
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr. , 22 April 2021
Sec. Nutrition and Metabolism
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.663560
This article is part of the Research Topic Mechanisms Linking Transport and Utilization of Metabolic Fuels to the Impact of Nutrition and Exercise upon Health View all 6 articles
After almost a century of misunderstanding, it is time to appreciate that lactate shuttling is an important feature of energy flux and metabolic regulation that involves a complex series of metabolic, neuroendocrine, cardiovascular, and cardiac events in vivo. Cell–cell and intracellular lactate shuttles in the heart and between the heart and other tissues fulfill essential purposes of energy substrate production and distribution as well as cell signaling under fully aerobic conditions. Recognition of lactate shuttling came first in studies of physical exercise where the roles of driver (producer) and recipient (consumer) cells and tissues were obvious. One powerful example of cell–cell lactate shuttling was the exchange of carbohydrate energy in the form of lactate between working limb skeletal muscle and the heart. The exchange of mass represented a conservation of mass that required the integration of neuroendocrine, autoregulatory, and cardiovascular systems. Now, with greater scrutiny and recognition of the effect of the cardiac cycle on myocardial blood flow, there brings an appreciation that metabolic fluxes must accommodate to pressure-flow realities within an organ in which they occur. Therefore, the presence of an intra-cardiac lactate shuttle is posited to explain how cardiac mechanics and metabolism are synchronized. Specifically, interruption of blood flow during the isotonic phase of systole is supported by glycolysis and subsequent return of blood flow during diastole allows for recovery sustained by oxidative metabolism.
The purported role of lactate in physiology and medicine is a century old, but understanding of the role has changed dramatically in the last three decades (1–7). No longer thought of as a dead-end metabolite, a fatigue agent, and metabolic poison, in contemporary physiology, lactate is seen as a major metabolic intermediate that has wide-ranging impacts in energy substrate distribution and utilization, gluconeogenesis, and cell signaling (1, 2, 5, 6). While extensive data have been presented to support the lactate shuttle hypothesis in humans in vivo, little had been written to describe the role of lactate in cardiac metabolism or the role of the heart in terms of overall, whole body–energy substrate balance. Beyond the important role of lactate in supporting cardiac functioning, examination of the role of lactate in cardiac metabolism is illustrative overall regulation of energy substrate partitioning in other body tissues and organs.
In terms of fuel energy substrate use, the heart is sometimes referred to as “omnivorous,” meaning it can simultaneously oxidize a variety of energy substrates including glucose, lactate, fatty acids, and ketones. The heart is also regarded as a “pay as you go” energy consumer because it relies heavily on exogenous as opposed to endogenous energy sources. As previously shown for skeletal muscle and whole-body metabolism (8), changes in cardiac work determine the rates and relative uses of energy substrates, with lesser work emphasizing lipid oxidation (9–12) and greater work emphasizing carbohydrate (i.e., glucose and lactate) energy sources (10, 12). The cardiac glycogen pool probably turns over, just as in skeletal muscle (13), but degradation in excess of synthesis is not as exaggerated as in working skeletal muscle (14). Hence, glycogen is not known to be a major energy source for the healthy heart. And strictly speaking in terms of the mobilization of endogenous energy sources, it is apparent that mobilization of intramuscular triglyceride can result in mobilization of fatty acids within the heart even though it is a net consumer of fatty acids from the systemic circulation (11).
A unique reason to emphasize cardiac lactate metabolism in this paper is that of the purported functions of lactate shuttling (energy substrate, gluconeogenic precursor, signaling molecule), studies indicate that the heart depends heavily on exogenous lactate as a fuel when cardiac work is elevated. In contrast to the liver and kidneys, the heart is not a gluconeogenic organ. And while the heart continuously receives neuro-endocrine signaling from the vagus and cardiac nerves as well as arterial blood, the heart also releases atrial natriuretic peptide (ANP), a unique endocrine signaling function of the heart induced by mechanical atrial stretching when blood volume is elevated. ANP secretion is not known to involve lactate signaling.
Before getting into specifics, it is advisable to introduce general concepts of lactate shuttling. Key to shuttling is that there exist lactate producing (driver) and recipient (consuming) cells and tissues (1, 6, 15). Experimentally, the shuttle concept was supported by findings that lactate flux between cells and tissue beds depends on metabolite and hydrogen ion (pH) concentration differences (16–19). Lactate exchanges between and among cells were shown to be facilitated by the presence of cell membrane lactate transport proteins termed monocarboxylate transporters (MCTs) (19, 20); MCTs are bidirectional symporters (16, 17, 21) sensitive to trans-stimulation by lactate and hydrogen ion gradients. Importantly, based on the results of studies on rat muscles after exercise (22–24), dog gracilis muscle preparations (25), and subsequently studies on resting and exercising human skeletal muscles (26, 27) and hearts (28, 29), the lactate shuttle concept emerged (1, 6, 15). Not surprisingly, we now know that driver and recipient cells can switch roles depending on conditions, and that some cells can exchange lactate through the interstitium and vascular beds. For instance, during exercise, fast white fibers can provide oxidizable substrate to red, oxidative fibers in the same tissue bed (22, 30). Conversely, postprandial glucose uptake in red fibers can provide substrate to the body corpus as in the glucose paradox (31) and postprandial lactate shuttle (32). By means of lactate shuttling, working muscle can fuel the beating heart (28), support cerebral executive functions (33–39), and provide gluconeogenic precursor to the splanchnic organs (40, 41).
Historically, investigators have been challenged in studying cardiac metabolism because substrate balance studies require making blood flow and arterial-venous difference (a-v) measurements. Arterial catheterization is considered to be invasive, but perhaps more invasive still is sampling venous output from the heart, which involves catheterization of the coronary sinus (CS), a vessel that empties into the right atrium. Hence, much of the data were obtained on patients with heart or other diseases receiving appropriate medications as contrasted to healthy young individuals (10, 42–44). Fortunately, there are studies on healthy humans (typically men) involving CS catheterization with blood flow and metabolite concentration measurements (28, 29, 45). Fortunately, there are also studies on healthy men and women that applied strict dietary controls required for interpretation of metabolism data (11, 12). An example of an experimental setting for cardiac and whole-body measurements of energy–substrate partitioning in a resting and exercising human subject is shown in Figure 1.
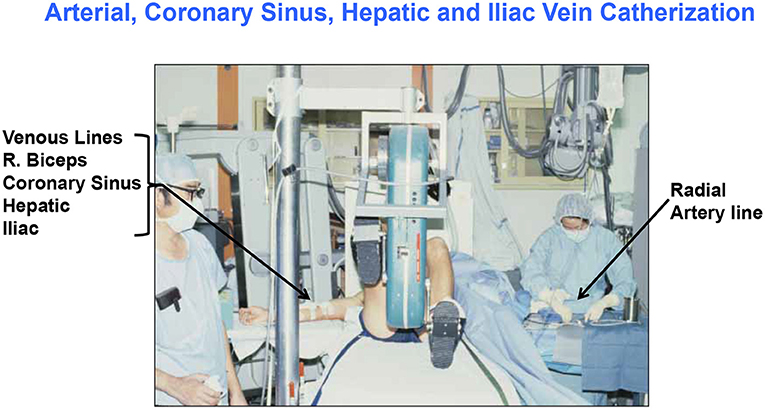
Figure 1. Illustration of the invasive and complicated methods to simultaneously determine cardiac and whole-body energy substrate partitioning in man. Setting for (26, 28, 29, 45–47); G. A. B. personal photo.
