- 1Department of Urology, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, Yantai, China
- 2Department of Urology, Beijing Tian Tan Hospital, Capital Medical University, Beijing, China
- 3Binzhou Medical University, Yantai, China
- 4Department of Allergy, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, Yantai, China
Background: Alternate-day fasting (ADF) method is becoming more and more popular among adults. This meta-analysis aims to evaluate the effects of ADF on adults.
Methods: Randomized controlled trials (RCTs) of ADF were searched using PubMed (1988 to March 2020), EMBASE (1995 to March 2020), and the Cochrane Controlled Trials Register. A systematic review was carried out using the Preferred Reporting Items for Systematic Reviews and Meta-analyses. The datum was calculated by RevMan version 5.3.0. The original references for relating articles were also reviewed.
Results: Seven randomized controlled trials involving 269 participants (152 in the ADF group and 117 in the control group) were studied. In this meta-analysis, compared with the control group, the ADF group showed statistically significant reductions in weight (p < 0.00001) and body mass index (p < 0.00001). Besides, the ADF group showed significant differences in terms of total cholesterol (p = 0.001), low-density lipoprotein (p = 0.01), triglycerides (p = 0.02), fat mass (p = 0.002), lean mass (p = 0.002), systolic blood pressure (p = 0.003), diastolic blood pressure (p = 0.007), and total calorie intake (p = 0.007). At the same time, the analysis demonstrated that the ADF group had a same effect compared with control group in aspects of high-density lipoprotein (p = 0.27), homeostasis model assessment-insulin resistance (p = 0.55), and fasting blood sugar (p = 0.09).
Conclusions: This meta-analysis suggests that ADF is a viable diet strategy for weight loss, and it has a substantial improvement in risk indicators for diseases in obese or normal people.
Introduction
It is well-established that obesity is a risk factor in some metabolic diseases, such as atherosclerosis, dyslipidemia, and nonalcoholic fatty liver disease, and the factor could be modified (1). The energy imbalance caused by increased calorie intake and decreased physical activity is an important reason for adult obesity (2). Among them, the long-term excessive carbohydrate intake has the most serious negative impact on the human body (3). Therefore, restricting calorie intake and increasing energy consumption are among the main methods to manage obesity. Meanwhile, people are investigating many manners of diet and exercise continuously for obese and overweight individuals (4–9). Some data showed that dietary restriction and physical exercise could maintain lean and weight loss. Of course, restrictive diets alone can also reduce lean body weight (4, 10, 11). Daily calorie restriction therapy is one of the most widely used diets for obese patients. This method can reduce energy intake by 15–40% every day (12, 13). Many people have lost weight successfully by using daily calorie restriction regimens; however, many obese people find it is difficult to insist on this approach because food intake must be limited every day. So, traditional daily calorie restriction therapy has poor compliance and long-term compliance (14, 15).
A recent study showed that caloric restriction could extend lifespan in rhesus monkeys (16, 17). In clinical trials, calorie restriction could reduce the risk factors of diseases and improve patient's health. However, a link has been found between continuous caloric restriction and harmful factors about human health (18). Overall, calorie restriction has many limitations, particularly on healthy humans. Alternate-day fasting (ADF), as a new calorie restriction method for obesity patients, has been proved to improve human health-related outcomes (9, 19–21). ADF involves a “fast day” where individuals consume 25% of energy needed, alternated with a “feed day” where subjects eat ad libitum, with days of eating freely and to appetite (ad libitum) (22). ADF may be an effective alternative for weight loss because this diet only requires calorie counting every other day (21, 23). In long-term human trials (2–3 months), ADF apparently lost body weight by about 3–7% and decreased the risk of artery disease and other biochemical indexes of the human body [low-density lipoprotein (LDL) cholesterol, triglycerides (TGs), and so on] (19, 24, 25).
According to relevant reports, obese patients with 25% calorie intake on fast days have been proved to be safe for at least 2 months; the characteristics of few adverse incidences have also been recognized by many people (26). In principle, some reports showed that adverse effects of ADF were minimal, such as mild headache or substantial hunger and light-headedness (27).
The ADF method has gotten more and more popular in the past decade. Some books of ADF became a bestseller; to some extent, these books also promote the popularity of weight-loss methods (28). Hitherto, it has been published more than a million copies in the United States and the United Kingdom (29). The body weight fluctuations made a great influence on people's physiology indexes. To evaluate the effects of ADF for adults, we performed a systematic review and meta-analysis of randomized controlled trials (RCTs).
Materials and Methods
Study Design
A systematic review of RCTs was carried out using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist (30).
Search Strategy
Our study searched PubMed (1988 to March 2020), EMBASE (1995 to March 2020), and the Cochrane Controlled Trials Register, looking for studies about the effect of ADF on the metabolism of the human body. The keywords and medical subjects are as follows: “alternate day fasting,” “calorie restriction,” “body weight,” “weight loss,” “obesity,” “non-obese humans,” “exercise,” “fasting,” “meal replacement,” and “RCT.” If necessary, we also contacted the author to obtain more information about their research. Furthermore, we also reviewed original references for including texts.
Inclusion and Exclusion Criteria
RCTs that met the following criteria were included: (a) the studies should have a connection with the topic: the effect of ADF on the metabolism of the human body (obese or nonobese humans), (b) full text of the study could be provided, or (c) precise data could be extracted, and there are similar indicators between the ADF group and the control group in every RCT.
The following studies were excluded: (a) not RCT, such as abstract, review, or comment or (b) incomplete data study.
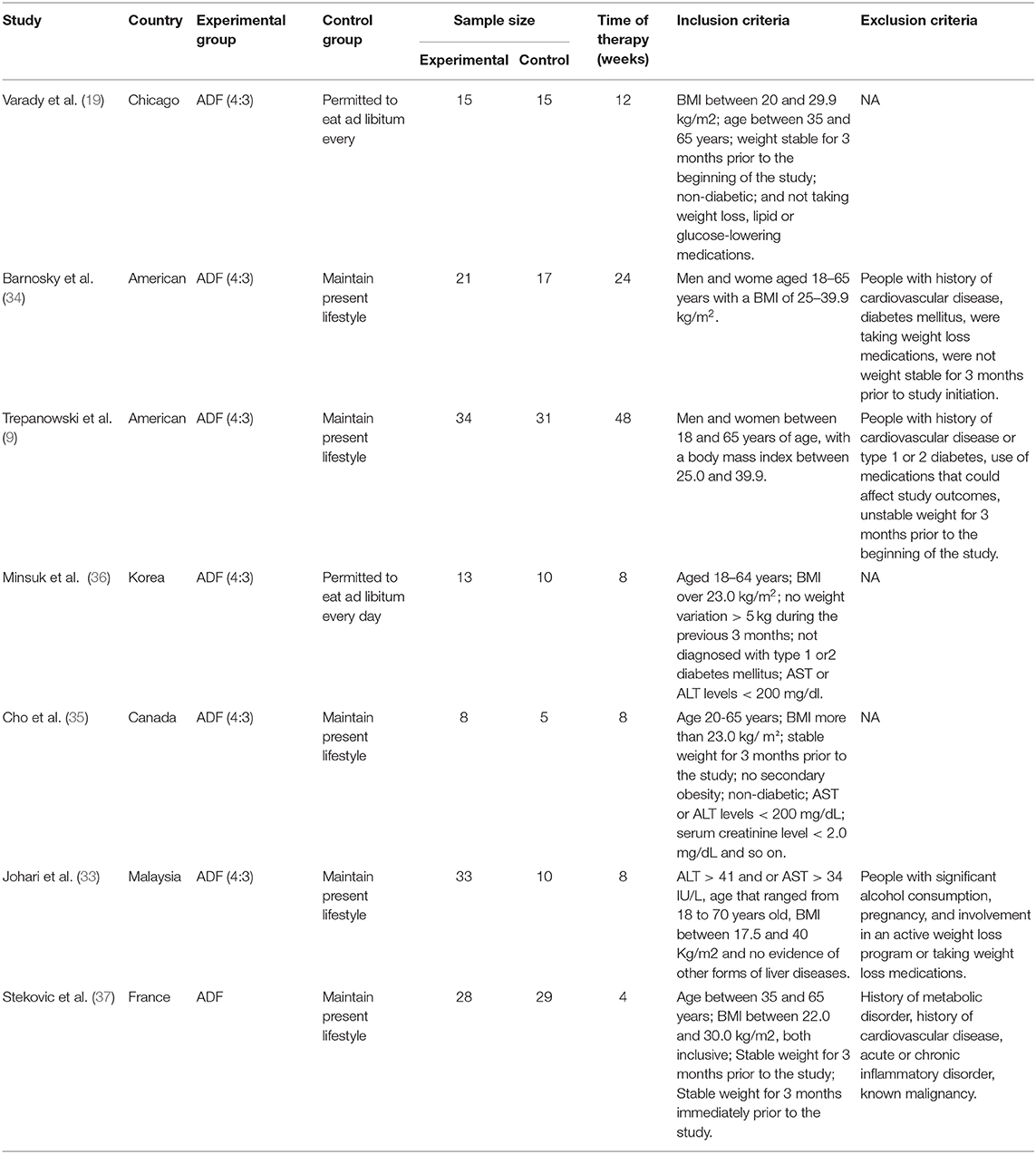
Furthermore, we included the most recently published study if studies described identical experiments. Besides, every study would be included if different measures were evaluated. Table 1 shows the specific inclusion and exclusion criteria.
