
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 31 May 2021
Sec. Nutrition and Microbes
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.648073
This article is part of the Research TopicAdvances in Microalgae NutritionView all 5 articles
 Yuichiro Nishimoto1
Yuichiro Nishimoto1 Tatsuhiro Nomaguchi1
Tatsuhiro Nomaguchi1 Yuka Mori1
Yuka Mori1 Masaki Ito1
Masaki Ito1 Yuya Nakamura1
Yuya Nakamura1 Masaki Fujishima2
Masaki Fujishima2 Shinnosuke Murakami1,3
Shinnosuke Murakami1,3 Takuji Yamada1,4*
Takuji Yamada1,4* Shinji Fukuda1,3,5,6*
Shinji Fukuda1,3,5,6*Recent studies have accumulated evidence that the intestinal environment is strongly correlated with host diet, which influences host health. A number of dietary products whose mechanisms of influence operate via the gut microbiota have been revealed, but they are still limited. Here, we investigated the dietary influence of Chlorella, a green alga commercially available as a dietary supplement. A randomised, double-blind, placebo-controlled crossover trial including 40 Japanese participants with constipation was performed. In this study, the primary outcome and secondary outcome were set as defecation frequency and blood folate level, respectively. In both outcomes, no significant differences were detected compared to the control intake. Therefore, we analysed the gut microbiome, gut metabolome, and blood parameters in an integrated manner as an exploratory analysis. We revealed that the consumption of Chlorella increased the level of several dicarboxylic acids in faeces. Furthermore, the analysis showed that individuals with low concentrations of faecal propionate showed an increase in propionate concentration upon Chlorella intake. In addition, increasing blood folate levels were negatively correlated with defecation frequency at baseline. Our study suggested that the effect of Chlorella consumption varies among individuals depending on their intestinal environment, which illustrates the importance of stratified dietary management based on the intestinal environment in individuals.
Chlorella is a genus that belongs to the class Chlorophyceae and consists of mostly green algae living in freshwater. Chlorella species are protein rich and are currently well known as a dietary supplement that is commercially available and consumed by various populations. Recent studies have reported that Chlorella has various beneficial effects, such as anti-inflammatory (1) and anti-allergic (2) effects, lipid metabolism improvement (3), and improvement of some markers of several cardiovascular risk factors (4). Chlorella species also include dietary fibre. It has been reported that dietary fibre has effects such as lowering blood glucose levels, lowering cholesterol levels and suppressing intestinal inflammation (5–7). However, dietary fibre has a variety of sugar compositions, and some of its positive effects on human health depend on this composition (8). It is important to investigate the effect of each food that contains dietary fibre. In addition, the effect of dietary fibre sometimes depends on the host gut microbiota, as the gut microbiota is involved in its mechanism of action. For instance, improvement in glucose metabolism by barley consumption is associated with increased abundance of the intestinal bacterial genus Prevotella (7). The improvement of glucose metabolism by succinate, which is produced from dietary fibre by Prevotella, has been proposed as the mechanism of action (9). Rat studies have reported that Chlorella intake improved blood lipid profiles, which was correlated with gut microbiota alteration (3, 10). However, the gut microbiota is known to differ between rodents and humans (11); thus, the effect of Chlorella intake into the intestinal environment in humans could differ from that in rats. A previous study reported that there are inter-individual differences in the response to Chlorella intake (12); however, while the study focused on genetic differences among consumers, individual differences in the intestinal environment were not considered. The intestinal environment differs among human individuals (13). In this interventional study, we recruited participants with a tendency for constipation to evaluate their responses to Chlorella intake. In particular, this study focused on their bowel movement, blood parameters and intestinal environment analysed by a metabologenomics approach which is an integrated approach of 16S rRNA gene-based microbiome and mass spectrometry-based metabolome analyses.
To assess the effects of Chlorella intake, a randomised, controlled crossover trial with two 4-week dietary intervention periods separated by a 4-week washout period was performed (Figure 1). A total of 40 Japanese participants with constipation tendencies passed the inclusion criteria, and all of the participants completed the two dietary intervention periods. Participants in two randomised blocks had similar clinical characteristics regarding the primary and secondary outcomes (defecation frequency and blood folate level) before the dietary intervention (Supplementary Table 1).
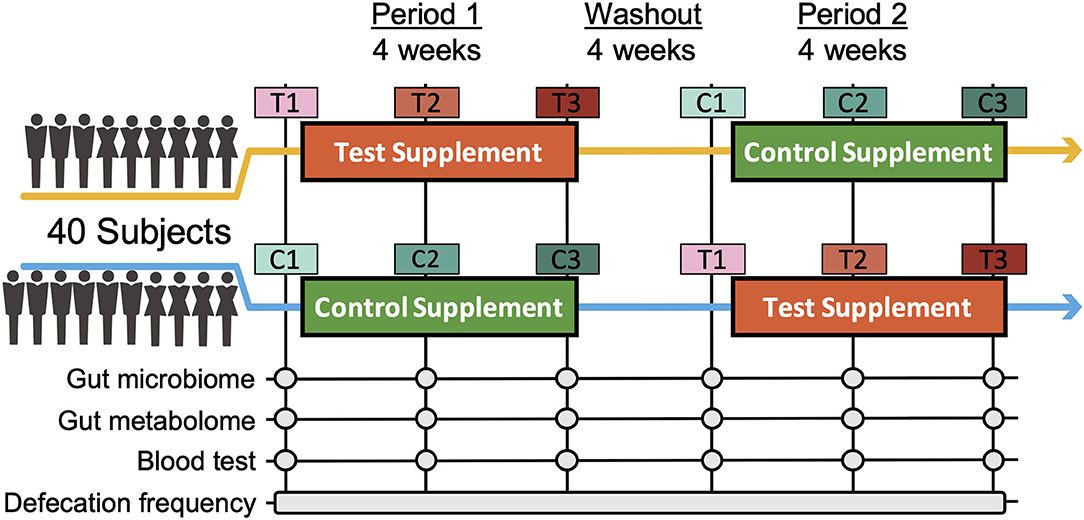
Figure 1. Timeline of the double-blind randomized crossover trial. Two 4-week dietary treatments were set in succession. The dietary intervention periods were interspaced by a 4-week washout period. Blood and faecal samples were collected beforehand and at week 2 and 4 of each intervention period. C and T indicate the control and test supplement intervention periods, respectively, and number 1-3 indicates the sampling timepoint in the period.
