
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 10 March 2021
Sec. Clinical Nutrition
Volume 7 - 2020 | https://doi.org/10.3389/fnut.2020.603370
This article is part of the Research Topic Nutritional Wasting and Cancer: Nutritional Interventions, Multimodal Management and Outcomes View all 6 articles
Malnutrition is prevalent in hospitalized cancer patients and has been associated with poor therapy response and unfavorable clinical outcome. While recent studies have shown a survival benefit through nutritional support in a hospitalized malnourished medical population including cancer patients, we aimed to investigate the association of nutritional support with in-hospital mortality and other clinical outcomes in a nationwide inpatient cancer population. In this population-based cohort study, using a large Swiss administrative claims database from April 2013 to December 2018, we created two cohorts of malnourished cancer patients on medical wards. We generated two pairwise cohorts of malnourished patients who received nutritional support by 1:1 propensity-score matching to patients not receiving nutritional support. The primary outcome was all-cause in-hospital mortality. Secondary outcomes were 30-days all-cause hospital readmission and discharge to a post-acute care facility. To account for disease activity, we stratified patients either admitted for cancer as main diagnosis or admitted with cancer as comorbidity. Among 1,851,498 hospitalizations on medical ward, we identified a total of 32,038 malnourished cancer patients. After matching, 11,906 (37%) cases were included in the “cancer main diagnosis cohort” and 5,954 (18.6%) in the “cancer comorbidity cohort.” Patients prescribed a nutritional support showed a lower in-hospital mortality in both cohorts as compared to their respective matched controls not receiving nutritional support [cancer main diagnosis cohort: 15.4 vs. 19.4 %, OR 0.76 (95% CI 0.69–0.83); cancer comorbidity cohort: 7.4 vs. 10.2%, OR 0.71 (95% CI 0.59–0.85)]. While we found no difference in 30-days readmission rates, discharge to a post-acute care facility was less frequent in the nutritional support group of both cohorts. In this large cohort study, nutritional support in hospitalized patients with either cancer as main diagnosis or comorbidity was associated with a lower risk of in-hospital mortality and discharge to a post-acute care facility.
In cancer patients, the combination of pathophysiological changes in appetite signals, treatment side effects, as well as physical limitations results in reduced food intake and consequently puts these patients at high risk for malnutrition (1). This state of chronic disease-related malnutrition is called cachexia (2). Cachexia is not only caused by anorexia but also by a state of metabolic derangements due to catabolic drivers such as inflammatory cytokines, which finally lead to a state of weight loss and sarcopenia.
Malnutrition and its consequences is more frequent in the population of patients with malignant diseases. Prevalences range from 20 to 70% in worldwide studies, depending on age, entity of the cancer, type of treatment and cancer stages (1). For example, a French study found an overall prevalence of 39% in cancer patients. There were even higher prevalence rates in patients with head neck cancer (48.9%), esophageal/stomac cancer (60.2%), and pancreatic cancer (66.7%) (3). In a recently published randomized controlled trial investigating the effect of nutritional support in medical inpatinets (4), cancer was the second most frequent admission diagnosis in patients at nutritional risk (18.5%).
The impact of malnutrition in cancer patients on health and financial factors had been shown in several studies: Malnourished cancer patients showed increased mortality rates (5–7), as well as longer length of hospital stay (5, 7, 8), higher health care cost (8), lower scores in QoL (9), and lower tolerance to chemotherapy (6).
Because of the strong impact of malnutrition on prognosis in cancer patients, the current ESPEN evidence-based nutrition guidelines (10) highlight the importance of recognizing and assessing malnutrition followed by treating it adequately with an individualized nutritional treatment concept. However, evidence for nutritional treatment to improve clinical outcomes in cancer patients is heterogenous. In 2012, a meta-analysis was showing that oral nutritional interventions increase the nutritional intake and improve some aspects of quality of life, but do not affect mortality risk (11). Focusing on patients with chemo(radio)therapy, another review revealed benefit of nutritional interventions on body weight but not regarding treatment toxicity or survival (12). A randomized controlled trial investigating long-term outcomes in patients with colorectal cancer showed a beneficial effect of individualized nutritional support counseling on nutritional status, survival as well as treatment toxicity (13).
To substantiate evidence in cancer patients, the ESPEN guidelines also recommend the performance of further trials to investigate the efficacy of nutritional interventions (10).
In Switzerland, screening, assessment, and treatment of malnutrition has remarkably increased during the recent years not only due to improved awareness of malnutrition being a predictor of adverse outcome but also due to reimbursement reasons1.