In addition to problems related to arterial and CS access and metabolite as well as blood flow measurements, other problems are related to the assessment of metabolites that undergo turnover within the tissue or organ of interest. Because glucose is taken up by, but not produced in, the myocardium, simple net chemical balance measurements, i.e., [(a-v) Metabolite (blood flow)], are adequate to assess cardiac glucose metabolism. However, such measurements are not adequate for lactate because it is simultaneously taken up, produced, and oxidized within the heart (12, 28). Also, the same problem applies to determinations of fatty acid metabolism, although to a lesser extent (11).
Using coronary sinus catheterization methodology, early investigators noted the omnivorous capabilities of human hearts, as well as the ability of one substrate, such as glucose, to suppress use of another, such as fatty acids (10, 48). Thus, early investigators making measurements of arterial-coronary sinus concentration differences, coronary flow, and oxygen extraction could determine energy substrate partitioning in human hearts. Subsequent experiments with isotopic tracer verified preferential myocardial FFA utilization (11, 45) and that FFA availability downregulates glucose utilization, and vice versa (45, 49). For the later experiments, investigators had the advantage of using radioactive and stable lactate isotope tracers that revealed significant intra-organ turnover. Moreover, studies using physical exercise (28, 29, 45) or cardiac pacing (11, 12) showed crossover from lipid to carbohydrate energy sources with increasing cardiac work. Thus, with measurements of cardiac metabolism in the setting of leg cycle ergometer exercise, it could be observed that lactate released from muscle and other driver sites became the major cardiac energy source in healthy young men as illustrated in Figure 2, an example of cell–cell lactate shuttling.
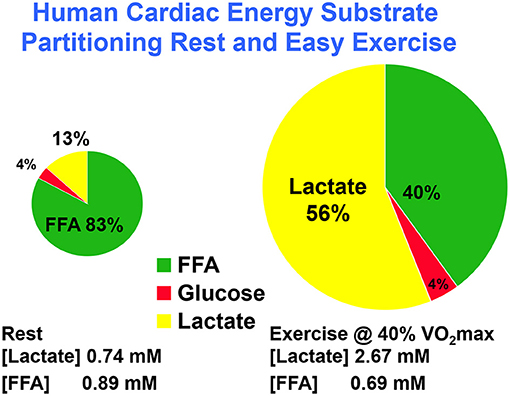
Figure 2. Illustration of substrate–substrate interactions in cardiac metabolism as determined in a man resting or engaged in easy, 40% VO2max exercise. Results show that the contribution of lactate to cardiac energy need depends on arterial lactate concentration and is significantly greater than that of glucose. Results also show crossover from fatty acid to lactate dependence in heart during exercise. Data from (28).
With few exceptions, extant data on resting humans give rise to the conclusion that fatty acids predominate over glucose as the major fuel energy course for cardiac metabolism in resting, healthy individuals. Hence, fatty acids and glucose are typically considered to be major fuel energy substrates for the heart. For instance, in overnight fasted healthy individuals following an American Heart Association (AHA) compliant diet, the cardiac respiratory quotient (RQ) and whole-body respiratory exchange ratio (RER = VCO2/VO2) averaged 0.83, or 58% lipid and 42% carbohydrate energy utilization. In this setting, beta-hydroxybutyrate and acetoacetate (i.e., “ketone bodies,” derivatives of lipid catabolism), and lactate (a derivative of glucose metabolism) are sometimes considered to be “alternative” energy substrates.
Initial studies of cardiac metabolism demonstrating utilization of FFA and ketones over glucose were conducted under carefully controlled conditions prudent considering invasiveness of the procedures and medical conditions of subjects (10, 48). However, supine rest is not typical of many activities of daily life such as requiring increased cardiac work. As illustrated in Figure 2, the notion of lactate as an alternative cardiac fuel energy source comes to question. During whole-body exercise, lactate is not an alternative fuel energy source, lactate is the main cardiac energy fuel the utilization of which downregulates fatty acid uptake and metabolism (28, 29, 45, 50). Interestingly, the same dominance of lactate over glucose and fatty acids as brain energy sources has been demonstrated in traumatic brain injury (TBI) patients and healthy controls (35, 51) as well as at the whole-body level during physical exercise (27, 52, 53).
Given the absolute and relative increase in cardiac lactate metabolism in the transition from rest to moderate-intensity supine physical exercise (Figure 2) (29, 54) or electrical pacing to heart rate 120 bpm (12), one wonders what energy substrate partitioning would be during hard, upright physical exercise for instance at 65–70% VO2max. At present, such data are unavailable, but given the known relationships among exercise intensity, circulating lactate level, and lactate uptake and oxidation determined across working muscle beds (27) and at the whole-body level (27, 46, 55, 56), a reasonable short-term prediction is that during hard exercise when arterial blood lactate concentration rises, myocardial lactate uptake and oxidation would rise also.
Beyond a predicted acute, short-term prediction of an effect of lactate myocardial energy substrate partitioning is an observed effect of lactate on MLOC constituent gene expression, in particular MCT1 and MCT4, as well as LDH that were observed in response to lactate incubation in perfused rat myocardium (57). As noted previously with L6 myocytes, lactate incubation appeared to work via PGC1α, ROS, and NRF mechanisms (58). Should repeated lactate exposures as would occur with regular exercise training, a long-term prediction would be an enhancement of myocardial capacity for lactate uptake, facilitated by increased MCT1 and MCT4 protein abundances, and lactate oxidation as facilitated by increased MMCT, LDH, and other MLOC protein levels.
Still, even in the absence of data obtained during hard, upright exercise, lactate may be considered to be the major fuel energy source for essential tissues such as brain, red skeletal muscle, and heart on conditions of increased energy demand.
Tracer studies and non-tracer (a-v) studies show that the healthy heart takes up lactate on a net basis because the highly expressed mitochondrial reticulum acts as a sink for substrate oxidation (12, 29). Also, studies on healthy humans show that the heart simultaneously extracts, produces, and oxidizes lactate especially when cardiac work is elevated (12, 28, 29). But, how can these apparently discordant results of simultaneous production and disposal be explained? This important question can be addressed in part by confirmative studies on heart and skeletal muscle preparations using 13C-lactate and nuclear magnetic resonance spectroscopy and hyperpolarized MRS (59–61). Given these observations, what are the mechanisms of glucose uptake, lactate formation, and lactate disposal?
The heart takes up and oxidizes glucose on a net basis because there is a need for energy substrate to support cardiac work, but also there appears to be a compartmental need, probably for excitation–contraction coupling (62). In the heart, glucose gives rise to lactate because it is the inevitable consequence of glycolysis (63), the minimal muscle L/P being 10 and rising to an order of magnitude or more when glycolytic flux is high (64). The heart produces lactate from glucose because the enzymes of glycolysis, including lactate dehydrogenase, are highly expressed in the myocardium and are up regulated in ischemic disease (65) and are associated with increased reliance of lactate as an energy source (29).
