- 1Department of Genetics and Plant Breeding, Faculty of Agriculture, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur, Bangladesh
- 2Laboratory of Field Science, Faculty of Applied Biological Sciences, Gifu University, Gifu, Japan
Four selected advance lines of salt-tolerant vegetable amaranth were evaluated for proximate, nutraceuticals, pigments, phytochemicals, and antioxidants components antioxidants activity in completely randomized block design (RCBD) design in three replicates. Salt-tolerant vegetable amaranth contained adequate carbohydrates, protein, moisture, and dietary fiber. The remarkable contents of iron, manganese, copper, zinc, sodium, molybdenum, boron, potassium, calcium, magnesium, phosphorus, sulfur, betacyanins, betalains, betaxanthins, chlorophylls, ascorbic acid, polyphenols, flavonoids, and antioxidant potentiality were found in salt-tolerant vegetable amaranth. The genotypes LS7 and LS9 had abundant proximate, nutraceuticals, pigments, phytochemicals, and antioxidants compared to the genotypes LS3 and LS5. Salt-tolerant vegetable amaranth demonstrated high content of flavonoid compounds including flavonols such as rutin, kaempferol, isoquercetin, myricetin, hyperoside, and quercetin; flavanol, such as catechin; flavone such as apigenin; and flavanone, such as naringenin. For the first time, we identified one flavonol such as myricetin; one flavanol, such as catechin; one flavone such as apigenin; and one flavanone, such as naringenin in salt-tolerant vegetable amaranth. Across six flavonols, rutin and quercetin were identified as the most prominent compounds followed by isoquercetin and myricetin in selected salt-tolerant vegetable amaranths. Across the genotypes, LS7 exhibited the highest flavonols such as rutin, kaempferol, isoquercetin, myricetin, hyperoside, and quercetin as well as the highest flavanols, such as catechin; flavones such as apigenin; and flavanones, such as naringenin. It revealed from the correlation study that antioxidant components of salt-tolerant vegetable amaranth genotypes exhibited good radical quenching capacity of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) and 2,2-diphenyl-1-picrylhydrazyl equivalent to Trolox. The two genotypes LS7 and LS9 of vegetable amaranth containing excellent sources of proximate, nutraceuticals, pigments, phytochemicals, and antioxidants components could be used as potent antioxidants to attaining nutrients and antioxidant sufficiency in the saline prone area of the globe. We can extract colorful juice from the genotypes LS7 and LS9 as drink purposes for consuming the nutraceuticals and antioxidant deficient community in the saline prone area around the world. However, further detail experimentation is required to confirm the standardization and stabilization of functional components of vegetable amaranth for extraction of juice as drinks.
Introduction
Foods' acceptability mostly depends on color, flavor, and taste. For this reason, recently coloring food products have been put forward as they considerably accepted the common interest of the people around the globe. These products interested the consumers in the safety, nutritional, and aesthetic aspects of foods. These products also increase the consumption of natural pigments including betacyanins, betaxanthins, betalains, anthocyanin, amaranthine, chlorophylls, and carotenoids. Vegetable amaranth is a unique source of betalains (betaxanthins and betacyanins) that has important free radical-scavenging activity (1). Betalains could be used as a food colorant in low-acid foods and it has higher pH stability than anthocyanins (2). Amaranthine, a major pigment of betacyanins in vegetable amaranth had very strong antioxidant potentials. It could be used as a substitute source for the well-known betanins from red beets in the food colorants and natural antioxidants (1). Vegetable amaranth has wide adaptability to different abiotic stresses like drought (3–6) and salinity (7–9).
Amaranth (belongs to the family Amaranthaceae) is C4 and a fast-growing plant with versatile uses such as for ornamental plants, vegetables, and grains. It has wider acclimatization and distributed in America, Africa, Australia, Asia, and Europe. Edible stems and leaves of vegetable amaranth are low-cost vegetables and have abundant protein with important amino acids including methionine and lysine, dietary fiber, carotenoids, vitamin C, minerals, such as calcium, magnesium, potassium, phosphorus, iron, zinc, copper, and manganese (10–15). This genus has many traditional medicinal uses, especially as antiviral, antimalarial, antidiabetic, antibacterial, antihelminthic, and snake antidote (16, 17). It has also abundant antioxidant pigments, such as betacyanins, anthocyanin, betaxanthins, betalains, carotenoids, and chlorophylls (18, 19); and antioxidant phytochemicals, such as vitamin C, phenolic acids, and flavonoids (20). These natural compounds have a remarkable contribution to the industry of food as they scavenge reactive oxygen species (ROS) in the human body and remedy several diseases like cardiovascular diseases, cancer, cataracts, atherosclerosis, retinopathy, arthritis, emphysema, and neurodegenerative diseases (21–24).
Morphologically amaranth is red and green in color (25). Red color amaranth has more pigments like amaranthine, betacyanins, anthocyanin, betaxanthins, carotenoids, and betalains than green color amaranth. Vegetable amaranth (Amaranthus gangeticus) has great variability and phenotypic diversity in Asia including Bangladesh and India (26) and has multipurpose uses. The selected genotypes are bright red-violet and maroon in color because of the presence of abundant betalains. It is popular and the cheapest leafy vegetables in Bangladesh and Asia. Its nutritional value, taste, and attractive leaf color has attracted people as a very popular vegetable in the Asian continent and elsewhere. In comparison to lettuce, Amaranth contains 18 times more vitamin A, 13 times more vitamin C, 20 times more calcium and 7 times more iron (27). Salt-tolerant vegetable amaranth leaves contain higher zinc and iron content than cassava leaves (28) and beach pea (29). Jimenez-Aguiar and Grusak (30) reported that potassium, calcium, magnesium, phosphorus, sulfur, manganese, iron, zinc, and copper in the leaves of amaranth were more pronounced than black nightshade, spinach, spider flower, black nightshade, and kale. In Bangladesh and India, Vegetable amaranth is grown year-round and even in the gaps of foliage crops between winter and hot summer (10, 11). Vegetable amaranth leaves inhibit the proliferation of colon (Caco-2) and breast (MCF-7) cancer cell lines and liver (HepG2) exhibit anticancer potential (31).
Recently, we have been exploring salt-tolerant vegetable amaranth genotypes containing high pigments, nutraceuticals, antioxidant phytochemicals, and phenolics of interest for making drinks for the sustainable health benefit of the consumers in the salinity-prone and coastal belt area of the globe. For this purpose, previously, we evaluated germplasms based on salt-tolerance, high yields, and antioxidant potential and four selected advance lines of salt-tolerant vegetable amaranths. It is the first attempt to study the pigments, nutraceuticals, antioxidant phytochemicals, phenolic and flavonoids, and antioxidant capacity in salt-tolerant vegetable amaranth. We ultimately study the possibility of the salt-tolerant genotypes for extracting colorful juice as drink purposes containing abundant pigments, nutraceuticals, antioxidant phytochemicals, phenolics, antioxidant capacity, and flavonoids.
Materials and Methods
Experimental Materials
This is the first report on phenolic profiles, antioxidant compositions, and antioxidant capacity in salt-tolerant vegetable amaranth. We previously evaluated several genotypes based on salt tolerance, antioxidant, and yield potentiality to select the best four high-yielding and antioxidant-enriched salt-tolerant genotypes for this experiment.
Design and Layout
We executed the experiment in three replicates following a completely randomized block design (RCBD) at Bangabandhu Sheikh Mujibur Rahman Agricultural University. Each genotype was grown in a 1 m2 experimental plot following 20 cm and 5 cm distances between rows and plants, respectively.
Intercultural Practices
Recommended compost doses, fertilizer, and appropriate cultural practices were maintained (32). For maintaining the exact spacing of plants in a row, proper thinning was executed. Weeds of experimental plots were regularly removed through proper weeding and hoeing. We provided regular irrigation in the experimental plots for maintaining the proper growth of vegetable amaranth. Leaves from 35-day-old plants were sampled for all biochemical analyses.
Solvent and Reagents
Solvent: Acetone, hexane, and methanol. Reagents: dithiothreitol (DTT), cesium chloride, HClO4, HNO3, H2SO4, ascorbic acid, standard compounds of pure Trolox (6-hydroxy-2, 5, 7, 8-tetramethyl-chroman-2-carboxylic acid), Folin-Ciocalteu reagent, gallic acid, DPPH, rutin, ABTS+, 2, 2-dipyridyl, aluminum chloride hexahydrate, potassium acetate, sodium carbonate, and potassium persulfate.
Estimation of Proximate Composition
ASAE standards were followed to estimate moisture content (3). In triplicates, the fresh samples of vegetable amaranth leaves were oven-dried for 72 h at 103°C. Then the samples were transferred to a desiccator and allowed to stand at room temperature for cooling. A Denver digital balance (USA) was used to record the weights of the samples. AOAC method described by Sarker and Oba (3) was followed to estimate the ash, crude fat, fiber, crude protein contents, and gross energy. The weight of leaf samples was recorded before and after heat treatment (550°C for 12 h) to estimate ash content. Crude fat content was determined according to AOAC method 960.39. Crude protein was assessed by the micro-Kjeldahl method described by Sarker and Oba (3). Finally, nitrogen was multiplied by 6.25 to measure crude protein (AOAC method 976.05). ISO method described by Sarker and Oba (3) was followed to determine fiber content. Powdered leaf samples were boiled for 30 min adding 0.255 M sulfuric acid. The insoluble residue was filtered again, washed, and boiled in 0.313 M sodium hydroxide. After filtering and washing the sample, it was dried at 130 ± 2°C for 2 h. At 350 ± 25°C temperature, the loss of weight was measured. Fiber content was expressed as fresh weight (FW). The total moisture, crude protein, ash, and crude fat (%) were subtracted from 100 for calculating carbohydrate (g 100 g−1 FW). A bomb calorimeter was used to measure gross energy according to ISO method 9831 method described by Sarker and Oba (3).
Estimation of Mineral Composition
The fresh leaf samples of salt-tolerant vegetable amaranth were dried in an oven at 70°C for 24 h. Dried samples were ground in a mill. We determined calcium, potassium, magnesium, phosphorus, sulfur, iron, manganese, copper, zinc, sodium, molybdenum, and boron from powdered leaves following the nitric-perchloric acid digestion method (3). For this digestion, in the presence of carborundum beads, 40 ml HClO4 (70%), 400 ml HNO3 (65%), and 10 ml H2SO4 (96%) were added to 0.5 g dried leaf sample. After digestion, the ascorbic acid method was followed to measure P through dilution of the solution appropriately in triplicate. We added ascorbic acid and antimony to the yellow-colored complex solution for converting it to a blue-colored phosphomolybdenum complex. The method of Sarker and Oba (3) was followed to read the absorbance by atomic absorption spectrophotometry (AAS) (Hitachi, Tokyo, Japan) at a wavelength of 285.2 nm (magnesium), 76 6.5 nm (potassium), 880 nm (phosphorus), 258.056 nm (sulfur), 248.3 nm (iron), 422.7 nm (calcium), 279.5 nm (manganese), 213.9 nm (zinc), 324.8 nm (copper), 589 nm (sodium), 313.3 nm (molybdenum), and 430 nm (boron).