The aim of this study was to investigate the association of nutritional support with in-hospital mortality and other clinical outcomes in malnourished cancer patients as treated in clinical routine using “real-world” data.
This was a retrospective cohort study using a nation-wide population-based database from Switzerland to investigate the association between nutritional support and clinical outcomes in malnourished cancer patients. We used administrative claims data from the Federal Statistical Office of Switzerland (“Medizinstatistik”)1 and observation period was from April 2013 to December 2018. The database contains all Swiss inpatient discharge records from acute care-, general-, and specialty hospitals, and provides information about in-hospital health-care use, main diagnosis and comorbidities, diagnostic tests and procedures. Data assessment was longitudinal with each patient having a unique code, so that re-hospitalization could be tracked and one patient could have more than one index hospitalization. Main diagnoses and comorbidities were assessed during index hospitalization and coded using the International Classification of Disease, version 10, German Modification (ICD-10-GM) codes; in-hospital therapeutic procedures were recorded using the “Swiss classification of operation” codes (CHOP).
The institutional review board of Northwestern Switzerland (AG/SO 2009/074 and EKNZ BASEC PB_2017-00449) approved the trial, including waiver of informed consent of the patients because data was de-identified. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines (14).
We included adult hospitalizations (≥18 years) on medical wards who were malnourished and had a diagnosis of cancer. Patients with an admission to intensive care unit in the course of hospitalization were excluded to increase specify of the study population. Malnutrition was defined as the presence of one of the following ICD-10-GM codes: E43 for unspecified severe protein-energy malnutrition, E44 for protein-energy malnutrition of moderate and mild degree, and E46 for unspecified protein-energy malnutrition2. Appendix Table 1 shows detailed information about the ICD-10 codes of malnutrition. For the assessment of cancer diagnosis and specific type of cancer we also used ICD-10-GM codes (C00-97). We stratified our cohort in two groups depending on whether the reason for admission cancer (“cancer main diagnosis cohort”) or whether cancer was coded as a comorbidity (“cancer comorbidity cohort”). Using the ICD-10-GM, we also sub classified the patients according to type of tumor: gastrointestinal (C15–C26), respiratory (C30–C39), hematological (C81–C96), urogenital (C51–C66), breast (C50), and others (all other ICD-10-GM codes for cancer; i.e., oropharyngeal, bone, skin, mesothelial, cerebral, endocrinological and unknown origin). Regarding current treatment of cancer we categorized the patients by means of CHOP codes: chemotherapy (99.25.xx), immunotherapy (99.28.xx), stem cell transplantation (41.0x.xx), radiotherapy (92.2x.xx, 92.3x.xx, 92.4x.xx).
We screened eligible hospitalizations of malnourished cancer patients for the presence of nutritional support during hospitalization. Again, we used CHOP codes to classify patients with nutritional support (“89.0A.32” and “89.0A.4x” for dietary advice and/or nutritional therapy, “96.6” for enteral tube-feeding of concentrated nutrients, and “99.15” for parenteral infusion of concentrated nutrient solutions). Dietary advice and nutritional therapy were not further classified; general practice in Switzerland is based on assessment and advice of a dietician and adjustment of hospital meals, food fortification, additional snacks or the use of oral nutritional supplements (ONS).
Our primary outcome was all-cause in-hospital mortality. Secondary outcomes were 30-days readmission rate from any cause as well as discharge to a post-acute care facility, defined as every discharge location except from home.
Before and after propensity-score matching we used descriptive statistics and standardized difference were applied to compare baseline characteristics of patients stratified by nutritional support. A standardized difference of <10% indicated adequate balance between groups (15).
We fitted different logistic regression models to control for confounding and to assess the robustness of our results. In a first step, we performed a unadjusted logistic regression analysis for primary and secondary outcomes comparing hospitalizations of patients on nutritional support with those not on nutritional support. Second, we fitted a multivariable logistic regression model adjusting for sociodemographic factors (age, gender, nationality, insurance status, month and year of admission, location before and mode of admission, hospital size, hospital site). Third, in the fully-adjusted model we included the sociodemographic factors as well as main diagnoses, comorbidities, entity of cancer, type of cancer therapy, severity of malnutrition, the total amount of hospitalizations per patient, any palliative treatment, Charlson Comorbidity Index, Hospital Frailty Risk Score, and length of hospital stay. Finally, we performed a propensity-score matched model by matching hospitalizations of patients receiving nutritional support 1:1 to a cohort of hospitalizations without nutritional support. Doing so, we used a multivariable logistic regression model to calculate the probability of receiving nutritional support and as covariates we used all parameters from the fully adjusted model (except for hospital site). The estimated propensity-score was used for the matching with nearest neighbor controls and the caliper size was 0.0005. Discrimination analysis revealed a c-index of 0.7545 for the final model. After propensity-score matching we performed logistic regressions, which were adjusted for hospital site.
