
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 09 April 2021
Sec. Nutritional Epidemiology
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.604296
This article is part of the Research TopicObjective Dietary Assessment in Nutrition Epidemiology StudiesView all 13 articles
 Ayaka Kotemori1,2
Ayaka Kotemori1,2 Norie Sawada1*
Norie Sawada1* Motoki Iwasaki1
Motoki Iwasaki1 Taiki Yamaji1
Taiki Yamaji1 Nitin Shivappa3,4,5
Nitin Shivappa3,4,5 James R. Hebert3,4,5
James R. Hebert3,4,5 Junko Ishihara2
Junko Ishihara2 Manami Inoue1
Manami Inoue1 Shoichiro Tsugane1
Shoichiro Tsugane1Background: Dietary components are known to affect chronic low-grade inflammation status. The dietary inflammatory index (DII®) was developed to measure the potential impact of a diet on an individual's inflammatory status, and it has been validated mainly in Western countries.
Objective: This study aimed to examine the validity of the energy-adjusted DII (E-DIITM) using high-sensitivity C-reactive protein (hs-CRP) concentration in Japanese men and women.
Methods: In total, 6,474 volunteers from a cancer-screening program (3,825 men and 2,649 women) completed a food frequency questionnaire (FFQ) and their hs-CRP concentrations were evaluated. E-DII scores were calculated on the basis of 30 food parameters derived from the FFQ. Higher E-DII scores reflect a greater pro-inflammatory potential of the diet. The associations between E-DII quartiles and hs-CRP concentration were assessed using regression models adjusted for age, body mass index, smoking status, and amount of physical activity.
Results: Mean E-DII in men and women was + 0.62 ± 1.93 and −1.01 ± 2.25, respectively. The proportion of men and women who had hs-CRP concentration >3 mg/L was 4.7 and 3.1%, respectively. A significant positive association was observed between E-DII score and hs-CRP concentration in men; geometric mean of hs-CRP concentration in the lowest and highest E-DII quartiles was 0.56 mg/L and 0.67 mg/L (Ptrend < 0.01), respectively. The odds ratio (95% confidence interval) of having an elevated hs-CRP concentration (>3 mg/L) was 1.72 (1.10–2.67) in the highest E-DII quartile (Ptrend = 0.03) in men. However, no association was observed between E-DII score and hs-CRP concentration in women, except in those not taking prescription medications.
Conclusions: DII was associated with inflammation status in Japanese men, but the association was limited in Japanese women.
Low-grade chronic inflammation promotes the development of lifestyle-related chronic diseases such as cancer (1–3), cardiovascular disease (4), diabetes (5), and depression (6). High-sensitivity C-reactive protein (hs-CRP) is a well-known inflammatory biomarker, and previous studies have reported that elevated concentrations of hs-CRP are associated with an increased risk of cancer and the incidence of other chronic diseases (1–3, 7, 8).
Dietary components are one of the key factors affecting an individual's inflammatory status (9). High intake of dietary fiber has been shown to be associated with low hs-CRP concentrations, whereas high intake of saturated fatty acids leads to elevation in hs-CRP concentration (10). In addition, a healthy dietary pattern, characterized by high intake of fruits, vegetables, and fish, has been associated with lower hs-CRP concentrations (11). Mediterranean diet also has been shown to reduce hs-CRP concentrations in randomized controlled trials (12, 13). In contrast, Western dietary patterns, characterized by high intake of red and processed meat, is associated with high hs-CRP concentrations (11). These reports suggest that dietary components and dietary patterns may have a contrasting effect on inflammatory status. Therefore, a comprehensive index is required to understand the potential impact of whole diet on inflammatory status.
The Dietary Inflammatory Index (DII®) is a literature-based dietary score that was developed to measure the potential impact of a diet on the inflammatory status of an individual; a high DII score reflects pro-inflammatory potential of the diet, whereas a low DII score reflects the anti-inflammatory potential of the diet (14, 15). To date, 30 validation studies have been performed between DII and various inflammatory markers, mainly in Western countries (15–18). In addition, the DII has also been shown to be associated with an increased risk of many chronic diseases including cancer (19–21). In Japan, one study reported that a higher DII score increased the risk of upper aerodigestive tract cancers (22). Among the 30 construct validations performed throughout the world, two have been performed in Japan (18, 23), the results of which are inconsistent with regard to sex-specific analyses. Dietary habits and inflammatory status are considerably different between Japanese and Western populations (23–26). Therefore, it is important to determine the utility of the DII to quantify the inflammatory potential of diet in Japanese men and women.
Previously, we conducted a cross-sectional validation study in a subsample from the Japan Public Health Center-based prospective (JPHC) study but could not identify a positive association between the DII and inflammatory status in women (23). The underlying reasons for the null result could be the small sample size of the study and the fact that women consume a more anti-inflammatory diet than do men (23). Therefore, the present study aimed to assess the associations between DII scores and hs-CRP concentrations in a large number of Japanese men and women.