That the heart takes up glucose and produces lactate may be in part due to cardiac anatomy and limits to intra-cardiac circulation imposed by the cardiac cycle. If it is true that even in a healthy heart systole interrupts coronary blood flow, especially in the endocardium, then the presence of a phasic intra-cardiac lactate shuttle is indicated. Assuming a resting heart rate of 60Bpm, there occurs 200ms of stopped flow during the isometric contraction phase of systole which is powered by glycolysis followed by 800ms of diastole for oxidative recovery and lactate clearance. Admittedly, the presence of an intra-cardiac lactate shuttle would be difficult to prove because even coronary sinus sampling would be insufficient to determine phasic events. Hopefully in the future, gated hyperpolarized MRS (60) or other technologies will allow for detection of lactate production, net uptake, and oxidative disposal within a cardiac cycle.
Even after endurance training, working muscle relies on glycolysis from glucose, and more importantly, from glycogen (27, 66, 67). In contrast, in the absence of ischemia or systemic anoxia or exsanguination, cardiac muscle glycogen does not appear to change. However, it may well be that glycogen synthesis is continuous (13) and that cardiac glycogen turnover involves a glycogen shunt as described by Robert Shulman and colleagues (68).
As illustrated for physical exercise (28, 29) or cardiac pacing (11, 12), increased cardiac work shifts substrate selection from lipid- to carbohydrate-derived energy sources. Also, this switch or crossover from lipid to carbohydrate-derived energy sources is seen in other stressful conditions such as heart failure (69), diabetes (70), and aging (71). Teleologically, the crossover can be attributed to increased oxygen efficiency of carbohydrate-derived fuel energy sources (12, 72).
Mechanisms at both ends of the pathways involved in lipid metabolism, from lipolysis in white adipose tissue to mitochondrial fatty acid uptake and oxidation, explain crossover from lipid- to carbohydrate-derived energy sources in the stressed heart.
Inverse relationships between blood (La−) and plasma free fatty acid concentration (FFA) and oxidation during hard exercise has long been recognized (8, 73–75), an effect attributed to hydrogen ions or lactate anions that overcome the stimulatory effect of epinephrine on adipose lipolysis.
The mechanism by which lactatemia suppresses circulating FFA is diagrammed in Figure 3. It is now known that there is suppression of adipose lipolysis by lactate working through receptor binding (77, 80–82). Moreover, it is now known that, independent of pH, lactate inhibits lipolysis in fat cells through activation of a previously orphan G-protein coupled receptor (GPR81), now termed hydroxycarboxylic acid receptor 1 (HCAR-1). The effect of lactate binding to HCAR-1 operates through cyclic-AMP (cAMP) and CREB (78, 83, 84). Ultimately, lack of substrate availability limits use of blood-borne fatty acids when the heart is stressed in the presence of lactatemia, with lactate being an oxygen-efficient fuel energy source and an inhibitor of fatty acid availability (85) as annotated in Figure 2.
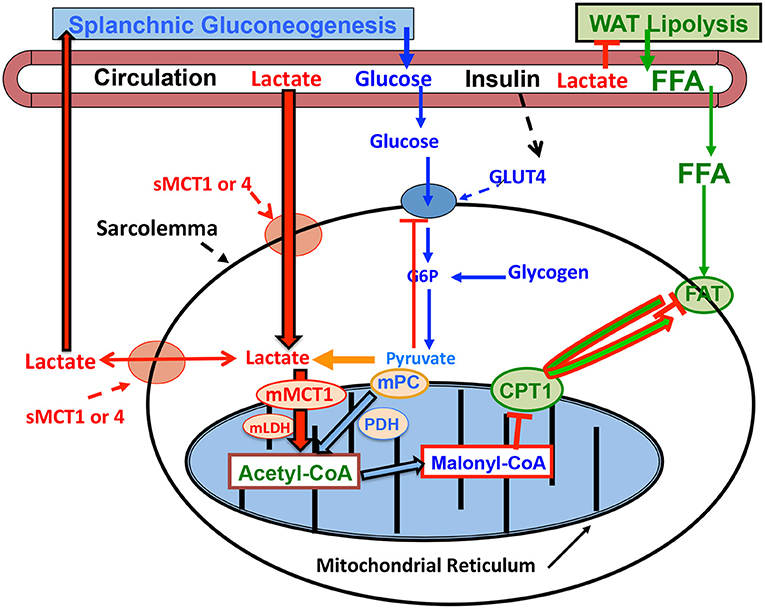
Figure 3. Illustration of how lactatemia affects crossover from lipid- to lactate-based metabolism in cardiac and skeletal muscle. Lactate is the inevitable consequence of glycolysis (63), the minimal muscle L/P being 10 and rising to an L/P >100 when glycolytic flux is high (64). Lactate availability as a myocardial energy source increases during physical exercise because arterial lactate concentration rises (27, 55), but also as the result of increased cardiac work and glycolytic flux during exercise (28, 29) or as the result of pacing (12). As the favored Oxidizable substrate, lactate catabolism results in product inhibition of glucose and FFA oxidation. As the products of glycolysis, lactate and pyruvate provide negative feedback inhibition of glucose disposal (blue dashed lines). Also as the predominant mitochondrial substrate, lactate gives rise to Acetyl-CoA, and in turn Malonyl-CoA. Acetyl-CoA inhibits β-ketothiolase, and hence β-oxidation, while Malonyl-CoA inhibits mitochondrial FFA-derivative uptake via CPT1 (T) (76). Moreover, lactate is the main gluconeogenic precursor raising glucose production and blood (glucose) (red lines). Via GPR81 binding, lactate inhibits lipolysis in WAT (T) depressing circulating (FFA) (77, 78). This model explains the paradoxical presence of lactatemia in high-intensity exercise and insulin-resistant states with limited ability to oxidize fat (green lines). Modified from Hashimoto et al. (79). CPT1, carnitine palmitoyl transporter-1; FFA, free fatty acid; FAT, fatty acid translocator composed of CD36 and FABPc; GLUT, glucose transporter; s, sarcolemmal; m, mitochondrial. Malonyl-CoA formed from exported TCA citrate controlled by the interactions of Malonyl-CoA decarboxylase (MCD) and acetyl-CoA carboxylase (ACC). MCT, monocarboxylate transporter; MPC, mitochondrial pyruvate transporter; PDH, pyruvate dehydrogenase; WAT, white adipose tissue; T, inhibition. Not shown is fatty acyl-Co (FA-CoA) that will accumulate if FFAs are taken up by myocytes, but blocked from mitochondrial entry by the effect of Malonyl-CoA on CPT1. Accumulated intracellular FA-CoA will give rise to intramyocellular triglyceride (IMTG) and the formulation of LC-FA, DAG, and ceramides via inhibition of PI3 kinase (PI3-k) and reducing GLUT4 translocation; from (1).