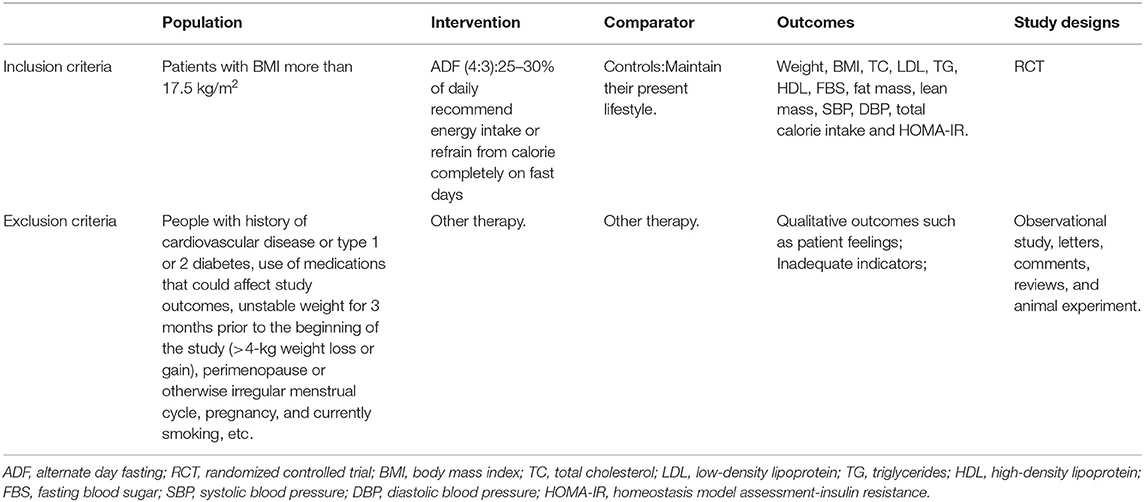
Table 1. Criteria for considering studies for the review based on the population, intervention, comparator, outcomes, and study designs (PICOS) structure.
Quality Assessment
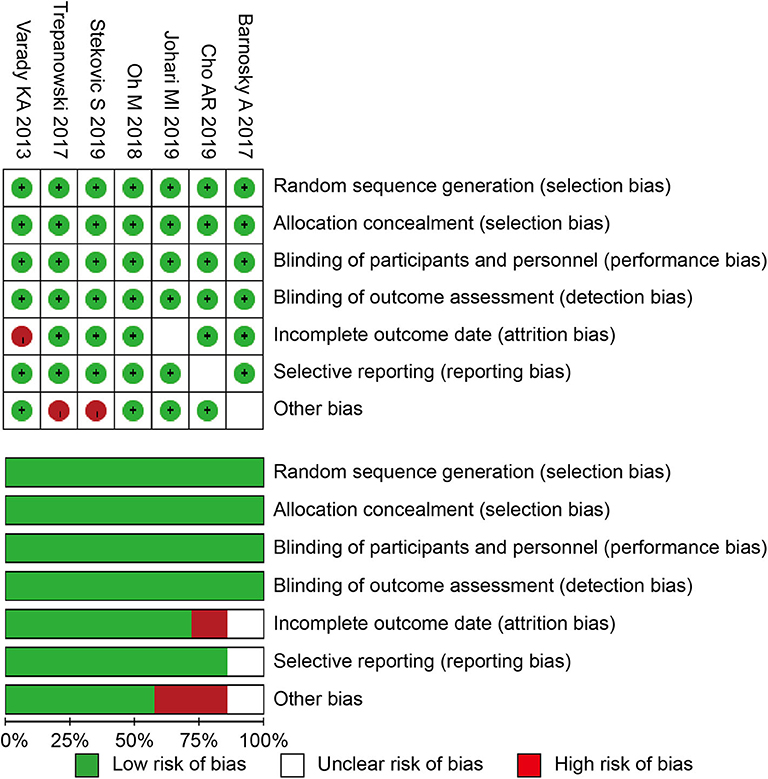
The Cochrane risk of bias tool was used to determine the quality of the retrieved RCTs (31). The quality items were selective outcome reporting, blinding, allocation concealment, incomplete outcome data, random sequence generation, and other sources of bias. A graph summarizing the risk of bias was generated based on discussions among the authors, as shown in Figure 1. Then, according to the guidelines published in the Cochrane Handbook for Systematic Reviews of Interventions v.5.3.0, the studies were classified qualitatively (32). All of the authors participated in the quality assessment of all RCTs and agreed with the results. Meanwhile, the differences between each RCT were bridged through discussion among authors. All reviewers independently assessed whether the study was suitable or not according to the criteria.
Data Extraction
The following information was collected: (a) the general data in the test; (b) name of the first author; (c) time of publication; (d) the design of study and size of the sample (e.g., the interventions between the different groups); (e) the efficacious data that changes in the following parameters, such as weight, body mass index (BMI), total cholesterol (TC), LDL, TG, high-density lipoprotein (HDL), fasting blood sugar (FBS), fat mass, lean mass, systolic blood pressure (SBP), diastolic blood pressure (DBP), total calorie intake, and homeostasis model assessment-insulin resistance (HOMA-IR). Meanwhile, our team cross-checked references and data of each included study to ensure there is no overlapping data.
