- 1Department of Community Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran
- 2Department of Geriatric Medicine, Ziaeian Hospital, Tehran University of Medical Sciences, Tehran, Iran
- 3Chronic Diseases Research Center (CDRC), Endocrinology and Metabolism Population Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran
- 4Obesity and Eating Habits Research Center, Endocrinology and Metabolism Molecular-Cellular Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran
- 5Department of Community Nutrition, Isfahan University of Medical Sciences, Isfahan, Iran
Background: Despite the associations between individual nutrients and sarcopenia, we are aware of no information about the link between patterns of nutrient intake and odds of sarcopenia and its components. The present study aimed to examine the association between nutrient-based dietary patterns and sarcopenia and its components among the Iranian adult population.
Methods: In this population-based, cross-sectional study, we enrolled 300 elderly adults (150 men and 150 women) aged ≥55 years by using a cluster random sampling method. Dietary intakes of the study population were assessed using a validated food frequency questionnaire. Principal component analysis was conducted to derive nutrient patterns based on a daily intake of 33 nutrients. Muscle mass, muscle strength, and gait speed were measured according to standard methods. Sarcopenia and its components were defined based on the European Working Group on Sarcopenia.
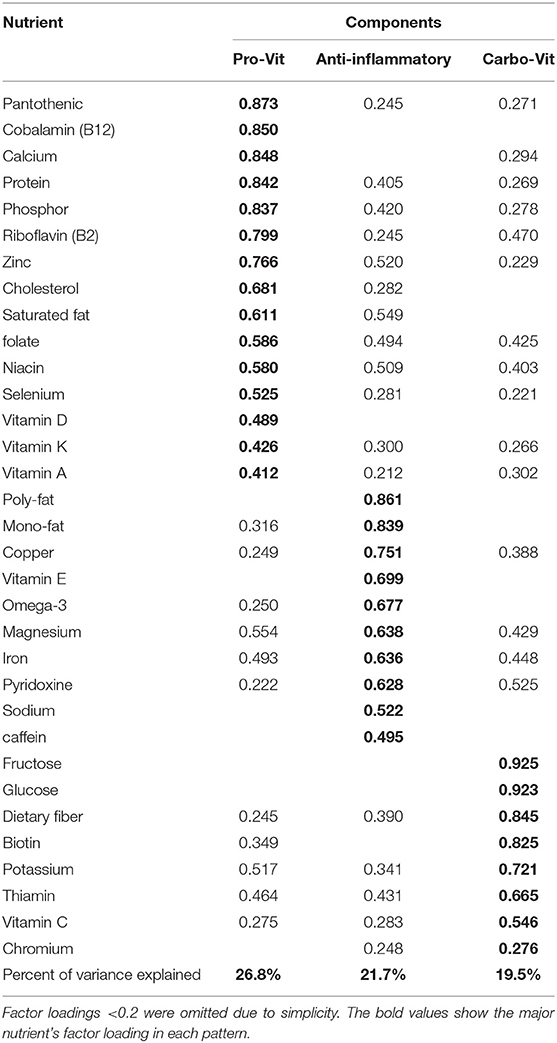
Results: Three major nutrient-based dietary patterns were identified: (1) the “pro-vit pattern” that was high in pantothenic (B5), cobalamin (B12), calcium, protein, phosphor, riboflavin (B2), zinc, cholesterol, saturated fat, folate, niacin (B3), selenium, vitamin D, vitamin K, and vitamin A; (2) the “anti-inflammatory” pattern, which was rich in polyunsaturated fat, monounsaturated fat, copper, vitamin E, omega-3, magnesium, iron, pyridoxine (B6), sodium, and caffeine; and (3) the “carbo-vit” patternm which is characterized by high intake of fructose, glucose, dietary fiber, biotin, potassium, thiamin (B1), vitamin C, and chromium. After adjusting for confounders, subjects in the top tertile of the anti-inflammatory pattern had lower odds of sarcopenia (OR 0.25; 95% CI 0.10–0.63) and low muscle strength (OR: 0.46; 95% CI: 0.22–0.96) than those in the bottom tertile. Greater adherence to the carbo-vit pattern was inversely associated with the odds of low gait speed (OR: 0.46; 95% CI: 0.235–0.93).
Conclusion: Major nutrient-based dietary patterns were significantly associated with sarcopenia and its components. Further studies are required to confirm our findings.
Introduction
Sarcopenia is a progressive muscle disease that is described as a combination of low muscle quality and physical performance. It is associated with chronic diseases, disability, falls, poor quality of life, and mortality (1, 2). Also, sarcopenia increases the costs of health care systems significantly (1). The prevalence of this disease varies according to the definition. It is prevalent in 16.5–32.5% of the Iranian population (3).
A low-quality diet is one of the major contributing factors to sarcopenia and muscle weakness (4). High intake of proteins, vitamin D, vitamin E, potassium, magnesium, phosphorus, iron, vitamin K, and omega-3 has been shown to preserve muscle mass (4–6). It must be kept in mind that people are not consuming single nutrients (7). Moreover, effects from single nutrients may be too small to be detectable, and assessing the pattern of nutrient intake that considers whole nutrient intake may show a significant association with the risk of chronic diseases (8, 9). Assessment of nutrient intake patterns may provide a better and more general insight into the diet–disease relationship (8). Despite earlier investigations on the relationship between some dietary patterns, including Mediterranean or healthy dietary patterns, and sarcopenia (7, 10), we are aware of no study linking the pattern of nutrient intake and sarcopenia. However, nutrient patterns have been assessed in relation to several other chronic conditions, including psychological disorders (11), obesity (12), metabolic syndrome (13), and some cancers (14).
Finding a nutritional approach to decline muscle wasting and maintain its performance in elderly people is of high priority. Given the growing number of elderly people in developing countries along with the nutritional transition from healthy foods to unhealthy diets and limited evidence about diet–sarcopenia associations, this cross-sectional study was done to identify major nutrient-based dietary patterns in relation to sarcopenia and its components in a group of Iranian people.
