- 1Centre for Applied Food Sustainability- and Biotechnology, Central University of Technology, Bloemfontein, South Africa
- 2Department of Food Science, University of Guelph, Guelph, ON, Canada
The COVID-19 pandemic has brought about a consideration of our understanding of transmission of the causal agent, SARS-CoV-2 to humans and its potential effect on food safety and food security. The main routes of transmission are reported to be person-to-person, by respiratory droplets and to a lesser degree, by fomites. Concerns have been raised on the possibility of transmission via food and food packaging and whether the virus poses a risk to food safety. The current contribution provides an exposé of updated literature and reports applicable to various components of food safety and its linkage to SARS-CoV-2. The article focuses on SARS-CoV-2 survival in food, on food contact materials and food packaging, and its categorization as a foodborne vs. respiratory virus, the possibility of fecal-oral transmission and the likelihood of infection via the gastro-intestinal system. The survival and inactivation of SARS-CoV-2 in food through thermal and non-thermal inactivation methods as well as the survival and inactivation on inanimate surfaces and effective disinfection of food contact surfaces, are discussed. Ultimately, the article endeavors to add to the body of knowledge pertaining to the role of SARS-CoV-2 in food safety and thereby contribute to an agile and robust fraternity that is equipped to absorb and weather the ongoing effects of the pandemic on the food sector.
Introduction
COVID-19 is caused by the SARS-CoV-2 virus (Severe Acute Respiratory Syndrome Coronavirus 2)—a member of the Coronaviridae Family generally comprising an enveloped, single-stranded RNA structure. A distinguishing characteristic of the Coronaviridae is the club- or petal-shaped surface projections or “spikes” which is reminiscent of the solar corona, hence the name coronaviruses (1). The virus particle of SARS-CoV-2 ranges from 50 to 200 nm and comprises a Spike (S), Envelope (E), Membrane (M), and Nucleocapsid (N) structural proteins, the latter containing the genome (RNA). The S, E, and M proteins constitute the viral envelope (2, 3).
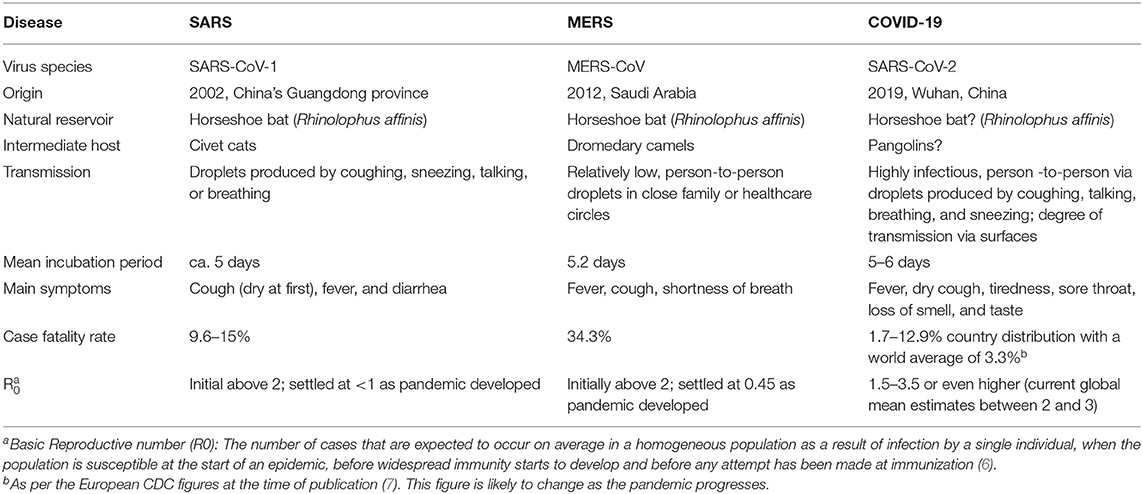
Coronaviruses mostly infect amphibians, birds, and mammals, with a number shown to be zoonotic (4). Four coronavirus genera (α, β, γ, and δ) have been identified so far, however, in the last 2 decades, three novel β coronaviruses (MERS-CoV, SARS-CoV-1, and SARS-CoV-2), now known to cause respiratory-related disease outbreaks in humans have been described, although each has displayed unique features (5). Although SARS (Severe Acute Respiratory Syndrome) and MERS (Middle-East Respiratory Syndrome) have significantly higher case-fatality rates than SARS-CoV-2, the latter is notably more infectious and spreads readily among humans. Table 1 provides a comparison of the fundamental traits of the post-2000 coronavirus outbreaks (8–10).
Data from various epidemiological studies report that COVID-19 is transmitted amongst humans mainly through respiratory droplets (breathing, sneezing, coughing, talking) or via the contact-oral route with contaminated objects and surfaces, although the latter seems to be less-significant (11, 12). A characteristic of SARS-CoV-2 infections that has played a pivotal role in the epidemiological spread is its ability to shed infective particles in the absence of symptoms. This is categorized as (1) after infection, but prior to showing symptoms (pre-symptomatic); (2) during infection, but not showing any symptoms (asymptomatic); and (3) after having recovered, but still shedding virulent particles (post-symptomatic). Research has indicated that the pre-symptomatic shedding (infective) window of COVID-19 typically ranges from 2 to 3 days after initial infection, followed by the asymptomatic phase over ca. 4–10/11 days and the post-symptomatic phase thereafter (from 11 up to 14 days or until the individual test is negative) (13).
