- 1Obesity Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2Nutrition and Endocrine Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Background: Hypertriglyceridemia (HTG) during pregnancy may be accompanied by acute pancreatitis, hyperviscosity syndrome, and preeclampsia. HTG during pregnancy should be managed by a multidisciplinary team; however, no clinical guidelines exist for severe gestational HTG.
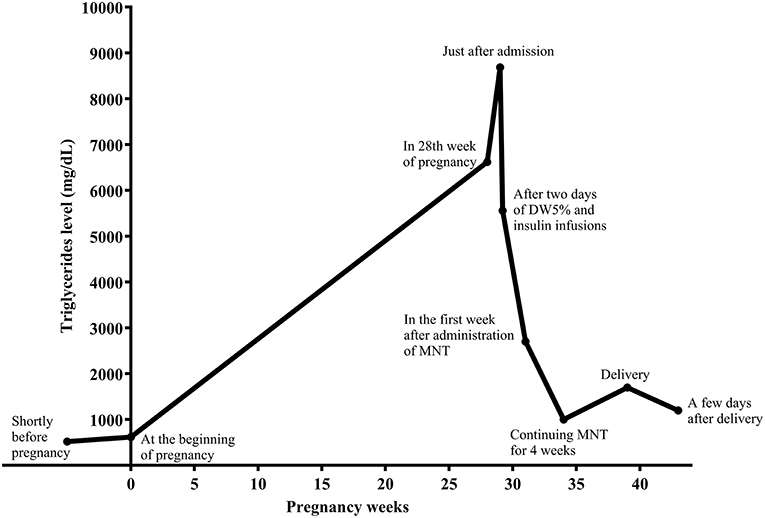
Case Presentation: We herein present a case of a 36-year-old in the first pregnancy (G1P0Ab0), with a history of severe HTG-induced necrotizing pancreatitis 9 years earlier. There was no family history of HTG. During these years, she did not follow any appropriate diet or medical therapy for HTG. She became pregnant in May 2019, without preconception counseling. Eruptive and tuberoeruptive xanthomas appeared in the 27th week of pregnancy. Serum triglycerides (TGs) and fasting blood sugar (FBS) were 6,620 and 124 mg/dL, respectively, indicating HTG and gestational diabetes (GDM). After admission for the management of severe HTG, she was put on parenteral nutrition with dextrose water 5% and infusion insulin therapy without receiving any enteral carbohydrate for 2 days. Following that, a very low-fat diet and omega-3 fatty acids (1,200 mg/day) were started. After 4 weeks, TG levels reached 1,000 mg/dL, and her self-monitoring blood glucose levels showed appropriate blood glucose for pregnancy. She underwent a successful elective cesarean section in the 39th of pregnancy.
Conclusion: This case report demonstrates that HTG during pregnancy could be managed by medical nutrition therapy (MNT).
Background
Hypertriglyceridemia (HTG) can arise from hepatic overproduction, decreased clearance of chylomicrons, and very-low-density lipoprotein (VLDL) remnant, ineffective lipolysis, or a combination of these factors (1). Severe HTG is defined as TG concentration >885 mg/dL, which is almost accompanied by chylomicronemia's pathological presence (2). Chylomicronemia manifested by eruptive xanthomas, lipemia retinalis, hepatosplenomegaly, and episodes of pancreatitis called chylomicronemia syndrome (3). Severe HTG is most often due to polygenic susceptibility interacting with secondary non-genetic factors (4), including consumption of high-fat foods, alcohol, estrogen-containing medication, pregnancy, obesity, diabetes, or medications that increase VLDL secretion (e.g., steroids and beta-blockers) (4, 5).
During pregnancy, significant alterations to lipid homeostasis occur (4) include an increase in TG and total cholesterol levels, which are mediated by estrogen, progesterone, and human placental lactogen (HPL) (6). The presence of HTG in pregnancy may cause other health-threatening risks, including acute pancreatitis, hyperviscosity syndrome, and preeclampsia (1).
There are no strict clinical guidelines for the treatment of HTG during pregnancy. Generally, during the prenatal period, a low-fat and low-glycemic diet with adequate nutrients to avoid essential fatty acid deficiency is advised. In refractory cases, hospitalization for parenteral nutrition or intravenous insulin therapy, fibrate use after the first trimester, and plasmapheresis are considered (1, 6). Unfortunately, only a few studies address the management of HTG during pregnancy through medical nutrition therapy (MNT).
