- 1Department of Microbiology and Immunology, Faculty of Medicine, University of Khartoum, Khartoum, Sudan
- 2Department of Physiology, Faculty of Medicine, Alneelain University, Khartoum, Sudan
- 3Department of Rheumatology, Military Hospital, Khartoum, Sudan
- 4Department of Physiology, Faculty of Medicine, University of Khartoum, Khartoum, Sudan
Background: Rheumatoid arthritis (RA) is an autoimmune disease that mainly affects the synovial joints with systemic manifestations. RA has a major impact on liver and kidney functions as part of the disease pathogenesis or as a sequel of disease medications or, mostly, both of them. The kidney and liver involvement increases the RA morbidity and mortality. Nowadays, dietary interventions are proposed as potential modifiers for disease severity. Gum Arabic (GA) is acacia senegal exudates; it is soluble fiber with prebiotic properties. GA has been discovered to be protective against experimental nephrotoxicity and hepatotoxicity, with comparable findings in human studies. This article addresses the effect of GA on hepatic and renal profile among RA patients.
Methods: Forty patients aged 18–70 received GA daily for 12 weeks as a single dose of 30 g. The liver enzymes, total protein level, serum albumin, serum globulin level, urea, creatinine, and serum electrolytes have been measured as a baseline after 4 weeks and by the end of the study. Cobas C311 (Roche, Germany) automated chemistry analyzer directly determined the values for total protein, albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and creatinine. The study ethically has been approved by the Ethical Committee of the National Medicines and Poisons Board. Trial Registration Identifier: NCT02804581.
Results: Regarding the liver enzymes, GA has significantly decreased the liver enzymes apart from alkaline phosphatase, which showed no significant change. In contrast, GA has increased the serum albumin level with a minor impact on the serum globulin level. Furthermore, GA has also significantly decreased the level of urea (P = 0.0001) and level of Sodium (P = 0.002) with nonsignificant change on creatinine and potassium concentrations.
Conclusion: GA presents hepatic and renal protective effects among RA patients, evidenced by the significant reduction of urea and liver enzymes. Thus, it can be recommended as a dietary supplement for RA patients. Nonetheless, we recommend further investigation to support our findings.
Introduction
Rheumatoid arthritis (RA) is an autoimmune rheumatic disorder, a type of systemic disease (1, 2) of unknown etiology (2, 3). Comorbid conditions are common in RA patients either associated with RA itself or related to treatment (RA) (4). The conventional definition of a comorbid condition is a medical condition other than the primary disease itself (5). Comorbidities contribute to a shortened lifespan and life expectancy in comparison to the general population (3). Therefore, comorbidity is a major issue regarding the care of rheumatic disease patients (5).
The most common manifestation of liver damage among RA patients is asymptomatic abnormal liver tests and elevated liver enzymes (4, 6). The main abnormalities are elevated levels of alkaline phosphatase (ALP) and serum gamma-glutamyl transferase (GGT) enzymes (4). The medications used in rheumatology are often hepatotoxic; thus, it is difficult to differentiate between the hepatic manifestations of the primary disease and suspected drug hepatotoxicity (6). Furthermore, patients with autoimmune rheumatic disorders are more susceptible to associated autoimmune liver disease (6), which causes liver damage. The latter may occasionally progress to cirrhosis (6). Rheumatoid arthritis patients have a low level of albumin that is not related to disease activity (7, 8). Hypoalbuminemia may have consequences from a hypercatabolic state secondary to inflammatory status (7), the latter being known as “rheumatoid cachexia” (9). Another supposed mechanism is abnormal albumin distribution between plasma and synovium (8, 10).
Renal disorders are a common cause of mortality among RA patients; however, they are less apparent as clinical manifestations (11). RA patients have a high prevalence of renal impairment with evidence of reduced glomerular filtration and tubular function (12). Nevertheless, renal involvement may remain unnoticed for a long period in a reversible subclinical stage (12). Causes of renal damage among RA patients with advanced stages is difficult to define because of the coexistence of multiple possible causes. Renal Biopsies from RA patients exhibit different histological findings. Most common was mesangial glomerulonephritis (13). Therefore, heterogeneous renal lesions may complicate advanced RA even if they are not clinically obvious (14).