In addition to the symptoms mentioned in Table 1, secondary complications of advanced COVID-19 disease can include breathing difficulty, chest pain or pressure, confusion, inability to stay awake, nausea, vomiting, diarrhea, and oxygen deprivation indicated by bluish lips or face. Ultimately, mortality manifests in an elevated immune response causing a cytokine over-production and severe pneumonia (14, 15). A number of co-morbidities and underlying health problems have been reported to impact the severity of the disease, amongst which are age, individuals suffering from chronic lung disease and asthma, heart conditions, hypertension, diabetes, obesity, and immunocompromised health (16). In children, the disease may manifest as a multisystem inflammatory syndrome, which can lead to serious and life-threatening consequences in previously healthy children and adolescents (17).
The mentioned underlying conditions are notably augmented in poor and under-developed communities, mostly due to compromising health practices, limited awareness and knowledge, lacking resources, and healthcare. The urban poor and rural residents, migrants, informal workers, those living in conflict-prone areas and individuals with co-morbidities have been identified as particularly at-risk (18). According to Guan and Liang (19), patients with comorbidities of over and under nutrition, hypertension and diabetes and those having malignancy yielded poorer clinical diagnoses than without. The observation that COVID-19 is especially fatal to individuals experiencing chronic or acute hunger or malnourishment constitute a particular risk in Africa with undernourishment affecting ca. 20% of the population—the highest incidence globally (20–22).
Amongst vulnerable groups, deficient water, sanitation, and housing infrastructure have presented a significant concern in light of the COVID-19 pandemic, as the provision of clean running water is a pivotal element of basic hygiene, especially considering proper WASH (water, sanitation, and hygiene) as the first line of defense against COVID-19 transmission. Currently, about 40% of people globally do not have basic handwashing facilities at home (23). Similarly, the densely populated domestic environment and ailing housing infrastructure eminent in squatter camps and informal settlements where one- or two-bedroom shacks are often occupied by 6 or more individuals, obviously challenges the principle of maintaining social distancing. The disruptive effect on health service delivery and the consequences for diseases such as malaria, tuberculosis (TB), and human immunodeficiency virus (HIV) in poor societies has also been a growing concern. By posing additional demands to already stretched healthcare systems, unexpected epidemics such as those caused by MERS and SARS-CoV-1 have shown to significantly undermine routine control efforts of TB, Malaria and HIV-AIDS, and have led to a considerable increase in, for example, malaria-related mortalities in a number of developing countries. Apart from resource concerns, the unique interaction between TB and HIV in that ca. 60% of TB patients are also HIV-positive, renders COVID-19 likely to be of particular concern for communities with high rates of TB and HIV, particularly where these illnesses are poorly controlled.
Transmission of SARS-CoV-2 by Food and Food Packaging
Several risk assessments have been conducted by food safety agencies on whether food, food contact materials and food packaging present a potential risk to food safety related to SARS-CoV-2. Considering that much was unknown about SARS-CoV-2 and its survival in food, on food contact materials or food packaging when the pandemic struck, scientists turned to published information on similar viruses. These included the first SARS virus (SARS-CoV-1), MERS and coronaviruses that cause the common cold (24–28). Establishing conservative risk estimates is possible, even when presented with significant data gaps. For a risk assessment of this nature, an intensive scrutiny of literature is required, followed by interrogation of that information to develop interim guidance for both policymakers and the public. As novel information emerges, such guidance is then updated accordingly.
According to authoritative literature, the consensus is that currently, there is no evidence that SARS-CoV-2 is a food safety risk. Therefore, from a hazard-risk perspective, the overall potential risk of acquiring COVID-19 from contaminated food or food packaging appears to be very low (27–33). SARS-CoV-2 is therefore, not considered a foodborne virus. It remains primarily a respiratory virus, which may also enter the bloodstream via mucous membranes in the eyes (34).
The French Agency for Food, Environmental and Occupational Health and Safety conducted one of the first risk assessments related to food safety and SARS-CoV-2 (27). Several questions were posed to the expert panel, one of which was whether there was a likelihood of contracting COVID-19, from ingesting the virus. Zhang et al. (35) found viral genetic material in anal swabs and blood taken from 178 patients. Considering that one of the symptoms of COVID-19 is diarrhea, these matters raised concerns on the likelihood of the virus being transmitted via the fecal-oral route. However, there are no reports to date showing fecal-oral transmission of the virus (36). A key question to address is how well SARS-CoV-2 survives during passage through the human stomach. It is known that SARS-CoV-2 uses angiotensin- converting enzyme 2 (ACE) as receptors, which are expressed on bronchial epithelial cells, mucosal, and epithelial cells in the intestine (37); however, we do not know the concentration of SARS-CoV-2 necessary to infect humans via the gastrointestinal route and whether SARS-CoV-2 can actually invade these cells and eventually enter into the bloodstream and/or if diarrhea is a main symptom. Lamers et al. (38) reported infection of enterocytes in human small intestine organoids by both SARS-CoV-1 and SARS-CoV-2 during in vitro studies and stated that SARS-CoV-2 replication was supported by intestinal epithelium. However, several studies have concluded that diarrhea is likely caused when the virus infiltrates the body through other mechanisms, during the severe stage of the illness and not to the virus being contracted from ingestion of contaminated food, via the gastro-intestinal tract (39–42). A further consideration affecting the likelihood of infection via the gastro-intestinal system, is that virus in food is likely to be at low concentration and may also be less available to host cells when contained within a food matrix in comparison to the virus being carried in respiratory droplets (43).
