
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 15 February 2021
Sec. Nutrition, Psychology and Brain Health
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.614149
 Roni Lotan1,2*
Roni Lotan1,2* Ithamar Ganmore2,3,4,5
Ithamar Ganmore2,3,4,5 Abigail Livny2,5,6
Abigail Livny2,5,6 Shahar Shelly2,7
Shahar Shelly2,7 Moran Zacharia2
Moran Zacharia2 Jaime Uribarri8
Jaime Uribarri8 Paul Beisswenger9
Paul Beisswenger9 Weijing Cai8
Weijing Cai8 Michal Schnaider Beeri2,8
Michal Schnaider Beeri2,8 Aron M. Troen1
Aron M. Troen1Introduction: Advanced glycation end products (AGEs) in diet and serum are positively correlated with chronic conditions such as type 2 diabetes and cognitive decline. Dietary reduction of AGEs was shown to reduce their level in serum and to have a beneficial effect on metabolic biomarkers. However, in part due to limitations of feasibility, clinical trials have not tested its effect on cognition in elderly. The current pilot study examines the feasibility of AGE reduction in elderly with diabetes in terms of recruitment and retention.
Methods: The design is a randomized controlled pilot trial of dietary AGEs in elderly with type 2 diabetes (clinicaltrials.gov NCT02739971). Recruitment followed two stages: we first recruited participants with mild cognitive impairment (MCI), and after expanding inclusion criteria, we later recruited cognitively normal participants with subjective memory complaints (SMCs). Participants were randomized to two arms. Participants in the control arm received standard of care (SOC) guidelines for good glycemic control; those in the experimental arm, in addition to SOC guidelines, were instructed to lower their dietary AGE intake, primarily by changing their cooking methods. Participants were closely followed for dietary adherence over 6 months and evaluated before and after the intervention for adherence to the assigned diet, blood tests, cognitive performance, and brain MRI.
Results: Seventy-five participants (52 with MCI and 23 cognitively normal with SMCs) were recruited primarily through mass mailing and advertising in social media websites. Seventy participants finished the study, and dropout was similar in both groups (7.5% in control vs. 5.7% in intervention, p = 0.757). The majority (57.5%) of participants in the AGEs-lowering arm showed very high adherence with the dietary guidelines.
Discussion: Targeting feasible lifestyle modifications in high-risk populations could prevent substantial cases of cognitive decline. Observational evidence supports that AGEs may contribute to cognitive decline; however, the cognitive effect of reducing AGEs exposure has yet to be evaluated in a randomized controlled trial (RCT). The results of our pilot trial delineate a methodology including effective recruitment strategies, population of choice, and ways to assure high adherence during lifestyle modifications, and significantly advance progress toward a definitive and well-powered future RCT.
The elderly are the fastest-growing segment of the Western population (1), and cognitive decline affects increasing numbers among them. Dementia, the clinical manifestation of severe progressive brain disease and cognitive decline, is projected to triple by 2050 and affect above 115 million people worldwide (2). Although Alzheimer's disease (AD) is the most common cause of dementia, vascular pathology, inflammation, and oxidative stress are prominent features of almost all types of dementia (3).
The failure of numerous clinical trials with candidate drugs to treat dementia has refocused attention on the potential of lifestyle interventions to delay or prevent the disease, including improvement of physical activity and diet, and management of risk factors for chronic cardiometabolic diseases like type 2 diabetes (T2D) (3). Given the consistent association of T2D with increased risk for dementia and AD (4, 5), vascular dementia (6), mild cognitive impairment (MCI) (7), and rapid cognitive decline (8), cognitive impairment and dementia are increasingly considered to be a serious complication of T2D in older adults (9).
Increased exposure to circulating advanced glycation end products (AGEs) in both T2D and AD offers a mechanism to explain these associations and suggests that lifestyle intervention could lower risk through reducing intake of AGEs (10). AGEs is a class of chemical compounds generated endogenously in the course of normal metabolism by the non-enzymatic reaction between reducing sugars or dicarbonyls and free amino groups of amino acids such as lysine and arginine. AGEs tend to accumulate with aging (11) and under conditions where the precursors for the reaction, such as glucose, are in excess. Thus, elderly individuals with T2D tend to have higher levels of circulating AGEs (12, 13). In addition to the endogenous creation of AGEs, exogenous sources such as pre-formed AGEs from food, particularly from animal-derived foods, also contribute significantly to the body's pool of AGEs. Cooking and exposure to high temperatures (i.e., grilling, broiling, roasting, searing, and frying) and long cooking times can greatly increase the amount of AGEs in food (14, 15).
Elevated circulating AGEs are correlated with a wide range of chronic diseases includingT2D and its complications (11, 16). Several clinical trials have demonstrated that by changing their cooking methods, people can reduce their circulating AGE levels and improve glucose metabolism, insulin resistance, and biomarkers of inflammation and oxidative stress (17–19). Growing evidence also suggests an association of AGEs with neurodegenerative disorders including AD (20). This association was shown in observational studies in humans (21) and in animal models (22). In older adults, higher levels of dietary or serum AGEs are associated with faster cognitive decline over time (21, 23). Evidence from animal studies showing that mice fed high-AGEs diet had lower performance scores on cognitive tests and higher hippocampal levels of insoluble amyloid also support a direct involvement of AGEs in the brain (24). Small post-mortem studies of AD brains found AGEs in neurons, astrocytes, and glial cells (25–28) as well as colocalization with the pathological hallmarks of AD, namely, amyloid plaques and neurofibrillary tangles (27). Recently, in humans, serum AGEs levels have been shown to be highly correlated with brain levels of AGEs (29). In addition to direct effects of AGEs on the brain and blood–brain barrier (30, 31), AGEs may also drive systemic inflammation and oxidative stress that play an important role in AD.
Given this biological plausibility and the results of clinical trials that have demonstrated that AGEs in serum are sensitive to dietary AGEs reduction, AGEs are likely a modifiable risk factor for cognitive decline.
We recently conducted a pilot randomized controlled trial (RCT) to develop methodology and evaluate the feasibility of intervening to lower dietary AGEs and of tracking the effect on cognitive performance and brain-related outcomes in older adults with T2D. The biological response to reducing dietary AGEs intake on circulating AGEs and other metabolic biomarkers is discussed in detail elsewhere (32).
Here, we focus on a detailed description and analysis of the study methods, including the dietary intervention, study participant selection, recruitment, and adherence strategies, so that these may be applied to future trials.