Statistical Analysis and Meta-Analysis
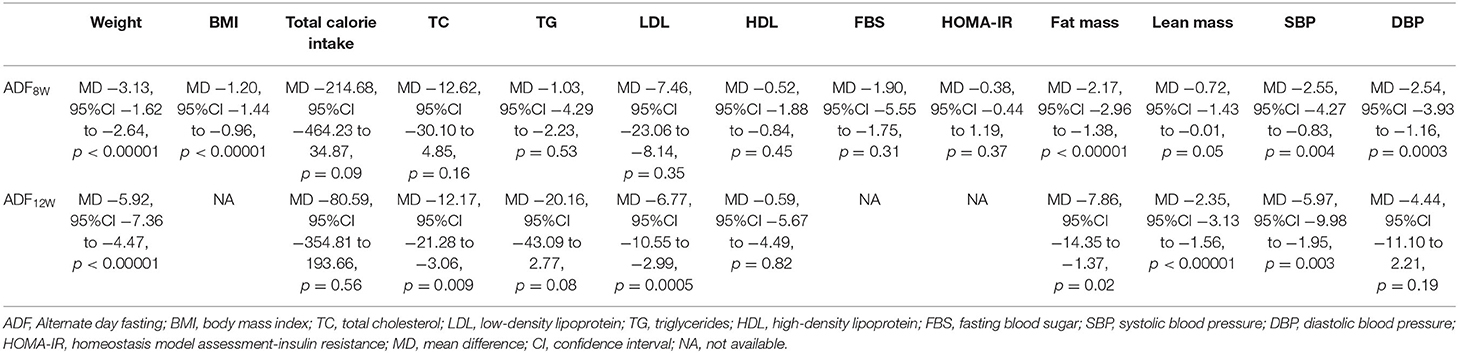
The data were carried out using the RevMan version 5.3.0 (Cochrane Collaboration, UK) (32). The changes in the weight, BMI, TC, LDL, TG, HDL, FBS, fat mass, lean mass, SBP, DBP, total calorie intake, and HOMA-IR were analyzed concerning the differences of each RCT between the entry and endpoint. This meta-analysis used mean difference (MD) to evaluate continuous data, and the odds ratio with 95% confidence intervals (CIs) was applied to evaluate dichotomous data. The fixed-effects model was used and considered to be homogeneous if the result was a p-value > 0.05. We utilized the I2 statistic to analyze inconsistent results, reflecting the proportion of heterogeneity across trials. In this meta-analysis, it is unnecessary to have ethical approval and patient consent because all of the data were acquired from articles that have already been published. A random-effects model would be used in the study when the results showed p < 0.05 and I2 > 50%. Moreover, if the results of the study showed p < 0.05, the result was deemed to be statistically significant. Meanwhile, a subgroup analysis was conducted according to the lengths of intervention time (ADF8W and ADF12W) in patients (Table 2).
Results
Characteristics of the Individual Studies
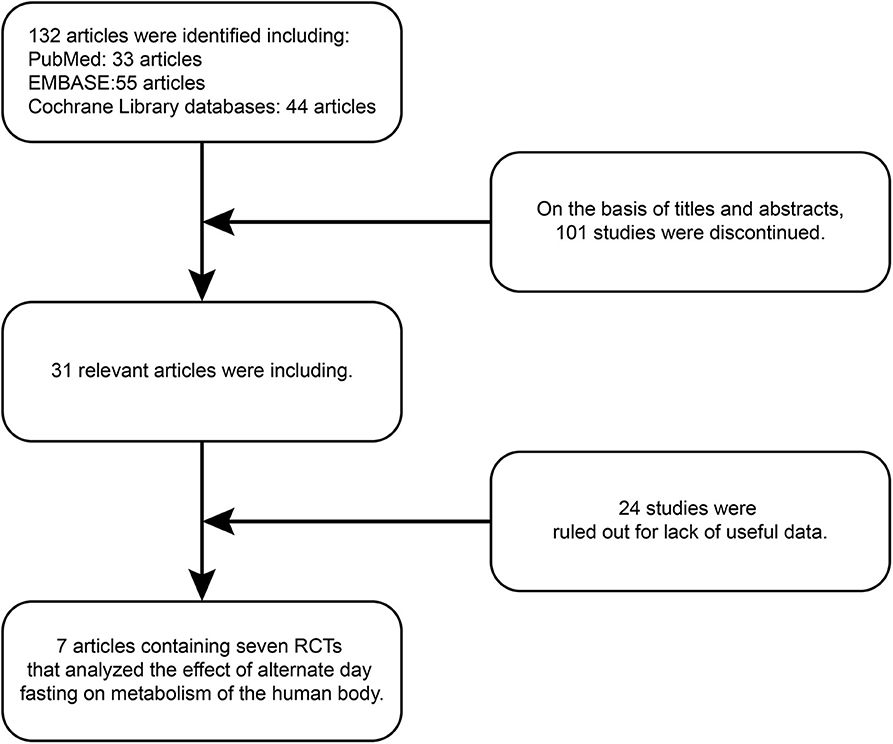
One hundred and thirty-two articles were discovered by retrieval in each database. After scrutinizing their abstracts and titles, 101 studies were discontinued. Twenty-four studies were ruled out for a lack of useful data. Finally, seven articles containing seven RCTs (9, 19, 33–37) that analyzed the effect of ADF on the metabolism of the human body were included in our analysis. A detailed flowchart showing the selection process is shown in Figure 2. Table 3 shows the baseline characteristics of the studies.
Quality of the Individual Studies
All of the seven studies included in the meta-analysis were RCT. Figure 1 presents a graphical summary of the risk bias. Besides, all of the studies described the randomization process. All articles had an appropriate number of participants to analyze. The funnel plot displayed the conclusion of a qualitative estimation of publication bias (Figure 3).
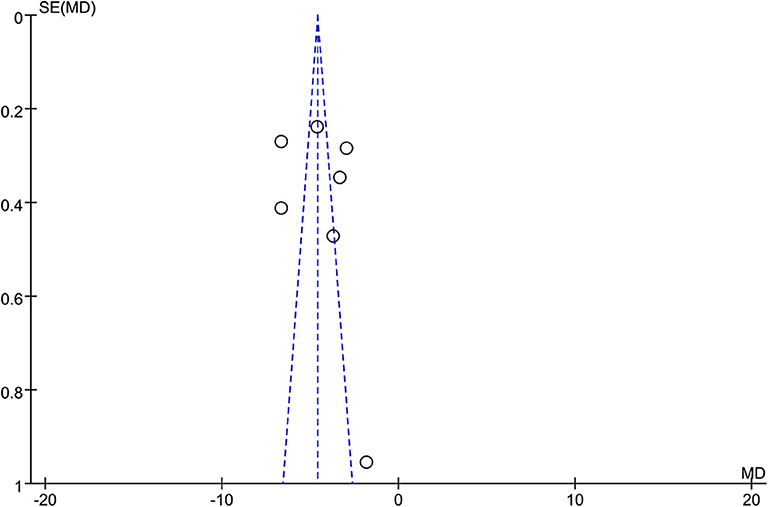
Figure 3. Funnel plot of the studies included in our meta-analysis. MD, mean difference; SE, standard error.
Efficacy
Weight
Seven RCTs involving 269 participants contained meaningful data on weight (152 in the ADF group and 117 in the control group). A random-effects model was adopted to evaluate changes between the two groups, showing an MD of −4.30, 95% CI: −5.54 to −3.05, P < 0.00001. It proved that compared with the control group (Figure 4), the ADF group showed statistically significant reductions in weight.