Gum arabic (GA) is a safe dietary fiber approved by the Food and Drug Administration (FDA) and World Health Organization (WHO) (15). It is a high-molecular weight non-starch polysaccharide retrieved from Acacia senegal and acacia seyal trees (15, 16). GA is mainly fermented by colonic bacteria instead of being digested by humans (17) and animals (15, 16). Moreover, GA fermentation produces short-chain fatty acids—mainly propionate and butyrate (15–17). The latter was discovered as a physiological modifier for different body functions such as the modulation of cell proliferation, apoptosis, regulation of angiogenesis, and inflammation (18). The anti-inflammatory effect of Gum Arabic has been investigated in various diseases and conditions (19–21). As reported earlier, the effect of GA is an anti-inflammatory agent among RA patients, since it decreases the level of TNF α and the disease severity score among RA patients (22).
Rheumatoid patients are at risk of multi-organ failure, primarily renal insufficiency and hepatic dysfunction even if they are on regular treatment and closely followed up. Anti-inflammatory and analgesic drugs have devastating effects on liver and kidney functions. Therefore, finding safe organ protective agents will be of great benefit for RA patients.
This study aims to investigate the possible ameliorative effect of GA on various parameters of renal and liver function among RA patients.
Methods and Materials
The study is a single-arm clinical Phase II trial made up of one group of patients. Patients' characteristics and background data have been reported previously (22). Inclusion and exclusion criteria have been described in details earlier (22). In all, 40 participants have completed the study and been included in the final analysis. The main outcome was the level of inflammatory markers and clinical severity score after 12 weeks of GA intake (22). This article documented secondary outcomes considering GA impact on renal and hepatic profile among RA patients.
Thirty grams of GA (acacia senegal exudate), fine powder packed as one sachet, was administered for 12 weeks as a single dose dissolved in water to be consumed in the early morning. Renal and liver function parameters were measured as a baseline, after week 4 and week 12 of GA oral ingestion.
Measurement of Blood Chemistry
Three ml were withdrawn from a lithium heparin container. Plasma was separated by centrifugation at 2,000 rpm for 10 min and then aliquoted and submitted for biochemical analysis.
Cobas C311 (Roche, Germany) automated chemistry analyzer was used to measure the total protein, albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, amylase, creatinine, total bilirubin, and direct bilirubin values. The machine applies absorption photometry to determine the absorbance amount in the blood that is used to calculate the concentration in the sample and apply the ion-selective electrode (ISE) to measure electrolytes level (23). The concept of measurement of ALT liver enzymes catalyzes the reaction between L-alanine and 2-oxoglutarate (24, 25). While AST catalyzes the transfer of an amino group between L-aspartate and 2-oxoglutarate to form oxaloacetate and L-glutamate (25). The ALP concept of measurementis that, p-nitrophenyl phosphate is cleaved by phosphatases into phosphate and p-nitrophenol in the presence of magnesium and zinc ions. The p-nitrophenol released is directly proportional to the catalytic ALP activity (26).
Data were analyzed using SPSS software package version 23; the quantitative data were expressed as mean, median, standard deviation, minimum, and maximum. Repeated measures ANalysis Of Variance (rANOVA) followed by multiple least significant difference (LSD) comparison tests to detect the changes in biomarker level, P ≤ 0.05 were considered significant.
Written informed consent was obtained voluntarily from all participants prior to participation in the study. The study was ethically approved from National Medicines and Poisons Board ERC and registered at ClinicalTrials.gov ID: NCT02804581 Prospective registration.
Results
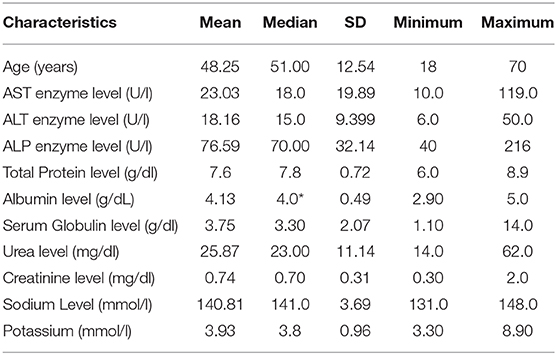
Table 1 represents baseline values of renal and hepatic profiles. As for the effect of GA on liver enzymes (Figure 1), there is a significant decrease in AST and ALT levels, with P-values 0.05 and 0.001, respectively. While there is no significant difference in ALP (P = 0.209), GA has significantly increased the albumin level (P = 0.019) with no effect on the total bilirubin and globulin levels (Figure 2).