The study was designed according to the CONSORT extension criteria for pilot trials (33) and was approved by the Helsinki Committee of the Sheba Medical Center, Israel. All participants signed informed consent. The study was registered with www.clinicaltrials.gov (NCT02739971).
This pilot initially aimed to study elderly participants with T2D and with MCI (stage 1). As the rate of recruitment was lower than expected, we included participants with subjective memory complaints (SMCs) who did not meet the criteria for MCI according to our screening tools, and they were classified with normal cognitive performance (stage 2). Recruitment strategies included:
1) Mass mailing to a list of 30,000 people who asked to be informed on health issues through the company “Infinity”;
2) Publication of the study in websites and forums for clinical trials, dementia, and diabetes;
3) Lectures at nursing homes on brain health;
4) Referral of participants with T2D from ongoing observational studies/screen failure from other RCTs at the Joseph Sagol Neuroscience Center at Sheba Medical Center;
5) Presenting the study to the health care staff at a diabetes clinic in “Maccabi,” a health medical organization with a request that they refer suitable candidates;
6) Publication of the study in the local newspapers, in the area of the hospital, with distribution of 30,000 units.
Interested people left their contact details through a voice machine or on a designated electronic landing page where they could answer questions related to inclusion criteria such as age and diagnosis of T2D.
Suitable candidates were contacted by phone to ascertain that eligibility criteria were met [e.g., age, diabetic subject, not suffering from dementia, have informant (a family member with a constant relationship with the candidate), not vegetarian (as vegetarians eat very low amounts of AGEs), and have SMCs].
Inclusion criteria for type 2 diabetic adults aged 65 years and older are summarized in Table 1. Eligible participants were asked to bring medical records to the screening meeting. Participants whose records indicated T2D, those taking medications for T2D, and those whose last two blood tests for fasting glucose were above 126 were considered as having T2D. Additional screening tools were used to ascertain cognition and dietary AGEs intake. The following screening tools were used:
• Clinical Dementia Rating (35) scale score of <1 (CDR = 0.5 at the first stage of study recruitment and CDR = 0 after normal controls became eligible),
• Montreal Cognitive Assessment (MoCA) (36) score of >18 (score between 18 and 26 in the first stage of the study recruitment and above 18 after normal controls became eligible),
• Geriatric Depression Scale (37) score of <6, confirming lack of mild or major depression (same score in both stages).
To assess AGEs' consumption, we used a Hebrew version of a validated 7-day AGEs-specific food frequency questionnaire with food items rich in AGEs. The English version of the AGEs questionnaire is attached in Supplement A. The information on participants' eating habits included he frequency at which such foods as meat, fish, poultry, cheese, egg yolk, fats, fast foods, and convenience breakfast and snack foods were consumed in the last 7 days; the portion sizes; and methods of cooking (boiling, roasting, broiling/grilling, frying, or canned). Each item is assigned a numerical value, multiplied by the number of entries; the sum of entries per day provides an AGEs score. The AGEs score from this questionnaire has been significantly correlated with AGEs data derived from a 3-day record and with serum AGEs (38). The AGEs questionnaire was developed based on a database of ~560 foods that list AGEs values expressed as AGEs Equivalents (Eq/day), AGEs equivalent = 1,000 kilounits (kU) (15). Participants with score above 13 kU, the cutoff used in other clinical trials for AGEs reduction, were eligible (39).
Participants enrolled in the study were randomly assigned to one of two diets by a computerized random sequence generator. Participants were asked to maintain the instruction for the study for 6 months and to avoid any new diet or lifestyle program during this period. At baseline, participants from both groups were guided face to face, by a registered dietitian, to follow guidelines to maintain good glycemic control (40). The main focus of the guidelines was on intake of carbohydrates including information on serving sizes, counting, and quality. Recommendations were given for carbohydrate intake from vegetables, fruits, whole grains, legumes, and low-fat dairy products while reducing the consumption of refined sugars. In addition, participants were advised to prefer food items rich in mono- and polyunsaturated fatty acids compared to saturated and trans fatty acid and to consume lean meat, low sodium, and low processed food items. Other guidelines for good glycemic control were individualized and were based on the participants' nutritional and metabolic needs, habits, preferences, and willingness and ability to make behavioral changes. Those assigned to the AGEs-lowering group were guided, in addition, on how to reduce their AGEs in diet mainly by modifying the cooking methods and lowering cooking time and temperature, without changing the quantity or nutrient composition of their eating habits. They were advised to boil, poach, stew, or steam and to avoid frying, baking, or grilling of animal-derived products (15). The participants received examples for dishes with relatively low AGEs content based on their eating habits (e.g., a subject who reported eating fried meatballs was advised to put the meatballs in tomato sauce without frying them first, and a subject who reported eating roast chicken was advised to make chicken soup, etc.) (15). All participants received written guidelines. Informants who shared a house and cooking with the subject were asked to join the nutritional counseling. If they could not attend, the dietitian instructed them via phone.
Participants from both groups were monitored on a weekly basis by telephone conversation to ascertain and encourage adherence with their diet. In the AGEs-lowering group, feedback was specifically given to maintain low AGEs intake. Participants were asked whether and how many times they cooked with the cooking methods causing higher AGEs since the last call. If a participant reported consuming food items rich in AGEs, the instructions to lower AGEs was repeated to both the subject and the informant. Participants from both groups were asked general questions about their adherence to the good glycemic control guidelines (e.g., “did you manage to follow the guidelines for your diabetes?” “did you have any occasions in which you did not manage to eat according to the guidelines”). The telephone conversation content was summarized by the study dietitian including the date of the conversation and main issues raised. Each following conversation included general questions to all participants as well as tailored questions and comments related to the prior conversations on difficulties that were raised (such as preventing hypoglycemia, handling holidays, and special events) and progress (such as self-monitoring of fasting glucose) of the specific participant. The telephone conversations lasted between 5 and 15 min, depending on how much was needed to re-discuss the recommendations for those who did not comply with their respective diet.
Diet adherence was categorized by the study dietitian at the end of the study and before the results of AGEs in serum or any analysis of outcomes to avoid bias. Following each phone conversation with the participants, the dietitian elicited answers to two questions: (1) Since our last call, did the participant use any cooking methods they were advised to avoid for certain food items? (yes/no for low AGEs only), (2) Since our last call, did the participant completely maintain the guidelines for good glycemic control? (yes/no for all participants). As carbohydrates intake is the key strategy in achieving glycemic control (40), the answer for maintaining guidelines for good glycemic control was focused on the intake of food items from this group.
