
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 28 October 2021
Sec. Nutritional Epidemiology
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.672462
This article is part of the Research Topic Analyzing the Relationship Between Dietary Patterns, Health Outcomes and Individual Food Choices View all 23 articles
Updated information on child feeding practices, nutritional status, and trends related to parental sociodemographic variables is required in developing countries. The objective of this study was to describe infant feeding practices and associated sociodemographic factors among Ethiopian children with an emphasis on complementary feeding (CF). Information on infant feeding and anthropometric measures was obtained from 1,054 mother-child pairs participating in a birth cohort study of children born between 2017 and 2020 prospectively followed in the city of Adama located in the Oromia region of central Ethiopia. Logistic regression models were used to identify sociodemographic and food groups associated with the initiation of CF. The introduction of complementary foods at 6 months of age was 84.7% (95% CI, 82.5, 86.8). Vegetables, cereals (teff, wheat, barley), and fruits were most often the earliest types of foods introduced. Wasting, stunting, underweight, and low body mass index (BMI) by age were found in 6.0, 16.9, 2.5, and 6.3%, respectively. Maternal age and occupation were the factors associated with timely initiation of CF [OR = 2.25, (95% CI, 1.14, 4.41)] and [OR = 0.68, (95% CI, 0.48, 0.97)], respectively. This study demonstrates that the majority of Ethiopian children in the Oromia region follow the recommendations of WHO on CF.
The introduction of complementary foods during weaning at the age of 6 months is generally recommended to ensure the growth, health, and development of children to reach their optimal growth potential during the first 2 years of childhood (1). However, several studies have demonstrated that infant feeding practices are not followed worldwide (2, 3). Early or late introduction of adequate complementary foods of suboptimal quality and quantity, as well as poor hygienic conditions, is still common in developing countries.
Early and delayed introduction of complementary feeding (CF) may result in poor nutritional status and increased morbidity in growing children (4, 5). As such, the WHO Infant and Young Child Feeding (IYCF) practices strategy was developed, which includes infant feeding recommendations for breastfeeding children under the age of 2 years, the introduction of solid and semisolid foods at the age of 6 months, and a gradual increase in the amount and frequency of different foods as the child grows older (6). Inappropriate feeding practices attributed to poor nutritional knowledge can, therefore, be a significant contributor to poor nutritional status and growth development, as well as long-term risk of developing chronic diseases in developing countries (7–9).
Several studies from the sub-Saharan regions, which include Ethiopia, Malawi, Benin, and the Democratic Republic of Congo, have identified cereals as the most common source of food in early childhood, but access is highly dependent on sociodemographic factors with significant cultural differences (10–12). However, there is a lack of recent studies exploring the paradigm shift in dietary patterns of infants in African countries with increasing wealth since the millennium.
In Ethiopia, most of the solid foods first introduced to infants between 6 and 23 months of age are homemade, since commercially ready-made foods are often beyond the reach of the low-income population. The most common solid foods are based on three major staples that are locally available, including (1) corn/enset/teff, (2) wheat/barley, and (3) sorghum/maize, all of which meet the nutritional contents recommended by WHO for children between 6 and 23 months of age (Supplementary Table 1) (13). Homemade solid foods for infants in Ethiopia are mainly based on cereals or starchy tubers such as corn (Zea mays), sorghum (Sorghum biocolor), millet (Panicum miliaceum), oats (Avena sativa), teff (Eragrostis tef ), rice (Oryza sativa), yam (Dioscorea), potato (Solanum tuberosum), barley (Hordeum vulgare), and legumes (Fabaceae). These foods are generally served as gruel, porridge, fetfet, kitta, and bread. However, the consumption of meat (beef, lamb, goat, poultry), fruits, and vegetables is very low (13).
Teff (Eragrostis tef ) is the most common staple food in Ethiopia and is an important part of the cultural heritage and national identity (14). Although teff is considered to be highly nutritious (15, 16), the 2019 Ethiopia Demographic and Health Survey (EDHS) report found that about 37% of children younger than 5 years of age were stunted (of whom 12% were severely stunted), 7% were wasted (of whom 1% were severely wasted), and 21% of the children were underweight (of whom 6% were severely underweight). The EDHS nutritional survey also found that undernutrition differed between regional location, sex, and age groups of children (17).