The National Cancer Center of Japan started a cancer-screening program in February 2004. The total number of participants in the present study was 7,919 healthy volunteers (4,664 men and 3,255 women) aged 40–69 years who had participated in the cancer-screening program from May 2009 to December 2013. The blood samples of participants were collected during cancer screening. They also answered self-administered questionnaires for demographic, lifestyle, and dietary information. This study was approved by the Institutional Review Board of the National Cancer Center, Tokyo, Japan (approval number G15-01 and 2016-165). The study aims and protocols were explained to all participants, and each participant provided written informed consent before enrolment in the study.
The self-administered questionnaire was designed to obtain participants' demographic, lifestyle, and dietary information, including information on height and weight at the time of examination, smoking status, physical activity, and past medical history of cancer, stroke, and myocardial infarction. Dietary information was obtained using a validated food frequency questionnaire (FFQ), which had questions on the consumption of 188 food and beverage items other than supplements. Energy and nutrient intakes were calculated by taking the sum of the products of eating frequency, portion size, and energy and nutrient content of each food, while referring to the Standard Tables of Food Composition in Japan, Fifth Revised and Enlarged Edition (27). A validation study for the energy and nutrient intake had already been conducted in a subsample of the examinees of the cancer screening program, wherein FFQ-derived data were compared with the data derived from four-day weighed dietary records. The correlation coefficients of energy intake in men and women were 0.53 and 0.34, respectively, and the median correlation coefficients of 45 nutrients were 0.57 and 0.47, respectively. More detailed information has been described elsewhere (28).
The DII is a literature-based dietary index calculated from 45 nutrients and food components to assess the potential impact of a diet on the inflammation status; a high score indicates pro-inflammatory potential of the diet and a low score indicates anti-inflammatory potential of the diet. The index was developed by reviewing and scoring 1,943 peer-reviewed publications, which included cell culture experiments, animal experiments, and human studies, and examined the associations between various dietary components and six inflammatory biomarkers [interleukin (IL)-1β, IL-4, IL-6, IL-10, Tumor Necrosis Factor α, and CRP] (14). Inflammatory effect scores for each of the DII components were determined by considering the direction of the effect on inflammation, weight of study design, and number of publications. The inflammatory effect scores are available from the publication (14). To calculate an individual's DII score, dietary intake of the DII components was standardized as a Z-score using global daily mean intake and converted into proportion scores in the study population. The global daily mean intake was calculated from a national database of dietary intake in eleven countries including Japan (14). Then, the individual's DII score was calculated as the sum of the products of the centered proportion score and the inflammatory effect score for each of the DII components.
In the present study, we computed the energy-adjusted DII (E-DIITM) using the energy-adjusted intake of the following 30 DII components (out of 45 possible components) (23): protein, total fat, saturated fatty acid, monounsaturated fatty acid, polyunsaturated fatty acid, n-3 fatty acid, n-6 fatty acid, cholesterol, carbohydrate, magnesium, iron, zinc, retinol equivalent, beta-carotene, vitamin D, alpha-tocopherol, vitamin B1, vitamin B2, niacin, vitamin B6, vitamin B12, folate, vitamin C, total dietary fiber, isoflavone, ethanol, onion, green or black tea, and caffeine. Some of the components (such as thyme or oregano and rosemary) are not commonly consumed by the Japanese (23). Energy adjustment was done using the density method, and the amount per 1,000 kcal was used for E-DII calculation.
Fasting blood samples were collected along with the self-administered questionnaire data before any cancer screening procedures on the first day of screening. Venous blood was drawn into a vacutainer tube without anticoagulant, and the samples were centrifuged to obtain serum.
Serum hs-CRP concentrations were measured using a commercial reagent kit (Nanopia CRP, Sekisui Medical Co., Ltd., Tokyo, Japan) with an automated analyzer JCA-BM6070 (Jeol Ltd., Tokyo, Japan) at National Cancer Center, Tokyo, Japan. The intra-assay coefficient of variation was 1.8% for 1 mg/L, 1.3% for 2 mg/L, and 0.7% for 82 mg/L of CRP concentration (n = 20 each) according to the manufacturer's data (29). The detection range of the kit was 0.2 mg/L−420 mg/L. In case the values were lower than the lower limit of quantification, we assigned a value of 0.1 mg/L.
Among the 7,919 participants, 1,445 were excluded on the basis of following criteria: BMI <14 or >40 kg/m2 (n = 13); missing data on smoking status (n = 1); missing data on metabolic equivalents (n = 1); participants with a history of any cancer, stroke, and myocardial infarction (n = 877); extreme energy intake identified in the upper and lower 2.5%-tile (n = 349); missing data on alcohol consumption (n = 120); and missing data on hs-CRP or hs-CRP concentration >10 mg/L (n = 84). Therefore, data from 6,474 participants (3,825 men and 2,649 women) were included in the statistical analysis. Cohen's effect size f 2 and noncentral F distribution F(dfReg, dfRes, λ) were used to calculate sample size n for multiple regression analysis; where , R2 is the coefficient of determination, dfReg is the degree of freedom for regression (= k), dfRes is the degree of freedom for residual (= n – k – 1), and the noncentral parameter λ is λ = f2n (30). When we set the effect size = 0.01, k = 12, power = 0.8, and significance level = 0.05, the sample size n was calculated to be 1,745.