Case Presentation
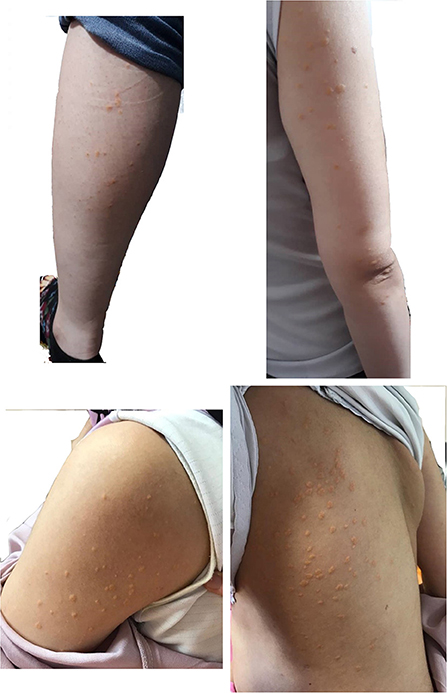
We herein present a case of a 36-year-old in the first pregnancy (G1P0Ab0), with a severe HTG presented in the second trimester of pregnancy. Eruptive xanthomas in the forearms, legs, back, and tuberoeruptive xanthomas on her elbows (Figure 1) occurred in the 27th week of pregnancy, and she was admitted to a hospital in November 2019. Her serum lipoprotein profile was as follows: fasting TG: 6,620 mg/dL, total cholesterol: 459 mg/dL, high-density lipoprotein cholesterol (HDL-C): 76 mg/dL, and serum amylase and lipase levels were within normal limits. Except for pregnancy, no other aggravating factors for HTG were found. After admission, TG level reached 8,683 mg/dL. Fasting blood sugar (FBS) was normal in early pregnancy. However, the glucose tolerance test (GTT) with 75 g anhydrase glucose showed: FBS: 124 mg/dL, 1-h glucose: 168 mg/dL, 2-h glucose: 130 mg/dL indicating the presence of gestational diabetes. There were no thyroid, liver, and renal function abnormalities. Abdominal ultrasound imaging was unremarkable with no evidence of pancreatitis and hepatosplenomegaly.
She had a hospital admission history 9 years ago with acute abdominal pain (acute necrotizing pancreatitis) and severe HTG >6,000 mg/dL. She had been discharged without symptoms. During her irregular medical visits, occasional fibrates were prescribed by her physician. She did not report severe HTG in her first and second-degree family. At the beginning of pregnancy, her serum TG level was 619 mg/dl with normal fasting blood glucose (FBG). Pre-pregnancy weight and body mass index (BMI) were 52 kg and 19.5 kg/m2, respectively. She still did not have a specific diet and did not consult an endocrinologist or dietitian for HTG because she had a low socioeconomic status. Considering the history of necrotizing pancreatitis as well as a lack of family history of HTG and access to genetic testing, our differential diagnosis based on clinical evidence was rare monogenic HTG.
To reduce TGs rapidly, the patient was put on dextrose 5% in water (D5W, 2,500 mL per day) and insulin regular (5–7 unit/hour) infusion, without any enteral intake, and with close monitoring for blood glucose and serum electrolytes, including sodium and potassium levels. After 2 days, TG level was 5,560 mg/dL. Insulin infusion was discontinued. A low-fat diet consisted of 10, 25, and 65% of energy from fat, protein, and carbohydrate, respectively, was prescribed by the dietician. The diet comprised four servings of skim-fat milk or yogurt, four servings of vegetables, six servings of fruits, one serving of simple carbohydrates such as sugar or honey, and 11 servings of grains. Three servings of meat and its substitutes were prescribed, including plant-based proteins such as legumes, one serving from egg white, and other servings from white meat, including chicken and turkey. Concurrently, seven capsules daily of omega-3 fatty acids containing 360 mg eicosapentaenoic acid (EPA) and 240 mg docosahexaenoic acid (DHA) per capsule (equal to 4,200 mg) were started in divided doses. Nevertheless, due to cost and availability, the patient received omega-3 at a dose of five capsules daily (equal to 3,000 mg) for 10 days and then two capsules daily (equal to 1,200 mg) for the rest of the pregnancy. She was followed by a multidisciplinary team consisting of an endocrinologist, obstetrician, and a dietitian. In the 1st week after MNT administration and omega-3 fatty acids, TG levels dropped to 2,700 mg/dL (Figure 2).
