of villus length to crypt depth of jejunum. Yeast culture was used as a foreign antigen to stimulate the development of intestinal tract. Some studies have shown that the addition of active yeast or yeast and its culture to the diet is beneficial to the development of intestinal mucosa and promote intestinal health (42, 43). In this study, it was found that YSE and CU increased the villus height of ileal mucosa, villus height/crypt depth, and decreased the crypt depth, indicating that YSE and CU can alleviate intestinal health problems caused by weaning stress. This is also one of the reasonable explanations for the reduction of diarrhea rate in the above study.
Our study showed that oral CU and dietary YSE increased Occludin concentration in jejunum and ileum and the β-definsin-2 concentration in jejunum, and increased the level of occludin in jejunum and ileum. The main mode of connection between intestinal mucosal epithelial cells is tight junction to maintain the integrity of the mechanical structure and function of the intestinal mucosal barrier. Tight junctions are composed of peripheral cytoplasmic proteins such as occludin, claudins, junction adhesion molecules, and closed small ring proteins, in which occludin is a transmembrane protein related to the integrity of tight junctions. Studies have found that probiotics inhibit the translocation of pathogenic bacteria on the intestinal surface by maintaining the integrity of intestinal mucosa and reducing intestinal mucosal permeability, preventing toxins and harmful substances from entering the blood circulation, and pathogens didn't further invade the body (44). Oral administration of Saccharomyces cerevisiae induced specific immune response in mice infected with Clostridium, significantly increased the level of intestinal mucosal IgA alleviated diarrhea and intestinal mucosal damage induced by endotoxin produced by Clostridium, indicating that probiotics such as yeast can enhance intestinal immune response and maintain intestinal health (45). The possible mechanisms of probiotics protecting intestinal mucosal barrier are: maintaining the balance of intestinal flora, protecting microbial barrier, promoting mucus secretion, promoting the expression of tight junction protein, strengthening intestinal mucosal mechanical barrier, and stimulating intestinal mucosal immune response and inhibition of intestinal epithelial cell apoptosis (46).
The Venn diagram is used to reflect the common and unique species among the samples. Our research showed that the four groups accounted for 84.53% of the total OTU, while the unique OTU control group, CU group, YSE group and CU+YSE group were 0.87, 0.44, 0.32, and 0.61%, respectively, indicating that the experimental treatment had little effect on the core species in the intestinal tract of weaned piglets. The intestinal microflora of piglets has been changing since birth until a stable top community is formed (47). The proportion of OTU shared by the four groups reached 84.63%, which may be related to the stable top community gradually formed after weaning.
Our research showed that at the gate level, the cecal microorganisms of weaned piglets were mainly composed of Bacteroides and Firmicutes, which was consistent with the results of previous studies (48, 49). YSE and CU had no significant effect on the relative abundance of Bacteroides and Firmicutes bacteria, indicating that the addition of them had no significant effect on the dominant flora of weaned piglets. Proteobacteria are gram-negative bacteria, and many of them are pathogenic bacteria (50). Our results also showed that the YSE had no significant effect on the relative abundance of Proteus, but CU significantly reduced the relative abundance of Proteus, and the effect was better than that of CU alone. Acidobacteria are related to denitrification and nitrogen metabolism, and are greatly affected by nitrogen sources and pH (51). Our results showed that the YSE decreased the relative abundance of the bacteria, which may be related to the decrease of ammonia in the intestine. Bacteroides can ferment glucose, fructose, galactose, lactose, sucrose, and dextrin to produce acid and gas, resulting in a waste of energy. Our study showed that the increase of the relative abundance of Bacteroides was mainly caused by the interaction between YSE and CU, indicating that the interaction between YSE and CU was not ideal in improving the relative abundance of Bacteroides. Lachnospiraceae and Ruminococcaceae can ferment cellulose to produce butyric acid to inhibit colitis, so we usually think that they are beneficial bacteria (52). Our study showed that oral CU and its culture increased the relative abundance of Lachnospiraceae and Ruminococcaceae in weaned piglets, which was beneficial to the maintenance of intestinal health. We believe that the effect of yeast and its culture on increasing beneficial bacteria and reducing harmful bacteria may be related to oligosaccharides and other active components in yeast culture. These active substances maintain gastrointestinal microecological balance, selectively promote the proliferation of beneficial flora and inhibit the reproduction of harmful bacteria.