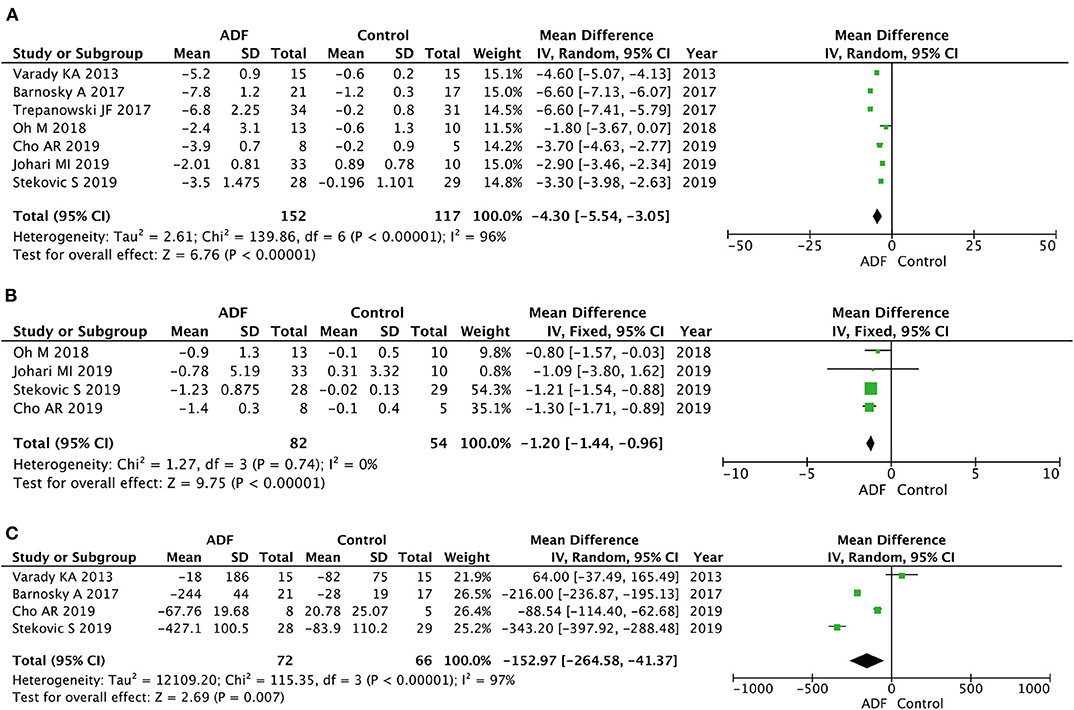
Figure 4. Forest plots showing changes between two groups in (A) weight, (B) body mass index (BMI), (C) total calorie intake; SD, standard deviation; IV, inverse variance; CI, confidence interval; df, degrees of freedom.
Body Mass Index
Four RCTs involving 136 participants contained meaningful data on BMI (82 in the ADF group and 54 in the control group). A fixed-effects model was adopted to evaluate changes between the two groups, showing an MD of −1.20, 95% CI: −1.44 to −0.96, P < 0.00001. The result proved that the ADF group showed statistical differences in BMI compared with the control group (Figure 4).
Total Calorie Intake
Four RCTs involving 138 participants contained meaningful data on total calorie intake (72 in the ADF group and 66 in the control group). A random-effects model was adopted to evaluate changes between the two groups, showing an MD of −152.97, 95% CI: −264.58 to −41.37, p = 0.007. It demonstrated that the ADF group showed statistically significant reductions in total calorie intake compared with the control group (Figure 4).
Total Cholesterol
Five RCTs involving 174 participants contained meaningful data on TC (103 in the ADF group and 71 in the control group). A random-effects model was chosen to evaluate changes between the two groups, showing an MD of −11.32, 95% CI: −18.20 to 4.44, P = 0.001. We found significant differences between the ADF group and the control group in the TC (Figure 5).
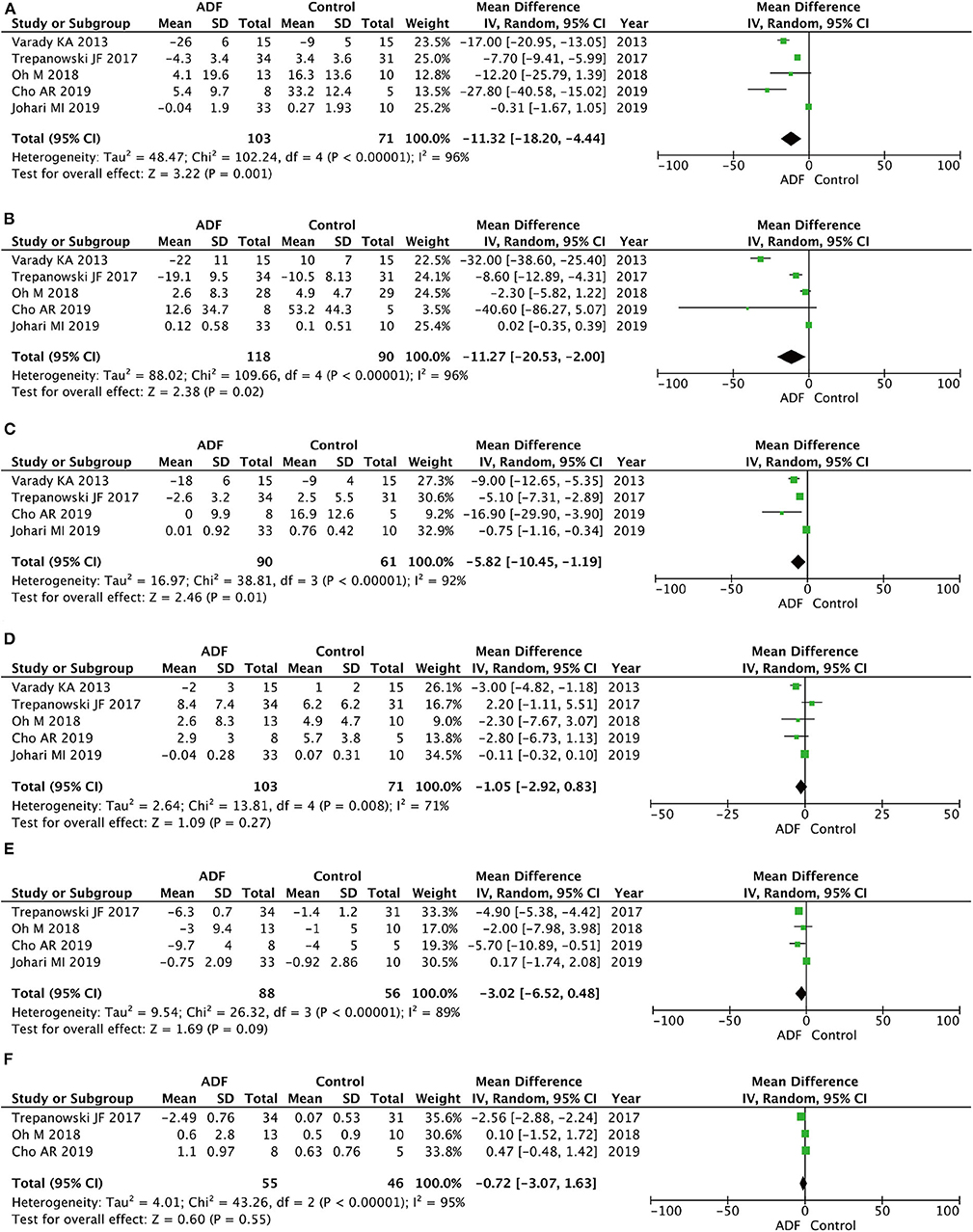
Figure 5. Forest plots showing changes between two groups in (A) total cholesterol (TC), (B) triglycerides (TG), (C) low-density lipoprotein (LDL), (D) high-density lipoprotein (HDL), (E) fasting blood sugar (FBS), (F) homeostasis model assessment-insulin resistance (HOMA-IR); SD, standard deviation; IV, inverse variance; CI, confidence interval; df, degrees of freedom.
Triglycerides
Five RCTs involving 208 participants contained meaningful data on TG (118 in the ADF group and 90 in the control group). A random-effects model was chosen to evaluate changes between the two groups, which showed an MD of −11.27, 95% CI: −20.53 to −2.00, P = 0.02. The result proved that the ADF group showed significant differences in TG compared with the control group (Figure 5).