For the secondary analyses and assessment of 30-days readmission and discharge to post-acute care facility, we excluded hospitalizations with an event of in-hospital death to address competing risk. Kaplan-Meier curves were used to illustrate the differences in time to in-hospital mortality and cox-regression model were used to calculate the hazard ratio.
In a sensitivity analysis, we explored the association between nutritional support and outcomes of interest in patients with cancer as main admission diagnosis only. We performed subgroup analyses to explore differences among cancer entities and treatments of cancer by calculating effect modification.
We used STATA, version 15.1 (StataCorp LLC), for all analyses. P-values are two-sided and values <0.05 are considered as significant.
In total, our database included 8,266,509 hospitalizations from April 2013 to December 2018 (Figure 1). Among them, we identified 1,892,131 hospitalizations of adult patients on medical wards. 6% (114,264) of them had a code for malnutrition and among the malnourished cohort 37.4% (42,755) had a malignancy. Nutritional support was provided in 71.3% (n = 30,501) of these cases, whereas 28.7% (n = 12,254) had no code for nutritional support. Even in the unmatched population most baseline characteristics were quite well-balanced. Importantly, there was a difference in the incidence of severe malnutrition (36.5% in the nutritional support group vs. 13.2% in the control group) and total length of hospital stay (median LOS 4 days longer in the nutritional support group).
After 1:1 propensity score matching, we paired 38.7% of the cases with nutritional support to 96.2% of the hospitalizations without nutritional support. All baseline characteristics were well-balanced (Table 1). Mean age was 70.3 years, 43.7% of the patients were female and most patients were treated in tertiary care hospitals (77.7%). 68.2% of the patients were admitted because of their malignancy. Burden of comorbidity was high as reflected by a mean Charlson Comorbidity index of 6.8 points (SD ± 3.1 points). 13.9% of the overall matched population were severely malnourished.
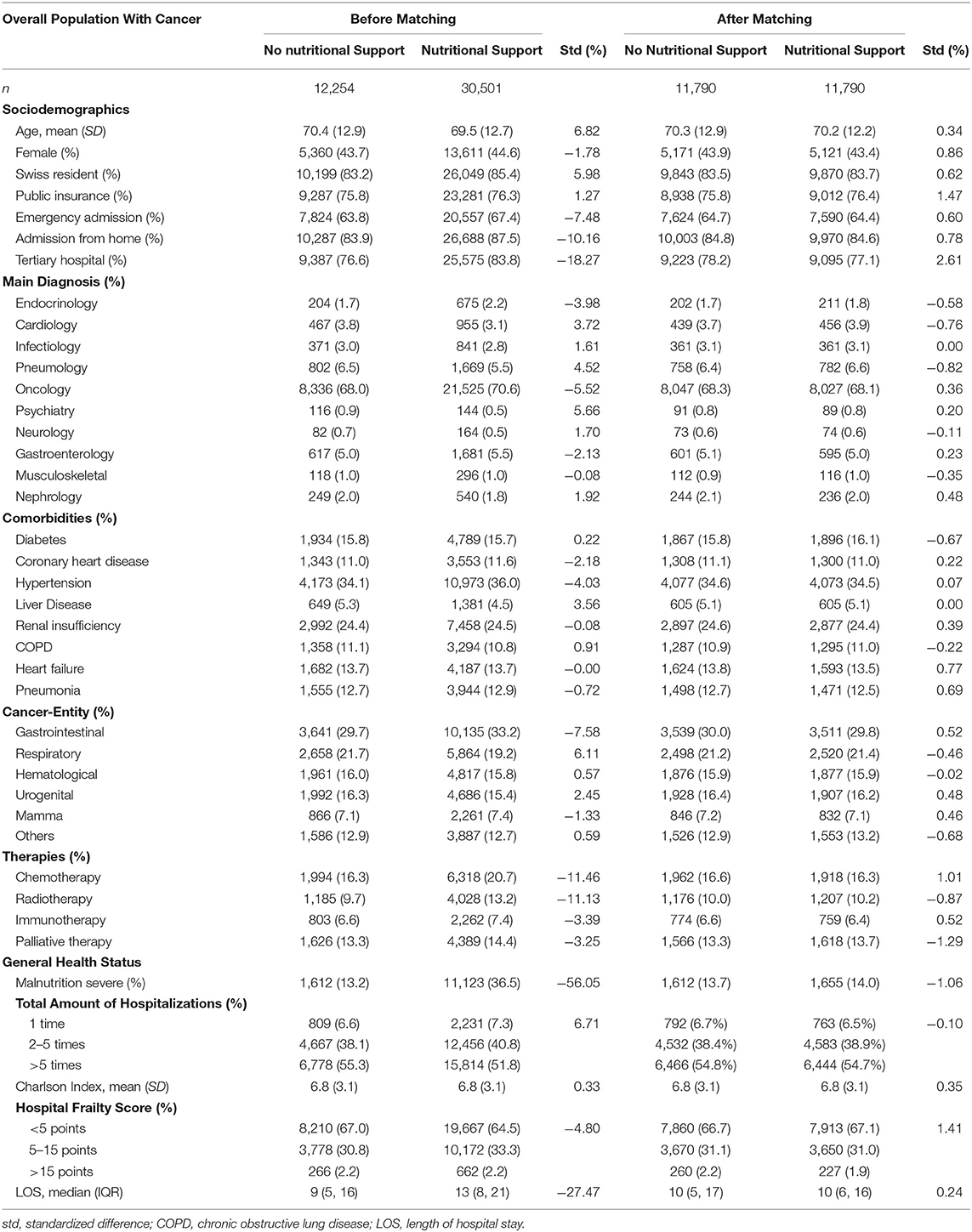
Table 1. Baseline characteristics before and after 1:1 propensity-score matching stratified by nutritional support.