Determination of Chlorophylls
Chlorophyll ab, chlorophyll b, and chlorophyll a were calculated by extracting the fresh leaves in acetone (80%) (3). A spectrophotometer (Hitachi, U-1800, Tokyo, Japan) was used to measure the absorbance at 646 nm for chlorophyll b and 663 nm for chlorophyll a, respectively. Chlorophylls were calculated as micrograms per gram of FW.
Betacyanins and Betaxanthins Content Measurement
The fresh leaves were extracted in 80% methyl alcohol having 50 mM ascorbate to measure betacyanins and betaxanthins according to the method of Sarker and Oba (33, 34). A spectrophotometer (Hitachi, U-1800, Tokyo, Japan) was used to measure the absorbance at 540 nm for betacyanins and 475 nm for betaxanthins, respectively. The data were calculated as the ng betanin equivalent per g of FW for betacyanins and ng indicaxanthin equivalent per gram of FW for betaxanthins.
Estimation of Ascorbic Acid
A Hitachi spectrophotometer (U-1800, Tokyo, Japan) was utilized to estimate ascorbic acid (AsA) and dehydroascorbic acid (DHA) from the fresh leaves. Dithiothreitol (DTT) was used for the sample pre-incubation and reduction of dehydroascorbic acid into ascorbic acid. Ascorbic acid reduced ferric ion to ferrous ion. Reduced ferrous ion forms complexes with 2, 2-dipyridyl (35, 36). We read the absorbance of Fe2+ complexes with 2, 2-dipyridyl at 525 nm for estimation of vitamin C through the spectrophotometer (Hitachi, U-1800, Tokyo, Japan). We calculated vitamin C in mg 100 g−1 FW.
Estimation of Total Polyphenols
Extraction of total polyphenols was carried out according to Jimenez-Aguilar and Grusak (30) using 25 mg of fresh sample in 2.5 mL of 1.2 M HCl containing methanol (90%) at 90°C for 2 h in a water bath. With readjusting the volume (2.5 mL), the leaf extract was centrifuged at 7,500 rpm for 20 min. The leaf extracts (100 μL) were added to the Folin-Ciocalteau reagent (2 N, 50 μL); after 5 min, 2 N Na2CO3 (400 μL) and water (1 mL). The leaf extracts were incubated for 90 min at 37°C. Finally, it was removed to a microplate (flat bottom). In a microplate reader, the absorbance was detected at 740 nm using gallic acid (GAE) as standard μg g−1 of FW.
Estimation of Total Flavonoids
Total flavonoids were extracted and quantfied according to the method described by Jimenez-Aguilar and Grusak (30). Dry leaf samples (100 mg) were mixed with 5 mL methanol (50%) in water and placed for 1 h with ultrasound. The leaf extracts were centrifuged for 10 min at 13,000 g (4°C). The supernatants were then recovered. Flavonoid extracts (400 μL) were homogenized with water (500 μL), 5% NaNO2 (60 μL), 10% AlCl3 (140 μL). After 10 min, 1 mM NaOH (400 μL) was added. The leaf extracts were incubated for 10 min at a normal temperature. Finally, it was removed to a flat bottom microplate. The absorbance was read at 500 nm in a microplate reader. Results are expressed in μg of rutin equivalents (RE) per gram of sample DW.
Radical Quenching Capacity Assay
Fresh leaves were harvested from 35-day-old plants. For the antioxidant capacity assay, the leaves were dried in the air in a shade; 40 ml aqueous methanol (90%) was utilized to extract ground dried leaves (1 g) from each cultivar in a capped bottle (100 ml). A Thomastant T-N22S (Thomas Kagaku Co. Ltd., Japan) shaking water bath was utilized to extract leaf samples for 1 h. An exactly 0.45 μm filter (MILLEX-HV syringe filter, Millipore Corporation, Bedford, MA, USA) was used to filter the homogenized mixture. After centrifugation for 15 min at 10,000 × g, the antioxidant capacity was estimated from the filtered extract.
Diphenyl-picrylhydrazyl (DPPH) radical degradation method (37, 38) was used to estimate the antioxidant activity. We added 1 ml DPPH solution (250 μM) to 10 μl extract (in triplicate) in a test tube. After adding 4 ml distilled water the extract was placed in the dark for 30 min. A Hitachi U1800 spectrophotometer (Hitachi, Tokyo, Japan) was used to measure the absorbance at 517 nm. The method of Khanam et al. (39) was followed for ABTS+ assay. To prepare two stock solutions separately an ABTS+ solution of 7.4 mM and potassium persulfate of 2.6 mM was used. We mixed both solutions in equal proportion to prepare the working solution at room temperature. The working solution was allowed to react in the dark for 12 h; a 150-μl extract was added to 2.85 ml of ABTS+ solution and allowed to react in the dark for 2 h. For the preparation of the solution, 1 ml of ABTS+ solution was mixed with 60 ml of methanol. A Hitachi spectrophotometer (U1800, Tokyo, Japan) was utilized to take the absorbance against methanol at 734 nm. The inhibition (%) of DPPH and ABTS+ corresponding with control was used to determine antioxidant capacity using the equation as follows:
Where, Abs. blank is the absorbance of the control reaction [10 μl methanol for TAC (DPPH), 150 μl methanol for TAC (ABTS+) instead of leaf extract] and Abs. sample is the absorbance of the test compound. Trolox was used as the reference standard, and the results were expressed as μg Trolox equivalent g−1 DW.
Samples Extraction for HPLC and LC-MS Analysis
The fresh leaf samples were extracted by adding 10 ml methanol (80%) containing acetic acid (1%) in 1 g leaves. The mixture was thoroughly homogenized. Then the mixture was kept in a test tube (50 ml) and capped tightly. The test tube was shaken in a shaker (Scientific Industries Inc., USA) for 15 h at 400 rpm. An exactly 0.45 μm filter (MILLEX®-HV syringe filter, Millipore Corporation, Bedford, MA, USA) was used to filter the homogenized mixture. We centrifuged the mixture at 10,000 × g for 15 min. The flavonols, flavanols, flavones, and flavanones were analyzed from the final filtrate. We performed all extractions in triplicate independent samples.
Flavonols, Flavanols, Flavones, and Flavanones Analysis Through HPLC
The method previously described by Sarker and oba (4, 33) was followed to determine flavonols, flavanols, flavones, and flavanones in the fresh leaf sample using HPLC. We equipped the Shimadzu SCL10Avp (Kyoto, Japan) HPLC with a binary pump (LC-10Avp), DGU-14A degasser, and a Shimadzu SPD-10Avp UV–vis detector. A CTO-10AC (STR ODS-II, 150 × 4.6 mm I.D. (Shinwa Chemical Industries, Ltd., Kyoto, Japan) column was used for the separation of flavonols, flavanols, flavones, and flavanones. The binary mobile phase was pumped with solvent A [6% (v/v) acetic acid] in water and solvent B (acetonitrile) at the flow rate of 1 ml/min for 70 min. HPLC system was run using a gradient program with 0–15% acetonitrile for 45 min, 15–30% for 15 min, 30–50% for 5 min, and 50–100% for 5 min; 35°C temperature in the column was maintained with a 10 μl volume of injection (29). We set the detector at 360, 370, and 280 nm, respectively, for continuous monitoring of flavonols, flavanols, flavones, and flavanones. For identification of the compound, we compared the retention time and UV–vis spectra with their respective standards. We confirmed the flavonols, flavanols, flavones, and flavanones through the mass spectrometry assay method. We estimated phenolic compounds as mg kg−1 FW. A mass spectrometer (AccuTOF JMS-T100LP, JEOL Ltd., Tokyo, Japan) was fitted with an Agilent 1100 Series HPLC system and a UV–vis detector coupled on-line with an ElectroSpray Ionization (ESI) source to analyze the mass spectrometry with negative ion mode with the column elutes in the range of m/z 0–1,000 and needle voltage at −2000 V. Extract constituents were identified by LC-MS-ESI analysis.
Quantification of Phenolic Compounds
We used the respective standards of calibration curves to quantify each flavonols, flavanols, flavones, and flavanones. We dissolved 9 flavonol, flavanol, flavone, and flavanone compounds in 80% methanol as stock solutions to the final concentration of 100 mg/ml. Respective standard curves (10, 20, 40, 60, 80, and 100 mg/ml) were used to quantify the individual flavonols, flavanols, flavones, and flavanones compounds with external standards. UV spectral characteristics, retention times, and co-chromatography of samples spiked with commercially available standards were utilized for identification and match the flavonols, flavanols, flavones, and flavanones.
Statistical Analysis
The Statistix 8 software was used to analyze the data for analysis of variance (ANOVA) (40, 41). Duncan's Multiple Range Test (DMRT) at 1% level of probability was used to compare the means. The results were reported as the mean ± SD of three separate replicates.
Results
The analysis of variance revealed a wide range of variability of the studied traits regarding selected drought-tolerant leafy vegetable amaranths.
Composition of Proximate
The composition of the proximate of salt-tolerant vegetable amaranth is shown in Figure 1. The moisture content ranged from 81.35 to 87.24 g 100 g−1 FW. The highest moisture content was recorded in LS5 (87.24 g 100 g−1 FW), while the lowest moisture content was found in LS9 (81.35 g 100 g−1 FW). Salt-tolerant vegetable amaranth leaves exerted significant and very much noticeable variations in protein content. The genotype LS7 showed the highest protein content (6.34 g 100 g−1) followed by LS9, whereas, the genotype LS3 had the lowest protein content (3.35 g 100 g−1). There were no significant variations in fat content in terms of four selected salt-tolerant vegetable amaranths. The range of fat content was 0.33–0.57 g 100 g−1 FW. The genotype LS9 had the highest carbohydrates content (7.93 g 100 g−1 FW) followed by LS3, while the carbohydrates content was the lowest in LS5 and LS7 (5.73 and 5.64 g 100 g−1 FW, respectively). The genotype LS9 had the highest energy (56.28 kcal 100 g−1 FW) followed by LS7, while the lowest energy was obtained from the genotype LS5 (39.28 kcal 100 g−1 FW). Ash content was the highest in LS7 (5.36 g 100 g−1 FW) followed by LS9, while the lowest ash content was noted in LS5 and LS3 (2.88 and 3.13 g 100 g−1 FW). Content of digestible fiber exhibited the least variations in four selected salt-tolerant vegetable amaranths studied. The accession LS5 and LS9 showed the highest dietary fiber content (8.06 and 7.95 g 100 g−1 FW) followed by LS3, whereas dietary fiber content was the lowest in LS7 (6.98 g 100 g−1 FW).
