
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 02 June 2021
Sec. Nutritional Immunology
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.608972
Background: Previous studies have suggested that maternal dietary protein was associated with allergic diseases in offspring, but few studies have evaluated the influence of dietary protein patterns. This study aimed to explore the prospective association between maternal dietary protein patterns during pregnancy and the risk of infant eczema.
Methods: A total of 713 mother-child pairs from a prospective cohort in Guangzhou, China were recruited. Maternal dietary protein was estimated using a validated face-to-face food frequency questionnaire at 20–28 weeks' gestation from 2017 to 2018. Dietary protein patterns were calculated based on the sources of protein. The data of infant eczema was assessed at 6 months of age using the symptom questionnaire of eczema. Logistic regression was carried out to examine the associations between maternal dietary protein patterns and infant eczema.
Results: The cumulative incidence of infant eczema at 6 months of age was 51.19%. Mothers of infants with eczema consumed more protein from poultry source during pregnancy than mothers of infants without eczema, while no statistical differences were observed in maternal intakes of protein from cereals and tubers, vegetables, fruits, red meat, fish and seafood, eggs, dairy, soybean, and nuts and seeds. Four dietary protein patterns were identified and termed poultry, plant, dairy and eggs, and red meat and fish. The cumulative incidence of eczema was 61.2, 45.8, 48.0, 51.4% for these four patterns, respectively. Compared to the poultry dietary pattern, the plant pattern and the dairy and eggs pattern were associated with a reduced risk of infant eczema, and the adjusted odds ratios (95% confidence interval) were 0.572 (0.330–0.992), 0.478 (0.274–0.837), respectively. No such association was observed for the red meat and fish dietary protein pattern.
Conclusion: This is the first study that focused on the association between maternal dietary protein during pregnancy from a whole-diet perspective and infant eczema. Compared with the poultry dietary protein pattern, the maternal plant pattern and the dairy and eggs pattern during pregnancy were associated with a reduced risk of infant eczema.
Eczema is one of the most frequent chronic inflammatory skin diseases and it most often occurs in early infancy (1). Infants with eczema tend to have an increased risk for food allergy, wheezing, asthma, allergic rhinitis, and subsequent psychosocial and behavioral issues (2–5). The pathogenesis of infant eczema is not yet well-understood but believed to be influenced by genetic factors and environmental exposures (1). In the past 30 years, the prevalence of eczema has increased globally (6). Environmental factors are likely to play an increasingly important role. According to the DOHaD (Developmental Origins of Health and Disease) hypothesis (7), non-inheritable exposures in utero can alter offspring programming of immune function and developing of allergic diseases (8, 9). Maternal diet during pregnancy can provide nutrients to fetal development, and also may impact fetal immune responses (10).
The impaired epidermal barrier is one of the hallmarks of eczema (11), enhancing penetration of the skin by allergen and microbes (12). Maternal dietary protein, as an important nutrient and major allergens, can influence fetal growth and development, such as epidermal structure and function as well as immune system (13, 14). The association between maternal dietary proteins and offspring allergic diseases has long been discussed. Previous studies suggested that maternal intake of some food allergens (e.g., milk and peanut) during pregnancy may reduce the risk of food allergy (15, 16). There is a strong association between eczema and food allergy, with approximately one-third of all patients with severe eczema documented for food allergy (17). Maternal ingestion of wheat during mid-pregnancy was associated with decreased childhood eczema (18), and higher intake of dairy products during pregnancy was associated with reduced risk of infant eczema and asthma (19). As nutritional support for fetal development, proteins are generally digested into amino acids and transport to the fetus through the placenta (20). Nevertheless, the intact major food allergens (from milk, eggs, fish, fruits, nuts, and wheat) were detected in maternal amniotic fluid during pregnancy (21), and experimental evidence in vitro prove the view of transplacental allergens transfer (22–24). Early-life exposure of nutritive allergens could modulate fetal immune development and affect immune responses to allergens exposed after birth (25).
Since the diet consists of various foods and complex nutrients, dietary pattern analysis would parallel more closely the actual situation (26). Dietary patterns can reflect the individual diet habit over a period of time. Since the foundations of contemporary dietary guidelines are based on dietary patterns (27) and dietary patterns of protein intake may influence the willingness of people to modify their dietary behavior (28), it is more practical to focus on proteins from the perspective of dietary patterns. Individual protein intake from different food sources is regarded as a whole and evaluated comprehensively. Therefore, it is practical and instructive to investigate people's dietary intake to determine their dietary patterns. To date, there is no available evidence for maternal dietary protein patterns and infant eczema. Therefore, the aim of current study is to investigate the association between maternal dietary protein patterns during pregnancy and the risk of infant eczema.
Data was drawn from an on-going prospective cohort study (ClinicalTrial.gov number: NCT03023293). At baseline, we recruited pregnant women (20–28 weeks' gestation) aged 20–45 years in a Maternal and Child Health Hospital in Guangzhou, China from March 2017 to November 2018. Individuals diagnosed with preexisting cardiovascular disease, thyroid disease, diabetes mellitus, hematological disease, polycystic ovary syndrome, or mental disorder before conception and those with pregnancy infection or multiple pregnancies were excluded. The cohort was followed at 6 months after delivery.
A total of 789 mother-infant pairs were enrolled. We further excluded those whose dietary data was incomplete (n = 35), or protein contributions from each food group were 5 standard deviations below or above the mean protein contributions (n = 41). Thus, a total of 713 mother-infant pairs were included in the final analysis. Ethical approval for this cohort was given by the Ethics Committee of the School of Public Health of Sun Yat-Sen University. Written informed consent was provided by all participants.
