- 1Department of Cell and Molecular Biology, John A. Burns School of Medicine, University of Hawaii at Manoa, Honolulu, HI, United States
- 2Pacific Biosciences Research Center, University of Hawaii at Manoa, School of Ocean and Earth Science and Technology, Honolulu, HI, United States
The essential micronutrient selenium (Se) provides antioxidant defense and supports numerous biological functions. Obtained through dietary intake, Se is incorporated into selenoproteins via the amino acid, selenocysteine (Sec). Mice with genetic deletion of the Se carrier, selenoprotein P (SELENOP), and the Se recycling enzyme selenocysteine lyase (SCLY), suffer from sexually dimorphic neurological deficits and require Se supplementation for viability. These impairments are more pronounced in males and are exacerbated by dietary Se restriction. We report here that, by 10 weeks of age, female Selenop/Scly double knockout (DKO) mice supplemented with 1 mg/ml sodium selenite in drinking water develop signs of hyper-adiposity not seen in male DKO mice. Unexpectedly, this metabolic phenotype can be reversed by removing Se from the drinking water at post-natal day 22, just prior to puberty. Restricting access to Se at this age prevents excess body weight gain and restriction from either post-natal day 22 or 37 reduces gonadal fat deposits. These results provide new insight into the sex-dependent relationship between Se and metabolic homeostasis.
Introduction
Selenium (Se) has been implicated in a wide range of biological functions that are critical for human health (1). This essential trace element is translationally incorporated into selenoproteins, as the amino acid, selenocysteine (Sec). Selenoproteins, in turn, comprise a major component of the antioxidant defense system of many different tissues. Se is acquired via dietary intake and utilized in particularly high levels by the liver, kidneys, brain, testes, and skeletal muscle (2). Distribution of Se throughout the body requires the combined actions of the Se carrier, Selenoprotein P (SELENOP) and the Se recycling enzyme selenocysteine lyase (SCLY). Following absorption by the gut and transport to the liver via the portal vein, Se is used to synthesize SELENOP, which contains multiple Sec residues. After being secreted into the bloodstream, SELENOP is taken up by target tissues to be catabolized intracellularly. Proper utilization of the delivered Sec residues is dependent on SCLY, which catalyzes the breakdown of Sec into selenide, to be used for de novo selenoprotein biosynthesis (3).
The role of Se in energy homeostasis is complicated, as clinical studies have correlated both Se deficiency and high Se intake with metabolic disease in humans (4). Hepatic SELENOP has been implicated in the development of hyperglycemia (5) and insulin resistance (6) in humans and mice, respectively. Mice with genetic knockout (KO) of Scly have increased susceptibility to metabolic syndrome (7) and diet-induced obesity (8), with more dramatic effects observed in male mice. Additionally, targeted deletion of specific selenoproteins causes differential metabolic disturbances in animal models (9–11), demonstrating the impact of not only dietary Se intake, but also proper Se utilization.
We previously bred Scly KO and Selenop KO mouse strains to produce double knockout (DKO) mice. DKO mice were found to suffer from severe neurological dysfunction (12), which was subsequently found to be attenuated by prepubescent castration (13). We recently reported that although female DKO mice display less severe neurological deficits than their male counterparts, the phenotype is worsened by the removal of Se supplementation during puberty (14). Here we provide preliminary data showing that female DKO mice exhibit a metabolic phenotype not seen in male DKO mice.