In the unmatched study population, in-hospital mortality rate was 16.9% (2,065/12,254) in the control group and 14.4% (4,397/30,501) in the nutritional intervention group corresponding to an odds ratio (OR) of 0.83 with 95% confidence interval (CI) of 0.79–0.88 (Table 2). The association between nutritional support and reduced mortality rate remained significant in the adjusted and the fully adjusted model (OR 0.82, 95%CI 0.77–0.86 and OR 0.82, 95%CI 0.69–0.79, respectively). After propensity score matching, the overall in-hospital mortality rate was 14.9%. 1,548 (13.1%) patients on nutritional support died during hospitalization compared to 1,970 (16.7%) patients in the control group (OR 0.73, 95%CI 0.68–0.79). Survival benefit over 30 days of hospitalization is graphically illustrated in Figure 2.
In the unadjusted analysis, 30-days readmission rate was significantly lower in the nutritional intervention group compared with the control group (25.0% in patients with nutritional support vs. 25.7% in patients without nutritional support, OR 0.92, 95%CI 0.88–0.97). However, there was no significant association in the fully adjusted analysis nor in the propensity-score matched analysis, respectively.
Considering discharge to post-acute care facility, there was no difference in the unmatched population but after full adjustment and in the propensity-score matched cohort, patients receiving nutritional support during hospitalization were less likely to be admitted to a post-acute care facility as compared to control patients.
Among hospitalizations with cancer as main admission diagnosis, nutritional support was prescribed in 21,525 (72.1%) patients, while 8,336 (27.9%) patients did not receive nutritional support. Baseline characteristics before and after 1:1 propensity-score matching of this population are shown in Appendix Table 2. Results for mortality, readmission rate, and discharge to post-acute care facility remained robust in this cohort of patients with cancer as admission diagnosis compared to the overall population (Table 3). In a subgroup analysis of this population we stratified for cancer entity, cancer treatment, and cancer stadium (Figure 3). The findings for in-hospital mortality remained robust with two exceptions: patient groups with hematological cancer and on chemotherapy both did not show significant survival benefit (OR 0.96, 95%CI 0.75–1.24, p for interaction 0.033; OR 1.04, 95%CI 0.79–1.36, p for interaction 0.025, respectively).