Maternal dietary information was collected via a validated food frequency questionnaire (FFQ) at baseline survey in the face-to-face interview. FFQ was often used in large-scale population-based dietary surveys and reported to be a useful method to determine maternal food intake during pregnancy (29). The FFQ consisted of 81 food items, covering the most common foods consumed in China, and had been shown to be valid and reproducible among Chinese women in Guangzhou (30). Participants were asked to recall their habitual diet in the past month, including their frequency of consumption (number of times per month, week, or day for each food item) and food portion sizes, and the field staff well-trained filled out the FFQ. The food intake portion per frequency was presented in grams (e.g., 100 g of cooked fish), natural units (e.g., 1 egg), or household measures (e.g., 1 spoon). To help participants quantify their food intake, food photo booklet with standard portion sizes was also provided.
The 2004 Chinese Food Composition Table (31) was applied to convert each food consumption into daily nutrients intake. Maternal daily intake of protein was adjusted for total energy intake using the regression residual method (32). According to the 2016 Chinese Dietary Guidelines (33), 81 food items were aggregated into 10 pre-defined food groups. These groups included cereals and tubers (including grains, mixed beans, and tubers), vegetables, fruits, red meat, poultry, fish and seafood, eggs, dairy and its products, soybeans and its products, and nuts and seeds. Individual daily protein intake from each food group was calculated. Then the percentage of total dietary protein for each food group was calculated for subsequent K-means cluster analysis to determine dietary protein patterns.
The information of infant eczema was collected by telephone interview when the infants were 6 months of age. The mothers of infants completed telephone interviews. Eczema was assessed by the symptom questionnaire of eczema, and infant eczema was defined according to the diagnostic criteria in practical pediatrics of Zhu Futang (34). All the telephone interviewers had medical research background and were trained in eczema diagnosis.
At baseline survey, the demographics and lifestyle factors during pregnancy were investigated by the face-to-face interview. The information included maternal age, gestational age, parity, educational level, monthly household income per capita, smoking, and alcohol consumption. Smoking status and alcohol use during pregnancy were divided into yes or no. Height (nearest 0.1 cm) was measured by trained clinical nurses, and pre-pregnancy body weight was self-reported. Pre-pregnancy body mass index (BMI, kg/m2) was calculated as pre-pregnancy body weight (kg) divided by height squared (m2).
Infant birth information was abstracted from hospital records, including infant sex, birth weight, and date of birth. Feeding patterns, breastfeeding duration, solids introduction in 6 months, maternal history of food allergy, family history of allergy diseases (at least one of the immediate family members had eczema, food allergy, drug allergy, asthma, allergic rhinitis, or urticaria), and family history of eczema were collected at 6 months of age by telephone interviews.
Characteristics of the study population and maternal dietary intakes of each food group by infant eczema were described using proportions for categorical variables or means and standard deviations for continuous variables, with differences tested using t-test and chi-square test. And multiple linear regression model was conducted to adjust for potential confounding factors, including maternal delivery age, pre-pregnancy BMI, monthly household income, educational level, maternal history of food allergy, family history of allergy diseases, family history of eczema, gestational age, parity, smoking during pregnancy, and alcohol use during pregnancy.
Dietary protein patterns were derived by K-means cluster analysis. This method was widely performed to analyze dietary patterns (35–37). Participants were categorized into mutually exclusive dietary pattern groups based on protein consumptions of each food group by cluster analysis. Cluster analysis was proved to have reasonable reproducibility and validity (38), and the K-means method has higher repeatability (39). Firstly, the number of clusters (k) was specified. In the present study, cluster analysis was performed varying from 3 to 5 to identify the optimal number of clusters. Then all subjects were assigned to k clusters by calculating Euclidean distances and updating the location of centroids in an iterative process. Finally, the four-cluster was selected because it distinguished meaningful separated clusters, and it presented a reasonable sample distribution in each cluster. These clusters were termed poultry, plant, dairy and eggs, and red meat and fish dietary protein patterns.
Logistic regression models were performed to determine the associations between maternal dietary protein patterns in pregnancy and infant eczema by calculating crude and adjusted odds ratios (ORs) and 95% confidence interval (95% CI). In model 1, we adjusted for maternal delivery age, pre-pregnancy BMI, monthly household income, and educational level. In model 2, we additionally adjusted for maternal history of food allergy, family history of allergy diseases, family history of eczema. In model 3, we further adjusted for gestational age, parity, smoking during pregnancy, alcohol use during pregnancy, daily dietary energy intake. In the last model, infant sex, birth weight, birth season, baby's feeding patterns, breastfeeding duration, and introducing solids in 6 months were further adjusted. To assess confounding arising from other dietary factors during pregnancy, we further adjusted separately for maternal n-3 polyunsaturated fatty acids (n-3 PUFAs), n-6 polyunsaturated fatty acids (n-6 PUFAs), dietary fiber, and Vitamin E intake in sensitivity analysis. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). P < 0.05 was considered statistically significant.
The participant characteristics according to infant eczema status are presented in Table 1. During the first 6 months after birth, 365 out of 713 infants (51.19%) had eczema. Compared to infants without eczema, infants with eczema were more likely to have a family history of allergy diseases (P < 0.0001). They also tended to have a positive family history of eczema (P = 0.0001). There was a higher proportion of underweight women (BMI < 18.50 kg/m2) among mothers of infants with eczema than those without eczema. There was no significant difference between eczema and non-eczema groups among other characteristics.