Food manufacturing businesses implement Food Safety Management Systems (FSMS), albeit at different levels of sophistication, depending on requirements in different countries. In all cases, however, development and implementation of a successful FSMS requires a solid foundation of Good Hygiene Practices (GHP). Such practices include washing hands with soap and water for a minimum of 20 s at specific times. In several cases, sanitizing hands is required after hand washing. There are additional hygienic practices listed in all such standards to ensure that food handlers practice proper hygiene to prevent transmission of any potential microbiological contamination to food (44).
Survival and Inactivation of SARS-CoV-2 in Food
Thermal (e.g., heat) and non-thermal (e.g., radiation and ultrasound) inactivation methods can be used to inactivate or reduce pathogens, including viruses, in the environment and on food (45, 46). Different thermal treatments have been used for the inactivation of foodborne viruses (e.g., human norovirus, hepatitis A and E viruses) on food matrices or liquids (47). Dry (hot air oven or incineration) and humid heat (steam, autoclave) are very effective methods for inactivating viruses and bacteria (45, 47, 48). After conducting an analysis of 10 different studies, Kampf et al. (48), reported that five different types of coronavirus suspended in liquid media, including SARS-CoV-1 and MERS-CoV, could be reduced by at least 4 logs using thermal treatments such as 60°C for 30 min, 65°C for 15 min or 80°C for 1 min. Chin et al. (49) found that SARS-CoV-2 was reduced by about 7 logs after a heat treatment of 70°C for 5 min. Furthermore, ANSES (27) considered the matter of sufficient heat treatment of food (27) and concluded that exposure of food to 63°C for 4 min would be adequate to kill the virus. In a qualitative risk assessment conducted by the Food Standards Agency of the United Kingdom, the organization acknowledged that despite several uncertainties, temperatures used for cooking should be sufficient to inactivate any virus present in food (28).
At refrigeration temperatures (4°C), Rabenau et al. (50) found no loss of infectious titer for SARS-CoV-1. A similar resistance to refrigeration was found by Chin et al. (49) when hardly any reduction in infectious SARS-CoV-2 was noted in transport medium held at 4°C for 14 days, which was the length of the experiment. It is therefore likely that the virus would survive for longer periods at refrigeration temperatures in specific media. Considering that freezing is generally not regarded as a destruction method for viruses in food, but rather as a method of preservation, it is likely that SARS-CoV-2 would survive freezing. In this regard, Fisher et al. (51) found that infectious SARS-CoV-2 did not decline in titer and was able to survive for 3 weeks in inoculated pieces of chicken, pork and salmon stored at 4, −20, and −80°C. Furthermore, Mullis et al. (52) demonstrated that a bovine coronavirus present on lettuce stored at 4°C retained its infectivity for at least 14 days. In addition, coronavirus 229E survived well for 2 days on lettuce stored at 4°C, before quickly decreasing in numbers, but did not survive on the surface of strawberries, potentially because of the acidity (53).
SARS-CoV-2 appears to be stable at different pH values (3–10) at room temperature, however, alkaline pH (>12) or acidic pH (<3) as well as heat, sunlight and UV light appear to be capable of inactivating the virus (49, 54, 55). Alternative methods of non-thermal physical disinfection include (i) ultraviolet (UV) light, (ii) pulsed light (iii) ionizing radiation, (iv) high pressure, (v) cold plasma, and (vi) high-intensity ultrasound (56), with some of these treatments limited to disinfecting surfaces.
Cold plasma treatment is a relatively new disinfection treatment that has attracted attention due to it being environmentally friendly, i.e., chemical free (57). This technology uses different inert gases that when submitted to high electricity, generate a large amount of a mixture of electrons, charged atoms and neutral atoms, which have the potential to inactivate microorganisms (57). The cold plasma technique uses cold gases to disinfect food contact surfaces as well as liquid and solid food products (56). Although it has been shown that cold plasma can effectively inactivate pathogenic viruses (e.g., human norovirus, adenovirus, and hepatitis A virus) on or in various matrices (57), further research is required to evaluate its effectiveness against SARS-CoV-2.
Survival and Inactivation of SARS-CoV-2 on Inanimate Surfaces
It is well-established that viruses causing respiratory infections, particularly SARS-CoV-2, can be transmitted by indirect contact (fomites) through the environment (58, 59), particularly when one touches contaminated surfaces and subsequently touches one's mouth, nose or eyes, without first washing hands. Warnes et al. (60), showed that human coronavirus 229E (HuCoV-229E), which is closely related to SARS-CoV-2, was able to persist on the surface of 5 different materials [e.g., polytetrafluoroethylene (Teflon), polyvinyl chloride (PVC), ceramic tiles, glass, and silicone rubber] for at least 5 days. In a review conducted by Kampf et al. (61) on the persistence of coronaviruses on inanimate surfaces, SARS-CoV-1, MERS coronavirus or the endemic human coronavirus (HuCoV-229E), persisted on inanimate surfaces like metal, glass or plastic for up to 9 days. At 30°C or greater, the duration of persistence of MERS was shorter, whereas persistence of the transmissible gastroenteritis virus (TGEV) on surfaces increased to over 28 days at 4°C. More recent research has shown that in an experimental setting at between 21 and 23°C at 40% humidity, SARS-CoV-2 could remain viable for up to 72 h on plastic and stainless steel, up to 4 h on copper, and up to 24 h on cardboard (12). Pezzotti et al. (62) demonstrated that silicon nitride can inactivate 99% of the SARS-CoV-2 virus after exposure for 1 min, which showed it to be as effective as copper. Therefore, the use of Si3N4 particles placed into the fabric of personal protective equipment, as well as other material, could be an effective way to decrease viral spread. In addition, Ratnesar-Shumate et al. (63) showed that SARS-CoV-2 is sensitive to artificial sunlight when suspended in simulated saliva on stainless steel coupons, i.e., 90% of the virus was inactivated in ~7 min.