For the primary and secondary outcomes, changes in defecation frequency and blood folate level, which are hypothesised to both be improved by Chlorella intake (12, 14), were analysed. Fasting blood samples were taken before and during each intervention period, and folate levels were biochemically analysed. Participants were asked to record their defecation frequency during the intervention periods. The defecation frequencies during days 14–28 in the control intervention period and the Chlorella intervention period were compared; however, no significant differences were observed in either defecation frequency or blood folate levels (Table 1).
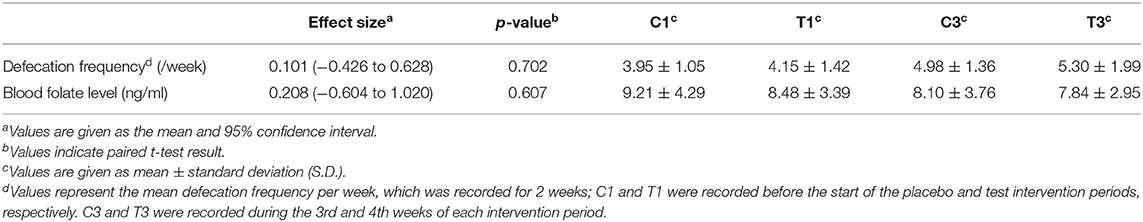
Table 1. Chlorella intake did not significantly increase defecation frequency or blood folate levels.
We next performed 16S rRNA-based microbiome analysis and capillary electrophoresis time-of-flight mass spectrometry (CE-TOFMS)-based metabolome analysis. In this study, 20 randomly selected subject samples were analysed. Faecal samples were obtained prior to intervention (C1 and T1 in Figure 1. C and T indicate the control supplement and test supplement intervention periods, respectively, and 1 indicates the first sampling timepoint during the intervention) and at ~14 days (C2 and T2) and 28 days (C3 and T3) into each intervention period (Figure 1). Multivariate analysis based on the Spearman rank correlation coefficient showed that the inter-individual distance in microbiome and metabolome profiles was significantly higher than the intra-individual distance (Figures 2A,C; p = 3.28 × 10−76 and 4.07 × 10−56 when using the microbiome and metabolome profiles, respectively; Wilcoxon rank sum test). This indicated that inter-individual variation in microbiome and metabolome profiles is more prominent than the effect caused by the intervention. Therefore, to evaluate the effect of Chlorella intake on the microbiome and metabolome profiles, it is necessary to compare variations within the individual. We compared intra-individual variation before and after placebo intake (C1 and C2, and C1 and C3) and intra-individual variation before and after Chlorella intake (T1 and T2, and T1 and T3), but no significant difference was detected (Figures 2B,D). These results indicated that Chlorella intake had no significant effect on the general profile of the microbiome and the metabolome.
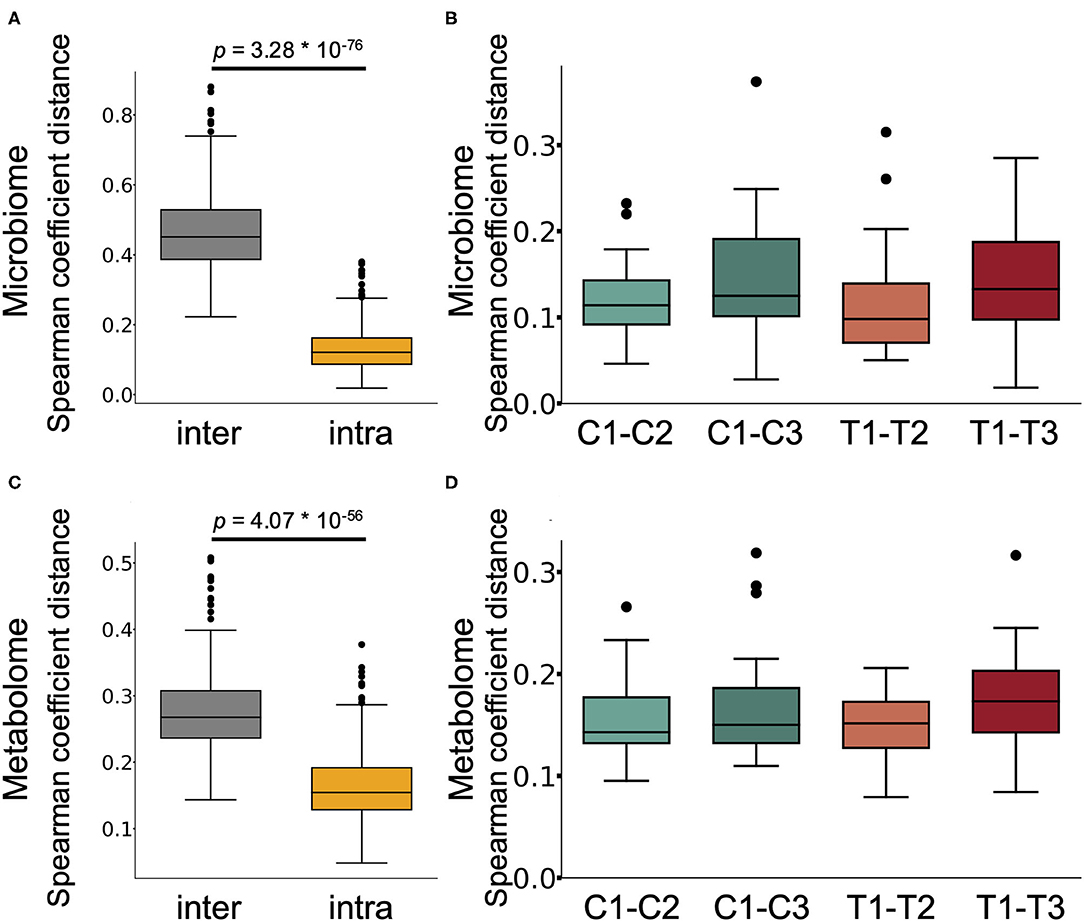
Figure 2. The influence of Chlorella consumption on the gut microbiome and metabolome profiles was not larger than that of control supplement consumption. (A,C) Box plot representing the distribution of Spearman coefficient distance for the gut microbiome (A) and metabolome profiles (C) among samples from different subjects at the first timepoint (inter) and the distance between samples from the same subject (intra). (B,D) The boxplots show the microbiome profile distance (B) and metabolome profile distance (D) within an individual between the labelled timepoints. A significant difference test was performed during the same intake period (T1–T2 and C1–C2, or T1–T3 and C1–C3), but no significant difference was detected (Wilcoxon signed-rank test).