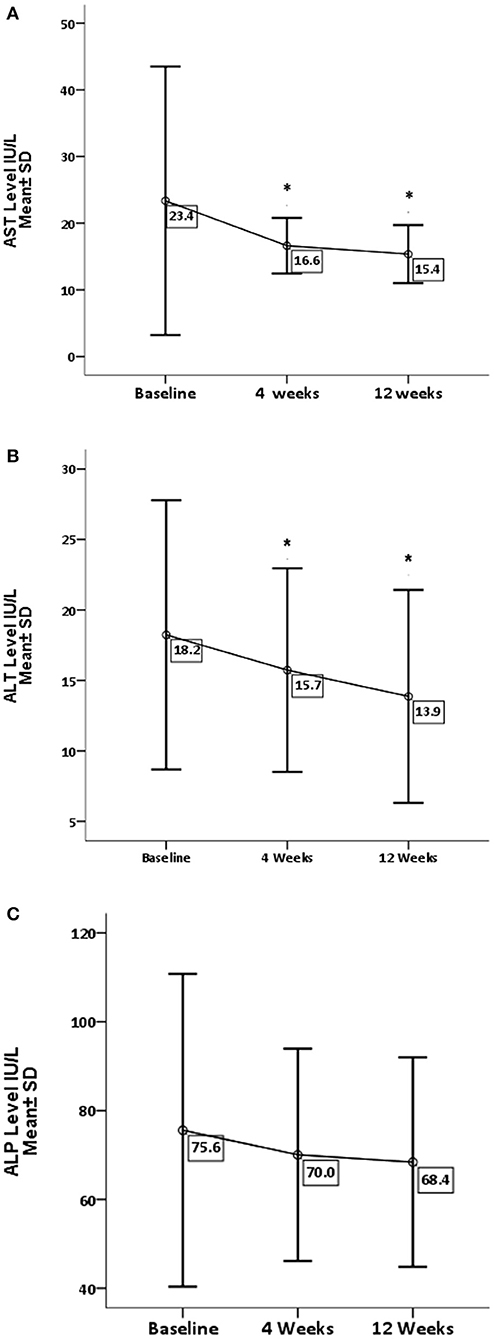
Figure 1. Effect of gum arabic on liver enzymes. *Indicates significant difference from baseline. (A) Effect of GA intake on AST Serum Level (P = 0.05). (B) Effect of GA intake on ALT Serum Level (P = 0.001). (C) Effect of GA intake on ALP Serum Level (P = 0.209).
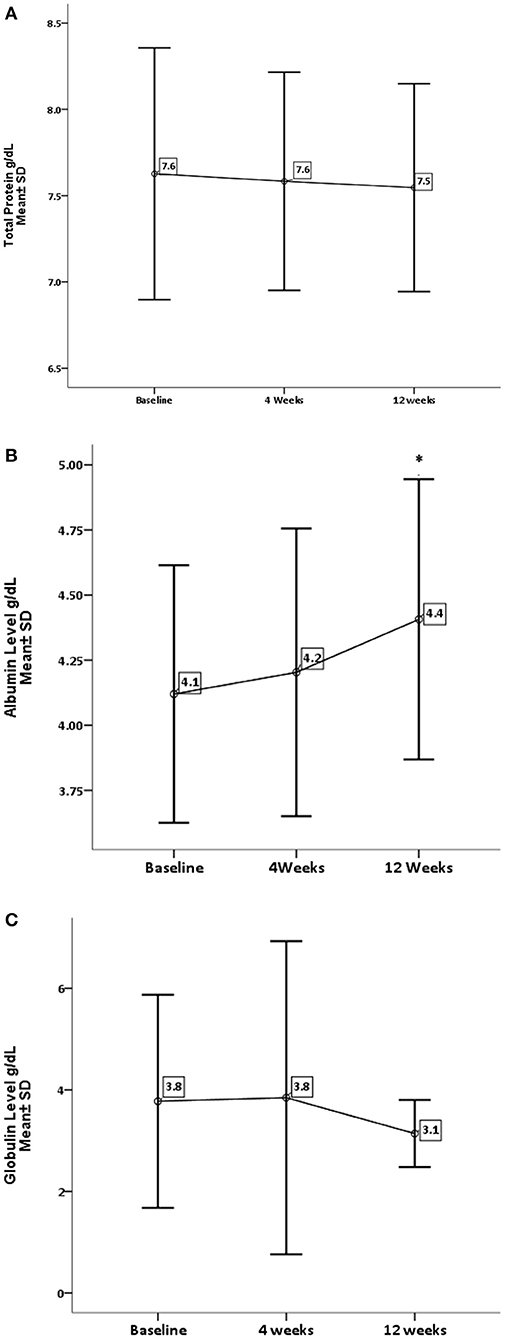
Figure 2. Effect of gum arabic on liver function test. *Indicates significant difference from baseline. (A) Effect of GA intake on total proteins level (P = 0.543). (B) Effect of GA intake on albumin level (P = 0.019). (C) Effect of GA intake on globulin level (P = 0.222).
