- 1School of Global Public Health, New York University, New York, NY, United States
- 2Department of Population Health, New York University Grossman School of Medicine, New York, NY, United States
- 3Department of Pediatrics, New York University Grossman School of Medicine, New York, NY, United States
- 4Department of Environmental Medicine, New York University Grossman School of Medicine, New York, NY, United States
- 5Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, New York University Langone Health, New York, NY, United States
Maternal diet, prior to and during pregnancy, plays an important role in the immediate and long-term health of the mother and her offspring. Our objectives were to assess diet quality among a large, diverse, urban cohort of pregnant women, and examine associations with sociodemographic and health behavior characteristics. Data were from 1,325 pregnant women enrolled in New York University Children's Health and Environment Study (NYU CHES). Diet quality was assessed using the Healthy Eating Index (HEI)-2015. Mean total HEI-2015 score was 74.9 (SD = 8.5); 376 (28%), 612 (46%), 263 (20%), and 74 (6%) of women had scores that fell into the grade range of A/B, C, D, and F, respectively. Mean HEI-2015 component scores were high for fruit and whole grains and low for protein-related, sodium, and fat-related components. In multivariable linear regression models, Hispanic women scored 1.65 points higher on the total HEI-2015 (95% CI: 0.21, 3.10) compared to non-Hispanic White women, while younger age (<30 years), parity, single status, pre-pregnancy obesity, smoking, pre-existing hypertension, moderate/severe depressive symptoms, not meeting physical activity recommendations, and not taking a vitamin before pregnancy were associated with ~1.5–5-point lower mean total HEI-2015 scores. Diet is a modifiable behavior; our results suggest a continued need for pre-conceptional and prenatal nutritional counseling.
Introduction
Maternal diet, both prior to conception and during pregnancy, plays an important role in the immediate and long-term health of the mother and her offspring (1, 2). Poor dietary quality before and during pregnancy is linked to maternal complications, such as gestational diabetes, hypertension, and post-partum depression (3–7). In offspring, in-utero exposure to malnutrition, defined as inadequate or excessive nutrient intakes, is associated with a range of adverse short- and long-term health outcomes, including fetal growth restriction, low or high birthweight, and obesity, as well as increased risk of chronic disease in adulthood (1, 8, 9).
It is critical to assess women's dietary quality prior to and during pregnancy with respect to current dietary guidelines and meeting pregnancy-specific recommendations for iron, folate, and other nutrients. Recently, Bodnar et al. described peri-conceptional dietary quality, during the 3 months around conception, of nulliparous women in the United States (U.S.) using the Healthy Eating Index (HEI)-2010 (10). Dietary quality was suboptimal; approximately one-third of women's energy intakes were from empty calories (e.g., added sugars and solid fats), with sugar-sweetened beverages, pasta, and grain desserts ranking among the top energy sources. Lower dietary quality was observed among non-Hispanic Black and Hispanic women, as well as women who did not graduate from college (10). Other studies also report that prenatal dietary quality (using various indices) differs by maternal characteristics and suggest younger age, pre-pregnancy obesity, parity, and smoking are associated with lower scores (2, 11–19). However, there currently remain limitations in the previous literature, including older cohort data collection, homogeneous populations, and lack of consideration of a range of maternal characteristics and health-related factors (2, 11–19).
Given the health implications for the mother and her offspring, there continues to be a need to study women's nutritional status before, during, and after pregnancy, particularly among socio-demographically and geographically diverse populations (19). We can use this information to identify subgroups of women who are at risk of malnutrition, as well as to recognize consumption of specific food groups or nutrients that may be below or exceed recommendations. Our objective in the current study was to assess diet quality using the most recent Dietary Guidelines for Americans 2015–2020 among a contemporary, urban cohort of pregnant women (20). Associations of women's dietary intakes with sociodemographic and health behavior characteristics were examined.
Materials and Methods
Study Sample
Data were from the New York University Children's Health and Environment Study (NYU CHES), a prospective birth cohort designed to evaluate the influences of prenatal non-persistent environmental chemical exposures on fetal and post-natal growth and development. The study is described in detail elsewhere (21). Briefly, beginning in 2016, adult women (ages 18 years and older) were enrolled at <18 weeks' gestation from three NYU School of Medicine affiliates in Manhattan and Brooklyn, March 2016–April 2019, post-natal follow-up of these women and their children is ongoing. Women were included if they were pregnant and planned to deliver at one of the study hospitals. All women gave written, informed consent and the study was approved by the institutional review board of the NYU School of Medicine (i15-00778). There were 2,193 women enrolled in NYU CHES; 2,000 women had live births, of which 1,384 completed the dietary assessment. We excluded women with implausible daily energy intakes of <500 kilocalories (n = 49) or more than 6,000 kilocalories (n = 10). This range was based on plausible intakes for non-pregnant and pregnant women used previously in the literature (22–24). The resulting analytic sample was 1,325 women.
Dietary Assessment
Women self-administered the electronic version of the Diet History Questionnaire II (DHQ-II, in English and translated to Spanish) to assess their usual dietary intakes during the previous year (25). The DHQ-II has not been validated, but the previous version (DHQ-I) was found to provide reasonable nutrient estimates in adults in validation studies (26). We used the DHQ-II because it captures intakes during the previous 12 months, spanning both prior to and during pregnancy. The DHQ-II is a publicly available food frequency questionnaire developed by the U.S. National Cancer Institute. It consists of 124 commonly consumed food items and includes frequency, portion size, and dietary supplement questions. The median (standard deviation, SD) gestational age of DHQ-II completion was 26 (8.6) weeks: 731 (55%) women completed the DHQ-II in the first/second trimester (<27 weeks' gestation), 512 (39%) women in the third trimester (27 to <40 weeks' gestation), and 82 (6%) women during peri-partum/early post-partum (≥40 weeks' gestation).