Chin et al. (49) found in an experimental setting, that no infectious virus was recovered from a number of surfaces after different times at 22°C. Notably, the virus was more stable on smooth surfaces with no infectious virus detected after 4 days on glass and banknotes and none detected after 7 days on plastic and stainless steel. However, it is important to note that such results were generated under experimental conditions and may not reflect the potential of picking up the virus from contact in a more realistic environment. The CDC (64) and other similar agencies and organizations (65), do not consider contracting SARS-CoV-2 via contaminated surfaces a main route of transmission. A report by the McKinsey Company seems to support this belief (66). This report states that approximately 90% of SARS-CoV-2 transmissions occur from symptomatic, pre-symptomatic and asymptomatic people, leaving 10% transmissions from the environment, which includes surfaces. Therefore, the greatest risk remains person-to-person transfer in a food environment, including manufacturing, retail and food service. This emphasizes the importance of wearing appropriate Personal Protective Equipment (PPE) and practicing proper hand hygiene and social distancing.
Disinfecting Food Contact Surfaces
Food contact surfaces include all areas that come into contact with food products during preparation (e.g., cutting boards, tables, utensils), production, processing and packaging and typically include stainless steel, plastic material, wood, rubber, ceramics, or glass (54, 60, 67). These surfaces could be contaminated with pathogenic bacteria and viruses, which can infect the food and/or people handling the food (68). Even though current consensus is that SARS-CoV-2 is not transmitted by food of food packaging material, it is very important to properly clean and sanitize food contact surfaces, since one of the modes of transmission of SARS-CoV-2 appears to be touching contaminated surfaces and then touching your mouth, nose or eyes (28, 69, 70).
The current WHO guidance states that “thoroughly cleaning environmental surfaces with water and detergent and applying commonly used hospital level disinfectants (such as sodium hypochlorite) are effective and sufficient procedures” (58). Disinfectants are very important in the control and inactivation of microorganisms on various inanimate surfaces (61, 71, 72). However, if not careful, they can leave harmful residues behind on food contact surfaces.
The use of UV light is a well-known method for inactivating viruses, mycoplasma, bacteria and fungi, especially on surfaces (45, 46). In particular, UV-C light, the shortest wavelength (100–280 nm), has been largely used in the food industry (46). Several studies have demonstrated that UV-C light may be an effective tool for inactivating SARS-CoV-1 after a treatment of 60 min (73–76).
If surfaces are dirty, they should be cleaned using a detergent or soap and water, prior to disinfection. The food industry generally uses sanitizers that are considered safe due to low toxicity and non-corrosiveness (45). Sanitizers used on food contact surfaces should be different from those used on non-food contact surfaces (77, 78). Food grade sanitizers are able to reduce or control specific bacteria, while food grade disinfectants are able to kill bacteria, viruses, and molds, however, it is necessary to rinse with water to eliminate residues (77, 79).
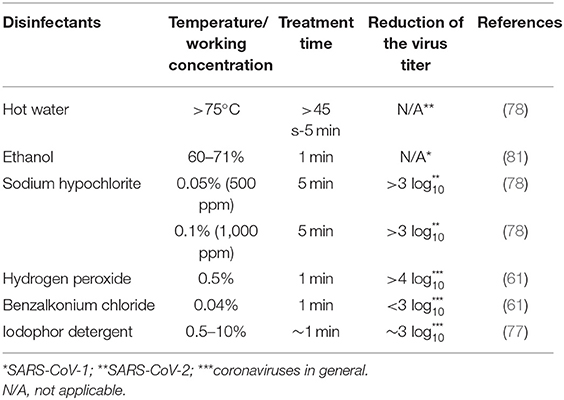
Although the biology of SARS-CoV-2 is not fully understood, it belongs to the coronavirus family of enveloped viruses, which makes them susceptible to detergents and a variety of other microbicides (24), even more so than fungi, vegetative bacteria and yeasts (80). On environmental surfaces, studies have shown that 0.1% sodium hypochlorite, 0.5% hydrogen peroxide, and 62–71% ethanol can significantly reduce coronavirus presence on surfaces after 1 min of exposure at room temperature [(61); see Table 2]; similar effects have been seen with SARS-CoV-2 (49, 58). Clean water, at a temperature of at least 77°C, for at least 45 s can also be used. The Environmental Protection Agency (82), Health Canada (83), as well as the European Union Open Data Portal (84) have a list of approved disinfectants for use against COVID-19. SARS-CoV-2, like other viruses, cannot multiply in food, therefore, over time, the number of infectious virions is expected to decrease if the virus happens to be present on the surface of a food product (54). Air disinfection could be considered in food-related environments and two practical methods include room air cleaners such as filters or UV light, as well as upper-room germicidal UV fixtures (85).
In summary, although the current evidence suggests that SARS-CoV-2 does not cause foodborne illness, the virus has caused major disruptions to the global food supply chain. Thus, issues such as food security and food sustainability have been brought to the forefront in this pandemic, as well as the health of food workers from farm through to retail and foodservice. Newer approaches/paradigms, such as the integration of One Health (e.g., creating a large team of specialists from many disciplines) along with systems thinking (i.e., a health crisis can rapidly spread to other systems that would normally appear unconnected), will be paramount to controlling pandemics in the future.