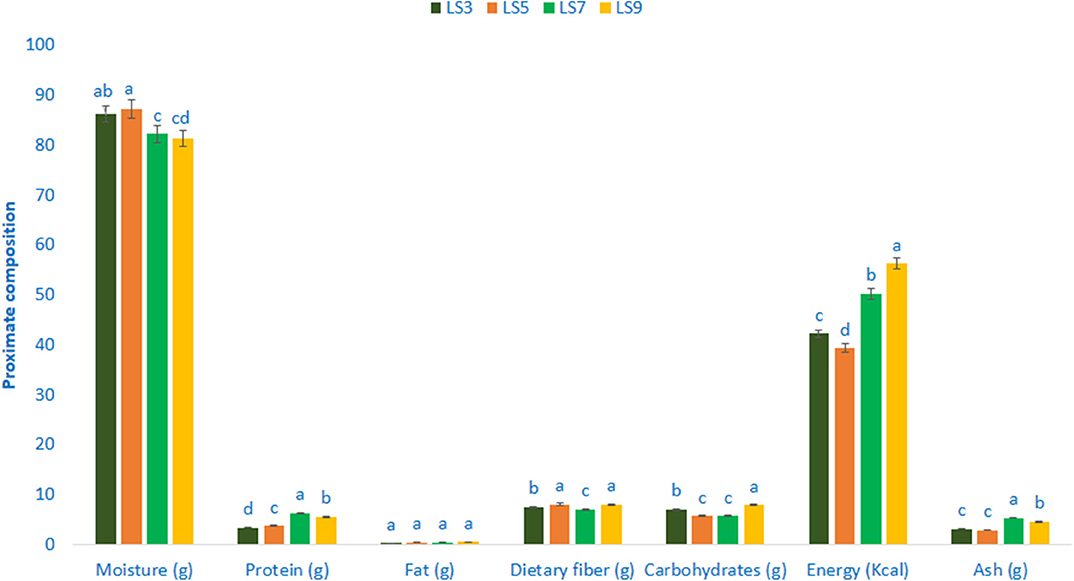
Figure 1. Proximate compositions (g 100 g−1 FW) in four selected salt-tolerant vegetable amaranths; different letters are differed significantly by Duncan multiple range test (P < 0.01), (n = 3).
Mineral Composition (Macroelements)
Mineral composition (macroelements) of salt-tolerant vegetable amaranth is shown in Figure 2. In this study, the range of potassium content was 4.66 mg g−1-7.54 mg g−1 FW. The genotypes LS7 had the highest potassium content, while genotype LS5 had the lowest potassium content. Calcium content ranged from 1.68 to 3.25 mg g−1 FW. The genotypes LS7 showed the highest calcium content, while the genotype LS9 had the lowest calcium content. Magnesium content was the highest in LS3 (3.59 mg g−1 FW) followed by LS5 and LS9. In contrast, the lowest magnesium was recorded in LS7 (2.49 mg g−1 FW). Phosphorus and sulfur content of vegetable amaranth leaves ranged from 0.65 to 1.75 and 0.51 to 1.27 mg g−1 FW. The genotype LS7 exhibited the highest phosphorus and sulfur content, while the genotype LS5 showed the lowest phosphorus and sulfur content.
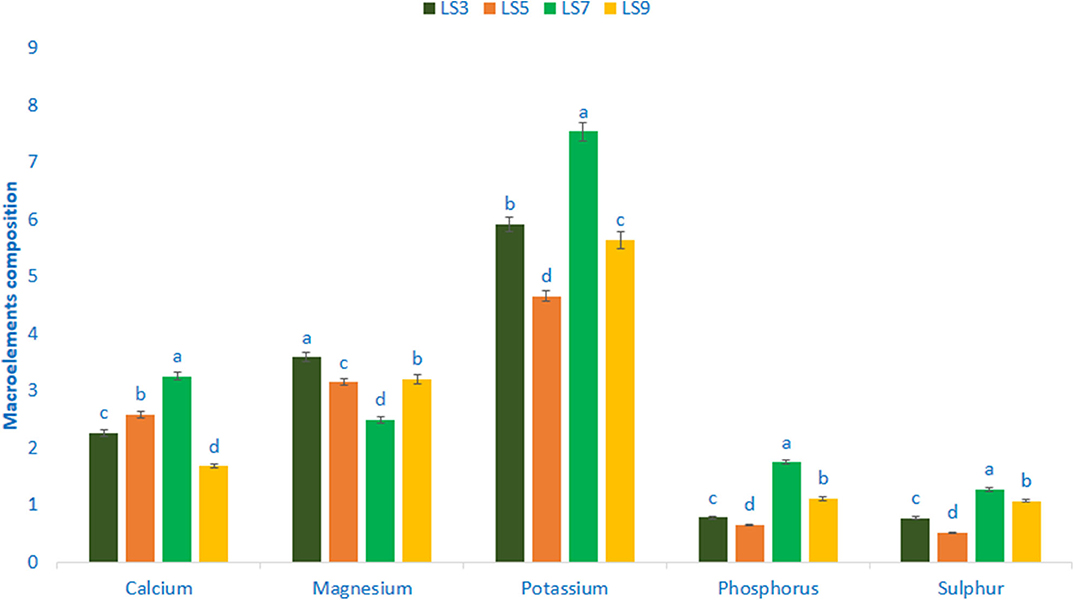
Figure 2. Mineral compositions (Macroelements mg g−1 FW,) in four selected salt-tolerant vegetable amaranths; different letters are differed significantly by Duncan multiple range test (P < 0.01), (n = 3).
Mineral Composition (Microelements)
Minerals composition (Microelements) of salt-tolerant vegetable amaranth is shown in Figure 3. Salt-tolerant vegetable amaranth showed remarkable iron and manganese content. The genotype LS9 had the highest iron content (17.35 μg g−1 FW) followed by LS7 and LS5, whereas the genotype LS3 showed the lowest iron content (12.99 μg g−1 FW). In this study, the range of manganese content was 12.25 μg g−1 FW and 16.77 μg g−1 FW. The genotype LS7 had the highest manganese content; however, the genotype LS3 had the lowest manganese content. The significant and notable variations of copper content were reported in salt-tolerant vegetable amaranth genotypes (1.27–2.26 μg g−1 FW). The copper content was the highest in LS7, followed by LS9; whereas the lowest copper content was obtained from the genotype LS3 and LS5, respectively. Salt-tolerant vegetable amaranth showed remarkable zinc, sodium, and boron content. Zinc, sodium, and boron content ranged from 11.33 to 14.61, 72.24 to 84.29, and 5.27 to 7.36 μg g−1 fresh weight, respectively. The genotypes LS7 had the highest zinc, sodium, and boron content, while LS3 showed the lowest zinc and sodium, and LS5 had the lowest boron content. Molybdenum content ranged from 0.26 to 0.57 μg g−1 FW. The genotypes LS7 had the highest molybdenum content, while LS3 showed the lowest molybdenum content.
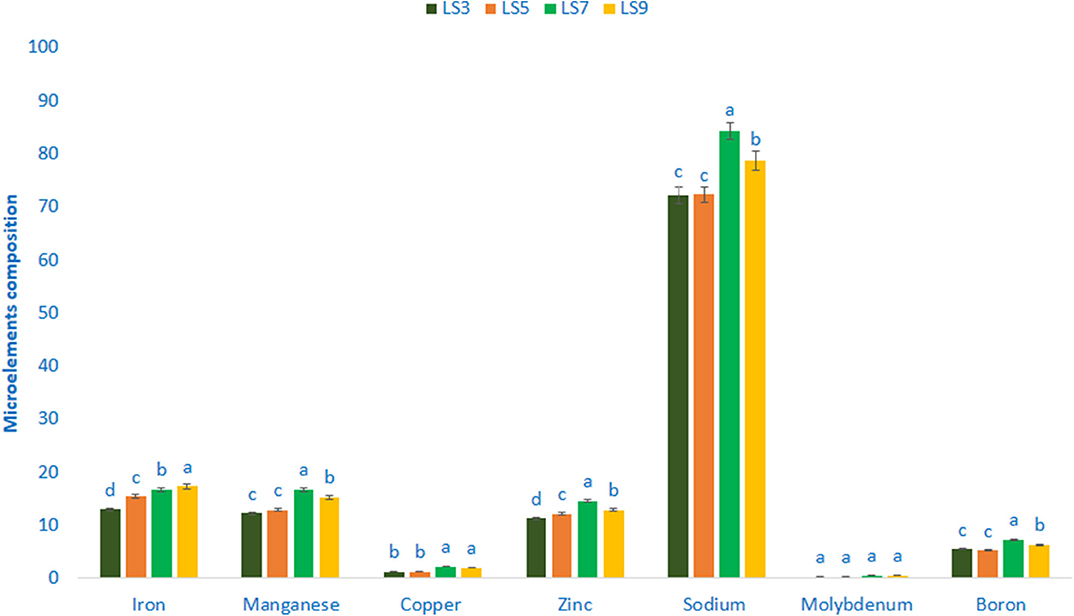
Figure 3. Mineral compositions (Microelements μg g−1 FW) in four selected salt-tolerant vegetable amaranths, different letters are differed significantly by Duncan multiple range test (P < 0.01), (n = 3).
Pigments Composition
Pigments of four selected salt-tolerant vegetable amaranths are shown in Figure 4. Betalains, betaxanthins, and betacyanins varied significantly and remarkably with the genotypes. Betalains, betaxanthins, and betacyanins ranged from 542.35 to 1,029.12, 355.98 to 517.12, and 185.02 to 512.06 ng g−1 FW, respectively. The genotype LS7 exhibited the highest betacyanins content, followed by LS9. Conversely, the genotype LS3 had the lowest betacyanins content. Among genotypes, considerable and significant variations were observed in betaxanthins content. Betalains and betaxanthins content were the highest in genotype LS7 followed by LS9. In contrast, genotype LS3 showed the lowest betaxanthins and betalains. The significant and notable variations were noticed for chlorophyll a content (156.09–545.06 μg g−1 FW). The genotype LS7 had the highest chlorophyll a content (545.06 μg g−1 FW), whereas the lowest chlorophyll a was recorded in LS3 (156.09 μg g−1 FW). Similar to chlorophyll a, significant and marked differences in chlorophyll b content were noted in vegetable amaranth genotypes (64.90–394.35 μg g−1 FW). LS9 had the highest chlorophyll b content (394.35 μg g−1 FW), followed by LS7. Conversely, LS3 had the lowest chlorophyll b (64.90 μg g−1 FW). Total chlorophyll content showed significant and noticeable variation (221.62–879.42 μg g−1 FW). LS7 and LS9 exhibited abundant total chlorophyll content, whereas, the lowest total chlorophyll content was obtained from LS3 (221.62 μg g−1 FW).