Low-Density Lipoprotein
Four RCTs involving 151 participants contained meaningful data on LDL (90 in the ADF group and 61 in the control group). A random-effects model was adopted to evaluate changes between the two groups, showing an MD of −5.82, 95% CI: −10.45 to −1.19, P = 0.01. The result proved that the ADF group showed significant differences in LDL compared with the control group (Figure 5).
High-Density Lipoprotein
Five RCTs involving 174 participants contained meaningful data on HDL (103 in the ADF group and 71 in the control group). A random-effects model was chosen to evaluate changes between the two groups, which showed an MD of −1.05, 95% CI: −2.92 to 0.83, P = 0.27. The result showed that it was no statistical difference in terms of HDL between the two groups (Figure 5).
Fasting Blood Sugar
Four RCTs involving 144 participants contained meaningful data on FBS (88 in the ADF group and 56 in the control group). A random-effects model was chosen to evaluate changes between the two groups, showing an MD of −3.02, 95% CI: −6.52 to 0.28, P = 0.09. The model showed no marked differences between the ADF group and the control group in the change of FBS (Figure 5).
Homeostasis Model Assessment—Insulin Resistance
Three RCTs involving 101 participants contained meaningful data on HOMA-IR (55 in the ADF group and 46 in the control group). A random-effects model was used to evaluate changes between the two groups, showing an MD of −0.72, 95% CI: −3.07 to 1.63, p = 0.55. Compared with the control group, the ADF group showed no meaningful difference in HOMA-IR (Figure 5).
Fat Mass and Lean Mass
Six RCTs involving 226 participants contained meaningful data on fat mass (119 in the ADF group and 107 in the control group). In terms of lean mass, five RCTs had an appropriate sample size of 172 patients (89 in the ADF group and 73 in the control group). A random-effects model showed statistical differences between the ADF group and the control group in the change of fat mass (MD −4.96, 95%CI −8.08 to 1.85, p = 0.002) and lean mass (MD −1.38, 95%CI −2.26 to −0.49, p = 0.002) (Figure 6).
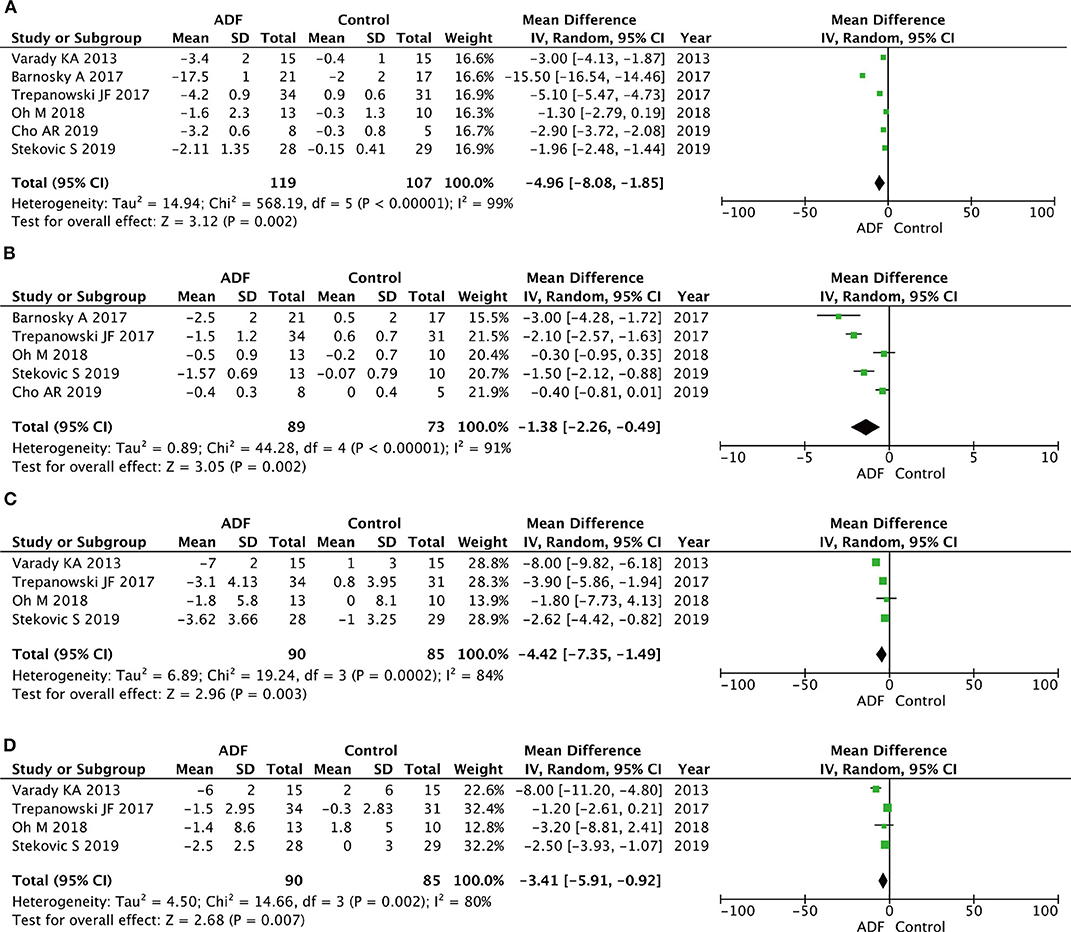
Figure 6. Forest plots showing changes between two groups in (A) fat mass, (B) lean mass, (C) systolic blood pressure (SBP), (D) diastolic blood pressure (DBP); total calorie intake; SD, standard deviation; IV, inverse variance; CI, confidence interval; df, degrees of freedom.
Systolic Blood Pressure and Diastolic Blood Pressure
Four RCTs involving 175 participants contained meaningful data on SBP and DBP (90 in the ADF group and 85 in the control group). A random-effects model was chosen to estimate changes between the two groups. The result demonstrated that the ADF group showed statistical differences in SBP (MD −4.42, 95%CI −7.35 to −1.49, p = 0.003) and DBP (MD −3.41, 95%CI −5.91 to −0.92, p = 0.007) compared with the control group (Figure 6).
Subgroup Analysis
In subgroup analyses, both ADF8W and ADF12W improved anthropometric outcomes, such as body weight (ADF8W: p < 0.00001; ADF12W: p < 0.00001), fat mass (ADF8W: p < 0.00001; ADF12W: p = 0.02), and SBP (ADF8W: p = 0.004; ADF12W: p = 0.003). Meanwhile, comparing ADF8W and ADF12W with control, ADF8W performed slightly better than ADF12W for total calorie intake (ADF8W: p = 0.09; ADF12W: p = 0.56) and DBP (ADF8W: p = 0.0003; ADF12W: p = 0.19), ADF12W performed slightly better for TC (ADF8W: p = 0.16; ADF12W: p = 0.009), lean mass (ADF8W: p = 0.05; ADF12W: p < 0.00001), and LDL (ADF8W: p = 0.35; ADF12W: p = 0.0005). No subgroup differences were observed for HDL (ADF8W: p = 0.45; ADF12W: p = 0.82) and TG (ADF8W: p = 0.53; ADF12W: p = 0.08) (Table 2).
Discussion
Recently, the most commonly used diet strategy for weight loss is calorie restriction. In many parts of the world, ADF is an age-old way that includes many forms. For example, Ramadan is a form of ADF and abided by Muslims all around the world (38). If someone wants to lose weight, it is generally recommended to restrict diets and exercises in clinical practice guidelines (39, 40). The traditional methods of losing weight (such as the daily calorie restriction), although the effect is affirmative, the control and compliance are poor (41). Based on this foundation, intermittent fasting regimens, especially ADF protocols, are being proposed in many pieces of literature (9, 19, 33–37). The important unsolved issue is whether the effects of ADF can play a role for people who want to lose weight.