Participants and Methods
Participants
We conducted this population-based cross-sectional study from May to October 2011 in Tehran, Iran. A detailed report on the sampling method and data-collection procedure has been published previously (15, 16). We used the formula suggested for cross-sectional studies. According to the study of Thomas the standard deviation of appendicular skeletal muscle mass (ASM) in women was 1.9 and in men was 3.4 kg (17). Therefore, the sample size was calculated to be 29 in women and 30 in men in each age group. Finally, 30 men and 30 women in each age category (55–59 y, 60–64 y, 65–69 y, 70–74 y, and over 75 y) were examined. Finally, a total sample of 300 people (150 women, 150 men) enrolled in the study (study power: 80%, design effect: 1.2, α = 5%). We used the cluster random sampling method to recruit individuals in district six of Tehran. The head of each cluster was selected based on a 10-digit postal code. We recruited participants aged ≥ 55 years with the ability to move without crutches, a walker, or assistive devices and those without any active cancers (based on self-reported data). Moreover, we did not include people with artificial limbs or limb prostheses and those with a history of debilitating disease (e.g., congestive heart failure) in our study. The study protocol was approved by the Tehran University of Medical Sciences Ethics' Committee. First, the study goals were clarified to all participants, and then they were requested to complete a written informed consent before data collection.
Dietary Intake Assessment
A block-format 117-item food frequency questionnaire (FFQ) was used to assess the usual dietary intakes of study participants; the validity and reliability of this questionnaire is reported in previous studies (15, 18). This FFQ included a list of foods with a specific portion size. Participants were able to report their consumption frequency based on a daily, weekly, or monthly basis for each food item. The questionnaire was filled in by a trained nutritionist through a face-to-face interview. Then, we converted the frequency of each food item to grams per day by considering the household measures of portion sizes. Finally, nutritionist IV software with a modified food composition database of the U.S. Department of Agriculture was used to calculate daily energy and nutrient intake of each participant. It should be noted that we did not use the food composition data set of Iran because it is from 40 years ago with an incomplete list of foods and nutrients. Therefore, the USDA food composition table was used; however, for Iranian local foods, we modified the database with the Iranian food composition data.
Assessment of Sarcopenia
Sarcopenia was defined by considering the combination of both low muscle mass and low muscle function (either strength or performance) according to the European Working Group on Sarcopenia (EWGSOP) definition (19). Muscle mass was measured as the ratio of an individual's total lean mass of legs and arms (also named ASM) to their squared height (ASM/height2) (20). We used a DXA scanner (Discovery W S/N 84430) to calculate ASM. Then, low muscle mass was defined as the amount of muscle mass <5.45 (kg/m2) for women and 7.26 (kg/m2) for men according to EWGSOP (19).
A handgrip test was used to assess muscle strength. The handgrip test was calculated by a pneumatic tool that is a squeeze bulb dynamometer (c7489-02 Rolyan) calibrated in pounds per square inch (psi). We measured the handgrip strength (maximum voluntary contractions) three times for each right and left hand with a 30-s rest in between measurements. The average measurements of both the participant's hands were considered as their muscle strength. Then, handgrip strength <30 kg for men and <20 kg for women was considered to be low muscle strength (21). A four-meter walk gait speed test was applied to calculate muscle performance (19). If participants had gait speeds <0.8 m/s, they were categorized as low muscle performance (19).
Assessment of Other Variables
A pretested questionnaire was used to collect general characteristics of participants, including age, sex, socioeconomic status, medical history, medication use, smoking habits, and alcohol consumption. A trained interviewer examined the physical activity level by using the short form of the International Physical Activity Questionnaire (IPAQ), which has been validated previously (18). Then, measures of physical activity for each participant were expressed as metabolic equivalent-hour per week (MET-h/week) according to IPAQ's guideline (22). A digital scale was used to measure weight while participants were minimally clothed. Height was measured by a wall tape meter in a standing position without shoes. Waist circumference was measured in the middle of the lower rib margin and iliac crest. Weight (kg) divided by height squared (m2) was used to calculate body mass index (BMI).
Statistical Analysis
Principal component analysis was conducted to determine major nutrient-based dietary patterns based on 33 components. The following nutrients were included in the factor analysis: protein, total dietary fiber, glucose, fructose, total saturated fatty acids (SFAs), total monounsaturated fatty acids (MUFAs), total polyunsaturated fatty acids (PUFAs), cholesterol, vitamin B12, vitamin A, vitamin D, vitamin E, vitamin K, thiamin, riboflavin, niacin, pantothenic acid, pyridoxine, biotin, folate, vitamin C, caffeine, sodium, potassium, phosphorus, magnesium, iron, selenium, calcium, chromium, copper, zinc, and omega-3. The Kaiser Meyer Olkin (KMO) test was used to determine whether the different nutrients were appropriate for principal components or not (KMO test = 0.88). Based on the scree plot, we retained factors with an eigenvalue higher than two as a major nutrient pattern. Varimax rotation was used to obtain independent, nutrient-based dietary patterns. Then, each participant received a factor score for each recognized pattern. To label each major nutrient-based dietary pattern, we considered the factor loading of 0.5 or above; however, we presented all nutrients with a factor loading of 0.2 or above in each nutrient pattern. Finally, we categorized participants into tertiles based on nutrient pattern scores instead of quartiles or quintiles due to avoiding a low number of people in each category. General characteristics of study participants across tertiles of nutrient-based dietary pattern scores were compared using one-way ANOVA and chi-square, where appropriate. The general linear model was used to compare age-, sex-, and energy-adjusted dietary intakes of participants across tertile categories of nutrient-based dietary pattern scores. Prevalence of sarcopenia (yes/no), low muscle mass (yes/no), low muscle strength (yes/no), and low gait speed (yes/no) across tertile categories of nutrient-based dietary patterns were assessed by chi-square. The general linear model was applied to compare means of muscle mass [ASM/h2] (kg), muscle strength (psi), and gait speed (m/s) across tertile categories of nutrient-based dietary pattern scores in crude and age-, sex-, and energy-adjusted models. Logistic regression assumptions, including assumptions of normality, homogeneity of variance, homogeneity of variance-covariance matrices, the absence of multicollinearity, and the absence of auto-correlation were met. Then, we conducted multivariable logistic regression to find the association between nutrient-based dietary patterns and sarcopenia (yes/no), low muscle mass (yes/no), low muscle strength (yes/no), and low gait speed (yes/no). In these analyses, we controlled for several confounders. Age (continuous), sex (male/female), and energy intake (kcal/d) were adjusted for in the first model. Then, further adjustments were done for physical activity (MET-h/wk), smoking (yes/no), alcohol consumption (yes/no), medication use (statin, corticosteroid, estrogen, testosterone), and positive history of chronic diseases (yes/no). In all these analyses, the lowest tertile was considered as the reference category, and the odds ratio for sarcopenia in other categories was calculated. We considered the tertile categories as an ordinal variable to calculate the linear trend of odds ratios across tertiles of the nutrient-based dietary pattern scores. All analyses were done using SPSS (version 26). P-values were considered significant at <0.05.
