
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 17 July 2020
Sec. Clinical Nutrition
Volume 7 - 2020 | https://doi.org/10.3389/fnut.2020.00104
This article is part of the Research Topic Ageing-Related Symptoms, Kampo Medicine and Treatment View all 16 articles
 Chayon Goswami1,2,3,4†
Chayon Goswami1,2,3,4† Katsuya Dezaki3,5†
Katsuya Dezaki3,5† Lei Wang1,2,3
Lei Wang1,2,3 Akio Inui6
Akio Inui6 Yutaka Seino1,7
Yutaka Seino1,7 Toshihiko Yada1,2,3,6*
Toshihiko Yada1,2,3,6*Appetite loss or anorexia substantially deteriorates quality of life in various diseases, and stand upstream of frailty. Neuropeptide Y (NPY) in the hypothalamic arcuate nucleus (ARC) and ghrelin released from stomach are potent inducers of appetite. We previously reported that Ninjin'yoeito, a Japanese kampo medicine comprising twelve herbs, restores food intake, and body weight in cisplatin-treated anorectic mice. Furthermore, Ninjin'yoeito increased cytosolic Ca2+ concentration ([Ca2+]i) in not only ghrelin-responsive but ghrelin-unresponsive NPY neurons in ARC. The cellular lineage/differentiation of ghrelin-unresponsive neuron is less defined but might alter along with aging and diet. This study examined the occupancy of ghrelin-unresponsive neurons among ARC NPY neurons in adult mice fed normal chow, and explored the mechanisms underlying Ninjin'yoeito-induced [Ca2+]i increases in ghrelin-unresponsive vs. ghrelin-responsive NPY neurons. Single ARC neurons were subjected to [Ca2+]i measurement and subsequent immunostaining for NPY. Ghrelin failed to increase [Ca2+]i in 42% of ARC NPY neurons. Ninjin'yoeito (10 μg/ml)-induced increases in [Ca2+]i were abolished in Ca2+ free condition in ghrelin-responsive and ghrelin-unresponsive ARC NPY neurons. Ninjin'yoeito-induced [Ca2+]i increases were inhibited by N-type Ca2+ channel blocker ω-conotoxin in the majority (17 of 20), while by L-type Ca2+ channel blocker nitrendipine in the minority (2 of 23), of ghrelin-responsive neurons. In contrast, Ninjin'yoeito-induced [Ca2+]i increases were inhibited by nitrendipine in the majority (14 of 17), while by ω-conotoxin in the minority (8 of 24), of ghrelin-unresponsive neurons. These results indicate that ghrelin-unresponsive neurons occur substantially among NPY neurons of ARC in adult mice fed normal chow. Ninjin'yoeito preferentially target N-type and L-type Ca2+ channels in the majority of ghrelin-responsive and ghrelin-unresponsive neurons, respectively, to increase [Ca2+]i. We suggest ARC N- and L-type Ca2+ channels as potential targets for activating, respectively, ghrelin-responsive, and unresponsive NPY neurons to treat anorexia.
Reduced appetite and body weight are associated with cancer, sarcopenia and frailty (1, 2), and deteriorate the quality of life (QOL) (3, 4). Hence, effective means to promote appetite have been awaited. Ninjin'yoeito, a Japanese traditional Kampo medicine, has been used clinically and demonstrated to be effective to treat anorexia, fatigue, anemia, cold limbs, persistent cough, mental disequilibrium, and to promote recovery from disease (5–7). Its ability to ameliorate this variety of symptoms is considered to result partly from counteraction of anorexia. Ninjin'yoeito comprises 12 crude drugs (Table 1). Some of these crude drugs are known to pass through the blood brain barrier (BBB) and hence possibly access to the hypothalamus including the arcuate nucleus (ARC), while ghrelin, an orexigenic gut hormone, freely accesses to the ARC neurons without crossing the BBB (8). Hence, oral administration of Ninjin'yoeito could act on ARC via one or both of these routes.
We previously reported that Ninjin'yoeito counteracted the anorexigenic and body weight-lowering effects of cisplatin, a chemo-therapy drug, in mice (9). In parallel, Ninjin'yoeito increased cytosolic Ca2+ concentration ([Ca2+]i) in single neurons isolated from ARC, and the majority (79%) of the Ninjin'yoeito-responsive neurons were immunoreactive (IR) to neuropeptide Y (NPY). The neuron coexpressing NPY and agouti-related protein (AgRP) (NPY/AgRP neuron) in ARC of hypothalamus is considered as the principal neuron for initiating feeding behavior (10–13). Selective activation of ARC NPY/AgRP neurons acutely and robustly triggers feeding (14, 15), while their selective deletion in adult mice markedly reduces feeding (16, 17), placing the ARC NPY/AgRP neuron as the necessary and adequate inducer of feeding.
We reported previously that Ninjin'yoeito increased [Ca2+]i in the ghrelin-responsive and ghrelin-unresponsive NPY neurons in ARC. Ghrelin, released from gut under fasted condition, stimulates feeding via activating NPY neurons (18–20). Hence, the ghrelin-responsive NPY neurons in ARC is considered to play a central role in stimulating physiological feeding. Compared to ghrelin-responsive NPY neurons, the role of the ghrelin-unresponsive NPY neurons is less defined. A possibility exists that ghrelin-responsive and ghrelin-unresponsive NPY neurons could be converted to each other depending on the diet/metabolic states and aging, including ghrelin resistance (21–23). The present study firstly investigated the occupancy (percentage) of the ghrelin-unresponsive neurons among ARC NPY neurons in adult mice fed normal chow. Notably, it has been documented that ghrelin-resistance occurs with aging and likely contributes to appetite reduction and resultant BW decrease and frailty. In this line, our previous finding that Ninjin'yoeito interacts with and recruits ghrelin-unresponsive NPY neurons to [Ca2+]i increases suggests its potential to restore appetite and counteract frailty. Hence, it is of relevance to elucidate the mechanisms underlying the [Ca2+]i responses to Ninjin'yoeito in ghrelin-unresponsive NPY neurons. The present study aimed to elucidate the Ca2+ channel type implicated in the [Ca2+]i response to Ninjin'yoeito in ghrelin-unresponsive NPY neurons in comparison with that in ghrelin-responsive NPY neurons.