Concerning renal function and electrolytes level, GA has significantly decreased the level of urea (P = 0.0001) and sodium (P = 0.002). On the other hand, GA shows no influence on creatinine and potassium levels, as shown in Figures 3, 4.
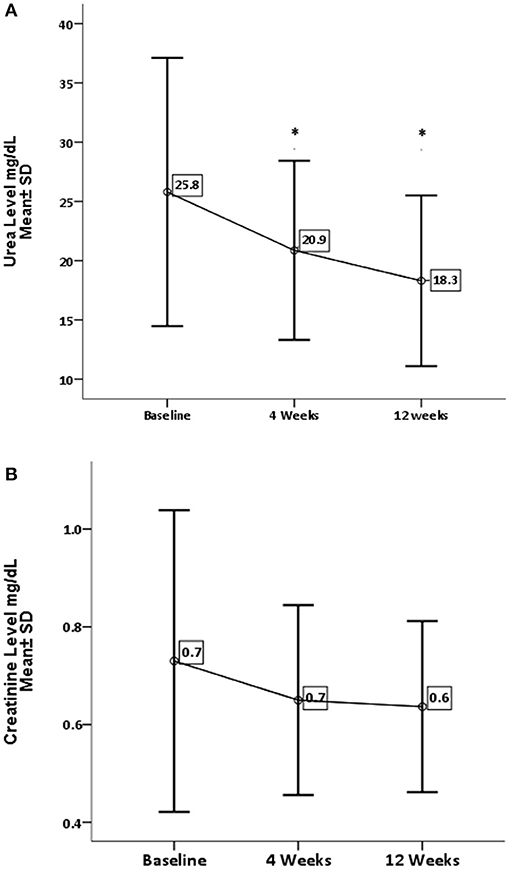
Figure 3. Effect of gum arabic on renal profile. *Indicates significant difference from baseline. (A) Effect of GA intake on urea serum level (P = 0.0001). (B) Effect of GA intake on creatinine level (P = 0.111).
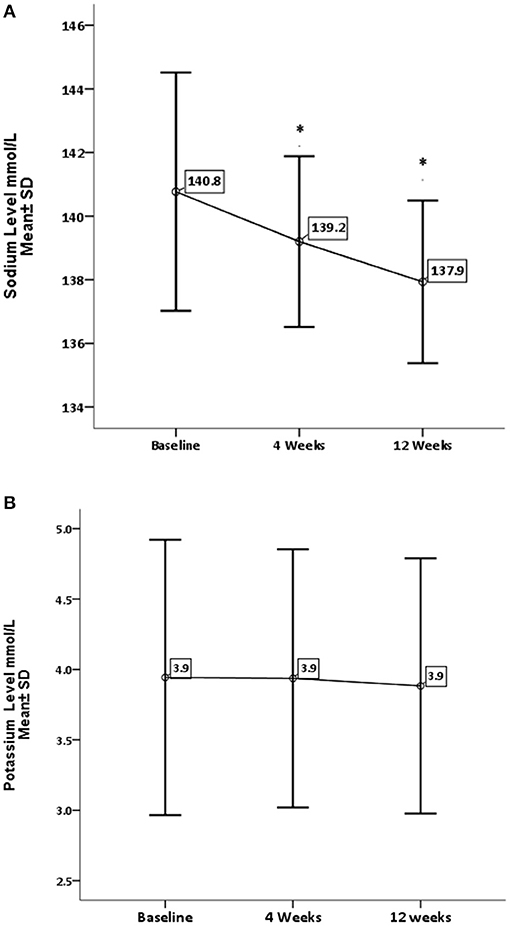
Figure 4. Effect of gum arabic on serum electrolytes. *Indicates significant difference from baseline. (A) Effect of GA intake on sodium level (P = 0.002). (B) Effect of GA intake on sodium level (P = 0.383).