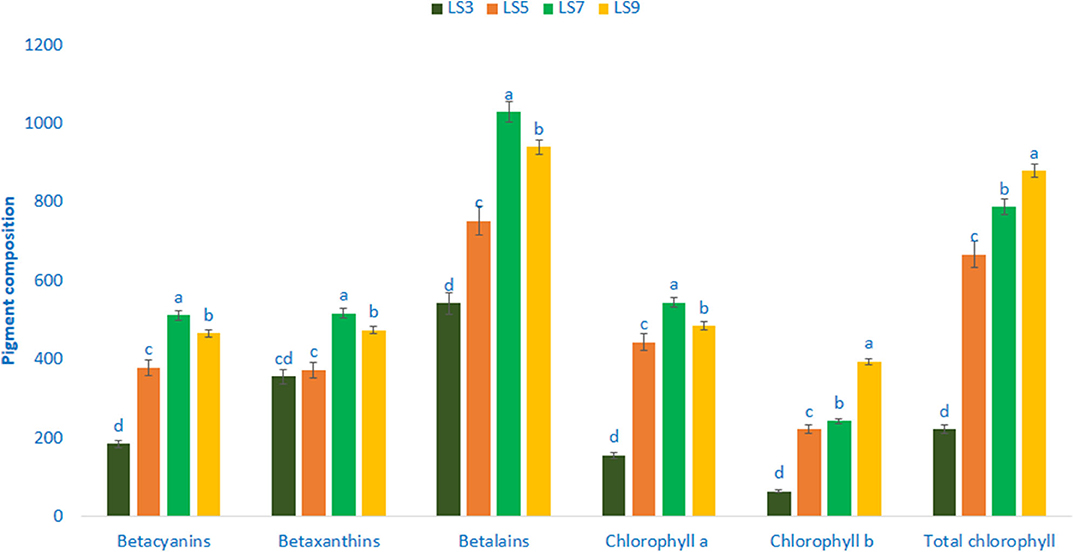
Figure 4. Pigment composition in four selected salt-tolerant vegetable amaranths, betacyanins (ng g−1 FW), chlorophyll a (μg g−1 FW), betaxanthins (ng g−1 FW), chlorophyll b (μg g−1 FW), betalains (ng g−1 FW), total chlorophyll (μg g−1 FW); different letters in the bar are differed significantly by Duncan multiple range test (P < 0.01), (n = 3).
Phytochemicals and Antioxidant Capacity
Polyphenols, flavonoids, ascorbic acid, and antioxidant capacity (AC) varied significantly among the studied salt-tolerant genotypes (Figure 5). Ascorbic acid content ranged from 72.45 mg 100 g−1 FW in the genotype LS3 to 152.96 mg 100 g−1 FW in the genotype LS7. Polyphenols ranged from 92.26 GAE μg g−1 FW (LS3) to 184.76 GAE μg g−1 FW (LS7). The genotype LS7 had the highest polyphenols followed by LS9. Flavonoids exhibited much noticeable variation in terms of genotypes, which ranged from 158.34 RE μg g−1 DW in the genotype LS5 to 282.87 RE μg g−1 DW in the genotype LS7. AC (DPPH) ranged from 13.35 TEAC μg g−1 DW (LS3) to 35.36 TEAC μg g−1 DW (LS7). The highest AC (DPPH) was recorded in the genotype LS7 followed by LS9 and LS5. In contrast, LS3 had the lowest AC (DPPH). AC (ABTS+) ranged from 27.62 TEAC μg g−1 DW to 70.24 TEAC μg g−1 DW. The salt-tolerant vegetable amaranth genotype LS7 had the highest AC (ABTS+) followed by LS9. In contrast, AC (ABTS+) was the lowest in LS3.
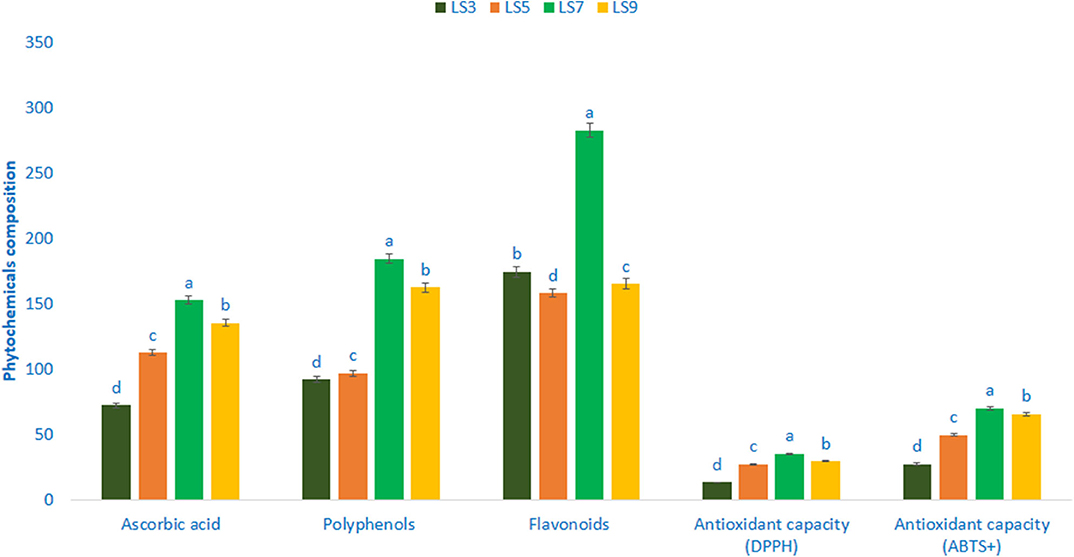
Figure 5. Phytochemical composition and free radical scavenging capacity in four selected salt-tolerant vegetable amaranths, ascorbic acid (mg 100 g−1 FW), polyphenols (GAE μg g−1 FW), flavonoids (RE μg g−1 DW), antioxidant capacity (DPPH) (TEAC μg g−1 DW), antioxidant capacity (ABTS+) (TEAC μg g−1 DW); different letters in the bar are differed significantly by Duncan multiple range test (P < 0.01), (n = 3).
Flavonols, Flavanols, Flavones, and Flavanones
Table 1 shows the data on main fragment ions in MS2, identified compounds, the molecular ion, λmax, and retention time. The liquid chromatography separated values of flavonols, flavanols, flavones, and flavanones compounds from four salt-tolerant leafy vegetable amaranths (LS3, LS5, LS7, and LS9) were compared with standard masses of flavonols, flavanols, flavones, and flavanones compounds through the respective peaks of the compounds. Nine flavonoids compounds were determined in salt-tolerant vegetable amaranth including six flavonols, such as rutin, kaempferol, isoquercetin, myricetin, hyperoside, and quercetin, one flavanol, such as catechin, one flavone such as apigenin, and one flavanone, such as naringenin. For the first time, we identified one flavonols such as myricetin, one flavanol, such as catechin, one flavone such as apigenin, and one flavanone, such as naringenin in salt-tolerant vegetable amaranth. Figure 6 showed the identified flavonols compounds and Figure 7 showed the identified flavanols, flavones, and flavanones compounds of leaves of four selected salt-tolerant vegetable amaranths. Across four principal groups of compounds, the most identified pronounced compounds in four selected salt-tolerant vegetable amaranths were observed in the following order: flavavanones > flavones > flavanols (Figures 6, 7). Across six flavonols, rutin and quercetin were identified as the most prominent compounds followed by isoquercetin and myricetin in selected salt-tolerant vegetable amaranths. Across the genotypes, LS7 exhibited the highest flavonols such as rutin, kaempferol, isoquercetin, myricetin, hyperoside, and quercetin. LS5 contained high total flavonols which were statistically similar to LS3, while LS9 demonstrated the lowest flavonols. Rutin, kaempferol, isoquercetin, myricetin, hyperoside, and quercetin of selected salt-tolerant vegetable amaranths varied from 6.75 to 9.62, 2.42 to 4.88, 3.45 to 6.58, 3.28 to 5.68, 1.25 to 2.58, and 3.55 to 6.62 mg kg−1 FW, respectively (Figure 6). LS7 exhibited the highest flavanols, such as catechin, flavones such as apigenin, and flavanones, such as naringenin followed by LS9 (Figure 7). In contrast, LS3 showed the minimum flavanols, such as catechin. LS5 showed the minimum flavones such as apigenin which was statistically similar to LS3. Similarly, LS3 showed the minimum flavanones, such as naringenin which as statistically similar to LS5 and LS9 (Figure 7).
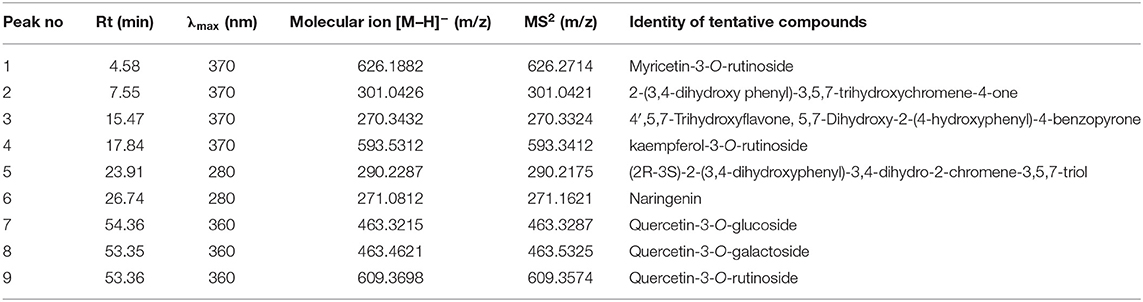
Table 1. Retention time (Rt), wavelengths of maximum absorption in the visible region (λmax), mass spectral data and tentative identification of flavonols, flavanols, flavones, and flavanones in four selected salt-tolerant vegetable amaranths.
