
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Nutr. , 21 April 2021
Sec. Clinical Nutrition
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.642306
 Brock A. Williams1,2
Brock A. Williams1,2 Cara Mayer1,2
Cara Mayer1,2 Heather McCartney3
Heather McCartney3 Angela M. Devlin2,3
Angela M. Devlin2,3 Yvonne Lamers1,2
Yvonne Lamers1,2 Suzanne M. Vercauteren2,4
Suzanne M. Vercauteren2,4 John K. Wu2,3
John K. Wu2,3 Crystal D. Karakochuk1,2*
Crystal D. Karakochuk1,2*Sickle cell disease (SCD) is an inherited hemoglobinopathy caused by a variant (rs344) in the HBB gene encoding the β-globin subunit of hemoglobin. Chronic hemolytic anemia and increased erythropoiesis and RBC turnover in individuals with SCD can result in increased needs for folate and other B-vitamins. We assessed B-vitamin status, and the distribution of folate forms, including unmetabolized folic acid (UMFA), in Canadian children with SCD supplemented with 1 mg/d folic acid (current routine practice). Non-fasted serum and plasma samples were analyzed for concentrations of folate, and vitamins B-2, B-6, and B-12. Eleven individuals (45% male; SCD type: HbSS n = 8, HbSC n = 2, HbSβ0-Thal n = 1), with a median (IQR) age of 14 (7, 18) years, were included. Total folate concentrations were 3–27 times above the deficiency cut-off (10 nmol/L), and 64% of children had elevated folate levels (>45.3 nmol/L). UMFA (>0.23 nmol/L) was detected in all children, and 36% of participants had elevated levels of UMFA (>5.4 nmol/L). All children were vitamin B-12 sufficient (>150 pmol/L), and the majority (55%) had sufficient B-6 status (>30 nmol/L). Among this sample of Canadian children with SCD, there was limited evidence of B-vitamin deficiencies, but UMFA was detectable in all children.
Sickle cell disease (SCD) is a rare, inherited hemoglobinopathy that occurs due to a missense variant (rs334) in the HBB gene encoding the β-globin subunit of hemoglobin (1, 2). This results in chronic hemolysis and a shortened RBC lifespan (1, 2). The increased production and turnover of RBCs in SCD is thought to increase the requirements of folate (vitamin B-9), a water-soluble family of compounds that are essential for erythropoiesis (1, 3). For this reason, individuals with SCD in Canada are routinely prescribed 1–5 mg/d of folic acid, the synthetic form of folate (4). Folic acid is commonly found in dietary supplements and folate-fortified foods, due to its stability, higher bioavailability, and oxidized state (5), while naturally occurring folate is commonly found in foods such as leafy green vegetables, oranges, beans, and legumes (6).
Beyond the role of folate in erythropoiesis, it also plays a vital role in one-carbon metabolism, a pathway which contributes to DNA and RNA synthesis, and the conversion of homocysteine to methionine in the methylation cycle (1, 6, 7). Vitamin B-2 (riboflavin), commonly found in animal products, green vegetables, and enriched grains (8), vitamin B-6, commonly found in animal products, grains, pulses, nuts and seeds (9), and vitamin B-12, found almost exclusively in animal products (10), also participate in one-carbon metabolism and are required for the production of S-adenosylmethionine (SAM), the universal methyl donor (11). Deficiencies of vitamins B-2, B-6, or B-12 can affect folate-dependent nucleic acid synthesis causing megaloblastic anemia, decrease cellular proliferation, and alter methylation reactions (12, 13).
A previous study of Canadian children with SCD supplemented with 1 mg/d folic acid (n = 92) reported that multiple serum nutrient insufficiencies/deficiencies were present in individuals, with zinc (<age- and sex-specific cut-offs) and vitamin D (<50 nmol/L) insufficiency being common (52 and 57%, respectively), vitamin B-6 deficiency (<20 nmol/L) being evident in a small minority of children (3%), but none of the children presenting with folate (<11 nmol/L) or vitamin B-12 deficiency (<133 pmol/L) (14).