Diet Quality
We assessed diet quality from dietary sources only (excluding vitamin or supplement use) using the HEI-2015 (25). The HEI was developed in 1995 by the U.S. Department of Agriculture and evaluates adherence to national dietary recommendations provided by the Dietary Guidelines for Americans, which are updated every 5 years (25, 27). We selected the HEI-2015 from among several indices available to evaluate diet quality because it reflects the most recent U.S. dietary recommendations for all individuals, the Dietary Guidelines for Americans, 2015–2020 (20). We calculated HEI-2015 scores using resources developed by the National Cancer Institute that are specific to the DHQ-II: the DHQ II Diet*Calc software and the HEI-2015 SAS program and required SAS macros (28). Higher HEI scores are associated with favorable infant anthropometric outcomes (e.g., fat mass) (12, 29), as well as reduced risks of chronic diseases and mortality in adults (30).
The HEI-2015 comprises thirteen components (maximum 100 points): nine adequacy components (60 points) and four moderation components (40 points). The nine adequacy components are: total vegetables (5 points), greens and beans (5 points), total fruit (5 points), whole fruit (5 points), whole grains (10 points), total dairy (10 points), total protein foods (5 points), seafood and plant protein (5 points), and fatty acid ratio (ratio of polyunsaturated fats and monounsaturated fats to saturated fat, 10 points). With the exception of fatty acid ratio, all of the adequacy components are scored based on nutrient density per 1,000 calories (e.g., cups of whole fruit per 1,000 calories, see Table 1). The four moderation components are: sodium (10 points), refined grains (10 points), saturated fat (10 points), and added sugars (10 points). Refined grains and sodium are scored based on nutrient density per 1,000 calories; calories from saturated fat and added sugars are scored as a percentage of total energy intake. Higher scores reflect better diet quality; i.e., for the adequacy components, a higher score reflects higher intakes of that component, while for the moderation components, a higher score reflects lower intakes of that component. The thirteen component scores are summed for an overall score (25). A grading approach (A through D and F) has been devised to interpret HEI-2015 scores: A = 90–100; B = 80–89; C = 70–79; D = 60–69; F = 0–59 (25). In NYU CHES, only 23 women had HEI-2015 scores of 90 or greater; therefore, the A and B grade categories were collapsed for statistical analyses. We also examined intakes from dietary sources only of selected nutrients that are needed in increased amounts during pregnancy: vitamin C, iron, folate, vitamin D, vitamin B1 (thiamin), vitamin B2 (riboflavin), vitamin B3 (niacin), vitamin B6 (pyroxidine), vitamin B12 (cobalamin), selenium, zinc, and docosahexanoic acid (DHA) (31).
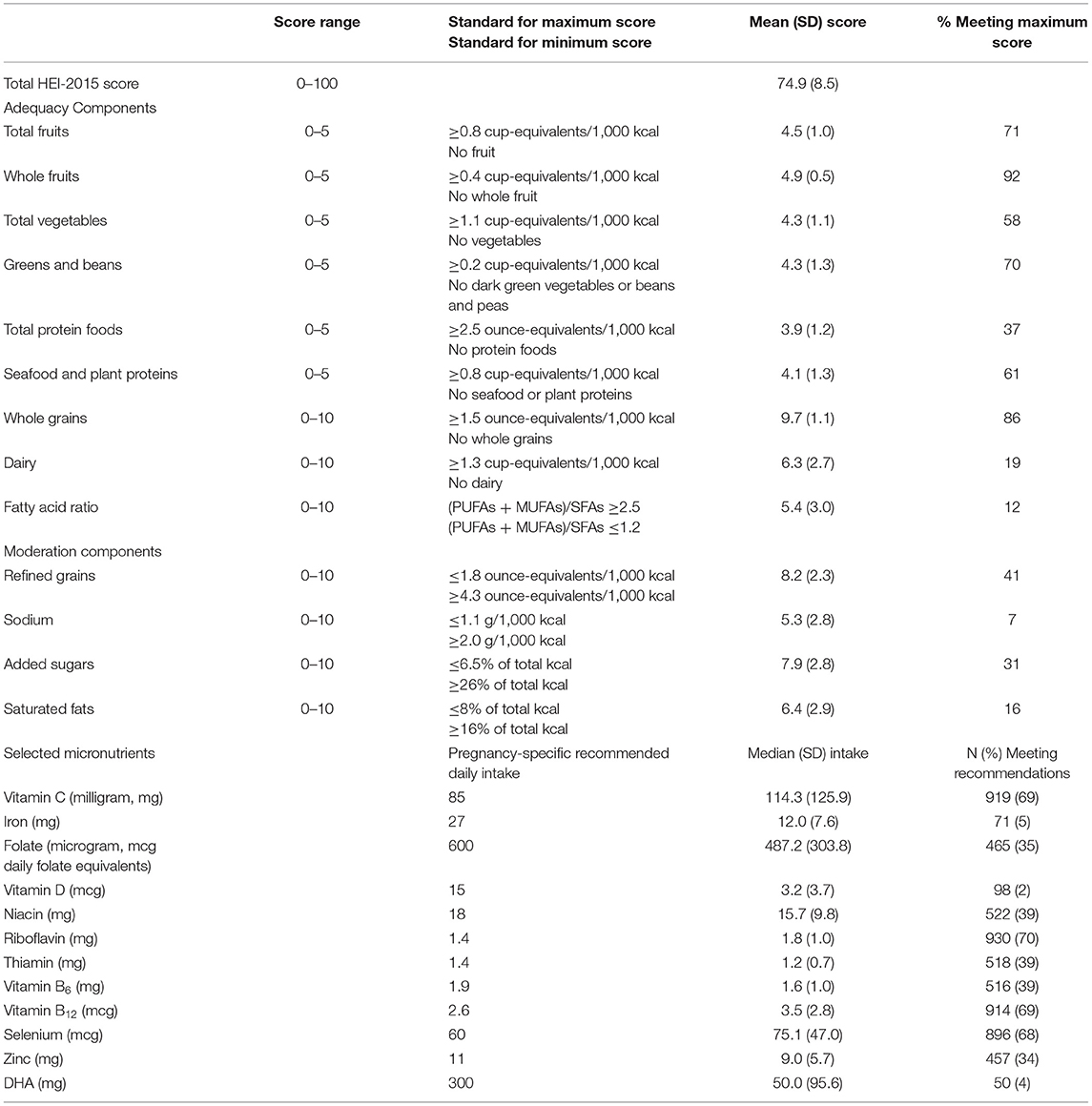
Table 1. Description of Healthy Eating Index-2015 (HEI-2015) scores and selected nutrient intakes among pregnant women participating in the New York University Children's Health and Environment Study (n = 1,325).