Materials and Methods
The data in this report were generated from mice used in our previous publication addressing the sex-specific neurological phenotype of DKO mice (14). Male and female C57/BL6N wild-type (WT) and DKO mice were generated as previously described [9]. Since supplementation with Se is critical for DKO mouse survival, all subjects in this study were maintained on standard mouse chow containing ~0.25 ppm Se and drinking water containing 1 mg/ml sodium selenite, Na2SeO3. Mice were given ad libitum access to food and water from weaning (~18 days of age) until the age of 10 weeks, at which point they were weighed, sacrificed via CO2 asphyxiation and tissues were harvested. Inguinal and gonadal white adipose tissue (WAT) deposits were removed and weighed on a benchtop analytical balance. For some groups, Se-supplemented drinking water was replaced with non-supplemented drinking water at either 22 days or 37 days post-natal (denoted as -NoSeP22 and -NoSeP37, respectively). All procedures and experimental protocols involving animals were approved by the University of Hawaii's Institutional Animal Care and Use Committee. Animal Care and Use Committee (IACUC) Protocol: “Mechanism of Selenoprotein Synthesis and Studies of Selenoprotein Functions:” APN 09-871-9, approved: 18 July 2019. Institutional Biosafety Committee (IBC) Protocol: “Mechanism of Selenoprotein Synthesis and Studies of Selenoprotein Functions:” IBC #18-10-544-02-4A-1R, approved: 23 October 2018.
Results
Female DKO mice had significantly higher total body weights compared to their WT counterparts at 10 weeks of age (Figure 1A). WAT deposits were also significantly heavier in female DKO mice compared to WT controls (Figures 1B,C), an effect that was not observed in male DKOSe mice. These results indicate a metabolic effect specific to female DKO mice.
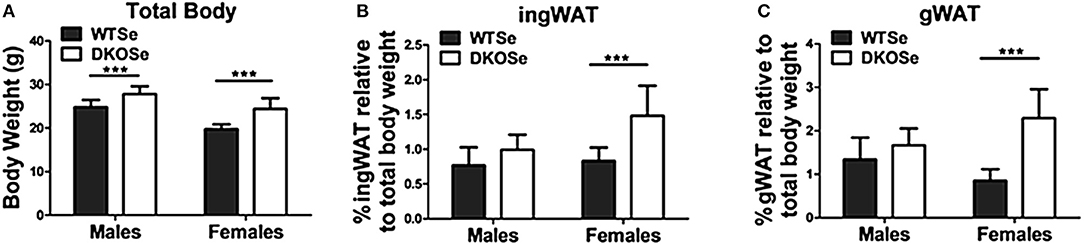
Figure 1. Body composition of wild-type (WT) and double knockout (DKO) mice of both sexes. (A) Total body weight, (B) percentage of inguinal white adipose tissue (ingWAT), and (C) percentage of gonadal white adipose tissue (gWAT) weight of male and female WT and DKO mice at 10 weeks of age. Two-way ANOVA: Total body weight Interaction NS, Genotype F(1,52) = 60.68, p = <0.0001, Sex F(1,52) = 75.15, p < 0.0001; ingWAT Interaction F(1,52) = 7.54, p = 0.0083, Genotype F(1, 52) = 31.51, p < 0.0001, Sex F(1,52) = 12.64 p = 0.0008; and gWAT Interaction F(1,52) = 18.64, p < 0.0001, Genotype F(1,52) = 47.51, p < 0.0001, Sex NS; n = 14 all groups. Bonferroni's multiple comparisons test: ***p < 0.001. All values are reported as mean ± SEM.
Previously, we demonstrated that removal of Se supplementation from female DKO mice prior to puberty at post-natal day 22 (P22) exacerbates the neurological phenotype to a similar level as male DKO mice without Se removal (14). We report here that, surprisingly, total body weight was significantly lower in female DKO when Se water was removed at P22 compared to female DKO mice with constant Se supplementation (Figure 2A). Regarding fat deposits, inguinal WAT weights trended lower in female DKO mice when Se was removed at P22 (Figure 2B). Removal of Se supplementation at either P22 or P37 significantly reduced gonadal WAT deposits in female DKO mice (Figure 2C). These data demonstrate that while female DKO mice develop a metabolic phenotype not seen in male DKO mice, this phenotype is partially attenuated by the removal of Se-supplemented drinking water.