Participants' characteristics were summarized using percentages for categorical variables; mean and standard deviation (SD), as well as median and interquartile range for continuous variables; and geometric mean and coefficient of variation (CV) for log-transformed hs-CRP. The CV was also calculated with the log-transformed value using the formula: CV = (eSD−1)1/2 (31). Comparison of characteristics between men and women was done using the chi-square test for categorical variables. The Mann–Whitney U test was used for all continuous variables, as the Kolmogorov–Smirnov test revealed non-normal distribution of all variables except men's height.
Multiple regression analyses were performed for the associations between the E-DII score and food group intake. Associations between the E-DII scores and hs-CRP concentration were evaluated using multivariable linear regression models. Linear trends across E-DII quartiles were calculated using natural log-transformed hs-CRP concentration as a dependent variable. To interpret a partial regression coefficient estimated using this model, e (Euler's number) should be exponentiated with that coefficient. The models were adjusted for age (years; continuous), BMI (kg/m2; continuous), smoking status (current, past, and never), regular prescription medicine use (yes or no), and daily total physical activity level (MET-h/d; continuous). Sensitivity analyses were performed stratified by age, BMI, and regular prescription medicine use. In addition, logistic regression models were applied to estimate the odds ratio (OR) of having elevated hs-CRP concentration (>3 mg/L) across quartiles of E-DII score because this is a clinically relevant cut-off point for chronic inflammation status (32). All statistical analyses were performed using Statistical Analysis Systems software, version 9.3 (SAS Institute Inc., Cary, NC, USA), and the significance level was set at p < 0.05.
Table 1 shows the characteristics of the participants stratified by sex. BMI and the proportion of smokers and regular prescription medicine users were significantly higher in men than in women. The amount of physical activity was significantly higher in women than in men. The E-DII score was significantly higher in men (+0.62 ± 1.93) than in women (−1.01 ± 2.25) (p < 0.001). Hs-CRP concentrations also were significantly higher in men than in women; mean (CV) was 0.58 mg/L (1.21) and 0.44 mg/L (1.24), respectively (p < 0.001). The proportion of participants who had hs-CRP concentration >3 mg/L was also significantly higher in men than in women (4.7 and 3.1%, respectively; p < 0.001). The comparisons between the prescription medication non-users and users are shown in Supplementary Table 1. In both sexes, users were older and had higher BMI, lower DII, and higher hs-CRP concentrations than non-users did.
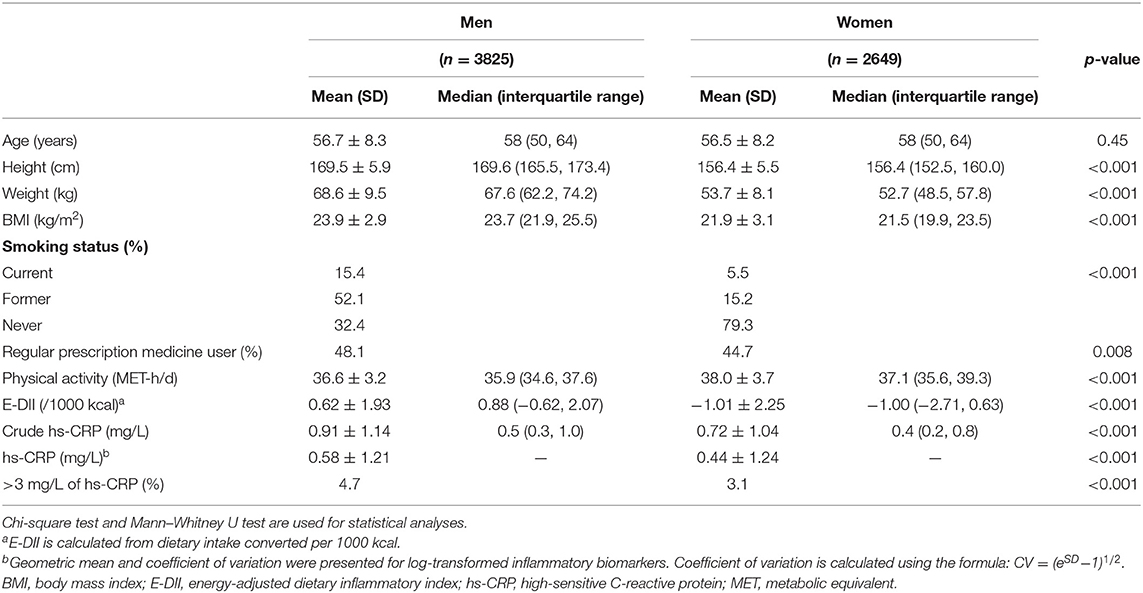
Table 1. Characteristics of the volunteers of the cancer-screening program from May 2009 to December 2013 at National Cancer Center Japan.
Table 2 shows the results of multiple regression analyses between E-DII score and food group intake, stratified by sex. The findings suggest that a higher E-DII score was associated with a higher intake of sugar, meat, and confectioneries in men, while a higher E-DII score was associated only with higher sugar intake in women.