The aim of this study was to describe recent infant feeding practices and associated sociodemographic factors in children under the age of 2 years who were born between 2017 and 2020 and lived in the city of Adama and the surrounding Oromia region in central Ethiopia.
The main aim of the Traditional Ethiopian Food (TEF) study is to investigate the genetic propensity, feeding practices, nutritional status, and gut microbiome on the risk of celiac disease in children living in the Oromia region in central Ethiopia (18). Between 2017 and 2020, 1,389 newborn children were approached from the general population of the city of Adama and the surrounding Oromia region of central Ethiopia to be enrolled in a 4-year follow-up study. The cohort consisted of women and their offspring participating in parallel research studies at three public health facilities in the city of Adama. Mothers were participating in the pregnancy tuberculosis study investigating the role of tuberculosis infection on pregnancy outcomes, while infants were participating in the TEF birth cohort study examining the prevalence of celiac disease in children (18, 19). A physical examination was performed, and baseline data were collected when the child was 6 weeks of age and then subsequently at 9, 18, 24, 36, and 48 months of age.
At the scheduled study visits, a legal guardian filled in structured questionnaires regarding the information on the early infant feeding practices of the participating child. The form was developed in English and translated to the local language used for the data collection. The food questionnaire was developed for this study to gather information on breastfeeding, time for the introduction of complementary foods, and dietary infant feeding habits.
For the present study, information about breastfeeding duration and age at first introduction to complementary foods was examined for 1,054 children whose mothers had completed the questionnaire at the 9 and 18 months of age visits. Baseline maternal sociodemographic data were collected during the antenatal care (ANC) follow-up of the mother and at the 6-week post-delivery visit for the pregnancy tuberculosis cohort (19).
Anthropometric measurements were done by trained nurses according to the WHO manual. Children were measured while lying down without wearing shoes using a calibrated length board for height. The weight of the children was measured with lightweight clothing and recorded to the nearest 0.1 kg using an infant weight scale. A Z-score <-2 SD in height-for-age was defined as stunted, <-2 SD in weight-for-height was defined as wasted, <-2SD in weight-for-age was defined as underweight, and <-2 SD in body mass index (BMI) for age was defined as low BMI (20).
Frequency distribution and descriptive characteristics were investigated for maternal age, marital status, education, occupation, family size, as well as the birth order, sex, and age of the child at the introduction of selected solid foods. Logistic regression models were used to identify sociodemographic variables associated with the timing of complementary food initiation. After the necessary adjustments, adjusted odds ratios (AOR) with 95% confidence intervals (CI) were used to assess the strength of association. P-values <0.05 were considered statistically significant. Data were analyzed using the Statistical Package for the Social Sciences (SPSS) software for Windows (version 25; SPSS Inc. Chicago, IL). Anthropometric data were analyzed by using WHO Anthro (version 3.2.2) and supported by SPSS.
A total of 1,054 mother-child (53.5% boys) pairs from the 9- and 18-month visits were included in the study (Table 1). The median age of mothers in the study was 25 (range 15–40) years. The distribution of the marital status of mothers showed that the majority of women (96.5%) were married. More than half of the mothers (68.8%) had 6 years of primary school education or longer. Most of the women identified their religious affiliation as Orthodox Christianity (60%), were housewives by occupation (63.8%), and had an average of three family members living in the household (72.6%). Information about the mode of the delivery indicated that most mothers gave birth by vaginal delivery (84.4%). A third of the participating infants (33.6%) was the first-born child, and the median age of the children at the time of data collection was 9 months (range 9–18).