Conversely, there is evidence of high folate concentrations in populations with national programs of refined-grain folic acid fortification and a high prevalence of supplement use, such as Canada and the United States. The 2011 Canadian Health Measures Survey reported that 40% of Canadians had high RBC folate concentrations (>1,360 nmol/L) (15), primarily related to supplement use (16). Unmetabolized folic acid (UMFA), folic acid that circulates in plasma when dihydrofolate reductase (DHFR) enzymatic capacity is limited or when the liver is fully saturated with folate (17), was detectable (> 0.3 nmol/L) in >95% of U.S. Americans in the 2007-8 National Health and Nutrition Examination Survey (NHANES) (18). Further, UMFA concentrations were significantly higher among supplement users (folic acid or folic acid-containing multivitamin), as compared to non-supplement users (1.54 vs. 0.79 nmol/L, respectively, P < 0.05) (18). Detectable UMFA concentrations in plasma have also been reported among Canadians, with 97% of pregnant women, the vast majority (≥90%) of whom consumed a prenatal supplement containing a median (IQR) of 1 (1-1) mg/d of folic acid, having detectable levels (19).
The presence of UMFA, which has been shown to occur at intake levels as low as 0.2 mg folic acid in adults (20), has not been previously investigated in children with SCD, who are routinely supplemented with high-dose folic acid (1 mg/d). This recommended dose exceeds the Tolerable Upper Intake Level (0.3–0.8 mg) for all ages between 1 and 18 years (21), and on average, the majority of Canadian children are predicted to meet the recommended dietary allowance (RDA) for folate from food sources alone in the era of folic acid food fortification (22). Further, the use of hydroxyurea, a medication which promotes fetal hemoglobin concentrations and thereby extends the average RBC lifespan in SCD (23), may reduce folate requirements. While the effects of UMFA in circulation are unknown, excess synthetic folic acid has been speculated to influence folate metabolism, DNA methylation, and gene expression (24–26).
Given the interrelationship between folate and the other B-vitamins (vitamins B-2, B-6, and B-12) in the folate and methylation cycles, and evidence of excess folic acid intake in folic acid supplemented individuals from countries with national programs of refined-grain folic acid fortification, we sought to determine B-vitamin status, and the distribution of folate forms, including UMFA, of Canadian children with SCD supplemented with prophylactic high-dose folic acid (1 mg/d).
Non-fasted serum and plasma samples from children aged 2–19 years of age diagnosed with SCD were obtained from BC Children's Hospital BioBank (Vancouver, Canada). Blood samples were collected in vacutainer tubes with EDTA or serum separator gel, following clinical protocols. For serum, blood samples were left at room temperature for 20–30 min to allow for clotting. Blood samples, subsequently, were stored at 4°C until centrifugation (1,500 × g for 10 min at 4°C) within 3 h of sample collection. Serum and plasma aliquots were stored at −80°C until analysis.
Samples were analyzed for vitamin B-6, vitamin B-2, and folate forms using electrospray ionization-liquid chromatography-tandem mass spectrometry (Agilent Technologies; ABSciex Pte), and for vitamin B-12 concentrations using chemiluminescent immunoassay (Architect i1000, Abbott Labs.). Sufficient plasma volumes were available for vitamin B-2 and vitamin B-6 analyzes for all participants, while plasma was only available for 10 out of 11 (91%) participants for vitamin B-12 analyses and 8 out of 11 (73%) for folate analyses. In these cases, serum was used for the remaining vitamin B-12 (n = 1) and folate analyses (n = 3). Concordance correlation coefficients were calculated, based on Lin's formula (27), for individuals with both plasma and serum total folate to determine agreement between measurements. For folate form analyses, the inter-assay CVs were 10% for 5-methyltetrahydrofolate (5-MTHF), 4% for folic acid, 8% for tetrahydrofolate (THF), 4% for 5-formyltetrahydrofolate (5-FoTHF), 7% for 5,10-methlyenetetrahydrofolate (5,10-MeTHF), and 9% for 5-methyltetrahydrofolate oxidation product (MeFox). This method has been externally validated (28). For PLP and vitamin B-2 (riboflavin) analyses, the inter-assay CVs were 6.8 and 11%, respectively.
The World Health Organization (WHO) cut-offs served to determine deficiencies of folate (<10 nmol/L) and vitamin B-12 (<150 pmol/L) (29). Elevated folate concentrations were defined as >45.3 nmol/L, according to WHO cut-offs (30). Plasma UMFA >0.23 nmol/L (i.e., the lowest limit of quantification) was defined as detectable levels of UMFA, which is similar to the level of 0.3 nmol/L applied for the US population ≥1 year of age (18). Elevated UMFA was defined as >5.4 nmol/L, according to the 95th percentile of values for folic acid containing supplement users >1 year of age who had fasted venous samples collected in the NHANES 2007–2008 survey (18).
The European Food Safety Authority Panel cut-off for pyridoxal 5′-phosphate (PLP) of <30 nmol/L served to determine deficiency for vitamin B-6, as PLP is the coenzyme and main transport form of vitamin B-6 (9). As no validated cut-offs for plasma/serum riboflavin concentration to determine serum vitamin B-2 deficiency exist (8), summaries of the data are primarily presented.