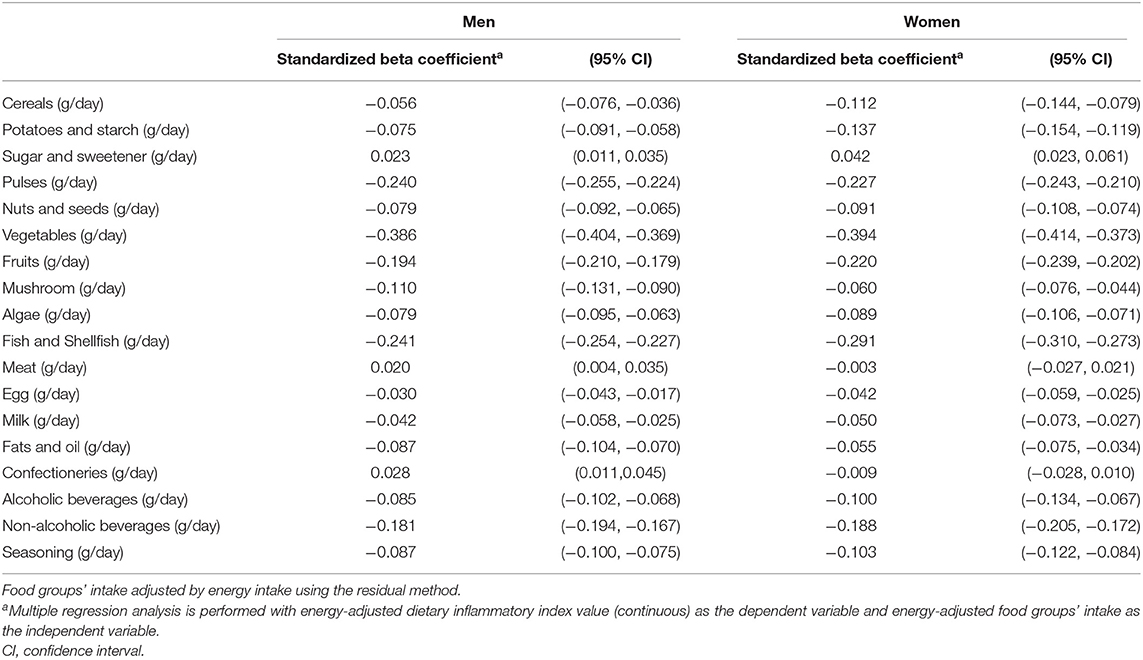
Table 2. Standardized beta coefficient with 95% confidence interval between E-DII score and food groups' intake by multiple regression analyses in Japanese men and women aged 40–69 years.
Table 3 shows the mean of E-DII score and the geometric mean of hs-CRP concentration according to the E-DII quartiles. The mean E-DII score increased from −2.04 to 2.85, and the geometric mean of hs-CRP concentration increased from 0.56 mg/L to 0.67 mg/L in men, according to the E-DII quartiles. For women, the mean E-DII score increased from −3.93 to 1.88, but the geometric mean of hs-CRP concentration remained unchanged across E-DII quartiles. A significant positive association was observed between E-DII quartiles and hs-CRP concentration in men (partial regression coefficient = 0.064, Ptrend < 0.01) but not in women (partial regression coefficient = 0.012, Ptrend = 0.44). This means that for every increase in E-DII quartiles in men, there was a 6.6% (e0.064 = 1.066) increase in the geometric mean concentration of hs-CRP.
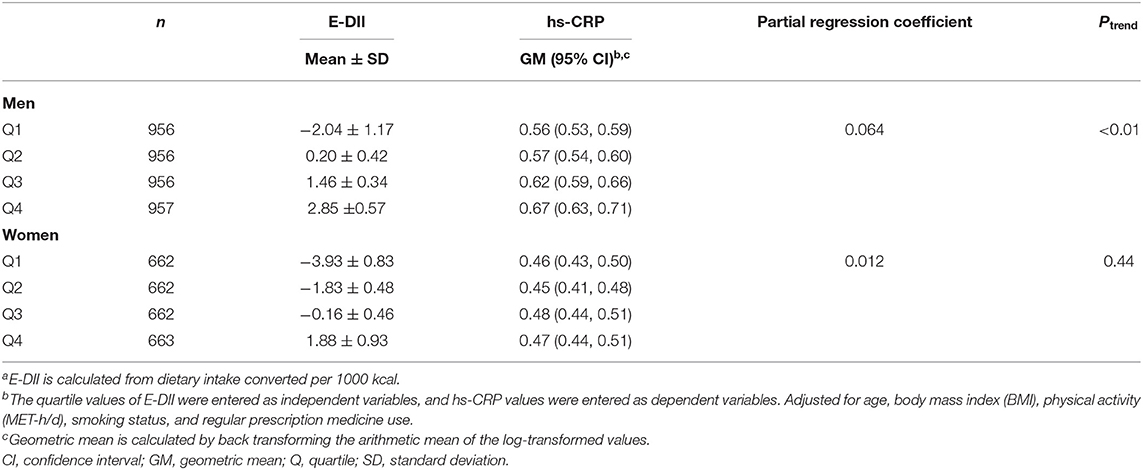
Table 3. Adjusted geometric mean and 95% confidence interval of high-sensitivity C-reactive protein (hs-CRP) concentration in serum (mg/L) according to quartile of energy-adjusted dietary inflammatory index (E-DII)a.