In addition, our study showed that the abundance and diversity of cecal microflora in weaned piglets increased with the addition of YSE or CU, alone or in combination. The increase of microbial diversity is usually positively correlated with the stability of microflora and the ability to resist the invasion of pathogens. Our results showed that dietary supplementation of YSE and CU could improve the diversity of cecal microflora and improve the health level of weaned piglets. Beta diversity analysis can support this result. YSE and CU had a certain effect on the structure of intestinal microflora of weaned piglets, which changed the sample distance between the control group and the experimental group in Beta diversity analysis. With the addition of YSE or CU, the change of microflora developed in the same direction, which showed that the samples of the three experimental groups were gathered together. It is suggested that the effects of YSE and CU on intestinal microorganisms may be consistent.
This study provides evidence that YSE and CU are expected to replace antibiotics in the production of weaned piglets. Our results demonstrate that YSE and CU can improve the growth performance of weaned piglets, reduce the diarrhea rate of weaned piglets, improve intestinal health, and increase the diversity and richness of cecal microflora of weaned piglets. At the same time, we provided the specific information of YSE and CU on the growth performance and healthy development of weaned piglets, thus providing information for the development of feed plans that can improve or resist the stress of weaning piglets.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
All experimental procedures in this study were approved by Sciences Animal Ethics Committee of Southwest University. Written informed consent was obtained from the owners for the participation of their animals in this study.
ZT and ZY designed the whole experiment. YW and TH performed the experiment, including chemical analysis, and statistical analysis. ZY, TH, and YW worked on the manuscript. ZS, GZ, LW, and WS verified the validity of experiment and checked the results. ZS, GZ, LW, and WS participated in the experiment design and gave valuable advice. All authors have read and approved the final version of this manuscript.
This study was funded in part by National Natural Science Foundation of China (31772610 and 31902167), Chongqing Natural Science Foundation (cstc2019jcyj-msxmX0524), the National Science Foundation for Post-doctoral Scientists of China (2018M640895), Chongqing key innovation project for overseas students (cx2017024), National Key R&D Program of China (2018YFD0501000), the Special Funding for Postdoctoral Research Projects in Chongqing (XMT 2081061), National Program on Key Basic Research Project of China (2013CB127303), and China Scholarship Council (201508505170).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Professor De Wu and Professor Guozhong Dong for their insightful suggestions on experimental design.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.685540/full#supplementary-material
1. Alagawany MM, Farag MR, Dhama K, El-Hack MEA, Alam GM. Mechanisms and beneficial applicationsof resveratrol as feed additive in animaland poultry nutrition: a review. Int J Pharmacol. (2015) 11:213–21. doi: 10.3923/ijp.2015.213.221
2. Alagawany MM, Farag MR, Dhama K. Nutritional and biological effects of turmeric (Curcuma longa) supplementation on performance, serum biochemical parameters and oxidative status of broiler chicks exposed to endosulfan in the diets. Asian J Anim Vet Adv. (2015) 10:86–96. doi: 10.3923/ajava.2015.86.96
3. Alagawany M, El-Hack MEA. The effect of rosemary herb as a dietary supplement on performance, egg quality, serum biochemical parameters, and oxidative status in laying hens. J Anim Feed Sci. (2015) 24:341–7. doi: 10.22358/jafs/65617/2015
4. Dhama K, Latheef SK, Mani S, Samad HA, Karthik K, Tiwari R, et al. Multiple beneficial applications and modes of action of herbs in poultry health and production-a review. Int J Pharmacol. (2015) 11:152–76. doi: 10.3923/ijp.2015.152.176