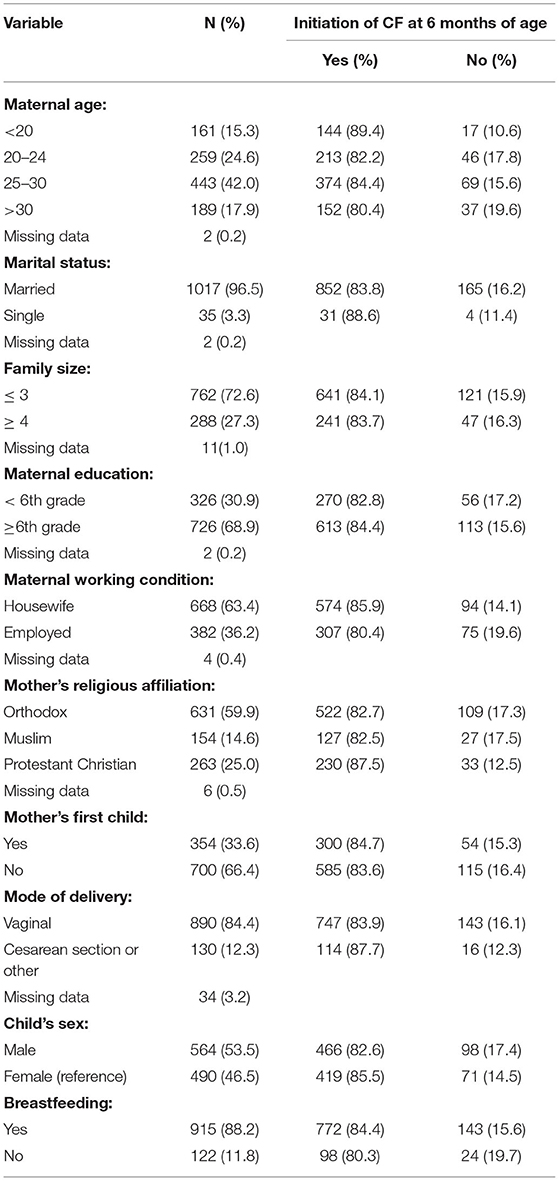
Table 1. Proportion of initiation of complementary feeding (CF) at 6 months of age by maternal sociodemographic characteristics.
At the age of 6 months, 86.1% of the children were still being breastfed and had started CF. The majority (84.0%) of children were introduced to solid, semi-solid, or liquid forms of complementary food, on an average at 6 months of age. Few children (3.5%) were introduced to complementary food before 6 months of age. A cow milk-based infant formula was among the first foods to be introduced, often initiated earlier than 2 months by some families. The most common foods introduced before 6 months were milk, vegetables, fruits, gluten-containing cereals, legumes, gluten-free cereals, while meat or eggs were the most common foods introduced at 6 months of age (Figure 1).
Gluten-containing cereals (wheat, barley, and rye) were introduced at a median age of 6.8 months (range 4–18), while a gluten-free cereal such as teff was introduced at a median age of 7.0 (range 5–16) months. The majority of children were fed with both gluten-containing foods (53.0%) and gluten-free cereal-based foods (52.0%) more than two times a day. Vegetables, fruits, gluten-containing grains, legumes, and milk were often introduced simultaneously at 6 months, whereas teff, meat, and eggs were introduced, on average, after 6 months of age.
Young maternal age ( < 20 years) was associated with timely initiation of CF (i.e., by age of 6 months) as compared to older mothers (> 20 years) [COR = 2.06, (95% CI, 1.11, 3.83) and AOR = 2.66, (95% CI, 1.28, 5.53)]. Mothers who were employed outside of the home were also associated with the time of initiation of CF compared with mothers who reported their occupation as a housewife [COR = 1.49, (95% CI, 1.07, 2.08) and AOR = 0.68, (95% CI, 0.48, 0.97)] (Table 2). None of the other sociodemographic factors (maternal education, marital status, religion, the birth order of the child, or gender) were associated with the timing of CF in this study.

Table 2. Multivariable logistic regression analysis of factors associated with the initiation of CF at 6 months of participating children.
The child anthropometry revealed that about 16.9% of the children were stunted (Figure 2). The corresponding numbers for wasting, underweight, and low BMI by age were 4.5, 2.5, and 6.3%, respectively (Figure 2). There was an association between a child's sex with stunting (95% CI, 0.12, 0.63) and underweight (95% CI, 0.08, 0.036), respectively (Figure 2).