In the sensitivity analysis stratified by age, although a positive association was not observed in participants aged 40–49 years (Ptrend = 0.31), significant positive associations were observed in those aged 50–59 years (Ptrend = 0.01) and 60–69 years (Ptrend < 0.01) in men. Upon stratification by BMI, positive associations were consistently observed in underweight (14 kg/m2 ≤ BMI < 18.5 kg/m2, Ptrend = 0.01), lower normal-weight (18.5 kg/m2 ≤ BMI < 22 kg/m2, Ptrend = 0.01), higher normal-weight (22 kg/m2 ≤ BMI <25 kg/m2, Ptrend < 0.01), and overweight and obese (25 kg/m2 ≤ BMI < 40 kg/m2, Ptrend = 0.08) men. Even when stratified by medication status, a significant association was observed in men (non-users, Ptrend < 0.01; users, Ptrend < 0.01). In contrast, no significant associations were observed between E-DII quartiles and hs-CRP concentration, stratified by age and BMI, in women. Similarly, when stratified by medication status in women, no association was observed in users; however, there was a significant positive association for women non-prescription-drug users (non-users, Ptrend = 0.034; users, Ptrend = 0.267, see the Supplementary Table 2).
Table 4 shows the association between E-DII quartiles and higher hs-CRP (>3 mg/L) concentration. Men in the highest quartile of E-DII had 72% higher odds of having CRP >3 mg/L than men in the lowest quartile of E-DII did [OR: 1.72, 95% confidence interval (CI): 1.10–2.67, Ptrend = 0.03]. In contrast, no significant association was observed among women (OR for the highest vs. lowest: 0.92, 95% CI: 0.47–1.82, Ptrend = 0.96).
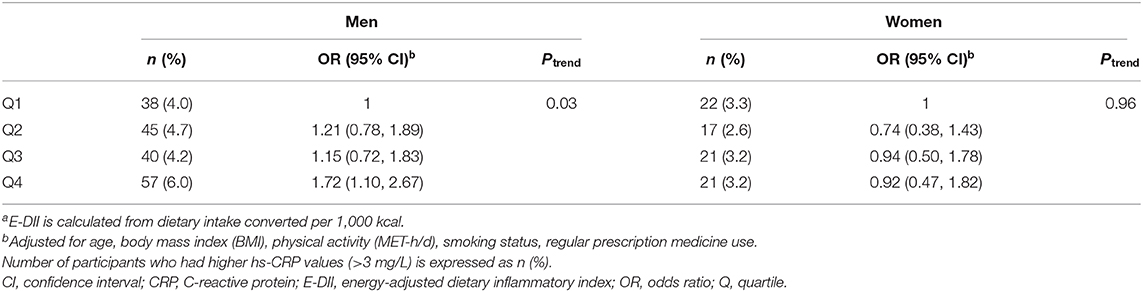
Table 4. Adjusted odds ratios and 95% confidence interval for the association between energy-adjusted dietary inflammatory index (E-DII) quartilea and >3 mg/L of high-sensitivity C-reactive protein (hs-CRP).
The DII was developed to evaluate the inflammatory potential of people's diets. The present study was conducted to validate the E-DII with a hs-CRP concentration in a large number of Japanese participants. The findings of our study suggest that there are significant positive associations in all men and only in women who are prescription drug non-users.
The positive association in Japanese men observed in the present study is consistent with the results of previous studies from Western countries (15–17) and Japan (18, 23). The dietary habits of the Japanese are different from those of the Western people, and the CRP concentrations of the Japanese (0.6 mg/L in the men of the present study) are considerably lower than those of Westerners [typically, they are approximately 2–3 mg/L (15, 17)]. Therefore, the positive association observed in Japanese men in the present study suggests that DII may apply to a diverse male population with considerably different dietary habits and a different range of inflammatory status.
As in previous studies, inconsistent results were observed between Japanese men and women in the present study. We could not detect any associations between E-DII scores and hs-CRP concentrations across women in this study. However, we did observe a positive association when we restricted the analyses to non-users of prescription medicine. The mean hs-CRP concentration was significantly lower in women than in men. In addition, the proportion of participants with hs-CRP concentration >3.0 mg/L was significantly lower in women than in men. Therefore, owing to the low concentration of hs-CRP in Japanese women in this study, detection of the association between E-DII score and hs-CRP concentration may be difficult. This may have also been responsible for the limited positive association between DII and hs-CRP concentration in non-users of prescription drugs among women. In this study, we excluded from the analysis subjects with a history of cancer, stroke, or myocardial infarction, which could be confounding. However, approximately half of the subjects were regular users of some prescription medication. It has been reported that hs-CRP concentrations are higher in hypertension patients than in healthy subjects (33) and that hs-CRP concentrations are higher in diabetes patients with high HbA1c concentrations (34). Considering these previous studies, hs-CRP concentrations may be high in populations with some disease taking prescribed medication. In fact, in the current study, prescription drug users of both sexes had higher hs-CRP concentrations than non-users did. The difference in the percentage of people with high hs-CRP concentration was particularly apparent in women (see Supplementary Table 1). Therefore, we suggest that in women, the association between DII and hs-CRP concentration may have been more clearly demonstrated in non-drug users only. Of note, studies in populations with low hs-CRP concentrations are limited; therefore, further studies are needed to determine the utility of DII in populations with low CRP concentrations, such as Japanese women.