While Chlorella intake had no significant effect on the entire microbiome and metabolome profiles, some of the specific intestinal bacteria or metabolites may have been affected. To assess the effect of intervention on each bacterium and metabolite, the relative abundance of each bacterium and scaled peak areas of each metabolite, which represent the amount of each metabolite in faeces, were analysed. During the analysis, we performed the Wilcoxon signed-rank test with two sets of pairs: comparison between T1 and T3 for comparison before and after Chlorella intake and comparison between T1–T3 and C1–C3 for comparison of the effect caused by control supplement and Chlorella intake (T1–T3 and C1–C3 represent the difference in values between the 3rd and 1st timepoints of the test supplement and the control supplement intervention periods, respectively, in each subject). Although significant differences in bacterial abundance were detected in either one comparison or the other, 10 metabolites showed significant differences in both comparisons (Figure 3). The functions of these metabolites were analysed using the KEGG BRITE database, and the results revealed that half of these metabolites were dicarboxylic acids (Table 2). Three metabolites, namely, azelaic acid, suberic acid, and 6-hydroxyhexanoic acid, remained significant after false discovery rate (FDR) adjustment by the Benjamini-Hochberg (BH) approach. Azelaic acid remained significant after FDR adjustment in the comparison between T1 and T3; suberic acid and 6-hydroxyhexanoic acid remained significant after FDR adjustment in the comparison between T1–T3 and C1–C3. Chlorella itself may contain these three metabolites (azelaic acid, suberic acid, and 6-hydroxyhexanoic acid). Therefore, CE-TOFMS metabolic profiling was performed to measure the nutritional contents in Chlorella. However, these three metabolites were not present in Chlorella (Supplementary Table 2).
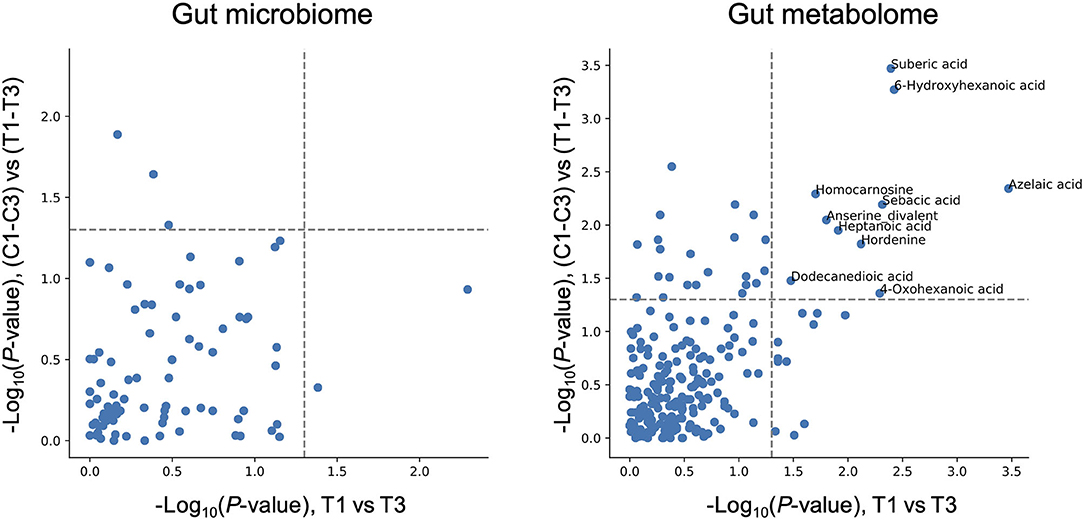
Figure 3. Chlorella intake significantly alters some intestinal metabolite levels. The X-axis indicates the logarithmic P-value of the Wilcoxon signed-rank test for each bacterial taxon/metabolite between T1 and T3. The Y-axis indicates the logarithmic P-value of the Wilcoxon signed-rank test for each bacterial taxon/metabolite between T1–T3 and C1–C3 (T1–T3 means differences between T1 and T3 within each subject treated as one group). Bacterial taxon/metabolite names are labelled if a significant difference was detected in both statistical tests.
Chlorella contains dietary fibre such as β-glucan that is metabolised to short-chain fatty acids (SCFAs) such as propionate and butyrate, which are known to have beneficial effects on host health, by the gut microbiota (15). However, a simple comparison showed no significant difference in the faecal abundances of propionate and butyrate before and after Chlorella intake in this study (Figures 4A,C). A previous study has reported that the effects of probiotics on SCFAs in faeces differ between individuals depending on their SCFA levels at baseline (16). We analysed the correlation between the effect size of Chlorella intake, which will be referred as the responder score (see section Methods for details), and the baseline faeces propionate and butyrate levels of each individual. The propionate responder score showed a negative correlation with the propionate levels at baseline (Figures 4A,B; Spearman r = −0.589; p = 0.00623). This indicates that subjects with low propionate levels in faeces before Chlorella consumption showed an increase in propionate after Chlorella intake. Butyrate also exhibited a similar tendency, but the correlation was not significant (Figures 4C,D; Spearman r = −0.362; p = 0.116).