Author Contributions
LA initiated and coordinated the article and wrote the sections on transmission of SARS-CoV-2 through food and food packaging as well as survival of the virus on surfaces. RL wrote the introduction, whilst VP authored the sections on disinfection of surfaces and inactivation of the virus in foods. JF contributed to all sections. All authors contributed to editing of the final article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Aronson JK. Corona viruses – a general introduction. Centre for Evidence-Based Medicine, Nuffield Department of Primary Care Health Sciences, University of Oxford. (2020). Available online at: https://www.cebm.net/covid-19/coronaviruses-a-general-introduction/ (accessed May 24, 2020).
2. Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. (2020) 5:562–9. doi: 10.1038/s41564-020-0688-y
3. Oberholzer M, Febbo P. What We Know Today About Coronavirus SARS-CoV-2 and Where Do We Go From Here. Genetic Engineering and Biotechnology News. Archived from the original on 14 March 2020 (2020). Available online at: https://www.genengnews.com/insights/what-we-know-today-about-coronavirus-sars-cov-2-and-where-do-we-go-from-here/ (accessed March 13, 2020).
4. Fox D. What you need to know about the Wuhan coronavirus. Nature. [Preprint]. (2020). doi: 10.1038/d41586-020-00209-y
5. Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. (2019) 17:181–92. doi: 10.1038/s41579-018-0118-9
6. Aronson JK, Brassey J, Mahtani KJ. When Will It Be Over? An Introduction to Viral Reproduction Numbers, R0 and Re. (2020). Available online at: https://www.cebm.net/covid-19/when-will-it-be-over-an-introduction-to-viral-reproduction-numbers-r0-and-re/ (accessed June 23, 2020).
7. European Centre for Disease Prevention and Control (ECDC). Case Fatality Rate of Covid-19. (2020). Available online at: https://github.com/owid/covid-19-data/tree/master/public/data (accessed September 9, 2020).
8. WHO (World Health Organization). Consensus Document on the Epidemiology of SARS. (2003). Available online at: https://apps.who.int/iris/handle/10665/70863 (accessed May 04, 2020).
9. Kakodkar P, Kaka N, Baig M. A comprehensive literature review on the clinical presentation, and management of the pandemic corona virus disease 2019 (COVID-19). Cureus. (2020) 12:e7560. doi: 10.7759/cureus.7560
10. Peeri NC, Shrestha N, Rahman MDS, Zaki R, Tan Z, Bibi S, et al. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats. What lessons have we learned? Int J Epidemiol. (2020) 49:717–26. doi: 10.1093/ije/dyaa033
11. Chan JF, Yuan S, Kok KH, ToK K, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel corona virus indicating person-to-person transmission: a study of a family cluster. Lancet. (2020) 395:514–23. doi: 10.1016/S0140-6736(20)30154-9
12. van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. (2020) 382:1564–7. doi: 10.1056/NEJMc2004973
13. He X, Lau EH, Wu P, Deng X, Wang J, Hao X, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. (2020) 26:672–5. doi: 10.1038/s41591-020-0869-5
14. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
15. Li J, You Z, Wang Q, Zhou Z, Qiu Y, Luo R, et al. The epidemic of 2019-novel-coronavirus (2019-nCoV) pneumonia and insights for emerging infectious diseases in the future. Microb Infect. (2020) 22:80–85. doi: 10.1016/j.micinf.2020.02.002
16. Wu D, Wu T, Liu Q, Yang Z. The SARS-CoV-2 outbreak: what we know. Int J Infect Dis. (2020) 94:44–8. doi: 10.1016/j.ijid.2020.03.004
17. Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. (2020) 383:334–46. doi: 10.1056/NEJMoa2021680
18. FAO (Food and Agriculture Organization). Committee on World Food Security (CFS). Impact of COVID-19 on Food Security and Nutrition (FSN). (2020). Available online at: http://www.fao.org/fileadmin/user_upload/hlpe/COVID-19/HLPE._Impact_of_COVID-19_on_FSN_-_2020-03-24_-_EN4.pdf (accessed April 16, 2020).
19. Guan W, Liang W. Comorbidity and its impact on 1590 patients with covid-19 in China: a nationwide analysis. Eur Respir J. (2020) 55:2000547. doi: 10.1183/13993003.00547-2020
20. Headly D, Ruel M. The COVID-19 Nutrition Crisis: What to Expect and How to Protect. CGIAR Research Program on Agriculture for Nutrition and Health (A4NH). (2020). Available online at: https://a4nh.cgiar.org/2020/04/23/the-covid-19-nutritional-crisis-what-to-expect-and-how-to-protect/ (accessed May 22, 2020).
21. UNICEF (United Nations Children's Fund). Nutrition Information Management, Surveillance and Monitoring in the Context of COVID-19 Global Nutrition Cluster, Global Technical Assistance Mechanism for Nutrition (GTAM). (2020). Available online at: https://www.unicef.org/media/68301/file/Nutrition-Information-Management-Surveillance-and-Monitoring-COVID19.pdf (accessed June 13, 2020).
22. WFP (World Food Programme). Risk of Hunger Pandemics as COVID-19 Set to Almost Double Acute Hunger by End of 2020. (2020). Available online at: https://insight.wfp.org/covid-19-will-almost-double-people-in-acute-hunger-by-end-of-2020-59df0c4a807 (accessed May 04, 2020).
23. Armah FA, Ekumah B, Yawson DO, Odoi JO, Afitiri AR, Nyieku FE. Access to improved water and sanitation in sub-Saharan Africa in a quarter century. Heliyon. (2018) 4:e00931. doi: 10.1016/j.heliyon.2018.e00931
24. Lai MYY, Cheng PKC, Lim WWL. Survival of SARS Coronavirus CID 2005:41 (1 October). e67 Survival of Severe Acute Respiratory Syndrome coronavirus (2005). Available online at: https://academic.oup.com/cid/article-abstract/41/7/e67/310340 (accessed April 29, 2020).