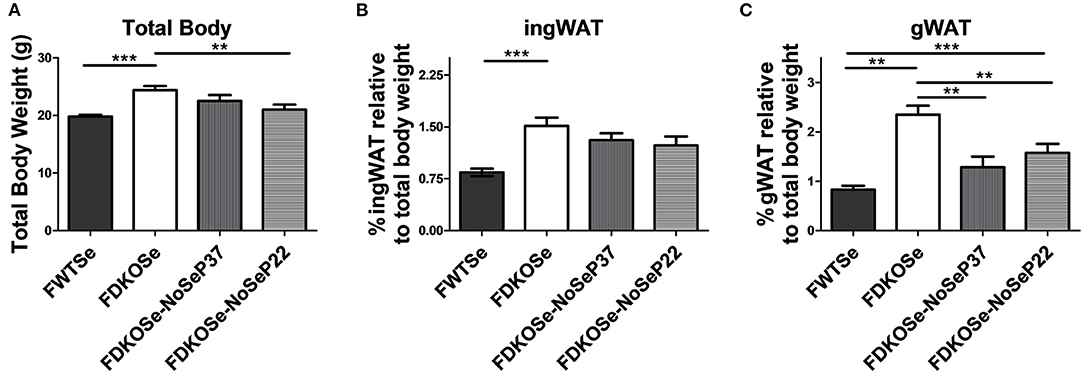
Figure 2. Effect of dietary Se removal on the body composition of female double knockout (DKO) mice. (A) Total body weight, (B) percentage of ingWAT, and (C) percentage of gWAT weight at 10 weeks of age of female wild-type (FWT), female double-knockout (FDKO), and female double knockout with Se supplementation removed at post-natal day 37 (FDKOSe-NoSeP37) or post-natal day 22 (FDKOSe-NoSeP22). One-way ANOVA: Total body weight F(3,39) = 8.46, p = 0.0002; %ingWAT F(3,39) = 7.29, p = 0.0005; %gWAT F(3,39) = 17.08, p < 0.0001; n = 13 all groups except P37 n = 4; Bonferroni's multiple comparisons test: **p < 0.01, ***p < 0.001. All values are reported as mean ± SEM.
Discussion
Although concurrent KO of Selenop and Scly in female mice causes a milder neurological phenotype compared to male DKO mice, we report here that female DKO exhibit an obesogenic phenotype marked by elevated WAT deposit weights. These results are consistent with past studies showing that Scly KO mice are predisposed to similar metabolic deficits (7, 8). This predisposition is more pronounced in male Scly KO mice, which contrasts with our current findings that female DKO mice exhibit hyper-adiposity while males do not. Considering that male DKO mice suffer from severe motor deficits and seizures, even while supplemented with Se, however, it is possible that these symptoms could mask a metabolic phenotype by affecting their ability to ambulate and eat. Thus, further characterization of DKO mice should involve analysis of physical activity and feeding behavior. Future studies should also evaluate core temperature, respiratory metabolism, and a more comprehensive analysis of adiposity, as Se has been shown to regulate adipose tissue thermogenesis (15) and lipid metabolism (16). Finally, a broad assessment of circulating hormones, such as plasma insulin, leptin, and thyroid hormones, as well as nutrients, such as glucose, triglycerides, and free fatty acids, would help detect changes in the endocrine regulation of body fat stores.