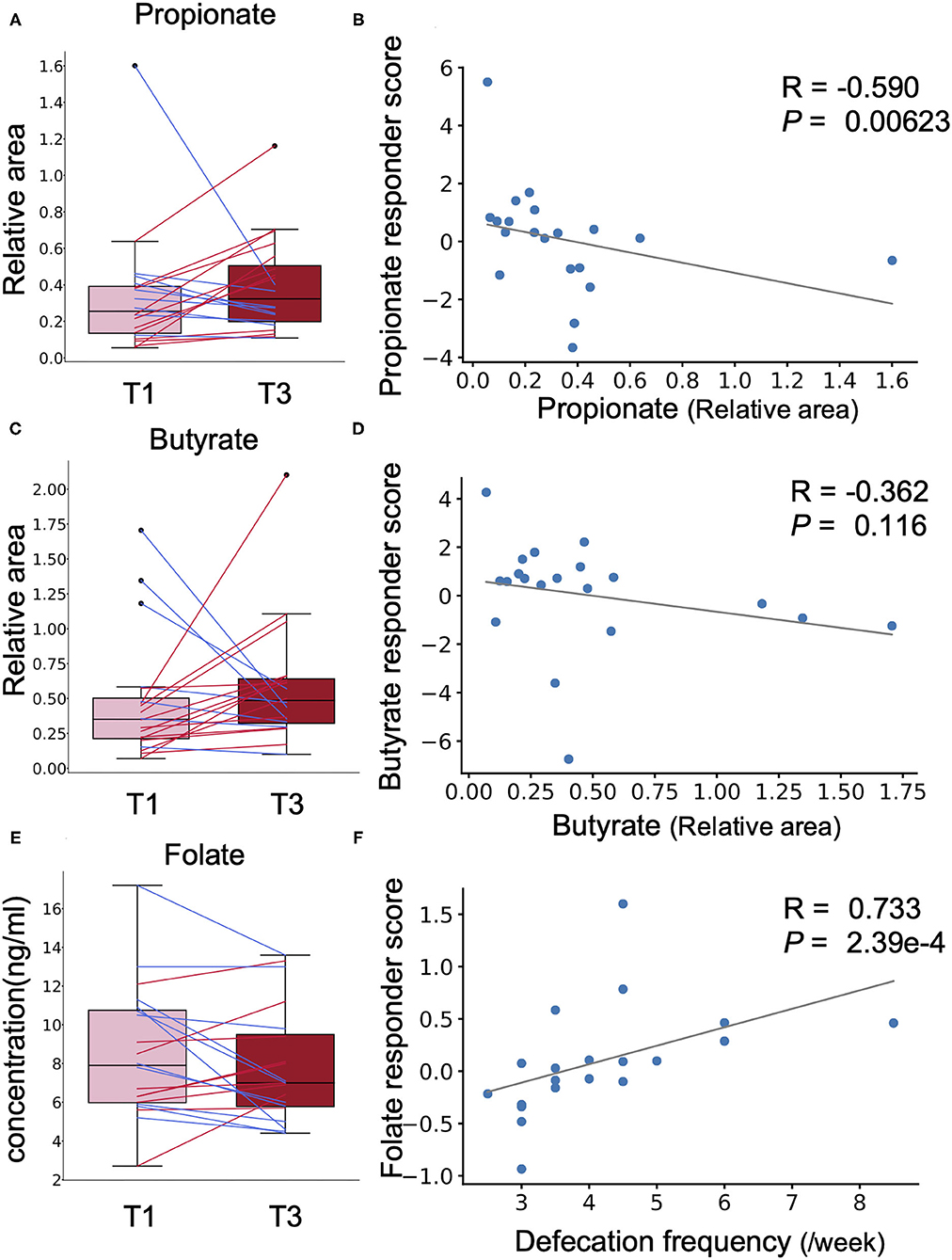
Figure 4. The effect of Chlorella intake depends on intestinal environmental factors at baseline. (A,C,E) Box plots representing the distribution of faecal propionate (A), butyrate (C), and blood folate (E) concentrations. The relative area shows the peak area ratio of samples compared to the internal standard, which represents the concentration of the corresponding metabolite. No significant difference was detected (Wilcoxon signed-rank test). (B,D,F) The X-axis indicates the value at baseline. The Y-axis indicates the responder score of each metabolite/blood test item. Correlation was determined using the Spearman coefficient. The grey line shows the approximated curve.
SCFAs are major faecal metabolites that are produced via the metabolism of dietary fibre by the gut microbiota. Therefore, to evaluate the bacteria contributing to the response, a correlation analysis was performed on the propionate and butyrate responder scores and the abundance of each intestinal bacterial taxon at baseline. The abundances of the two bacteria (Ruminiclostridium 9 and Butyricimonas) were significantly correlated with both propionate and butyrate responder scores (Supplementary Table 3). Bacteria that were correlated significantly with propionate or butyrate responder scores are listed in Supplementary Table 3.
Next, we performed the same analysis with primary and secondary outcomes (defecation frequency and blood folate level) to evaluate whether the effect on these outcomes was also dependent on prior individual intestinal environments. To evaluate whether the effects on defecation frequency and blood folate level were dependent on the intestinal environment, correlation analysis was performed for defecation frequency responder score, blood folate responder score, and intestinal environment factors, including intestinal bacteria and metabolite abundances and defecation frequency at baseline. A strong positive correlation was found between blood folate responder score and defecation frequency at baseline (Figures 4E,F; Spearman r = 0.733; p = 2.39 × 10−4). Other intestinal environmental factors that were significantly correlated with defecation frequency or blood folate responder score are listed in Supplementary Table 4.