Participants in the AGEs-lowering arm were scored twice; once for their adherence to the AGEs reduction and once for their adherence for good glycemic control. Participants in the standard of care (SOC) arm were scored only for their adherence with the guidelines for good glycemic control. Phone calls with “yes” answers (reflecting full compliance with guidelines for T2D or AGEs reduction) were granted a score of 1. If participants reported they had not followed instructions for good glycemic control (focused on carbohydrates) or had used cooking methods that promote AGEs creation in animal-derived products (applied for AGEs-lowering arm) even once since the last call, the score of the conversation was 0. The scores for phone conversation (0/1) were then summed for each subject and were divided by the average number of phone conversations. For example, if the average number of phone conversation was 17, and the subject reported in 12 conversations that they had faithfully maintained the dietary instructions, then adherence was scored as 12/17*100 = 70%.
Table 2 displays the characteristics for each score for both arms. Adherence was measured on a 1–5 Likert scale based on the participants' report if they maintained AGEs or T2D recommendations: 1 for very high adherence, 2 for good adherence, 3 for partial adherence, 4 for lack of adherence but intention to adhere more in the future, and 5 for lack of adherence and no intention to adhere more (e.g., desire to withdraw).
The following outcomes were assessed upon inclusion in the study (baseline), and 6 months later, immediately after participants completed the intervention (post intervention).
Fasting blood samples were drawn and analyzed locally for glucose, insulin, HbA1C%, lipid profile, complete blood count, urea, and creatinine. In addition, blood tubes were centrifuged and serum was separated into tubes stored at −80°C for future analysis.
AGEs were measured in serum samples by liquid chromatography–mass spectrometry (LC-MS) using internal stable heavy isotope substituted standards (Prevent AGE Healthcare Technology). Analysis was performed in duplicates in a blinded fashion on the serum filtrate following centrifugation through 10-K cutoff Amicon filter. This fraction contains free AGE as well as peptides of various sizes, and the analytical method measured the free products. An Agilent model 6490 Triple Quadrupole MS system with a 1290 Rapid Resolution LC system was used to detect analytes. All AGEs were separated and analyzed using Waters X-select HSS T3 column (2.5 mm, 2.1 3 150 mm) with a mobile phase gradient of methanol and water with 0.20% heptafluorobutyric acid. Total analysis time ran 19 min. Five dicarbonyl-derived AGEs were assessed: Ne-carboxymethyl lysine (CML), Ne-carboxyethyl lysine (CEL), glyoxalhydroimidazolone (G-H1), methylglyoxal hydroimidazolone (MG-H1), and 3-deoxyglucosone hydroimidazolone (3DG-H1).
Nutritional assessment included Food Frequency Questionnaires (FFQ) and AGEs questionnaires (described above in the Face-to-face screening section). The FFQ questionnaire was specifically designed to assess nutrition in the elderly and is commonly used in this population (41). The questionnaire includes 126 items and was administrated by the study dietitian.
A neuropsychological assessment was performed by an experienced neuropsychologist specializing in the cognitive assessment of older adults. A paper-and-pencil battery, administered in face-to-face sessions, included commonly used cognitive tests presenting a range of cognitive domains including attention, executive functions, language, and memory (Supplement B). Neuropsychological test scores were transformed into Z scores using the baseline mean and standard deviation and averaged for each domain (42). An overall cognition measure averaged the scores of all four domains. We also invited participants to repeat cognitive testing at 12, 6 months after the intervention.
Scans were acquired on a 3-T Philips Ingenia scanner using a 32-channel head radio frequency (RF) coil. Participants underwent brain MRI for cerebral blood flow (CBF) quantification using an Artierial Spin Labeling (ASL) sequence (43). Further details on the MRI acquisition can be found in Supplement C.
Weight, height, and waist circumference were measured by the study dietitian using the same equipment, and BMI was calculated [height (m)2/weight (kg)]. Participants wore their shoes and clothing to avoid discomfort. Waist circumference was measured in centimeters at the level of the umbilicus. Smoking habits, demographics, physical activity (Minnesota Leisure Time Physical Activity Questionnaire) (44), independence (IADL questionnaire) (45), and activities of daily living (ADL questionnaire) (46) were assessed.
Although allocation to the diet intervention was randomly assigned, the participant and study dietitian could not be blinded to diet. All other staff members involved in measurement of any outcome were blind to the assignments, including the neuropsychologist, the MRI technician, and the lab technician where the blood tests were analyzed. Data entry was done by a research assistant who was also blinded to group assignments.
In keeping with the pilot nature of this study, and according to CONSORT guidelines for pilot and feasibility studies, we did not calculate a formal sample size (33). Currently, there are no published data on AGEs-reduction interventions on cognition in older adults. Various authors have discussed the minimal sample size required for pilot studies (33). Here, we followed Whitehead et al. (47) who recommend at least 25 people per arm in the pilot study if the intention is to power a future trial with 90% power where the expected effect size is small. A recent meta-analysis of nutritional interventions for cognition found that the overall effect size (measured by standardized mean differences) for verbal and visual memory domains was small (=0.19) (48). With respect to attrition, a recent trial of AGEs reduction of participants with metabolic syndrome found 20 and 35% attrition rate in the regular AGEs arm and low-AGEs arm, respectively (19). Thus, for a 30% average attrition, we targeted 72 participants at baseline. Our aim was to provide initial estimation of the effect size to allow robust power calculations for a future definitive trial.
Alpha of 0.05 was used for significance levels. SPSS version 23 (IBM Corp., Armonk, NY, USA) was used for all analyses. Descriptive statistics were used to assess recruitment and attrition rates. Baseline demographic and clinical characteristics are presented descriptively as proportions or as means with standard deviations.
The study will be analyzed by an intent-to-treat (ITT) primary analysis, with a secondary per-protocol (PP) analysis. The ITT analysis will include all randomized participants, regardless of any protocol deviation including non-adherence, adverse events, or loss of follow-up. To be considered fully compliant in the PP analysis, participants must have reported at least 80% good compliance with low AGEs cooking modifications or following standard T2D nutritional guidance for good glycemic control in weekly phone conversations.
AGEs and other biomarkers reduction in serum from baseline to 6 months will be analyzed using two-sample t-test or Mann–Whitney. χ2-test will be used for categorical outcomes.