Complete blood count results were collected from patient charts. Whole blood samples were analyzed for complete blood counts, including measurement of hemoglobin (Hb) concentration (g/L), hematocrit (Hct; %), mean corpuscular volume (MCV; fL), red cell distribution width (RDW; % of red blood cell), and counts of reticulocytes, platelets and neutrophils (×109/L), using an automated hematology analyzer (Sysmex, Sysmex Corp.) at the BC Children's and Women's Hospital Laboratory. Megaloblastic changes were defined as a MCV increase >3 fL and a reticulocyte count <100 × 109/L and/or unexplained neutropenia and thrombocytopenia. Thrombocytopenia was defined as platelets <100 × 109/L (31), and neutropenia was defined as neutrophils <1.5 × 109/L (32).
Patient age, sex, use of hydroxyurea, folic acid supplementation recommendations, and sickle cell genotype were gathered from patient charts. Sickle cell genotypes were classified as homozygous hemoglobin SS (βSβS), hemoglobin SC (βSβC) genotype, and hemoglobin Sβ-thalassemia genotypes.
Medians with interquartile ranges (IQR) are presented. Data were analyzed with Stata IC/16.0 for Mac (Stata Corp.). All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Clinical Research Board of the University of British Columbia (#H18-01471).
In total, 11 children and adolescents with SCD (5 males; 6 females) were included in this study. Median (IQR) age of participants was 14 (7, 18) years. Overall, 8 (73%) individuals had a diagnosis of homozygous hemoglobin SS, 2 (18%) had the hemoglobin SC genotype, and 1 (9%) had the hemoglobin Sβ0-thalassemia genotype.
A total of 6 (55%) individuals were prescribed hydroxyurea, with a median (IQR) dose of 20 (15, 26) mg/kg/d. All individuals were recommended prophylactic folic acid supplementation (1 mg/d); adherence or duration of folic acid supplementation data was unavailable.
Megaloblastic changes were not detected in any participant (Table 1). Only 1 participant was noted to have neutropenia (neutrophils <1.5 × 109) (Table 1), however, benign neutropenia has been noted to occur in individuals of African descent (32, 33).
Concordance analysis completed for individuals with both plasma and serum total folate results (n = 8) illustrated a correlation coefficient (95% CI) of 0.984 (0.930, 0.996), indicating very strong agreement between serum and plasma results.
Folate deficiency (total folate <10 nmol/L) was not detected in any participant, and 7 participants had elevated folate concentrations (>45.3 nmol/L) (Table 2). Overall, folate concentrations were 3–27 times above the cut-off for deficiency. UMFA (>0.23 nmol/L; the lowest level of quantification) was detected in all 11 participants, and elevated UMFA (>5.4 nmol/L) was present in 4 participants (Table 2). UMFA as a percentage of total folate concentrations was a median (IQR) of 2.6 (2.1, 27.7) %.
There was no evidence of vitamin B-12 deficiency in this population, however, 5 individuals were vitamin B-6 deficient (PLP <30 nmol/L) (Table 2 and Figure 1).
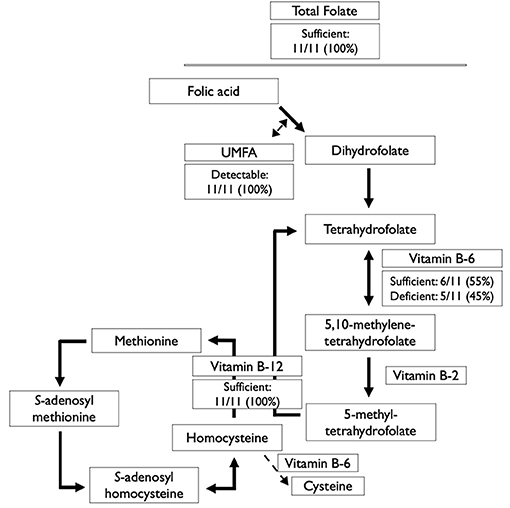
Figure 1. Serum/plasma status of B-vitamins involved in the folate and methionine cycles among 11 Canadian children with sickle cell disease. UMFA, unmetabolized folic acid.