Sociodemographic and Health Behavior Characteristics
We used electronic health records and questionnaires [administered at <18 weeks' gestation (baseline), at 18–25 weeks' gestation, and after birth] to collect information on sociodemographic characteristics, health behaviors, medical history, mental health, and other factors. In the current analyses, we considered baseline: maternal age (years); highest attained education level (high school graduate or less, some college, college graduate, or graduate/professional degree); household income (< $30,000, $30,000–$99,999, and ≥$100,000); current employment status (yes or no); parity (number of previous births); marital status (single or living with partner/married); smoking status (non-smoker or ever smoking prior to/during pregnancy); consumption of alcoholic beverages (never used, used before pregnancy but not during, used during pregnancy); self-reported pre-existing diabetes (yes or no); self-reported pre-existing hypertension (yes or no); and multi-/prenatal vitamin and/or folic acid supplement use in the month before pregnancy and during pregnancy (vitamin use, yes or no). Self-reported race/ethnicity was categorized as non-Hispanic White, non-Hispanic Black, Hispanic, Asian, and other. Women reporting multiple race (n = 33) or other (n = 10) were categorized as other. Pre-pregnancy body mass index (BMI, kilograms/meters2, kg/m2) was calculated using height and self-reported pre-pregnancy weight (from electronic health records and, if missing, from questionnaires) and categorized as underweight (<18.5 kg/m2), normal weight (18.5 to <25 kg/m2), overweight (25 to <30 kg/m2), and obese (≥30 kg/m2). There were only 12 underweight women; we included these women with normal weight women for analyses.
The Patient Health Questionnaire-9 (PHQ-9, total points = 27) assessed depressive symptoms and was administered at least once during pregnancy (32). For analyses, we considered only responses from the first administered PHQ-9 [mean (SD) gestational age, 12.7 (5.8) weeks]. We categorized PHQ-9 scores as none/minimal depressive symptoms (0–4 points), mild depressive symptoms (5–9 points), and moderate/severe depressive symptoms (≥10 points) (32). The International Physical Activity Questionnaire-Short Form (IPAQ-SF) queried about physical activity during the previous 7 days (33). The IPAQ-SF was administered up to three times during pregnancy and only responses from the first administration were included in analyses [median (SD) gestational age, 10 (5.5) weeks, 80% within 1st trimester]. Ten women (0.8%) completed the IPAQ after delivering their baby and were instructed to recall their physical activity during their first trimester. We dichotomized women's responses according to meeting the minimal recommended amount of weekly physical activity for pregnant women of at least 150 min of moderate-intensity aerobic activities, including walking (yes or no) (34). We calculated average sleep duration using the difference between usual sleep and wake times based on responses to the following questions, “In the 3 months before you became pregnant, what time did you go to sleep on an average weekday?” and “In the 3 months before you became pregnant, what time did you go to sleep on an average weekday?”. We categorized sleep duration as <7 h, 7 to <9 h, and ≥9 h based on cut-points from previous literature (35). Sleep quality was assessed using the following question, “In the 3 months before you became pregnant, how would you rate the quality of your sleep overall?”, with response choices of very good, fairly good, fairly bad, and very bad. Lastly, we assessed perceived social support using the 5-item ENRICHD Social Support Index (ESSI): “Is there someone available to you whom you can count on to listen to you when you need to talk?”, “Is there someone available to give you good advice about a problem?”, “Is there someone available to you who shows you love and affection?”, “Can you count on anyone to provide you with emotional support (talking over problems or helping you make a difficult decision)?”, and “Do you have as much contact as you would like with someone you feel close to, someone in whom you can trust and confide?” (36, 37). Responses were summed using a point system: 1, none of the time; 2, a little of the time; 3, some of the time; 4, most of the time; and 5, all of the time. Low social support (yes or no) was defined as a response of 3 or lower on at least two of the five items or a total score of 18 or lower (38).
Statistical Analysis
Data analyses were performed using STATA 15.0 (StataCorp LLC, College Station, TX). Bivariate analyses estimated differences in mean total and component HEI-2015 scores using independent t-tests (or F-tests from one-way analysis of variance). We examined differences in total HEI-2015 by timing of DHQ-II completion: 1st/2nd trimester, 3rd trimester, or peri-/post-partum (Supplementary Table 1). Missing data were observed for several maternal characteristics: race/ethnicity (n = 2), education (n = 12), household income (n = 309), insurance (n = 9), employment status (n = 6), pre-pregnancy BMI (n = 10), pre-existing diabetes (n = 174), pre-existing hypertension (n = 174), depressive symptoms (n = 21), sleep duration (n = 172), sleep quality (n = 179), vitamin use before pregnancy (n = 3), vitamin use during pregnancy (n = 3), and social support (n = 226). Missing data were assumed to be missing at random and were imputed using chained equations (mi impute chained command in Stata). We included all maternal characteristics and total HEI scores in the imputation model. During the imputation procedures, pre-pregnancy BMI and depressive symptoms were treated as continuous variables (using predictive mean matching); education and household income were treated as ordinal variables; race/ethnicity, sleep duration, and sleep quality were treated as nominal variables; and employment status, insurance, pre-existing diabetes, pre-existing hypertension, vitamin use, and social support were treated as binary variables. We created 20 imputed data sets. Unadjusted and multivariable linear regression analyses estimated mean differences in total HEI-2015 scores for the maternal characteristics using the imputed data sets (n = 1,325). All variables were included in multivariable analyses (Adjusted Model). Regression analyses were repeated using the complete cases for comparison (n = 777).
We compared distributions of the selected characteristics of women who were included in analyses (n = 1,325) to those who were excluded because of missing or implausible diet data (n = 675, Supplementary Table 2). We conducted sensitivity analyses to examine the potential impact for selection bias by estimating inverse probability weights and applying them to regression analyses. We used probit regression with the selected maternal characteristics (Supplementary Table 2; age, race/ethnicity, education, income, marital status, parity, insurance, employment, alcohol use, depressive symptoms, sleep quality, and vitamin use before and during pregnancy) and the resulting predicted probabilities were inverted to determine the final weight (mean 2.00, SD = 1.39). Results from linear regression models with and without weighting did not substantively differ in magnitude or statistical significance. Presented models are unweighted and use multiple imputation.