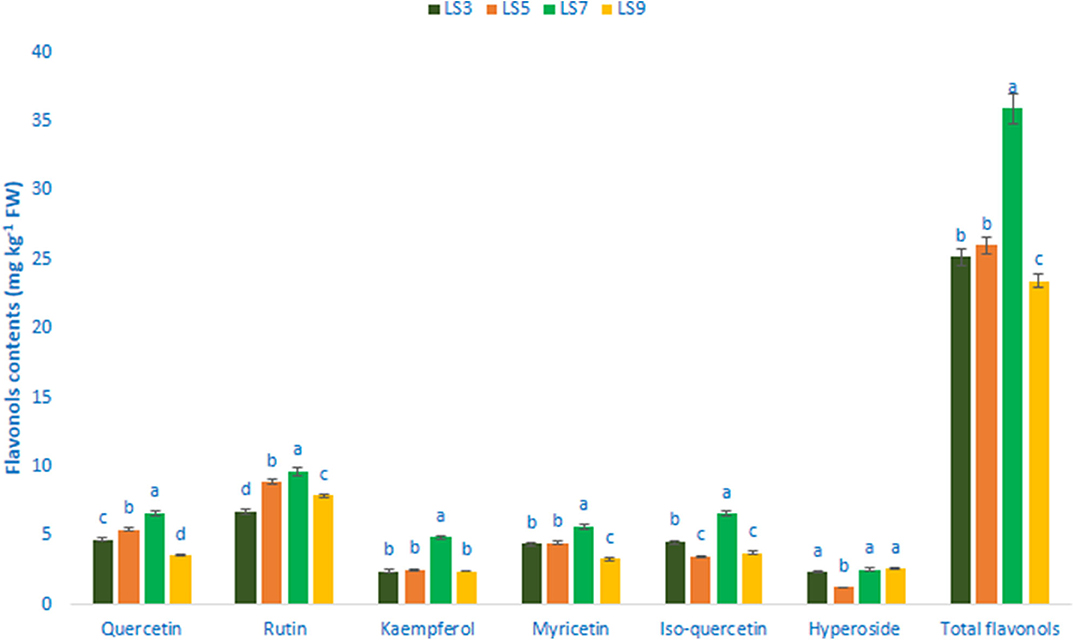
Figure 6. Flavonol contents (mg kg−1 FW) in four selected salt-tolerant vegetable amaranths; different letters in the bar are differed significantly by Duncan multiple range test (P < 0.01), (n = 3).
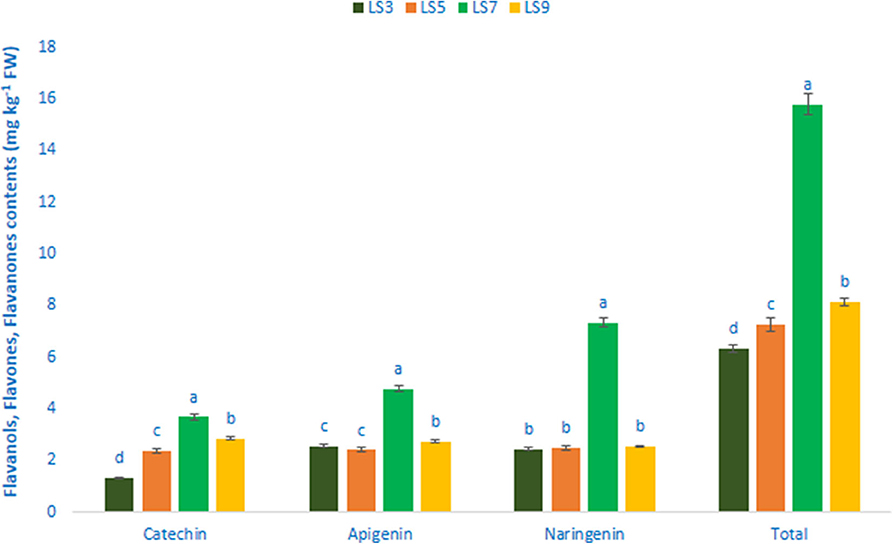
Figure 7. Flavanol, flavone, and flavanone content (mg kg−1 FW) in four selected salt-tolerant vegetable amaranths; different letters in the bar are differed significantly by Duncan multiple range test (P < 0.01), (n = 3).
Correlation Coefficient Analysis
Correlation of antioxidant pigments and phytochemicals of salt-tolerant vegetable amaranth are shown in Table 2. Highly significant positive associations of betalains, betaxanthins, betacyanins, total chlorophyll, chlorophyll b, and chlorophyll a were exhibited among pigments and with AC (ABTS+), AC (DPPH), ascorbic acid, polyphenols, and flavonoids. Ascorbic acid exerted significant associations with all traits along with AC (ABTS+) and AC (DPPH). The significant associations of polyphenols and flavonoids were observed with AC (ABTS+) and AC (DPPH). Similarly, a significant relationship of AC (ABTS+) with AC (DPPH) validated antioxidant activity measurement of different methods in salt-tolerant vegetable amaranth.
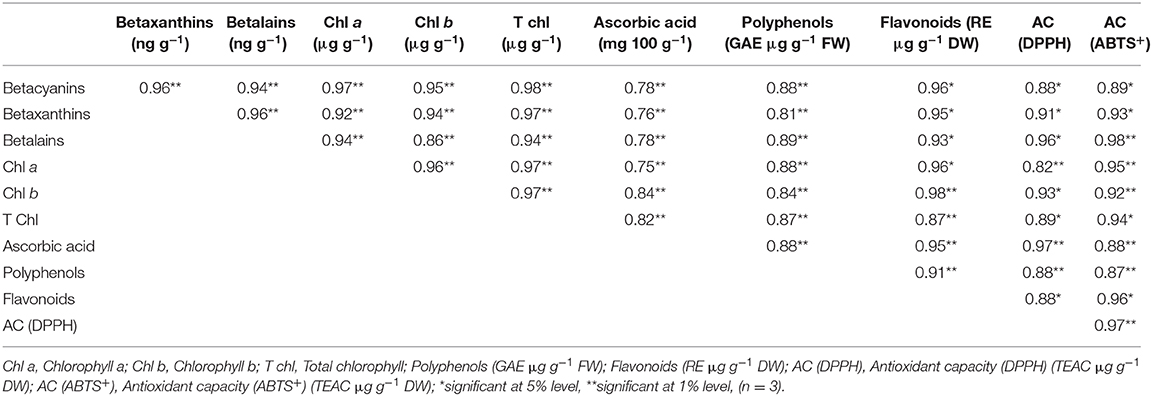
Table 2. The correlation coefficient for pigments, phytochemicals, and antioxidant capacity in four selected salt-tolerant vegetable amaranths.
Discussion
The analysis of variance revealed a wide range of variability of the studied traits regarding selected drought-tolerant leafy vegetable amaranths. A wide range of variability was also reported in red and green color amaranth (33), rice (42–56), maize (57–59), and coconut (60, 61). The lowest moisture content was noted in the salt-tolerant vegetable amaranth genotypes LS9 and LS7. As leaf higher dry matter obtained from lower moisture contents, two genotypes (19–18% dry matter) had considerable dry biomass. The maturity is directly interrelated to the moisture content of leaves. The results obtained in this study were corroborated to the reports of A. tricolor and sweet potato leaves by Sarker and Oba (3) and Sun et al. (62), respectively. Salt-tolerant vegetable amaranth leaves exerted significant and very much noticeable variations in protein content. Poor people and vegetarians of the developing countries mainly depend on vegetable amaranth for their protein source. The protein content of salt-tolerant vegetable amaranth was much higher as compared to A. tricolor (1.26%) in our earlier study (11). There were no significant variations in fat content in terms of four selected salt-tolerant vegetable amaranths. Our results were corroborated with the results of Sarker and Oba (3) and Sun et al. (62) in A. tricolor and leaves of sweet potato, respectively. They reported that fat influences the cell function, covering the organs of the body, and upholding the temperature of the body. Fats have abundant omega-6 and omega-3 fatty acids. Fats play a significant role in digestion, absorption, and transport of vitamins E, D, A, and K that are soluble in fats.
The salt-tolerant vegetable amaranth genotypes showed considerable dietary fiber. Dietary fiber remarkably contributed to the cure of constipation, increment of digestibility, and palatability (13). It revealed from our results that leaves of salt-tolerant vegetable amaranth have abundant protein, moisture, carbohydrates, and digestible fiber. The results of this study corroborated with the results of our earlier study (3). The digestible fiber and carbohydrates contents obtained from the advance lines were corroborated with our previous studies of red morph amaranth (63), weedy amaranth (64), green morph amaranth (65), stem amaranth (66), and A. blitum (67). However, dry matter contents of these advance lines were greater than the dry matter contents of red morph amaranth (63), weedy amaranth (64), green morph amaranth (65), stem amaranth (66), and A. blitum (67). Except for weedy amaranth, protein contents of these advance lines were greater than the protein contents of red morph amaranth (63), green morph amaranth (65), stem amaranth (66), and A. blitum (67).
In our present study, we found remarkable potassium (7.54 mg g−1), calcium (3.25 mg g−1) magnesium (3.59 mg g−1) phosphorus (1.75 mg g−1), and sulfur (1.27 mg g−1) in salt-tolerant vegetable amaranth. Chakrabarty et al. (15) in A. lividus and Sarker and Oba (3) in A. tricolor also observed similar results. Jimenez-Aguiar and Grusak (30) noted abundant potassium, calcium, magnesium, phosphorus, and sulfur in different amaranths. They also noticed that amaranth potassium, calcium, magnesium, phosphorus, and sulfur were much pronounced than black nightshade, spinach, spider flower, and kale. Salt-tolerant vegetable amaranth leaves contained higher zinc and iron content than the cassava leaves (28) and beach pea (29). In this study, we found remarkable iron (17.35 μg g−1), manganese (16.77 μg g−1), copper (2.26 μg g−1), zinc (14.61 μg g−1), sodium (84.29 μg g−1), molybdenum (0.57 μg g−1), and boron (7.36 μg g−1) in salt-tolerant vegetable amaranth. Jimenez-Aguiar and Grusak (30) noted abundant iron, manganese, copper, zinc sodium, molybdenum, and boron in different amaranths. They also noticed that manganese, iron, zinc, and copper in the leaves of amaranth were pronounced than black nightshade, spinach, spider flower, black nightshade, and kale. Potassium contents obtained from these advance lines were corroborated with our previous studies of green morph amaranth (65), while calcium contents recorded in these advance lines were greater than red morph amaranth (63), stem amaranth (66), and A. blitum (67). We observed high phosphorus and sodium in the current investigation than our weedy amaranth (64). Similarly, iron, zinc, and magnesium obtained from the current investigation were much pronounced than our earlier investigation in red morph amaranth (63), green morph amaranth (65), stem amaranth (66), and A. blitum (67). We obtained high copper than our earlier study of green morph amaranth (65) and high manganese than weedy amaranth (64), green morph amaranth (65). Hence, these selected salt-tolerant advance lines could contribute as high minerals enriched genotypes than our previously tested amaranth genotypes.