The crossover from lipid- to lactate-based myocardial lactate fueling during exercise illustrated in Figure 2 is explained as follows. When glycolysis is accelerated during skeletal muscle contraction, arterial lactate (L) and pyruvate (P) concentrations rise because of net release and raise the L/P an order of magnitude from a nominal value of 10 (86). Also, because of increased cardiac work and glycolytic flux during whole-body physical exercise (28, 29), or as the result of pacing (12), lactate availability to the cardiac muscle mitochondrial reticulum rises. Then, because of the relatively greater abundance of lactate compared to pyruvate, lactate becomes the main precursor of acetyl-Co-A formation (76, 87, 88) and, thereby, malonyl-CoA (Figure 3). The rise in malonyl-CoA inhibits the entry of activated FFAs into the mitochondrial matrix by inhibiting carnitine-palmitoyl transferase-1 (CPT1) (76, 89). Also, the accumulation of acetyl-CoA downregulates β-ketothiolase, the terminal and rate-limiting enzyme of the mitochondrial β-oxidation pathway. Moreover, increased cardiac frequency and work stimulates glycolysis, and by changing the L/P, inversely the NAD+/NADH, and consequently cytosolic redox. Hence, by mass action, allosteric binding, and effects on cell redox, lactate acts to shut the gates of activated fatty acids into matrix of the mitochondrial reticulum. Suppression of cardiac capacity of lipid oxidation may also be attributable to downregulation of enzymes of fatty acid oxidation (90).
Extant data show simultaneous lactate uptake, production, and disposal in the healthy human heart. A question then is how much of whole-body lactate turnover is attributable to heart metabolism? For this, we have one complete study using electrically stimulated atrial cardiac pacing (i.e., pacing) as well as the ability to piece together results from a limited number of disparate physical exercise studies.
For cardiac pacing, Bergman and colleagues studied subjects with primed continuous infusions of [3, 3, 3-2H] lactate and [6, 6-2H] glucose (12). Subjects were healthy, of moderate fitness (VO2max = 35.5 ± 3 ml/kg/min), were on an AHA-compliant diet, fed supper, and slept in the university General Clinical Research Center and studied in the morning after an overnight fast. Arterial and coronary sinus blood sampling and measurements of CS blood flow were made and allowed for simultaneous calculation of whole-body and cardiac metabolite flux rates. Compared with resting (≈70 beats min/min), heart rate increased (≈111 beats min/min) due to pacing. From rest, myocardial blood flow (≈200 ml/min) doubled while myocardial oxygen consumption at rest (22 ml/min) increased three times due to atrial pacing. Resting mean blood pressure (MAP≈100 mmHg) and cardiac output (5.3 L/min) did not change significantly from rest during pacing. Arterial levels of catecholamines, insulin, and glucagon were unchanged due to cardiac pacing. Stroke volume during rest (77 ml) declined 31% because cardiac output was unaffected by pacing.
There was no arterial-coronary sinus difference (a-CS) for glucose isotopic enrichment across the heart during rest or pacing verifying the absence of glucose production in the organ. Thus, the product of (a-CS) for glucose and blood flow [(a-CS) Glucose (Blood Flow)] gave net glucose uptake. Whole-body and cardiac RQs (≈0.83) were predictable considering the controlled diet and fast, indicating a 58–42% lipid–CHO fuel mix that did not change due to pacing. Glucose fractional extraction during rest (9%) was maintained during pacing, but blood flow doubled indicating that net cardiac glucose uptake (≈14 mg/min) increased 60% due to pacing (12).
The arterial-coronary sinus difference (a-CS, 0.35mMol/L) was maintained in the transition from rest to pacing, indicating myocardial net lactate uptake under both resting and paced conditions. Isotopically measured fractional lactate extraction (≈65%) was maintained during pacing, but isotopic enrichment in CS blood declined 50% indicating major intra-organ lactate production. Consequently, myocardial lactate production during pacing increased 230% (i.e., was 3.3-fold over rest values). Thus, the heart accounted for a significantly greater percentage of whole-body lactate Disposal during atrial pacing (15%) compared with rest (5%) (12). Those percentages were similar to those found previously (29, 47). In Bergman et al., investigators used deuterated as opposed to 13C- or 14C-lactate tracer previously (27). Consequently, lactate oxidation rate could not be determined. However, in previous studies using carbon-labeled tracers on the heart (29) or skeletal muscle (27), most lactate taken up was oxidized in situ.
Atrial pacing studies serve to illustrate dynamics under tightly controlled conditions. However, during physical exercise, far greater changes in whole-body metabolism, MAP, cardiac output, circulating catecholamines, and glucoregulatory hormones occur, making an atrial pacing–whole-body exercise comparison interesting, but probably not completely adequate to estimate the role of the heart in glucose or lactate disposal during moderate- to hard-intensity exercise.
At the whole-body level, comparisons of blood glucose and lactate fluxes on a mass basis (mg/kg/min) indicate that lactate rate of appearance (Ra) ~40% of that of glucose disappearance (Rd) in resting subjects (47). However, as exercise power output increases, glucose Rd increases, but lactate Ra increases relatively more, such that the two flux rates are about equal at an exercise power output that elicits about 40% VO2max (29, 47). At higher exercise power outputs, such as those approaching the lactate threshold (LT), the gain in whole-body lactate Ra outstrips that in glucose Rd in by almost 2-fold (Figure 4) (55, 91). Lactate Ra in excess of glucose Rd reflects the additional contribution of glycogen to glycolytic flux.
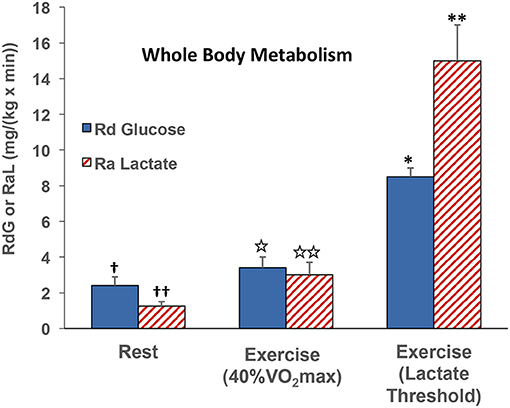
Figure 4. Illustration of the relationships between whole-body glucose rate of disappearance (RdG) and lactate appearance (RAL) during rest, exercise at 40% VO2max (47), and lactate threshold (55, 91).
Reiterating from above, when isotope tracers of metabolites are administered, it is possible to determine whole-body metabolic flux rates. Also, if blood flow and arterial and venous drainage blood from discrete organs can be sampled, such as the jugular bulb for studies of cerebral metabolism (35), and the coronary sinus for cardiac metabolism (11, 12, 28, 29), then metabolism in those organs can be determined. For the present, we are fortunate to have data allowing simultaneous comparisons of whole-body and cardiac glucose as well as lactate flux rates in human subjects at rest and subsequently during mild to moderate-intensity (40% VO2max) exercise. Whole-body glucose lactate comparisons are shown in Figure 4 whereas simultaneously determined cardiac glucose–lactate comparisons are shown in Figure 5. We know of no whole-body vs. cardiac metabolism comparisons at greater exercise power outputs as assessed by either % VO2max or LT. Still, from the data at hand, it is apparent that the contribution of lactate to cardiac energy need is similar to or greater than that of glucose (Figure 5). Results portrayed in Figure 2 are from one subject in the same set of studies (29, 47), except that in Figure 2 the role of fatty acids is given showing crossover from cardiac fatty acid to lactate dependence in exercise.