It has been reported that N-type (CaV2.2) and L-type (CaV1.3) voltage-dependent Ca2+ channels (VDCCs) participate in depolarization-induced release of NPY in rat medium eminence-ARC preparation (24). In this report, 23 mM KCl-induced NPY release was blunted by nitrendipine, a blocker of L-type Ca2+ channels, and 45 mM KCl-induced NPY release was markedly attenuated by ω-conotoxin, a blocker of N-type Ca2+ channels, but not by nitrendipine. These data may indicate differential roles of N-type and L-type Ca2+ channels depending on the magnitude of depolarization by KCl. In addition, N-type and/or L-type Ca2+ channels in ARC neurons are involved in the [Ca2+]i responses to several substances including ghrelin (25, 26). Hence, the present study explored the link of Ninjin'yoeito to N-type and/or L-type VDCCs in ghrelin-responsive vs. unresponsive neurons. Single neurons were isolated from ARC of adult mice fed normal chow, subjected to Ca2+ imaging to determine their responsiveness to ghrelin and Ninjin'yoeito, and subsequently immunostained for NPY. In the ghrelin-responsive and ghrelin-unresponsive NPY neurons, the effects of Ninjin'yoeito on [Ca2+]i and their modulation by N-type and L-type Ca2+ channel blockers were examined.
Ninjin'yoeito is an herbal supplement composed of 12 crude drugs (Table 1). Ninjin'yoeito extract was supplied by Kracie Co. (Tokyo, Japan). Ninjin'yoeito extract was mixed with distilled water to prepare the stock solution.
For [Ca2+]i imaging, Ninjin'yoeito solution was diluted at the concentrations used for superfusion in HEPES-buffered Krebs-Ringer bicarbonate buffer (HKRB) solution composed of (in mM) 129 NaCl, 5.0 NaHCO3, 4.7 KCl, 1.2 KH2PO4, 1.8 CaCl2, 1.2 MgSO4, and 10 HEPES with pH adjusted at 7.4 using NaOH. Ghrelin was purchased from Peptide Institute (Osaka, Japan). Fresh solution of Ninjin'yoeito and ghrelin were prepared before each experiment.
Male C57BL/6J mice aged 4–6 weeks were obtained from Japan SLC (Shizuoka, Japan) and housed for at least 1 week under conditions of controlled temperature (23 ± 1°C), humidity (55 ± 5%) and lighting (light phase 7:30–19:30). Food and water were available ad libitum. Animal experiments were carried out after receiving approval from the Institutional Animal Experiment Committee and in accordance with the Institutional Regulation for Animal Experiments at Jichi Medical University (IACUC approval number; 17-229) and Kobe University (IACUC approval number; 30-10-06-R1).
The ARC was isolated from the brain of mice aged 5–7 weeks and single neurons were prepared as reported previously (25). Briefly, mice were anesthetized with intraperitoneal injection of urethane (ethyl carbamate; 1 g/kg, ip) or inhalation administration of isoflurane and decapitated, and their brain was removed. Brain slices containing ARC were prepared, and the whole ARC of the left and right sides was punched out. The dissected tissues were incubated in HKRB supplemented with 20 units/ml papain (Sigma Aldrich, St. Louis, MO), 0.015 mg/ml deoxyribonuclease, and 0.75 mg/ml BSA for 16 min at 36°C in a shaking water bath, followed by gentle mechanical trituration for 5–10 min. The cell suspension was centrifuged at 100 × g for 5 min. The pellet was resuspended in HKRB and distributed onto coverslips. The cells were kept at 30°C in moisture-saturated dishes till [Ca2+]i measurements for up to 6 h.
At 2–10 h after cell preparation, [Ca2+]i was measured by ratiometric fura-2 fluorescence imaging as previously reported (25). Briefly, following incubation with 2 μM fura-2AM (DOJINDO, Kumamoto, Japan) for 30 min at 30°C, the cells were mounted in a chamber and superfused at 1 ml/min with HKRB containing 2.5 mM glucose at 30°C. Data were taken from the single cells that were identified as neurons by the criteria reported previously (25); relatively large diameter (≥10 μm), clear and round cell bodies on phase-contrast microscopy. Ninjin'yoeito (10 μg/ml) and ghrelin (10−8 M) were administered under superfusion conditions. Fluorescence ratio (F340/F380) images were produced by Aquacosmos ver. 2.5 (Hamamatsu Photonics, Shizuoka, Japan). When [Ca2+]i increases took place within 10 min of superfusion with agents and their amplitudes were at least twice larger than fluctuations of baseline, they were considered responses. In all experiments, neurons from at least three separate preparations were analyzed.
After [Ca2+]i measurements, cells were fixed with 4% paraformaldehyde, pretreated with 3% H2O2 for 1 h, and blocked in 10% normal goat serum and in 0.1 M PBS for 1 h at room temperature. Cells were incubated overnight at 4°C with primary antiserum to NPY (DiaSorin, Stillwater, MN) diluted 1:10,000 in PBS containing 1.5% normal goat serum. After rinsing, cells were incubated with biotinylated secondary antibody raised against rabbit IgG (Vector Laboratories Inc., Burlingame, CA; diluted 400-fold) for 1 h at room temperature. After rinsing, the sections were labeled with avidin-peroxidase complex (ABC kit, Vector) for 1 h and color-developed with 3, 3'-diaminobenzidine (DAB). [Ca2+]i and immunocytochemical data were correlated to each other, based on the photographs of the single neurons subjected to [Ca2+]i measurements in the microscopic field (25).
Single neurons isolated from ARC were superfused with HKRB containing 2.5 mM glucose for [Ca2+]i imaging. After [Ca2+]i was stabilized at baseline, administration of ghrelin (10−8 M) increased [Ca2+]i in some single neurons that were subsequently shown to be immunoreactive (IR) to NPY by immunocytochemistry (Figure 1A). In contrast, administration of ghrelin failed to increase [Ca2+]i in other single neurons that were IR to NPY (Figure 1B). Among 43 ARC NPY neurons, 25 (58%) neurons responded to ghrelin and 18 (42%) neurons did not respond to ghrelin (Figure 1C). These results indicated that ghrelin-unresponsive neurons occur substantially among NPY neurons of ARC in adult mice fed normal chow.