For the exploratory aim of estimating effect size for future RCTs, global and domain-specific cognitive outcomes will each be analyzed as z-scores using a linear regression model with time of assessment (baseline or 6 months) as the within-participants factor, treatment group (dietary AGEs reduction vs. control) as the between-participants factor, and baseline value of the outcome measure as the covariate. CBF will be analyzed similarly with the linear regression model.
Recruitment of the study began in July 2016 and ended in December 2018. A flow chart describing the study recruitment and follow-up is given below (Figure 1). Nine hundred candidates have shown interest to participate in the study, of whom 100 gave information that do not meet the eligibility criteria (e.g., age, impaired fasting glucose, and not T2D) and received an email informing them of their ineligibility. Out of the 800 candidates that were contacted by phone to ascertain eligibility, 148 were invited for a face-to-face screening. The most common reason for ineligibility at this stage was that the cognitive complaints were limited to a SMC (65%) that focused only on forgetting names or locating household items. Such complaints, on their own, do not indicate a clinically detectable cognitive impairment but rather normal aging. Other reasons for ineligibility at this stage are specified in Figure 1. The first stage of recruitment (i.e., recruitment of only MCI) was 27 months (between July 2016 and September 2018) and 52 participants were eligible; 23 participants were excluded due to inconsistent MOCA and CDR results impeding a clear ascertainment of cognitive status. Because it took 27 months to recruit 52 eligible participants under the original eligibility criteria (1.9 participants per month), we expanded the eligibility criteria to include cognitively normal diabetics. At the second stage of recruitment (October 2018–December 2018), we approached previously screened candidates who were originally ineligible for enrollment in the trial because their cognition was normal. Twenty-four candidates that were screened were approached in the order in which they had been screened. All of those invited (100%) agreed to participate. In total, 75 participants were randomized.
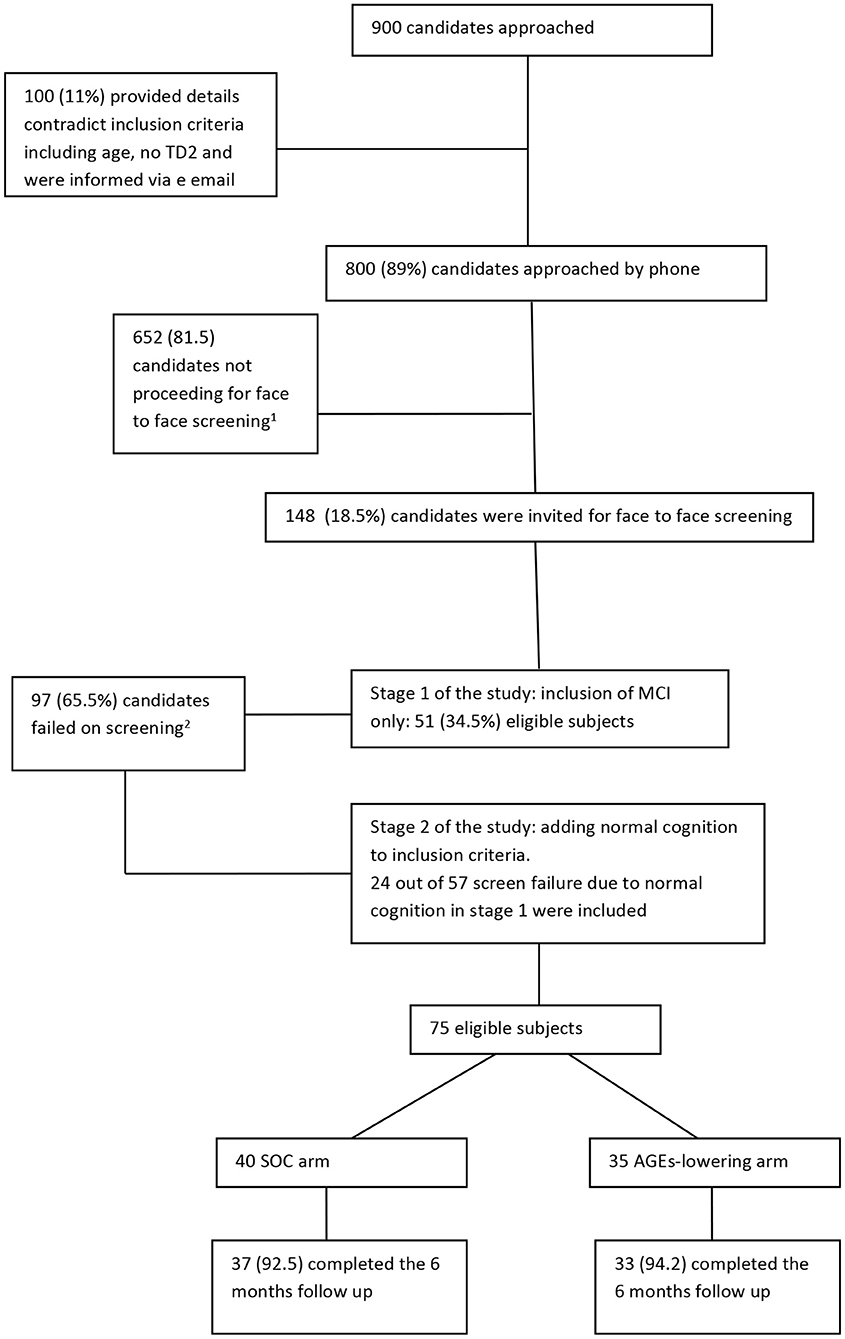
Figure 1. Flow chart of study recruitment and follow-up. 1Main reasons for not proceeding to a face to face screening were (order by prevalence): not reporting to have memory decline other than forgetting names, not having type 2 diabetes but impaired fasting glucose, no care giver available, portable issues, vegetarian, participating in other clinical trials, not interested, having other medical condition that unable them to participate such as CKD, active cancer or other neurological condition. 2Main reasons for screen failure (n = 97) were: 57 had normal cognitive status, 13 with mild depression, 9 had other health conditions that are not eligible such as brain surgery in the last year, chronic renal failure, 8 changed their mind or informant declined to cooperate, 4 had very low consumption of AGEs, 2 were advised to take medication for memory problems, 2 with CDR=1, 1 with major anxiety, 1 not fluent in Hebrew for cognitive tests.