This quantitative meta-analysis summarized the evidence from RCTs. We performed this meta-analysis from seven studies, including 269 participants, to evaluate the effect of ADF on weight loss for at least 1 month. In this meta-analysis, compared with the control group, the ADF group showed statistically significant reductions in weight (p < 0.00001) and BMI (p < 0.00001). In this case, we selected some meaningful indexes of many diverse indicators to illustrate this difference between the two groups. Here, the ADF group showed significant differences in terms of TC (p = 0.001), LDL (p = 0.01), TG (p = 0.02), fat mass (p = 0.002), lean mass (p = 0.002), SBP (p = 0.003), DBP (p = 0.007), and total calorie intake (p = 0.007) compared with the control group. At the same time, the analysis demonstrated that the ADF group had a similar effect compared with the control group in aspects of HDL (P = 0.27) and FBS (P = 0.09). Based on our results, ADF was the positive influential method on the physiology, body composition, and parameters for obesity or a normal human.
For the first time, this meta-analysis suggested that ADF is a potentially superior alternative to daily calorie restriction in normal-weight and overweight subjects. Just the way we assumed, the ADF strategy was effectively reduced body-related biomarkers, such as weight, BMI, and so on. Compared with the complete calorie restriction method, ADF had a lower capacity for weight management. In animal experiments, weight loss can redistribute fat in the ADF group without losing lean mass (42). New research showed that exercise plus ADF would experience the largest reductions in cardiometabolic risk factors, with the least decrease in lean mass compared with ADF alone (36). Also, it could be suggested as an alternative option for daily calorie restriction (CR) in treating nonalcoholic fatty liver disease (33). On the one hand, the decrease of liver enzymes might be explained by an improvement in visceral fat or steatosis of the liver in animal and human experiments. On the other hand, the liver enzymes have an increased anomaly in “fast days” and reduced in “feast days,” so this is an irreversible process (43). The phenomenon showed that ADF could promote hepatocyte restorative process when transient autophagy occurred to liver cells (44). However, the exact molecular mechanisms that underlie fasting and liver autophagy need to be further studied and established (45).
The research suggested that ADF had effects on cardiovascular improvements. As is known to all, alterations in cholesterol metabolism were known to be powerful predictors of developing cardiovascular events, even in the early stages of atherosclerosis (46). For instance, abnormal cholesterol metabolism, including low intestinal cholesterol absorption and elevated cholesterol biosynthesis, played an important role in metabolic syndrome, obesity, and diabetes (47). This diet strategy may also have cardioprotective effects in participants by reducing triacylglycerol and increasing LDL particle size and adiponectin concentration. To our knowledge, the Mediterranean and certain low carbohydrate diets help maintain a healthy weight and reduce the risks of coronary heart disease. If ADF were combined with a Mediterranean diet or a low-carbohydrate diet, it would be meaningful to observe how it affects weight loss and cardiovascular outcomes in future studies (48, 49). To improve lipid, the combination of diet and exercise is more effective than diet or exercise alone (50). This diet strategy may also have cardioprotective effects in participants by reducing triacylglycerol and increasing LDL particle size and adiponectin concentration. Previous research has already demonstrated that glucose and insulin have been associated with obesity. Thus, it is important to manage and control glucose levels and insulin resistance (51). At the same time, the significant decrease in fasting insulin may potentially be attributed in part to the decline in body weight and the reduction in total body fat (52). We found a significant reduction in lean mass in the ADF group. This serves as a caution for patient populations at risk for sarcopenia because ADF could exacerbate muscle loss. Some studies have shown that adequate/excessive protein consumption during weight loss can mitigate losses in lean mass (53).
Some studies indicated that ADF is the most beneficial diet strategy for lowering fasting insulin, glucose, and HOMA-IR. However, there was no significant difference in insulin resistance between the two groups. It suggested that ADF plus exercise might reduce insulin resistance, which needs to be further elucidated (36). Meanwhile, ADF did not result in a decline in bone mineral density or white blood cell count. It might even have a trend to increase bone mineral density values for a long period (54–56). It was worth mentioning that no effect was found in some studies: (a) the weight of participants lost <10%; (57, 58) (b) the sample size of RCTs that we selected was weak; (c) the duration of the maintenance phase was absent; (d) others, such as the time for blood sampling and so on. In a recent study we founded, ADF likely makes little differences compared with continuous energy restriction, but ADF probably slightly reduces body weight and fat mass. In additional analyses, no important differences were detected when comparing different types of ADF (4:3 vs. 5:2; consecutive vs. nonconsecutive days) (59).
Randomized control trials suggested that ADF is effective for weight loss, weight maintenance, and improving certain metabolic disease risk factors such as LDL cholesterol, blood pressure, and fasting insulin after 6 months (60). However, all studies were limited by a lower number of included RCTs and were often limited by also including short-term trials (<12 weeks) (61, 62).
ADF had some physiological benefits with similar daily calorie restriction (63). Recently, true ADF that had a limited capacity intake completely on a fast day was demonstrated and reduced fat cell size by 35–55% in both visceral and subcutaneous adipose tissue depots after 4 weeks in mice (64). ADF might lead to fat redistribution from visceral to subcutaneous depots in female mice (42). Evidence suggested that plasma adiponectin was inversely proportional to visceral fat accumulation (65, 66). Thus, the redistribution in body fat by ADF may be linked to increases in plasma adiponectin observed. All in all, ADF may improve body fat distribution and circulating adiponectin; conversely, the diet strategy may take precautions against the development of obesity-related diseases whether these effects can be reproduced in clinical trials.
Several adverse events were reported in the study. Its incidences were significantly low and slight. A small number of participants experienced mild headaches or light-headedness in the early days of the trial. Others reported constipation during weeks 1 and 2 of the trial (27). This phenomenon may or may not be linked with dietary therapy. The participants were proposed to be consuming more fruits and vegetables on feed days. Those clinical manifestations would gradually disappear in the course of the experiment.
This meta-analysis included seven RCTs and concentrating on the efficacy of ADF in participants. Compared with previous studies, our study had some advantages; the data were derived from randomized, double-blind, controlled trials. However, this study also has some limitations, which reflect the common limitations of other systematic reviews and meta-analyses. First of all, this article did not include numerous RCTs such as unpublished studies, which limit evidence to affect study quality. Second, RCTs had low methodological rigor and short intervention; more appropriate high-quality trials are needed to improve the accuracy of results.
Conclusions
In summary, this meta-analysis suggests that ADF is a viable diet strategy for weight loss, and it has a substantial improvement in risk indicators for diseases in obese or normal people. Therefore, adults, whether healthy or not, should perform ADF with recommendations of clinical physicians to prevent adverse effects.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
Author Contributions
YL: literature search. ZG: study design and data collection. TC: data interpretation. JW and YZ: writing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Nature Science Foundation of China (nos. 81870525, 81801429, and 81572835), Taishan Scholars Program of Shandong Province (no. tsqn201909199), Beijing Municipal Administration of Hospitals' Ascent Plan, code: DFL20190502, and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support, code: ZYLX201820.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ADF, alternate day fasting; RCTs, randomized controlled trials; TC, total cholesterol; BMI, body mass index; LDL, low-density lipoprotein; LDL, low-density lipoprotein; TG, triglycerides; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment-insulin resistance; FBS, fasting blood sugar; MD, mean difference; OR, odds ratio; CI, confidence intervals; CR, calorie restriction.