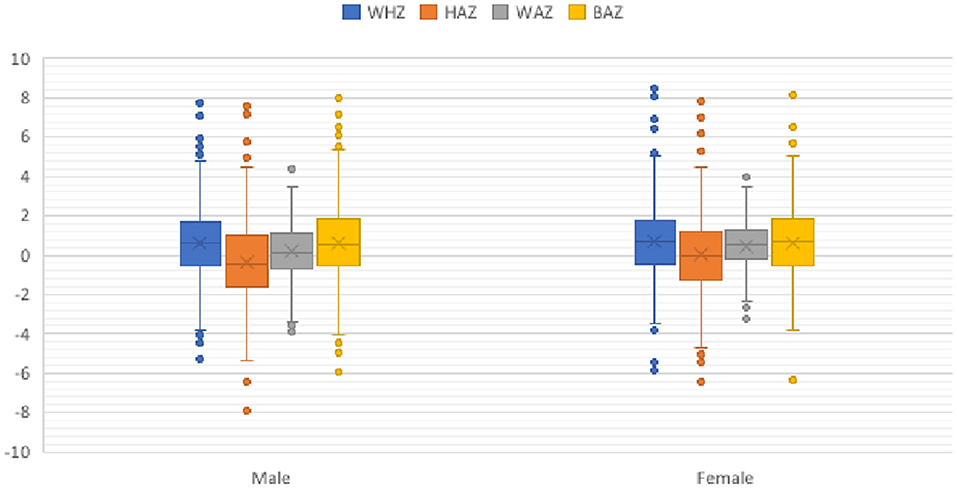
Figure 2. Child anthropometrics by sex. BAZ, body mass index for age z-score (<-2 SD, low BMI); HAZ, height-for-age z-score (<-2 SD, stunted); WAZ, weight-for-age z-score (<-2 SD, underweight); WHZ, weight-for-height z-score (<-2 SD, wasted).
The first 2 years of life are a critical time window for optimal growth, development, and health of a child (21). The introduction of an adequate, appropriate, and safe dietary intake at the age of 6 months following exclusive breastfeeding is recommended by WHO since inadequate nutrient intake during this period increases the risk for underweight or overweight with potentially lifelong health problems.
The present study included children under the age of 2 years living in the city of Adama and the surrounding area in the Oromia region in central Ethiopia. The median age of initiation of CF was 6 months of age and only 3.6% of the parents started CF earlier than the WHO recommendations (22). Results of this study are in line with a study conducted in Addis Ababa, in which 83% followed the WHO infant feeding recommendations (10). On the other hand, the proportion of children following the recommendations is higher than studies conducted in other parts of the country, including Mekele in northern Ethiopia (62%) (23), Arsi Negelle (72%) (24), and in Sodo town in southern Ethiopia (71%) (25). The proportion is also much higher than the finding from the national prevalence in Ethiopia (56%) (26), Tahatay Maichew (53%) (27), Gonder town in northern Ethiopia (47%) (28), Damot Weydie (50.6%) (29), and Kamba in southern Ethiopia (40.6%) (30).
Disparities in the timely initiation of CF between different parts of Ethiopia could be explained by the variation in maternal occupations, awareness of recommended WHO guidelines, urban vs. rural residency of the study participants, data collection approaches, the identified religious affiliation of a population, and/or socioeconomic status. Location can also contribute to varied access to health services and information. All mothers who were enrolled in the present study attended both ANC and postnatal care at health institutes where the study was conducted. The variables are both important indicators of successful infant and young children feeding practices.
The majority of children in the study received foods made from vegetables (96%) and gluten-free cereals (92%) by 18 months of age. In contrast, foods made from legumes and dairy products are received by relatively low numbers of children. These results are in accordance with previous studies in Ethiopia regarding foods made from cereals and dairy products but are higher than other studies, which usually found low consumption of vegetables (31). Discrepancies in types of foods consumed might be explained by a variation in the socioeconomic status of the study participants or differences among parents' awareness of the importance of the variety of food items recommended by WHO.
Being a mother younger than age 20 years was one of the factors associated with following the complementary infant feeding recommendations of WHO, which is in contrast with studies from Southwest of Ethiopia (30). The other associated factor with the time of infant feeding was maternal occupation (32). These findings are in contrast with those of previous studies that demonstrated an association between time of infant feeding and religious affiliation (33) or mode of delivery (34, 35). The disparities in CF habits in mothers by age compared with previous studies from other regions of Ethiopia may be severalfold; this study indicates the associations with the employment of the mother outside the home and having access to health services during ANC and postnatal care.