Results
We identified three major nutrient-based dietary patterns using principal component analysis (Table 1). The KMO test was 0.88, showing the adequacy of the sample size. The pro-vit nutrient pattern was high in pantothenic (B5), cobalamin (B12), calcium, protein, phosphorous, riboflavin (B2), zinc, cholesterol, saturated fat, folate, niacin (B3), selenium, vitamin D, vitamin K, and vitamin A. The anti-inflammatory nutrient pattern was characterized by high intakes of polyunsaturated fat, monounsaturated fat, copper, vitamin E, omega-3, magnesium, iron, pyridoxine (B6), sodium, and caffeine. The carbo-vit nutrient pattern was characterized by high consumption of fructose, glucose, dietary fiber, biotin, potassium, thiamin (B1), vitamin C, and chromium. Overall, these nutrient-based dietary patterns explained 68% of the variance.
General characteristics of study participants across tertiles of nutrient-based dietary pattern scores are indicated in Table 2. Subjects with the greatest adherence to the pro-vit nutrient pattern were more likely to be physically active than those with the lowest adherence. Individuals in the highest tertile of the anti-inflammatory nutrient pattern were more likely to be physically active than smokers and those who use alcohol. Greater adherence to the carbo-vit nutrient pattern was associated with higher levels of physical activity. There were no other significant differences across tertiles of nutrient-based dietary pattern scores.
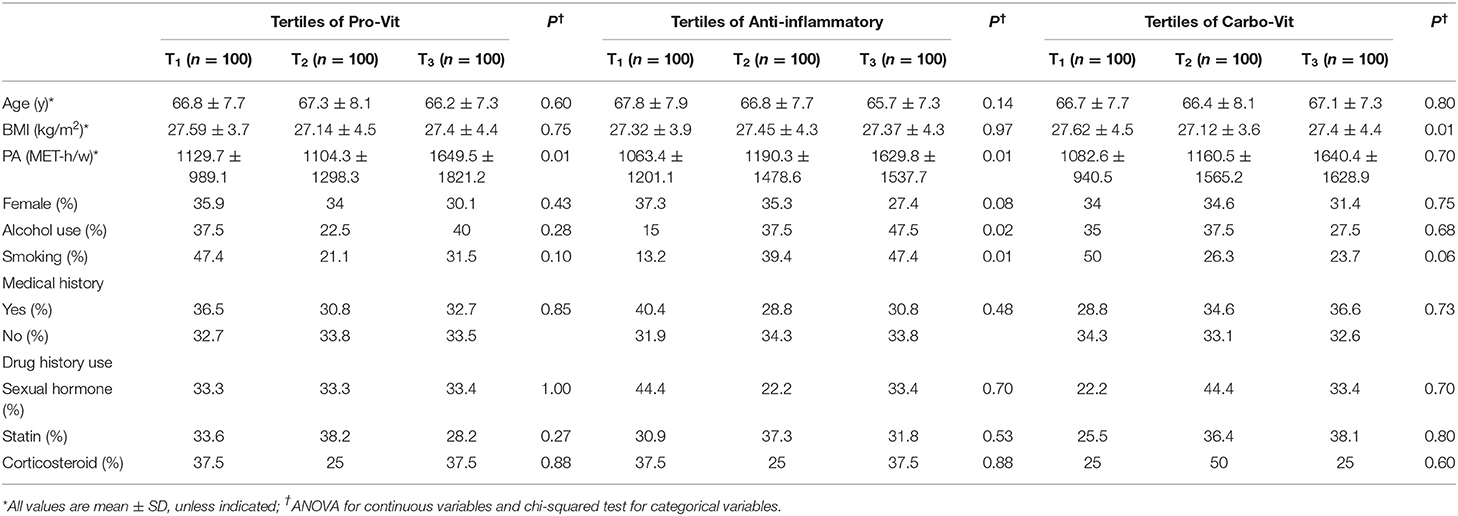
Table 2. General characteristics of the study participants across categories of nutrient-based dietary pattern scores.
Table 3 shows age-, gender-, and energy-adjusted dietary intakes of study participants across categories of nutrient-based dietary pattern scores. Subjects in the top tertile of the pro-vit pattern had a higher intake of vegetables, dairy products, whole grains, red and white meats, energy, protein, calcium, and folate and a lower intake of carbohydrate and vitamin E than those in the bottom tertile. Greater adherence to the anti-inflammatory nutrient pattern was associated with a higher intake of legumes and nuts, vegetable oils, red meats, energy, fat, vitamin E, and folate and a lower intake of carbohydrates, dairy products, and calcium. Individuals in the highest tertile of the carbo-vit pattern had higher intakes of fruits, vegetables, energy, carbohydrates, dietary fiber, vitamin B6, and folate and a lower intake of sweets and desserts, red meats, fat, and vitamin E than those in the lowest tertile.