Routine high-dose (1–5 mg/d) folic acid supplementation in SCD is a practice that is intended to prevent the occurrence of folate deficiency and the potential for megaloblastic anemia in this hemolytic condition. In children with SCD, this practice is largely based on a limited body of evidence consisting of one randomized control trial conducted in Jamaican children in 1983, which provided evidence of higher serum folate concentrations but no changes in hematological measures when children were supplemented with 5 mg/d of folic acid (1). The results of our study suggest that folate deficiency and megaloblastic changes were not present in this sample of Canadian children with SCD supplemented with folic acid, and that 64% of individuals had elevated folate concentrations. Further, detectable levels of UMFA were present in all participants, and there was evidence of elevated levels of UMFA in 36% of participants.
Previous studies have identified detectable UMFA and elevated folate concentrations in adult populations with hemolytic blood disorders, specifically hereditary spherocytosis (34) and β-thalassemia (35), that consumed folic acid-fortified refined grains and were supplemented with 5 mg/d of folic acid. This is the first study, to our knowledge, to report this observation in folic acid-supplemented children with sickle cell disease, which is of note as UMFA has been proposed to be a biomarker of excess folic acid intake (36). A recent National Institutes of Health (NIH) Workshop (2020) on understanding the metabolic and clinical effects of excess folates/folic acid has identified that the evidence on possible adverse effects of excess folate and/or UMFA remains inconclusive, but there is an emerging need to address these gaps in the literature (37). Previous studies have reported an association between excess folic acid intakes and colorectal (38) and prostate (39) cancer in adults, impaired cellular immunity [reduced natural killer cell cytotoxicity in adults, and potential effects on the mucosal immune tissue of the gut in children (40, 41)], and adverse effects on metabolic health during development (42, 43). This may be of significant importance for children in SCD who may be exposed to UMFA, which can occur at doses of folic acid as low as 0.2 mg/d (20), chronically from life-long folic acid supplementation of ≥1 mg/d.
As folate does not work in isolation in one-carbon metabolism, we also determined the status of other key B-vitamins (vitamins B-6 and B-12) in this supplemented population. In this sample, there was no evidence of vitamin B-12 deficiency; however, vitamin B-6 deficiency was evident in 45% of participants (n = 5). Previous cross-sectional studies have suggested that among US children with SCD, not treated with hydroxyurea, that 77% (n = 88/109) of individuals were vitamin B-6 deficient (44), while in Canadian children with SCD, the majority of whom were treated with hydroxyurea, that 19% (n = 15/80) were deficient (14). Of note, in both studies, a PLP concentration of <20 nmol/L was used to determine deficiency of vitamin B-6, and no participants in this study had PLP concentrations below that cut-off. Thus, the 45% of children with PLP concentration between 20 and 30 nmol/L classified as insufficient in our study, would be classified as having marginal B-6 status using previously applied adult categories (20–30 nmol/L) (45).
We present a descriptive summary of plasma vitamin B-2 for this sample of children, as no well-established cut-offs exist for determining deficiency of vitamin B-2 using plasma or serum concentrations (8). Vitamin B-2 status is most often determined using the erythrocyte glutathione reductase activation coefficient (EGRac), a functional measure of B-2 status (46), which is only available in a select few research institutions and requires washed red blood cells as biospecimen, which was not available for this sample of children with SCD. Emerging research has sought to establish the usefulness of plasma vitamin B-2 as a more-widely available indicator of B-2 status. Preliminary data among healthy women has identified a plasma concentration of 26.5 nmol/L of vitamin B-2 as the change point between plasma riboflavin and an EGRac of 1.25, indicative of functional B-2 deficiency, and a range of 6.7–64.2 nmol/L as the lower and upper limits of the central 95% reference interval, respectively (47). Among participants in our study, 73% (n = 8) had plasma riboflavin concentration below the change point of 26.5 nmol/L, indicative of functional B-2 deficiency. Further study which establishes the utility of plasma vitamin B-2 for the determination of B-2 status may help to provide a holistic understanding of B-vitamin status in children with hemolytic hemoglobinopathies. This is of value as adequate vitamin B-2 is important for folate cycle activity, specifically the formation of 5-MTHF, as well as for the conversion of dietary vitamin B-6 to its coenzyme form PLP (8).