In the present study, a lower proportion of the children experienced wasting, stunting, underweight, and low BMI for their age compared with studies from other areas of Ethiopia (26, 36). This discrepancy might be related to the difference in the culture of feeding, parents' awareness of WHO feeding recommendations, or socioeconomic variation, access to information or methods of measures on IYCF practices. Another plausible explanation could be a selection bias in study participants, in which those attending health services are also more likely to participate in a research study. Although these may all confound the findings, stunting and underweight were more common in males compared with females, which confirms previous reports (37, 38).
A strength of the present study is the study design in which data were continuously and prospectively collected close to birth with information about the health status of both the mother and the child. A limitation is that data were collected in only one city with close surroundings, which may not be representative for all surrounding rural areas or other urban cities of Ethiopia and, therefore, may not fully represent the community in the Oromia region. Moreover, information on CF habits was only presented at the 9- and 18-month visits, which may not be represented as the children grow older. Other potential limitations include missing data on the socioeconomic status of the mother and only including one child per family to participate in the study. It cannot be ignored that CF habits differ in families with more than one child in the household and families with different socioeconomic backgrounds. Also, the study focuses on the age of initiation of CF practices and does not investigate CF indicators, such as minimum dietary diversity, meal frequency, and recommended diets. Finally, the quality and quantity of the food types were not investigated.
In conclusion, the present study from the city of Adama in the Oromia region in central Ethiopia demonstrates that a majority of mothers follow the WHO recommendations in introducing CF to infants at the age of 6 months. Gluten-free cereals are among the most consumed food types by children in the study area. Mothers younger than 20 years old and the employment of mothers outside of the home were factors associated with the timely initiation of the CF of the infant. This finding is noteworthy since it gives updated information on infant feeding and stimulates more research into the frequency, quality, and quantity of food consumed by infants.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Armauer Hansen Research Institute, National Research Ethics Committee of Ethiopia, Lund University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
AG conducted the research and statistical analysis and drafted the manuscript. DA and CA contributed to the result interpretation and writing of the manuscript. DA contributed to the study design, editing of the manuscript, and overall supervision of the study. CA and TT contributed to the critical reading and editing of the manuscript. All authors accepted the final version of the manuscript.
This research was aided by the funds provided by the SUS Stiftelser och Fonder, Region Skåne FoU-medel, Swedish Celiac Disease Foundation, Håkanssons Stiftelse, and Pålssons Stiftelse.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank Sarah Austin-Gonzalez for the English proofreading of the manuscript, the staff at the Adama site, study participants, and their families and acknowledge the nutritionist who was consulted for the anthropometric data analysis.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.672462/full#supplementary-material
1. World Health Organization. Complementary Feeding: Report of the Global Consultation and Summary of Guiding Principles for Complementary Feeding of the Breastfed Child. Geneva: World Health Organization (2003). Available online at: https://apps.who.int/iris/handle/10665/42739