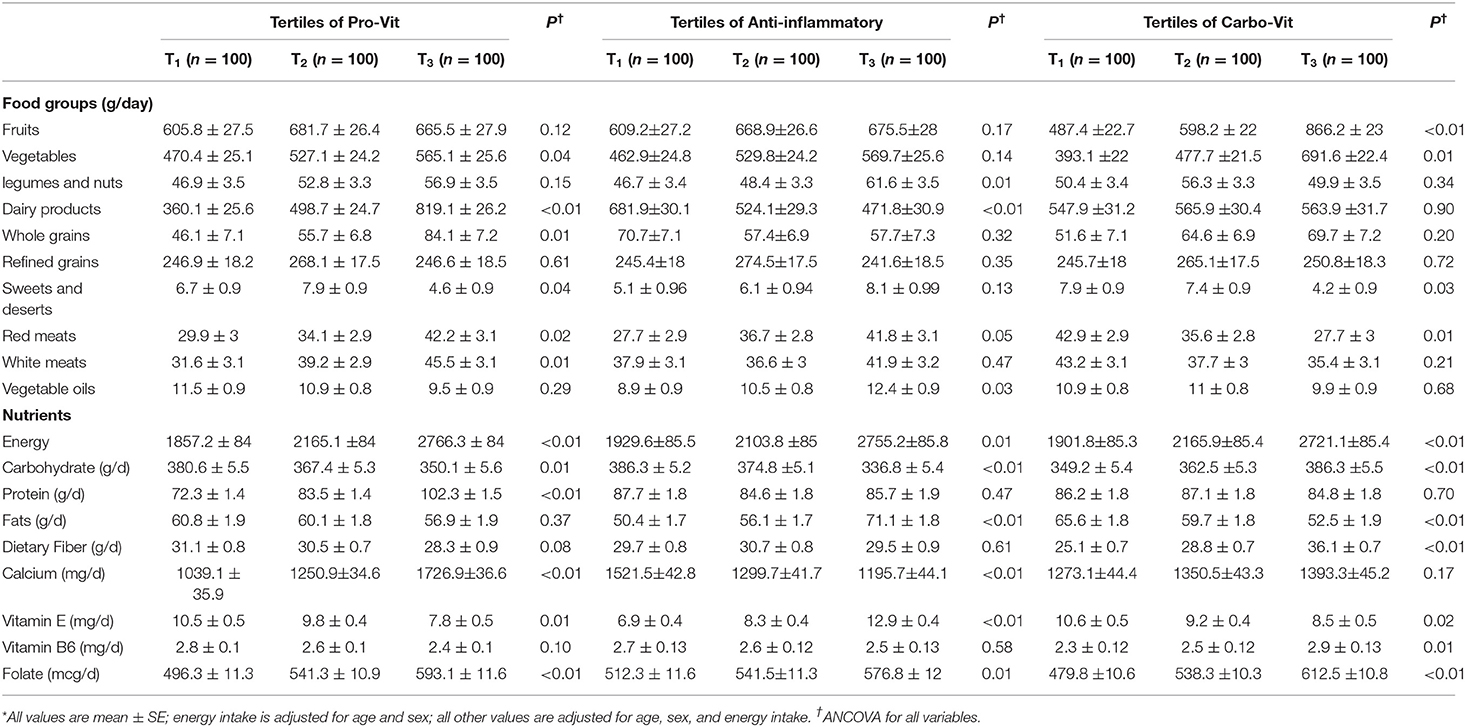
Table 3. Dietary intakes of study participants across categories of nutrient-based dietary pattern scores*.
The prevalence of sarcopenia and its components across tertile categories of nutrient-based dietary pattern scores are depicted in Table 4. There were no significant differences in the prevalence of sarcopenia and its components across tertiles of the pro-vit and carbo-vit patterns; however, subjects with the greatest adherence to the anti-inflammatory nutrient pattern were less likely to have sarcopenia (P = 0.02) and low handgrip strength (P = 0.05) than those with the lowest adherence.
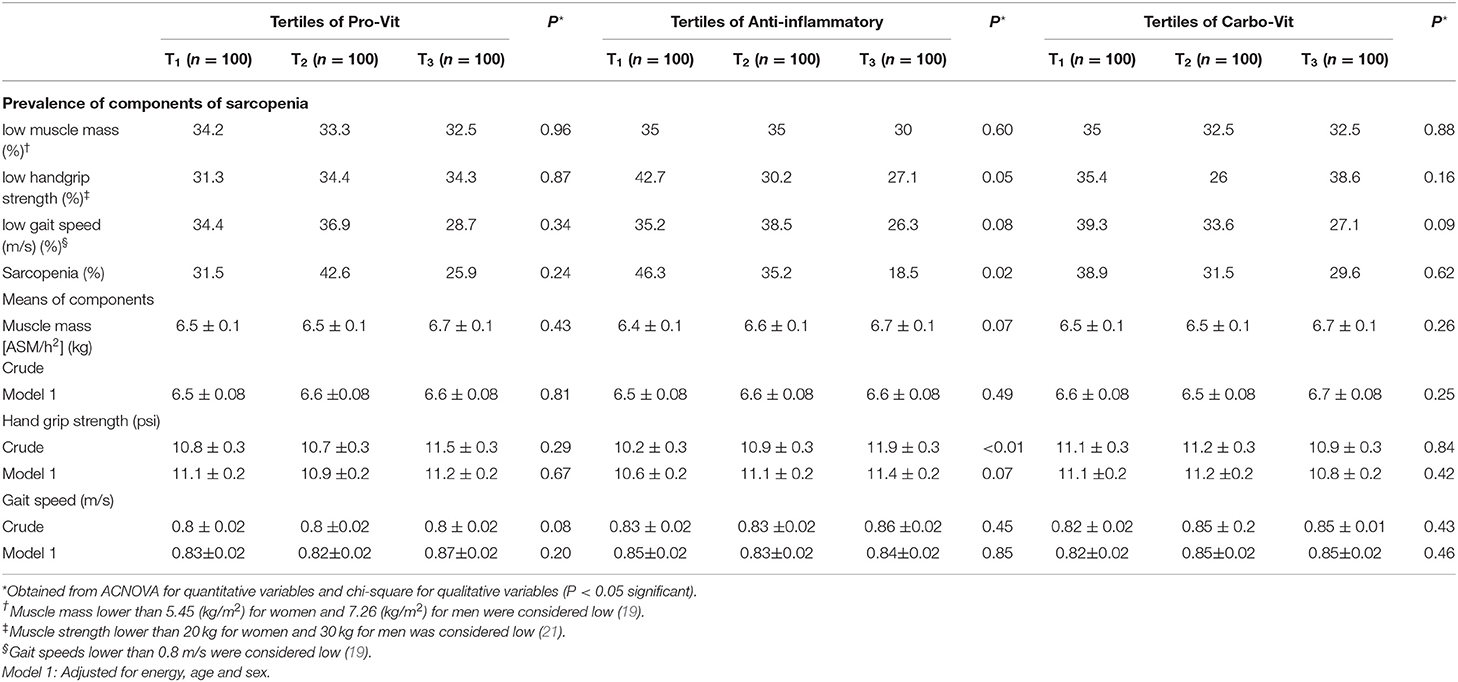
Table 4. Prevalence of sarcopenia and its components across tertile categories of nutrient-based dietary pattern patterns.