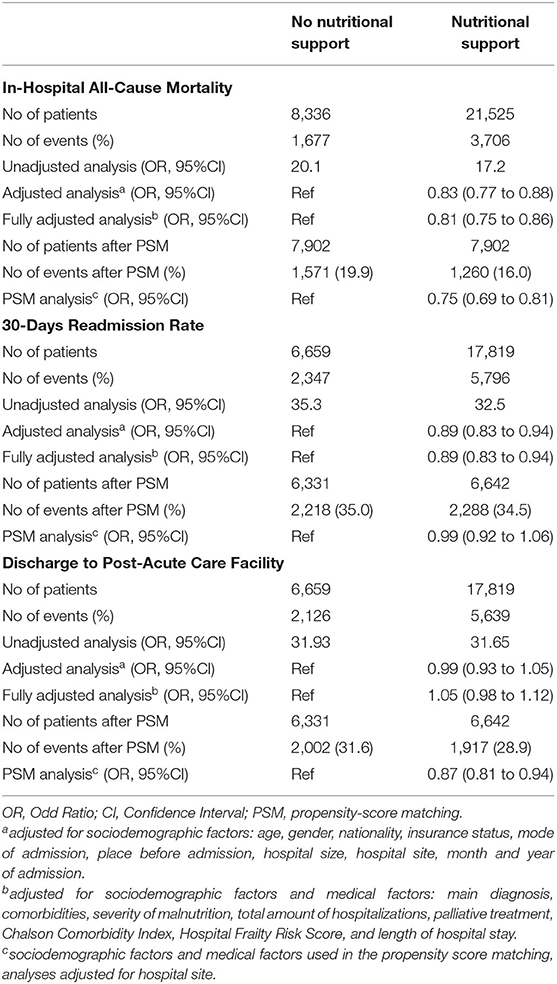
Table 3. Association of nutritional support with clinical outcomes in patients admitted for an oncological diagnosis.
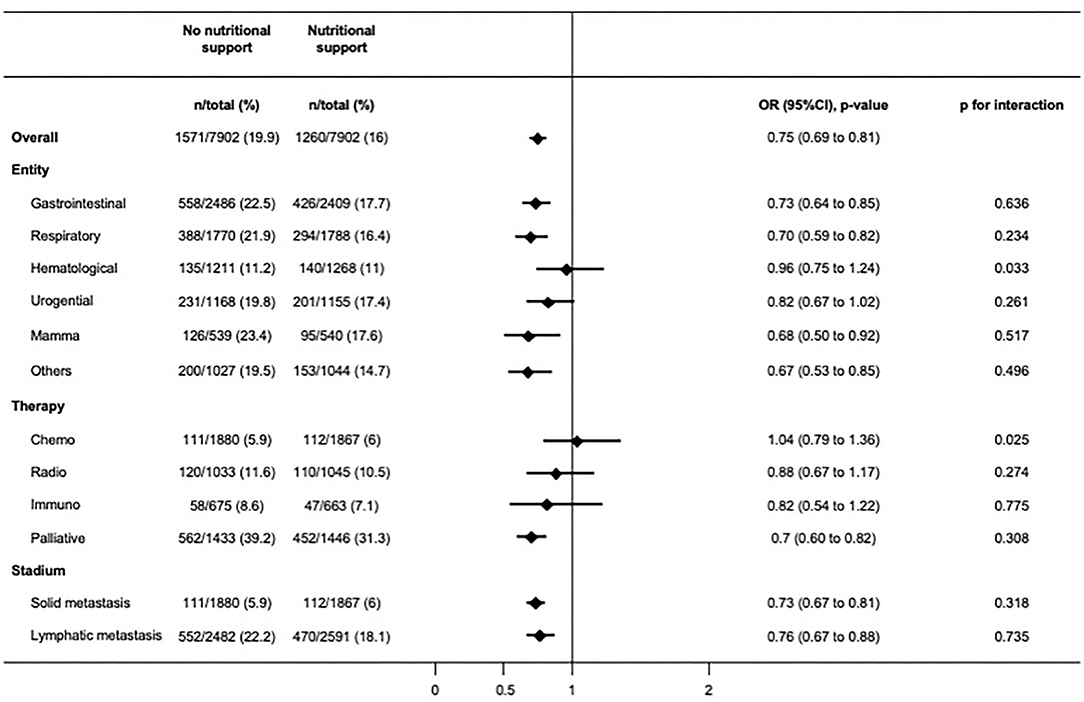
Figure 3. Subgroup analyses showing odds ratios for primary outcome after matching in patients admitted for an oncological diagnosis.
This large population-based cohort study revealed two key findings. First, inhospital mortality rate was lower among malnourished cancer patients who received nutritional support as compared to those not receiving additional nutritional support. Results were consistent in patients mainly admitted for cancer per se. Second, the findings of this study remained robust among the different cancer entities and treatment options except for patients suffering from a hematological cancer or undergoing chemotherapy.