In our study, no significant differences in defecation frequency or blood folate level were observed between timepoints C3 and T3. One possible reason was the placebo effect caused by the intervention. When we compared the defecation frequency between T1 and T3 with a statistical pairwise test, a significant difference was detected. However, there was also a significant difference observed between C1 and C3, which correspond to times before and after control supplement intake, respectively. This suggests that placebo effects appeared during both intake periods; thus, the effect caused by Chlorella intake became difficult to detect when C1–C3 and T1–T3 were compared.
Multivariate analysis showed that Chlorella intake had no significant effect on the general profiles of either the gut microbiome or metabolome. This is the same tendency as that found in our previous research (17). While the general microbiome and metabolome profiles were not significantly affected, there were several metabolites that showed significant changes in abundance after Chlorella intervention. Azelaic acid showed an especially clear effect, since it was detected as a significantly increased metabolite even after FDR adjustment (T1 and T3 comparison, q < 0.10). Azelaic acid is a commonly contained nutrient in grain such as barley. Azelaic acid can improve glucose tolerance in a mouse model (18). This effect is consistent with the result in a previous barley study showing that barley can also improve glucose tolerance (7). Previous studies with rodents also reported that Chlorella intake improves glucose tolerance (19, 20), possibly via an increase in intestinal azelaic acid. In this study, fasting blood glucose levels did not improve significantly. Tests showing glucose tolerance, such as oral glucose tolerance tests, are needed to clarify the effect of Chlorella consumption.
Dicarboxylic acids, which were significantly enriched metabolites in this study, were not reported to be present in Chlorella. Indeed, metabolome analysis revealed that azelaic acid and suberic acid were not detected in Chlorella (Supplementary Table 2). Therefore, the observed increase was likely derived from the metabolism of other molecules originally present in Chlorella. Chlorella and many algae are known to produce and accumulate various long-chain fatty acid molecules (21). Such long-chain fatty acids are metabolised to dicarboxylic acids by ω-oxidation, which was also shown in an in vivo study in which fatty acids were administered to animals or humans that then excreted high levels of dicarboxylic acids in their urine (22). These reports suggest that the fatty acids in Chlorella may be metabolised to dicarboxylic acids, the levels of which increase in the blood. Dicarboxylic acids have been reported to have beneficial effects, such as antiketogenic effects; therefore, the increase in dicarboxylic acids caused by Chlorella intake would provide additional benefits other than the improvement of glucose tolerance discussed previously (23).
The effect derived from food consumption varies among individuals, and recent studies have pointed out that the intestinal environment is an important factor determining individual dependency. For instance, barley-induced improvement in glucose metabolism is associated with an increase in the gut microbial Prevotella/Bacteroides ratio (7). Therefore, it is important to stratify the population into groups depending on their response to the intervention and to study those groups separately to understand the truly important elements responsible for the effect. In this study, we performed a correlation analysis with our defined responder scores and discovered that subjects with a low concentration of SCFAs, especially propionate, had increased faecal SCFA levels. Although there were differences in whether the test supplement was probiotic or prebiotic, previous studies have shown the same tendency as our Chlorella intervention study (16). Propionate has beneficial effects, such as anti-inflammatory activity (24) and body weight maintenance (25). Since our data suggested that individuals with low concentrations of propionate showed an increase in propionate concentration upon Chlorella intake, there is a possibility that Chlorella intake reduces the risk of inflammation and obesity in individuals with low concentrations of propionate. We also analysed features of the gut microbiota and metabolites in subjects with increased propionate levels (Supplementary Table 3). In particular, the abundances of Ruminiclostridium 9 and Butyricimonas showed positive and negative correlations, respectively, with butyrate and propionate responder scores. There is a possibility that gut microbes that have a positive correlation with propionate and butyrate responder scores may be related to the production of propionate and butyrate and that those with a negative correlation may inhibit propionate and butyrate production. The genus Ruminiclostridium includes species such as Ruminiclostridium cellulolyticum, which is known as a cellulolytic bacterium (26). Therefore, efficient Chlorella-derived cellulose degradation by Ruminiclostridium 9 may have been involved in the production of short-chain fatty acids.
When we focused on the parameters related to blood folate levels, we discovered that the defecation frequency was positively correlated with the folate responder score. A previous study has shown that individuals with constipation tendencies have a lower absorption rate of nutrients than healthy individuals (27). This study showed that folate is also associated with constipation tendency. Our results may indicate that the absorbance of Chlorella-derived folate depends on defecation frequency.
The following limitations should be considered for our study. First, we analysed multivariate data, which included abundances of hundreds of intestinal bacterial taxa and metabolites. FDR correction is necessary in statistical hypothetical tests. However, the FDR correction was strict, as many items were comprehensively observed. Therefore, we discuss the factor detected by the multiple comparison without FDR adjustment. Since the factors detected by multiple comparisons without FDR adjustment possibly include false positives, validation with animal models or humans is crucial to confirm the findings. Second, this study was human intervention research that aimed to evaluate the effects of Chlorella intervention and elucidate the mechanism. To clarify the detailed mechanism, further study with animal models and molecular biological experiments are necessary. Third, the human gut microbiota is influenced by the host diet, but there was no assessment of the background diet in the present study. Although the intake of prebiotics and probiotics, which are known to affect the gut environment, was restricted during the trial, the possible influence of diet on the effects of Chlorella should be assessed in future studies.
In conclusion, we showed that the consumption of Chlorella increases the levels of several faecal dicarboxylic acids, such as azelate, which may improve glucose tolerance. On the other hand, the blood folate level, which has been reported to be increased by Chlorella consumption, was increased in only specific individuals with high defecation frequency. The data suggest that Chlorella intake with simultaneous intervention for improving bowel movement may enhance the effect of Chlorella consumption. In addition to the blood folate level, we discovered that the faecal propionate concentration was also improved by Chlorella consumption in individuals with low concentrations of propionate. Our results suggested that the effects derived from Chlorella consumption differ by individual based on their intestinal environments prior to intake. Such inter-individual differences in the effect of supplementation indicate the importance of a stratified healthcare approach that involves estimating the optimal nutrition for each individual to maximise the effects of diet.