Another underlying reason for the null finding among Japanese women may be the differences in dietary habits between men and women. The women's diets had significantly lower inflammatory potential, indicated by the negative value of mean E-DII score, than those of men, who showed a positive value of mean E-DII score. This is in line with the results of our previous study, wherein the E-DII scores of women were much lower than those of men, and no associations were observed in women in that study either (23). Further, similar results have been reported in a previous study conducted in postmenopausal women from USA, wherein mean E-DII score was a negative value (−0.62 ± 2.69), and a null association was observed between E-DII score and hs-CRP concentration despite having comparatively higher hs-CRP concentration (mean 1.36 mg/L) (35). Taking into consideration the aforementioned reports, it is possible that if the target population eats a predominantly anti-inflammatory diet, the association between DII score and hs-CRP concentration may be difficult to detect.
The dietary patterns that constitute E-DII may vary among populations. Among men with an association between E-DII and inflammatory markers in this study, the food groups positively associated with E-DII were meat and confectioneries. Similarly, in NIPPON DATA, wherein the association with inflammatory markers was confirmed, meat and confectioneries, as well as cereals and fats, were positively associated with E-DII (18). Contrastingly, in the male population of the JPHC-FFQ validation study, meat and confectioneries were not associated with E-DII. The higher the E-DII, the lower the intake of potatoes, legumes, vegetables, and seaweed (23). The study period of the current study (2009–2013) is similar to that of the NIPPON DATA2010 study (18), but it differs from that of the JPHC-FFQ validation study (1990–1993) (23). Thus, the differences in the dietary habits due to cohort effects may contribute to the differences in the dietary patterns involved in E-DII. Further studies in various populations are needed to determine the types of dietary patterns involved.
This study has several limitations. First, the participants of the present study were individuals who had voluntarily undergone cancer screening. These participants, especially women, may be particularly health conscious as indicated by their smoking rate, which was considerably lower than that observed in the National Health and Nutrition Survey in Japan: approximately 15% men and 5% women in the current study compared with 30% men and 10% women in the national survey (36). This selection bias might have contributed to the null association between E-DII score and hs-CRP concentration, observed in women. Second, because this is a cross-sectional study, we could not account for the temporality requirement for assessing causality. Despite these limitations, the present study is a relatively large-scale study, and the results indicated gender differences in E-DII validity and reaffirmed validity in Japanese men. Thus, this justifies the use of E-DII in Japanese men.
To conclude, we conducted a validation study of E-DII using hs-CRP concentration in Japanese men and women and observed a positive association between E-DII scores and hs-CRP concentration in Japanese men, even after adjusting for age, BMI, smoking status, regular prescription medicine use, and physical activity. This indicates the utility of the E-DII in Japanese men who have different dietary habits and considerably lower-grade inflammation status than those of the Western population. However, we could not detect any association between E-DII scores and hs-CRP concentration in Japanese women, except for prescription medication non-users. Therefore, further studies are needed to clarify the utility of the E-DII in Japanese women.
We cannot publicly provide individual data due to participant privacy, according to ethical guidelines in Japan. Additionally, the informed consent we obtained does not include a provision for publicly sharing data. Qualifying researchers may apply to access a minimal dataset by contacting Dr. Shoichiro Tsugane, Principal Investigator, Epidemiology and Prevention group, Center for Public Health Sciences, National Cancer Center, Tokyo, Japan, at c3RzdWdhbmVAbmNjLmdvLmpw. Or, please contact the Office of the Research Center for Cancer Prevention and Screening Program at dHlhbWFqaUBuY2MuZ28uanA=.
This study was approved by the Institutional Review Board of the National Cancer Center, Tokyo, Japan (approval number G15-01 and 2016-165). The study aims and protocols were explained to all participants, and each participant provided written informed consent before enrolment in the study.
AK, NSa, MIw, JI, and MIn designed the study. ST, JI, NSa, MIw, and MIn arranged the field survey. AK, NSa, MIw, and TY contributed to the blood analysis. NSh and JH conducted DII calculation and provided critical input to the manuscript. AK performed statistical analysis, interpreted the results, and drafted the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Ministry of Health, Labour and Welfare of Japan (Grant-in-Aid for the 3rd Term Comprehensive 10-Year-Strategy for Cancer Control), the National Cancer Center Research and Development Fund (23-A-1, 26-A-1, 29-A-1), Practical Research for Innovative Cancer Control from Japan Agency for Medical Research and Development (AMED; No. JP19ck0106266h003), and JSPS KAKENHI (Grant Number: 20K15488).