While SCD is the most common monogenic disease worldwide (48), in the Canadian population it is a relatively rare disease estimated to occur in up to ~1 in 5,600 births in some regions of the country (49). We acknowledge the inherent limitation of our small sample size given the rarity of this disease, especially in Western Canada; however, in British Columbia, our sample of children with SCD represents ~20% of the total pediatric SCD population. We acknowledge a few other limitations of our study: a lack of dietary data and biomarkers such as RBC folate concentrations for the determination of longer-term folate status and EGRac for the determination of B-2 status. In general, RBC folate concentrations are considered to be reflective of longer term folate status, previous 3–4 months in healthy RBCs, whereas serum/plasma folate reflects recent status or dietary/supplementary intake (50). It has, however, been suggested that steady state RBC folate concentrations can appear higher in those with HbSS and HbSC genotypes, in comparison to healthy controls (HbAA; normal hemoglobin) supplemented with the same dose of folic acid (5 mg/d) (51). This results from the shorter RBC half-life in SCD, as RBC folate concentrations are established during erythropoiesis and subsequentially decrease in circulation (51). In this population with a higher proportion of young RBCs, a comprehensive approach to assessment of folate status, which includes both serum/plasma and RBC folate concentrations, may aid in this interpretation.
In this study, the high plasma/serum folate concentrations in non-fasting samples measured in some participants may have resulted from recent folic acid supplementation consumption. The vast majority of participants (n = 8) had a percentage of total folate concentrations resulting from UMFA <10%, while three participants had concentrations above that threshold (27.7, 56.8, and 71.7%, respectively), likely indicating recent ingestion of a significant amount of folic acid.
The analysis of vitamin B-12 using chemiluminescent immunoassay in SCD, a hemolytic hemoglobinopathy, may have also potentially underestimated total B-12 concentrations. One sample in our study had significant hemolysis and there is evidence to suggest that greater degrees of hemolysis can lead to lower measured vitamin B-12 in plasma when using the Abbott Architect instrument (52). The inclusion of other biomarkers, such as holotranscobalamin, the form of vitamin B-12 in circulation that is taken up by tissues, and/or methylmalonic acid, which is elevated in vitamin B-12 deficiency, may have provided a more accurate estimation of vitamin B-12 status than just plasma total vitamin B-12 alone (53).
This cross-sectional study presents preliminary evidence of detectable unmetabolized folic acid and limited B-vitamin deficiencies among this sample of Canadian children with SCD supplemented with prophylactic high-dose folic acid. Future rigorously designed, and comprehensive research in which RBC and serum folate concentrations, folate-related metabolites, such as S-adenosylmethionine, S-adenosylhomocysteine, and total homocysteine concentrations, other biomarkers for B-vitamins involved in folate metabolism, including vitamins B-6 and B-12, and biomarkers of inflammation and oxidation are measured during periods with and without high-dose folic acid supplementation for pre-defined study time periods in children with SCD may support the findings presented, allow for detailed analysis of one-carbon metabolites in these individuals, and provide further insight into the utility of routine folic acid supplementation. Larger, sufficiently powered trials are also warranted to determine if B-vitamin status differs by sickle cell genotype, the degree of RBC hemolysis, and/or the use of hydroxyurea.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Clinical Research Board of the University of British Columbia. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
CK, BW, HM, SV, and JW: conceptualization. CM and HM: assistance with study preparation. YL: sample analysis. BW and CK: writing—original draft preparation. CM, HM, AD, YL, SV, and JW: writing—review and editing. CK: supervision and funding acquisition. All authors have read and agreed to the published version of the manuscript.
This funding support was provided by The Rare Disease Foundation (#2263) and BC Children's Hospital Foundation. BW was supported by a Canadian Institutes of Health Research (CIHR) Frederick Banting and Charles Best Canada Graduate Scholarship Doctoral Award (#434962). AD was supported by an Investigator Grant from BC Children's Hospital Research Institute. CK was supported by a Michael Smith Foundation for Health Research Scholar Award. Funding agencies had no role in the design of the study, collection, analysis, and interpretation of data, or in the writing of manuscripts.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Veronica Chow and Adam Velenosi (BC Children's BioBank) for their assistance with data and sample retrieval. We also thank Roger Dyer and Janette King (BC Children's Hospital Research Institute), and Shujun Lin and Nadia Moran-Garcia (University of British Columbia) for their expertise and assistance with B-vitamin analyses.
1. Dixit R, Nettem S, Madan SS, Soe HHK, Abas ABL, Vance LD, et al. Folate supplementation in people with sickle cell disease. Cochrane Database Syst Rev. (2018) 3:CD011130. doi: 10.2139/ssrn.3297902
2. Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. (2010) 376:2018–31. doi: 10.1016/S0140-6736(10)61029-X
3. Koury MJ, Ponka P. New insights into erythropoiesis: the roles of folate, vitamin B12, and iron. Annu Rev Nutr. (2004) 24:105–31. doi: 10.1146/annurev.nutr.24.012003.132306
4. The Canadian Haemoglobinopathy Association. Consensus Statement on the Care of Patients with Sickle Cell Disease in Canada. The Canadian Haemoglobinopathy Association (2015).