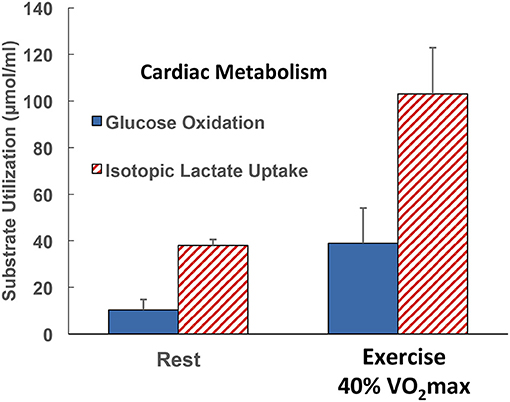
Figure 5. Illustration of the contributions of glucose and lactate to cardiac metabolism in healthy men studied at rest and during supine leg cycle ergometry at 40% VO2max (see Figure 1) (29). Results are to be compared with those in the same subjects resting and exercising at 40% of VO2max (see Figure 4). Results are also to be compared with those on one subject, the author, but in Figure 2 the role of fatty acids in supplying energy for cardiac metabolism is depicted. Results show that the contribution of lactate to cardiac energy need depends on arterial lactate concentration and is significantly greater than that of glucose. Results in Figure 2 also show crossover from fatty acid to lactate dependence in heart during exercise. Data from (28).
In this and probably every other piece on cardiac metabolism, it is either understood or explicitly stated that the hard and enduring work of the heart is a most impressive feature of mammalian physiology (72). With data at hand as portrayed in Figure 5 and the original source (29), results were that lactate uptake was disposed of as oxidation whereas glucose oxidation approximated 50% of uptake in resting subjects and 70% during exercise. For resting subjects, the difference between cardiac glucose uptake and oxidation could be attributed to glycogen synthesis. However, the glucose uptake–oxidation difference during exercise is harder to explain. Could the difference be attributed to the presence of cardiac glycogen turnover and net utilization during exercise as seen in studies on laboratory rodents (92)? Or, could another amazing feature of cardiac metabolism have been operant; is it possible that cardiac glycogen storage occurs during physical exercise? Regrettably, there were no means to measure cardiac glycogen content or turnover under the conditions studied, so veracity of the alternative explanations could not be assessed.
Lactate shuttling between producer (driver) and consumer (recipient) cells requires the presence of cell–cell and intracellular lactate shuttles that fulfill at least three purposes: lactate is (1) a major energy source, (2) the major gluconeogenic precursor, and (3) a signaling molecule. Lactate production occurs during rest and exercise under fully aerobic conditions (26, 28, 60, 93) and increases exponentially as exercise power output increases (55). There is no evidence that oxygen inadequacy gives rise to lactate production and accumulation in resting or exercising subjects, even in the hypoxia of high altitude (54). Rather, there is abundant evidence that lactate production occurs in fully aerobic tissues and organs (26, 28, 35, 51, 60, 93). Importantly, results of studies on cardiac metabolism in human subjects using coronary sinus catheterization and isotope tracer studies showed simultaneous cardiac lactate consumption, production, and oxidation at rest and during cardiac work induced by atrial pacing or physical exercise. Simultaneous measurements of working muscle, whole-body, and cardiac metabolism show lactate shuttling between working muscle and heart (12, 26, 27, 29, 46, 47). As predicted from studies on skeletal muscle (8), [(a-CS) Metabolite (Blood Flow)] measurements show crossover from lipid (fatty acid) to carbohydrate (lactate)–based metabolism with increments in cardiac work (Figure 2). The contribution of cardiac to whole-body metabolism appears to scale to whole-body metabolic rate and represents 5% at rest to 15% during atrial pacing (12) or easy (40% VO2max) exercise (29, 47). The contribution of cardiac to whole-body metabolism during hard exercise has yet to be determined, but is likely not much greater than during easy exercise because increasing muscle mass, recruitment of type 2 glycolytic fibers, and catecholamine stimulation during hard and intense exercise (55) likely overwhelm the heart's ability to clear lactate from the circulation. Lactate shuttling from muscle to heart fuels it during rest or exercise when lactate is the dominant energy source (Figures 2, 5). Although limited by small relative mass, the heart plays a significant role during rest and exercise.
Original articulation of the lactate shuttle (6, 15) was based on the contemporaneous observations of muscle cell heterogeneity and posited the exchange of lactate between driver and recipient cells, organs, and tissues. In view of data on cardiac blood flow during the cardiac cycle, it is now possible to posit presence of a cardiac or intra-cardiac lactate shuttle in which interruption of blood flow and rapid glycolysis occur during the isotonic phase of systole with the return of flow and oxidative metabolism during diastole. Whether the hypothesis of a phasic cardiac lactate shuttle is viable remains to be determined. Regardless, it is clear that lactate released from working muscles (47) or released from the integument (94) as a consequence of sympathetic nervous system activation and catecholamine-stimulated glycogenolysis (1) can fuel the heart (Figure 2). And lastly, as in the instances of whole-body exercise (8) or working muscle (27), increases in cardiac work cause a crossover (shift) from lipid- to carbohydrate-derived fuel energy sources (Figures 2, 5).
The paper was conceived and written by GB.
This study was supported by NIH 1 R01 AG059715-01, Pac-12 Conference Grant # 3-02-Brooks-17 and the UCB Center for Research and Education on Aging (CREA).
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Adam D. Osmond, Robert G. Leija, Casey C. Curl, and Jose A. Arevalo are thanked for reading and commenting on the article. Ashley Tovar is thanked for reading and commenting on the article as well as for rendering Figures 3, 4.
1. Brooks GA. The science and translation of lactate shuttle theory. Cell Metab. (2018) 27:757–85. doi: 10.1016/j.cmet.2018.03.008
2. Brooks GA. Lactate production under fully aerobic conditions: the lactate shuttle during rest and exercise. Fed Proc. (1986) 45:2924–9.