JH owns a controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the dietary inflammatory index (DII), from the University of South Carolina, to develop computer and smartphone applications for patient counseling and dietary intervention in the clinical setting. NSh is an employee of CHI. The subject matter of this paper will have no direct bearing on the work of CHI, nor has any CHI-related activity exerted any influence on this project. MI was the beneficiary of a financial contribution from the AXA Research Fund as a chair holder of the AXA Department of Health and Human Security at The University of Tokyo. The AXA Research Fund had no role in the design, data collection, analysis, interpretation, manuscript drafting, or in the decision to submit the manuscript for publication.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to all the participants of the study and to the doctors, nurses, and administrative staff at the Research Center for Cancer Prevention and Screening who were involved in the study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.604296/full#supplementary-material
BMI, body mass index; CI, confidence interval; CV, coefficient of variation; DII, dietary inflammatory index; E-DII, energy-adjusted dietary inflammatory index; FFQ, food frequency questionnaire; hs-CRP, high-sensitivity C-reactive protein; MET, metabolic equivalent; OR, odds ratio; SD, standard deviation.
1. Guo YZ, Pan L, Du CJ, Ren DQ, Xie XM. Association between C-reactive protein and risk of cancer: a meta-analysis of prospective cohort studies. Asian Pac J Cancer Prev. (2013) 14:243–8. doi: 10.7314/APJCP.2013.14.1.243
2. Sasazuki S, Inoue M, Sawada N, Iwasaki M, Shimazu T, Yamaji T, et al. Plasma levels of C-reactive protein and serum amyloid a and gastric cancer in a nested case-control study: Japan public health center-based prospective study. Carcinogenesis. (2010) 31:712–8. doi: 10.1093/carcin/bgq010
3. Otani T, Iwasaki M, Sasazuki S, Inoue M, Tsugane S. Japan public health center-based prospective study group. Plasma C-reactive protein and risk of colorectal cancer in a nested case-control study: Japan public health center-based prospective study. Cancer Epidemiol Biom Prev. (2006) 15:690–5. doi: 10.1158/1055-9965.EPI-05-0708
4. Sarwar N, Thompson AJ, Di Angelantonio E. Markers of inflammation and risk of coronary heart disease. Dis Markers. (2009) 26:217–25. doi: 10.1155/2009/851962
5. Black PH. The inflammatory response is an integral part of the stress response: implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav Immun. (2003) 17:350–64. doi: 10.1016/S0889-1591(03)00048-5
6. Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. (2015) 172:1075–91. doi: 10.1176/appi.ajp.2015.15020152
7. Calabro P, Golia E, Yeh ET. CRP and the risk of atherosclerotic events. Semin Immunopathol. (2009) 31:79–94. doi: 10.1007/s00281-009-0149-4
8. Mora S, Musunuru K, Blumenthal RS. The clinical utility of high-sensitivity C-reactive protein in cardiovascular disease and the potential implication of JUPITER on current practice guidelines. Clin Chem. (2009) 55:219–28. doi: 10.1373/clinchem.2008.109728
9. Smidowicz A, Regula J. Effect of nutritional status and dietary patterns on human serum C-reactive protein and interleukin-6 concentrations. Adv Nutr. (2015) 6:738–47. doi: 10.3945/an.115.009415
10. King DE, Egan BM, Geesey ME. Relation of dietary fat and fiber to elevation of C-reactive protein. Am J Cardiol. (2003) 92:1335–9. doi: 10.1016/j.amjcard.2003.08.020
11. Wood AD, Strachan AA, Thies F, Aucott LS, Reid DM, Hardcastle AC, et al. Patterns of dietary intake and serum carotenoid and tocopherol status are associated with biomarkers of chronic low-grade systemic inflammation and cardiovascular risk. Br J Nutr. (2014) 112:1341–52. doi: 10.1017/S0007114514001962
12. Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. (2004) 292:1440–6. doi: 10.1001/jama.292.12.1440
13. Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C. Adherence to the mediterranean diet attenuates inflammation and coagulation process in healthy adults: the ATTICA study. J Am Coll Cardiol. (2004) 44:152–8. doi: 10.1016/j.jacc.2004.03.039
14. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96. doi: 10.1017/S1368980013002115
15. Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS. A population-based dietary inflammatory index predicts levels of C-reactive protein in the seasonal variation of blood cholesterol study (SEASONS). Public Health Nutr. (2014) 17:1825–33. doi: 10.1017/S1368980013002565
16. Shivappa N, Wirth MD, Hurley TG, Hebert JR. Association between the dietary inflammatory index (DII) and telomere length and C-reactive protein from the national health and nutrition examination survey-1999-2002. Mol Nutr Food Res. (2017) 61:1–7. doi: 10.1002/mnfr.201600630