5. Lamers Y, MacFarlane AJ, O'Connor DL, Fontaine-Bisson B. Periconceptional intake of folic acid among low-risk women in Canada: summary of a workshop aiming to align prenatal folic acid supplement composition with current expert guidelines. Am J Clin Nutr. (2018) 108:1357–68. doi: 10.1093/ajcn/nqy212
7. Lamers Y. Indicators and methods for folate, vitamin B-12, and vitamin B-6 status assessment in humans. Curr Opin Clin Nutr Metab Care. (2011) 14:445–54. doi: 10.1097/MCO.0b013e328349f9a7
8. EFSA Panel on Dietetic Products Nutrition and Allergies, Turck D, Bresson J, Burlingame B, Dean T, Fairweather-Tait S, et al. Scientific opinion on dietary reference values for riboflavin. EFSA J. (2017) 15:1–65. doi: 10.2903/j.efsa.2017.4919
9. EFSA Panel on Dietetic Products Nutrition and Allergies, Turck D, Bresson J-L, Burlingame B, Dean T, Fairweather-Tait S, et al. Dietary reference values for vitamin B6. EFSA J. (2016) 14:e04485. doi: 10.2903/j.efsa.2016.4485
10. Watanabe F. Vitamin B12 sources and bioavailability. Exp Biol Med. (2007) 232:1266–74. doi: 10.3181/0703-MR-67
11. Ued FV, Mathias MG, Toffano RBD, Barros TT, Almada MO, Salomao RG, et al. Vitamin B2 and folate concentrations are associated with ARA, EPA, and DHA fatty acids in red blood cells of Brazilian children and adolescents. Nutrients. (2019) 11:1–17. doi: 10.3390/nu11122918
12. Selhub J. Folate, vitamin B12 and vitamin B6 and one carbon metabolism. J Nutr Health Aging. (2002) 6:39–42.
13. Ducker GS, Rabinowitz JD. One-carbon metabolism in health and disease. Cell Metab. (2017) 25:27–42. doi: 10.1016/j.cmet.2016.08.009
14. Martyres DJ, Vijenthira A, Barrowman N, Harris-Janz S, Chretien C, Klaassen RJ. Nutrient insufficiencies/deficiencies in children with sickle cell disease and its association with increased disease severity. Pediatr Blood Cancer. (2016) 63:1060–4. doi: 10.1002/pbc.25940
15. Colapinto CK, O'Connor DL, Tremblay MS. Folate status of the population in the Canadian health measures survey. CMAJ. (2011) 183:E100–6. doi: 10.1503/cmaj.100568
16. Colapinto CK, O'Connor DL, Dubois L, Tremblay MS. Prevalence and correlates of high red blood cell folate concentrations in the Canadian population using 3 proposed cut-offs. Appl Physiol Nutr Metab. (2015) 40:1025–30. doi: 10.1139/apnm-2015-0191
17. Patanwala I, King MJ, Barrett DA, Rose J, Jackson R, Hudson M, et al. Folic acid handling by the human gut: implications for food fortification. Am J Clin Nutr. (2014) 100:593–9. doi: 10.3945/ajcn.113.080507
18. Pfeiffer CM, Sternberg MR, Fazili Z, Yetley EA, Lacher DA, Bailey RL, et al. Unmetabolized folic acid is detected in nearly all serum samples from US children, adolescents, and adults. J Nutr. (2015) 145:520–31. doi: 10.3945/jn.114.201210
19. Plumptre L, Masih SP, Ly A, Aufreiter S, Sohn K-J, Croxford R, et al. High concentrations of folate and unmetabolized folic acid in a cohort of pregnant Canadian women and umbilical cord blood. Am J Clin Nutr. (2015) 102:848–57. doi: 10.3945/ajcn.115.110783
20. Kelly P, McPartlin J, Goggins M, Weir DG, Scott JM. Unmetabolized folic acid in serum: acute studies in subjects consuming fortified food and supplements. Am J Clin Nutr. (1997) 65:1790–5. doi: 10.1093/ajcn/65.6.1790
21. Otten JJ, Hellwig JP, Meyers L, editor. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, DC: National Academies Press (2006).