5. Sahoo SP, Kaur D, Sethi APS, Sharma A, Chandra M. Evaluation of yucca schidigera extract as feed additive on performance of broiler chicks in winter season. Vet World. (2015) 8:556–60. doi: 10.14202/vetworld.2015.556-560
6. Štochmalová A, Kadasi A, Alexa R, Grossman R, Sirotkin A. The effect of yucca on proliferation, apoptosis, and steroidogenesis of porcine ovarian granulosa cells. Potravinarstvo. (2014) 8:87–91. doi: 10.5219/357
7. Wang JP, Kim IH. Effect of caprylic acid and yucca schidigera extract on production performance, egg quality, blood characteristics, and excreta microflora in laying hens. Br Poultry Sci. (2011) 52:711–7. doi: 10.1080/00071668.2011.635638
8. Gebhardt JT, Woodworth JC, Tokach MD, Derouchey JM, Goodband RD, Loughmiller JA, et al. Determining the influence of chromium propionate and yucca schidigera on growth performance and carcass composition of pigs housed in a commercial environment1. Trans Anim Sci. (2019) 3:1275–85. doi: 10.1093/tas/txz117
9. Marek K, Katarzyna B, Javier JL, Anna MK, Joanna S. Metabolic response of the yeast candida utilis during enrichment in selenium. Int J Mol Sci. (2020) 21:5287–304. doi: 10.3390/ijms21155287
10. Wang Y H, Xu M, Wang FN, ZP Yu, Yao JH, Zan LS, et al. Effect of dietary starch on rumen and small intestine morphology and digesta pH in goats. Livest Sci. (2010) 122:48–52. doi: 10.1016/j.livsci.2008.07.024
11. Edgar R C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nature Methods. (2013) 10:996–8. doi: 10.1038/nmeth.2604
12. Anna ST, Tomasz H, Magorzata K, Bogusaw F. Dietary Supplementation of a yeast-whey preparation for weaned piglets. Acta Vet. (2020) 70:126–35. doi: 10.2478/acve-2020-0009
13. Piyum AK, Justin M, Nhuan PN, Kevin BH, David GS. Conversion of deoxynivalenol to 3-acetyldeoxynivalenol in barley-derived fuel ethanol co-products with yeast expressing trichothecene 3-O-acetyltransferases. Biotechnol Biofuels. (2011) 4:26–38. doi: 10.1186/1754-6834-4-26
14. Wüthrich D, Irmler S, Berthoud H, Guggenbühl B, Eugster E, Bruggmann R. Conversion of methionine to cysteine in lactobacillus paracasei depends on the highly mobile cysK-ctl-cysE gene cluster. Front Microbiol. (2018) 9:2415. doi: 10.3389/fmicb.2018.02415
15. Shen YB, Piao XS, Kim SW, Wang L, Liu P, Yoon I, et al. Effects of yeast culture supplementation on growth performance, intestinal health, and immune response of nursery pigs. J Anim Sci. (2009) 87:2614–24. doi: 10.2527/jas.2008-1512
16. Zhang SH, Wu ZH, Heng JH, Song HQ, Tian M, Chen F, et al. Combined yeast culture and organic selenium supplementation during late gestation and lactation improve preweaning piglet performance by enhancing the antioxidant capacity and milk content in nutrient-restricted sows. Anim Nutr. (2020) 6:160–7. doi: 10.1016/j.aninu.2020.01.004
17. Zhaxi YP, Meng XQ, Wang WH, Wang L, Pu WX. A Yeast probiotic, improves intestinal mucosa integrity and immune function in weaned piglets. Sci Rep. (2020) 10:4556–68. doi: 10.1038/s41598-020-61279-6
18. Lv LK, Zhang H, Liu ZY, Lei L, Feng Z, Zhang DD, et al. Comparative study of yeast selenium vs.sodium selenite on growth performance, nutrient digestibility, anti-inflammatory and anti-oxidative activity in weaned piglets challenged by Salmonella typhimurium. Innate Immun. (2020) 26:258–68. doi: 10.1177/1753425919888566
19. Kiros TG, Derakhshani H, Pinloche E, D'Inca R, Marshall J, Auclair E, et al. Effect of live yeast Saccharomyces cerevisiae (Actisaf Sc 47) supplementation on the performance and hindgut microbiota composition of weanling pigs. Sci Rep. (2018) 8:5315–27. doi: 10.1038/s41598-018-23373-8
20. Khan SA, Zhang MZ, Liu L, Dong LH, Ma YX, Wei ZC, et al. Co-culture submerged fermentation by lactobacillus and yeast more effectively improved the profiles and bioaccessibility of phenolics in extruded brown rice than single-culture fermentation. Food Chem. (2020) 326:126985–92. doi: 10.1016/j.foodchem.2020.126985