To the best of our knowledge, there is only few data investigating the association of nutritional intervention and mortality in a heterogenous cancer patient population specifically. Previous studies have focused rather on general medical patients including cancer patients, but evidence still remains scarce. For example, the EFFORT trial, which reported an overall 35% relative risk reduction for 30-days mortality in patients receiving nutritional support vs. standard care did not find any significant risk reduction in the subgroup of cancer patients (4). In contrast to our study, most of the studies in cancer patients are from the outpatient setting. For example a smaller study—specifically investigating colorectal cancer patients—reported that patients with individualized nutritional counseling or with nutritional supplement therapy showed a long-term survival benefit as compared with the control group receiving usual care (13). Considering parenteral nutrition, there are few randomized controlled trials but results are heterogenous. No beneficial or even detrimental effects (more adverse events) were found in a study with advanced colorectal cancer patients (16), whereas in another study with a similar population there was an effect on fat-free mass and quality of life (17). Nonetheless, no studies reported from real-world cancer inpatients as treated in clinical routine, limiting the comparability of the studies.
We found no significant reduction in readmission rates associated with nutritional support. Similarly, Britton et al. (18) did not find any significant benefit regarding readmission rates in a population of head and neck cancer patients comparing a psychological intervention to improve nutritional intake to standard dietician treatment. In contrast, a retrospective study also investigating head and neck cancer patients showed a significantly lower readmission rate in patients undergoing prophylactic gastrostomy to improve nutritional status (18). However, their intervention was more invasive and the retrospective design might introduce confounding by indication. Regarding discharge to post-acute care facilities, we found the patients on nutritional support being less frequently discharged to a post-acute care facility. Taking into account the limited places in those facilities, the increasing health care costs and patients preferences, this finding may be of particular interest for physicians, policy makers and patients. So far, no further studies have investigated this outcome.
Assessing the association of nutritional support among patients primarily hospitalized for an oncological reason, the in-hospital mortality benefit of nutritional support was present in most of the different cancer entities and treatment options, with, however, two exceptions, hematological malignancies, and chemotherapy. Most of the previous trials investigating hematological malignancies were performed in patients on chemotherapy. A meta-analysis published in 2018 included 11 trial of cancer patients with chemo(radio)therapy and assessed the effect of nutritional interventions on body weight (12). They found a significant effect of nutritional interventions including dietary advice, ONS and ONS enriched with protein and n-3 polyunsaturated fatty acids (PUFA) on body weight (1.31 kg, 95% CI 0.24–2.38, P 0.02, heterogeneity Q = 21.1, P = 0.007), mainly driven by high-protein n-3 PUFA-enriched ONS. But they did not show any effect on survival. Limiting factors of this meta-analysis were high heterogeneity in treatment effects, low to moderate quality of the included studies, and inclusion of different settings as well as different interventions (19). Another nutritional treatment option for patients with chemotherapy is glutamine. In the review of Kuhn et al. they reported that eight of 24 studies using oral, and six of 12 studies using parenteral glutamine found a clinical benefit (i.e., decreased risks of high-dose chemotherapy and radiation). But, according to the current ESPEN guidelines (10) the role of glutamine in chemotherapy patients remains controversial.
In summary, evidence in patients with hematological cancer entities and on chemotherapy is still poor and leads to the suggestion that provision of the right nutrients with the right application form may be crucial. This underlines the importance of future well-designed clinical trials for nutritional interventions in (hematological) cancer patients undergoing chemotherapy.
The strengths and limitations of this study has to be interpreted in the context of the study design. The main strength of this study is that it includes a nation-wide sample of all hospitalized cancer patients in Switzerland between 2013 and 2018. Because of the heterogeneity of the population and in regard to robust results in the sensitivity and subgroup analyses, our findings reveal high external validity. Additionally, we were able to address multiple possible confounding variables by including them into our adjusted or propensity-score matched analyses. The definition of nutritional support in this study is highly specific, but due to the possibility of missed coding for nutritional support, there could be some patients in the control group also receiving nutritional support, mirroring decreased sensitivity. We thus might argue that the observed findings could be even underestimated.