Of the different recruitment strategies, the mass mailing for people who wanted to receive information on health issues ultimately yielded the most study participants (n = 57). Publication of the study in websites and forums for clinical trials and diabetes yielded 11 participants, and referring participants from other observational or screen failure clinical trials yielded the rest (n = 7). Participants recruited through mass mailing were more educated than those recruited from other studies or websites and forums combined (14.8 ± 2.5 vs. 13.2 ± 3.1, Pv = 0.04). We did not find any differences in age or sex between the recruitment strategies. The total cost of advertising the study in social media and websites was 5,800 USD.
Seventy participants (93%) completed 6 months of follow up. Three withdrew from the SOC arm and two withdrew from the AGEs-lowering arm. The withdrawal rate was not statistically different between the two arms (p = 0.757). Reasons for withdrawal from the trial in the SOC arm were deterioration in angina pectoris symptoms in one subject who could not attend the 6-month visit (the neuropsychology assessment alone was done at the participant's home); sickness of one subject's husband; and one lost to follow-up, who showed very low adherence to diet and regularly failed to answer the phone. In the AGEs-lowering arm, there were two lost to follow up; one participant showed very low adherence as reflected in the weekly phone conversation and another participant reported very high adherence during the trial but did not want to come to the follow-up visit. The average number of phone conversation made during the 6 months of follow up was 16.52. On average, a call was made every 11 days and not on a weekly basis as initially planned due to unanswered calls and holidays.
Adherence to T2D dietary guidelines was similar with very high adherence, good adherence, and partial adherence, respectively, for the two arms (p = 0.962): 43.2, 45.9, and 10.8% for the AGEs-lowering arm and 42.9, 47.1, and 10% for the SOC arm. Of the 33 participants who completed the study in the AGEs-lowering arm, 19 (57.5%) were scored with very high adherence, 10 (30.3%) with good adherence, and 4 (12.2%) with partial adherence for the low-AGEs adherence scale. Cognition status (MCI vs. normal cognition) was not associated with adherence to T2D (Pv = 0.338) or low-AGEs dietary guidelines (Pv = 0.352). None of the participants who finished the 6 months follow-up scored 4/5, representing no adherence.
Participants' baseline characteristics are provided in Table 3. The mean age was 71.6 ± 4.07; 74.7% were males. Similarly to other elderly participants in prospective cohorts (49), our sample represented educated and motivated participants with an average of 14.4 ± 2.74 years of education and an average HbA1C% of 6.67 ± 0.67, reflecting good glycemic control. Participants' consumption of macronutrients show that carbohydrates account for <50% of the daily consumption. Participants reported AGEs consumption with a mean of 22.65 ± 6.47 kU/day. There were no significant differences between the groups at baseline on any of the variables, indicating that randomization was successful.
The current paper describes the methodology, recruitment, adherence measurements, and the sample baseline characteristics of a pilot study assessing dietary AGEs reduction in older adults with T2D. Our study population is different from that of previous trials in two aspects. First, we have tested older adults (mean age, 71.6) with T2D while the age of the participants in previous trials of AGEs reduction in diabetics ranged 60–62 (18, 50). As age is positively correlated with circulating AGEs (11) and negatively with kidney function (51), a decade difference may be significant for the AGEs' pool due to both higher accumulation and decreased disposal. As such, we hypothesized that older adults with T2D may respond differently to a low-AGEs diet. Second, our trial is the first to recruit participants with cognitive impairment for intervention to reduce AGEs. The results showed that adherence to the dietary guidelines was similar in participants with impaired and normal cognition, supporting the feasibility of the program in this population. In terms of feasibility of the dietary intervention per se, the high adherence to dietary AGEs reduction among 57.5% of participants suggests that older adults with T2D population is able and willing to commit to a low-AGEs diet that is closely supervised by a nutritionist. We focused the study on older adults with T2D as we anticipated this as an optimal population to target for AGEs modification to benefit cognition, since T2D individuals have greater accumulation of AGEs due to hyperglycemia (12) and are also at higher risk for cognitive decline and dementia (9). Our original intention was to recruit only participants with MCI. Under the eligibility criteria in the first stage of recruitment, the overall screen failure rate for 900 participants screened was 17 for every eligible subject, emphasizing the great challenge in recruiting MCI with T2D. The main reason for ineligibility through telephone screening was that what candidates reported as memory deficit was actually normative. In the face-to-face screening, 65% did not meet the inclusion criteria and 58% of those screen failures were due to normal cognition. Low rates of recruitments of MCI participants from both community (52) and clinic settings (53) and the low sensitivity of SMCs alone to find eligible participants have been shown in prior studies. A community-based trial found that only 44% of those who applied with memory concerns following newspaper advertisement had MCI while 47% were classified with normal cognition in the tests with an average MOCA of 27 (52). Kirsebom et al. showed that cognitive performance of participants referred by memory clinics compared to self-referral is significantly lower in MCI (54). In other words, previous studies support our finding that SMCs from the general population do not distinguish participants with MCI and that involving several memory clinics may be a more successful strategy for identifying a large number of eligible participants with MCI. Since several studies indicated that use of a mutual informant with self-report of cognitive decline may better represent cognitive changes, our screening process also included an informant (51, 52).
We found that the most productive recruitment tool, which was the source of 76% of our participants, was mass mailing to a list of 30,000 people who indicated they wished to receive email information about health. We sent this email eight times at 3-month intervals, allowing enough time for updating the list. We also advertised in relevant internet forums and websites and used referrals from other studies as previously suggested (55), but those yielded a relatively small number of eligible participants. We found that although potential participants who approached us from different sources did not differ in age or sex, participants from mass mailing for people interested in receiving information about health had higher education and thus may not have been representative of the general older adult population. Thus, it is likely that they represented motivated participants wishing to improve their health and therefore more willing to volunteer for such trials.