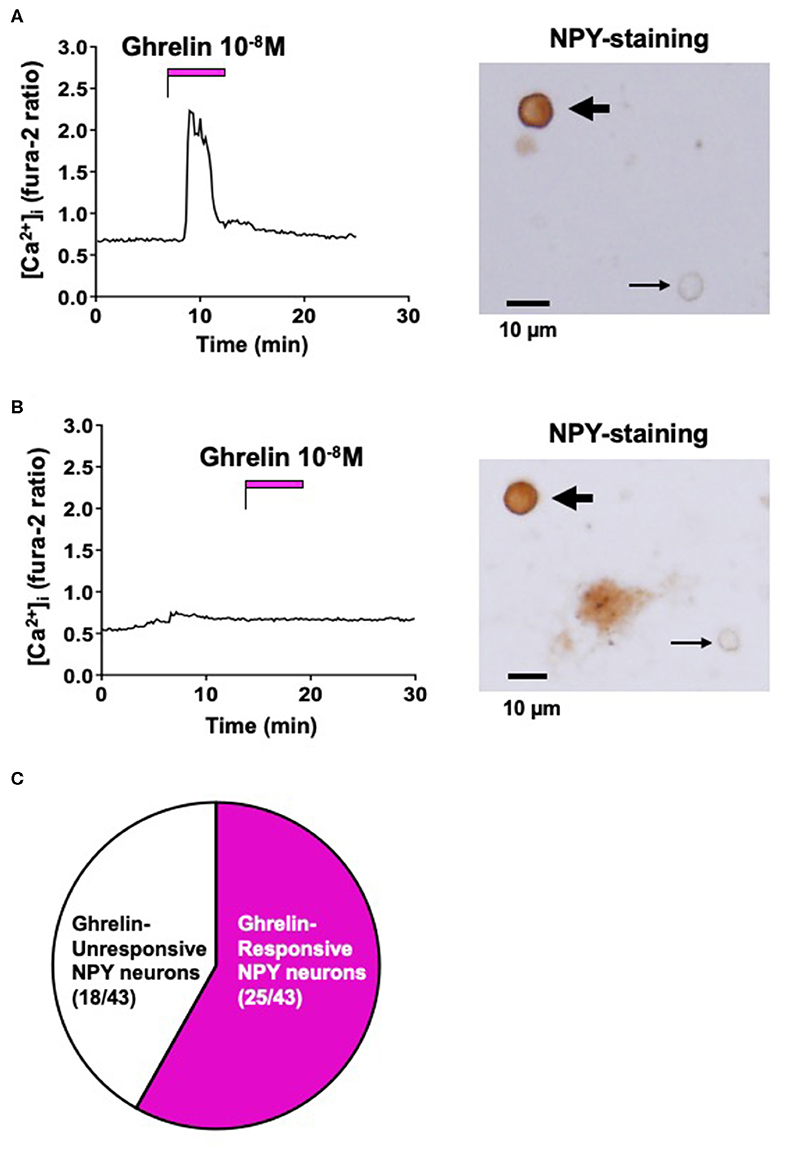
Figure 1. Effect of ghrelin on [Ca2+]i in ARC NPY neurons. (A) Ghrelin (10−8 M) increased [Ca2+]i in a single ARC neuron that was subsequently shown to be IR to NPY, indicated by the thick arrow in right. (B) Ghrelin (10−8 M) failed to increase [Ca2+]i in a single ARC neuron that was subsequently shown to be IR to NPY, indicated by the thick arrow in right. Thin arrows in right of (A,B) indicate neurons that were not IR to NPY. Scale bars in right of (A,B) show 10 μm. (C) Incidence of ghrelin-responsive and ghrelin-unresponsive ARC NPY neurons: 25 of 43 (58%) neurons responded to ghrelin and 18 of 43 (42%) neurons did not respond to ghrelin. Glucose concentration was 2.5 mM. These data were obtained from 3 preparations of single neurons from 3 mice.
The effect of Ninjin'yoeito (10 μg/ml) on [Ca2+]i in ARC NPY neurons that responded to ghrelin were examined. Under superfusion with HKRB without added Ca2+ and with 0.1 mM EGTA (Ca2+-free HKRB), administration of Ninjin'yoeito (10 μg/ml) for 10–12 min did not increase [Ca2+]i, while it subsequently increased [Ca2+]i in HKRB with 2 mM Ca2+ (normal HKRB) in a single neuron that subsequently responded to ghrelin with [Ca2+]i increase (Figure 2A). In nine ghrelin-responsive neurons, none responded to Ninjin'yoeito in Ca2+-free HKRB (Figure 2A, Right). This result indicated that the [Ca2+]i response to Ninjin'yoeito in ghrelin-responsive neurons requires the presence of extracellular Ca2+.
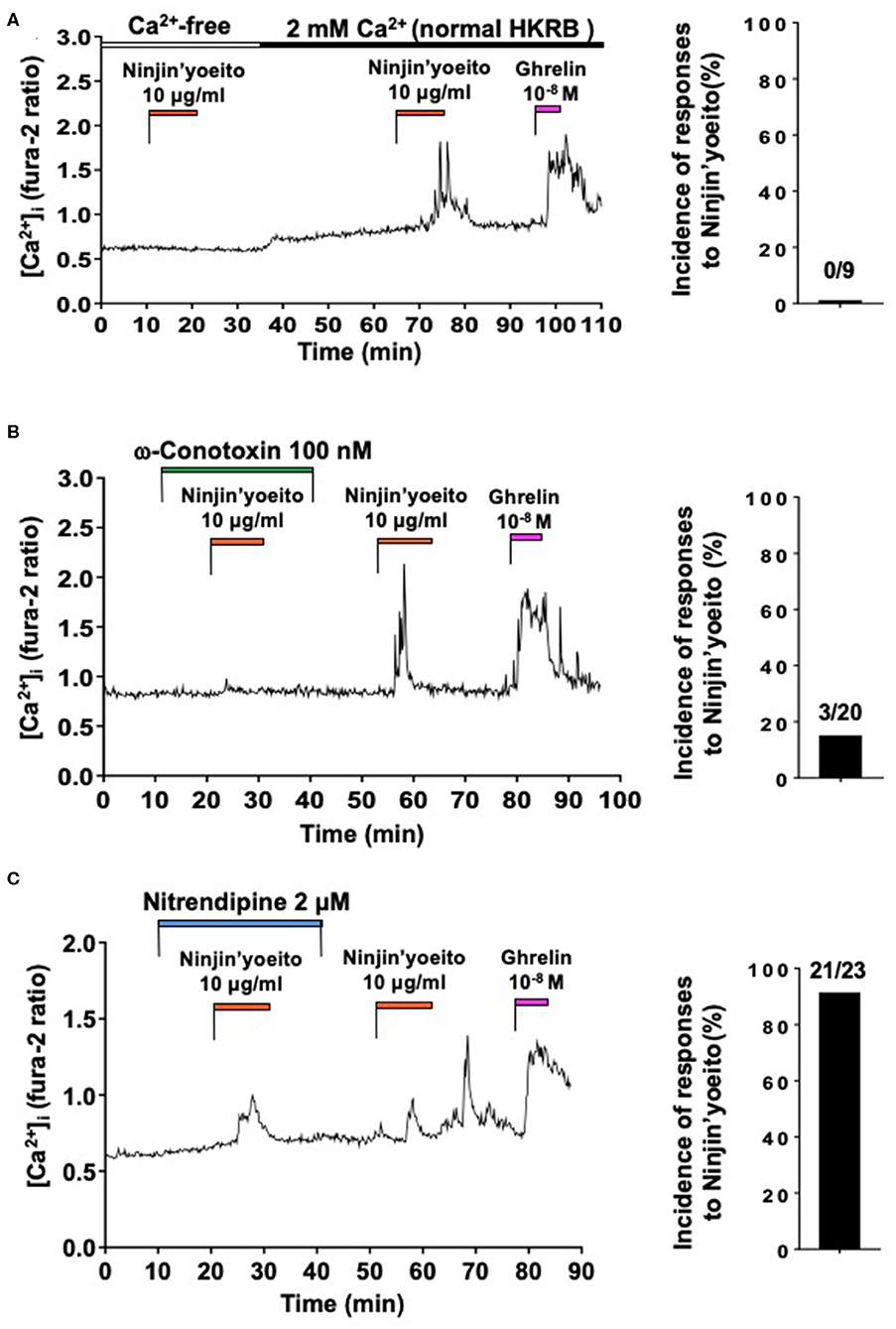
Figure 2. Ninjin'yoeito-induced [Ca2+]i increases were inhibited in Ca2+ free condition and preferentially by N-type Ca2+ channel blocker in the majority of ghrelin-responsive ARC NPY neurons. ARC NPY neurons that responded to Ninjin'yoeito (10 μg/ml) and ghrelin (10−8 M) with increases in [Ca2+]i were studied. Glucose concentration was 2.5 mM. (A) The [Ca2+]i response to Ninjin'yoeito was inhibited under superfusion with Ca2+ free HKRB. Right: incidence (%) of responses to Ninjin'yoeito in Ca2+ free HKRB. Numbers above bar indicate number of neurons responding to Ninjin'yoeito over that examined. Data were from five preparations of single neurons from four mice. (B) An N-type Ca2+ channel