2. Demilew YM, Tafere TE, Abitew DB. Infant and young child feeding practice among mothers with 0-24 months old children in Slum areas of Bahir Dar City, Ethiopia. Int Breastfeed J. (2017) 12:26. doi: 10.1186/s13006-017-0117-x
3. Saha KK. Appropriate infant feeding practices result in better growth of infants and young children in rural Bangladesh. Am J Clin Nutr. (2008) 87:1852–9. doi: 10.1093/ajcn/87.6.1852
4. Pearce J, Taylor M, Langley-Evans S. Timing of the introduction of complementary feeding and risk of childhood obesity: a systematic review. Int J Obes. (2013) 37:1295–306. doi: 10.1038/ijo.2013.99
5. Biks GA, Tariku A, Wassie MM, Derso T. Mother's Infant and Young Child Feeding (IYCF) knowledge improved timely initiation of complementary feeding of children aged 6–24 months in the rural population of northwest Ethiopia. BMC Res Notes. (2018) 11:1–7. doi: 10.1186/s13104-018-3703-0
6. Teji Roba K. Infant and Young Child Feeding (IYCF) practices among mothers of children aged 6–23 months in two agro-ecological zones of rural Ethiopia. Int J Nutr Food Sci. (2016) 5:185–94. doi: 10.11648/j.ijnfs.20160503.16
7. Sadauskaite-Kuehne V, Ludvigsson J, Padaiga Ž, Jašinskiene E, Samuelsson U. Longer breastfeeding is an independent protective factor against development of type 1 diabetes mellitus in childhood. Diabetes Metabol Res Rev. (2004) 20:150–7. doi: 10.1002/dmrr.425
8. Akobeng AK, Ramanan AV, Buchan I, Heller RF. Effect of breast feeding on risk of coeliac disease: a systematic review and meta-analysis of observational studies. Arch Dis Childhood. (2006) 91:39–43. doi: 10.1136/adc.2005.082016
9. Klement E, Cohen RV, Boxman J, Joseph A, Reif S. Breastfeeding and risk of inflammatory bowel disease: a systematic review with meta-analysis. Am J Clin Nutr. (2004) 80:1342–52. doi: 10.1093/ajcn/80.5.1342
10. Mohammed S, Getinet T, Solomon S, Jones AD. Prevalence of initiation of complementary feeding at 6 months of age and associated factors among mothers of children aged 6 to 24 months in Addis Ababa, Ethiopia. BMC Nutr. (2018) 4:54. doi: 10.1186/s40795-018-0264-5
11. Teshale AB, Tesema GA. Timely initiation of breastfeeding and associated factors among mothers having children less than two years of age in sub-Saharan Africa: a multilevel analysis using recent demographic and health surveys data. PLoS ONE. (2021) 16:e0248976. doi: 10.1371/journal.pone.0248976
12. Nkoka O, Mhone TG, Ntenda PAM. Factors associated with complementary feeding practices among children aged 6-23 mo in Malawi: an analysis of the demographic and health survey 2015-2016. Int Health. (2018) 10:466–79. doi: 10.1093/inthealth/ihy047
13. Abeshu MA, Lelisa A, Geleta B. Complementary feeding: review of recommendations, feeding practices, and adequacy of homemade complementary food preparations in developing countries–lessons from Ethiopia. Front Nutr. (2016) 3:41. doi: 10.3389/fnut.2016.00041
14. Lee H. Teff, a rising global crop: current status of teff production and value chain. Open Agric J. (2018) 12:185–93. doi: 10.2174/1874331501812010185
15. Yilmaz HO, Arslan M. Teff: nutritional compounds and effects on human health. Acta Sci Med Sci. (2018) 2:15–8.
16. Shumoy H, Raes K. Tef: The rising ancient cereal: what do we know about its nutritional and health benefits? Plant Foods Hum Nutr. (2017) 72:335–44. doi: 10.1007/s11130-017-0641-2
17. Institute EPH ICF. Ethiopia Mini Demographic and Health Survey 2019: Key Indicators. EPHI and ICF (2019).
18. Gudeta AN, Ramelius A, Balcha TT, Girma A, Ilonen J, Agardh D. Distribution of HLA-DQ risk genotypes for celiac disease in Ethiopian children. HLA. (2020) 96:681–7. doi: 10.1111/tan.14119
19. Björkman P. Tuberculosis Infection in Women of Reproductive Age and Their Infants. (2017). Available online at: https://clinicaltrials.gov/ct2/show/NCT03305991 (accessed March 17, 2021).