Our findings demonstrate that it is possible to achieve high compliance with our study protocol as shown by the excellent telephone contact (mean of 16.52) and low withdrawal rate (6%). Other AGEs-lowering clinical trials (19) experienced a much higher withdrawal rate (27%) over the course of 1 year. Similarly, another behavioral intervention study in elderly with MCI that included cognitive training, yoga support groups, and wellness education reported a 17% dropout at 1 year (53). Our low attrition rate may be due to the intense follow up during the 6-month study, which helped participants maintain the guidelines and to ask questions about their interpretation of the guidelines (e.g., “can I use the oven if I cover the food?” “what food should I choose to eat if I have an event next week,” etc.). Along with a high retention rate, participants' adherence with the instructions was high as well; 57.5% of participants from the AGEs-lowering arm reported that they followed the guidelines to reduce AGEs in at least 80% of the phone conversations. Another 30.3% reported that they maintained the guidelines 60–80% of the time and only 12.2% reported maintaining the guidelines <60% of the time. Nevertheless, measuring adherence to dietary guidelines is a challenge, and we cannot rule out self-reporting bias. Questionnaires for diet adherence such as the MEDI score questionnaire (56) or self-reported scale of adherence with T2D recommendations (57) usually assess the intake of certain food items. Our study did not ask the participants to score their own adherence, as self-reported adherence may be biased. Instead, we used an adherence score built on the dietitian perspective based on numerous phone conversations, suggesting that the adherence measure is less vulnerable to information bias. To our knowledge, this study is the first to use a dietitian's perspective scale for adherence measurement. In future studies, both self-reported and dietician-based adherence assessments should be collected and their correlations should be examined to validate the advantages of one over the other as adherence scales may indicate in real time who needs a higher rate of monitoring in order to increase adherence. The high adherence to the SOC guidelines for T2D in both arms might have contributed to a partial reduction in serum AGEs, which was observed also in the “active” SOC control arm. This may have limited our ability to assess the true effect of decreasing circulating AGEs compared to no change or increase over time that would be expected in a passive control. We have recently showed that that the reduction of serum AGEs in the intervention arm was not attributed to any metabolic benefits other than reduced AGEs intake (32). However, a future RCT may require a passive intervention arm to definitively determine the benefit of the intervention. While this may pose ethical challenges regarding equipoise, it may be acceptable to randomize participants to a waiting list, to receive SOC guidance at baseline without any further involvement during the follow up, or to use a crossover design with passive and active interventions in random order.
While our study highlights the feasibility of AGEs-lowering diet for cognitive protection in older diabetics, it also has several limitations. An unavoidable limitation of the design (overt dietary interventions) is that neither the investigators nor the patients were blind to the intervention. We attempted to minimize this effect by providing both arms tailored from dietary recommendations and followed both groups equally. In addition, the average HbA1C of 6.6% ± 0.6% suggests that our participants maintain good health in general so that their adherence to such a diet may not be generalized to the population of older adults with T2D at large. In addition, the successful recruitment through mass mailing of people wishing to learn about health issues also raise the potential of selection bias. A sample including more severe diabetics or non-diabetics with other metabolic conditions that do not follow regularly dietary guidelines may benefit more from such intervention and should be investigated in the future.
In conclusion, we described the methods, recruitment, and adherence of a pilot study of low-AGEs diet in elderly with T2D and brain-related outcomes. The pilot study emphasizes the challenge in recruiting MCI participants from the general population and shows that mass mailing and social media advertising are a good strategy for recruiting candidates. We found that including an informant and an objective global cognition tool (MoCA) rather than relying on subjective complaints alone was important for MCI screening. Lastly, our findings suggest that an intensive follow-up during the intervention might help reduce attrition in a study aimed at promoting health-related behavioral change.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Sheba medical center approval number 2206-15-SMC. The patients/participants provided their written informed consent to participate in this study.
RL conducted the trial, analyzed the study data, and wrote the initial draft. IG and SS were the trial physicians and signed up the participants on informed consent. AL managed the neuro-imaging section of the trial and participated in writing of the manuscript. JU and WC served as scientific advisors and participated in writing or technical editing of the manuscript. PB was responsible for the analysis of advanced glycation end products. MZ coordinated the trial and was responsible for the data entry. MS and AT initiated the trial plan, analyzed data, and revised and finalized the manuscript. All authors contributed to the article and approved the submitted version.
Partially funded by the research grant from the Katzin Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.614149/full#supplementary-material
1. Jaul E, Barron J. Age-related diseases and clinical and public health implications for the 85 years old and over population. Front Public Health. (2017) 5:335. doi: 10.3389/fpubh.2017.00335
2. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. (2013) 9:63–75.e2. doi: 10.1016/j.jalz.2012.11.007
3. Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol. (2011) 10:819–28. doi: 10.1016/S1474-4422(11)70072-2
4. Schnaider Beeri M, Goldbourt U, Silverman JM, Noy S, Schmeidler J, Ravona-Springer R, et al. Diabetes mellitus in midlife and the risk of dementia three decades later. Neurology. (2004) 63:1902–7. doi: 10.1212/01.WNL.0000144278.79488.DD