Surprisingly, challenging female DKO mice with the removal of Se supplementation in drinking water partially prevented the development of excess weight gain and hyper-adiposity. These results are in contrast with our previous findings that Scly KO mice develop signs of metabolic syndrome, including obesity, when raised on a Se-deficient diet (7). This implies that the added effect of Selenop deletion, which limits the ability of the body to distribute Se, alters the metabolic response to dietary Se restriction in mice lacking Scly. It is possible that the baseline redox environment in DKO mice is dramatically different from Scly KO mice, thus altering the compensatory mechanisms implemented in response to changes in antioxidant availability. These changes are likely complex as Se and selenoproteins have shown a capacity to differentially regulate energy metabolism through a variety of physiological processes, such as thyroid hormone metabolism (17) and insulin activity (18), as well as tissues that closely regulate energy metabolism including liver (19), pancreas (20), and the hypothalamus (21). This phenomenon is somewhat reminiscent of the observation that there appears to be a relatively narrow range of Se intake that is beneficial in humans as both low and high levels of Se status have been connected to an increased risk for type 2 diabetes (4). Thus, it is possible that the beneficial window is somehow lowered in female mice under the conditions of Selenop/Scly double KO, and that the combination of Se intake from both food and water surpasses the upper limit of that window. This could possibly explain why, in regard to metabolic phenotype, removal of Se water, while maintaining some Se intake via food consumption, appears to have a beneficial effect on female DKO mice. There are multiple pathways through which Se may affect adiposity by regulating lipid metabolism, including cholesterol synthesis and insulin signaling (7). For example, SCLY-mediated selenoprotein activity may negatively regulate the ability of insulin to induce lipogenesis in the liver by promoting protein tyrosine phosphatase 1B (PTP1B) antagonism of insulin signaling. On the other hand, SELENOP may regulate glucose metabolism in both the liver and skeletal muscle by actin on AMP-activated protein kinase (AMPK) (6). Thus, the deletion of both the Selenop and Scly genes may disrupt multiple pathways affecting adiposity. The possible intersection between Se metabolism and lipid metabolism is described in Figure 3.
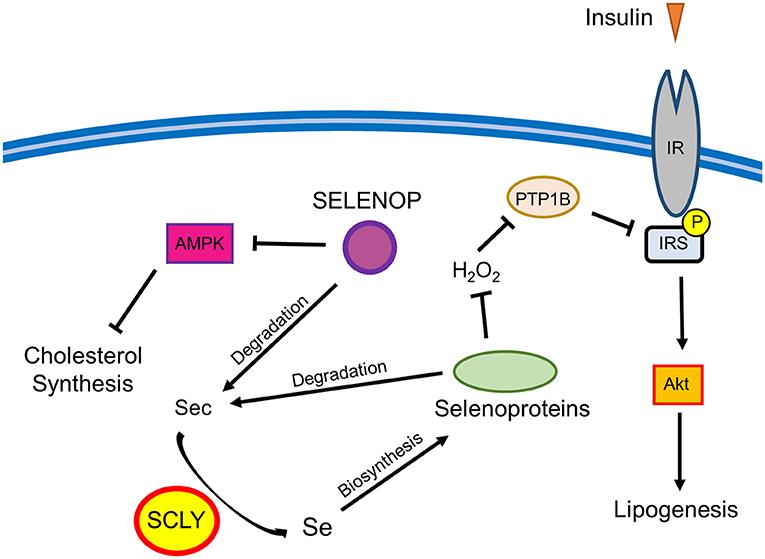
Figure 3. Schematic representation of the possible interactions between Se metabolism and lipid metabolism. Selenoprotein P (SELENOP) has shown an ability to de-activate AMP-activated protein kinase (AMPK) and inhibit insulin signaling in liver and skeletal muscle (6). AMPK limits cholesterol synthesis in the liver, thus representing a potential node through which SELENOP may regulate cholesterol. SELENOP and other selenoproteins can be degraded to produce selenocysteine (Sec) residues that are further metabolized through a process involving selenocysteine lyase (SCLY) to produce Se. This Se can then be used for de-novo synthesis of selenoproteins. The antioxidant activity of selenoproteins may promote the negative regulation of insulin signaling by preventing the oxidative inactivation of protein tyrosine phosphatase 1B (PTP1B) by hydrogen peroxide (H2O2). PTP1B de-phosphorylates the IR and insulin receptor substrate (IRS), which, in turn, reduces protein kinase B (AKT)-mediated lipogenesis. Through affecting this pathway, SCLY could work to limit lipogenesis (7).