25. Otter JA, Donskey C, Yezli S, Douthwaite S, Goldenberg SD, Weber DJ. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. (2016) 92:235–50. doi: 10.1016/j.jhin.2015.08.027
26. Azhar EI, Hui DSC, Memish ZA, Drosten C, Zumla A. The middle east respiratory syndrome (MERS). Infect Dis Clin N Am. (2019) 33:891–905. doi: 10.1016/j.idc.2019.08.001
27. ANSES. (French Agency for Food, Environmental and Occupational Health and Safety). Opinion on an Urgent Request to Assess Certain Risks Associated with COVID-19. (2020). Available online at: https://www.anses.fr/en/system/files/SABA2020SA0037-1.pdf (accessed April 30, 2020).
28. FSA (Food Standards Agency UK). Qualitative Risk Assessment: What is the Risk of Food or Food Contact Materials Being A Source or Transmission Route of SARS-CoV-2 for UK Consumers? (2020). Available online at: https://www.food.gov.uk/research/research-projects/qualitative-risk-assessment-on-the-risk-of-food-or-food-contact-materials-as-a-transmission-route-for-sars-cov-2 (accessed May 21, 2020).
29. EFSA (European Food Safety Authority). Novel Coronavirus – Where to Find Information. (2020). Available online at: https://www.efsa.europa.eu/en/news/novel-coronavirus-where-find-information (accessed May 15, 2020).
30. FDA (Food and Drug Administration USA). Food Safety and Covid-19. (2020). Available online at: https://www.fda.gov/food/food-safety-during-emergencies/food-safety-and-coronavirus-disease-2019-covid-19 (accessed May 01, 2020).
31. FSAI (Food Safety Authority of Ireland). COVID-19 (Coronavirus). (2020). Available online at: https://www.fsai.ie/faq/coronavirus.html (accessed April 29, 2020).
32. FSANZ (Food Standards Australia New Zealand) New Coronavirus and Food Safety. (2020). Available online at: https://www.foodstandards.gov.au/consumer/safety/Pages/NOVEL-CORONAVIRUS-AND-FOOD-SAFETY.aspx (accessed April 29, 2020).
33. WHO (World Health Organization). COVID-19 and Food Safety: Guidance for Food Businesses: Interim Guidance, 07 April 2020. (2020). Available online at: https://www.who.int/publications/i/item/covid-19-and-food-safety-guidance-for-food-businesses (accessed April 10, 2020).
34. Colavita F, Lapa D, Carletti F, Lalle E, Bordi L, Marsella P, et al. SARS-CoV-2 isolation from ocular secretions of a patient with COVID-19 in Italy with prolonged viral RNA detection. observation: brief research report. Ann Intern Med. (2020) 17:M20–1176. doi: 10.7326/M20-1176
35. Zhang W, Du RH, Li B, Zheng XS, Yang XL, Hu B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implications of multiple shedding routes. Emerg Microbes Infect. (2020) 9:386–9. doi: 10.1080/22221751.2020.1729071
36. Wang S, Tu J, Sheng Y. Clinical characteristics and fecal–oral transmission potential of patients with COVID19. medRxiv [Preprint]. (2020). doi: 10.1101/2020.05.02.20089094
37. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. (2004) 203:631–7. doi: 10.1002/path.1570
38. Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, et al. SARS-CoV-2 productively infects human gut enterocytes. Science. (2020) 1:eabc1669. doi: 10.1126/science.abc1669
39. Amirian SE. Potential faecal transmission of SARS-CoV-2: current evidence and implications for public health. Int J Infect Dis. (2020) 95:363–70. doi: 10.1016/j.ijid.2020.04.057
40. Wadman M, Couzin-Frankel J, Kaiser J, Matacic C. How Does Coronavirus Kill? Science. (2020). Available online at: https://www.sciencemag.org/news/2020/04/how-does-coronavirus-kill-clinicians-trace-ferocious-rampage-through-body-brain-toes?utm_source=Nature+Briefing# (accessed April 30, 2020).
41. Wang X, Zhou Y, Jiang N, Zhou Q, Ma WL. Persistence of intestinal SARS-CoV-2 infection in patients with COVID-19 leads to re-admission after pneumonia resolved. Int J Infect Dis. (2020) 95:433–5. doi: 10.1016/j.ijid.2020.04.063
42. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. (2020) 158:1831–3. doi: 10.1053/j.gastro.2020.02.055
43. FSAI (Food Safety Authority of Ireland). Qualitative Risk Assessment on the Development of COVID-19 Illness from the Consumption of Bivalve Molluscs. (2020). Available online at: https://www.fsai.ie/news_centre/COVID19_risk_assessment_03072020.html (accessed July 06, 2020).
44. CAC (Codex Alimentarius Commission). General Principles of Food Hygiene CAC/RCP 1-1969. Available online at: http://www.fao.org/fao-who-codexalimentarius/en/ Fourth edition WHO/FAO: Rome (2009).
45. Dvorak G. Disinfection 101. Center for Food Security and Public Health (2008). Available online at: http://www.cfsph.iastate.edu/Disinfection/Assets/Disinfection101.pdf (accessed May 18, 2020).