Multivariable-adjusted logistic regression analysis indicating the link between nutrient-based dietary patterns and odds of sarcopenia and its components is provided in Table 5. We did not find any association between adherence to the pro-vit nutrient pattern and odds of sarcopenia either before (OR: 0.79; 95% CI: 0.36–1.71) or after controlling for potential confounders (OR: 0.74; 95% CI: 0.31–1.76). Also, no significant associations were seen between this nutrient pattern and components of sarcopenia. Subjects in the top tertile of the anti-inflammatory nutrient pattern were 67% less likely to have sarcopenia (OR: 0.33; 95% CI: 0.15–0.74) than those in the bottom tertile. The association strengthened in the fully adjusted model (OR 0.25; 95% CI 0.10–0.63). Also, participants in the top tertile of this pattern were less likely to have low handgrip strength in the crude (OR: 0.50; 95% CI: 0.27–0.92) and fully adjusted models (OR: 0.46; 95% CI: 0.22–0.96). Adherence to the carbo-vit nutrient pattern was significantly associated with 47% decreased odds of low gait speed in the crude model (OR: 0.53; 95% CI: 0.30–0.94). This protective association remained significant even after controlling for all potential confounders (OR: 0.46; 95% CI: 0.235–0.93).
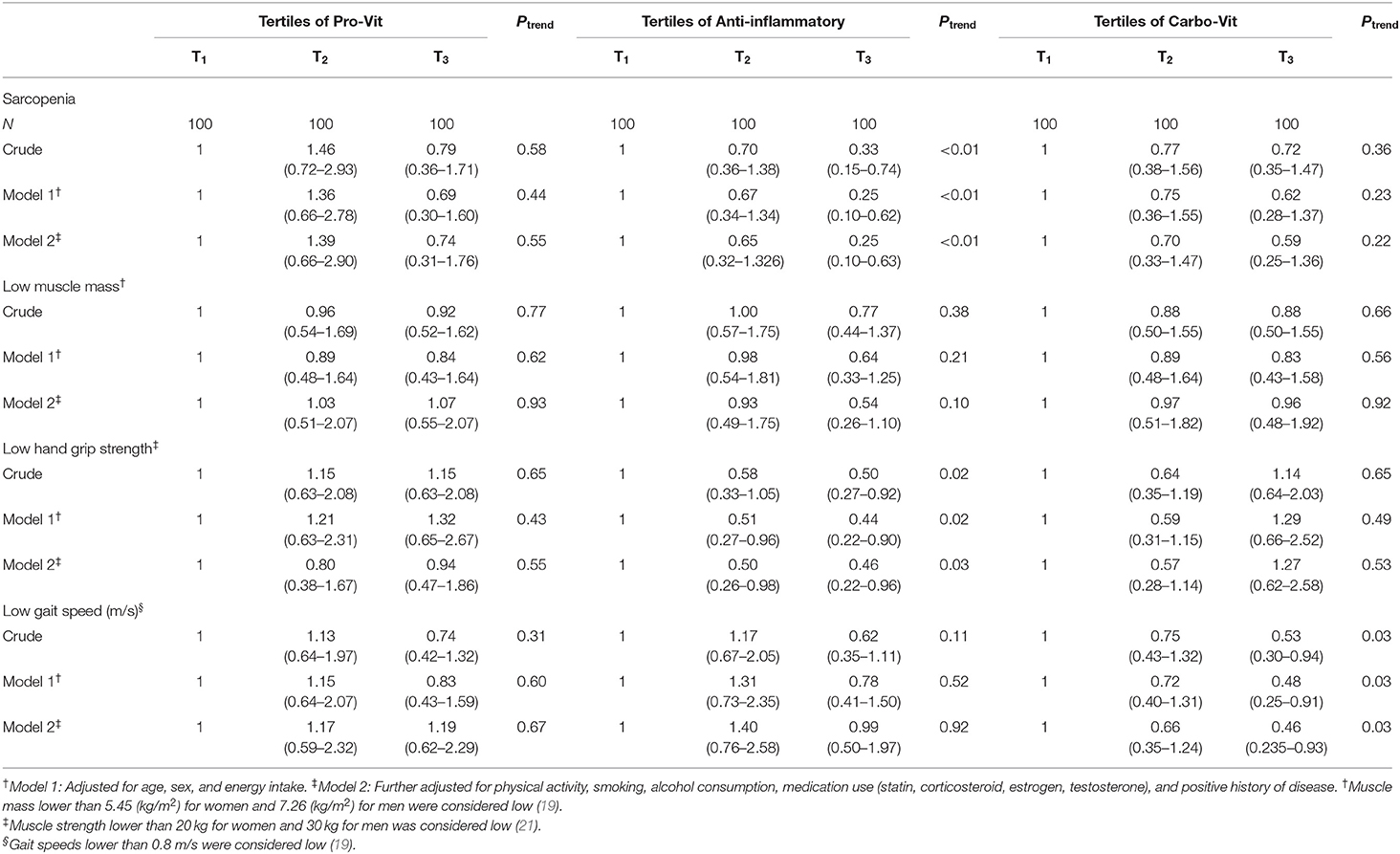
Table 5. Multivariable-adjusted odds ratios (95% CIs) for sarcopenia and its components across tertile categories of nutrient-based dietary patterns.
Discussion
In this cross-sectional study, we found a significant inverse association between adherence to anti-inflammatory nutrient-based dietary patterns and odds of sarcopenia and low muscle strength. Also, the carbo-vit pattern was significantly associated with decreased odds of low gait speed. To the best of our knowledge, this is the first study investigating the association between nutrient patterns and sarcopenia.