22. Shakur YA, Garriguet D, Corey P, O'Connor DL. Folic acid fortification above mandated levels results in a low prevalence of folate inadequacy among Canadians. Am J Clin Nutr. (2010) 92:818–25. doi: 10.3945/ajcn.2010.29696
23. Ballas SK, Marcolina MJ, Dover GJ, Barton FB. Erythropoietic activity in patients with sickle cell anaemia before and after treatment with hydroxyurea. Br J Haematol. (1999) 105:491–6. doi: 10.1111/j.1365-2141.1999.01339.x
24. Smith AD, Kim Y-I, Refsum H. Is folic acid good for everyone? Am J Clin Nutr. (2008) 87:517–33. doi: 10.1093/ajcn/87.3.517
25. Sie KKY, Li J, Ly A, Sohn K, Croxford R, Kim Y. Effect of maternal and postweaning folic acid supplementation on global and gene-specific DNA methylation in the liver of the rat offspring. Mol Nutr Food Res. (2013) 57:677–85. doi: 10.1002/mnfr.201200186
26. Barua S, Chadman KK, Kuizon S, Buenaventura D, Stapley NW, Ruocco F, et al. Increasing maternal or post-weaning folic acid alters gene expression and moderately changes behavior in the offspring. PLoS ONE. (2014) 9:e101674. doi: 10.1371/journal.pone.0101674
27. Lin L, Torbeck LD. Coefficient of accuracy and concordance correlation coefficient: new statistics for methods comparison. PDA J Pharm Sci Technol. (1998) 52:55–9.
28. Fazili Z, Sternberg MR, Paladugula N, Pfeiffer CM. Two international round-robin studies showed good comparability of 5-methyltetrahydrofolate but poor comparability of folic acid measured in serum by different high-performance liquid chromatography-tandem mass spectrometry methods. J Nutr. (2017) 147:1815–25. doi: 10.3945/jn.117.254144
29. de Benoist B. Conclusions of a WHO technical consultation on folate and vitamin B12 deficiencies. Food Nutr Bull. (2008) 29:238–44. doi: 10.1177/15648265080292S129
30. World Health Organization. Serum and Red Blood Cell Folate Concentrations for Assessing Folate Status in Populations. (2012). Available online at: http://apps.who.int/iris/bitstream/10665/75584/1/WHO_NMH_NHD_EPG_12.1_eng.pdf (accessed November 20, 2020).
31. Ault KA, Rinder HM, Mitchell J, Carmody MB, Vary CPH, Hillman RS. The significance of platelets with increased RNA content (reticulated platelets) a measure of the rate of thrombopoiesis. Am J Clin Pathol. (1992) 98:637–46. doi: 10.1093/ajcp/98.6.637
32. Haddy TB, Rana SR, Castro O. Benign ethnic neutropenia: what is a normal absolute neutrophil count? J Lab Clin Med. (1999) 133:15–22. doi: 10.1053/lc.1999.v133.a94931
33. Thobakgale CF, Ndung'u T. Neutrophil counts in persons of African origin. Curr Opin Hematol. (2014) 21:50–7. doi: 10.1097/MOH.0000000000000007
34. Paniz C, Lucena MR, Bertinato JF, Lourenço FR, Barros BCA, Gomes GW, et al. Daily supplementation with 5 mg of folic acid in Brazilian patients with hereditary spherocytosis. J Investig Med. (2019) 67:1110–17. doi: 10.1136/jim-2019-001025
35. Paniz C, Lucena MR, Bertinato JF, dos Santos MNN, Gomes GW, Figueiredo MS, et al. Serum folate and cytokines in heterozygous β-thalassemia. Int J Lab Hematol. (2020) 42:718–26. doi: 10.1111/ijlh.13287
36. Sulistyoningrum D, Green T, Palmer D, Sullivan T, Wood S, Makrides M, et al. Study protocol for a randomised controlled trial evaluating the effect of folic acid supplementation beyond the first trimester on maternal plasma unmetabolised folic acid in late gestation. BMJ Open. (2020) 10:e040416. doi: 10.1136/bmjopen-2020-040416
37. Maruvada P, Stover PJ, Mason JB, Bailey RL, Davis CD, Field MS, et al. Knowledge gaps in understanding the metabolic and clinical effects of excess folates/folic acid: a summary, and perspectives, from an NIH workshop. Am J Clin Nutr. (2020) 112:1390–403. doi: 10.1093/ajcn/nqaa259
38. Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. (2007) 297:2351–9. doi: 10.1001/jama.297.21.2351
39. Figueiredo JC, Grau MV, Haile RW, Sandler RS, Summers RW, Bresalier RS, et al. Folic acid and risk of prostate cancer: results from a randomized clinical trial. J Natl Cancer Inst. (2009) 101:432–5. doi: 10.1093/jnci/djp019