blocker, ω-conotoxin, inhibited the [Ca2+]i response to Ninjin'yoeito. Right: incidence of responses to Ninjin'yoeito in the presence of ω-conotoxin. Numbers above bar indicate number of neurons responding over examined. Data were from six preparations of single neurons from three mice. (C) An L-type Ca2+ channel blocker, nitrendipine, failed to inhibit the [Ca2+]i response to Ninjin'yoeito. Right: incidence of responses to Ninjin'yoeito in the presence of nitrendipine. Numbers above bar indicate number of neurons responding over examined. Data were from 9 preparations of single neurons from four mice.
We next examined whether particular type of VDCCs could be implicated in the [Ca2+]i response to Ninjin'yoeito in ghrelin-responsive neurons. The effects of N-type Ca2+ channel blocker, ω-conotoxin, and L-type Ca2+ channel blocker, nitrendipine, were examined. In the presence of ω-conotoxin (100 nM) Ninjin'yoeito did not increase [Ca2+]i, while it subsequently increased [Ca2+]i after washing out ω-conotoxin in the majority of single neurons (Figure 2B). In the presence of ω-conotoxin Ninjin'yoeito increased [Ca2+]i in only 3 of 20 (15.0%) ghrelin-responsive neurons (Figure 2B, Right). By contrast, in the presence of nitrendipine (2 μM) Ninjin'yoeito increased [Ca2+]i in the majority of single neurons (Figure 2C), and this response occurred in 21 of 23 (91.3%) of ghrelin-responsive neurons (Figure 2C, Right). These results indicated that Ninjin'yoeito increases [Ca2+]i in the majority of ghrelin-responsive neurons via Ca2+ influx to which N-type Ca2+ channel has greater contribution than L-type Ca2+ channel.
The effect of Ninjin'yoeito (10 μg/ml) on [Ca2+]i in ARC NPY neurons that did not respond to ghrelin were examined. Under superfusion with Ca2+ free KRB, Ninjin'yoeito did not increase [Ca2+]i while it subsequently increased [Ca2+]i in 2 mM Ca2+ KRB in a single neuron that subsequently failed to respond to ghrelin with [Ca2+]i increase (Figure 3A). In nine ghrelin-unresponsive neurons, none responded to Ninjin'yoeito in Ca2+-free HKRB (Figure 3A, Right). This result indicated that the [Ca2+]i response to Ninjin'yoeito in ghrelin-unresponsive neurons requires the presence of extracellular Ca2+.