Table 2 summarizes the maternal dietary intakes of total energy, animal protein, plant protein, and protein from different food sources, comparing infants with or without eczema. Mothers of infants with eczema consumed more poultry protein sources during pregnancy (P = 0.005). No statistical differences were observed in maternal intakes of total energy, animal protein, plant protein or protein from other food sources (cereals and tubers, vegetables, fruits, red meat, fish and seafood, eggs, dairy, soybean, and nuts and seeds) between different eczema status categories.
Participants were divided into four different dietary protein pattern groups, named by their predominant protein intake sources. Percentage contribution of protein intake from each food group across four dietary patterns is listed in Figure 1. The results of Analysis of Variance showed that there were significant differences among the percentage contribution of protein intake from each food group between four dietary pattern groups (P < 0.05, data not shown). Compared to other groups, women who exhibited poultry dietary protein pattern consumed relatively higher protein from poultry (mean percentage contribution of protein intake from poultry: 14.15%, n = 147). The plant dietary protein pattern (n = 179) was characterized by a relatively higher protein intake from cereals and tubers (32.57%), vegetables (6.91%), soybean (7.76%), and nuts and seeds (5.55%). The dairy and eggs dietary protein pattern (n = 171) presented with higher protein consumption from dairy (16.08%) and eggs (11.97%). The red meat and fish dietary protein pattern (n = 216) was characterized by higher protein intake from red meat (33.53%) and fish and seafood (11.49%).
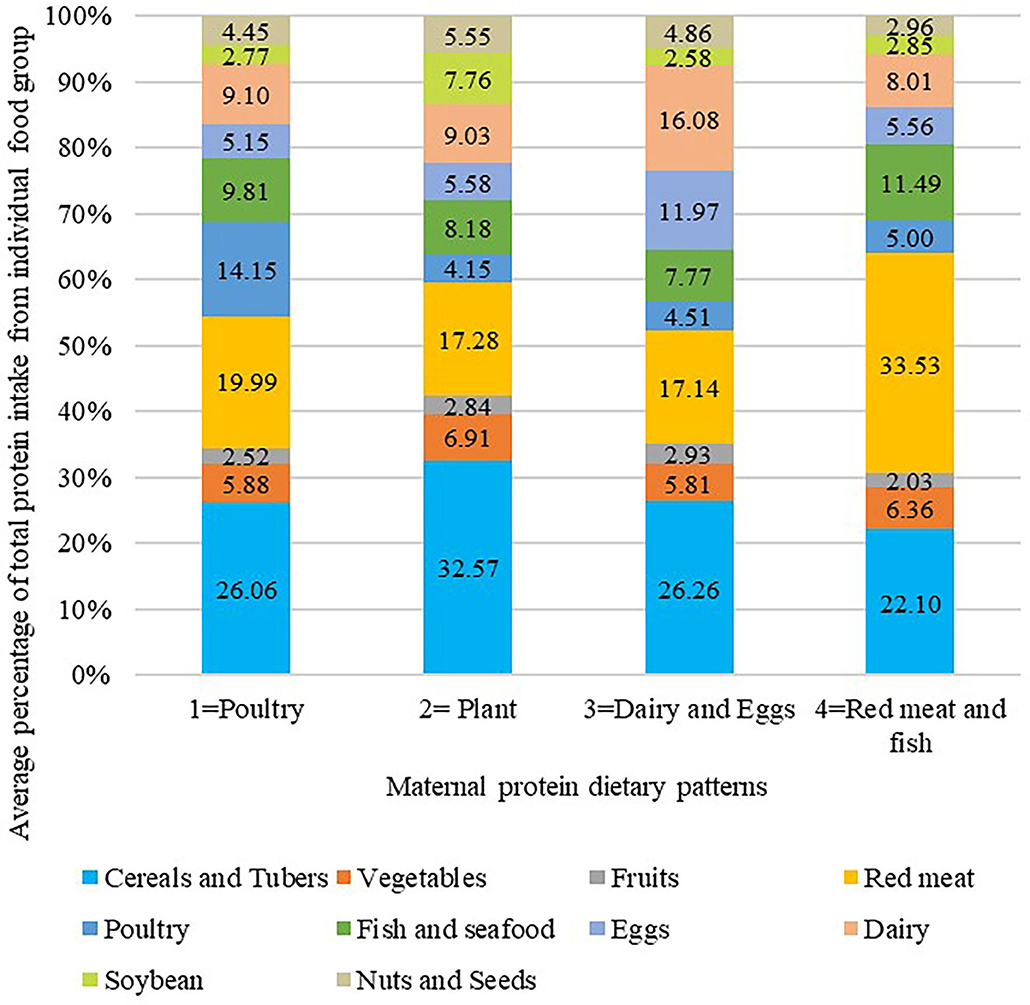
Figure 1. Average percentage of total protein intake from individual food group across protein food cluster analysis. A K-means cluster analysis was used to classify participants into mutually exclusive groups. Naming of clusters was determined by the value which represent the highest consumption of one or two food groups compared with other clusters. The results of Analysis of Variance showed that there were significant differences among the percentage contribution of protein intake from each food group between four dietary pattern groups (P < 0.05).