In this study, we found remarkable total chlorophyll (879.45 μg g−1 FW), chlorophyll a (545.06 μg g−1 FW), and chlorophyll b (394.35 μg g−1 FW) in salt-tolerant vegetable amaranth, whereas, Khanam and Oba (68) observed comparatively lower chlorophyll content in A. tricolor. On the other hand, Khanam and Oba (68) observed a more or less similar trend in betacyanin, betalain, chlorophyll, and betaxanthin content of red and green amaranth. The genotype LS7 and LS9 had abundant betacyanin, betalain, chlorophyll, and betaxanthin content indicating the presence of the high antioxidant activity. The genotype LS7 and LS9 had abundant betacyanins, betalains, chlorophylls, and betaxanthins among leafy vegetables that have important free radical-scavenging activity (1). Presence of high betacyanins, betalains, chlorophylls, and betaxanthins in vegetable amaranth genotype LS7 and LS9 is an important parameter for consumers, having an essential role in detoxification of ROS in the human body and preventing many degenerative human diseases and antiaging (22, 24). Total chlorophyll, chlorophyll a, chlorophyll b, betacyanins, betalains, and betaxanthins content obtained from these advance lines were greater than red morph amaranth (63), green morph amaranth (65), stem amaranth (66), weedy amaranth (64), and A. blitum (67). Hence, these selected salt-tolerant advance lines could contribute as high antioxidant pigments enriched genotypes than our previously tested amaranth genotypes.
Salt-tolerant vegetable amaranth genotype LS7 and LS9 had high polyphenols, flavonoids, ascorbic acid, and antioxidant capacity (AC). Our results were corroborated with the results of Khanam and Oba (68) where they observed higher polyphenols, flavonoids, and AC content in the red amaranth genotype compared to green amaranth. Salt-tolerant vegetable amaranth LS7 and LS9 contained higher ascorbic acid, polyphenols, flavonoids, and AC compared to the genotype LS3 and LS5. Hence, these antioxidant phytochemicals of salt-tolerant vegetable amaranth genotypes could be an important parameter for consumers, playing a crucial role in detoxification of ROS in the human body and preventing antiaging and many degenerative human diseases (22, 24). Our result showed that salt-tolerant vegetable amaranth genotypes contained antioxidant phytochemicals such as ascorbic acid, polyphenols, flavonoids, and AC among leafy vegetables that have the important scavenging activity of free radicals (1). The ascorbic acid and total polyphenols obtained from these advance lines were greater than our previous studies of green morph amaranth (65) and weedy amaranth (64), while total flavonoids recorded in these advance lines were greater than our previous studies of green morph amaranth (65) and red morph amaranth (63). Antioxidant capacity in DPPH obtained from these advance lines was greater than our previous studies of red morph amaranth (63) and antioxidant capacity in ABTS+ obtained from these advance lines was greater than our previous studies of red morph amaranth (63), green morph amaranth (65), and stem amaranth (66). Hence, these selected salt-tolerant advance lines could contribute as high vitamin C, polyphenols, flavonoids, and antioxidants enriched genotypes than our previously tested amaranth genotypes.
We observed considerable pigments including betacyanins, betalains, betaxanthins, chlorophylls, and antioxidant phytochemicals such as ascorbic acid, polyphenols, antioxidant potentiality, and flavonoids in salt-tolerant vegetable amaranth genotypes. Our results were fully in agreement to the results of Khanam and Oba (68) where they observed higher AC, betacyanins, flavonoids, betalains, betaxanthins, and polyphenols content in the red amaranth genotype compared to green amaranth. Pigments such as betalains (1,029.12 ng g−1), betacyanins (512.06 ng g−1), betaxanthins (517.12 ng g−1), total chlorophyll (879.45 μg g−1 FW), chlorophyll a (545.06 μg g−1 FW), chlorophyll b (394.35 μg g−1 FW), and antioxidant phytochemicals such as AC (ABTS+) (70.24 TEAC μg g−1 DW), flavonoids (282.87 RE μg g−1 DW), and AC (DPPH) (35.36 TEAC μg g−1 DW) obtained in this study, more or less similar to the findings in A. tricolor of Khanam et al. (39), whereas polyphenols obtained in our study were much prominent than the findings in A. tricolor of Khanam et al. (39). The genotypes LS7 and LS9 had high pigments such as betacyanins, betalains, betaxanthins, chlorophylls, and antioxidant phytochemicals such as ascorbic acid, polyphenols, flavonoids, and AC.
In this study, we found plentiful protein, carbohydrates, nutraceuticals, and digestible fiber, moisture, remarkable pigments profile such as betacyanins, betalains, betaxanthins, chlorophylls, antioxidant phytochemicals such as ascorbic acid, polyphenols, flavonoids, and antioxidant potential in salt-tolerant vegetable amaranth genotypes. We obtained corroborative results compared to the results of Khanam et al. (39) where they observed higher AC, betacyanin, flavonoid, betalain, betaxanthin, and polyphenols content in the red amaranth. The genotypes LS7 and LS9 had abundant carbohydrates, protein, moisture, and dietary fiber, nutraceuticals, pigments, antioxidant phytochemicals, flavonoids, and antioxidant potentials. The genotypes LS7 and LS9 could be used as antioxidant profile enriched high-yielding varieties as drink purposes. It revealed from the investigation that these two genotypes contained adequate polyphenols, flavonoids, ascorbic acid, pigments, and antioxidant potentials that have prospects for extracting colorful juice for drinking purposes as well as for consuming the nutraceuticals and antioxidant-deficient community in the saline prone area of the world.
Salinity stress induces the ROS accumulation in plants that alters the biosynthesis of flavonoids (69) to mitigate the damaging effects of ROS and to adjust unfavorable stress conditions (70). These flavonoid compounds act as non-enzymatic antioxidants to alleviate the negative effect of ROS in plants through quenching these free radicals (71). Abiotic stress facilitates flavonoid biosynthesis in higher concentration to adjust oxidative stress in plants because of their high antioxidant potentiality (72). Furthermore, salinity stress highly accelerates the activity of biosynthesis pathway genes such as TT3, TT4, TT5, TT6, TT7, TT8, TT9, TT18, FLS, and F60H1 that are involved in the biosynthesis of flavanone, flavone, flavonol, anthocyanin, and its derivatives (73–75). In addition, the regulatory gene TT8 stimulates many flavonoid biosynthesis pathways genes and facilitates the accumulation of more antioxidant flavonoid compounds in plants (76). The data of foxtail millet roots showed that 17 flavonoid biosynthesis genes were significantly up-regulated 2–11-fold under salinity. In keeping with gene expressions, the over-accumulation of 27 flavonoids was obtained under salt tolerance (77).
Nine flavonoid compounds were determined in salt-tolerant vegetable amaranth including six flavonols, such as rutin, kaempferol, isoquercetin, myricetin, hyperoside, and quercetin; one flavanol, such as catechin; one flavone such as apigenin; and one flavanone, such as naringenin. For the first time, we identified one flavonol such as myricetin; one flavanol, such as catechin; one flavone such as apigenin; and one flavanone, such as naringenin in salt-tolerant vegetable amaranth. Khanam et al. (39) and Khanam and Oba (68) noticed three flavonols such as isoquercetin, rutin, and hyperoside in red and green amaranth. In the leaf, stalks, flowers, sprouts, and the seed of A. cruentus, A. caudatus, and A. hypochondriacus, Li et al. (78) observed three flavonols, such as kaempferol, rutin, and quercetin. Three flavonoids including isovitexin, vitexin, and rutin were reported in the seeds and sprouts of A. cruentus (79). Across four principal groups of compounds, the most identified pronounced compounds in four selected salt-tolerant vegetable amaranths were observed in the following order: flavonols > flavanones > flavones > flavanols. Across six flavonols, rutin and quercetin were identified as the most prominent compounds followed by isoquercetin and myricetin in selected salt-tolerant vegetable amaranths. Across the genotypes, LS7 exhibited the highest flavonols such as rutin, kaempferol, isoquercetin, myricetin, hyperoside, and quercetin. LS5 contained high total flavonols which were statistically similar to LS3, while LS9 demonstrated the lowest flavonols. Quercetin and hyperoside of our selected salt-tolerant leafy vegetable amaranths were higher than the content of quercetin and hyperoside reported by Khanam et al. (39) in A. tricolor genotypes. The varietal differences, and differential geographic locations, climatic and edaphic conditions, and cultural managements may have played a major contribution in securing higher quercetin and hyperoside in our salt-tolerant vegetable amaranth genotypes in comparison with the results of Khanam et al. (39). LS7 exhibited the highest flavanols, such as catechin; flavones such as apigenin; and flavanones, such as naringenin followed by LS9.
Highly significant positive associations of betalains, betaxanthins, betacyanins, total chlorophyll, chlorophyll b, and chlorophyll a were exhibited among pigments and with AC (ABTS+), AC (DPPH), ascorbic acid, polyphenols, and flavonoids. Pigments of salt-tolerant vegetable amaranth (betalains, betaxanthins, chlorophylls, and betacyanins) showed strong antioxidant activity as all the pigments exhibited significant associations with AC (ABTS+) and AC (DPPH). Ascorbic acid exerted significant associations with all traits along with AC (ABTS+) and AC (DPPH). The significant positive associations of ascorbic acid with AC (ABTS+) and AC (DPPH) also suggested a strong antioxidant activity. The significant associations of polyphenols and flavonoids were observed with AC (ABTS+) and AC (DPPH) indicating the strong antioxidant capacity of phenolics and flavonoids in salt-tolerant vegetable amaranth. The TPC, TFC, and TAC of salt-induced purslane and amaranth corroborated with the results of the present investigation (80, 81). Similarly, the significant relationship of AC (ABTS+) with AC (DPPH) validated the antioxidant activity measurement of different methods in salt-tolerant vegetable amaranth.
Conclusion
Salt-tolerant vegetable amaranth genotypes contained ample proximate, pigments, nutraceuticals, and phytochemicals such as protein, carbohydrates, moisture, dietary fiber, polyphenols, minerals, betaxanthins, flavonoids, betacyanins, betalains, and chlorophylls. Salt-tolerant vegetable amaranth genotypes LS7 and LS9 had greater proximate, nutraceuticals, pigments, antioxidant phytochemicals, and antioxidant activity compared to the genotype LS3 and LS5. Nine flavonoids compounds were determined in salt-tolerant vegetable amaranth including six flavonols, such as rutin, kaempferol, isoquercetin, myricetin, hyperoside, and quercetin; one flavanol, such as catechin; one flavone such as apigenin; and one flavanone, such as naringenin. For the first time, we identified one flavonol such as myricetin; one flavanol, such as catechin; one flavone such as apigenin; and one flavanone, such as naringenin in salt-tolerant vegetable amaranth. Across six flavonols, rutin and quercetin were identified as the most prominent compounds followed by isoquercetin and myricetin in selected salt-tolerant vegetable amaranths. Across the genotypes, LS7 exhibited the highest flavonols such as rutin, kaempferol, isoquercetin, myricetin, hyperoside, and quercetin as well as the highest flavanols, such as catechin; flavones such as apigenin; and flavanones, such as naringenin. The correlation study revealed that all antioxidant constituents of salt-tolerant vegetable amaranth had strong antioxidant activity. It revealed from the study that salt-tolerant vegetable amaranth genotypes LS7 and LS9 exhibited excellent sources of proximate, nutraceuticals, pigments, antioxidant phytochemicals, and antioxidant activity that offered huge prospects for nutritional and health-boosting effects. We can extract colorful juice from the genotypes LS7 and LS9 as drink purposes for consuming the nutraceuticals and antioxidant deficient community in the saline prone area around the world. However, further details experimentation is required to confirm the standardization and stabilization of functional components of vegetable amaranth for extraction of juice as drinks.