On a final note, it is possible that the reduction of the female DKO mouse hyper-adiposity phenotype brought on by Se water removal may be affected by worsening neurological symptoms (14). It is important to note, however, that while gonadal WAT deposits were reduced in female DKO mice by Se removal at either P22 (just prior to puberty) or P37 (latter stages of puberty), the neurological phenotype of female DKO mice was shown to be aggravated only by Se removal at P22, not at P37 (14). Since female DKO mice with Se removed at P37 show no changes in neuromotor function as a result, it is, thus, likely that the reduced gonadal WAT deposits result from a distinct mechanism central to energy homeostasis. Taken together, these data implicate Selenop/Scly DKO mice as a useful model for investigation of these relationships and warrant comprehensive metabolic characterization of these mice and interrogation of underlying mechanisms.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by University of Hawaii's Institutional Animal Care and Use Committee.
Author Contributions
PK, AH, and MB were involved in conceptualization, experimental design, obtaining resources, and interpretation of data. PK and AH were involved in data acquisition and analysis. PK was involved in writing the initial drafts. DT was involved in data validation, interpretation of data, writing the initial drafts, and manuscript revisions. MB was involved in manuscript revisions and providing funding. All authors read and approved of the final manuscript.
Funding
This research was funded by The National Institutes of Health, grant numbers R01DK047320 (MB), G12MD007601 and U54MD007601 which supported core facilities, a Research Supplements to Promote Diversity in Health-Related Research, R01DK047320-19S1 to PK and a Ruth L. Kirschstein National Research Service Award F32DK124963-02 to DT.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to Matthew Pitts for insightful input on data interpretation.
References
1. Fairweather-Tait SJ, Bao Y, Broadley MR, Collings R, Ford D, Hesketh JE, et al. Selenium in human health and disease. Antioxid Redox Signal. (2011) 14:1337–83. doi: 10.1089/ars.2010.3275
2. Burk RF, Hill KE. Selenoprotein P-expression, functions, and roles in mammals. Biochim Biophys Acta. (2009) 1790:1441–7. doi: 10.1016/j.bbagen.2009.03.026
3. Esaki N, Nakamura T, Tanaka H, Soda K. Selenocysteine lyase, a novel enzyme that specifically acts on selenocysteine. Mammalian distribution and purification and properties of pig liver enzyme. J Biol Chem. (1982) 257:4386–91. doi: 10.1016/S0021-9258(18)34734-3
4. Ogawa-Wong AN, Berry MJ, Seale LA. Selenium and metabolic disorders: an emphasis on type 2 diabetes risk. Nutrients. (2016) 8:80. doi: 10.3390/nu8020080
5. Oo SM, Misu H, Saito Y, Tanaka M, Kato S, Kita Y, et al. Serum selenoprotein P, but not selenium, predicts future hyperglycemia in a general Japanese population. Sci Rep. (2018) 8:16727. doi: 10.1038/s41598-018-35067-2
6. Misu H, Takamura T, Takayama H, Hayashi H, Matsuzawa-Nagata N, Kurita S, et al. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. (2010) 12:483–95. doi: 10.1016/j.cmet.2010.09.015
7. Seale LA, Hashimoto AC, Kurokawa S, Gilman CL, Seyedali A, Bellinger FP, et al. Disruption of the selenocysteine lyase-mediated selenium recycling pathway leads to metabolic syndrome in mice. Mol Cell Biol. (2012) 32:4141–54. doi: 10.1128/MCB.00293-12