The univariate and multivariate regression analysis for the association between maternal dietary protein pattern during pregnancy and risk of infant eczema were listed in Table 3. The cumulative incidence of infant eczema in the poultry, plant, dairy and eggs, and red meat and fish dietary protein patterns was 61.2, 45.8, 48.0, 51.4%, respectively. After controlling for potential confounders, compared to the poultry dietary protein pattern, the multivariable-adjusted ORs (95% CI) for infant eczema were 0.572 (0.330–0.992) and 0.478 (0.274–0.837) in the plant pattern and dairy and eggs pattern, respectively. For the red meat and fish dietary protein pattern, the association was not statistically significant.
In a sensitivity analysis, compared to the red meat and fish dietary protein pattern, no association was observed between other dietary protein patterns and risk of infant eczema after adjusting for potential confounders (see Supplementary Table 1). Additional separate adjustment for maternal intake of n-3 PUFAs, n-6 PUFAs, dietary fiber, and Vitamin E during pregnancy did not substantially alter the main findings (see Supplementary Table 2).
In this prospective mother-infant cohort study, maternal consumption of poultry protein during pregnancy was higher in the eczema group than the control group. Four dietary protein patterns among pregnant women were identified, labeled the poultry, plant, dairy and eggs, and red meat and fish dietary protein patterns. Compared with the poultry dietary pattern, the plant pattern, and dairy and eggs pattern were associated with reduced risk of infant eczema at 6 months of age. This is the first prospective study to investigate the association between maternal dietary protein patterns during pregnancy and infant eczema, and this study provides epidemiological evidence to support the associations between maternal diet and offspring allergic diseases.
Evaluating the association between maternal dietary protein and infant eczema from a whole-diet perspective is necessary. Antigens composition and amino acid profiles vary in different dietary protein patterns. The mechanisms of divergent associations between maternal dietary intake and infant eczema remain unclear. Maternal dietary protein patterns may influence infant eczema through several pathways below. Firstly, there is growing evidence that maternal microbiota influences allergic diseases in offspring (40, 41). Animal experiments have shown that the composition and function of microbiota, especially in the gastrointestinal tract, can be modulated by dietary proteins (42, 43), including protein sources diversity and protein level (44). Recent studies have indicated that maternal gut microbiota could be transferred to the fetus (45, 46), and microbes are detected in human placenta and amniotic fluid (47). Prenatal microbial exposures are necessary to establish and develop a healthy nascent microbiome (48), which may have major impacts on immune system maturation (49). Secondly, epigenetic mechanisms provide a new explanation for the development of allergic diseases (50–52). Amino acids play an important role in the epigenetic mechanisms, especially DNA methylation. Some amino acids (such as methionine, serine), as dietary methyl donors, can alter epigenetic characteristics (53, 54). And amino acids profile and transport capacity from mother to fetus are influenced by the composition and content of maternal dietary proteins (55). Immune development in utero, including T-helper type 1 (Th)1 and Th2 differentiation patterns, regulatory T cell differentiation, and Th17 development, is under epigenetic control, indicating marked plasticity in early T cell differentiation (50).
In our study, the poultry dietary protein pattern had the highest incidence of infant eczema. We also found that mothers of infants with eczema consumed more protein from poultry food source during pregnancy than mothers of infants without eczema. This result was in line with a previous study in Australia demonstrating that maternal pre-conception poultry intake was positively associated with offspring eczema (56). In the adult literature, two studies also found that diets with high intake of poultry were associated with allergic diseases (57, 58). Because of the potential adverse association between poultry protein intake and eczema, the poultry dietary protein pattern, consisting of more poultry protein, was used as a benchmark for comparison.
We did not find significant difference between the red meat and fish pattern and the poultry dietary pattern on risk of infant eczema. Participants in the red meat and fish dietary pattern consumed a relatively higher protein intake from red meat and fish (even more than 1/3 of protein intake from red meat). In a previous prospective study in Japan, higher maternal meat intake during pregnancy was independently related to an increased risk of infant eczema, whereas no relationship was observed between maternal intake of fish and the risk of eczema (59). However, the effect of maternal fish intake on offspring allergic diseases remains controversial. A pooled analysis of 18 birth cohorts found that maternal fish and seafood intake during pregnancy was not associated with offspring wheeze, asthma, and allergic rhinitis (60). Similarly, a meta-analysis indicated that fish intake during pregnancy had no effect on the risk of eczema (61). However, several epidemiological studies showed protective effects from fish consumption in pregnancy on childhood allergic diseases (62–64). Fish, especially oily fish, is the main dietary source of n-3 PUFAs, which have been suggested to have anti-inflammatory properties and may decrease the risk of allergic diseases (65). In sensitivity analysis of the current study, compared to the red meat and fish pattern, other dietary protein patterns were not associated with a reduced risk of infant eczema, that might be due to the interaction of various food ingredients in this dietary pattern.