21. Bertsch A, Roy D, LaPointe G. Fermentation of wheat bran and whey permeate by mono-cultures of Lacticaseibacillus rhamnosus strains and co-culture with yeast enhances bioactive properties. Front Bioeng Biotech. (2020) 8:956–63. doi: 10.3389/fbioe.2020.00956
22. Hong JW, Kim IH, Moon TH, Kwon OS, Lee SH, Kim YG. Effects of yucca extract and (or) far infrared emitted materials supplementation on the growth performance, serum characteristics and ammonia production of growing and finishing pigs. Asian Austral J Anim. (2001) 14:1299–303. doi: 10.5713/ajas.2001.1299
23. Colina JJ, Lewis AJ, Miller PS, Fischer RL. Dietary manipulation to reduce aerial ammonia concentrations in nursery pig facilities. J Anim Sci. (2001) 79:3096–103. doi: 10.2527/2001.79123096x
24. Sun D, Jin X, Shi B, Tong M, Yan S. Dietary yucca schidigera extract improved growth performance and liver antioxidative function in broilers. Ital J Anim Sci. (2017) 16:1–8. doi: 10.1080/1828051X.2017.1302826
25. Sun H, Tang JW, Yao XH, Wu YF, Wang X, Feng J. Effects of dietary inclusion of fermented cottonseed meal on growth, cecal microbial population, small intestinal morphology, and digestive enzyme activity of broilers. Trop Anim Health Pro. (2013) 45:987–93. doi: 10.1007/s11250-012-0322-y
26. Su JL, Shi BL, Zhang PF, Sun DS, Li TY, Yan SM. Effects of yucca extract on feed efficiency, immune and antioxidative functions in broilers. Braz Arch Biol Technol. (2016) 59:e16150035–42. doi: 10.1590/1678-4324-2016150035
27. Luiz GG, Gabriela MG, Hiam JM, Eduardo AB, Aleksandro SDS. Effects of yucca extract and organic chromium on growth performance and health of lactating lambs. Small Ruminant Res. (2020) 91:106172–8. doi: 10.1016/j.smallrumres.2020.106172
28. Oelschlager ML, Rasheed MSA, Smith BN, Rincker MJ, Dilger RN. Effects of yucca schidigera-derived saponin supplementation during a mixed Eimeria challenge in broilers. Poultry Sci. (2019) 98:3212–22. doi: 10.3382/ps/pez051
29. Theo R, Hanna S, Jessica T, Jochen W. Formation and stability of emulsions stabilised by Yucca saponin extract. Int J Food Sci Tech. (2018) 53:1381–8. doi: 10.1111/ijfs.13715
30. Tian LX, Shi BL, Fu XZ, Li TY, Zhao QL, Yue YX, et al. Effects of yucca extract on dairy cattle artificial ruminal fermentation parameters. Cereal Feed Industry. (2014) 6:53–60. doi: 10.7633/j.issn.1003-6202.2014.06.014
31. Killeen GF, Connolly CR, Walsh GA, Duffy CF, Headon DR, Power RF. The effects of dietary supplementation with yucca schidigera extract or fractions thereof on nitrogen metabolism and gastrointestinal fermentation processes in the rat. J Sci Food Agr. (2015) 76:91–9. doi: 10.1002/(SICI)1097-0010(199801)76:1 <91::AID-JSFA926>3.0.CO;2-H
32. Dos RJS, Zangerônimo MG, Ogoshi RCS, França J, Costa AC, Almeida TN, et al. Inclusion of Yucca schidigera extract in diets with different protein levels for dogs. Anim Sci J. (2016) 87:1019–27. doi: 10.1111/asj.12535
33. Nuray O. The effects of yucca schidigera on blood glucose and lipid levels in diabetic rats. African J Bioch Res. (2013) 7:179–83. doi: 10.5897/AJBR.9000237
34. Giovanni T. How treatments with endocrine and metabolic drugs influence pituitary cell function. Endocr Connect. (2020) 9:14–27. doi: 10.1530/EC-19-0482
35. Lee KT, Sohn IC, Kim DH, Choi JW, Kwon SH, Park HJ. Hypoglycemic and hypolipidemic effects of tectorigenin and kaikasaponin III in the streptozotocin-induced diabetic rat and their antioxidant activity in vitro. Arch Pharm Res. (2000) 23:461–6. doi: 10.1007/BF02976573
36. Olgun O, Alp ÖY. Effect of dietary supplementation of essential oils mixture on performance, eggshell quality, hatchability, and mineral excretion in quail breeders. Environ Sci Pollut Res. (2014) 21:13434–9. doi: 10.1007/s11356-014-3285-x
37. Liu CL, Li ZQ. Effect of levels of yucca schidigera extract on ruminal fermentation parameters, digestibility of nutrients and growth performance in sheep. Adv Mat Res. (2012) 343–4:655–60. doi: 10.4028/www.scientific.net/AMR.343-344.655