Results
Mean (SD) usual energy intake was 1,686 (834) calories, with 51% (10%) of calories derived from carbohydrates, 34% (8%) from fats, and 15% (3%) from proteins. Mean total HEI-2015 score was 74.9 (SD = 8.5, Range = 37.4–94.8); 28, 46, 20, and 6% of women had scores that fell into the grade range of A/B, C, D, and F, respectively (Table 1). Mean HEI-2015 component scores were high for the fruit and whole grains components and low for protein-related, dairy, sodium, added sugars, and fat-related components. Approximately a third of women or fewer met the maximum HEI-2015 component score for total protein foods (37%), dairy (19%), fatty acid ratio (12%), sodium (7%), added sugars (31%), or saturated fats (16%). Mean dietary intakes were above pregnancy-specific recommendations for vitamin C, riboflavin, B12, and selenium but below recommendations for iron, vitamin D, and DHA, with 5% or fewer of women meeting recommended intakes for these nutrients (Table 1).
Differences were observed for most of the maternal characteristics between women who were included and excluded from analyses (p < 0.05, Supplementary Table 2). Among women included in analyses (n = 1,325), more than 80% self-reported as Hispanic (45%) or non-Hispanic White (38%) (Table 2). The majority of women were 30 years or older (67%), under/normal weight (50%), married (90%), currently employed (68%), at least college educated (61%), and nulliparous (52%). Only 11% met physical activity guidelines during pregnancy of 150 min per week. Twelve percent of women reported moderate/severe depressive symptoms during pregnancy and 9% reported sleeping <7 h per night prior to pregnancy. Approximately half of women (51%) reported taking a prenatal vitamin in the month prior to pregnancy, while 87% reported taking a vitamin during pregnancy (Table 2).

Table 2. Unadjusted and multivariable linear regression analyses estimating associations of selected maternal characteristics and total Healthy Eating Index-2015 scores among pregnant women in the New York University Children's Health and Environment Study.
Total HEI-2015 scores varied by sociodemographic characteristics (Table 2, Supplementary Table 3). Younger maternal age (<30 years), single status, pre-pregnancy obesity, any smoking, pre-existing hypertension, reporting moderate to severe depressive symptoms, not meeting physical activity guidelines, self-reported fairly/very bad sleep quality, and not taking a vitamin before pregnancy were associated with lower mean total HEI-2015 scores in unadjusted models. There were no differences in total HEI-2015 and component scores by timing of DHQ-II completion (1st/2nd trimester, 3rd trimester, and peri-/ post-partum), with the exception that women who completed the DHQ-II during pregnancy had slightly higher mean scores for total fruits and sodium, but lower mean scores for total protein foods and saturated fats (Supplementary Table 1).
In multivariable linear regression models (Table 2), Hispanic ethnicity was associated with an ~2-point higher total HEI-2015 score compared to non-Hispanic White (1.65, 95% CI: 0.21, 3.10). Parity, pre-pregnancy obesity, moderate to severe depressive symptoms during pregnancy, not meeting physical activity guidelines, ever smoking, and not using vitamins before pregnancy were each associated with an ~1.5–2.5-point lower total HEI-2015 score. Younger age (<30 years), single status, and pre-existing hypertension were each associated an ~3–5-point lower total HEI-2015 score (Table 2). Results from the regression models restricted to women with complete data were generally of similar direction and magnitude to those using the imputed data.
Discussion
In this large, diverse, urban cohort of pregnant women, usual dietary intakes within the previous year did not adhere to the most recent recommendations in the Dietary Guidelines for Americans 2015–2020. The average total HEI-2015 score was 74.9, corresponding to a C grade. Although the majority of women achieved high scores for intakes of fruits, vegetables, seafood/plant proteins, and whole grains, the suboptimal total dietary score was reflective of lower scores on components related to dairy, fats, sodium, refined grains, and added sugars. Dietary quality varied by several maternal characteristics. In adjusted models, maternal age <30 years, parity, single status, pre-pregnancy obesity, pre-existing hypertension, ever smoking, moderate/severe depressive symptoms, not meeting physical activity guidelines, and not taking a vitamin prior to pregnancy were associated with lower diet quality scores, with differences ranging from 1.5 to 5 points.
Disparities in dietary quality (assessed using various dietary quality indices) by sociodemographic and lifestyle characteristics are commonly reported among pregnant populations (2, 10–15, 17–19, 39, 40). Consistent with findings from the current study, dietary quality is often lower among women who are low-income, less-educated, or smokers (10–15, 39), while dietary quality is higher among women who are older or nulliparous (13, 15). Maternal obesity is also associated with poor diet quality (12, 14, 17, 18, 39, 40) and there is some evidence that diet quality among women with obesity may worsen throughout pregnancy and the post-partum period (40). In the current study, women with pre-pregnancy obesity had lower total HEI-2015 scores. This is of concern because maternal obesity is an independent risk factor for micronutrient deficiencies (e.g., iron and folate), as well as pregnancy complications and adverse health outcomes, such as gestational diabetes, birth defects, macrosomia, and childhood obesity (8, 9, 41). Nutritional counseling may help to at least partially alleviate many of these outcomes by improving women's dietary intakes prior to and during pregnancy (42). Additionally, we found that pre-existing hypertension, but not diabetes, was associated with lower HEI-2015 scores. Lower dietary quality is often linked with increased risk of these conditions (43–45). Women with diabetes prior to pregnancy may be more likely to modify their diets as part of a treatment program, compared to women with hypertension, who may be more reliant on blood pressure lowering medications to control their condition.
Evidence supporting maternal race/ethnicity as an independent predictor of diet quality is inconsistent among non-White race/ethnic groups (10, 12, 13, 15, 17, 39), but studies often lack adequately diverse populations needed to make comparisons (11, 14, 18). Among a large, multi-site pregnancy cohort, Bodnar et.al. found that non-Hispanic Black and Hispanic women had lower peri-conceptional HEI-2010 scores than non-Hispanic White women, which was attributed to greater consumption of sugar-sweetened beverages and lower consumption of nutrient-dense foods, such as fruits, vegetables, whole grains, and dairy products (10). Other studies, among participants of a large birth cohort in North Carolina (using the Diet Quality Index for Pregnancy) and among participants of the National Health and Nutrition Examination Surveys (2003–2012, using the HEI-2010), reported similar or better diet quality among non-White women compared to White women (15, 17, 39). In the current study, mean total HEI-2015 score was nearly 2 points higher among Hispanic women compared to non-Hispanic White women in adjusted models, with no differences observed for non-Hispanic Black, Asian, or other race/ethnic groups. Discrepancies in findings between studies likely highlight underlying differences in study populations related to maternal social, behavioral, environmental, or cultural characteristics (e.g., urban vs. rural settings or level of acculturation), which are strongly associated with race/ethnicity and not always captured in statistical analyses (13).