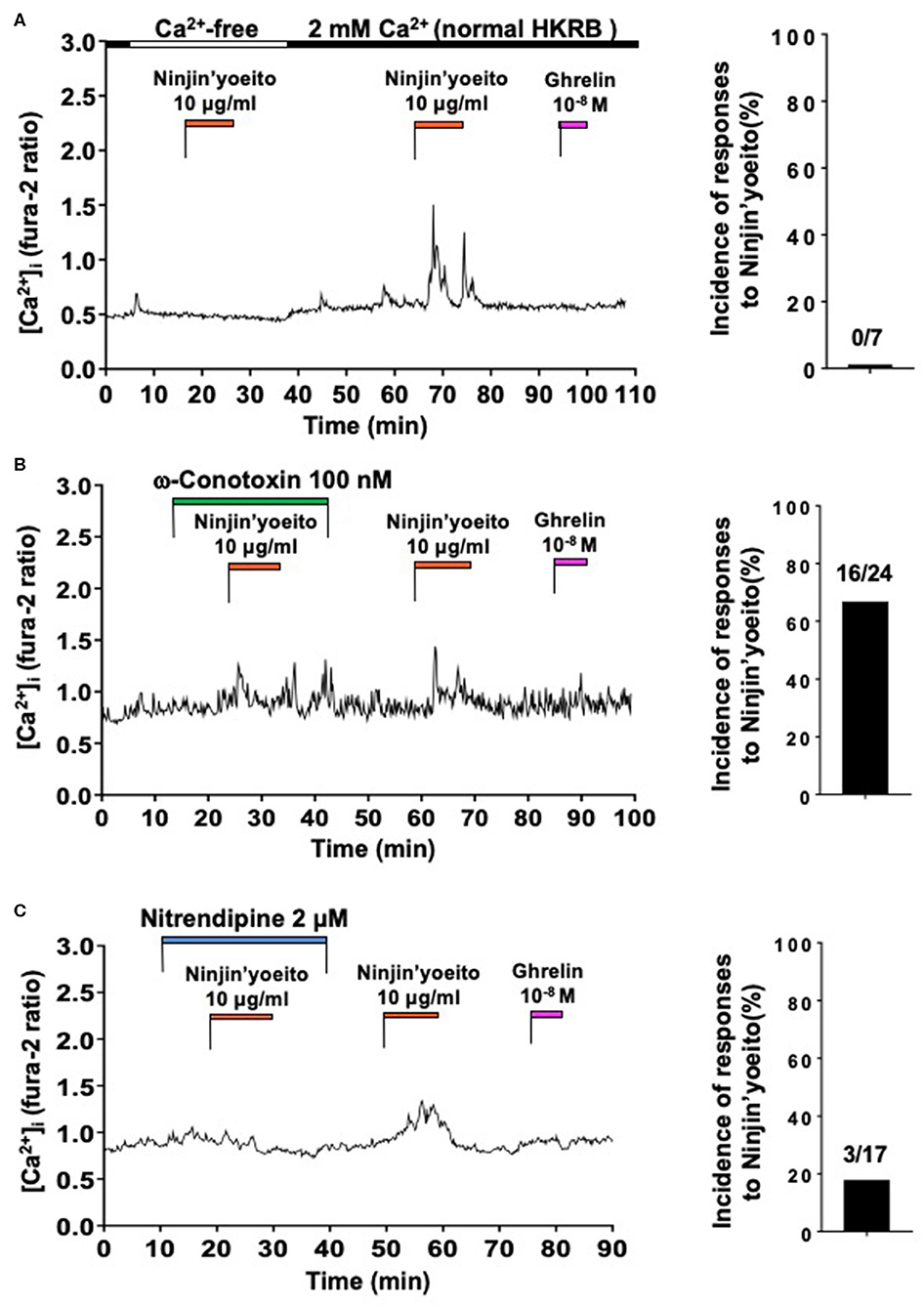
Figure 3. Ninjin'yoeito-induced [Ca2+]i increases were inhibited in Ca2+ free condition and preferentially by L-type Ca2+ channel blocker in the majority of ghrelin-unresponsive ARC NPY neurons. ARC NPY neurons that responded to Ninjin'yoeito (10 μg/ml) but not to ghrelin (10−8 M) with increases in [Ca2+]i were studied. Glucose concentration was 2.5 mM. (A) The [Ca2+]i response to Ninjin'yoeito was inhibited under superfusion with Ca2+ free HKRB. Right: incidence (%) of responses to Ninjin'yoeito in Ca2+ free HKRB. Numbers above bar indicate number of neurons responding to Ninjin'yoeito over that examined. Data were from five preparations of single neurons from four mice. (B) An N-type Ca2+ channel blocker, ω-conotoxin, failed to inhibit the [Ca2+]i response to Ninjin'yoeito. Right: incidence of responses to Ninjin'yoeito in the presence of ω-conotoxin. Numbers above bar indicate number of neurons responding over examined. Data were from six preparations of single neurons from three mice. (C) An L-type Ca2+ channel blocker, nitrendipine, inhibited the [Ca2+]i response to Ninjin'yoeito. Right: incidence of responses to Ninjin'yoeito in the presence of nitrendipine. Numbers above bar indicate number of neurons responding over examined. Data were from nine preparations of single neurons from four mice.
We examined involvement of particular type of VDCC in the [Ca2+]i response to Ninjin'yoeito in ghrelin-unresponsive neurons. Ninjin'yoeito induced increases in [Ca2+]i both in the presence of and after washing out ω-conotoxin (100 nM) in the majority of single neurons (Figure 3B). The pattern and amplitude of [Ca2+]i increases in response to Ninjin'yoeito in the presence and absence of ω-conotoxin were comparable (Figure 3B). In the presence of ω-conotoxin, Ninjin'yoeito increased [Ca2+]i in 16 of 24 ghrelin-unresponsive neurons (66.7%) (Figure 3B, Right). By contrast, in the presence of nitrendipine (2 μM) Ninjin'yoeito failed to increase [Ca2+]i in the majority of single neurons (Figure 3C), while it subsequently increased [Ca2+]i after washing out this drug, showing a reversible inhibition. In the presence of nitrendipine Ninjin'yoeito increased [Ca2+]i in only 3 of 17 ghrelin-unresponsive neurons (17.6%) (Figure 3C, Right). These results indicated that Ninjin'yoeito increases [Ca2+]i in ghrelin-unresponsive neurons via Ca2+ influx to which L-type Ca2+ channel has greater contribution than N-type Ca2+ channel.
The present study employed [Ca2+]i measurement in ARC single neurons combined with immunocytochemistry and found that among 43 ARC NPY neurons, 25 (58%) neurons responded to ghrelin and 18 (42%) neurons did not respond to ghrelin. This incidence of ghrelin-responsive neurons (58%) is not far from that in previous reports, considering different experimental conditions used among studies. The extracellular single unit recordings from in vitro slices indicated that ghrelin excited 73% of neurons in the ventromedial ARC, where NPY neurons are dominant, in adult rats (27). Ex vivo whole-cell patch-clamp recordings showed that ghrelin depolarized 40% of GFP-labeled arcuate NPY neurons in brain slices from 8–12 week-old male NPY-humanized Renilla reniformis green fluorescent protein transgenic mice (28). Ghrelin increased [Ca2+]i in 59% of single ARC NPY neurons (9) and in 21–41% of single ARC neurons (12, 25) in 5–7 week-old male mice. The present study demonstrated that ghrelin-unresponsive neurons occur substantially among NPY neurons of ARC in 5–7 week-old male mice fed normal chow. Ninjin'yoeito-induced [Ca2+]i increase was blunted in Ca2+-free condition in both ghrelin-responsive and ghrelin-unresponsive neurons. Ninjin'yoeito-induced [Ca2+]i increases were inhibited by N-type Ca2+ channel blocker ω-conotoxin in the majority (17 of 20), while by L-type Ca2+ channel blocker nitrendipine in the minority (2 of 23), of ghrelin-responsive neurons. In contrast, Ninjin'yoeito-induced [Ca2+]i increases were inhibited by nitrendipine in the majority (14 of 17), while by ω-conotoxin in the minority (8 of 24), of ghrelin-unresponsive neurons. Both N- and L-type Ca2+ channels are reportedly expressed and functioning in ARC (24, 25, 29). Our results demonstrate that Ninjin'yoeito increases [Ca2+]i via Ca2+ influx, to which N-type Ca2+ channels have greater contribution in ghrelin-responsive neurons and L-type Ca2+ channels have greater contribution in ghrelin-unresponsive neurons. The [Ca2+]i increase often results from membrane excitation and/or stimulated signal transduction and results in exocytosis, transport, gene expression, and/or protein regulation. Thus, the [Ca2+]i increase, in general, reflects the neuronal activation. The present findings place N-type and L-type Ca2+ channels in ARC as potential molecular targets for Ninjin'yoeito to preferentially activate ghrelin-responsive and ghrelin-unresponsive NPY neurons, respectively, in ARC.