5. Ravona-Springer R, Luo X, Schmeidler J, Wysocki M, Lesser M, Rapp M, et al. Diabetes is associated with increased rate of cognitive decline in questionably demented elderly. Dement Geriatr Cogn Disord. (2010) 29:68–74. doi: 10.1159/000265552
6. Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PLoS ONE. (2009) 4:e4144. doi: 10.1371/journal.pone.0004144
7. Luchsinger JA, Reitz C, Patel B, Tang M, Manly J, Mayeux R. Relation of diabetes to mild cognitive impairment. Arch Neurol. (2007) 64:570–5. doi: 10.1001/archneur.64.4.570
8. Hsu FC, Raffield LM, Hugenschmidt CE, Cox A, Xu J, Carr J, et al. Relationships between cognitive performance, neuroimaging and vascular disease: the DHS-MIND study. Neuroepidemiology. (2015) 45:1–11. doi: 10.1159/000435775
9. Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes–systematic overview of prospective observational studies. Diabetologia. (2005) 48:2460–9. doi: 10.1007/s00125-005-0023-4
10. Ravona-Springer R, Schnaider-Beeri M. The association of diabetes and dementia and possible implications for nondiabetic populations. Expert Rev Neurother. (2011) 11:1609–17. doi: 10.1586/ern.11.152
11. Uribarri J, Cai W, Peppa M, Goodman S, Ferrucci L, Striker G, et al. Circulating glycotoxins and dietary advanced glycation endproducts: two links to inflammatory response, oxidative stress, and aging. J Gerontol A Biol Sci Med Sci. (2007) 62:427–33. doi: 10.1093/gerona/62.4.427
12. Poulsen MW, Hedegaard R, Andersen J, Courten B, Bügel S, Nielsen J, et al. Advanced glycation endproducts in food and their effects on health. Food Chem Toxicol. (2013) 60:10–37. doi: 10.1016/j.fct.2013.06.052
13. Kellow NJ, Coughlan MT. Effect of diet-derived advanced glycation end products on inflammation. Nutr Rev. (2015) 73:737–59. doi: 10.1093/nutrit/nuv030
14. Goldberg T, Cai W, Peppa M, Dardaine V, Baliga BS, Uribarri J, et al. Advanced glycoxidation end products in commonly consumed foods. J Am Diet Assoc. (2004) 104:1287–91. doi: 10.1016/j.jada.2004.05.214
15. Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. (2010) 110:911–16.e12. doi: 10.1016/j.jada.2010.03.018
16. Koska J, Saremi A, Howell S, Bahn G, Courten B, Ginsberg H, et al. Advanced glycation end products, oxidation products, and incident cardiovascular events in patients with type 2 diabetes. Diabetes Care. (2018) 41:570–6. doi: 10.2337/dc17-1740
17. Uribarri J, Cai W, Pyzik R, Goodman S, Chen X, Zhu L, et al. Suppression of native defense mechanisms, SIRT1 and PPARgamma, by dietary glycoxidants precedes disease in adult humans; relevance to lifestyle-engendered chronic diseases. Amino Acids. (2014) 46:301–9. doi: 10.1007/s00726-013-1502-4
18. Uribarri J, Cai W, Pyzik R, Goodman S, Chen X, Zhu L, et al. Restriction of advanced glycation end products improves insulin resistance in human type 2 diabetes: potential role of AGER1 and SIRT1. Diabetes Care. (2011) 34:1610–6. doi: 10.2337/dc11-0091
19. Vlassara H, Cai W, Tripp E, Pyzik R, Yee K, Goldberg L, et al. Oral AGE restriction ameliorates insulin resistance in obese individuals with the metabolic syndrome: a randomised controlled trial. Diabetologia. (2016) 59:2181–92. doi: 10.1007/s00125-016-4053-x
20. Salahuddin P, Rabbani G, Khan RH. The role of advanced glycation end products in various types of neurodegenerative disease: a therapeutic approach. Cell Mol Biol Lett. (2014) 19:407–37. doi: 10.2478/s11658-014-0205-5
21. West RK, Moshier E, Lubitz I, Schmeidler J, Godbold J, Cai W, et al. Dietary advanced glycation end products are associated with decline in memory in young elderly. Mech Ageing Dev. (2014) 140:10–2. doi: 10.1016/j.mad.2014.07.001
22. Cai W, Uribarri U, Zhu L, Chen X, Swamy S, Zhao Z, et al. Oral glycotoxins are a modifiable cause of dementia and the metabolic syndrome in mice and humans. Proc Natl Acad Sci U S A. (2014) 111:4940–5. doi: 10.1073/pnas.1316013111
23. Beeri MS, Moshier E, Schmeidler J, Godbold J, Uribarri J, Reddy S, et al. Serum concentration of an inflammatory glycotoxin, methylglyoxal, is associated with increased cognitive decline in elderly individuals. Mech Ageing Dev. (2011) 132:583–7. doi: 10.1016/j.mad.2011.10.007
24. Lubitz I, Ricny J, Atrakchi-Baranes D, Shemesh C, Kravitz E, Liraz-Zaltsman S, et al. High dietary advanced glycation end products are associated with poorer spatial learning and accelerated Abeta deposition in an Alzheimer mouse model. Aging Cell. (2016) 15:309–16. doi: 10.1111/acel.12436
25. Luth HJ, Ogunlade V, Kuhla B, Kientsch-Engel R, Stahl P, Julie Webster, et al. Age- and stage-dependent accumulation of advanced glycation end products in intracellular deposits in normal and Alzheimer's disease brains. Cereb Cortex. (2005) 15:211–20. doi: 10.1093/cercor/bhh123
26. Dei R, Takeda A, Niwa H, Li M, Nakagomi Y, Watanabe M, et al. Lipid peroxidation and advanced glycation end products in the brain in normal aging and in Alzheimer's disease. Acta Neuropathol. (2002) 104:113–22. doi: 10.1007/s00401-002-0523-y
27. Takeda A, Wakai M, Niwa H, Dei R, Yamamoto M, Li M, et al. Neuronal and glial advanced glycation end product [Nepsilon-(carboxymethyl)lysine]] in Alzheimer's disease brains. Acta Neuropathol. (2001) 101:27–35. doi: 10.1007/s004010000256
28. Girones X, Guimerà A, Cruz-Sánchez C, Ortega A, Sasaki N, Makita Z, et al. N epsilon-carboxymethyllysine in brain aging, diabetes mellitus, and Alzheimer's disease. Free Radic Biol Med. (2004) 36:1241–7. doi: 10.1016/j.freeradbiomed.2004.02.006
29. Beeri MS, Uribarri J, Cai W, Buchman A, Haroutunian V, et al. Human brain and serum advanced glycation end products are highly correlated: preliminary results of their role in Alzheimer disease and type 2 diabetes. Endocr Pract. (2020) 26:576–7. doi: 10.4158/1934-2403-26.5.576
30. Akhter F, Chen D, Akhter A, Sosunov AA, Chen A, McKhann GM, et al. High dietary advanced glycation end products impair mitochondrial and cognitive function. J Alzheimers Dis. (2020) 76:165–78. doi: 10.3233/JAD-191236
31. Dobi A, Rosanaly S, Devin A, Baret P, Meilhac O, Harry GJ, d'Hellencourt CL, et al. Advanced glycation end-products disrupt brain microvascular endothelial cell barrier: the role of mitochondria and oxidative stress. Microvasc Res. (2021) 133:104098. doi: 10.1016/j.mvr.2020.104098
32. Lotan R, Ganmore I, Shelly S, Zacharia M, Uribarri J, Beisswenger P, et al. Long term dietary restriction of advanced glycation end-products (AGEs) in older adults with type 2 diabetes is feasible and efficacious-results from a pilot RCT. Nutrients. (2020) 12:E3143. doi: 10.3390/nu12103143