In addition to smoking, we identified two other health behaviors that were associated with lower diet quality scores: not meeting minimum physical activity recommendations and not taking a multivitamin or supplement before pregnancy. Only 11% of women met the minimum recommendations for physical activity during pregnancy of 150 min per week, equivalent to brisk walking for 30 min on most days of the week. A growing body of evidence demonstrates that exercise during pregnancy is safe and provides numerous health benefits for both mother and baby, including lower risks of pregnancy complications, poor birth outcomes, maternal weight retention, and post-partum depression (34). Exercise may encourage healthy eating and is positively related to nutrient intakes in pregnant (18) and non-pregnant populations (46), which is consistent with our findings in pregnant women and suggests that exercise is an important target for interventions. Similar to physical activity, multivitamin use before pregnancy may also serve as an indicator of women's overall health behaviors and be used to identify women who are at risk for inadequate nutrient stores and intakes during pregnancy (39). In our study, only half of women reported taking a vitamin or supplement before pregnancy. This is concerning given that mean dietary intakes of iron, folate, vitamin D, and DHA, which are critical for optimal fetal development, were below pregnancy-specific recommendations, with only one third of women meeting recommendations for folate (47). Recent national data show that multivitamin use remains low among women ages 18–44 years; ~38 and 21% of women who report trying to get pregnant and not trying to get pregnant, respectively, take a daily multivitamin (48). Contrary to studies in non-pregnant populations, sleep duration and sleep quality were not associated with diet quality in our study (35). In unadjusted models, women with short (<7 h) and long (≥9 h) weekday sleep durations had lower HEI-2015 scores, but these associations were attenuated in multivariable models. Few studies have examined this association among women around the time of pregnancy; however, studies among pregnant women (49, 50) and mothers (51) suggest a relationship between poor sleep characteristics and unhealthy dietary intakes. Pre-conceptional messaging from health care providers and public health practitioners regarding these modifiable health behaviors is necessary to encourage women to improve their nutritional and overall health status, especially among those who are considering pregnancy.
We also found that reporting moderate to severe depressive symptoms during pregnancy was associated with lower dietary quality. Our findings for depressive symptoms are consistent with previous, though limited, literature suggesting that maternal mental health, including depression and anxiety, is associated with unhealthy dietary intakes (using various dietary assessments) (52, 53). This is of concern because both poor maternal mental health and poor dietary intakes increase the risk of adverse birth outcomes, such as preterm birth and low birth weight (54, 55), and later childhood health outcomes (56, 57). In non-pregnant populations, consumption of healthy foods with low dietary glycemic index/load, particularly those containing omega-3 fatty acids (e.g., DHA) and antioxidants, may prevent or lessen depressive symptoms (58, 59). Although evidence during pregnancy is limited, women with a history of depression or reporting depressive symptoms should be a priority for prenatal nutrition counseling given the benefits for mother and baby (60). Lastly, we did not observe an association between social support (which excluded spousal/partner support) and diet quality. Results from previous studies of pregnant women are inconsistent (18, 61, 62) but there is notable variability in their assessment methods, statistical analyses, and study population characteristics, which may account for discrepant findings. Social support is likely inter-related with socioeconomic and cultural factors. More research is needed to understand how social support may influence diet and other health behaviors among high-risk groups of women, such as those who are single or living in low resource settings.
Main strengths of this study include the large, urban population of pregnant women that was heterogeneous across a range of maternal sociodemographic and health behavior characteristics, as well as the use of the most recent dietary recommendations. Limitations of this study include our dietary assessment method. The DHQ-II assessed dietary intakes for the previous year, capturing usual intakes prior to and during pregnancy for the majority of women, which precluded our ability to distinguish specific timeframes for women's dietary intakes. It is not clear how dietary assessments completed during pregnancy may influence the recall of intakes during a time period that spans both preconception and pregnancy; to our knowledge, there are no studies that examine the validity of a 12-month dietary assessment tool self-administered during pregnancy. There is little evidence that women greatly alter or improve their dietary intakes during pregnancy (63–66), with the exception of increases in fruit intakes (64, 66). We compared diet quality scores by timing of DHQ-II completion and observed no differences in total HEI-2015 score and the majority of component scores among women who completed the DHQ-II during the peri-/post-partum period compared to those who completed it during pregnancy. Demographic characteristics of women with live deliveries enrolled in NYU CHES were very similar to those of pregnant women in New York City (21); however, we did observe differences in characteristics of women who did and did not complete the DHQ-II. Although there were no substantive differences in our results after weighting them, we cannot exclude the possibility that our results may be biased and have limited generalizability. Several of the maternal characteristics, including younger age and single status, were associated with lower diet quality, potentially underestimating differences in dietary quality based on these variables. It should also be noted that the IPAQ-SF only queried physical activities during the previous week and has limited validity compared to objective measures of physical activity, particularly among pregnant women (67). We dichotomized reported weekly duration of physical activities according to current recommendations, but misclassification is still possible, which would underestimate our results.
Conclusion
Consistent with previous literature, evidence from the current study suggests that sub-optimal dietary quality is common among women prior to and during pregnancy and that several maternal characteristics, such as young maternal age, parity, smoking, not taking a vitamin before pregnancy, and reporting depressive symptoms, may be associated with lower diet quality scores. Pregnancy (and lactation) substantially increase nutritional demands to an extent that may compromise even women who enter pregnancy well-nourished. This is of clinical and public health concern because poor nutrient intakes during pregnancy are associated with a range of adverse health outcomes in both the mother and her child. Diet represents a modifiable behavior and continued emphasis should be placed on the provision of pre-conceptional and prenatal nutritional counseling, particularly among high-risk women who are in need of dietary intervention.