Discussion
Evaluation of renal and hepatic function is necessary since many antirheumatic agents cause renal or hepatic toxicity and may be contraindicated if these organs are impaired (1). So these parameters were measured regularly during the clinical trial.
The current study reveals that GA can significantly decrease liver enzymes apart from ALP (Figure 1). These findings are consistent with previous animals and human studies, and earlier animal experiments had documented the favorable effect of GA on liver enzymes (27, 28). Furthermore, GA has decreased ALT level among healthy subjects and sickle patients (29, 30). Nowadays, there is strong evidence confirming the significant role of the liver in the modulation of immune response in autoimmune and chronic inflammatory disorders (6). GA showed no effect on ALP, which is consistent with earlier research (30). Elevated ALP is hepatic in origin among at least one-third of patients (6), while ALT is a well-recognized biomarker for drug-induced hepatotoxicity (31). These findings confirm the hepatoprotective effect of GA consumption, which increases albumin levels with no significant increase in globulin or total protein as seen in Figure 2. These results contradict a previous study when GA did not alter the albumin level among sickle cell patients (30). The elevation of albumin levels could be secondary to the anti-inflammatory effect of GA (22), which may decrease the catabolic state among RA patients. The latter was proposed as a cause of hypoalbuminemia among RA patients (7). With respect to other liver function parameters, GA did not alter total protein and globulin levels, which is consistent with the previous study (30).
RA has a destructive effect on kidney functions, chronic kidney disease is a common presentation among RA patients (32, 33). The inflammation proposed as a risk factor for CKD among rheumatoid patients (32, 33). The beneficial effect of GA on renal functions is one of the early and documented studies among both humans and rats (34). GA in the current study has significantly reduced urea levels with no impact on creatinine levels (see Figure 3). These findings are comparable to the GA effect among sickle cell patients (30). GA increased nitrogen excretion in stool reducing serum urea levels (34, 35); probably this is what reduced the urea levels in our study, in addition to the reduction in inflammatory status due to regular GA consumption (22). The creatinine clearance was found to be low among RA patients compared with healthy subjects because of muscle atrophy and lower creatinine production (11). Moreover, most of our patients' creatinine levels were within normal limits, as shown in Table 1, which might explain why GA did not have an effect on creatinine in the current research (Figure 3). GA improved renal profiles among chronic renal failure patients and corrected renal dysfunction secondary to induced nephrotoxicity (36–38). Besides, GA acts as an anti-inflammatory and antioxidant among end-stage renal patients (21). As for serum electrolytes, GA significantly reduced sodium levels with no significant effect on potassium (see Figure 4). The reduction in sodium may protect against the development of hypertension, since RA patients are at risk of developing hypertension, which is underdiagnosed (39) and associated with asymptomatic organ damage (40). An experimental study revealed that GA intake increased sodium excretion in urine without affecting serum level (41). Previous clinical trials showed GA did not affect electrolyte levels (29, 30). An earlier study showed that the rheumatoid patients had lower salivary potassium levels compared with healthy subjects (42).
RA is an inflammatory disease affecting mainly synovial joints; nevertheless, the disease pathogenesis and treatment side effects take a tremendous toll on liver and kidney functions. GA, which is a soluble dietary fiber, exhibits potent anti-inflammatory activity among rheumatoid patients; also, it displays strong renal and hepatic protective effect evidenced by a significant decrease in liver enzymes and urea levels. The limitations of our study are that it was a single-arm and short-time study (only 12 weeks). Since it was conducted, it is the first study ever to investigate GA organ protective properties among RA patients.
Conclusion
In conclusion, our results document the potential efficacy and safety of Gum Arabic (A. senegal) as a nutritional supplementary agent for the management of RA supported by the reduction of ALT, AST, and urea serum level and improved level of albumin. Nevertheless, our findings should be validated further by a long-term randomized placebo-controlled trial to establish its utilization in the clinical setting.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Consent for Publication
The principal investigator obtained informed consent from each participant to publish the data without breaching confidentiality.
Ethics Statement
The studies involving human participants were reviewed and approved by National Medicines and Poisons Board Ethics Research Committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
EK, LK, and AS participated in study design. EK and LK, were involved in all aspects of the study conduct. EK and LK participated in the data collection process. EK and LK analyzed the data. LK and EK participated in the writing and reviewing the manuscript. AA was significant clinical contributor to the study. All authors approved final version of manuscript.