The human rights of the subjects who participated in this study were protected at all times, and the study observed the Helsinki Declaration and the Ethical Guidelines on Epidemiological Research in Japan referring to standards for clinical trials of drugs. This trial was conducted with the approval of the clinical trial ethics review committee of the Chiyoda Paramedical Care Clinic and reported to https://www.umin.ac.jp/ (Identifiers: UMIN000041648).
In this study, a randomised double-blind placebo-controlled crossover trial in Japanese participants was performed for 3 months between December 2016 and April 2017 (Figure 1; Supplementary Figure 1; Supplementary Table 5). The sample size was estimated based on a previous study (14). Eight subjects were required per group (significance level was 5% and statistical power was 80%). In total, 80 participants were recruited in this study. The participants fulfilled the following criteria: women aged between 20 and 60 years old with constipation (defecation frequency of 3–5 times per week). The detailed inclusion criteria were as indicated: (1) females aged 20–60 years; (2) defecation frequency of 3–5 times per week; (3)subjects who could visit the designated facility on the scheduled date; (4) understanding of the study procedures and agreement with participation in the study by written informed consent prior to the study. The detailed exclusion criteria were as indicated: (1) continuing to receive medical treatment; (2) constantly taking oral medicines, functional foods, and/or supplements that could affect the test results; (3) currently pregnant, lactating, or having the possibility of pregnancy; (4) donating over 200 mL of blood and/or blood components within the last 4 weeks to the current study; (5) donating over 400 mL of blood within the last 16 weeks to the current study; (6) over 800 mL of blood was collected when the amounts sampled within the last 12 months were added to the planned sampling amounts of this study; (7) participating in the other clinical test within 4 weeks before the start of the test; (8) having pain or bleeding during defecation; (9) having abdominal surgical operation within 6 months before the start of the test; (10) taking antibiotics within 6 months before the start of the test; (11) consuming an excessive amount of alcohol or smoking heavily; (12) having irregular dining habits and/or working midnight or irregular shifts; (13) planning a large change in lifestyle during the test period; (14) having a tendency for chronic diarrhoea; (15-a) having heart disease, liver disease, kidney disease, or diabetes (including the complication of other diseases); (15-b) having a medical history of cardiovascular diseases; (15-c) having an allergy to the test supplement; (16) determined ineligible by principal investigator or subinvestigator. Based on the inclusion/exclusion criteria, 40 subjects were selected for the main trial. Randomisation in this trial was performed using the block stratified randomisation method. Subjects were stratified by age and defecation frequency. Details regarding randomisation were as previously reported (13). The study included 4-week dietary intervention periods in which the subjects ingested the test supplement [3 g of Chlorella pyrenoidosa (Sun Chlorella A Tablets®, Sun Chlorella Corp., Kyoto, Japan) twice daily] and control supplement (3 g of digestible dextrin and pigment twice daily) in random order and separated by a 4-week washout period. Both the test supplement and control supplement were provided as tablets with similar appearances.
As this trial was designed as a crossover trial, all 40 volunteers consumed both the test and control supplements (Figure 1). The volunteers were divided into two groups (20 volunteers in each group) with stratified random sampling. Volunteers in each group consumed either test or control supplements during the first period and then consumed the other supplement during the second period. The order of consumption depended on the group to which the volunteers were assigned. At each timepoint, volunteers were asked to collect faecal samples three times: (1) before starting consumption, (2) 2 weeks after the start of consumption, and (3) 4 weeks after consumption. Timepoints were named [C1, C2, and C3] or [T1, T2, and T3], where C and T indicate the control and test supplements that the volunteers were consuming at that point. The collected faecal samples were frozen at −20°C until processing. Clinical blood tests were performed following 12 h of fasting at the same timepoint. In the clinical blood test, folate was measured by a chemiluminescence immunoassay as the key secondary outcome. As other outcomes from blood tests, total protein, albumin, aspartate aminotransferase, alanine transaminase, lactate dehydrogenase, total bilirubin, alkaline phosphatase, γ-glutamyl transpeptidase, creatine phosphate enzyme, urea nitrogen, creatinine, uric acid, sodium, chlorine, potassium, calcium, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, neutral fat, glucose, white blood cells, red blood cells, haemoglobin, haematocrit, platelet, folate, vitamin B12, and homocysteine were measured. All subjects completed the trial, and microbiome and metabolome analyses were performed for 20 out of the 40 people for financial reasons. The primary outcome was defecation frequency, and the key secondary outcomes were faecal 16S rRNA metagenomic analysis, metabolome analysis and blood folate concentration.
DNA extraction from faecal samples was performed as previously reported (28). After extraction, the V1–V2 variable region of the 16S rRNA gene was amplified using the bacterial universal primers 27F-mod (5′-AGRGTTTGATYMTGGCTCAG-3′) and 338R (5′-TGCTGCCTCCCGTAGGAGT-3′) with Tks Gflex DNA polymerase (TaKaRa Bio Inc., Japan) (29). The amplicon DNA was sequenced using MiSeq (Illumina, USA) according to the manufacturer's protocol. Extraction of metabolites from faecal samples was performed as previously reported (30). The obtained sequence data are available from DRA010607, and the metabolome data are available in Supplementary Table 6.
CE-TOFMS metabolic profiling was performed to measure the nutritional contents in Chlorella. Fifty milligrammes of powdered Chlorella was vortexed with Milli-Q water for one minute and then agitated at 500 rpm and 37°C for 1 h. Samples were then centrifuged at 9,100 × g for 5 min. A total of 250 μL of supernatant was ultrafiltrated using an Ultrafree 5 kDa MWCO centrifugal philtre unit (Millipore) at 9,100 × g for 80 min. The internal standard was added to the filtrate and analysed using CE-TOFMS in both positive and negative modes. As a blank sample, a sample without Chlorella powder was also prepared and analysed.