Nutrient pattern analysis is a new approach in nutritional epidemiology that is designed to consider all nutrient interactions in a single exposure (23). Although several studies assess the association between major dietary patterns and risk of sarcopenia (24), there is no information available on the link between patterns of nutrient intake and sarcopenia. Sarcopenia and its components are closely related to nutritional status (25). It is important to note that sarcopenia is associated with adverse health-related outcomes, including fall, fracture, and mortality (1, 2). Moreover, low handgrip strength is related to insulin resistance, hypertension, and mortality (26–28). We identified an inverse association between adherence to an anti-inflammatory nutrient pattern that was high in healthy oils, antioxidants, and anti-inflammatory nutrients and odds of sarcopenia and low handgrip strength. A cross-sectional study in Belgium revealed that sarcopenic subjects had a lower intake of lipid, iron, magnesium, and potassium (4). In the Maastricht Sarcopenia Study on adults aged 65 years or older, subjects with sarcopenia had 10–18% lower intake of n-3 fatty acids, vitamin B6, vitamin E, and magnesium compared with non-sarcopenic subjects (29). A cross-sectional study from UK Biobank cohort data, which was conducted on 68,002 adults age ≥60, indicated a significant positive link between dietary intake of oily fish and magnesium in either gender as well as dietary intake of iron and vitamin E in women and handgrip strength (30). In contrast to our findings, in Tasmanian older adults, dietary intakes of protein, iron, magnesium, and zinc were associated with muscle mass but not muscle strength (31). Overall, it seems that intake of anti-inflammatory nutrients may have a muscle protective role.
We found no association between the pro-Vit nutrient pattern and sarcopenia and its components. Although protein intake has been loaded in this pattern, we failed to find any significant association with sarcopenia. However, based on this finding, it is not true to claim that protein intake is not associated with sarcopenia because protein is only one component of this nutrient-based dietary pattern, and the combined effects of other nutrients loaded in this dietary pattern should be considered. For instance, lack of a significant association might be attributed to the loading of SFAs and cholesterol in this pattern. A longitudinal study in UK elderly people found that the “traditional British dietary pattern” characterized by a high intake of butter, red meat, gravy, and potato was associated with an increased risk of sarcopenia even when overall protein intake was good (32). However, a cross-sectional study in Belgium found that people with sarcopenia had significantly lower intakes of protein (4). As a conclusion, the meaning of a high-protein, nutrient-based dietary pattern can be different from the studies in which protein intake, as a sole nutrient, has been examined in relation to sarcopenia or its components.
In this study, adherence to the carbo-vit nutrient pattern that was characterized by high intakes of healthy carbohydrate, biotin, potassium, thiamin (B1), vitamin C, and chromium was associated with lower low gait speed. Low walking speed was related to cognitive impairment, dementia, cardiovascular diseases, mortality, disability, and hospitalization in earlier studies (33–36). In a cross-sectional analysis of community-dwelling Australian older adults aged ≥50 years with type 2 diabetes, greater adherence to the Mediterranean diet was significantly associated with better gait speed (37). In a cross-sectional study on 380 Spanish older adults aged 55–80, the investigators reported a positive association between the Mediterranean dietary pattern and walking speed and a negative link between the Western dietary pattern and walking speed (38). Therefore, dietary intake of some nutrients may have a beneficial role in muscle performance and gait speed.
Several mechanisms have been proposed for the role of nutrients in sarcopenia and its components. Oxidative stress has an important effect on the pathogenesis of sarcopenia (39). It causes atrophy, loss of myocytes, and muscle fibers by accumulation of mitochondrial and nuclear DNA damage (40, 41). Moreover, oxidative stress through increasing expression of inflammatory cytokines, such as interleukin- (IL-) 1, tumor necrosis factor (TNF), and IL-6 might damage muscle tissue (42, 43). Based on this background, antioxidant and anti-inflammatory nutrients, including vitamin E and C, MUFA, PUFA, omega-3, fiber, magnesium, caffeine, chromium, and copper, can play an important role against sarcopenia. Thiamine, pyridoxine, biotin, and iron are part of the coenzyme involved in energy production (44). Carbohydrates are the first source of energy for the muscles (44). Dietary intake of healthy carbohydrates high in fiber may have a beneficial effect on muscles. However, due to low data on nutrient intake and sarcopenia, additional investigation is needed in this area.
This study has several strengths. To our knowledge, this is the first study examining the association between nutrient-based dietary patterns and odds of sarcopenia and its components. Various confounding factors were adjusted for in the present analysis. Additionally, for the assessment of dietary intakes, a validated FFQ was used. Finally, valid definitions and valid tools were used for the diagnosis of sarcopenia. Along with strengths, some limitations should also be kept in mind. The cross-sectional design is the main limitation of our study, which prohibits us from conferring a causal relationship. The misclassification of study subjects and measurement error due to the use of FFQ is another limitation. It should also be kept in mind that muscle loss is different in men and women. One might be interested in a gender-stratified analysis to accurately examine the association of nutrient-based dietary patterns and low muscle mass. However, due to the small sample size and given that separating genders in the analysis resulted in a small number of people in each category, we did not perform such analysis. Last, because of financial limitations and inadequate access to the DEXA device in Tehran, the study was conducted on a small sample (maximum 300 cases). Thus, the generalization of these results to the whole Iranian population should be done with caution.
In conclusion, we observed that adherence to the anti-inflammatory nutrient pattern that is characterized by high consumption of polyunsaturated fat, monounsaturated fat, copper, vitamin E, omega-3, magnesium, iron, pyridoxine (B6), sodium, and caffeine might decrease the odds of sarcopenia and low muscle strength. Furthermore, the carbo-vit nutrient pattern with high amounts of fructose, glucose, dietary fiber, biotin, potassium, thiamin (B1), vitamin C, and chromium was associated with lower odds of low gait speed. Prospective studies are required to confirm these findings.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, on reasonable request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Tehran University of Medical Sciences Ethics' Committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AB, RHa, RHe, AM, and AE contributed to the conception, design, data collection, statistical analyses, data interpretation, manuscript drafting, approval of the final version of the manuscript, and agreed for all aspects of the work. All authors contributed to the article and approved the submitted version.