38. Li M, Chai JQ, Sun YF, Guo HJ, Zhang W, Liu YJ, et al. The optimization of feed-grade beer yeast culture conditions. Southwest China J Agric Sci. (2008) 21:829–32. doi: 10.16213/j.cnki.scjas.2008.03.067
39. Espinosa C, Esteban MÁ. Effect of dietary supplementation with yeast Saccharomyces cerevisiae on skin, serum and liver of gilthead seabream (Sparus aurata L). J Fish Biol. (2020) 97:869–81. doi: 10.1111/jfb.14449
40. Shonyela SM, Feng B, Yang WT, Yang GL, Wang CF. The regulatory effect of Lactobacillus rhamnosus GG on T lymphocyte and the development of intestinal villi in piglets of different periods. Amb Express. (2020) 10:76–86. doi: 10.1186/s13568-020-00980-1
41. Qi W, Wen J, Bo X. Catch-up growth in intrauterine growth-restricted piglets associated with the restore of pancreatic and intestinal functions via porcine glucagon-like peptide-2 microspheres. Arch Anim Nutr. (2020) 74:462–75. doi: 10.1080/1745039X.2020.1833598
42. Van CMC, Jansman AJM, Smidt H, Yoon I. Effects of yeast culture on performance, gut integrity, and blood cell composition of weanling pigs. J Anim Sci. (2007) 85:3099–109. doi: 10.2527/jas.2007-0110
43. Bontempo V, Di GA, Savoini G, Dell'Orto V, Domeneghini C. Live yeast dietary supplementation acts upon intestinal morpho-functional aspects and growth in weanling piglets. Anim Feed Sci Tech. (2006) 129:224–36. doi: 10.1016/j.anifeedsci.2005.12.015
44. Alexandre Y, Le BG, Boisramé GS, Le GF, Héry AG, Gouriou S, et al. Probiotics: a new way to fight bacterial pulmonary infections?. Med Maladies Infect. (2014) 44:9–17. doi: 10.1016/j.medmal.2013.05.001
45. Qamar A, Aboudola S, Warny M, Michetti P, Pothoulakis C, LaMont JT, et al. Saccharomyces boulardii stimulates intestinal immunoglobulin A immune response to Clostridium difficile toxin A in mice. Infect Immun. (2001) 69:2762–5. doi: 10.1128/IAI.69.4.2762-2765.2001
46. Chorawala MR, Chauhan S, Patel R, Shah G. Cell wall contents of probiotics (Lactobacillus species) protect against lipopolysaccharide (LPS)-induced murine colitis by limiting immuno-inflammation and oxidative stress. Probiotics Antimicrob. (2021) 4:1–13. doi: 10.1007/s12602-020-09738-4
47. Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. (2008) 6:e280–91. doi: 10.1371/journal.pbio.0060280
48. Isaacson R, Kim HB. The intestinal microbiome of the pig. Anim Health Res Rev. (2012) 13:100–9. doi: 10.1017/S1466252312000084
49. Looft T, Allen HK, Cantarel BL, Levine UY, Bayles DO, Alt DP, et al. Bacteria, phages and pigs: the effects of in-feed antibiotics on the microbiome at different gut locations. ISME J. (2014) 8:1566–76. doi: 10.1038/ismej.2014.12
50. Hawkey PM, Warren RE, Livermore DM, McNulty CAM, Enoch DA, Otter JA, et al. Treatment of infections caused by multidrug-resistant Gram-negative bacteria: report of the British Society for Antimicrobial Chemotherapy/Healthcare Infection Society/British Infection Association Joint Working Party. J Antimicrob Chemother. (2018) 73:2–78. doi: 10.1093/jac/dky027
51. Liu CX, Dong YH, Hou LY, Deng N, Jiao RZ. Acidobacteria community responses to nitrogen dose and form in Chinese Fir plantations in Southern China. Curr Microbiol. (2017) 74:396–403. doi: 10.1007/s00284-016-1192-8
Keywords: yucca schidigera extract, Candida utilis, occludin, β-definsin-2, microflora
Citation: Yang Z, Wang Y, He T, Ziema Bumbie G, Wu L, Sun Z, Sun W and Tang Z (2021) Effects of Dietary Yucca Schidigera Extract and Oral Candida utilis on Growth Performance and Intestinal Health of Weaned Piglets. Front. Nutr. 8:685540. doi: 10.3389/fnut.2021.685540
Received: 25 March 2021; Accepted: 22 April 2021;
Published: 26 May 2021.
Edited by:
Silvia Turroni, University of Bologna, ItalyReviewed by:
Jiashun Chen, Hunan Agricultural University, ChinaCopyright © 2021 Yang, Wang, He, Ziema Bumbie, Wu, Sun, Sun and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenguo Yang, Z3VvZ3VvMDAwMDJAMTYzLmNvbQ==; Zhiru Tang, dGFuZ3poaXJ1MjMyNkBzaW5hLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.