Data Availability Statement
The datasets generated for this article are not readily available because data may be shared pursuant to ECHO program regulations. Requests to access the datasets should be directed to LT, bGVvbmFyZG8udHJhc2FuZGVAbnl1bGFuZ29uZS5vcmc=.
Ethics Statement
The studies involving human participants were reviewed and approved by institutional review board of the NYU School of Medicine (i15-00778). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AD conducted the secondary analyses and wrote the first draft of the manuscript. AG, LK, YA, SM-L, SB, and LT contributed to the design of the study and acquisition of the data. All authors contributed revisions to the manuscript and approved the final manuscript for submission.
Funding
NYU CHES was supported by institutional funds of NYU Grossman School of Medicine as well as the NIH Office of the Director (UG3/UH3OD023305) and NIH K99ES030403.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.639425/full#supplementary-material
References
1. Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. (2008) 359:61–73. doi: 10.1056/NEJMra0708473
2. Martin JC, Zhou SJ, Flynn AC, Malek L, Greco R, Moran L. The assessment of diet quality and its effects on health outcomes pre-pregnancy and during pregnancy. Semin Reprod Med. (2016) 34:83–92. doi: 10.1055/s-0036-1571353
3. Pham NM, Van Do V, Lee AH. Polyphenol-rich foods and risk of gestational diabetes: a systematic review and meta-analysis. Eur J Clin Nutr. (2019) 73:647–56. doi: 10.1038/s41430-018-0218-7
4. Mijatovic-Vukas J, Capling L, Cheng S, Stamatakis E, Louie J, Cheung N, et al. Associations of diet and physical activity with risk for gestational diabetes mellitus: a systematic review and meta-analysis. Nutrients. (2018) 10:698. doi: 10.3390/nu10060698
5. Yamamoto JM, Kellett JE, Balsells M, García-Patterson A, Hadar E, Solà I, et al. Gestational diabetes mellitus and diet: a systematic review and meta-analysis of randomized controlled trials examining the impact of modified dietary interventions on maternal glucose control and neonatal birth weight. Diabetes Care. (2018) 41:1346–61. doi: 10.2337/dc18-0102
6. Schoenaker DA, Soedamah-Muthu SS, Callaway LK, Mishra GD. Prepregnancy dietary patterns and risk of developing hypertensive disorders of pregnancy: results from the Australian longitudinal study on women's health. Am J Clin Nutr. (2015) 102:94–101. doi: 10.3945/ajcn.114.102475
7. Gur EB, Gokduman A, Turan GA, Tatar S, Hepyilmaz I, Zengin EB, et al. Mid-pregnancy vitamin D levels and postpartum depression. Eur J Obstet Gynecol Reprod Biol. (2014) 179:110–6. doi: 10.1016/j.ejogrb.2014.05.017
8. Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VW, Eriksson JG, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. (2017) 5:53–64. doi: 10.1016/S2213-8587(16)30107-3
9. Catalano Pa, Ehrenberg H. The short-and long-term implications of maternal obesity on the mother and her offspring. BJOG. (2006) 113:1126–33. doi: 10.1111/j.1471-0528.2006.00989.x
10. Bodnar LM, Simhan HN, Parker CB, Meier H, Mercer BM, Grobman WA, et al. Racial or ethnic and socioeconomic inequalities in adherence to national dietary guidance in a large cohort of US pregnant women. J Acad Nutr Diet. (2017) 117:867–77.e3. doi: 10.1016/j.jand.2017.01.016
11. Thomson JL, Tussing-Humphreys LM, Goodman MH, Olender S. Baseline demographic, anthropometric, psychosocial, and behavioral characteristics of rural, Southern women in early pregnancy. Matern Child Health J. (2016) 20:1980–8. doi: 10.1007/s10995-016-2016-y
12. Shapiro AL, Kaar JL, Crume TL, Starling AP, Siega-Riz AM, Ringham BM, et al. Maternal diet quality in pregnancy and neonatal adiposity: the healthy start study. Int J Obes. (2016) 40:1056–62. doi: 10.1038/ijo.2016.79
13. Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Oken E, Gillman MW. Dietary quality during pregnancy varies by maternal characteristics in project viva: a US cohort. J Am Diet Assoc. (2009) 109:1004–11. doi: 10.1016/j.jada.2009.03.001
14. Emond JA, Karagas MR, Baker ER, Gilbert-Diamond D. Better diet quality during pregnancy is associated with a reduced likelihood of an infant born small for gestational age: an analysis of the prospective new hampshire birth cohort study. J Nutr. (2018) 148:22–30. doi: 10.1093/jn/nxx005
15. Bodnar LM, Siega-Riz AM. A diet quality index for pregnancy detects variation in diet and differences by sociodemographic factors. Pub Health Nutr. (2002) 5:801–9. doi: 10.1079/PHN2002348
16. Pinto E, Barros H, dos Santos Silva I. Dietary intake and nutritional adequacy prior to conception and during pregnancy: a follow-up study in the north of Portugal. Pub Health Nutr. (2009) 12:922–31. doi: 10.1017/S1368980008003595
17. Shin D, Lee K, Song W. Pre-pregnancy weight status is associated with diet quality and nutritional biomarkers during pregnancy. Nutrients. (2016) 8:162. doi: 10.3390/nu8030162
18. Nash DM, Gilliland JA, Evers SE, Wilk P, Campbell MK. Determinants of diet quality in pregnancy: sociodemographic, pregnancy-specific, and food environment influences. J Nutr Educ Behav. (2013) 45:627–34. doi: 10.1016/j.jneb.2013.04.268
19. Doyle I-M, Borrmann B, Grosser A, Razum O, Spallek J. Determinants of dietary patterns and diet quality during pregnancy: A systematic review with narrative synthesis. Pub Health Nutr. (2017) 20:1009–28. doi: 10.1017/S1368980016002937
20. Department of Health Human Services U.S. Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th ed. (2015). Available online at: http://health.gov/dietaryguidelines/2015/guidelines/ (accessed December 15, 2020).