Funding
The financial support for this study comes from the Tehran Endocrine and Metabolism Research Center and the Tehran University of Medical Science.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We wish to thank all individuals who kindly participated in our study.
References
1. Bloom I, Shand C, Cooper C, Robinson S, Baird J. Diet quality and sarcopenia in older adults: a systematic review. Nutrients. (2018) 10:308. doi: 10.3390/nu10030308
2. Chen L-K, Liu L-K, Woo J, Assantachai P, Auyeung T-W, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. (2014) 15:95–101. doi: 10.1016/j.jamda.2013.11.025
3. Shafiee G, Heshmat R, Ostovar A, Nabipour I, Larijani B. Sarcopenia disease in Iran: an overview. J Diabetes Metab Disord. (2019):1–10. doi: 10.1007/s40200-019-00452-9
4. Beaudart C, Locquet M, Touvier M, Reginster J-Y, Bruyère O. Association between dietary nutrient intake and sarcopenia in the SarcoPhAge study. Aging Clin Exp Res. (2019) 31:815–24. doi: 10.1007/s40520-019-01186-7
5. Kim MK, Baek KH, Song K-H, Il Kang M, Park CY, Lee WY, et al. Vitamin D deficiency is associated with sarcopenia in older Koreans, regardless of obesity: the Fourth Korea National Health and Nutrition Examination Surveys (KNHANES IV) 2009. J Clin Endocrinol Metab. (2011) 96:3250–6. doi: 10.1210/jc.2011-1602
6. Smith GI, Julliand S, Reeds DN, Sinacore DR, Klein S, Mittendorfer B. Fish oil–derived n– 3 PUFA therapy increases muscle mass and function in healthy older adults. Am J Clin Nutr. (2015) 102:115–22. doi: 10.3945/ajcn.114.105833
7. Chan R, Leung J, Woo J. A prospective cohort study to examine the association between dietary patterns and sarcopenia in Chinese community-dwelling older people in Hong Kong. J Am Med Dir Assoc. (2016) 17:336–42. doi: 10.1016/j.jamda.2015.12.004
8. Newby P, Tucker KL. Empirically derived eating patterns using factor or cluster analysis: a review. Nutr Rev. (2004) 62:177–203. doi: 10.1111/j.1753-4887.2004.tb00040.x
9. De Stefani E, Boffetta P, Fagundes RB, Deneo-Pellegrini H, Ronco AL, Acosta G, et al. Nutrient patterns and risk of squamous cell carcinoma of the esophagus: a factor analysis in uruguay. Anticancer Res. (2008) 28:2499–506.
10. Granic A, Sayer AA, Robinson SM. Dietary patterns, skeletal muscle health, and sarcopenia in older adults. Nutrients. (2019) 11:745. doi: 10.3390/nu11040745
11. Salehi-Abargouei A, Esmaillzadeh A, Azadbakht L, Keshteli AH, Afshar H, Feizi A, et al. Do patterns of nutrient intake predict self-reported anxiety, depression and psychological distress in adults? SEPAHAN study. Clin Nutr. (2019) 38:940–7. doi: 10.1016/j.clnu.2018.02.002
12. Salehi-Abargouei A, Esmaillzadeh A, Azadbakht L, Keshteli AH, Feizi A, Feinle-Bisset C, et al. Nutrient patterns and their relation to general and abdominal obesity in Iranian adults: findings from the SEPAHAN study. Eur J Nutr. (2016) 55:505–18. doi: 10.1007/s00394-015-0867-4
13. Iwasaki Y, Arisawa K, Katsuura-Kamano S, Uemura H, Tsukamoto M, Kadomatsu Y, et al. Associations of nutrient patterns with the prevalence of metabolic syndrome: results from the baseline data of the Japan multi-institutional collaborative cohort study. Nutrients. (2019) 11:990. doi: 10.3390/nu11050990
14. Edefonti V, La Vecchia C, Di Maso M, Crispo A, Polesel J, Libra M, et al. Association between nutrient-based dietary patterns and bladder cancer in Italy. Nutrients. (2020) 12:1584. doi: 10.3390/nu12061584
15. Hashemi R, Heshmat R, Motlagh AD, Payab M, Esmaillzadeh A, Baigy F, et al. Sarcopenia and its determinants among Iranian elderly (SARIR): study protocol. J Diabetes Metab Disord. (2012) 11:23. doi: 10.1186/2251-6581-11-23
16. Bagheri A, Soltani S, Hashemi R, Heshmat R, Motlagh AD, Esmaillzadeh A. Inflammatory potential of the diet and risk of sarcopenia and its components. Nutr J. (2020) 19:1–8. doi: 10.1186/s12937-020-00649-2
17. Thomas DR. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr. (2007) 26:389–99. doi: 10.1016/j.clnu.2007.03.008
18. Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. (2010) 13:654–62. doi: 10.1017/S1368980009991698
19. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosisReport of the European Working Group on Sarcopenia in Older People. Age Ageing. (2010) 39:412–23. doi: 10.1093/ageing/afq034
20. Heymsfield SB, Smith R, Aulet M, Bensen B, Lichtman S, Wang J, et al. Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr. (1990) 52:214–8. doi: 10.1093/ajcn/52.2.214
21. Merkies I, Schmitz P, Samijn J, Meché FVD, Toyka K, Van Doorn P. Assessing grip strength in healthy individuals and patients with immune-mediated polyneuropathies. Muscle Nerve. (2000) 23:1393–401. doi: 10.1002/1097-4598(200009)23:9<1393::aid-mus10>3.0.co;2-o
22. Committee IR. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ)-Short and Long Forms. (2005). Available online at: http://www.ipaq.ki.se/
23. Willett W. Nutritional Epidemiology. Oxford: Oxford University Press (2012). doi: 10.1093/acprof:oso/9780199754038.001.0001
24. Mohseni R, Aliakbar S, Abdollahi A, Yekaninejad MS, Maghbooli Z, Mirzaei K. Relationship between major dietary patterns and sarcopenia among menopausal women. Aging Clin Exp Res. (2017) 29:1241–8. doi: 10.1007/s40520-016-0721-4