Compared to the poultry dietary protein pattern, the plant pattern, characterized by higher protein intake from cereals and tubers, vegetables, soybean, and nuts and seeds, was associated with a reduced risk of infant eczema. And such association was observed in the dairy and eggs pattern, which was characterized by higher protein intake from dairy and eggs. A previous ecological study based on the International Study of Asthma and Allergies in Childhood (ISAAC) reported an inverse relationship between protein from cereals, nuts, and vegetables and childhood eczema (66). A cross-sectional study in Japan demonstrated that higher intake of soy protein was associated with a reduced prevalence of allergic rhinitis among pregnant women (67). In addition, our results were consistent with those of previous studies that indicated a protective effect of maternal intake of vegetables, fruits, and dairy products on infant eczema (19, 68, 69). These foods were rich in antioxidant (such as vitamin C and β-carotene), vitamin D and calcium, which might have potential protective effects on eczema (69, 70). Furthermore, it is well-known that immune system development begins in utero, and immune dysfunction in children who have allergic diseases has been manifest at birth (10). Evidence is accumulating that exposure to food allergens [commonly present in milk, fruit, eggs, nuts, wheat, and fish (21)] during pregnancy may reduce the risk of allergic diseases in offspring (18, 19, 68, 69, 71). And encounter with food allergens in utero via maternal dietary could induce immune tolerance rather than sensitization (10, 18), which may explain that the plant and dairy and eggs dietary protein patterns are associated with a reduced risk of infant eczema compared with the poultry dietary protein pattern.
There are several limitations that should be considered in our study. Firstly, we measured maternal dietary intake via FFQ, which might lead to recall bias and imprecise quantification of dietary intake (29). However, reliability and validity of FFQ among Chinese women have been validated to be good overall (30). And visual aids such as food photographs of portion sizes were provided to help them quantify their food intake. Secondly, data of infant eczema was based on parent-reported by telephone interview, which might lead to misdiagnose and misclassification. However, this misclassification of the outcome was likely to be non-differential in four dietary protein patterns, and the bias effect estimates was expected toward the null. And well-trained investigators can help mothers determine whether their offspring have ever had eczema. Thirdly, although we adjusted numerous potential confounders, residual confounding may also influence the development of infant eczema. Finally, it remains to be seen whether associations between maternal dietary protein patterns during pregnancy and infant eczema persists beyond the age of 6 months. Therefore, continued follow-up of our cohort is required to observe the long-term effects of maternal diet during pregnancy on childhood eczema.
The current prospective cohort study suggested that maternal dietary protein during pregnancy may influence the development of infant eczema at the age of 6 months. Compared with the poultry dietary protein pattern, the maternal plant pattern and dairy and eggs dietary protein pattern were associated with a reduced risk of infant eczema. Optimizing the composition of proteins from different food sources during pregnancy may be a promising strategy for reducing the risk of infant eczema, and further researches regarding biological mechanisms and interventional trials are necessary to confirm our findings.
The datasets analyzed in this article are not publicly available. Requests to access the datasets should be directed to LC, Y2FpbGk1QG1haWwuc3lzdS5lZHUuY24=.
The studies involving human participants were reviewed and approved by the Ethics Committee of the School of Public Health of Sun Yat-Sen University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
LC, JJ, WW, and JZ conceived the study. JZ and WW extracted data and performed all statistical analyses. JZ prepared the manuscript draft. LC, JJ, and WW revised the initial manuscript. LC, NT, and WW critically edited language. LC and JJ supervised the study. LC obtained funding and material support. JZ, WW, NT, LC, JJ, and YC performed the investigation. All authors critically revised drafts of the manuscript and approved the final manuscript.
This work was supported by the Key Realm R&D Program of Guangdong Province (2019B030335001) and the Natural Science Foundation of Guangdong Province (No. 2016A030310150).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank all the participating families and the research assistants involved with our cohort.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.608972/full#supplementary-material
1. Weidinger S, Novak N. Atopic dermatitis. Lancet. (2016) 387:1109–22. doi: 10.1016/S0140-6736(15)00149-X
2. Illi S, von Mutius E, Lau S, Nickel R, Gruber C, Niggemann B, et al. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J Allergy Clin Immunol. (2004) 113:925–31. doi: 10.1016/j.jaci.2004.01.778
3. van der Hulst AE, Klip H, Brand PL. Risk of developing asthma in young children with atopic eczema: a systematic review. J Allergy Clin Immunol. (2007) 120:565–9. doi: 10.1016/j.jaci.2007.05.042
4. Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. (2013) 24:476–86. doi: 10.1111/pai.12095
5. Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. (2013) 131:428–33. doi: 10.1016/j.jaci.2012.10.041