References
1. Wilson PW, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. (2002) 162:1867–72. doi: 10.1001/archinte.162.16.1867
2. Popkin BM, Gordon-Larsen P. The nutrition transition: worldwide obesity dynamics and their determinants. Int J Obes Relat Metab Dis. (2004) 28:S2. doi: 10.1038/sj.ijo.0802804
3. Kroemer G, López-Otín C, Madeo F, De Cabo R. Carbotoxicity—noxious effects of carbohydrates. Cell. (2018) 175:605–614. doi: 10.1016/j.cell.2018.07.044
4. Curioni CC, LourenO PM. Long-term weight loss after diet and exercise: a systematic review. Int J Obes. (2005) 29:1168–74. doi: 10.1038/sj.ijo.0803015
5. Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. (2011) 364:1218–29. doi: 10.1056/NEJMoa1008234
6. Miller WC, Koceja DM, Hamilton EJ. A meta-analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int J Obes Relat Metab Disord. (1997) 21:941–7. doi: 10.1038/sj.ijo.0800499
7. Wu T, Gao X, Chen M, Dam RMV. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: a meta-analysis. Obes Rev. (2009) 10:313–23. doi: 10.1111/j.1467-789X.2008.00547.x
8. Wood PD, Stefanick ML, Williams PT, Haskell WL. The effects on plasma lipoproteins of a prudent weight-reducing diet, with or without exercise, in overweight men and women. J Cardiopulm Rehabil. (1991) 325:461–6. doi: 10.1056/NEJM199108153250703
9. Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, Hoddy KK, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults. JAMA Intern Med. (2017) 177:930–8. doi: 10.1001/jamainternmed.2017.0936
10. Hansen D, Dendale P, Berger J, van Loon LJC. The effects of exercise training on fat-mass loss in obese patients during energy intake restriction. Sports Med. (2007) 37:31–46. doi: 10.2165/00007256-200737010-00003
11. Ross R, Rissanen J, Pedwell H, Clifford J, Shragge P. Influence of diet and exercise on skeletal muscle, and visceral adipose tissue in men. J Appl Physiol. (1996) 81:2445–55. doi: 10.1152/jappl.1996.81.6.2445
12. Fock KM, Khoo J. Diet and exercise in management of obesity and overweight. J Gastroenterol Hepatol. (2013) 28:59–63. doi: 10.1111/jgh.12407
13. Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. aha/acc/tos guideline for the management of overweight and obesity in adults. J Am Coll Cardiol. (2014) 63:2985–3023. doi: 10.1016/j.jacc.2013.11.004
14. Moreira EAM, Most M, Howard J, Ravussin E. Dietary adherence to long-term controlled feeding in a calorie-restriction study in overweight men and women. Nutr Clin Pract. (2011) 26:309–15. doi: 10.1177/0884533611405992
15. Moroshko I, Brennan L, O"Brien P. Predictors of dropout in weight loss interventions: a systematic review of the literature. Obes Rev. (2011) 12:912–34. doi: 10.1111/j.1467-789X.2011.00915.x
16. Antoni R, Johnston KL, Collins AL, Robertson MD. Intermittent v. continuous energy restriction: differential effects on postprandial glucose and lipid metabolism following matched weight loss in overweight/obese participants. Br J Nutr. (2018) 119:507–16. doi: 10.1017/S0007114517003890
17. Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS, et al. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun. (2017) 8:14063. doi: 10.1038/ncomms14063
18. Redman LM, Heilbronn LK, Martin CK, de Jonge L, Williamson DA, Delany JP, et al. Metabolic and behavioral compensations in response to caloric restriction: implicationsfor the maintenance of weight loss. PLoS ONE. (2009) 4:e4377. doi: 10.1371/journal.pone.0004377
19. Varady KA, Klempel MC, Kroeger CM, Trepanowski JF, et al. Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr J. (2013) 12:1–8. doi: 10.1186/1475-2891-12-146
20. Varady KA, Church EC, Klempel MC. Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr. (2000) 5:1138–43. doi: 10.3945/ajcn.2009.28380
21. Klempel MC, Kroeger CM, Trepanowski JF, Varady KA. Intermittent fasting combined with calorie restriction is effective for weight loss and cardio-protection in obese women. Nutr J. (2012) 11:98. doi: 10.1186/1475-2891-11-98
22. Varady KA, Hellerstein MK. Alternate-day fasting and chronic disease prevention: a review of human and animal trials. Am J Clin Nutr. (2007) 86:7–13. doi: 10.1093/ajcn/86.1.7
23. Luigi F. Aging, adiposity, and calorie restriction. JAMA. (2007) 297:986. doi: 10.1001/jama.297.9.986
24. Tinsley GM, La BPM. Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr Rev. 73:661–74. doi: 10.1093/nutrit/nuv041
25. Varady KA, Church EC, Klempel MC. Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr. (2000) 5.
26. Hoddy KK, Kroeger CM, Trepanowski JF, Barnosky A, Bhutani S, Varady KA. Meal timing during alternate day fasting: impact on body weight and cardiovascular disease risk in obese adults. Obesity. (2014) 22:2524–31. doi: 10.1002/oby.20909
27. Ganesan K, Habboush Y, Sultan S. Intermittent fasting: the choice for a healthier lifestyle. Cureus. (2018) 10:e2947. doi: 10.7759/cureus.2947
28. Mosley M, Spencer M. The Fastdiet: Lose Weight, Stay Healthy, and Live Longer With the Simple Secret of Intermittent Fasting. Atria Books. Britain (2013).
30. Moher D. Corrigendum to: preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007
31. Vader JP. Randomised controlled trials: a user's guide. BMJ. (1998) 317:1258. doi: 10.1136/bmj.317.7167.1258
32. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
33. Johari MI, Yusoff K, Haron J, Nadarajan C, Ibrahim KN, Wong MS, et al. A randomised controlled trial on the effectiveness and adherence of modified alternate-day calorie restriction in improving activity of non-alcoholic fatty liver disease. Sci Rep. (2019) 9:11232. doi: 10.1038/s41598-019-47763-8
34. Barnosky A, Kroeger CM, Trepanowski JF, Klempel MC Bhutani, et al. Effect of alternate day fasting on markers of bone metabolism: an exploratory analysis of a 6-month randomized controlled trial. Nutr Healthy Aging. (2017) 4:255–263. doi: 10.3233/NHA-170031
35. Cho AR, Moon JY, Kim S, An KY, Oh M, Jeon JY, et al. Effects of alternate day fasting and exercise on cholesterol metabolism in overweight or obese adults: a pilot randomized controlled trial. Metab Clin Exp. (2019) 93:52–60. doi: 10.1016/j.metabol.2019.01.002
36. Minsuk O, Sue K, Ki-Yong A, Jihee M, In YH, Junga L, et al. Effects of alternate day calorie restriction and exercise on cardio-metabolic risk factors in overweight and obese adults: an exploratory randomized controlled study. BMC Public Health. (2018) 18:1124. doi: 10.1186/s12889-018-6009-1
37. Stekovic S, Hofer SJ, Tripolt N, Aon MA, Royer P, Pein L, et al. Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metab. (2020) 31:878–81. doi: 10.1016/j.cmet.2020.02.011
38. Mohsen M, Peyman R, Owais C, Mohsen N. The effect of Ramadan fasting on cardiovascular risk factors and anthropometrics parameters: a systematic review. Pak J Med Sci. (2015) 31:1250–5. doi: 10.12669/pjms.315.7649