33. Eldridge SM, Chan C, Campbell M, Bond C, Hopewell S, Thabane L, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. (2016) 355:i5239. doi: 10.1136/bmj.i5239
34. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. (1976) 16:31–41. doi: 10.1159/000180580
35. Morris JC, Ernesto C, Schafer K, Coats M, Leon S, Sano M, et al. Clinical dementia rating training and reliability in multicenter studies: the Alzheimer's Disease Cooperative Study experience. Neurology. (1997) 48:1508–10. doi: 10.1212/WNL.48.6.1508
36. Lifshitz M, Dwolatzky T, Press Y. Validation of the Hebrew version of the MoCA test as a screening instrument for the early detection of mild cognitive impairment in elderly individuals. J Geriatr Psychiatry Neurol. (2012) 25:155–61. doi: 10.1177/0891988712457047
37. Bass DS, Attix D, Phillips-Bute B, Monk T. An efficient screening tool for preoperative depression: the Geriatric Depression Scale-Short Form. Anesth Analg. (2008) 106:805–9, table of contents. doi: 10.1213/ane.0b013e318163fa75
38. Uribarri J, Peppa M, Cai W, Goldberg T, Lu M, Baliga S, et al. Dietary glycotoxins correlate with circulating advanced glycation end product levels in renal failure patients. Am J Kidney Dis. (2003) 42:532–8. doi: 10.1016/s0272-6386(03)00779-0
39. Vlassara H, Cai W, Goodman S, Pyzik R, Yong A, Chen X, et al. Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: role of the antiinflammatory AGE receptor-1. J Clin Endocrinol Metab. (2009) 94:4483–91. doi: 10.1210/jc.2009-0089
40. Evert AB, Boucher J, Cypress M, Dunbar S, Franz M, Mayer-Davis E, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. (2014) 37(Suppl. 1):S120–43. doi: 10.2337/dc14-S120
41. Shahar D, Fraser D, Shai I, Vardi V Development of a food frequency questionnaire (FFQ) for an elderly population based on a population survey. J Nutr. (2003) 133:3625–9. doi: 10.1093/jn/133.11.3625
42. Beeri MS, Ravona-Springer R, Moshier E, Schmeidler J, Godbold J, Karpati T, et al. The Israel Diabetes and Cognitive Decline (IDCD) study: design and baseline characteristics. Alzheimers Dement. (2014) 10:769–78. doi: 10.1016/j.jalz.2014.06.002
43. Birdsill AC, Carlsson C, Willette A, Okonkwo O, Johnson S, Xu G, et al. Low cerebral blood flow is associated with lower memory function in metabolic syndrome. Obesity. (2013) 21:1313–20. doi: 10.1002/oby.20170
44. Richardson MT, Leon A, Jacobs D Jr, Ainsworth B, Serfass R. Comprehensive evaluation of the Minnesota Leisure Time Physical Activity Questionnaire. J Clin Epidemiol. (1994) 47:271–81. doi: 10.1016/0895-4356(94)90008-6
45. Graf C. The Lawton instrumental activities of daily living scale. Am J Nurs. (2008) 108:52–62; quiz 62–3. doi: 10.1097/01.NAJ.0000314810.46029.74
46. Shinar D, Gross CR, Bronstein KS, Licata-Gehr EE, Eden DT, Cabrera AR, et al. Reliability of the activities of daily living scale and its use in telephone interview. Arch Phys Med Rehabil. (1987) 68:723–8.
47. Whitehead AL, Julious S, Cooper C, Campbell M. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res. (2016) 25:1057–73. doi: 10.1177/0962280215588241
48. Radd-Vagenas S, Duffy S, Naismith S, Brew B, Flood V, Fiatarone Singh M. Effect of the Mediterranean diet on cognition and brain morphology and function: a systematic review of randomized controlled trials. Am J Clin Nutr. (2018) 107:389–404. doi: 10.1093/ajcn/nqx070
49. Guerrero-Berroa E, Ravona-Springer R, Schmeidler J, Silverman J, Sano M, Koifmann K, et al. Age, gender, and education are associated with cognitive performance in an older Israeli sample with type 2 diabetes. Int J Geriatr Psychiatry. (2014) 29:299–309. doi: 10.1002/gps.4008
50. Cai W, Cijiang He J, Zhu L, Peppa M, Lu C, Uribarri J, et al. High levels of dietary advanced glycation end products transform low-density lipoprotein into a potent redox-sensitive mitogen-activated protein kinase stimulant in diabetic patients. Circulation. (2004) 110:285–91. doi: 10.1161/01.CIR.0000135587.92455.0D
51. Denic A, Glassock RJ, Rule AD. Structural and functional changes with the aging kidney. Adv Chronic Kidney Dis. (2016) 23:19–28. doi: 10.1053/j.ackd.2015.08.004
52. Dube JB, Lin T, Best S, Truemner J, Sargeant P, Borrie M, et al. The case-finding study: a novel community-based research recruitment approach for engaging participants with early cognitive decline. Alzheimers Dement. (2019) 5:501–7. doi: 10.1016/j.trci.2019.07.009
53. Chandler MJ, Locke D, Crook J, Fields J, Ball C, Phatak V, et al. Comparative effectiveness of behavioral interventions on quality of life for older adults with mild cognitive impairment: a randomized clinical trial. JAMA Netw Open. (2019) 2:e193016. doi: 10.1001/jamanetworkopen.2019.3016
54. Kirsebom BE, Espenes R, Waterloo K, Hessen E, Johnsen S, Bråthen G, et al. Screening for Alzheimer's disease: cognitive impairment in self-referred and memory clinic-referred patients. J Alzheimers Dis. (2017) 60:1621–31. doi: 10.3233/JAD-170385
55. Bracken K, Askie L, Keech A, Hague W, Wittert G. Recruitment strategies in randomised controlled trials of men aged 50 years and older: a systematic review. BMJ Open. (2019) 9:e025580. doi: 10.1136/bmjopen-2018-025580
56. Zaragoza-Marti A, Cabañero-Martínez MJ, Hurtado-Sánchez JA, Laguna-Pérez A, Ferrer-Cascales R. Evaluation of Mediterranean diet adherence scores: a systematic review. BMJ Open. (2018) 8:e019033. doi: 10.1136/bmjopen-2017-019033
Keywords: mild cognitive impairment, type 2 diabetes, diet, randomized controlled trial, pilot, feasibility, advanced glycation end products
Citation: Lotan R, Ganmore I, Livny A, Shelly S, Zacharia M, Uribarri J, Beisswenger P, Cai W, Schnaider Beeri M and Troen AM (2021) Design and Feasibility of a Randomized Controlled Pilot Trial to Reduce Exposure and Cognitive Risk Associated With Advanced Glycation End Products in Older Adults With Type 2 Diabetes. Front. Nutr. 8:614149. doi: 10.3389/fnut.2021.614149
Received: 05 October 2020; Accepted: 04 January 2021;
Published: 15 February 2021.
Edited by:
David Vauzour, University of East Anglia, United KingdomReviewed by:
Eglantine Balland, Monash University, AustraliaCopyright © 2021 Lotan, Ganmore, Livny, Shelly, Zacharia, Uribarri, Beisswenger, Cai, Schnaider Beeri and Troen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roni Lotan, cm9uaS5sb3RhbkBtYWlsLmh1amkuYWMuaWw=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.