The mechanisms underlying the link of Ninjin'yoeito to distinct VDCCs in ghrelin-responsive and ghrelin-unresponsive neurons remain to be elucidated. It has been documented that ghrelin and/or GHSR influence the activities of VDCCs (30–36). Since ghrelin increases [Ca2+]i via Ca2+ influx primarily through N-type Ca2+ channels in NPY neurons (25), it is speculated that Ninjin'yoeito could interact with the GHSR and/or downstream signaling linked to N-type Ca2+ channels in ghrelin-responsive NPY neurons. In consistent with this, it was previously reported that GHSR coexpression with dopamine type 2 receptor (D2R) reduces the inhibition of N-type VDCC currents by D2R activation (35). However, different results were also reported that N-type VDCC currents is inhibited by constitutive GHSR activity in mouse hippocampal cultures (34) and hypothalamic neurons (33). Though the cause for the apparent discrepancy among previous documents and our finding remains unknown, we investigated the effect of short-term (~5 min) administration of ghrelin in the current and previous studies (25), while some of previous documents observed the effect of constitutive GHSR activation (33, 34). Hence, ghrelin and GHSR signaling may have dual, acute stimulatory and chronic inhibitory, actions on N-type Ca2+ channel activity. Compared to ghrelin-responsive NPY neurons, properties of ghrelin-unresponsive ARC NPY neurons are less characterized. However, it was reported that a ligand for the taste receptor T1R2/T1R3 increased [Ca2+]i in ARC neurons, the majority of which did not respond to ghrelin (26), and that this [Ca2+]i increase was inhibited by nitrendipine, but not ω-conotoxin. Hence, T1R2/T1R3 is possibly linked to L-type Ca2+ channels in ghrelin-unresponsive ARC neurons and this pathway could be involved in the action of Ninjin'yoeito.
Though the Ninjin'yoeito's cellular signaling is less defined, it could interact with the excitatory signaling pathways in NPY neurons, which include the orexin—OX1R—phospholipase C pathway and low glucose—Na+,K+-ATPase suppression—depolarization pathway (37, 38). Notably, it was shown that AMPK activator AICAR increased [Ca2+]i in two types of ARC NPY neurons, one with and the other without [Ca2+]i responses to ghrelin (39), and that the AICAR-induced [Ca2+]i increases were blunted in Ca2+-free conditions (40). Thus, Ninjin'yoeito and AICAR share the common properties: they stimulate Ca2+ influx in both ghrelin-responsive and unresponsive NPY neurons in ARC. In line with this, expression of carnitine palmitoyltransferase 1 (CPT1), a signaling molecule of AMPK, is regulated by metabolic conditions (41). Taken together, Ninjin'yoeito may elicit intracellular AMPK signaling pathway for activating ghrelin-responsive and ghrelin-unresponsive NPY neurons. However, further studies are definitely required to elucidate intracellular signaling mechanisms of Ninjin'yoeito.
The functional role of the Ninjin'yoeito-regulated VDCCs in ARC NPY neurons remains to be clarified. In ghrelin-responsive neurons, administration of Ninjin'yoeito interacts with N-type Ca2+ channels to enhance and/or cooperate with the action of ghrelin, possibly leading to efficacious activation of ghrelin-responsive NPY neurons and consequent stimulation of appetite. Our study places ARC N-type Ca2+ channel as a potential mediator and integrator of the actions of Ninjin'yoeito and ghrelin in ghrelin-responsive NPY neurons. On the other hand, the ghrelin-unresponsive ARC NPY neuron has been less defined for its physiological property and cellular lineage/differentiation. It has been documented that metabolic/feeding conditions induce dynamic remodeling of NPY/AgRP neurons and differentiation of feeding related neurons in ARC (42). Hence, the ghrelin-responsive neurons and ghrelin-unresponsive neurons could be converted to each other, depending on metabolic/feeding conditions and aging. Our finding that the type of VDCC correlates with ghrelin responsiveness in NPY neurons suggests that expression of specific VDCC type may be related to remodeling of NPY neurons. In this line, the ghrelin resistance, the phenomenon that ghrelin administration cannot stimulate feeding, occurs in association with aging and diet-induced obesity (21–23). This ghrelin resistance reportedly takes place in ARC NPY neurons (21), which could result in reductions in NPY neuronal activity and appetite. The transformation of ghrelin-responsive to ghrelin-unresponsive NPY neurons may underly ghrelin resistance. Of note, we found that Ninjin'yoeito preferentially target L-type VDCC to activate ghrelin-unresponsive NPY neurons in ARC. This action of Ninjin'yoeito could serve to compensate for the ghrelin resistance and restore appetite.
The present study demonstrated that Ninjin'yoeito activates the majority of ghrelin-responsive ARC NPY neurons preferentially via N-type VDCC while the majority of ghrelin-unresponsive NPY neurons preferentially via L-type VDCCs. We suggest ARC N-type VDCC as a target for activating ghrelin-responsive NPY neurons and promoting feeding while L-type VDCC as a target for activating ghrelin-unresponsive NPY neurons and possibly compensating for ghrelin-resistance to restore appetite.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Animal experiments were carried out after receiving approval from the Institutional Animal Experiment Committee and in accordance with the Institutional Regulation for Animal Experiments at Jichi Medical University and Kobe University.
TY designed the study. CG, KD, and LW conducted experiments. AI and YS participated in discussion. TY and CG wrote the manuscript. TY supervised the work. All authors contributed to the article and approved the submitted version.
This work was supported by Grant-in-Aid for Scientific Research (B) (19H04045) and Challenging Exploratory Research (19K22611) from Japan Society for the Promotion of Science (JSPS) to TY. TY was supported by the Advanced Research and Development Programs for Medical Innovation (AMED-CREST) 2015-2020 from Japan Agency for Medical Research and development (AMED).