39. European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD) European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. (2016) 64:1388–402. doi: 10.1016/j.jhep.2015.11.004
40. Chitturi S, Wong WS, Chan WK, Wong LH, Wong KH, et al. The asia-pacific working party on nonalcoholic fatty liver disease guidelines 2017 part 2: management and special groups. J Gastroenterol Hepatol. (2017) 33:86–98. doi: 10.1111/jgh.13856
41. Jeffery RW, Drewnowski A, Epstein LH, Stunkard AJ, Hill DR. Long-term maintenance of weight loss: current status. Health Psychol. (2000) 19 (1 Suppl.):5–16. doi: 10.1037/0278-6133.19.Suppl1.5
42. Varady KA, Allister CA, Roohk DJ, Hellerstein MK. Improvements in body fat distribution and circulating adiponectin by alternate-day fasting versus calorie restriction. J Nutr Biochem. (2010) 21:188–95. doi: 10.1016/j.jnutbio.2008.11.001
43. Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. (2015) 22:86–99. doi: 10.1016/j.cmet.2015.05.012
44. Allaire M, Rautou PE, Codogno P Lotersztajn, et al. Autophagy in liver diseases: time for translation? J Hepatol. (2019) 70:985–98. doi: 10.1016/j.jhep.2019.01.026
45. Saito T. Autophagy regulates lipid metabolism through selective turnover of ncor1. Nat Commun. (2019) 10:1567. doi: 10.1038/s41467-019-08829-3
46. Son HH, Moon JY, Seo HS, Kim HH, Chung BC, Choi MH. High-temperature GC-MS-based serum cholesterol signatures may reveal sex differences in vasospastic angina. J Lipid Res. (2014) 55:155–62. doi: 10.1194/jlr.D040790
47. Wilund KR, Feeney LA, Tomayko EJ, Weiss EP, Hagberg JM. Effects of endurance exercise training on markers of cholesterol absorption and synthesis. Physiol Res. (2009) 58:545–52. doi: 10.1109/TMAG.2004.838741
48. Kris-Etherton P, Eckel RH, Howard BV, St Jeor S, Bazzarre TL, Nutrition Committee Population Science Committee and Clinical Science Committee of the American Heart Association. AHA science advisory: lyon diet heart study. Benefits of a mediterranean-style, National Cholesterol Education Program/American Heart Association Step I dietary pattern on cardiovascular disease. Circulation. (2001) 103:1823–5. doi: 10.1161/01.CIR.103.13.1823
49. Schwingshackl L, Hoffmann G. Low-carbohydrate diets and cardiovascular risk factors. Obes Rev. (2013) 14:183–4. doi: 10.1111/j.1467-789X.2012.01060.x
50. Kelley GA, Kelley KS, Roberts S, Haskell W. Comparison of aerobic exercise, diet or both on lipids and lipoproteins in adults: a meta-analysis of randomized controlled trials. Clin Nutr. (2012) 31:156–67. doi: 10.1016/j.clnu.2011.11.011
51. Abbasi F, Brown BW, Lamendola C, Mclaughlin T, Reaven GM. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol. (2002) 40:937–43. doi: 10.1016/S0735-1097(02)02051-X
52. Harris L, Hamilton S, Azevedo LB, Olajide J, Ells L. Intermittent fasting interventions for treatment of overweight and obesity in adults: a systematic review and meta-analysis. JBI Database Syst Rev Implem Rep. (2018) 16:507–47. doi: 10.11124/JBISRIR-2016-003248
53. Lowe DA, Wu N, Rohdin-Bibby L, et al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern Med. (2020) 180:1–9. doi: 10.1001/jamainternmed.2020.4153
54. Schafer AL. Decline in bone mass during weight loss: a cause for concern? J Bone Miner Res. (2016) 31:36–9. doi: 10.1002/jbmr.2754
55. Villareal DT, Fontana L, Das SK, Redman L, Smith SR, Saltzman E, et al. Effect of two-year caloric restriction on bone metabolism and bone mineral density in non-obese younger adults: a randomized clinical trial. J Bone Miner Res. (2016) 31:40–51. doi: 10.1002/jbmr.2701
56. Wiklund P, Toss F, Weinehall L, Hallmans G, Franks PW, NordstrM A, et al. Abdominal and gynoid fat mass are associated with cardiovascular risk factors in men and women. J Clin Endocrinol Metab. (2008) 93:4360–6. doi: 10.1210/jc.2008-0804
57. Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. (1992) 56:320–8. doi: 10.1093/ajcn/56.2.320
58. Jae SY, Fernhall B, Heffernan KS, Jeong M, Chun EM, Sung J, et al. Effects of lifestyle modifications on c-reactive protein: contribution of weight loss and improved aerobic capacity. Metab Clin Exp. (2006) 55:825–31. doi: 10.1016/j.metabol.2006.02.010
59. Schwingshackl L, Zähringer J, Nitschke K, Torbahn G, Lohner S, Kühn T, et al. Impact of intermittent energy restriction on anthropometric outcomes and intermediate disease markers in patients with overweight and obesity: systematic review and meta-analyses. Crit Rev Food Sci Nutr. (2020) 1–12. doi: 10.1080/10408398.2020.1757616
60. Kalam F, Gabel K, Cienfuegos S, Wiseman E, Ezpeleta M, Steward M, et al. Alternate day fasting combined with a low-carbohydrate diet for weight loss, weight maintenance, and metabolic disease risk reduction. Obesity science practice. (2019) 5:531–9. doi: 10.1002/osp4.367
61. Cho Y, Hong N, Kim KW, Cho SJ, Lee M, Lee YH, et al. The effectiveness of intermittent fasting to reduce body mass index and glucose metabolism: a systematic review and meta-analysis. J Clin Med. (2019) 8:1645. doi: 10.3390/jcm8101645
62. Cioffi I, Evangelista A, Ponzo V, Ciccone G, Soldati L, Santarpia L, et al. Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: a systematic review and meta-analysis of randomized controlled trials. J Transl Med. (2018) 16:371. doi: 10.1186/s12967-018-1748-4
63. Varady KA, Roohk DJ, McEvoyHein BK, Gaylinn BD, Thorner MO, Hellerstein MK. Modified alternate-day fasting regimens reduce cell proliferation rates to a similar extent as daily calorie restriction in mice. FASEB J. (2008) 22:2090. doi: 10.1096/fj.07-098178
64. Varady KA, Roohk DJ, Loe YC, Mcevoy-Hein BK, Hellerstein MK. Effects of modified alternate-day fasting regimens on adipocyte size, triglyceride metabolism, and plasma adiponectin levels in mice. J Lipid Res. (2007) 48:2212–9. doi: 10.1194/jlr.M700223-JLR200
65. Asayama K, Hayashibe H, Dobashi K. Decrease in serum adiponectin level due to obesity and visceral fat accumulation in children. Obes Res. (2003). 11:1072–9. doi: 10.1038/oby.2003.147
Keywords: alternate day fasting, calorie restriction (CR), body weight, weight loss, meta-analysis, randomized controlled trials (RCT)
Citation: Cui Y, Cai T, Zhou Z, Mu Y, Lu Y, Gao Z, Wu J and Zhang Y (2020) Health Effects of Alternate-Day Fasting in Adults: A Systematic Review and Meta-Analysis. Front. Nutr. 7:586036. doi: 10.3389/fnut.2020.586036
Received: 07 August 2020; Accepted: 21 October 2020;
Published: 24 November 2020.
Edited by:
Donato Angelino, University of Teramo, ItalyReviewed by:
Francesco Sofi, University of Florence, ItalyMarialaura Bonaccio, Istituto Neurologico Mediterraneo Neuromed (IRCCS), Italy
Copyright © 2020 Cui, Cai, Zhou, Mu, Lu, Gao, Wu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jitao Wu, Z3psMTk5NTAyMTlAMTYzLmNvbQ==; Yong Zhang, MzMyODU4Njc1QHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yuanshan Cui
Yuanshan Cui Tong Cai
Tong Cai Zhongbao Zhou2†
Zhongbao Zhou2†