Funding
The supporting fund was obtained from University of Khartoum Sudan to principal Investigator EK. Funding bodies did not interfere in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Dar Savanna Ltd., Khartoum, Sudan (http://www.darsavanna.net/) for providing Gum Arabic as a gift to the study. In addition, we thank the Department of Clinical Chemistry, Central Laboratory Military Hospital, Omdurman, Sudan for their contribution to laboratory work.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.552049/full#supplementary-material
Abbreviations
ALP, Alkaline Phosphatase; ALT, Alanine Transaminase; AST, Aspartate Transaminase; GA, Gum Arabic; LFT, Liver Function Test; RA, Rheumatoid Arthritis; RFT, Renal Function Test.
References
1. Guidelines ACoRSoRA. Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum. (2002) 46:328–46. doi: 10.1002/art.10148
2. Alwarith J, Kahleova H, Rembert E, Yonas W, Dort S, Calcagno M, et al. Nutrition interventions in rheumatoid arthritis: the potential use of plant-based diets. A review. Front Nutr. (2019) 6:141. doi: 10.3389/fnut.2019.00141
3. Innala L, Sjöberg C, Möller B, Ljung L, Smedby T, Södergren A, et al. Co-morbidity in patients with early rheumatoid arthritis-inflammation matters. Arthritis Res Ther. (2016) 18:33. doi: 10.1186/s13075-016-0928-y
4. Gullick NJ, Scott DL. Co-morbidities in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. (2011) 25:469–83. doi: 10.1016/j.berh.2011.10.009
5. Wolfe F, Michaud K, Li T, Katz RS. Chronic conditions and health problems in rheumatic diseases: comparisons with rheumatoid arthritis, noninflammatory rheumatic disorders, systemic lupus erythematosus, and fibromyalgia. J Rheumatol. (2010) 37:305–15. doi: 10.3899/jrheum.090781
6. Radovanović-Dinić B, Tešić-Rajković S, Zivkovic V, Grgov S. Clinical connection between rheumatoid arthritis and liver damage. Rheumatol Int. (2018) 38:715–24. doi: 10.1007/s00296-018-4021-5
7. Ballantyne FC, Fleck A, Dick WC. Albumin metabolism in rheumatoid arthritis. Ann Rheum Dis. (1971) 30:265. doi: 10.1136/ard.30.3.265
8. Wilkinson P, Jeremy R, Brooks FP, Hollander JL. The mechanism of hypoalbuminemia in rheumatoid arthritis. Ann Intern Med. (1965) 63:109–14. doi: 10.7326/0003-4819-63-1-109
9. Masuko K. A potential benefit of “balanced diet” for rheumatoid arthritis. Front Med. (2018) 5:141. doi: 10.3389/fmed.2018.00141
10. Brown D, Cooper A, Bluestone R. Exchange of IgM and albumin between plasma and synovial fluid in rheumatoid arthritis. Ann Rheum Dis. (1969) 28:644. doi: 10.1136/ard.28.6.644
11. Boers M, editor. Renal disorders in rheumatoid arthritis. Semin Arthritis Rheum. (1990) 20:57–68. doi: 10.1016/0049-0172(90)90095-W
12. Pedersen LM, Nordin H, Svensson B, Bliddal H. Microalbuminuria in patients with rheumatoid arthritis. Ann Rheum Dis. (1995) 54:189–92. doi: 10.1136/ard.54.3.189
13. Helin HJ, Korpela MM, Mustonen JT, Pasternack AI. Renal biopsy findings and clinicopathologic correlations in rheumatoid arthritis. Arthritis Rheum. (1995) 38:242–7. doi: 10.1002/art.1780380213
14. Koseki Y, Terai C, Moriguchi M, Uesato M, Kamatani N. A prospective study of renal disease in patients with early rheumatoid arthritis. Ann Rheum Dis. (2001) 60:327–31. doi: 10.1136/ard.60.4.327
15. Phillips GO, Ogasawara T, Ushida K. The regulatory and scientific approach to defining gum arabic (Acacia senegal and Acacia seyal) as a dietary fibre. Food Hydrocolloids. (2008) 22:24–35. doi: 10.1016/j.foodhyd.2006.12.016