The main limitations of this study involve, first, a certain risk of misclassification and underreporting of tumor entities, therapy forms, and malnutrition per se as we used ICD-10 codes. In our cohort of malnourished medical inpatients, the prevalence rate of malignant diseases was 30% which was similar to the results of the large multi-center EFFORT trial (4). Regarding the prevalence of malnutrition defined by ICD-10 code in the overall population of Swiss medical inpatients (6%), it was remarkably lower compared to trials using clinical screening tools [i.e., 28% in the study of Felder et al. (20)]. As shown in a former Swiss study investigating the diagnostic accuracy of malnutrition codes, sensitivity was low (30%) but specificity was high (93.4%) meaning that mostly all patients with a coding for malnutrition really were malnourished but a relevant proportion of patients with malnutrition might not be included in our study (21). Similar, we were not able to assess activity and severity of the malignant disease nor the treatment strategy in detail. But malignancies which did not affect the actual state of health or which were considered as cured had to be coded with a different ICD-10-GM code, which we did not include in our analysis. Also, the high average inhospital mortality rate of 15.6% could be an indicator that our population in fact included patients with more severe neoplastic diseases. Additionally, our sensitivity analysis only including patients with an oncological admission diagnosis showed robust results. Second, type of nutritional intervention was not defined in detail by the CHOP codes and the attribution of the nutritional intervention was in discretion of the treating physician. Third, unmeasured or unmeasurable confounding cannot be excluded, and whether more palliative patients did not qualify for an additional therapy anymore, should be critically discussed. However, we included several surrogate variables concerning general health status (Hospital Frailty Index, presence of a code for palliative treatment, burden of comorbidities) into our statistical models to better account for potential residual confounding.
In this large nationwide cohort study of malnourished cancer patients, we found nutritional support as prescribed in clinical practice to be associated with a decrease in in-hospital mortality. These findings support the current guidelines, which recommend a standardized screening and multidisciplinary management of malnutrition in cancer patients. By broadening the use of a validated screening tool [i.e., NRS 2002 (22)], the awareness of physicians and nurses will further increase and consequently more patients at risk for malnutrition could benefit from an early treatment performed by trained dieticians. Further high-quality studies are needed to investigate optimal disease and treatment-specific nutritional support to individualize the treatment of malnourished cancer patients.
The datasets generated for this study are available on request to the corresponding author.
The studies involving human participants were reviewed and approved by Institutional review board of Northwestern Switzerland (AG/SO 2009/074 and EKNZ BASEC PB_2017-00449). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
NK-B and AK designed the study, wrote the manuscript, and analyzed the data. AK had full access to all the data. All authors were responsible for the decision to submit the manuscript, provided substantial comments on drafts, and approved the final report.
This study was supported in part by the Swiss National Science Foundation (SNSF, National Research Program (NRP 74), 407440_167376), the research council (Grant 3071410.000.086) and the Wissenschaft & Weiterbildung (W&W) Fonds (140.000.495) of the Kantonsspital Aarau AG, and the Hugo und Elsa Isner Foundation of the Argovian Department of Health and Social Affairs and Funds from the Argovia Professur of the Medical Faculty of the University of Basel.
The funding sources had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the Swiss Federal Office for Statistics (Bundesamt für Statistik, Neuchâtel, Switzerland) for the acquisition and provision of data.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2020.603370/full#supplementary-material
1. ^Medizinische Statistik der Krankenhäuser. (2020). Available online at: https://www.bfs.admin.ch/bfs/de/home/statistiken/gesundheit/erhebungen/ms.assetdetail.7369.html (accessed July 11, 2020).
2. ^Online Definitionshandbuch. (2020). Available online at: https://manual50.swissdrg.org (accessed July 11, 2020).
1. Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NEP, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. (2017) 36:1187–96. doi: 10.1016/j.clnu.2017.06.017
2. Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. (2017) 36:49–64. doi: 10.1016/j.clnu.2016.09.004
3. Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. J Parenter Enteral Nutr. (2014) 38:196–204. doi: 10.1177/0148607113502674
4. Schuetz P, Fehr R, Baechli V, Geiser M, Deiss M, Gomes F, et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet. (2019) 393:2312–21. doi: 10.1016/S0140-6736(18)32776-4
5. Maasberg S, Knappe-Drzikova B, Vonderbeck D, Jann H, Weylandt KH, Grieser C, et al. Malnutrition predicts clinical outcome in patients with neuroendocrine Neoplasia. Neuroendocrinology. (2017) 104:11–25. doi: 10.1159/000442983
6. Aaldriks AA, van der Geest LGM, Giltay EJ, le Cessie S, Portielje JEA, Tanis BC, et al. Frailty and malnutrition predictive of mortality risk in older patients with advanced colorectal cancer receiving chemotherapy. J Geriatr Oncol. (2013) 4:218–26. doi: 10.1016/j.jgo.2013.04.001
7. Pressoir M, Desné S, Berchery D, Rossignol G, Poiree B, Meslier M, et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br J Cancer. (2010) 102:966–71. doi: 10.1038/sj.bjc.6605578
8. Planas M, Álvarez-Hernández J, León-Sanz M, Celaya-Pérez S, Araujo K, García de Lorenzo A, et al. Prevalence of hospital malnutrition in cancer patients: a sub-analysis of the PREDyCES® study. Support Care Cancer. (2016) 24:429–35. doi: 10.1007/s00520-015-2813-7
9. Gellrich N-C, Handschel J, Holtmann H, Kruskemper G. Oral cancer malnutrition impacts weight and quality of life. Nutrients. (2015) 7:2145–60. doi: 10.3390/nu7042145
10. Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. (2017) 36:11–48. doi: 10.1016/j.clnu.2016.07.015
11. Baldwin C, Spiro A, Ahern R, Emery PW. Oral nutritional interventions in malnourished patients with cancer: a systematic review and meta-analysis. J Natl Cancer Inst. (2012) 104:371–85. doi: 10.1093/jnci/djr556
12. de van der Schueren MAE, Laviano A, Blanchard H, Jourdan M, Arends J, Baracos VE. Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: current evidence and guidance for design of future trials. Ann Oncol. (2018) 29:1141–53. doi: 10.1093/annonc/mdy114
13. Ravasco P, Monteiro-Grillo I, Camilo M. Individualized nutrition intervention is of major benefit to colorectal cancer patients: long-term follow-up of a randomized controlled trial of nutritional therapy. Am J Clin Nutr. (2012) 96:1346–53. doi: 10.3945/ajcn.111.018838
14. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. (2014) 12:1495–9. doi: 10.1016/j.ijsu.2014.07.013
15. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. (2009) 28:3083–107. doi: 10.1002/sim.3697
16. Bouleuc C, Anota A, Cornet C, Grodard G, Thiery-Vuillemin A, Dubroeucq O, et al. Impact on health-related quality of life of parenteral nutrition for patients with advanced cancer cachexia: results from a randomized controlled trial. Oncologist. (2020) 25:e843–51. doi: 10.1634/theoncologist.2019-0856
17. Obling SR, Wilson BV, Pfeiffer P, Kjeldsen J. Home parenteral nutrition increases fat free mass in patients with incurable gastrointestinal cancer. Results of a randomized controlled trial. Clin Nutr. (2019) 38:182–90. doi: 10.1016/j.clnu.2017.12.011
18. Britton B, Baker AL, Wolfenden L, Wratten C, Bauer J, Beck AK, et al. Eating As Treatment (EAT): a stepped-wedge, randomized controlled trial of a health behavior change intervention provided by dietitians to improve nutrition in patients with head and neck cancer undergoing radiation therapy (TROG 12.03). Int J Radiat Oncol Biol Phys. (2019) 103:353–62. doi: 10.1016/j.ijrobp.2018.09.027
19. McGeer AJ, Detsky AS, O'Rourke K. Parenteral nutrition in cancer patients undergoing chemotherapy: a meta-analysis. Nutrition. (1990) 6:233–40.
20. Felder S, Lechtenboehmer C, Bally M, Fehr R, Deiss M, Faessler L, et al. Association of nutritional risk and adverse medical outcomes across different medical inpatient populations. Nutrition. (2015) 31:1385–93. doi: 10.1016/j.nut.2015.06.007
21. Khalatbari-Soltani S, Waeber G, Marques-Vidal P. Diagnostic accuracy of undernutrition codes in hospital administrative discharge database: improvements needed. Nutrition. (2018) 55–56:111–5. doi: 10.1016/j.nut.2018.03.051
Keywords: malnutrition, nutritional support, cancer, oncology, mortality
Citation: Kaegi-Braun N, Schuetz P, Mueller B and Kutz A (2021) Association of Nutritional Support With Clinical Outcomes in Malnourished Cancer Patients: A Population-Based Matched Cohort Study. Front. Nutr. 7:603370. doi: 10.3389/fnut.2020.603370
Received: 06 September 2020; Accepted: 14 October 2020;
Published: 10 March 2021.
Edited by:
Zeno Stanga, Bern University Hospital, SwitzerlandReviewed by:
Peter Ballmer, Independent Researcher, Winterthur, SwitzerlandCopyright © 2021 Kaegi-Braun, Schuetz, Mueller and Kutz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander Kutz, a3V0ei5hbGV4YW5kZXJAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.