Self-monitoring of blood glucose revealed a mean level of FBS and 2-h plasma glucose equal to 74 mg/dL and 96 mg/dL, respectively. She was discharged with a 10% fat-restricted diet with medium-chain TGs (MCT) 15 g daily. Continuing MNT for 4 weeks, TG levels reached 1,000 mg/dL, and all SMBGs were in the recommended ranges for the pregnancy. From the time of admission to the specialists and up to labor, she had regular weekly contacts with her endocrinologist and dietician. She underwent elective cesarean section at 39th week of pregnancy with TG level of 1,700 mg/dL. She delivered a healthy baby girl weighing 2,750 g. At the end of her pregnancy, the body weight was 58 kg (equal to 6 kg weight gain). The patient had an uncomplicated postoperative course and was discharged and recommended to continue her diet. A few days after delivery, her TG level dropped to 1,200 mg/dL. She was advised to breastfeed her baby. No complications or health problems related to HTG was found up to 2nd month of postpartum.
Discussion
Our patient was a 36-year-old pregnant woman presenting chylomicronemia syndrome during pregnancy, which seemed to be managed successfully by MNT. The widely used term familial chylomicronemia syndrome is synonymous with monogenic chylomicronemia (7). The absence of secondary factors and HTG diagnosis at young adulthood suggests monogenic chylomicronemia, mainly if hypertriglyceridemia is severe and associated with pancreatitis (8).
Guidelines advise a combination of restricting dietary fat intake with drug treatment (e.g., fibrates, niacin, or omega-3 fatty acids) to manage severe HTG in non-pregnant patients. However, no clear clinical guidelines exist for severe gestational HTG to maintain the balance between maternal and fetal needs. We suggest that a multidisciplinary team should manage women with gestational HTG (8, 9), as it is associated with many clinical challenges (4).
A few case reports are expressing MNT to manage severe HTG among pregnant women. Shenhav et al. treated a pregnant woman admitted at 33rd weeks of pregnancy with TG concentration of 13,805 mg/dL with a diet consisting of intravenous hydration with 3,000 mL of 5% dextrose and a hypocaloric low-carbohydrate, low-fat diet. The serum concentration of TG was reduced to 1,035 mg/dL after 27 days of the intervention (10). Furthermore, Kleess et al. reported a case of a female aged 38-year in the 21st week of pregnancy with GDM and severe HTG (TGs and FPG 7,812 and 300 mg/dL, respectively). She had received insulin glargine and lispro and a diet consisting of low-fat (<20% of calories) and low-carbohydrate. At the delivery, the serum concentration of TGs was <2,000 mg/dL (11). None of the previous studies indicated the MNT in detail, as we have provided in the current study.
Many factors and presenting symptoms are involved in the admission of patients with HTG (6). The patient was admitted due to rapid TG increase, poor dietary compliance, previous pancreatitis due to HTG despite the absence of present pancreatitis and presence of GDM (6). Short-term hospitalizations can be used proactively to reduce TG levels through parenteral nutrition (PN) (10, 12), or intravenous insulin therapy (13) if GTT is also impaired, injection of heparin (14, 15), and plasmapheresis (16–18) (Table 1). The literature showed that MNT's effect on severe HTG is acceptable; however, its effect would not be acute, and serum concentration of TG cannot rapidly decrease.