For 16S rRNA gene analysis, QIIME2 (version 2019.10) was used (31). In the analytical pipeline, sequence data were processed by using the DADA2 pipeline for quality filtering and denoising (options: –p-trim-left-f 20 –p-trim-left-r 19 –p-trunc-len-f 240 –p-trunc-len-r 140) (32). The filtered output sequences were assigned to taxa by using the “qiime feature-classifier classify-sklearn” command with the default parameters. Silva SSU Ref Nr 99 (version 132) was used as a reference database for taxonomy assignment. In statistical analysis, the paired t-test and paired Cohen's d in the R package effsize were used for pairwise comparisons of primary and secondary outcomes. Other statistical analyses were performed with in-house Python scripts (version 3.7.3). For pairwise comparison of the relative abundances of intestinal bacterial taxa and the relative areas of intestinal metabolites, the Wilcoxon signed-rank test with Benjamini-Hochberg false discovery rate (FDR-BH) correction was used (scipy version 1.3.1 and statsmodels 0.10.1 were used for the Wilcoxon signed-rank test and FDR-BH correction, respectively). During the comparison, bacterial taxa with a mean relative abundance below 0.001 and metabolites not detected in 75% of samples were excluded.
In this study, the test supplement effect size was defined as the responder score and used to evaluate whether effects depended on individual basal characteristics. The response score was calculated with the following equation:
The original contributions presented in the study are publicly available. This data can be found here: The obtained 16S rRNA gene sequence data are available in the DDBJ DRA (DRA accession number: DRA010607).
The studies involving human participants were reviewed and approved by Chiyoda Paramedical Care Clinic. The patients/participants provided their written informed consent to participate in this study.
MF, TY, and SF: conceptualisation. YNi: data curation. YNi and YNa: formal analysis and visualisation. YM and MI: investigation. MF and SM: methodology. SF: project administration. YNi and TN: writing – original draft. YNi, TN, YM, MI, YNa, MF, SM, TY, and SF: writing – review and editing. All authors contributed to the article and approved the submitted version.
This study was funded by Sun Chlorella Corp., and Metabologenomics, Inc.
YNi, TN, YM, MI, YNa, and SM are employee of Metabologenomics, Inc.; TY and SF are founders of Metabologenomics, Inc., and MF is an employee of Sun Chlorella Corp.
The supercomputing resource was provided by the Human Genome Centre, the Institute of Medical Science, and the University of Tokyo. We would like to thank CPCC Co., Ltd., who conducted the clinical trial. Additionally, we would like to thank the staff at Metabologenomics, Inc., who participated in the discussion of this research.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.648073/full#supplementary-material
Supplementary Figure 1. Flow diagram of the phases of the randomized double-blind placebo-controlled crossover study.
Supplementary Table 1. Difference in primary/secondary outcome in baseline.
Supplementary Table 2. Relative area of metabolome in chlorella.
Supplementary Table 3. List of microbial taxa significantly correlated with SCFAs responder score.
Supplementary Table 4. List of microbial taxa significantly correlated with folate and defecation frequency responder score.
Supplementary Table 5. CONSORT checklist.
Supplementary Table 6. Relative area of metabolome in faeces.
1. Sibi G, Rabina S. Inhibition of pro-inflammatory mediators and cytokines by Chlorella vulgaris extracts. Pharmacognosy Res. (2016) 8:118–22. doi: 10.4103/0974-8490.172660
2. Bae M-J, Shin HS, Chai OH, Han J-G, Shon D-H. Inhibitory effect of unicellular green algae (Chlorella vulgaris) water extract on allergic immune response. J Sci Food Agric. (2013) 93:3133–6. doi: 10.1002/jsfa.6114
3. Wan X, Li T, Liu D, Chen Y, Liu Y, Liu B, et al. Effect of marine microalga Chlorella pyrenoidosa ethanol extract on lipid metabolism and gut microbiota composition in high-fat diet-fed rats. Mar Drugs. (2018) 16:498. doi: 10.3390/md16120498
4. Fallah AA, Sarmast E, Habibian Dehkordi S, Engardeh J, Mahmoodnia L, Khaledifar A, et al. Effect of Chlorella supplementation on cardiovascular risk factors: a meta-analysis of randomized controlled trials. Clin Nutr. (2018) 37:1892–901. doi: 10.1016/j.clnu.2017.09.019
5. Ripsin CM, Keenan JM, Jacobs DR, Elmer PJ, Welch RR, Van Horn L, et al. Oat products and lipid lowering. A meta-analysis. JAMA. (1992) 267:3317–25. doi: 10.1001/jama.1992.03480240079039
6. Martínez I, Lattimer JM, Hubach KL, Case JA, Yang J, Weber CG, et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. (2013) 7:269–80. doi: 10.1038/ismej.2012.104
7. Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, et al. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of prevotella. Cell Metab. (2015) 22:971–82. doi: 10.1016/j.cmet.2015.10.001
8. Deehan EC, Yang C, Perez-Muñoz ME, Nguyen NK, Cheng CC, Triador L, et al. Precision microbiome modulation with discrete dietary fiber structures directs short-chain fatty acid production. Cell Host Microbe. (2020) 27:389–404.e6. doi: 10.1016/j.chom.2020.01.006
9. De Vadder F, Kovatcheva-Datchary P, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metab. (2016) 24:151–7. doi: 10.1016/j.cmet.2016.06.013