TY received grant support from Kracie Pharma Ltd. provided Ninjin-yoeito but was not involved in the conducting of the current study at any stage.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Ryuji Takahashi and Nina Fujita for valuable discussion, Ms. Maya Ikeda for secretarial assistance, and Drs. Wanxin Han and Yanan Zhao for technical support.
VDCC, voltage-dependent Ca2+ channel; NPY, Neuropeptide Y; ARC, arcuate nucleus.
1. Raghavendran HR, Rekha S, Shin JW, Kim HG, Wang JH, Park HJ, et al. Effects of Korean ginseng root extract on cisplatin-induced emesis in a rat-pica model. Food Chem Toxicol. (2011) 49:215–21. doi: 10.1016/j.fct.2010.10.019
2. Morley JE. Pathophysiology of the anorexia of aging. Curr Opin Clin Nutr Metab Care. (2013) 16:27–32. doi: 10.1097/MCO.0b013e328359efd7
3. Hiura Y, Takiguchi S, Yamamoto K, Takahashi T, Kurokawa Y, Yamasaki M, et al. Effects of ghrelin administration during chemotherapy with advanced esophageal cancer patients: a prospective, randomized, placebo-controlled phase 2 study. Cancer. (2012) 118:4785–94. doi: 10.1002/cncr.27430
4. Landi F, Calvani R, Tosato M, Martone AM, Ortolani E, Savera G, et al. Anorexia of aging: risk factors, consequences, and potential treatments. Nutrients. (2016) 8:69. doi: 10.3390/nu8020069
5. Yamasaki A, Sugahara K, Takemoto T, Ikeda T, Yamashita H. Effect of ninjin-yoei-to (Rensheng-Yangrong-Tang) on olfactory behavior after olfactory nerve transection. Phytomedicine. (2008) 15:358–66. doi: 10.1016/j.phymed.2007.08.006
6. Suzuki T, Yamamoto A, Ohsawa M, Motoo Y, Mizukami H, Makino T. Ninjin'yoeito and ginseng extract prevent oxaliplatin-induced neurodegeneration in PC12 cells. J Nat Med. (2015) 69:531–7. doi: 10.1007/s11418-015-0921-9
7. Takaku S, Shimizu M, Takahashi H. Japanese kampo medicine ninjin'yoeito synergistically enhances tumor vaccine effects mediated by CD8(+) T cells. Oncol Lett. (2017) 13:3471–8. doi: 10.3892/ol.2017.5937
8. Perello M, Cabral A, Cornejo MP, De Francesco PN, Fernandez G, Uriarte M. Brain accessibility delineates the central effects of circulating ghrelin. J Neuroendocrinol. (2019) 31:e12677. doi: 10.1111/jne.12677
9. Goswami C, Dezaki K, Wang L, Inui A, Seino Y, Yada T. Ninjin-yoeito activates ghrelin-responsive and unresponsive NPY neurons in the arcuate nucleus and counteracts cisplatin-induced anorexia. Neuropeptides. (2019) 75:58–64. doi: 10.1016/j.npep.2019.03.001
10. Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. (2000) 404:661–71. doi: 10.1038/35007534
11. Zhang L, Bijker MS, Herzog H. The neuropeptide Y system: pathophysiological and therapeutic implications in obesity and cancer. Pharmacol Ther. (2011) 131:91–113. doi: 10.1016/j.pharmthera.2011.03.011
12. Kohno D, Yada T. Arcuate NPY neurons sense and integrate peripheral metabolic signals to control feeding. Neuropeptides. (2012) 46:315–9. doi: 10.1016/j.npep.2012.09.004
13. Yada T, Kohno D, Maejima Y, Sedbazar U, Arai T, Toriya M, et al. Neurohormones, rikkunshito and hypothalamic neurons interactively control appetite and anorexia. Curr Pharm Des. (2012) 18:4854–64. doi: 10.2174/138161212803216898
14. Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. (2011) 14:351–5. doi: 10.1038/nn.2739
15. Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. (2011) 121:1424–8. doi: 10.1172/JCI46229
16. Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci. (2005) 8:1289–91. doi: 10.1038/nn1548
17. Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. (2005) 310:683–5. doi: 10.1126/science.1115524
18. Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, et al. A role for ghrelin in the central regulation of feeding. Nature. (2001) 409:194–8. doi: 10.1038/35051587
19. Kohno D, Nakata M, Maekawa F, Fujiwara K, Maejima Y, Kuramochi M, et al. Leptin suppresses ghrelin-induced activation of neuropeptide Y neurons in the arcuate nucleus via phosphatidylinositol 3-kinase- and phosphodiesterase 3-mediated pathway. Endocrinology. (2007) 148:2251–63. doi: 10.1210/en.2006-1240
20. Zhang L, Yagi M, Herzog H. The role of NPY and ghrelin in anorexia nervosa. Curr Pharm Des. (2012) 18:4766–78. doi: 10.2174/138161212803216988
21. Briggs DI, Enriori PJ, Lemus MB, Cowley MA, Andrews ZB. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology. (2010) 151:4745–55. doi: 10.1210/en.2010-0556
22. Miyazaki Y, Kaneko K, Iguchi S, Mizushige T, Kanamoto R, Yoshikawa M, et al. Orally administered delta opioid agonist peptide rubiscolin-6 stimulates food intake in aged mice with ghrelin resistance. Mol Nutr Food Res. (2014) 58:2046–52. doi: 10.1002/mnfr.201400100
23. Amitani M, Amitani H, Cheng KC, Kairupan TS, Sameshima N, Shimoshikiryo I, et al. The role of ghrelin and ghrelin signaling in aging. Int J Mol Sci. (2017) 18:1511. doi: 10.3390/ijms18071511
24. Sahu A, Crowley WR, Kalra SP, Kalra PS. Role of multiple voltage-sensitive calcium channels in depolarization-induced release of neuropeptide y and luteinizing hormone-releasing hormone from rat median eminence-arcuate nucleus. Mol Cell Neurosci. (1993) 4:492–8. doi: 10.1006/mcne.1993.1061
25. Kohno D, Gao HZ, Muroya S, Kikuyama S, Yada T. Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca2+ signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes. (2003) 52:948–56. doi: 10.2337/diabetes.52.4.948