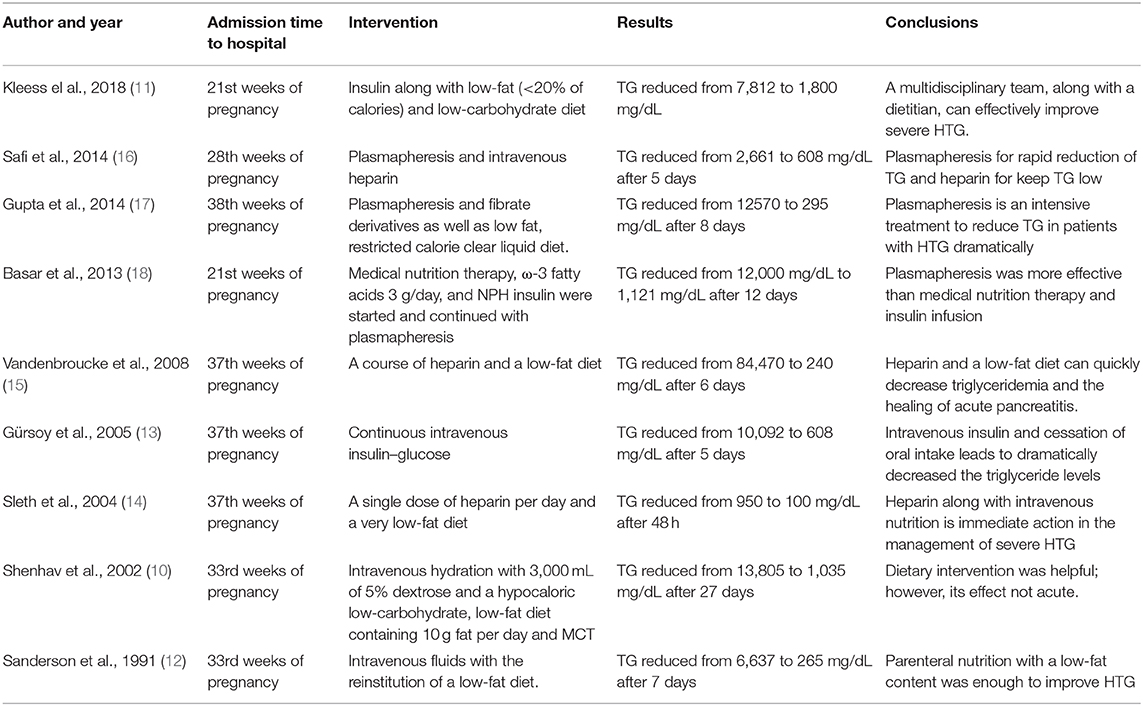
Table 1. Literature review in case reports preenting a therapy in a pregnant female with severe hypertriglyceridemia (HTG).
Starting PN is effective because lipids' systemic delivery bypasses the portal system, allowing for peripheral metabolism and trans-placental passage of fats (1). Moreover, insulin is a rapid and potent activator of LPL. It can be used to treat severe HTG because it increases the removal of TGs from the plasma. Given the risk of hypoglycemia, it is usually co-administrated with glucose infusion (4). Therefore, after the patient was hospitalized, we prescribed D5W and regular insulin infusion through two separate veins. Nevertheless, no significant drop in TG levels was observed after 2 days (Figure 2).
In non-pregnant individuals, higher doses of omega-3 fatty acids that contain EPA and DHA (4 g/day total EPA+DHA) have been shown to reduce ongoing metabolic control, the concentration of VLDL, and possible chylomicron secretion (2, 19). Omega-3 fatty acids are the cornerstone of safe therapy for mother and child in the long-term (4). As previously mentioned, due to cost and limited availability, our patient received only 1,200 mg of omega-3 daily. Therefore, the triglyceride drop does not appear to be related to omega-3.
Dietary intervention with fat <20% (4), ideally, <10% of calories should be immediately started (5). However, adherence to such a regimen is extremely challenging for most patients (5). In addition, restricted fat intake can lead to maternal and, more importantly, fetal deficiency of omega-3 and omega-6 essential fatty acids (EFA) (including alpha-linolenic acid (ALA) and linoleic acid (LA)) as well as the vital long-chain omega-3 PFA (including EPA and DHA). Although symptoms of EFA deficiency are relatively mild for the mother (typically skin dryness and desquamation) (4), fetal EFA and long-chain polyunsaturated fatty acids deficiency may end in impaired fetal brain and visual development (4). The diet should include a minimum of 300 mg of EPA and DHA (4). The use of omega-3- fatty acids and medium-chain triglycerides (MCTs) will provide calories while preventing plasma TGs increase (4, 5). Although MCTs' safety has not been specifically evaluated in the fetus (20), in a randomized, double-blind intervention by Rayyan et al. MCT- containing emulsion was safe and well-tolerated by preterm infants (20). The patient was discharged with a low-fat diet, containing mostly medium-chain triglyceride foods. In addition to attention to the fat in a diet, avoidance of refined carbohydrates, especially simple sugar, should be a part of a dietary intervention (18). Compelling evidence indicated that foods contain simple sugars and fructose supply substrates for TG production, which substantially are enhancing TG levels in susceptible people (21, 22). Therefore, dietary intake of the patients was limited to only one serving per day simple sugar and the rest of carbohydrate was provided by whole-grain foods. We strongly encouraged her to refrain from eating any food with added sugar by educating food labeling.