3. Laboratory investigations in clinical immunology: methods pitfalls and clinical indications. A second IUIS/WHO report. Clin Immunol Immunopathol. (1988) 49:478–97. doi: 10.1016/0090-1229(88)90134-1
4. Gladden LB. Lactate metabolism: a new paradigm for the third millennium. J Physiol. (2004) 558:5–30. doi: 10.1113/jphysiol.2003.058701
5. Brooks GA. Lactate as a fulcrum of metabolism. Redox Biol. (2020) 35:101454. doi: 10.1016/j.redox.2020.101454
6. Brooks GA. Glycolytic end product and oxidative substrate during sustained exercise in mammals–the “lactate shuttle. Com Physiol Biochem. (1984) 208–18. doi: 10.1007/978-3-642-70610-3_15
7. Brooks GA. Anaerobic threshold: review of the concept and directions for future research. Med Sci Sports Exerc. (1985) 17:22–34. doi: 10.1249/00005768-198502000-00005
8. Brooks GA, Mercier J. Balance of carbohydrate and lipid utilization during exercise: the “crossover” concept. J Appl Physiol. (1994) 76:2253–61. doi: 10.1152/jappl.1994.76.6.2253
9. Lopaschuk GD. Metabolic modulators in heart disease: past, present, and future. Can J Cardiol. (2017) 33:838–49. doi: 10.1016/j.cjca.2016.12.013
10. Bing RJ, Siegel A, Vitale A, Balboni F, Sparks E, Taeschler M, et al. Metabolic studies on the human heart in vivo. I. Studies on carbohydrate metabolism of the human heart. Am J Med. (1953) 15:284–96. doi: 10.1016/0002-9343(53)90082-5
11. Bergman BC, Tsvetkova T, Lowes B, Wolfel EE. Myocardial FFA metabolism during rest and atrial pacing in humans. Am J Physiol Endocrinol Metab. (2009) 296:E358–66. doi: 10.1152/ajpendo.90747.2008
12. Bergman BC, Tsvetkova T, Lowes B, Wolfel EE. Myocardial glucose and lactate metabolism during rest and atrial pacing in humans. J Physiol. (2009) 587:2087–99. doi: 10.1113/jphysiol.2008.168286
13. Azevedo Jr JL, Linderman JK, Lehman SL, Brooks GA. Training decreases muscle glycogen turnover during exercise. Eur J Appl Physiol Occup Physiol. (1998) 78:479–86. doi: 10.1007/s004210050449
15. Brooks GA. Lactate shuttles in nature. Biochem Soc Trans. (2002) 30:258–64. doi: 10.1042/bst0300258
16. Roth DA, Brooks GA. Lactate transport is mediated by a membrane-bound carrier in rat skeletal muscle sarcolemmal vesicles. Arch Biochem Biophys. (1990) 279:377–85. doi: 10.1016/0003-9861(90)90505-S
17. Roth DA, Brooks GA. Lactate and pyruvate transport is dominated by a pH gradient-sensitive carrier in rat skeletal muscle sarcolemmal vesicles. Arch Biochem Biophys. (1990) 279:386–94. doi: 10.1016/0003-9861(90)90506-T
18. Watt PW, MacLennan PA, Hundal HS, Kuret CM, Rennie MJ. L(+)-lactate transport in perfused rat skeletal muscle: kinetic characteristics and sensitivity to pH and transport inhibitors. Biochim Biophys Acta. (1988) 944:213–22. doi: 10.1016/0005-2736(88)90434-8
19. Garcia CK, Goldstein JL, Pathak RK, Anderson RG, Brown MS. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell. (1994) 76:865–73. doi: 10.1016/0092-8674(94)90361-1
20. Garcia CK, Brown MS, Pathak RK, Goldstein JL. cDNA cloning of MCT2, a second monocarboxylate transporter expressed in different cells than MCT1. J Biol Chem. (1995) 270:1843–9. doi: 10.1074/jbc.270.4.1843
21. Brown MA, Brooks GA. Trans-stimulation of lactate transport from rat sarcolemmal membrane vesicles. Arch Biochem Biophys. (1994) 313:22–8. doi: 10.1006/abbi.1994.1353
22. Hooker AM, Baldwin KM. Substrate oxidation specificity in different types of mammalian muscle. Am J Physiol. (1979) 236:C66–9. doi: 10.1152/ajpcell.1979.236.1.C66
23. Baldwin KM. Effects of chronic exercise on biochemical and functional properties of the heart. Med Sci Sports Exerc. (1985) 17:522–8. doi: 10.1249/00005768-198510000-00004
24. Baldwin KM, Campbell PJ, Cooke DA. Glycogen, lactate, and alanine changes in muscle fiber types during graded exercise. J Appl Physiol. (1977) 43:288–91. doi: 10.1152/jappl.1977.43.2.288
25. Stainsby WN, Welch HG. Lactate metabolism of contracting dog skeletal muscle in situ. Am J Physiol. (1966) 211:177–83. doi: 10.1152/ajplegacy.1966.211.1.177
26. Stanley WC, Gertz EW, Wisneski JA, Morris DL, Neese RA, Brooks GA. Systemic lactate kinetics during graded exercise in man. Am J Physiol. (1985) 249:E595–602. doi: 10.1152/ajpendo.1985.249.6.E595
27. Bergman BC, Wolfel EE, Butterfield GE, Lopaschuk GD, Casazza GA, Horning MA, et al. Active muscle and whole body lactate kinetics after endurance training in men. J Appl Physiol. (1999) 87:1684–96. doi: 10.1152/jappl.1999.87.5.1684
28. Gertz EW, Wisneski JA, Neese R, Bristow JD, Searle GL, Hanlon JT. Myocardial lactate metabolism: evidence of lactate release during net chemical extraction in man. Circulation. (1981) 63:1273–9. doi: 10.1161/01.CIR.63.6.1273
29. Gertz EW, Wisneski JA, Stanley WC, Neese RA. Myocardial substrate utilization during exercise in humans. Dual carbon-labeled carbohydrate isotope experiments. J Clin Invest. (1988) 82:2017–25. doi: 10.1172/JCI113822
30. Baldwin KM, Klinkerfuss GH, Terjung RL, Mole PA, Holloszy JO. Respiratory capacity of white, red, and intermediate muscle: adaptative response to exercise. Am J Physiol. (1972) 222:373–8. doi: 10.1152/ajplegacy.1972.222.2.373
31. Foster DW. Banting lecture 1984. From glycogen to ketones–and back. Diabetes. (1984) 33:1188–99. doi: 10.2337/diab.33.12.1188
32. Brooks GA, Arevalo JA, Osmond AD, leija RG, Curl C, Tovar A. Lactate in contemporary biology: a phoenix risen. J Physiol. (2021). doi: 10.1113/JP280955. [Epub ahead of print].
33. Steinman MQ, Gao V, Alberini CM. The role of lactate-mediated metabolic coupling between astrocytes and neurons in long-term memory formation. Front Integr Neurosci. (2016) 10:10. doi: 10.3389/fnint.2016.00010
34. Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. (2011) 144:810–23. doi: 10.1016/j.cell.2011.02.018
35. Glenn TC, Martin NA, Horning MA, McArthur DL, Hovda D, Vespa PM, et al. Lactate: brain fuel in human traumatic brain injury. A comparison to normal healthy control subjects. J Neurotrauma. (2015) 32:820–832. doi: 10.1089/neu.2014.3483
36. Hashimoto T, Tsukamoto H, Takenaka S, Olesen ND, Petersen LG, Sorensen H, et al. Maintained exercise-enhanced brain executive function related to cerebral lactate metabolism in men. FASEB J. (2018) 32:1417–27. doi: 10.1096/fj.201700381RR
37. El Hayek L, Khalifeh M, Zibara V, Abi Assaad R, Emmanuel N, Karnib N, et al. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J Neurosci. (2019) 39:2369–82. doi: 10.1523/JNEUROSCI.1661-18.2019
38. Schurr A. Lactate: a major and crucial player in normal function of both muscle and brain. J Physiol. (2008) 586:2665–6. doi: 10.1113/jphysiol.2008.155416
39. Schurr A. Cerebral glycolysis: a century of persistent misunderstanding and misconception. Front Neurosci. (2014) 8:360. doi: 10.3389/fnins.2014.00360
40. Gerich JE, Meyer C, Woerle HJ, Stumvoll M. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care. (2001) 24:382–91. doi: 10.2337/diacare.24.2.382
41. Bergman BC, Horning MA, Casazza GA, Wolfel EE, Butterfield GE, Brooks GA. Endurance training increases gluconeogenesis during rest and exercise in men. Am J Physiol Endocrinol Metab. (2000) 278:E244–51. doi: 10.1152/ajpendo.2000.278.2.E244
42. Goodale WT, Eckenhoff JE. The study of myocardial metabolism and coronary blood flow by coronary sinus catheterization. J Clin Invest. (1948) 27:536.