6. Deckers IA, McLean S, Linssen S, Mommers M, van Schayck CP, Sheikh A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PLoS ONE. (2012) 7:e39803. doi: 10.1371/journal.pone.0039803
7. Barker DJ. The fetal and infant origins of adult disease. BMJ. (1990) 301:1111. doi: 10.1136/bmj.301.6761.1111
8. Grieger JA, Clifton VL, Tuck AR, Wooldridge AL, Robertson SA, Gatford KL. In utero programming of allergic susceptibility. Int Arch Allergy Immunol. (2016) 169:80–92. doi: 10.1159/000443961
9. Khan TK, Palmer DJ, Prescott SL. In-utero exposures and the evolving epidemiology of paediatric allergy. Curr Opin Allergy Clin Immunol. (2015) 15:402–8. doi: 10.1097/ACI.0000000000000209
10. West CE, D'Vaz N, Prescott SL. Dietary immunomodulatory factors in the development of immune tolerance. Curr Allergy Asthma Rep. (2011) 11:325–33. doi: 10.1007/s11882-011-0200-0
11. Eyerich K, Eyerich S, Biedermann T. The multi-modal immune pathogenesis of atopic eczema. Trends Immunol. (2015) 36:788–801. doi: 10.1016/j.it.2015.10.006
12. Kezic S, Novak N, Jakasa I, Jungersted JM, Simon M, Brandner JM, et al. Skin barrier in atopic dermatitis. Front Biosci. (2014) 19:542–56. doi: 10.2741/4225
13. Mousa A, Naqash A, Lim S. Macronutrient and micronutrient intake during pregnancy: an overview of recent evidence. Nutrients. (2019) 11:443. doi: 10.3390/nu11020443
14. He ZX, Sun ZH, Yang WZ, Beauchemin KA, Tang SX, Zhou CS, et al. Effects of maternal protein or energy restriction during late gestation on immune status and responses to lipopolysaccharide challenge in postnatal young goats. J Anim Sci. (2014) 92:4856–64. doi: 10.2527/jas.2014-7904
15. Tuokkola J, Luukkainen P, Tapanainen H, Kaila M, Vaarala O, Kenward MG, et al. Maternal diet during pregnancy and lactation and cow's milk allergy in offspring. Eur J Clin Nutr. (2016) 70:554–9. doi: 10.1038/ejcn.2015.223
16. Frazier AL, Camargo CA, Malspeis S, Willett WC, Young MC. Prospective study of peripregnancy consumption of peanuts or tree nuts by mothers and the risk of peanut or tree nut allergy in their offspring. JAMA Pediatr. (2014) 168:156–62. doi: 10.1001/jamapediatrics.2013.4139
17. Werfel T, Ballmer-Weber B, Eigenmann PA, Niggemann B, Rance F, Turjanmaa K, et al. Eczematous reactions to food in atopic eczema: position paper of the EAACI and GA2LEN. Allergy. (2007) 62:723–8. doi: 10.1111/j.1398-9995.2007.01429.x
18. Bunyavanich S, Rifas-Shiman SL, Platts-Mills TA, Workman L, Sordillo JE, Camargo CA, et al. Peanut, milk, and wheat intake during pregnancy is associated with reduced allergy and asthma in children. J Allergy Clin Immunol. (2014) 133:1373–82. doi: 10.1016/j.jaci.2013.11.040
19. Miyake Y, Tanaka K, Okubo H, Sasaki S, Arakawa M. Maternal consumption of dairy products, calcium, and vitamin D during pregnancy and infantile allergic disorders. Ann Allergy Asthma Immunol. (2014) 113:82–7. doi: 10.1016/j.anai.2014.04.023
20. Kang CM, Chiang BL, Wang LC. Maternal nutritional status and development of atopic dermatitis in their offspring. Clin Rev Allergy Immunol. (2020). doi: 10.1007/s12016-020-08780-y. [Epub ahead of print].
21. Pastor-Vargas C, Maroto AS, Diaz-Perales A, Villalba M, Esteban V, Ruiz-Ramos M, et al. Detection of major food allergens in amniotic fluid: initial allergenic encounter during pregnancy. Pediatr Allergy Immunol. (2016) 27:716–20. doi: 10.1111/pai.12608
22. Szepfalusi Z, Loibichler C, Pichler J, Reisenberger K, Ebner C, Urbanek R. Direct evidence for transplacental allergen transfer. Pediatr Res. (2000) 48:404–7. doi: 10.1203/00006450-200009000-00024
23. Edelbauer M, Loibichler C, Witt A, Gerstmayr M, Putschogl B, Urbanek R, et al. Dose-dependent and preterm- accentuated diaplacental transport of nutritive allergens in vitro. Int Arch Allergy Immunol. (2003) 130:25–32. doi: 10.1159/000068373
24. Loibichler C, Pichler J, Gerstmayr M, Bohle B, Kisst H, Urbanek R, et al. Materno-fetal passage of nutritive and inhalant allergens across placentas of term and pre-term deliveries perfused in vitro. Clin Exp Allergy. (2002) 32:1546–51. doi: 10.1046/j.1365-2222.2002.01479.x
25. Vance GH, Lewis SA, Grimshaw KE, Wood PJ, Briggs RA, Thornton CA, et al. Exposure of the fetus and infant to hens' egg ovalbumin via the placenta and breast milk in relation to maternal intake of dietary egg. Clin Exp Allergy. (2005) 35:1318–26. doi: 10.1111/j.1365-2222.2005.02346.x
26. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. (2002) 13:3–9. doi: 10.1097/00041433-200202000-00002
27. Tapsell LC, Neale EP, Satija A, Hu FB. Foods, nutrients, and dietary patterns: interconnections and implications for dietary guidelines. Adv Nutr. (2016) 7:445–54. doi: 10.3945/an.115.011718
28. de Gavelle E, Davidenko O, Fouillet H, Delarue J, Darcel N, Huneau JF, et al. The willingness to modify portion sizes or eat new protein foods largely depends on the dietary pattern of protein intake. Nutrients. (2019) 11:1556. doi: 10.3390/nu11071556
29. Venter C, Higgins B, Grundy J, Clayton CB, Gant C, Dean T. Reliability and validity of a maternal food frequency questionnaire designed to estimate consumption of common food allergens. J Human Nutrition Dietetics. (2006) 19:129–38. doi: 10.1111/j.1365-277X.2006.00677.x
30. Zhang CX, Ho SC. Validity and reproducibility of a food frequency Questionnaire among Chinese women in Guangdong province. Asia Pac J Clin Nutr. (2009) 18:240–50. doi: 10.6133/apjcn.2009.18.2.13
31. YX Y. Chinese Food Composition Table 2004. Beijing: Peking University Medical Press (2005). p. 75–215.
32. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. (1997) 65:1220S–8S; discussion 9S-31S. doi: 10.1093/ajcn/65.4.1220S
33. Chinese Nutrition Society. The Dietary Guidelines for Chinese Residents 2016. Beijing: People's Medical Publishing House (2016). p. 271.