16. Patel S, Goyal A. Applications of natural polymer gum arabic: a review. Int J Food Prop. (2015) 18:986–98. doi: 10.1080/10942912.2013.809541
17. Cherbut C, Michel C, Raison V, Kravtchenko T, Severine M. Acacia gum is a bifidogenic dietary fibre with high digestive tolerance in healthy humans. Microb Ecol Health Dis. (2003) 15:43–50. doi: 10.1080/08910600310014377
18. Smith JG, Yokoyama WH, German JB. Butyric acid from the diet: actions at the level of gene expression. Crit Rev Food Sci. (1998) 38:259–97. doi: 10.1080/10408699891274200
19. Kaddam LA, Kaddam AS. Effect of gum arabic (Acacia senegal) on C-reactive protein level among sickle cell anemia patients. BMC Res Notes. (2020) 13:162. doi: 10.1186/s13104-020-05016-2
20. Elamin S, Alkhawaja MJ, Bukhamsin AY, Idris MA, Abdelrahman MM, Abutaleb NK, et al. Gum arabic reduces C-reactive protein in chronic kidney disease patients without affecting urea or indoxyl sulfate levels. Int J Nephrol. (2017) 2017:9501470. doi: 10.1155/2017/9501470
21. Ali NE, Kaddam LA, Alkarib SY, Kaballo BG, Khalid SA, Higawee A, et al. Gum arabic (Acacia senegal) augmented total antioxidant capacity and reduced C-reactive protein among haemodialysis patients in phase II trial. Int J Nephrol. (2020) 2020:7214673. doi: 10.1155/2020/7214673
22. Kamal E, Kaddam LA, Dahawi M, Osman M, Salih MA, Alagib A, et al. Gum arabic fibers decreased inflammatory markers and disease severity score among rheumatoid arthritis patients, phase II trial. Int J Rheumatol. (2018) 2018:4197537. doi: 10.1155/2018/4197537
23. Palmer SM, Kaufman RA, Salamone SJ, Blake-Courtney J, Bette W, Wahl H-P, et al. Cobas Integra: clinical laboratory instrument with continuous and random-access capabilities. Clin Chem. (1995) 41:1751–60. doi: 10.1093/clinchem/41.12.1751
24. Huang X-J, Choi Y-K, Im H-S, Yarimaga O, Yoon E, Kim H-S. Aspartate aminotransferase (AST/GOT) and alanine aminotransferase (ALT/GPT) detection techniques. Sensors. (2006) 6:756–82. doi: 10.3390/s6070756
25. Sherwin JE. Liver function. In: Kaplan L, Pesce A, editors. Clinical Chemistry, Theory, Analysis, and Correlation. St louis: Mosby (1984), p. 420–38.
26. Tietz NW, Burtis CA, Duncan P, Ervin K, Petitclerc CJ, Rinker AD, et al. A reference method for measurement of alkaline phosphatase activity in human serum. Clin Chem. (1983) 29:751–61. doi: 10.1093/clinchem/29.5.751
27. Ahmed AA, Fedail JS, Musa HH, Kamboh AA, Sifaldin AZ, Musa TH. Gum Arabic extracts protect against hepatic oxidative stress in alloxan induced diabetes in rats. Pathophysiology. (2015) 22:189–94. doi: 10.1016/j.pathophys.2015.08.002
28. Gamal el-din AM, Mostafa AM, Al-Shabanah OA, Al-Bekairi AM, Nagi MN. Protective effect of arabic gum against acetaminophen-induced hepatotoxicity in mice. Pharmacol Res. (2003) 48:631–5. doi: 10.1016/S1043-6618(03)00226-3
29. Ross AM, Eastwood MA, Brydon WG, Anderson J, Anderson DM. A study of the effects of dietary gum arabic in humans. Am J Clin Nutr. (1983) 37:368–75. doi: 10.1093/ajcn/37.3.368
30. Kaddam LA, Fdl-Elmula I, Eisawi OA, Abdelrazig HA, Elnimeiri MK, Saeed AM. Biochemical effects and safety of Gum arabic (Acacia Senegal) supplementation in patients with sickle cell anemia. Blood Res. (2019) 54:31–7. doi: 10.5045/br.2019.54.1.31
31. Ozer JS, Chetty R, Kenna G, Palandra J, Zhang Y, Lanevschi A, et al. Enhancing the utility of alanine aminotransferase as a reference standard biomarker for drug-induced liver injury. Regul Toxicol Pharmacol. (2010) 56:237–46. doi: 10.1016/j.yrtph.2009.11.001