43. Murashige D, Jang C, Neinast M, Edwards JJ, Cowan A, Hyman MC, et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science. (2020) 370:364–8. doi: 10.1126/science.abc8861
44. Goodale WT, Olson RE, Hackel DB. Myocardial glucose, lactateand pyruvate metabolism of normal and failing hearts studied by coronary sinus catheterization in man. Federation Proc. (1950) 9:49.
45. Wisneski JA, Gertz EW, Neese RA, Mayr M. Myocardial metabolism of free fatty acids. Studies with 14C-labeled substrates in humans. J Clin Invest. (1987) 79:359–66. doi: 10.1172/JCI112820
46. Stanley WC, Gertz EW, Wisneski JA, Neese RA, Morris DL, Brooks GA. Lactate extraction during net lactate release in legs of humans during exercise. J Appl Physiol. (1986) 60:1116–20. doi: 10.1152/jappl.1986.60.4.1116
47. Stanley WC, Wisneski JA, Gertz EW, Neese RA, Brooks GA. Glucose and lactate interrelations during moderate-intensity exercise in humans. Metabolism. (1988) 37:850–8. doi: 10.1016/0026-0495(88)90119-9
48. Bing RJ, Siegel A, Ungar I, Gilbert M. Metabolism of the human heart. II. Studies on fat, ketone and amino acid metabolism. Am J Med. (1954) 16:504–15. doi: 10.1016/0002-9343(54)90365-4
49. Wisneski JA, Gertz EW, Neese RA, Gruenke LD, Morris DL, Craig JC. Metabolic fate of extracted glucose in normal human myocardium. J Clin Invest. (1985) 76:1819–27. doi: 10.1172/JCI112174
50. Smith D, Pernet A, Hallett WA, Bingham E, Marsden PK, Amiel SA. Lactate: a preferred fuel for human brain metabolism in vivo. J Cereb Blood Flow Metab. (2003) 23:658–64. doi: 10.1097/01.WCB.0000063991.19746.11
51. Glenn TC, Martin NA, McArthur DL, Hovda DA, Vespa P, Johnson ML, et al. Endogenous nutritive support after traumatic brain injury: peripheral lactate production for glucose supply via gluconeogenesis. J Neurotrauma. (2015) 32:811–9. doi: 10.1089/neu.2014.3482
52. Bergman BC, Butterfield GE, Wolfel EE, Casazza GA, Lopaschuk GD, Brooks GA. Evaluation of exercise and training on muscle lipid metabolism. Am J Physiol. (1999) 276:E106–17. doi: 10.1152/ajpendo.1999.276.1.E106
53. Bergman BC, Butterfield GE, Wolfel EE, Lopaschuk GD, Casazza GA, Horning MA, et al. Muscle net glucose uptake and glucose kinetics after endurance training in men. Am J Physiol. (1999) 277:E81–92. doi: 10.1152/ajpendo.1999.277.1.E81
54. Brooks GA, Butterfield GE, Wolfe RR, Groves BM, Mazzeo RS, Sutton JR, et al. Decreased reliance on lactate during exercise after acclimatization to 4,300 m. J Appl Physiol. (1991) 71:333–41. doi: 10.1152/jappl.1991.71.1.333
55. Messonnier AL, Emhoff CW, Fattor JA, Horning MA, CT J, Brooks GA. Lactate kinetics at the lactate threshold in trained and untrained men. J Appl Physiol. (2013) 114:1593–602. doi: 10.1152/japplphysiol.00043.2013
56. Mazzeo RS, Brooks GA, Schoeller DA, Budinger TF. Disposal of blood [1-13C]lactate in humans during rest and exercise. J Appl Physiol. (1986) 60:232–41. doi: 10.1152/jappl.1986.60.1.232
57. Gabriel-Costa D, da Cunha TF, Bechara LR, Fortunato RS, Bozi LH, Coelho Mde A, et al. Lactate up-regulates the expression of lactate oxidation complex-related genes in left ventricular cardiac tissue of rats. PLoS ONE. (2015) 10:e0127843. doi: 10.1371/journal.pone.0127843
58. Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. Faseb J. (2007) 21:2602–12. doi: 10.1096/fj.07-8174com
59. Bertocci LA, Jones JG, Malloy CR, Victor RG, Thomas GD. Oxidation of lactate and acetate in rat skeletal muscle: analysis by 13C-nuclear magnetic resonance spectroscopy. J Appl Physiol 1985. (1997) 83:32–9. doi: 10.1152/jappl.1997.83.1.32
60. Park JM, Josan S, Mayer D, Hurd RE, Chung Y, Bendahan D, et al. Hyperpolarized 13C NMR observation of lactate kinetics in skeletal muscle. J Exp Biol. (2015) 218:3308–18. doi: 10.1242/jeb.123141
61. Bertocci LA, Lujan BF. Incorporation and utilization of [3-13C]lactate and [1,2-13C]acetate by rat skeletal muscle. J Appl Physiol. (1999) 86:2077–89. doi: 10.1152/jappl.1999.86.6.2077
62. Weiss J, Hiltbrand B. Functional compartmentation of glycolytic versus oxidative metabolism in isolated rabbit heart. J Clin Invest. (1985) 75:436–47. doi: 10.1172/JCI111718
63. Rogatzki MJ, Ferguson BS, Goodwin ML, Gladden LB. Lactate is always the end product of glycolysis. Front Neurosci. (2015) 9:22. doi: 10.3389/fnins.2015.00022
64. Henderson GC, Horning MA, Wallis GA, Brooks GA. Pyruvate metabolism in working human skeletal muscle. Am J Physiol Endocrinol Metab. (2007) 292:E366. doi: 10.1152/ajpendo.00363.2006
65. Hammond GL, Nadal-Ginard B, Talner NS, Markert CL. Myocardial LDH isozyme distribution in the ischemic and hypoxic heart. Circulation. (1976) 53:637–43. doi: 10.1161/01.CIR.53.4.637
66. Brooks GA, Wolfel EE, Butterfield GE, Cymerman A, Roberts AC, Mazzeo RS, et al. Poor relationship between arterial [lactate] and leg net release during exercise at 4,300 m altitude. Am J Physiol. (1998) 275:R1192–201. doi: 10.1152/ajpregu.1998.275.4.R1192
67. Brooks GA, Wolfel EE, Groves BM, Bender PR, Butterfield GE, Cymerman A, et al. Muscle accounts for glucose disposal but not blood lactate appearance during exercise after acclimatization to 4,300 m. J Appl Physiol. (1992) 72:2435–45. doi: 10.1152/jappl.1992.72.6.2435
68. Shulman RG, Rothman DL. The “glycogen shunt” in exercising muscle: a role for glycogen in muscle energetics and fatigue. Proc Natl Acad Sci USA. (2001) 98:457–61. doi: 10.1073/pnas.98.2.457
69. Neglia D, De Caterina A, Marraccini P, Natali A, Ciardetti M, Vecoli C, et al. Impaired myocardial metabolic reserve and substrate selection flexibility during stress in patients with idiopathic dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. (2007) 293:H3270–8. doi: 10.1152/ajpheart.00887.2007
70. Peterson LR, Saeed IM, McGill JB, Herrero P, Schechtman KB, Gunawardena R, et al. Sex and type 2 diabetes: obesity-independent effects on left ventricular substrate metabolism and relaxation in humans. Obesity. (2012) 20:802–10. doi: 10.1038/oby.2011.208
71. Kates AM, Herrero P, Dence C, Soto P, Srinivasan M, Delano DG, et al. Impact of aging on substrate metabolism by the human heart. J Am Coll Cardiol. (2003) 41:293–9. doi: 10.1016/S0735-1097(02)02714-6
72. Brooks GA, Fahey TD, Baldwin KM. Exercise Physiology: Human Bioenergetics and Its Applications. Lexington, KY; Kindle Direct Publishing. (2019).