34. Yamei H, Zaifang J, Futang Z. Practical Pediatric of ZHU Futang, The 7th Edn. Beijing: People's Medical Publishing House (2002). p. 642–3.
35. Chen L, Zhu H, Gutin B, Dong Y. Race, gender, family structure, socioeconomic status, dietary patterns, and cardiovascular health in adolescents. Curr Dev Nutr. (2019) 3:nzz117. doi: 10.1093/cdn/nzz117
36. Gonzalez-Gil EM, Tognon G, Lissner L, Intemann T, Pala V, Galli C, et al. Prospective associations between dietary patterns and high sensitivity C-reactive protein in European children: the IDEFICS study. Eur J Nutr. (2018) 57:1397–407. doi: 10.1007/s00394-017-1419-x
37. Monjardino T, Lucas R, Ramos E, Lopes C, Gaio R, Barros H. Associations between a posteriori defined dietary patterns and bone mineral density in adolescents. Eur J Nutr. (2015) 54:273–82. doi: 10.1007/s00394-014-0708-x
38. Funtikova AN, Benitez-Arciniega AA, Fito M, Schroder H. Modest validity and fair reproducibility of dietary patterns derived by cluster analysis. Nutr Res. (2015) 35:265–8. doi: 10.1016/j.nutres.2014.12.011
39. Lo Siou G, Yasui Y, Csizmadi I, McGregor SE, Robson PJ. Exploring statistical approaches to diminish subjectivity of cluster analysis to derive dietary patterns: the tomorrow project. Am J Epidemiol. (2011) 173:956–67. doi: 10.1093/aje/kwq458
40. Vuillermin PJ, Macia L, Nanan R, Tang ML, Collier F, Brix S. The maternal microbiome during pregnancy and allergic disease in the offspring. Semin Immunopathol. (2017) 39:669–75. doi: 10.1007/s00281-017-0652-y
41. Tanabe H, Sakurai K, Kato T, Kawasaki Y, Nakano T, Yamaide F, et al. Association of the maternal microbiome in Japanese pregnant women with the cumulative prevalence of dermatitis in early infancy: A pilot study from the Chiba study of Mother and Child Health birth cohort. World Allergy Organ J. (2019) 12:100065. doi: 10.1016/j.waojou.2019.100065
42. Rist VTS, Weiss E, Sauer N, Mosenthin R, Eklund M. Effect of dietary protein supply originating from soybean meal or casein on the intestinal microbiota of piglets. Anaerobe. (2014) 25:72–9. doi: 10.1016/j.anaerobe.2013.10.003
43. Zhu Y, Shi X, Lin X, Ye K, Xu X, Li C, et al. Beef, chicken, and soy proteins in diets induce different gut microbiota and metabolites in rats. Front Microbiol. (2017) 8:1395. doi: 10.3389/fmicb.2017.01395
44. Ma N, Tian Y, Wu Y, Ma X. Contributions of the interaction between dietary protein and gut microbiota to intestinal health. Curr Protein Pept Sci. (2017) 18:795–808. doi: 10.2174/1389203718666170216153505
45. Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med. (2016) 22:713–22. doi: 10.1038/nm.4142
46. Jenmalm MC. The mother-offspring dyad: microbial transmission, immune interactions and allergy development. J Intern Med. (2017) 282:484–95. doi: 10.1111/joim.12652
47. Collado MC, Rautava S, Aakko J, Isolauri E, Salminen S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep. (2016) 6:23129. doi: 10.1038/srep23129
48. Chu DM, Meyer KM, Prince AL, Aagaard KM. Impact of maternal nutrition in pregnancy and lactation on offspring gut microbial composition and function. Gut Microbes. (2016) 7:459–70. doi: 10.1080/19490976.2016.1241357
49. Neu J. The microbiome during pregnancy and early postnatal life. Semin Fetal Neonatal Med. (2016) 21:373–9. doi: 10.1016/j.siny.2016.05.001
50. Martino DJ, Prescott SL. Silent mysteries: epigenetic paradigms could hold the key to conquering the epidemic of allergy and immune disease. Allergy. (2010) 65:7–15. doi: 10.1111/j.1398-9995.2009.02186.x
51. Martino D, Prescott S. Epigenetics and prenatal influences on asthma and allergic airways disease. Chest. (2011) 139:640–7. doi: 10.1378/chest.10-1800
52. Prescott S, Saffery R. The role of epigenetic dysregulation in the epidemic of allergic disease. Clin Epigenetics. (2011) 2:223–32. doi: 10.1007/s13148-011-0028-4
53. McGee M, Bainbridge S, Fontaine-Bisson B. A crucial role for maternal dietary methyl donor intake in epigenetic programming and fetal growth outcomes. Nutr Rev. (2018) 76:469–78. doi: 10.1093/nutrit/nuy006
54. Maddocks ODK, Labuschagne CF, Adams PD, Vousden KH. Serine metabolism supports the methionine cycle and DNA/RNA methylation through de novo ATP synthesis in cancer cells. Molecular Cell. (2016) 61:210–21. doi: 10.1016/j.molcel.2015.12.014
55. Brown LD, Green AS, Limesand SW, Rozance PJ. Maternal amino acid supplementation for intrauterine growth restriction. Front Biosci. (2011) 3:428–44. doi: 10.2741/s162