32. Kochi M, Kohagura K, Shiohira Y, Iseki K, Ohya Y. Inflammation as a risk of developing chronic kidney disease in rheumatoid arthritis. PLoS One. (2016) 11:e0160225. doi: 10.1371/journal.pone.0160225
33. Chiu H-Y, Huang H-L, Li C-H, Chen H-A, Yeh C-L, Chiu S-H, et al. Increased risk of chronic kidney disease in rheumatoid arthritis associated with cardiovascular complications-a national population-based cohort study. PLoS One. (2015) 10:e0136508. doi: 10.1371/journal.pone.0136508
34. Kaddam L, Fdl-Elmula I, Saeed A. 17 - Gum arabic beneficial effects, clinical applications, and future prospective. In: Mariod AA, editor. Gum Arabic. Academic Press (2018). p. 211–20. doi: 10.1016/B978-0-12-812002-6.00017-8
35. Khalid SA, Musa AM, Saeed AM, Abugroun EA, Ahmed EOS, Ghalib MB, et al. Manipulating dietary fibre: gum arabic making friends of the colon and the kidney. Bioact Carbohyd Diet Fibre. (2014) 3:71–6. doi: 10.1016/j.bcdf.2014.01.005
36. Nasir O. Renal and extrarenal effects of gum arabic (Acacia senegal)-what can be learned from animal experiments? Kidney Blood Press Res. (2013) 37:269–79. doi: 10.1159/000350152
37. Ali AA, Ali KE, Fadlalla AE, Khalid KE. The effects of gum arabic oral treatment on the metabolic profile of chronic renal failure patients under regular haemodialysis in Central Sudan. Nat Prod Res. (2008) 22:12–21. doi: 10.1080/14786410500463544
38. Bliss DZ, Stein TP, Schleifer CR, Settle RG. Supplementation with gum arabic fiber increases fecal nitrogen excretion and lowers serum urea nitrogen concentration in chronic renal failure patients consuming a low-protein diet. Am J Clin Nutr. (1996) 63:392–8. doi: 10.1093/ajcn/63.3.392
39. van Breukelen-van der Stoep DF, van Zeben D, Klop B, van de Geijn G-JM, Janssen HJ, van der Meulen N, et al. Marked underdiagnosis and undertreatment of hypertension and hypercholesterolaemia in rheumatoid arthritis. Rheumatology. (2016) 55:1210–6. doi: 10.1093/rheumatology/kew039
40. Midtbø H, Gerdts E, Kvien TK, Olsen IC, Lønnebakken MT, Davidsen ES, et al. The association of hypertension with asymptomatic cardiovascular organ damage in rheumatoid arthritis. Blood Press. (2016) 25:298–304. doi: 10.3109/08037051.2016.1172867
41. Nasir O, Umbach AT, Rexhepaj R, Ackermann TF, Bhandaru M, Ebrahim A, et al. Effects of gum arabic (Acacia senegal) on renal function in diabetic mice. Kidney Blood Press Res. (2012) 35:365–72. doi: 10.1159/000336359
Keywords: rheumatoid arthritis, gum acacia, renal function, hepatic profile, dietary fibers, clinical trial
Citation: Kamal E, Kaddam LA, Alagib A and Saeed A (2021) Dietary Fibers (Gum Arabic) Supplementation Modulates Hepatic and Renal Profile Among Rheumatoid Arthritis Patients, Phase II Trial. Front. Nutr. 8:552049. doi: 10.3389/fnut.2021.552049
Received: 15 April 2020; Accepted: 05 February 2021;
Published: 10 March 2021.
Edited by:
Jun Lu, Auckland University of Technology, New ZealandReviewed by:
Frank Mojiminiyi, Usmanu Danfodiyo University, NigeriaReza Nemati, Canterbury Health Laboratories, New Zealand
Symon Mahungu, Egerton University, Kenya
Copyright © 2021 Kamal, Kaddam, Alagib and Saeed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lamis AbdelGadir Kaddam, bGFtaXNrYWRkYW1AaG90bWFpbC5jb20=
 Ebtihal Kamal1
Ebtihal Kamal1 Lamis AbdelGadir Kaddam
Lamis AbdelGadir Kaddam