Data Availability Statement
All the data supporting the conclusions of this article is provided within the article.
Author Contributions
US initiated the research work, conceived the study, performed biochemical analysis, statistical analysis, drafted, edited, interpreted data, and prepared the manuscript. US, MH, and MI performed the experiments. SO edited the manuscript, provided valuable suggestions during the experiment. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Cai Y, Sun M, Corke H. Antioxidant activity of betalains from plants of the Amaranthaceae. J Agric Food Chem. (2003) 51:2288–94. doi: 10.1021/jf030045u
2. Stintzing FC, Carle R, Betalains-emerging prospects for food scientists. Trends Food Sci Technol. (2007) 18:514–25. doi: 10.1016/j.tifs.2007.04.012
3. Sarker U, Oba S. Response of nutrients, minerals, antioxidant leaf pigments, vitamins, polyphenol, flavonoid and antioxidant activity in selected vegetable amaranth under four soil water content. Food Chem. (2018) 252:72–83. doi: 10.1016/j.foodchem.2018.01.097
4. Sarker U, Oba S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biol. (2018) 18:258. doi: 10.1186/s12870-018-1484-1
5. Sarker U, Oba S. Drought stress effects on growth, ROS markers, compatible solutes, phenolics, flavonoids, and antioxidant activity in Amaranthus tricolor. Appl Biochem Biotechnol. (2018) 186:999–1016. doi: 10.1007/s12010-018-2784-5
6. Sarker U, Oba S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci Rep. (2018) 8:16496. doi: 10.1038/s41598-018-34944-0
7. Sarker U, Oba S. Augmentation of leaf color parameters, pigments, vitamins, phenolic acids, flavonoids and antioxidant activity in selected Amaranthus tricolor under salinity stress. Sci Rep. (2018) 8:12349. doi: 10.1038/s41598-018-30897-6
8. Sarker U, Oba S. Salinity stress enhances color parameters, bioactive leaf pigments, vitamins, polyphenols, flavonoids and antioxidant activity in selected Amaranthus leafy vegetables. J Sci Food Agric. (2019) 99:2275–84. doi: 10.1002/jsfa.9423
9. Sarker U, Islam MT, Oba S. Salinity stress accelerates nutrients, dietary fiber, minerals, phytochemicals and antioxidant activity in Amaranthus tricolor leaves. PLoS ONE. (2018) 13:0206388. doi: 10.1371/journal.pone.0206388
10. Sarker U, Islam MT, Rabbani MG, Oba S. Genotypic variability for nutrient, antioxidant, yield and yield contributing traits in vegetable amaranth. J Food Agri Environ. (2014) 12:168–74. Available online at: https://www.wflpublisher.com/Abstract/5378
11. Sarker U, Islam MT, Rabbani MG, Oba S. Variability, heritability and genetic association in vegetable amaranth (Amaranthus tricolor). Span J Agric Res. (2015) 13:1–8. doi: 10.5424/sjar/2015132-6843
12. Sarker U, Islam MT, Rabbani MG, Oba S. Genotype variability in composition of antioxidant vitamins and minerals in vegetable amaranth. Genetika. (2015) 47:85–96. doi: 10.2298/GENSR1501085S
13. Sarker U, Islam MT, Rabbani MG, Oba S. Genetic variation and interrelationship among antioxidant, quality and agronomic traits in vegetable amaranth. Turk J Agric For. (2016) 40:526–35. doi: 10.3906/tar-1405-83
14. Sarker U, Islam MT, Rabbani MG, Oba S. Genotypic diversity in vegetable amaranth for antioxidant, nutrient and agronomic traits. Indian J Genet Pl Breed. (2017) 77:173–6. doi: 10.5958/0975-6906.2017.00025.6
15. Chakrabarty T, Sarker U, Hasan M, Rahman MM. Variability in mineral compositions, yield and yield contributing traits of stem amaranth (Amaranthus lividus). Genetika. (2018) 50:995–1010. doi: 10.2298/GENSR1803995C
16. Vardhana H. In vitro antibacterial activity of Amaranthus spinosus root extracts. Pharmacophore. (2011) 2:266–70.
17. Kumar A, Lakshman BS, Jayaveera K, Nandeesh KN, Manoj N, Ranganayakulu D. Comparative in vitro atihelminthic activity of three plants from Amaranthaceae family. Biol Sci. (2010) 62:185–9. doi: 10.2298/ABS1001185K
18. Sarker U, Islam MT, Rabbani MG, Oba S. Variability in total antioxidant capacity, antioxidant leaf pigments and foliage yield of vegetable amaranth. J Integrative Agric. (2018) 17:1145–53. doi: 10.1016/S2095-3119(17)61778-7
19. Sarker U, Islam MT, Rabbani MG, Oba S. Antioxidant leaf pigments and variability in vegetable amaranth. Genetika. (2018) 50:209–20. doi: 10.2298/GENSR1801209S
20. Sarker U, Islam MT, Rabbani MG, Oba S. Phenotypic divergence in vegetable amaranth for total antioxidant capacity, antioxidant profile, dietary fiber, nutritional and agronomic traits. Acta Agric Scand Section B Soil Plant Sci. (2018) 68:67–76. doi: 10.1080/09064710.2017.1367029
21. Isabelle M, Lee BL, Lim MT, Koh WP, Huang D, Ong CN. Antioxidant activity and profiles of common fruits in Singapore. Food Chem. (2010) 123:77–84. doi: 10.1016/j.foodchem.2010.04.002
22. Repo-Carrasco-Valencia R, Hellstrom JK, Pihlava JM, Mattila PH. Flavonoids and other phenolic compounds in Andean indigenous grains: Quinoa (Chenopodium quinoa), kaniwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Food Chem. (2010) 120:128–33. doi: 10.1016/j.foodchem.2009.09.087
23. Steffensen SK, Rinnan A, Mortensen AG, Laursen B, Troiani RM, Noellemeyer EJ, et al. Variations in the polyphenol content of seeds of field grown Amaranthus genotypes. Food Chem. (2011) 129:131–8. doi: 10.1016/j.foodchem.2011.04.044
24. Venskutonis PR, Kraujalis P. Nutritional components of amaranth seeds and vegetables: a review on composition, properties, and uses. Comp Rev Food Sci Food Saf. (2013) 12:381–412. doi: 10.1111/1541-4337.12021
25. Shukla S, Bhargava A, Chatterjee A, Pandey AC, Mishra B. Diversity in phenotypic and nutritional traits in vegetable amaranth (A. tricolor), a nutritionally underutilized crop. J Sci Food Agric. (2010) 90:139–44. doi: 10.1002/jsfa.3797
26. Rajan S, Markose BL. horticultural science series-6. In: Peter KMV, editor. Propagation of Horticultural Crops. New Delhi, India: New India Publishing Agency (2007). p. 110–3.
27. Guillet D. Grain Amaranthus, History and Nutrition. Koko-pelli Seed Foundation (2004). Available online at: http://www.kokopelli-seed-foundation.com/amaranths.htm (accessed October 01, 2020).
28. Madruga MS, Camara FS. The chemical composition of “Multimistura” as a food supplement. Food Chem. (2000) 68:41–4. doi: 10.1016/S0308-8146(99)00152-1
29. Shahidi F, Chavan UD, Bal AK, McKenzie DB. Chemical composition of beach pea (Lathyrus maritimus L.) plant parts. Food Chem. (1999) 64:39–44. doi: 10.1016/S0308-8146(98)00097-1
30. Jimenez-Aguilar DM, Grusak MA. Minerals, vitamin C, phenolics, flavonoids and antioxidant activity of Amaranthus leafy vegetables. J Food Compos Anal. (2017) 58:33–9. doi: 10.1016/j.jfca.2017.01.005
31. Sani HA, Rahmat A, Ismail M, Rosli R, Endrini S. Potential anticancer effect of red spinach (Amaranthus gangeticus) extract. Asia Pacific J Clinical Nutri. (2004) 13:396–400.
32. Rashad MMI, Sarker U. Genetic variations in yield and yield contributing traits of green amaranth. Genetika. (2020) 52:391–404. doi: 10.2298/GENSR2001393R
33. Sarker U, Oba S. Antioxidant constituents of three selected red and green color Amaranthus leafy vegetable. Sci Rep. (2019) 9:18233. doi: 10.1038/s41598-019-52033-8
34. Sarker U, Oba S. Leaf pigmentation, its profiles and radical scavenging activity in selected Amaranthus tricolor leafy vegetables. Sci Rep. (2020) 10:18617. doi: 10.1038/s41598-020-66376-0
35. Sarker U, Oba S. Phenolic profiles and antioxidant activities in selected drought-tolerant leafy vegetable amaranth. Sci Rep. (2020) 10:18287. doi: 10.1038/s41598-020-71727-y
36. Sarker U, Oba S. Nutritional and bioactive constituents and scavenging capacity of radicals in Amaranthus hypochondriacus. Sci Rep. (2020) 10:19962. doi: 10.1038/s41598-020-71714-3
37. Sarker U, Oba S. The response of salinity stress-induced A. tricolor to growth, anatomy, physiology, non-enzymatic and enzymatic antioxidants. Front Plant Sci. (2020). doi: 10.3389/fpls.2020.559876
38. Sarker U, Oba S. Polyphenol and flavonoid profiles and radical scavenging activity in selected leafy vegetable Amaranthus gangeticus. BMC Plant Biol. (2020) 20:499. doi: 10.1186/s12870-020-02700-0
39. Khanam UKS, Oba S, Yanase E, Murakami Y. Phenolic acids, flavonoids and total antioxidant capacity of selected leafy vegetables. J Funct Foods. (2012) 4:979–87. doi: 10.1016/j.jff.2012.07.006
40. Hasan-Ud-Daula M, Sarker U. Variability, heritability, character association, and path coefficient analysis in advanced breeding lines of rice (Oryza sativa L.). Genetika. (2020) 52:711–26. doi: 10.2298/GENSR2002711H
41. Hasan MJ, Kulsum MU, Majumder RR, Sarker U. Genotypic variability for grain quality attributes in restorer lines of hybrid rice. Genetika. (2020) 52.