21. Trasande L, Ghassabian A, Kahn LG, Jacobson MH, Afanasyeva Y, Liu M, et al. The NYU children's health and environment study. Eur J Epidemiol. (2020) 35:305–20. doi: 10.1007/s10654-020-00623-6
22. Radesky JS, Oken E, Rifas-Shiman SL, Kleinman KP, Rich-Edwards JW, Gillman MW. Diet during early pregnancy and development of gestational diabetes. Paediatr Perinat Epidemiol. (2008) 22:47–59. doi: 10.1111/j.1365-3016.2007.00899.x
23. Lamb MM, Myers MA, Barriga K, Zimmet PZ, Rewers M, Norris JM. Maternal diet during pregnancy and islet autoimmunity in offspring. Pediatr Diabetes. (2008) 9:135–41. doi: 10.1111/j.1399-5448.2007.00311.x
24. Willett W. Nutritional Epidemiology. Oxford: Oxford University Press (2012). doi: 10.1093/acprof:oso/9780199754038.001.0001
25. Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, et al. Update of the healthy eating index: HEI-2015. J Acad Nutr Diet. (2018) 118:1591–602. doi: 10.1016/j.jand.2018.05.021
26. National Institutes of Health National Cancer Institute. Diet History Questionnaire Version 2.0. Availabe online at: https://epi.grants.cancer.gov/dhq2/about/validation.html (accessed December 15, 2020).
27. Reedy J, Lerman JL, Krebs-Smith SM, Kirkpatrick SI, Pannucci TE, Wilson MM, et al. Evaluation of the healthy eating index-2015. J Acad Nutr Diet. (2018) 118:1622–33. doi: 10.1016/j.jand.2018.05.019
28. National Cancer Institute Diet History Questionnaire II: Calculating Healthy Eating Index (HEI) Scores Using Diet*Calc Output. Availabe online at: https://epi.grants.cancer.gov/dhq2/dietcalc/output.html (accessed December 15, 2020).
29. Tahir MJ, Haapala JL, Foster LP, Duncan KM, Teague AM, Kharbanda EO, et al. Higher maternal diet quality during pregnancy and lactation is associated with lower infant weight-for-length, body fat percent, and fat mass in early postnatal life. Nutrients. (2019) 11:632. doi: 10.3390/nu11030632
30. Panizza C, Shvetsov Y, Harmon B, Wilkens L, Le Marchand L, Haiman C, et al. Testing the predictive validity of the healthy eating index-2015 in the multiethnic cohort: is the score associated with a reduced risk of all-cause and cause-specific mortality? Nutrients. (2018) 10:452. doi: 10.3390/nu10040452
31. Kominiarek MA, Rajan P. Nutrition recommendations in pregnancy and lactation. Med Clin North Am. (2016) 100:1199–215. doi: 10.1016/j.mcna.2016.06.004
32. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J General Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
33. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. (2003) 35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB
34. Birsner ML, Gyamfi-Bannerman C. Physical activity and exercise during pregnancy and the postpartum period ACOG committee opinion summary, number 804. Obstet Gynecol. (2020) 135:E178–88. doi: 10.1097/AOG.0000000000003772
35. Chaput JP. Sleep patterns, diet quality and energy balance. Physiol Behav. (2014) 134:86–91. doi: 10.1016/j.physbeh.2013.09.006
36. Mitchell PH, Powell L, Blumenthal J, Norten J, Ironson G, Pitula CR, et al. A short social support measure for patients recovering from myocardial infarction: the ENRICHD social support inventory. J Cardiopulm Rehabil. (2003) 23:398–403. doi: 10.1097/00008483-200311000-00001
37. Vaglio J Jr, Conard M, Poston WS, O'Keefe J, Haddock CK, House J, et al. Testing the performance of the ENRICHD Social Support Instrument in cardiac patients. Health Qual Life Outcomes. (2004) 2:24. doi: 10.1186/1477-7525-2-24
38. Gan Y, Xiong R, Song J, Xiong X, Yu F, Gao W, et al. The effect of perceived social support during early pregnancy on depressive symptoms at 6 weeks postpartum: a prospective study. BMC Psychiatry. (2019) 19:232. doi: 10.1186/s12888-019-2188-2
39. Laraia BA, Bodnar LM, Siega-Riz AM. Pregravid body mass index is negatively associated with diet quality during pregnancy. Pub Health Nutr. (2007) 10:920–6. doi: 10.1017/S1368980007657991
40. Moran L, Sui Z, Cramp C, Dodd J. A decrease in diet quality occurs during pregnancy in overweight and obese women which is maintained post-partum. Int J Obes. (2013) 37:704–11. doi: 10.1038/ijo.2012.129
41. Sen S, Iyer C, Meydani S. Obesity during pregnancy alters maternal oxidant balance and micronutrient status. J Perinatol. (2014) 34:105–11. doi: 10.1038/jp.2013.153
42. Farpour-Lambert NJ, Ells LJ, Martinez de Tejada B, Scott C. Obesity and weight gain in pregnancy and postpartum: an evidence review of lifestyle interventions to inform maternal and child health policies. Front Endocrinol. (2018) 9:546. doi: 10.3389/fendo.2018.00546
43. Schwingshackl L, Hoffmann G. Diet quality as assessed by the healthy eating index, the alternate healthy eating index, the dietary approaches to stop hypertension score, and health outcomes: a systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. (2015) 115:780–800.e5. doi: 10.1016/j.jand.2014.12.009
44. Fanelli SM, Jonnalagadda SS, Pisegna JL, Kelly OJ, Krok-Schoen JL, Taylor CA. Poorer diet quality observed among us adults with a greater number of clinical chronic disease risk factors. J Prim Care Community Health. (2020) 11:2150132720945898. doi: 10.1177/2150132720945898
45. Fung TT, McCullough M, van Dam RM, Hu FB. A prospective study of overall diet quality and risk of type 2 diabetes in women. Diabetes Care. (2007) 30:1753–7. doi: 10.2337/dc06-2581
46. Jayawardene WP, Torabi MR, Lohrmann DK. Exercise in young adulthood with simultaneous and future changes in fruit and vegetable intake. J Am Coll Nutr. (2016) 35:59–67. doi: 10.1080/07315724.2015.1022268
47. Christian P, Stewart CP. Maternal micronutrient deficiency, fetal development, and the risk of chronic disease. J Nutr. (2010) 140:437–45. doi: 10.3945/jn.109.116327
48. Wong EC, Rose CE, Flores AL, Yeung LF. Trends in multivitamin use among women of reproductive age: United States, 2006-2016. J Womens Health. (2019) 28:37–45. doi: 10.1089/jwh.2018.7075
49. van Lee L, Chia AR, Loy SL, Colega M, Tham EKH, Cai S, et al. Sleep and dietary patterns in pregnancy: findings from the GUSTO cohort. Int J Environ Res Public Health. (2017) 14:1409. doi: 10.3390/ijerph14111409
50. Chang MW, Brown R, Nitzke S, Smith B, Eghtedary K. Stress, sleep, depression and dietary intakes among low-income overweight and obese pregnant women. Matern Child Health J. (2015) 19:1047–59. doi: 10.1007/s10995-014-1604-y
51. Xiao RS, Moore Simas TA, Pagoto SL, Person SD, Rosal MC, Waring ME. Sleep duration and diet quality among women within 5 years of childbirth in the United States: a cross-sectional study. Matern Child Health J. (2016) 20:1869–77. doi: 10.1007/s10995-016-1991-3
52. Baskin R, Hill B, Jacka FN, O'Neil A, Skouteris H. The association between diet quality and mental health during the perinatal period. A systematic review. Appetite. (2015) 91:41–7. doi: 10.1016/j.appet.2015.03.017
53. Boutté AK, Turner-McGrievy GM, Wilcox S, Liu J, Eberth JM, Kaczynski AT. Associations of maternal stress and/or depressive symptoms with diet quality during pregnancy: a narrative review. Nutr Rev. (2020). doi: 10.1093/nutrit/nuaa019. [Epub ahead of print].
54. Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch General Psychiatry. (2010) 67:1012–24. doi: 10.1001/archgenpsychiatry.2010.111
55. Chia AR, Chen LW, Lai JS, Wong CH, Neelakantan N, van Dam RM, et al. Maternal dietary patterns and birth outcomes: a systematic review and meta-analysis. Adv Nutr. (2019) 10:685–95. doi: 10.1093/advances/nmy123
56. Borge TC, Aase H, Brantsæter AL, Biele G. The importance of maternal diet quality during pregnancy on cognitive and behavioural outcomes in children: a systematic review and meta-analysis. BMJ Open. (2017) 7:e016777. doi: 10.1136/bmjopen-2017-016777
57. Gentile S. Untreated depression during pregnancy: short- and long-term effects in offspring. A systematic review. Neuroscience. (2017) 342:154–66. doi: 10.1016/j.neuroscience.2015.09.001
58. Lassale C, Batty GD, Baghdadli A, Jacka F, Sánchez-Villegas A, Kivimäki M, et al. Healthy dietary indices and risk of depressive outcomes: a systematic review and meta-analysis of observational studies. Mol Psychiatry. (2018) 24:1094. doi: 10.1038/s41380-018-0299-7
59. Salari-Moghaddam A, Saneei P, Larijani B, Esmaillzadeh A. Glycemic index, glycemic load, and depression: a systematic review and meta-analysis. Eur J Clin Nutr. (2019) 73:356. doi: 10.1038/s41430-018-0258-z
60. Sparling TM, Henschke N, Nesbitt RC, Gabrysch S. The role of diet and nutritional supplementation in perinatal depression: a systematic review. Matern Child Nutr. (2017) 13:e12235. doi: 10.1111/mcn.12235
61. Fowles ER, Stang J, Bryant M, Kim S. Stress, depression, social support, and eating habits reduce diet quality in the first trimester in low-income women: a pilot study. J Acad Nutr Diet. (2012) 112:1619–25. doi: 10.1016/j.jand.2012.07.002
62. Berube LT, Messito MJ, Woolf K, Deierlein A, Gross R. Correlates of prenatal diet quality in low-income Hispanic women. J Acad Nutr Diet. (2019) 119:1284–95. doi: 10.1016/j.jand.2019.02.004
63. Crozier SR, Robinson SM, Borland SE, Godfrey KM, Cooper C, Inskip HM, et al. Do women change their health behaviours in pregnancy? Findings from the Southampton women's survey. Paediatr Perinat Epidemiol. (2009) 23:446–53. doi: 10.1111/j.1365-3016.2009.01036.x
64. Crozier SR, Robinson SM, Godfrey KM, Cooper C, Inskip HM. Women's dietary patterns change little from before to during pregnancy. J Nutr. (2009) 139:1956–63. doi: 10.3945/jn.109.109579
65. Rifas-Shiman SL, Rich-Edwards JW, Willett WC, Kleinman KP, Oken E, Gillman MW. Changes in dietary intake from the first to the second trimester of pregnancy. Paediatr Perinat Epidemiol. (2006) 20:35–42. doi: 10.1111/j.1365-3016.2006.00691.x
66. Skreden M, Bere E, Sagedal LR, Vistad I, Øverby NC. Changes in fruit and vegetable consumption habits from pre-pregnancy to early pregnancy among Norwegian women. BMC Preg Childbirth. (2017) 17:107. doi: 10.1186/s12884-017-1291-y
Keywords: diet, pregnancy, healthy eating index, health behavior, sociodemographic characteristics
Citation: Deierlein AL, Ghassabian A, Kahn LG, Afanasyeva Y, Mehta-Lee SS, Brubaker SG and Trasande L (2021) Dietary Quality and Sociodemographic and Health Behavior Characteristics Among Pregnant Women Participating in the New York University Children's Health and Environment Study. Front. Nutr. 8:639425. doi: 10.3389/fnut.2021.639425
Received: 08 December 2020; Accepted: 11 March 2021;
Published: 09 April 2021.
Edited by:
Ikuko Kashino, National Center for Global Health and Medicine, JapanReviewed by:
Rosa Casas Rodriguez, Institut de Recerca Biomèdica August Pi i Sunyer (IDIBAPS), SpainKeisuke Kuwahara, Teikyo University, Japan
Copyright © 2021 Deierlein, Ghassabian, Kahn, Afanasyeva, Mehta-Lee, Brubaker and Trasande. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea L. Deierlein, YWxkOEBueXUuZWR1
 Andrea L. Deierlein
Andrea L. Deierlein Akhgar Ghassabian2,3,4
Akhgar Ghassabian2,3,4