Although metabolic control is ongoing, TG concentrations may rapidly rebound at any time (2). The risk of pancreatitis is always present, and more effective therapies are required (2). Close surveillance of plasma TG concentrations during pregnancy is essential and should be followed up at least on a monthly basis. As pregnancy progresses, TG levels may need to be monitored every 1–2 weeks (4); we carried this out with our patient. Strong consideration should be given toward induction once fetal maturity is established (i.e., at 36th weeks), especially in women whose TG levels show a steep upward trend in the third trimester (4). However, as previously discussed, due to the low fetal growth for her gestational age, she underwent an elective cesarean section with meticulous fetal and maternal monitoring at the 39th week of pregnancy.
In the postpartum period, the decision to resume these medications will depend on the latest blood results and trend of the serum TGs as well as maternal breastfeeding plans (4). In the postpartum period, considering the relative reduction of TGs, our patient continued to receive individualized MNT.
Conclusion
In the current study, the case with severe gestational HTG, who was at high risk for pancreatitis, seemed to be treated with MNT successfully. The management led to the termination of a safe pregnancy and the birth of a healthy baby girl. Hopefully, we will see more case studies and in the future, so we can begin to provide recommendations on MNTs that could be acceptable for pregnant women.
Data Availability Statement
The original contributions generated for this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The patient provided her written informed consent to participate in this study. Written informed consent was obtained from the patient for the publication of any potentially identifiable images or data included in this article.
Author Contributions
MZ and FH interpreted in the diagnosis of disease, medical treatment, and follow-up of the patient. GA and PM contributed to MNT. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors express their appreciation to S. Ghaemi for her continual cooperation and support as a case of this manuscript. We also thank Dr. Laleh A. Marvasti for her meticulous English editing of the manuscript.
Abbreviations
HTG, Hypertriglyceridemia; TGs, Serum triglycerides; FBS, Fasting blood sugar; GDM, Gestational diabetes; MNT, Medical nutrition therapy; VLDL, Very low-density lipoprotein; WHO ICD, World Health Organization International Classification of Diseases; HPL, Human placental lactogen; BMI, Body mass index; HDL-C, High-density lipoprotein cholesterol; GTT, Glucose tolerance test; D5W, Dextrose 5% in water; EPA, Eicosapentaenoic acid; DHA, Docosahexaenoic acid; EFA, Essential fatty acids; ALA, Alpha-linolenic acid; LA, linoleic acid; MCTs, Medium-chain triglycerides.
References
1. Falko JM. Familial chylomicronemia syndrome: a clinical guide for endocrinologists. Endocr Pract. (2018) 24:756–63. doi: 10.4158/EP-2018-0157
2. Hegele RA, Borén J, Ginsberg HN, Arca M, Averna M, Binder CJ, et al. Rare dyslipidaemias, from phenotype to genotype to management: a European Atherosclerosis Society task force consensus statement. Lancet Diabetes Endocrinol. (2020) 8:50–67. doi: 10.1016/S2213-8587(19)30264-5
3. Stroes E, Moulin P, Parhofer KG, Rebours V, Löhr JM, Averna M. Diagnostic algorithm for familial chylomicronemia syndrome. Atheroscler Suppl. (2017) 23:1–7. doi: 10.1016/j.atherosclerosissup.2016.10.002
4. Wong B, Ooi TC, Keely E. Severe gestational hypertriglyceridemia: a practical approach for clinicians. Obstet Med. (2015) 8:158–67. doi: 10.1177/1753495X15594082
5. Brahm AJ, Hegele RA. Chylomicronaemia–current diagnosis and future therapies. Nat Rev Endocrinol. (2015) 11:352–62. doi: 10.1038/nrendo.2015.26
6. Cao S, Dao N, Roloff K, Valenzuela GJ. Pregnancies complicated by familial hypertriglyceridemia: a case report. AJP Rep. (2018) 8:e362–4. doi: 10.1055/s-0038-1676832
7. Dron JS, Wang J, Cao H, McIntyre AD, Iacocca MA, Menard JR, et al. Severe hypertriglyceridemia is primarily polygenic. J Clin Lipidol. (2019) 13:80–8. doi: 10.1016/j.jacl.2018.10.006
8. Berglund L, Brunzell JD, Goldberg AC, Goldberg IJ, Sacks F, Murad MH, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. (2012) 97:2969–89. doi: 10.1210/jc.2011-3213
9. Karalis DG. A review of clinical practice guidelines for the management of hypertriglyceridemia: a focus on high dose Omega-3 fatty acids. Adv Ther. (2017) 34:300–23. doi: 10.1007/s12325-016-0462-y
10. Shenhav S, Gemer O, Schneider R, Harats D, Segal S. Severe hyperlipidemia-associated pregnancy: prevention in subsequent pregnancy by diet. Acta Obstet Gynecol Scand. (2002) 81:788–90. doi: 10.1034/j.1600-0412.2002.810819.x
11. Kleess LE, Janicic N. Severe hypertriglyceridemia in pregnancy: a case report and review of the literature. AACE Clin Case Rep. (2019) 5:e99–103. doi: 10.4158/ACCR-2018-0168
12. Sanderson SL, Iverius PH, Wilson DE. Successful hyperlipemic pregnancy. JAMA. (1991) 265:1858–60. doi: 10.1001/jama.265.14.1858
13. Gürsoy A, Kulaksizoglu M, Sahin M, Ertugrul DT, Ozer F, Tutuncu NB, et al. Severe hypertriglyceridemia-induced pancreatitis during pregnancy. J Natl Med Assoc. (2006) 98:655–7.