8. Seale LA, Gilman CL, Hashimoto AC, Ogawa-Wong AN, Berry MJ. Diet-induced obesity in the selenocysteine lyase knockout mouse. Antioxid Redox Signal. (2015) 23:761–74. doi: 10.1089/ars.2015.6277
9. de Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim SW, Harney JW, et al. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest. (2001) 108:1379–85. doi: 10.1172/JCI200113803
10. Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S, et al. Reactive oxygen species enhance insulin sensitivity. Cell Metab. (2009) 10:260–72. doi: 10.1016/j.cmet.2009.08.009
11. Pitts MW, Reeves MA, Hashimoto AC, Ogawa A, Kremer P, Seale LA, et al. Deletion of selenoprotein M leads to obesity without cognitive deficits. J Biol Chem. (2013) 288:26121–34. doi: 10.1074/jbc.M113.471235
12. Byrns CN, Pitts MW, Gilman CA, Hashimoto AC, Berry MJ. Mice lacking selenoprotein P and selenocysteine lyase exhibit severe neurological dysfunction, neurodegeneration, and audiogenic seizures. J Biol Chem. (2014) 289:9662–74. doi: 10.1074/jbc.M113.540682
13. Pitts MW, Kremer PM, Hashimoto AC, Torres DJ, Byrns CN, Williams CS, et al. Competition between the brain and testes under selenium-compromised conditions: insight into sex differences in selenium metabolism and risk of neurodevelopmental disease. J Neurosci. (2015) 35:15326–38. doi: 10.1523/JNEUROSCI.2724-15.2015
14. Kremer PM, Torres DJ, Hashimoto AC, Berry MJ. Disruption of selenium handling during puberty causes sex-specific neurological impairments in mice. Antioxidants. (2019) 8:40110. doi: 10.3390/antiox8040110
15. Jedrychowski MP, Lu GZ, Szpyt J, Mariotti M, Garrity R, Paulo JA, et al. Facultative protein selenation regulates redox sensitivity, adipose tissue thermogenesis, and obesity. Proc Natl Acad Sci USA. (2020) 117:10789–96. doi: 10.1073/pnas.2001387117
16. Tinkov AA, Ajsuvakova OP, Filippini T, Zhou JC, Lei XG, Gatiatulina ER, et al. Selenium and selenoproteins in adipose tissue physiology and obesity. Biomolecules. (2020) 10:40658. doi: 10.3390/biom10040658
17. Schomburg L. Selenium, selenoproteins and the thyroid gland: interactions in health and disease. Nat Rev Endocrinol. (2011) 8:160–71. doi: 10.1038/nrendo.2011.174
18. Ezaki O. The insulin-like effects of selenate in rat adipocytes. J Biol Chem. (1990) 265:1124–8. doi: 10.1016/S0021-9258(19)40166-X
19. Iizuka Y, Sakurai E, Maeda K, Hikichi N. Effects of selenium on the glycolysis and gluconeogenesis system in rat liver. Yakugaku Zasshi. (1993) 113:525–31. doi: 10.1248/yakushi1947.113.7_525
20. Saito Y. Selenoprotein P as a significant regulator of pancreatic beta cell function. J Biochem. (2020) 167:119–24. doi: 10.1093/jb/mvz061
Keywords: selenium, selenoproteins, sex differences, selenocysteine lyase, metabolic syndrome
Citation: Kremer PM, Torres DJ, Hashimoto AC and Berry MJ (2021) Sex-Specific Metabolic Impairments in a Mouse Model of Disrupted Selenium Utilization. Front. Nutr. 8:682700. doi: 10.3389/fnut.2021.682700
Received: 19 March 2021; Accepted: 07 April 2021;
Published: 10 May 2021.
Edited by:
Cristiane Cominetti, Universidade Federal de Goiás, BrazilReviewed by:
Paul Copeland, Rutgers Biomedical and Health Sciences, United StatesNikolay Solovyev, Institute of Technology, Sligo, Ireland
Copyright © 2021 Kremer, Torres, Hashimoto and Berry. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel J. Torres, ZGp0b3JyQGhhd2FpaS5lZHU=
†These authors have contributed equally to this work and share first authorship
 Penny M. Kremer1†
Penny M. Kremer1† Daniel J. Torres
Daniel J. Torres Marla J. Berry
Marla J. Berry