26. Kohno D, Koike M, Ninomiya Y, Kojima I, Kitamura T, Yada T. Sweet taste receptor serves to activate glucose- and leptin-responsive neurons in the hypothalamic arcuate nucleus and participates in glucose responsiveness. Front Neurosci. (2016) 10:502. doi: 10.3389/fnins.2016.00502
27. Riediger T, Traebert M, Schmid HA, Scheel C, Lutz TA, Scharrer E. Site-specific effects of ghrelin on the neuronal activity in the hypothalamic arcuate nucleus. Neurosci Lett. (2003) 341:151–5. doi: 10.1016/S0304-3940(02)01381-2
28. Torz LJ, Osborne-Lawrence S, Rodriguez J, He Z, Cornejo MP, Mustafá ER, et al. Metabolic insights from a GHSR-A203E mutant mouse model. Mol Metab. (2020) 39:101004. doi: 10.1016/j.molmet.2020.101004
29. Ishii M, Hiller AJ, Pham L, McGuire MJ, Iadecola C, Wang G. Amyloid-beta modulates low-threshold activated voltage-gated L-type calcium channels of arcuate neuropeptide Y neurons leading to calcium dysregulation and hypothalamic dysfunction. J Neurosci. (2019) 39:8816–25. doi: 10.1523/JNEUROSCI.0617-19.2019
30. Falls HD, Dayton BD, Fry DG, Ogiela CA, Schaefer VG, Brodjian S, et al. Characterization of ghrelin receptor activity in a rat pituitary cell line RC-4B/C. J Mol Endocrinol. (2006) 37:51–62. doi: 10.1677/jme.1.01943
31. Erriquez J, Bernascone S, Ciarletta M, Filigheddu N, Graziani A, Distasi C. Calcium signals activated by ghrelin and D-Lys(3)-GHRP-6 ghrelin antagonist in developing dorsal root ganglion glial cells. Cell Calcium. (2009) 46:197–208. doi: 10.1016/j.ceca.2009.07.003
32. Hashiguchi H, Sheng Z, Routh V, Gerzanich V, Simard JM, Bryan J. Direct versus indirect actions of ghrelin on hypothalamic NPY neurons. PLoS ONE. (2017) 12:e0184261. doi: 10.1371/journal.pone.0184261
33. Mustafá ER, López Soto EJ, Martínez Damonte V, Rodríguez SS, Lipscombe D, Raingo J. Constitutive activity of the ghrelin receptor reduces surface expression of voltage-gated Ca2+ channels in a CaVβ-dependent manner. J Cell Sci. (2017) 130:3907–17. doi: 10.1242/jcs.207886
34. Martínez Damonte V, Rodríguez SS, Raingo J. Growth hormone secretagogue receptor constitutive activity impairs voltage-gated calcium channel-dependent inhibitory neurotransmission in hippocampal neurons. J Physiol. (2018) 596:5415–28. doi: 10.1113/JP276256
35. Cordisco Gonzalez S, Mustafá ER, Rodriguez SS, Perello M, Raingo J. Dopamine receptor type 2 and ghrelin receptor coexpression alters CaV2.2 modulation by G protein signaling cascades. ACS Chem Neurosci. (2020) 11:3–13. doi: 10.1021/acschemneuro.9b00426
36. Mustafá ER, Cordisco Gonzalez S, Raingo J. Ghrelin selectively inhibits CaV3.3 subtype of low-voltage-gated calcium channels. Mol Neurobiol. (2020) 57:722–35. doi: 10.1007/s12035-019-01738-y
37. Muroya S, Funahashi H, Yamanaka A, Kohno D, Uramura K, Nambu T, et al. Orexins (hypocretins) directly interact with neuropeptide Y, POMC and glucose-responsive neurons to regulate Ca2+ signaling in a reciprocal manner to leptin: orexigenic neuronal pathways in the mediobasal hypothalamus. Eur J Neurosci. (2004) 19:1524–34. doi: 10.1111/j.1460-9568.2004.03255.x
38. Kurita H, Xu KY, Maejima Y, Nakata M, Dezaki K, Santoso P, et al. Arcuate Na+,K+-ATPase senses systemic energy states and regulates feeding behavior through glucose-inhibited neurons. Am J Physiol Endocrinol Metab. (2015) 309:E320–33. doi: 10.1152/ajpendo.00446.2014
39. Kohno D, Sone H, Minokoshi Y, Yada T. Ghrelin raises [Ca2+]i via AMPK in hypothalamic arcuate nucleus NPY neurons. Biochem Biophys Res Commun. (2008) 366:388–92. doi: 10.1016/j.bbrc.2007.11.166
40. Kohno D, Sone H, Tanaka S, Kurita H, Gantulga D, Yada T. AMP-activated protein kinase activates neuropeptide Y neurons in the hypothalamic arcuate nucleus to increase food intake in rats. Neurosci Lett. (2011) 499:194–8. doi: 10.1016/j.neulet.2011.05.060
41. Yasrebi A, Hsieh A, Mamounis KJ, Krumm EA, Yang JA, Magby J, et al. Differential gene regulation of GHSR signaling pathway in the arcuate nucleus and NPY neurons by fasting, diet-induced obesity, and 17beta-estradiol. Mol Cell Endocrinol. (2016) 422:42–56. doi: 10.1016/j.mce.2015.11.007
42. Cabral A, Fernandez G, Tolosa MJ, Rey Moggia A, Calfa G, De Francesco PN, et al. Fasting induces remodeling of the orexigenic projections from the arcuate nucleus to the hypothalamic paraventricular nucleus, in a growth hormone secretagogue receptor-dependent manner. Mol Metab. (2020) 32:69–84. doi: 10.1016/j.molmet.2019.11.014
Keywords: Ninjin-yoeito, anorexia, arcuate nucleus, neuropeptide Y, ghrelin, N-type Ca2+ channel, L-type Ca2+ channel
Citation: Goswami C, Dezaki K, Wang L, Inui A, Seino Y and Yada T (2020) Ninjin'yoeito Targets Distinct Ca2+ Channels to Activate Ghrelin-Responsive vs. Unresponsive NPY Neurons in the Arcuate Nucleus. Front. Nutr. 7:104. doi: 10.3389/fnut.2020.00104
Received: 02 April 2020; Accepted: 08 June 2020;
Published: 17 July 2020.
Edited by:
Sergueï O. Fetissov, Université de Rouen, FranceReviewed by:
Mario Perello, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), ArgentinaCopyright © 2020 Goswami, Dezaki, Wang, Inui, Seino and Yada. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Toshihiko Yada, dG9zaGloaWtvLnlhZGFAa2VwbXJpLm9yZw==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.