56. Grieger JA, Pelecanos AM, Hurst C, Tai A, Clifton VL. Pre-conception maternal food intake and the association with childhood allergies. Nutrients. (2019) 11:1851. doi: 10.3390/nu11081851
57. McKeever TM, Lewis SA, Cassano PA, Ocké M, Burney P, Britton J, et al. Patterns of dietary intake and relation to respiratory disease, forced expiratory volume in 1 s, and decline in 5-y forced expiratory volume. Am J Clin Nutr. (2010) 92:408–15. doi: 10.3945/ajcn.2009.29021
58. Rosenkranz RR, Rosenkranz SK, Neessen KJ. Dietary factors associated with lifetime asthma or hayfever diagnosis in Australian middle-aged and older adults: a cross-sectional study. Nutr J. (2012) 11:84. doi: 10.1186/1475-2891-11-84
59. Saito K, Yokoyama T, Miyake Y, Sasaki S, Tanaka K, Ohya Y, et al. Maternal meat and fat consumption during pregnancy and suspected atopic eczema in Japanese infants aged 3-4 months: the Osaka Maternal and Child Health Study. Pediatr Allergy Immunol. (2010) 21:38–46. doi: 10.1111/j.1399-3038.2009.00897.x
60. Stratakis N, Roumeliotaki T, Oken E, Ballester F, Barros H, Basterrechea M, et al. Fish and seafood consumption during pregnancy and the risk of asthma and allergic rhinitis in childhood: a pooled analysis of 18 European and US birth cohorts. Int J Epidemiol. (2017) 46:1465–77. doi: 10.1093/ije/dyx007
61. Zhang GQ, Liu B, Li J, Luo CQ, Zhang Q, Chen JL, et al. Fish intake during pregnancy or infancy and allergic outcomes in children: A systematic review and meta-analysis. Pediatr Allergy Immunol. (2017) 28:152–61. doi: 10.1111/pai.12648
62. Sausenthaler S, Koletzko S, Schaaf B, Lehmann I, Borte M, Herbarth O, et al. Maternal diet during pregnancy in relation to eczema and allergic sensitization in the offspring at 2 y of age. Am J Clin Nutr. (2007) 85:530–7. doi: 10.1093/ajcn/85.2.530
63. Romieu I, Torrent M, Garcia-Esteban R, Ferrer C, Ribas-Fito N, Anto JM, et al. Maternal fish intake during pregnancy and atopy and asthma in infancy. Clin Exp Allergy. (2007) 37:518–25. doi: 10.1111/j.1365-2222.2007.02685.x
64. Willers SM, Devereux G, Craig LC, McNeill G, Wijga AH, Abou El-Magd W, et al. Maternal food consumption during pregnancy and asthma, respiratory and atopic symptoms in 5-year-old children. Thorax. (2007) 62:773–9. doi: 10.1136/thx.2006.074187
65. Calder PC, Kremmyda LS, Vlachava M, Noakes PS, Miles EA. Is there a role for fatty acids in early life programming of the immune system? Proc Nutr Soc. (2010) 69:373–80. doi: 10.1017/S0029665110001552
66. Ellwood P, Asher MI, Bjorksten B, Burr M, Pearce N, Robertson CF. Diet and asthma, allergic rhinoconjunctivitis and atopic eczema symptom prevalence: an ecological analysis of the International Study of Asthma and Allergies in Childhood (ISAAC) data. ISAAC Phase One Study Group. Eur Respir J. (2001) 17:436–43. doi: 10.1183/09031936.01.17304360
67. Miyake Y, Sasaki S, Ohya Y, Miyamoto S, Matsunaga I, Yoshida T, et al. Soy, isoflavones, and prevalence of allergic rhinitis in Japanese women: the Osaka Maternal and Child Health Study. J Allergy Clin Immunol. (2005) 115:1176–83. doi: 10.1016/j.jaci.2005.02.016
68. Miyake Y, Sasaki S, Tanaka K, Hirota Y. Consumption of vegetables, fruit, and antioxidants during pregnancy and wheeze and eczema in infants. Allergy. (2010) 65:758–65. doi: 10.1111/j.1398-9995.2009.02267.x
69. Miyake Y, Sasaki S, Tanaka K, Hirota Y. Dairy food, calcium and vitamin D intake in pregnancy, and wheeze and eczema in infants. Eur Respir J. (2010) 35:1228–34. doi: 10.1183/09031936.00100609
70. West CE, Dunstan J, McCarthy S, Metcalfe J, D'Vaz N, Meldrum S, et al. Associations between maternal antioxidant intakes in pregnancy and infant allergic outcomes. Nutrients. (2012) 4:1747–58. doi: 10.3390/nu4111747
Keywords: pregnancy, protein, dietary pattern, food sources, infant eczema
Citation: Zeng J, Wu W, Tang N, Chen Y, Jing J and Cai L (2021) Maternal Dietary Protein Patterns During Pregnancy and the Risk of Infant Eczema: A Cohort Study. Front. Nutr. 8:608972. doi: 10.3389/fnut.2021.608972
Received: 22 September 2020; Accepted: 11 May 2021;
Published: 02 June 2021.
Edited by:
Brandt D. Pence, University of Memphis, United StatesReviewed by:
Kate J. Claycombe, United States Department of Agriculture (USDA), United StatesCopyright © 2021 Zeng, Wu, Tang, Chen, Jing and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Cai, Y2FpbGk1QG1haWwuc3lzdS5lZHUuY24=; Jin Jing, amluZ2ppbkBtYWlsLnN5c3UuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.