20. De Onis M, Habicht JP. Anthropometric reference data for international use: recommendations from a World Health Organization Expert Committee. Am J Clin Nutr. (1996) 64:650–8. doi: 10.1093/ajcn/64.4.650
21. Victora CG, De Onis M, Hallal PC, Blössner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. (2010) 125:e473–e80. doi: 10.1542/peds.2009-1519
22. World Health Organization. Strengthening Action to Improve Feeding of Infants and Young Children 6-23 Months of Age in Nutrition and Child Health Programmes: Report of Proceedings. Geneva: World Health Organization (2008). Available online at: https://apps.who.int/iris/handle/10665/44034
23. Shumey A, Demissie M, Berhane Y. Timely initiation of complementary feeding and associated factors among children aged 6 to 12 months in Northern Ethiopia: an institution-based cross-sectional study. BMC Public Health. (2013) 13:1050. doi: 10.1186/1471-2458-13-1050
24. Kassa T, Meshesha B, Haji Y, Ebrahim J. Appropriate complementary feeding practices and associated factors among mothers of children age 6–23 months in Southern Ethiopia, 2015. BMC Pediatr. (2016) 16:131. doi: 10.1186/s12887-016-0675-x
25. Chane T, Bitew S, Mekonnen T, Fekadu W. Initiation of complementary feeding and associated factors among children of age 6-23 months in Sodo town, Southern Ethiopia: cross-sectional study. Pediatr Rep. (2017) 9:7240. doi: 10.4081/pr.2017.7240
26. Amare ZY, Ahmed ME, Mehari A. Determinants of nutritional status among children under five age in Ethiopia: a further analysis of the Ethiopian Demographic and Health Survey (EDHS) 2016 Data. bioRxiv. (2019) 2019:698308. doi: 10.1101/698308
27. Reda EB, Teferra AS, Gebregziabher MG. Time to initiate complementary feeding and associated factors among mothers with children aged 6–24 months in Tahtay Maichew district, Northern Ethiopia. BMC Res Notes. (2019) 12:17. doi: 10.1186/s13104-019-4061-2
28. Bazezew K, Worku W, Abebe Z. Timely initiation of complementary feeding practices in Gondar town Northwest Ethiopia: a cross-sectional Study. Ecol Food Nutr. (2020) 59:329–41. doi: 10.1080/03670244.2020.1733994
29. Epheson B, Birhanu Z, Tamiru D, Feyissa GT. Complementary feeding practices and associated factors in Damot Weydie District, Welayta zone, South Ethiopia. BMC Public Health. (2018) 18:1–7. doi: 10.1186/s12889-018-5245-8
30. Agedew E, Demissie M, Misker D, Haftu D. Early initiation of complementary feeding and associated factors among 6 months to 2 years young children. Kamba Woreda, South West Ethiopia: a community–based cross-sectional study. J Nutr Food Sci. (2014) 4:314. doi: 10.4172/2155-9600.1000314
31. Yonas F. Infant and young child feeding practice status and associated factors among mothers of under 24-month-old children in Shashemene Woreda, Oromia region, Ethiopia. Open Access Libr J. (2015) 2:1. doi: 10.4236/oalib.1101635
32. Tamiru D, Aragu D, Belachew T. Survey on the introduction of complementary foods to infants within the first six months and associated factors in rural communities of Jimma Arjo. Int J Nutr Food Sci. (2013) 2:77–84. doi: 10.11648/j.ijnfs.20130202.18
33. Oladejo T A, Elizabeth T A, Isaac O A, Lavalle T. Role of religion on knowledge, attitude and practices of lactating mothers on infant feeding. Int J Nutr. (2019) 4:14–25. doi: 10.14302/issn.2379-7835.ijn-19-2876
34. Saeed G, Fakhar S, Imran T, Abbas LK. The effect of modes of delivery on infants' feeding practices. Iran J Med Sci. (2011) 36:131.
35. Saaka M, Hammond AY. Caesarean section delivery and risk of poor childhood growth. J Nutr Metab. (2020) 2020:6432754. doi: 10.1155/2020/6432754
36. Ahmadi D, Amarnani E, Sen A, Ebadi N, Cortbaoui P, Melgar-Quiñonez H. Determinants of child anthropometric indicators in Ethiopia. BMC Public Health. (2018) 18:626. doi: 10.1186/s12889-018-5541-3
37. Woldeamanuel BT, Tesfaye TT. Risk factors associated with under-five stunting, wasting, and underweight based on Ethiopian demographic health survey datasets in Tigray region, Ethiopia. J Nutr Metabol. (2019) 2019:6967170. doi: 10.1155/2019/6967170
Keywords: children, complementary feeding, Ethiopia, gluten, malnutrition, teff
Citation: Gudeta AN, Andrén Aronsson C, Balcha TT and Agardh D (2021) Complementary Feeding Habits in Children Under the Age of 2 Years Living in the City of Adama in the Oromia Region in Central Ethiopia: Traditional Ethiopian Food Study. Front. Nutr. 8:672462. doi: 10.3389/fnut.2021.672462
Received: 25 February 2021; Accepted: 24 September 2021;
Published: 28 October 2021.
Edited by:
Francesco Sofi, Università degli Studi di Firenze, ItalyReviewed by:
Vimlendu Bhushan Sinha, Sharda University, IndiaCopyright © 2021 Gudeta, Andrén Aronsson, Balcha and Agardh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Agardh, ZGFuaWVsLmFnYXJkaEBtZWQubHUuc2U=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.