10. Hua P, Xiong Y, Yu Z, Liu B, Zhao L. Effect of Chlorella pyrenoidosa protein hydrolysate-calcium chelate on calcium absorption metabolism and gut microbiota composition in low-calcium diet-fed rats. Mar Drugs. (2019) 17:348. doi: 10.3390/md17060348
11. Pan H, Guo R, Zhu J, Wang Q, Ju Y, Xie Y, et al. A gene catalogue of the Sprague-Dawley rat gut metagenome. Gigascience. (2018) 7:giy055. doi: 10.1093/gigascience/giy055
12. Uchiyama-Tanaka Y, Fujishima M, Okumura E. A Case Study on the Influence of Chlorella pyrenoidosa on Subjects of MTHFR C677T Polymorphism. Diagn Pathol Open Access. (2018) 3:144. doi: 10.4172/2476-2024.1000144
13. Nakamura Y, Suzuki S, Murakami S, Higashi K, Watarai N, Nishimoto Y, et al. Metabologenomics identified fecal biomarkers for bowel movement regulation by Bifidobacterium longum capsules: an RCT. medRxiv [Preprint]. (2020). doi: 10.1101/2020.03.23.20041400
14. Fujiwara Y, Sinpo K, Imae Y, Nonomura M, Hirakawa K. Effect of Chlorella vulgaris strain CK-5 on the frequency of bowel movement in humans. Jpn J Nutr Diet. (1998) 56:253–63. doi: 10.5264/eiyogakuzashi.56.253
15. Ramos-Romero S, Torrella JR, Pagès T, Viscor G, Torres JL. Edible microalgae and their bioactive compounds in the prevention and treatment of metabolic alterations. Nutrients. (2021) 13:563. doi: 10.3390/nu13020563
16. Ferrario C, Taverniti V, Milani C, Fiore W, Laureati M, De Noni I, et al. Modulation of fecal clostridiales bacteria and butyrate by probiotic intervention with Lactobacillus paracasei DG varies among healthy adults. J Nutr. (2014) 144:1787–96. doi: 10.3945/jn.114.197723
17. Nishimoto Y, Mizuguchi Y, Mori Y, Ito M, Miyazato S, Kishimoto Y, et al. Resistant maltodextrin intake reduces virulent metabolites in the gut environment: randomized control study in a Japanese cohort. medRxiv [Preprint]. (2020). doi: 10.1101/2020.05.25.20112508
18. Muthulakshmi S, Saravanan R. Protective effects of azelaic acid against high-fat diet-induced oxidative stress in liver, kidney and heart of C57BL/6J mice. Mol Cell Biochem. (2013) 377:23–33. doi: 10.1007/s11010-013-1566-1
19. Noguchi N, Konishi F, Kumamoto S, Maruyama I, Ando Y, Yanagita T. Beneficial effects of Chlorella on glucose and lipid metabolism in obese rodents on a high-fat diet. Obes Res Clin Pract. (2013) 7:e95–e105. doi: 10.1016/j.orcp.2013.01.002
20. Lee HS, Kim MK. Effect of Chlorella vulgaris on glucose metabolism in Wistar rats fed high fat diet. J Med Food. (2009) 12:1029–37. doi: 10.1089/jmf.2008.1269
21. Harwood J. Algae: critical sources of very long-chain polyunsaturated fatty acids. Biomolecules. (2019) 9:708. doi: 10.3390/biom9110708
22. Miura Y. The biological significance of ω-oxidation of fatty acids. Proc Jpn Acad Ser B Phys Biol Sci. (2013) 89:370–82. doi: 10.2183/pjab.89.370
23. Wada F, Usami M. Studies on fatty acid omega-oxidation. Antiketogenic effect and gluconeogenicity of dicarboxylic acids. Biochim Biophys Acta. (1977) 487:361–8. doi: 10.1016/0005-2760(77)90002-9
24. Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. (2013) 341:569–73. doi: 10.1126/science.1241165
25. Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SEK, et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. (2015) 64:1744–54. doi: 10.1136/gutjnl-2014-307913
26. Fosses A, Maté M, Franche N, Liu N, Denis Y, Borne R, et al. A seven-gene cluster in Ruminiclostridium cellulolyticum is essential for signalization, uptake and catabolism of the degradation products of cellulose hydrolysis. Biotechnol Biofuels. (2017) 10:250. doi: 10.1186/s13068-017-0933-7
27. Kobayashi A, Nobuta Y, Suganuma H, Aoe S, The effect of bowel movement on urinary/serumal nutrition concentration and urinary oxidative-stress markers. In: Annual Meeting of the Japan Society for Bioscience, Biotechnology, and Agrochemistry, 2014; Tokyo, Japan. Jpn Soc Biosci Biotechnol Agrochem. (2014). p. 3B05p14.
28. Murakami S, Goto Y, Ito K, Hayasaka S, Kurihara S, Soga T, et al. The consumption of bicarbonate-rich mineral water improves glycemic control. Evid Based Complement Altern Med. (2015) 2015:824395. doi: 10.1155/2015/824395
29. Kim SW, Suda W, Kim S, Oshima K, Fukuda S, Ohno H, et al. Robustness of gut microbiota of healthy adults in response to probiotic intervention revealed by high-throughput pyrosequencing. DNA Res. (2013) 20:241–53. doi: 10.1093/dnares/dst006
30. Kim Y-G, Sakamoto K, Seo S-U, Pickard JM, Gillilland MG 3rd, Pudlo NA, et al. Neonatal acquisition of Clostridia species protects against colonization by bacterial pathogens. Science. (2017) 356:315–9. doi: 10.1126/science.aag2029
31. Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. (2019) 37:852–7. doi: 10.1038/s41587-019-0209-9
Keywords: prebiotics (sources:MeSH), gut microbiome, gut metabolome, dietary fibre, Chlorella
Citation: Nishimoto Y, Nomaguchi T, Mori Y, Ito M, Nakamura Y, Fujishima M, Murakami S, Yamada T and Fukuda S (2021) The Nutritional Efficacy of Chlorella Supplementation Depends on the Individual Gut Environment: A Randomised Control Study. Front. Nutr. 8:648073. doi: 10.3389/fnut.2021.648073
Received: 23 March 2021; Accepted: 06 May 2021;
Published: 31 May 2021.
Edited by:
Clara G. De Los Reyes-Gavilan, Consejo Superior de Investigaciones Científicas (CSIC), SpainReviewed by:
Wendy J. Dahl, University of Florida, United StatesCopyright © 2021 Nishimoto, Nomaguchi, Mori, Ito, Nakamura, Fujishima, Murakami, Yamada and Fukuda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takuji Yamada, eWFtYWRhQG1ldGFnZW4uY28uanA=; Shinji Fukuda, c2Z1a3VkYUBtZXRhZ2VuLmNvLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.