17. Shivappa N, Bonaccio M, Hebert JR, Di Castelnuovo A, Costanzo S, Ruggiero E, et al. Association of proinflammatory diet with low-grade inflammation: results from the moli-sani study. Nutrition. (2018) 54:182–8. doi: 10.1016/j.nut.2018.04.004
18. Yang Y, Hozawa A, Kogure M, Narita A, Hirata T, Nakamura T, et al. Dietary inflammatory index positively associated with high-sensitivity C-reactive protein level in Japanese from NIPPON DATA2010. J Epidemiol. (2020) 30:98–107. doi: 10.2188/jea.JE20180156
19. Shivappa N, Godos J, Hebert JR, Wirth MD, Piuri G, Speciani AF, et al. Dietary inflammatory index and colorectal cancer risk—a meta-analysis. Nutrients. (2017) 9:1–17. doi: 10.3390/nu9091043
20. Fowler ME, Akinyemiju TF. Meta-analysis of the association between dietary inflammatory index (DII) and cancer outcomes. Int J Cancer. (2017) 141:2215–27. doi: 10.1002/ijc.30922
21. Phillips CM, Chen LW, Heude B, Bernard JY, Harvey NC, Duijts L, et al. Dietary inflammatory index and non-communicable disease risk: a narrative review. Nutrients. (2019) 11:1–32. doi: 10.3390/nu11081873
22. Abe M, Shivappa N, Ito H, Oze I, Abe T, Shimizu Y, et al. Dietary inflammatory index and risk of upper aerodigestive tract cancer in Japanese adults. Oncotarget. (2018) 9:24028–40. doi: 10.18632/oncotarget.25288
23. Kotemori A, Sawada N, Iwasaki M, Yamaji T, Shivappa N, Hebert JR, et al. Validating the dietary inflammatory index using inflammatory biomarkers in a Japanese population: a cross-sectional study of the JPHC-FFQ validation study. Nutrition. (2020) 69:110569. doi: 10.1016/j.nut.2019.110569
24. Willcox DC, Scapagnini G, Willcox BJ. Healthy aging diets other than the Mediterranean: a focus on the Okinawan diet. Mech Ageing Dev. (2014) 136–137:148–62. doi: 10.1016/j.mad.2014.01.002
25. Oda E, Kawai R. Tentative cut point of high-sensitivity C-reactive protein for a component of metabolic syndrome in Japanese. Circ J. (2009) 73:755–9. doi: 10.1253/circj.CJ-08-0848
26. Kubota Y, Moriyama Y, Yamagishi K, Tanigawa T, Noda H, Yokota K, et al. Serum vitamin C concentration and hs-CRP level in middle-aged Japanese men and women. Atherosclerosis. (2010) 208:496–500. doi: 10.1016/j.atherosclerosis.2009.07.052
27. Standard Tables of Food Composition in Japan; Fifth Revised and Enlarged Edition. Tokyo: Ministry of Education, Culture, Sports, Science and Technology (2005).
28. Takachi R, Ishihara J, Iwasaki M, Hosoi S, Ishii Y, Sasazuki S, et al. Validity of a self-administered food frequency questionnaire for middle-aged urban cancer screenees: comparison with 4-day weighed dietary records. J Epidemiol. (2011) 21:447–58. doi: 10.2188/jea.JE20100173
29. Reference Data for C-Reactive Protein Kit; Nanopia CRP. Available online at: https://www.sekisuimedical.jp/english/business/diagnostics/biochemistry/crp/data.html (accessed April 14, 2020).
30. Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York, NY: Lawrence Erlbaum Associates (1988).
31. Quan H, Zhang J. Estimate of standard deviation for a log-transformed variable using arithmetic means and standard deviations. Stat Med. (2003) 22:2723–36. doi: 10.1002/sim.1525
32. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the american heart association. Circulation. (2003) 107:499–511. doi: 10.1161/01.CIR.0000052939.59093.45
33. Chrysohoou C, Pitsavos C, Panagiotakos DB, Skoumas J, Stefanadis C. Association between prehypertension status and inflammatory markers related to atherosclerotic disease: the ATTICA study. Am J Hypertens. (2004) 17:568–73. doi: 10.1016/j.amjhyper.2004.03.675
34. Seo YH, Shin HY. Relationship between hs-CRP and HbA1c in diabetes mellitus patients: 2015-2017 korean national health and nutrition examination survey. Chonnam Med J. (2021) 57:62–7. doi: 10.4068/cmj.2021.57.1.62
35. Tabung FK, Steck SE, Zhang J, Ma Y, Liese AD, Agalliu I, et al. Construct validation of the dietary inflammatory index among postmenopausal women. Ann Epidemiol. (2015) 25:398–405. doi: 10.1016/j.annepidem.2015.03.009
36. National Health and Nutrition Survey in 2013. Ministry of Health, Labour, and Welfare, Japan. Available online at: https://www.mhlw.go.jp/bunya/kenkou/eiyou/dl/h25-houkoku-06.pdf (accessed August 31, 2020).
Keywords: dietary inflammatory index, food frequency questionnaire, inflammatory biomarker, high-sensitivity C-reactive protein, Japanese
Citation: Kotemori A, Sawada N, Iwasaki M, Yamaji T, Shivappa N, Hebert JR, Ishihara J, Inoue M and Tsugane S (2021) Dietary Inflammatory Index Is Associated With Inflammation in Japanese Men. Front. Nutr. 8:604296. doi: 10.3389/fnut.2021.604296
Received: 09 September 2020; Accepted: 09 March 2021;
Published: 09 April 2021.
Edited by:
Megan A. McCrory, Boston University, United StatesReviewed by:
Roberta Masella, National Institute of Health (ISS), ItalyCopyright © 2021 Kotemori, Sawada, Iwasaki, Yamaji, Shivappa, Hebert, Ishihara, Inoue and Tsugane. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Norie Sawada, bnNhd2FkYUBuY2MuZ28uanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.