40. Troen AM, Mitchell B, Sorensen B, Wener MH, Johnston A, Wood B, et al. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J Nutr. (2006) 136:189–94. doi: 10.1093/jn/136.1.189
41. Taneja S, Strand TA, Kumar T, Mahesh M, Mohan S, Manger MS, et al. Folic acid and vitamin B-12 supplementation and common infections in 6-30-mo-old children in India: a randomized placebo-controlled trial. Am J Clin Nutr. (2013) 98:731–7. doi: 10.3945/ajcn.113.059592
42. Henderson AM, Tai DC, Aleliunas RE, Aljaadi AM, Glier MB, Xu EE, et al. Maternal folic acid supplementation with vitamin B12 deficiency during pregnancy and lactation affects the metabolic health of adult female offspring but is dependent on offspring diet. FASEB J. (2018) 32:5039–50. doi: 10.1096/fj.201701503RR
43. Yajnik CS, Deshpande SS, Jackson AA, Refsum H, Rao S, Fisher DJ, et al. Vitamin B 12 and folate concentrations during pregnancy and insulin resistance in the offspring: the pune maternal nutrition study. Diabetologia. (2008) 51:29–38. doi: 10.1007/s00125-007-0793-y
44. Nelson MC, Zemel BS, Kawchak DA, Barden EM, Frongillo EAJ, Coburn SP, et al. Vitamin B6 status of children with sickle cell disease. J Pediatr Hematol Oncol. (2002) 24:11. doi: 10.1097/00043426-200208000-00011
45. da Silva VR, Rios-Avila L, Lamers Y, Ralat MA, Midttun Ø, Quinlivan EP, et al. Metabolite profile analysis reveals functional effects of 28-day vitamin B-6 restriction on one-carbon metabolism and tryptophan catabolic pathways in healthy men and women. J Nutr. (2013) 143:1719–27. doi: 10.3945/jn.113.180588
46. McAuley E, McNulty H, Hughes C, Strain JJ, Ward M. Riboflavin status, MTHFR genotype and blood pressure: current evidence and implications for personalised nutrition. Proc Nutr Soc. (2016) 75:405–14. doi: 10.1017/S0029665116000197
47. Tan A, Zubair M, Ho C, McAnena L, McNulty H, Ward M, et al. Plasma riboflavin concentration as novel indicator for vitamin-B2 status assessment: suggested cutoffs and its association with vitamin-B6 status in women. Proc Nutr Soc. (2020) 79:E658. doi: 10.1017/S0029665120006072
48. Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med. (2017) 376:1561–73. doi: 10.1056/NEJMra1510865
49. Lieberman L, Kirby M, Ozolins L, Mosko J, Friedman J. Initial presentation of unscreened children with sickle cell disease: the toronto experience. Pediatr Blood Cancer. (2009) 53:397–400. doi: 10.1002/pbc.22023
50. WHO. Serum and Red Blood Cell Folate Concentrations for Assessing Folate Status in Populations. Geneva: World Health Organization (2015).
51. Muskiet FAJ, van der Dijs FPL, Fokkema MR, Muskiet FD. Erythrocyte folate does not accurately reflect folate status in sickle cell disease. Clin Chem. (2000) 46:2015–6. doi: 10.1093/clinchem/46.12.2015
52. Hasanato R, Brearton S, Alshebani M, Bailey L, Aldugashim S, Alothaim A, et al. Effects of serum indices interference on hormonal results from the Abbott Architect i2000 immunoassay analyser. Br J Biomed Sci. (2015) 72:151–5. doi: 10.1080/09674845.2015.11665744
Keywords: sickle cell disease, pediatrics, folate, unmetabolized folic acid, B-vitamins, nutrition
Citation: Williams BA, Mayer C, McCartney H, Devlin AM, Lamers Y, Vercauteren SM, Wu JK and Karakochuk CD (2021) Detectable Unmetabolized Folic Acid and Elevated Folate Concentrations in Folic Acid-Supplemented Canadian Children With Sickle Cell Disease. Front. Nutr. 8:642306. doi: 10.3389/fnut.2021.642306
Received: 15 December 2020; Accepted: 26 March 2021;
Published: 21 April 2021.
Edited by:
Sergio Davinelli, University of Molise, ItalyReviewed by:
Marilda De Souza Gonçalves, Oswaldo Cruz Foundation (Fiocruz), BrazilCopyright © 2021 Williams, Mayer, McCartney, Devlin, Lamers, Vercauteren, Wu and Karakochuk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Crystal D. Karakochuk, Y3J5c3RhbC5rYXJha29jaHVrQHViYy5jYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.