46. Skara T, Rosnes JT. Emerging methods and principles in food contact surface decontamination/prevention environmental factors in infectious disease in: Innovation and Future Trends in Food Manufacturing and Supply Chain Technologies, Woodhead Publishing Series in Food Science. Technol Nutr. (2016) 151–72. doi: 10.1016/B978-1-78242-447-5.00006-X
47. Bosch A, Gkogka E, Le Guyader FS, Loisy-Hamon F, Lee A, van Lieshout L, et al. Foodborne viruses: detection, risk assessment, and control options in food processing. Int J Food Microbiol. (2018) 285:110–28. doi: 10.1016/j.ijfoodmicro.2018.06.001
48. Kampf G, Voss A, Scheithauer S. Inactivation of coronaviruses by heat. J Hosp Infect. (2020) 105:348–9. doi: 10.1016/j.jhin.2020.03.025
49. Chin AWH, Chu JTS, Perera MRA, Hui KPY, Yen HL, Chan MCW, et al. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. (2020) 1:e10. doi: 10.1016/S1473-3099(20)30113-4
50. Rabenau HF, Cinatl J, Morgenstern B, Bauer G, Preiser W, Doerr HW. Stability and inactivation of SARS coronavirus. Med Microbiol Immunol. (2005) 194:1–6. doi: 10.1007/s00430-004-0219-0
51. Fisher D, Reilly A, Kang Eng Zheng A, Cook AR, Anderson DE. Seeding of outbreaks of COVID-19 by contaminated fresh and frozen food. bioRxiv [Preprint]. (2020). doi: 10.1101/2020.08.17.255166
52. Mullis L, Saif LJ, Zhang Y, Zhang X, Azevedo MSP. Stability of bovine coronavirus on lettuce surfaces under household refrigeration conditions. Food Microbiol. (2012) 30:180–6. doi: 10.1016/j.fm.2011.12.009
53. Yépiz-Gómez MS, Gerba CP, Bright KR. Survival of respiratory viruses on fresh produce. Food Environ Virol. (2013) 5:150–6. doi: 10.1007/s12560-013-9114-4
54. Pressman P, Naidu AS, Clemens R. COVID-19 and Food Safety: Risk Management and Future Considerations. Wolters Kluwer (2020). Available online at: https://journals.lww.com/nutritiontodayonline/Documents/Pressman%20Naidu%20%20Clemens%20-%20COVID-19%20%20Food%20Safety%20(005).pdf (accessed April 29, 2020).
55. WHO (World Health Organization). Water, Sanitation, Hygiene and Waste Management for COVID- 19: Technical Brief, 03 March 2020. (2020). Available online at: https://www.who.int/publications-detail/water-sanitation-hygiene-and-waste-management-for-covid-19 (accessed April 10, 2020).
56. Deng LZ, Mujumdar AS, Pan Z, Vidyarthi SK, Xu J, Zielinska M, et al. Emerging chemical and physical disinfection technologies of fruits and vegetables: a comprehensive review. Crit Rev Food Sci Nutr. (2019) 60:2481–508. doi: 10.1080/10408398.2019.1649633
57. Filipić A, Gutierrez-Aguirre I, Primc G, Mozetič M, Dobnik D. Cold plasma, a new hope in the field of virus inactivation. Trends Biotechnol. (2020) 38:1278–91. doi: 10.1016/j.tibtech.2020.04.003
58. WHO (World Health Organization). Infection Prevention and Control During Health Care When Novel Coronavirus (nCoV) Infection is Suspected: Interim Guidance, 25 January 2020. (2020). https://www.who.int/publications-detail/infection-prevention-and-control-during-health-care-when-novel-coronavirus-(ncov)-infection-is-suspected-20200125 (accessed February 20, 2020).
59. CDC (Centers for Disease Control and Prevention). Updates COVID-19 Transmission Webpage to Clarify Information About Types of Spread. (2020). Available online at: https://www.cdc.gov/media/releases/2020/s0522-cdc-updates-covid-transmission.html (accessed May 20, 2020).
60. Warnes SL, Little ZR, Keevil CW. Human coronavirus 229E remains infectious on common touch surface materials. MBio. (2015) 6:e01691-15. doi: 10.1128/mBio.01697-15
61. Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. (2020) 104:246–51. doi: 10.1016/j.jhin.2020.01.022
62. Pezzotti G, Ohgitani E, Shin-Ya M, Adachi TD, Marin E, Boschetto F, et al. Rapid inactivation of SARS-CoV-2 by silicon nitride, copper, and aluminum nitride. bioRxiv [Preprint]. (2020). doi: 10.1101/2020.06.19.159970
63. Ratnesar-Shumate S, Williams G, Green B, Krause M, Holland B, Wood S, et al. Simulated sunlight rapidly inactivates SARS-CoV-2 on surfaces. J Infect Dis. (2020) 222:214–22. doi: 10.1093/infdis/jiaa274/5841129
64. CDC (Centers for Disease Control and Prevention). How COVID-19 Spreads. (2020). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html (accessed May 21, 2020).
65. NZFSSRC (New Zealand Food Safety Science and Research Centre). Potential for Foodborne Transmission of Covid-19: Literature Review Update. (2020). Available online at: https://www.nzfssrc.org.nz/covid19 (accessed May 21, 2020).
66. Bode M, Craven M, Leopoldseder M, Rutten P, Wilson M. Contact Tracing for Covid-19: New Considerations for Its Practical Application. (2020). Available online at: https://www.mckinsey.com/industries/public-sector/our-insights/contact-tracing-for-covid-19-new-considerations-for-its-practical-application (accessed May 21, 2020).