14. Sleth JC, Lafforgue E, Servais R, Saizy C, Pluskwa F, Huet D, et al. A case of hypertriglycideremia-induced pancreatitis in pregnancy: value of heparin. Ann Fr Anesth Reanim. (2004) 23:835–7. doi: 10.1016/j.annfar.2004.06.006
15. Vandenbroucke L, Seconda S, Lassel L, Le Bouar G, Poulain P. Acute pancreatitis induced by major hypertriglyceridemia during pregnancy. A case report. J Gynecol Obstet Biol Reprod (Paris). (2009) 38:436–9. doi: 10.1016/j.jgyn.2009.04.006
16. Safi F, Toumeh A, Abuissa Qadan MA, Karaz R, AlAkdar B, Assaly R. Management of familial hypertriglyceridemia-induced pancreatitis during pregnancy with therapeutic plasma exchange: a case report and review of literature. Am J Ther. (2014) 21:e134–6. doi: 10.1097/MJT.0b013e31825b9e98
17. Gupta N, Ahmed S, Shaffer L, Cavens P, Blankstein J. Severe hypertriglyceridemia induced pancreatitis in pregnancy. Case Rep Obstet Gynecol. (2014) 2014:485493. doi: 10.1155/2014/485493
18. Basar R, Uzum AK, Canbaz B, Dogansen SC, Kalayoglu-Besisik S, Altay-Dadin S, et al. Therapeutic apheresis for severe hypertriglyceridemia in pregnancy. Arch Gynecol Obstet. (2013) 287:839–43. doi: 10.1007/s00404-013-2786-z
19. Skulas-Ray AC, Wilson PWF, Harris WS, Brinton EA, Kris-Etherton PM, Richter CK, et al. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American heart association. Circulation. (2019) 140:e673–91. doi: 10.1161/CIR.0000000000000709
20. Rayyan M, Devlieger H, Jochum F, Allegaert K. Short-term use of parenteral nutrition with a lipid emulsion containing a mixture of soybean oil, olive oil, medium-chain triglycerides, and fish oil: a randomized double-blind study in preterm infants. JPEN J Parenter Enteral Nutr. (2012) 36:81s–94s. doi: 10.1177/0148607111424411
21. Havel PJ Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. (2005) 63:133–57. doi: 10.1111/j.1753-4887.2005.tb00132.x
Keywords: medical nutrition therapy, hypertriglyceridemia, pregnant women, GDM, chylomicronemia syndrome
Citation: Zahedi M, Asghari G, Mirmiran P and Hosseinpanah F (2021) Case Report: Management of a Patient With Chylomicronemia Syndrome During Pregnancy With Medical Nutrition Therapy. Front. Nutr. 8:602938. doi: 10.3389/fnut.2021.602938
Received: 04 September 2020; Accepted: 25 January 2021;
Published: 05 March 2021.
Edited by:
Simonetta Friso, University of Verona, ItalyReviewed by:
Charikleia Stefanaki, National and Kapodistrian University of Athens, GreeceKlaus Parhofer, LMU Munich University Hospital, Germany
Copyright © 2021 Zahedi, Asghari, Mirmiran and Hosseinpanah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Farhad Hosseinpanah, Zmhvc3BhbmFoQGVuZG9jcmluZS5hYy5pcg==; Golaleh Asghari, YXNnaGFyaUBlbmRvY3JpbmUuYWMuaXI=
 Maryam Zahedi1
Maryam Zahedi1 Parvin Mirmiran
Parvin Mirmiran Farhad Hosseinpanah
Farhad Hosseinpanah