73. Issekutz BJ, Miller H. Plasma free fatty acids during exercise and the effect of lactic acid. Proc Soc Exp Biol Med. (1962) 110:237–9. doi: 10.3181/00379727-110-27478
74. Issekutz Jr B, Miller HI, Paul P, Rodahl K. Aerobic work capacity and plasma FFA turnover. J Appl Physiol. (1965) 20:293–6. doi: 10.1152/jappl.1965.20.2.293
75. Gold M, Miller HI, Issekutz Jr B, Spitzer JJ. Effect of exercise and lactic acid infusion on individual free fatty acids of plasma. Am J Physiol. (1963) 205:902–4. doi: 10.1152/ajplegacy.1963.205.5.902
76. Saddik M, Gamble J, Witters LA, Lopaschuk GD. Acetyl-CoA carboxylase regulation of fatty acid oxidation in the heart. J Biol Chem. (1993) 268:25836–45. doi: 10.1016/S0021-9258(19)74465-2
77. Liu C, Wu J, Zhu J, Kuei C, Yu J, Shelton J, et al. Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J Biol Chem. (2009) 284:2811–22. doi: 10.1074/jbc.M806409200
78. Hoque R, Farooq A, Ghani A, Gorelick F, Mehal WZ. Lactate reduces liver and pancreatic injury in toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology. (2014) 146:1763–74. doi: 10.1053/j.gastro.2014.03.014
79. Hashimoto T, Hussien R, Cho HS, Kaufer D, Brooks GA. Evidence for the mitochondrial lactate oxidation complex in rat neurons: demonstration of an essential component of brain lactate shuttles. PLoS ONE. (2008) 3:e2915. doi: 10.1371/journal.pone.0002915
80. Ahmed K, Tunaru S, Tang C, Muller M, Gille A, Sassmann A, et al. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metab. (2010) 11:311–9. doi: 10.1016/j.cmet.2010.02.012
81. Cai TQ, Ren N, Jin L, Cheng K, Kash S, Chen R, et al. Role of GPR81 in lactate-mediated reduction of adipose lipolysis. Biochem Biophys Res Commun. (2008) 377:987–91. doi: 10.1016/j.bbrc.2008.10.088
82. Ge H, Weiszmann J, Reagan JD, Gupte J, Baribault H, Gyuris T, et al. Elucidation of signaling and functional activities of an orphan GPCR, GPR81. J Lipid Res. (2008) 49:797–803. doi: 10.1194/jlr.M700513-JLR200
83. Bergersen LH. Lactate transport and signaling in the brain: potential therapeutic targets and roles in body-brain interaction. J Cereb Blood Flow Metab. (2015) 35:176–85. doi: 10.1038/jcbfm.2014.206
84. Lauritzen KH, Morland C, Puchades M, Holm-Hansen S, Hagelin EM, Lauritzen F, et al. Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolism. Cerebral Cortex. (2014) 24:2784–95. doi: 10.1093/cercor/bht136
85. Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. (2010) 90:207–58. doi: 10.1152/physrev.00015.2009
86. Henderson GC, Horning MA, Lehman SL, Wolfel EE, Bergman BC, Brooks GA. Pyruvate shuttling during rest and exercise before and after endurance training in men. J Appl Physiol. (2004) 97:317–25. doi: 10.1152/japplphysiol.01367.2003
87. Brooks GA, Brown MA, Butz CE, Sicurello JP, Dubouchaud H. Cardiac and skeletal muscle mitochondria have a monocarboxylate transporter MCT1. J Appl Physiol. (1999) 87:1713–8. doi: 10.1152/jappl.1999.87.5.1713
88. Passarella S, de Bari L, Valenti D, Pizzuto R, Paventi G, Atlante A. Mitochondria and L-lactate metabolism. FEBS Lett. (2008) 582:3569–76. doi: 10.1016/j.febslet.2008.09.042
89. McGarry JD, Mannaerts GP, Foster DW. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest. (1977) 60:265–70. doi: 10.1172/JCI108764
90. Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. (1996) 94:2837–42. doi: 10.1161/01.CIR.94.11.2837
91. Emhoff CA, Messonnier LA, Horning MA, Fattor JA, Carlson TJ, Brooks GA. Gluconeogenesis and hepatic glycogenolysis during exercise at the lactate threshold. J Appl Physiol. (2013) 114:297–306. doi: 10.1152/japplphysiol.01202.2012
92. Goldfarb AH, Bruno JF, Buckenmeyer PJ. Intensity and duration effects of exercise on heart cAMP, phosphorylase, and glycogen. J Appl Physiol 1985. (1986) 60:1268–73. doi: 10.1152/jappl.1986.60.4.1268
93. Richardson RS, Noyszewski EA, Leigh JS, Wagner PD. Lactate efflux from exercising human skeletal muscle: role of intracellular PO2. J Appl Physiol. (1998) 85:627–34. doi: 10.1152/jappl.1998.85.2.627
Keywords: heart, lactate, glucose, fatty acids, ketones, exercise, metabolism, muscle
Citation: Brooks GA (2021) Role of the Heart in Lactate Shuttling. Front. Nutr. 8:663560. doi: 10.3389/fnut.2021.663560
Received: 03 February 2021; Accepted: 05 March 2021;
Published: 22 April 2021.
Edited by:
Gregory C. Henderson, Purdue University, United StatesReviewed by:
Todd Hagobian, California Polytechnic State University, United StatesCopyright © 2021 Brooks. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: George A. Brooks, Z2Jyb29rc0BiZXJrZWxleS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.