67. Ren S-Y, Wang W-B, Hao Y-G, Zhang H-R, Wang Z-C, Chen Y-L, et al. Stability and infectivity of coronaviruses in inanimate environments. World J Clin Cases. (2020) 8:1391–9. doi: 10.12998/wjcc.v8.i8.1391
68. Rose DU, De Reposi MP, Amadio P, Auriti C, Dall'Oglio I, Corsetti T, et al. Use of disinfectant wipes to sanitize milk's containers of human milk bank during COVID-19 pandemic. J Hum Lact. (2020) 36:547–9. doi: 10.1177/0890334420924639
69. Sohrabi C, Alsafi Z, O'Neill N, Khan M, Kerwan A, Al-Jabir A, et al. World health organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19). Int J Surg. (2020) 76:71–76. doi: 10.1016/j.ijsu.2020.02.034
70. USDA (United States Department of Agriculture). Coronavirus Disease (COVID-19). (2020). Available online at: https://www.usda.gov/coronavirus (accessed April 24, 2020).
71. Kapoor A, Saha R. Hand washing agents and surface disinfectants in times of coronavirus (Covid-19) outbreak. Indian J. Commun Health. (2020) 32:225–27. doi: 10.47203/IJCH.2020.v32i02SUPP.008
72. Suman R, Javaid M, Haleem A, Vaishya R, Bahl S, Nandan D. Sustainability of coronavirus on different surfaces. J Clin Exp Hepatol. (2020) 10:386–90. doi: 10.1016/j.jceh.2020.04.020
73. Duan SM, Zhao XS, Wen RF, Huang JJ, Pi GH, Zhang SX, et al. Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomed Environ Sci. (2003) 16:246–55.
74. Darnell MER, Subbarao K, Feinstone SM, Taylor DR. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J Virol Methods. (2004) 121:85–91. doi: 10.1016/j.jviromet.2004.06.006
75. Walker CM, Ko G. Effect of ultraviolet germicidal irradiation on viral aerosols. Environ Sci Technol. (2007) 41:5460–5. doi: 10.1021/es070056u
76. Eickmann M, Gravemann U, Handke W, Tolksdorf F, Reichenberg S, Uller TH, et al. Inactivation of three emerging viruses – severe acute respiratory syndrome coronavirus, Crimean–Congo haemorrhagic fever virus and Nipah virus – in platelet concentrates by ultraviolet C light and in plasma by methylene blue plus visible light. Vox Sang. (2020) 115:146–51. doi: 10.1111/vox.12888
77. Gaulin C, Lê M, Shum M, Fong D. Disinfectants and Sanitizers for Use on Food Contact Surfaces: General Information About. Revised August 2011, 1–15. (2011). Availabe online at: http://www.ncceh.ca/sites/default/files/Food_Contact_Surface_Sanitizers_Aug_2011.pdf (accessed May 15, 2020).
78. Chen T. Reducing COVID-19 Transmission Through Cleaning and Disinfecting Household Surfaces: Appropriate Use of Cleaners, Sanitizers and Disinfectants Against SARS-CoV-2. National Collaborating Centre for Environmental Health, 1–18. (2020). Available online at: https://ncceh.ca/documents/guide/reducing-covid-19-transmission-through-cleaning-and-disinfecting-household-surfaces (accessed May 20, 2020).
79. BCCDC. Food Business. (2020). Available online at: http://www.bccdc.ca/health-info/diseases-conditions/covid-19/employers-businesses/food-businesses (accessed April 29, 2020).
80. Ijaz MK, Sattar S, Rubino J, Nims R, Gerba C. Combating SARS-CoV-2: leveraging microbicidal experiences with other emerging/re-emerging viruses. OSF [Preprint]. (2020). doi: 10.31219/osf.io/wjzuq
81. Fathizadeh H, Maroufi P, Momen-Heravi M, Dao S, Köse S, Ganbarov K, et al. Protection and disinfection policies against SARS-CoV-2 (COVID-19). Infez Med. (2020) 28:185–91.
82. EPA (Environmental Protection Agency). United States of America. (2020). Available online at: https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2 (accessed May 20, 2020).
83. Health Canada. Hard-Surface Disinfectants and Hand Sanitizers (COVID-19): List of Disinfectants with Evidence for Use Against COVID-19. (2020). Available online at: https://www.canada.ca/en/health-canada/services/drugs-health-products/disinfectants/covid-19/list.html (accessed May 14, 2020).
84. EU ODP (European Union Open Data Portal). (2020). Available online at: https://data.europa.eu/euodp/en/data/dataset/biocidal-products-lists-of-disinfectant-active-substances-and-products (accessed May 20, 2020).
Keywords: SARS-CoV-2, COVID-19, food safety, food packaging, surfaces, survival, inactivation
Citation: Anelich LECM, Lues R, Farber JM and Parreira VR (2020) SARS-CoV-2 and Risk to Food Safety. Front. Nutr. 7:580551. doi: 10.3389/fnut.2020.580551
Received: 14 July 2020; Accepted: 14 October 2020;
Published: 02 November 2020.
Edited by:
Angel Gil-Izquierdo, Spanish National Research Council, SpainReviewed by:
Artur Rzezutka, National Veterinary Research Institute (NVRI), PolandAna Allende, Consejo Superior de Investigaciones Científicas (CSIC), Spain
Copyright © 2020 Anelich, Lues, Farber and Parreira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lucia E. C. M. Anelich, bGFAYW5lbGljaGNvbnN1bHRpbmcuY28uemE=
 Lucia E. C. M. Anelich
Lucia E. C. M. Anelich Ryk Lues
Ryk Lues Jeffrey M. Farber
Jeffrey M. Farber Valeria R. Parreira
Valeria R. Parreira