25. Flood A, Chung A, Parker H, Kearns V, O'Sullivan TA. The use of hand grip strength as a predictor of nutrition status in hospital patients. Clin Nutr. (2014) 33:106–14. doi: 10.1016/j.clnu.2013.03.003
26. Jochem C, Leitzmann M, Volaklis K, Aune D, Strasser B. Association between muscular strength and mortality in clinical populations: a systematic review and meta-analysis. J Am Med Dir Assoc. (2019) 20:1213–23. doi: 10.1016/j.jamda.2019.05.015
27. Li S, Zhang R, Pan G, Zheng L, Li C. Handgrip strength is associated with insulin resistance and glucose metabolism in adolescents: Evidence from National Health and Nutrition Examination Survey 2011 to 2014. Pediatr Diabetes. (2018) 19:375–80. doi: 10.1111/pedi.12596
28. Ji C, Zheng L, Zhang R, Wu Q, Zhao Y. Handgrip strength is positively related to blood pressure and hypertension risk: results from the National Health and nutrition examination survey. Lipids Health Dis. (2018) 17:1–7. doi: 10.1186/s12944-018-0734-4
29. ter Borg S, de Groot LC, Mijnarends DM, de Vries JH, Verlaan S, Meijboom S, et al. Differences in nutrient intake and biochemical nutrient status between sarcopenic and nonsarcopenic older adults—results from the Maastricht Sarcopenia Study. J Am Med Dir Assoc. (2016) 17:393–401. doi: 10.1016/j.jamda.2015.12.015
30. Gedmantaite A, Celis-Morales CA, Ho F, Pell JP, Ratkevicius A, Gray SR. Associations between diet and handgrip strength: a cross-sectional study from UK Biobank. Mech Ageing Dev. (2020) 189:111269. doi: 10.1016/j.mad.2020.111269
31. Scott D, Blizzard L, Fell J, Giles G, Jones G. Associations between dietary nutrient intake and muscle mass and strength in community-dwelling older adults: the Tasmanian Older Adult Cohort study. J Am Geriatr Soc. (2010) 58:2129–34. doi: 10.1111/j.1532-5415.2010.03147.x
32. Granic A, Mendonça N, Sayer AA, Hill TR, Davies K, Siervo M, et al. Effects of dietary patterns and low protein intake on sarcopenia risk in the very old: The Newcastle 85+ study. Clin Nutr. (2020) 39:166–73. doi: 10.1016/j.clnu.2019.01.009
33. Veronese N, Stubbs B, Volpato S, Zuliani G, Maggi S, Cesari M, et al. Association between gait speed with mortality, cardiovascular disease and cancer: a systematic review and meta-analysis of prospective cohort studies. J Am Med Dir Assoc. (2018) 19:981–8.e987. doi: 10.1016/j.jamda.2018.06.007
34. Peel NM, Alapatt LJ, Jones LV, Hubbard RE. The association between gait speed and cognitive status in community-dwelling older people: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci. (2019) 74:943–8. doi: 10.1093/gerona/gly140
35. Kim M, Won CW. Sarcopenia is associated with cognitive impairment mainly due to slow gait speed: results from the Korean Frailty and Aging Cohort Study (KFACS). Int J Environ Res Public Health. (2019) 16:1491. doi: 10.3390/ijerph16091491
36. Perera S, Patel KV, Rosano C, Rubin SM, Satterfield S, Harris T, et al. Gait speed predicts incident disability: a pooled analysis. J Gerontol A Biol Sci Med Sci. (2016) 71:63–71. doi: 10.1093/gerona/glv126
37. McClure R, Villani A. Greater adherence to a Mediterranean Diet is associated with better gait speed in older adults with type 2 diabetes mellitus. Clin Nutr ESPEN. (2019) 32:33–9. doi: 10.1016/j.clnesp.2019.05.009
38. Bibiloni MdM, Julibert A, Argelich E, Aparicio-Ugarriza R, Palacios G, Pons A, et al. Western and Mediterranean dietary patterns and physical activity and fitness among Spanish older adults. Nutrients. (2017) 9:704. doi: 10.3390/nu9070704
39. Rondanelli M, Faliva M, Monteferrario F, Peroni G, Repaci E, Allieri F, et al. Novel insights on nutrient management of sarcopenia in elderly. Biomed Res Int. (2015) 2015:524948. doi: 10.1155/2015/524948
40. McKenzie D, Bua E, McKiernan S, Cao Z, Wanagat J, Aiken JM. Mitochondrial DNA deletion mutations: a causal role in sarcopenia. Eur J Biochem. (2002) 269:2010–5. doi: 10.1046/j.1432-1033.2002.02867.x
41. Carmeli E, Coleman R, Reznick AZ. The biochemistry of aging muscle. Exp Gerontol. (2002) 37:477–89. doi: 10.1016/S0531-5565(01)00220-0
42. Morley JE, Baumgartner RN. Cytokine-Related Aging Process. Oxford: Oxford University Press (2004).
43. Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. (2006) 119:526.e529–17. doi: 10.1016/j.amjmed.2005.10.049
Keywords: nutrient patterns, sarcopenia, muscle mass, muscle strength, gait speed
Citation: Bagheri A, Hashemi R, Heshmat R, Motlagh AD and Esmaillzadeh A (2021) Patterns of Nutrient Intake in Relation to Sarcopenia and Its Components. Front. Nutr. 8:645072. doi: 10.3389/fnut.2021.645072
Received: 22 December 2020; Accepted: 15 March 2021;
Published: 27 April 2021.
Edited by:
Esther Molina-Montes, University of Granada, SpainCopyright © 2021 Bagheri, Hashemi, Heshmat, Motlagh and Esmaillzadeh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmad Esmaillzadeh, YS1lc21haWxsemFkZWgmI3gwMDA0MDt0dW1zLmFjLmly; Rezvan Hashemi, cl9oYXNoZW1pJiN4MDAwNDA7dHVtcy5hYy5pcg==
 Amir Bagheri
Amir Bagheri Rezvan Hashemi2*
Rezvan Hashemi2* Ramin Heshmat
Ramin Heshmat Ahmad Esmaillzadeh
Ahmad Esmaillzadeh