42. Ganapati RK, Rasul MG, Mian, MAK, Sarker U. Genetic variability and character association of T-Aman rice (Oryza sativa L.). Int J Plant Biol Res. (2014) 2:1–4.
43. Rai PK, Sarker U, Roy PC, Islam AKMS. Character association in F4 generation of rice (Oryza sativa L.). Bangladesh J Plant Breed Genet. (2013) 26:39–44. doi: 10.3329/bjpbg.v26i2.23848
44. Karim D, Sarker U, Siddique MNA, Mian MAK, Hasnat MZ. Variability and genetic parameter analysis in aromatic rice. Int J Sustain Crop Prod. (2007) 2:15–8.
45. Karim D, Sarker U, Siddique MNA, Mian MAK, Hasnat MZ. Phenotypic and genotypic correlation co-efficient of quantitative characters and character association of aromatic rice. J Biosci Agric Res. (2014) 1:34–46. doi: 10.18801/jbar.010114.05
46. Biswas PS, Sarker U, Bhuiyan MAR, Khatun S. Genetic divergence in cold tolerant irrigated rice (Oryza sativa L.). Agriculturists. (2006) 4:15–20.
47. Sarker U, Mian MAK. Genetic variations and correlations between floral traits in rice. Bangladesh J Agric Res. (2004) 29:553–8.
48. Sarker U, Mian MAK. Genetic variability, character association and path analysis for yield and its components in rice. J Asiat Soc Bangladesh Sci. (2003) 29:47–54.
49. Sarker U, Biswas PS, Prasad B, Mian MAK. Correlated response, relative selection efficiency and path analysis in cold tolerant rice. Bangladesh J Plant Breed Genet. (2001) 14:33–6.
50. Nath JK, Sarker U, Mian MAK, Hossain T. Genetic divergence in T. Aman rice. Ann Bangladesh Agric. (2008) 12:51−60.
51. Ali MA, Sarker U, Mian MAK, Islam MA, Fatema-Tuj-Johora. Estimation of genetic divergence in boro rice (Oryza sativa L.). Int J BioRes. (2014) 16:28–36.
52. Hasan MR, Sarker U, Mian MAK, Hossain T, Mahmud MNH. Genetic variation in micronutrient dense rice and its implication in breeding for higher yield. Ecofriend Agric J. (2012) 5:175–82.
53. Hasan MR, Sarker U, Islam MR, Mahmud MNH. Genetic diversity in micronutrient dense rice and its implication in breeding program. Ecofriend Agric J. (2012) 5:183–5.
54. Siddique MNA, Sarker U, Mian MAK. Genetic diversity in restorer line of rice. In: Bhuiyan MSR, Rahman L, editors. Proceedings of the International Conference on Plant Breeding and Seed for Food Security. Plant Breeding and Genetics Society of Bangladesh (2009). p. 137–42.
55. Rahman MH, Sarker U, Main MAK. Assessment of variability of floral and yield traits; II maintainer lines of rice. Ann Bangladesh Agric. (2007) 11:95–102.
56. Rahman MH, Sarker U, Main MAK. Assessment of variability of floral and yield traits; I restorer lines of rice. Ann Bangladesh Agric. (2007) 11:87–94.
57. Biswas A, Sarker U, Banik BR, Rohman MM, Mian MAK. Genetic divergence study in salinity stress tolerance maize (Zea mays L.). Bangladesh J Agric Res. (2014) 39:621–30. doi: 10.3329/bjar.v39i4.22540
58. Azam MG, Sarker U, Mahmud JA, Maniruzzam M, Banik BR. Genetic variability of yield and its contributing characters of CIMMYT maize inbreds in stress condition. Bangladesh J Agric Res. (2014) 39:419–26. doi: 10.3329/bjar.v39i3.21985
59. Azam MG, Sarker U, Mian MAK, Banik BR, Talukder MZA. Genetic divergence on quantitative characters of exotic maize inbreds (Zea mays L.). Bangladesh J Plant Breed Genet. (2013) 26:09–14. doi: 10.3329/bjpbg.v26i2.23844
60. Talukder MZA, Sarker U, Khan ABMMM, Moniruzzaman M, Zaman MM. Genetic variability and correlation coefficient of coconut (Cocos nacifera L.) in Barisal region. Int J Bio Res. (2011) 11:15–21.
61. Talukder MZA, Sarker U, Harun-Or-Rashid, Mian MAK, Zakaria M. Genetic diversity of coconut (Cocos nucifera L.) in Barisal Region. Ann Bangladesh Agric. (2015) 19:13−21.
62. Sun H, Mu T, Xi L, Zhang M, Chen J. Sweet potato (Ipomoea batatas L.) leaves as nutritional and functional foods. Food Chem. (2014) 156:380–9. doi: 10.1016/j.foodchem.2014.01.079
63. Sarker U, Oba S. Protein, dietary fiber, minerals, antioxidant pigments and phytochemicals, and antioxidant activity in selected red morph Amaranthus leafy vegetable. PLoS ONE. (2019) 14:0222517. doi: 10.1371/journal.pone.0222517
64. Sarker U, Oba S. Nutraceuticals, antioxidant pigments, and phytochemicals in the leaves of Amaranthus spinosus and Amaranthus viridis weedy species. Sci Rep. (2019) 9:20413. doi: 10.1038/s41598-019-50977-5
65. Sarker U, Hossain MM, Oba S. Nutritional and antioxidant components and antioxidant capacity in green morph Amaranthus leafy vegetable. Sci Rep. (2020) 10:1336. doi: 10.1038/s41598-020-57687-3
66. Sarker U, Oba S, Daramy MA. Nutrients, minerals, antioxidant pigments and phytochemicals, and antioxidant capacity of the leaves of stem amaranth. Sci Rep. (2020) 10:3892. doi: 10.1038/s41598-020-60252-7
67. Sarker U, Oba S. Nutrients, minerals, pigments, phytochemicals, and radical scavenging activity in Amaranthus blitum leafy vegetables. Sci Rep. (2020) 10:3868. doi: 10.1038/s41598-020-59848-w
68. Khanam UKS, Oba S. Bioactive substances in leaves of two amaranth species, Amaranthus lividus and A. hypochondriacus. Can J Plant Sci. (2013) 93:47–58. doi: 10.4141/cjps2012-117
69. Colla G, Rouphael Y, Cardarelli M, Svecova E, Rea E, Lucini L. Effects of saline stress on mineral composition, phenolic acids and flavonoids in leaves of artichoke and cardoon genotypes grown in floating system. J Sci Food Agric. (2013) 93:1119–27. doi: 10.1002/jsfa.5861
70. D'Maris, Amick Dempsey AC, Vlot MCW, Daniel FK. Salicylic acid biosynthesis and metabolism. Am Soc Plant Biol. (2011) 9:e0156. doi: 10.1199/tab.0156
71. Syvertsen JP, Garcia-Sanchez F. Multiple abiotic stresses occurring with salinity stress in citrus. Environ Exp Bot. (2014) 103:128–37. doi: 10.1016/j.envexpbot.2013.09.015
72. Arbona V, Manzi M, Ollas C, Gómez-Cadenas A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int J Mol Sci. (2013) 14:4885–911. doi: 10.3390/ijms14034885
73. Winkel-Shirley B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. (2001) 126:485–93. doi: 10.1104/pp.126.2.485
74. Petrussa E, Braidot E, Zancani M, Peresson C, Bertolini A, Patui S, et al. Plant flavonoids–biosynthesis, transport and involvement in stress responses. Int J Mol Sci. (2013) 14:14950–73. doi: 10.3390/ijms140714950
75. Wang F, Xu Z, Fan X, Zhou Q, Cao J, Ji G, et al. Transcriptome analysis reveals complex molecular mechanisms underlying UV tolerance of wheat (Triticum aestivum L.). J Agric Food Chem. (2019) 67:563–77. doi: 10.1021/acs.jafc.8b05104
76. Gonzalez A, Brown M, Hatlestad G, Akhavan N, Smith T, Hembd A, et al. TTG2 controls the developmental regulation of seed coat tannins in Arabidopsis by regulating vacuolar transport steps in the proanthocyanidin pathway. Dev Biol. (2016) 419:54–63. doi: 10.1016/j.ydbio.2016.03.031
77. Pan J, Li Z, Dai S, Ding H, Wang Q, Li, et al. Integrative analyses of transcriptomics and metabolomics upon seed germination of foxtail millet in response to salinity. Sci Rep. (2020) 10:13660. doi: 10.1038/s41598-020-70520-1
78. Li H, Deng Z, Liu R, Zhu H, Draves J, Marcone M, et al. Characterization of phenolics, betacyanins and antioxidant activities of the seed, leaf, sprout, flower and stalk extracts of three Amaranthus species. J Food Compos Anal. (2015) 37:75–81. doi: 10.1016/j.jfca.2014.09.003
79. Pasko P, Sajewicz M, Gorinstein S, Zachwieja Z. Analysis of selected phenolic acids and flavonoids in Amaranthus cruentus and Chenopodium quinoa seeds and sprouts by HPLC. Acta Chromatogr. (2008) 20:661–72. doi: 10.1556/AChrom.20.2008.4.11
Keywords: nutraceuticals, pigments, polyphenols and flavonoids, vitamin C, antioxidant activity, HPLC, LC-MS-ESI, abiotic stress-tolerant underutilized vegetables
Citation: Sarker U, Hossain MN, Iqbal MA and Oba S (2020) Bioactive Components and Radical Scavenging Activity in Selected Advance Lines of Salt-Tolerant Vegetable Amaranth. Front. Nutr. 7:587257. doi: 10.3389/fnut.2020.587257
Received: 25 July 2020; Accepted: 19 October 2020;
Published: 30 November 2020.
Edited by:
Dharini Sivakumar, Tshwane University of Technology, South AfricaReviewed by:
Yogendra Nayak, Manipal University, IndiaJanet Alejandra Gutierrez-Uribe, Monterrey Institute of Technology and Higher Education (ITESM), Mexico
Copyright © 2020 Sarker, Hossain, Iqbal and Oba. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Umakanta Sarker, dW1ha2FudGFAYnNtcmF1LmVkdS5iZA==; orcid.org/0000-0002-6814-8816
 Umakanta Sarker
Umakanta Sarker Md. Nazmul Hossain
Md. Nazmul Hossain Md. Asif Iqbal
Md. Asif Iqbal Shinya Oba2
Shinya Oba2