- 1Geisinger Commonwealth School of Medicine, Scranton, PA, United States
- 2Neuroscience Institute, Geisinger Health System, Danville, PA, United States
- 3Clinical Nutrition, Geisinger Health System, Danville, PA, United States
- 4NIMML Institute, Blacksburg, VA, United States
- 5Department of Molecular and Functional Genomics, Geisinger Health System, Danville, PA, United States
Nutrition plays a vital role in health and the recovery process. Deficiencies in macronutrients and micronutrients can impact the development and progression of various disorders. However, malnutrition screening tools and their utility in the clinical setting remain largely understudied. In this study, we summarize the importance of nutritional adequacy and its association with neurological, cardiovascular, and immune-related disorders. We also examine general and specific malnutrition assessment tools utilized in healthcare settings. Since the implementation of the screening process in 2016, malnutrition data from hospitalized patients in the Geisinger Health System is presented and discussed as a case study. Clinical data from five Geisinger hospitals shows that ~10% of all admitted patients are acknowledged for having some form of nutritional deficiency, from which about 60–80% of the patients are targeted for a more comprehensive assessment. Finally, we conclude that with a reflection on how technological advances, specifically machine learning-based algorithms, can be integrated into electronic health records to provide decision support system to care providers in the identification and management of patients at higher risk of malnutrition.
Introduction
Malnutrition and Health
Nutrition, a vital component of proper growth and development, plays an integral role in preventing disease, maintaining health, and facilitating faster recovery. Undernutrition remains a major public health concern for people of all ages. Nutritional deficiencies of essential macronutrients, including proteins, fats, and carbohydrates, in addition to micronutrients, including vitamins and minerals (as set by the US Department of Agriculture) (1), result in various physical and psychological impairments. Current studies show that malnutrition is diagnosed in ~3–5% of hospitalized patients, but estimates indicate that the rates may be as high as 30–60% (2–5). Improvements in hospital malnutrition recognition strategies as well as collaboration between nutritionists and health care providers, could lead to better identification of patients with malnutrition. The development of targeted programs could reduce disease burden, while at the same time, improve recovery following an event, such as major surgery. One such example is the Geisinger Health System's Proven Recovery program that improves patient recovery following surgical procedures. This innovative initiative, which focuses on proper nutrition, pain management, and early mobility has seen an estimated 18% decrease of opioid use in cardiac, bariatric, spine, and joint surgical care, decrease in hospital stays by up to 50%, and an average saving of $4,556 per case for colorectal surgery patients (6). Geisinger Health System has also enhanced its approach to medicine by establishing the Fresh Food Farmacy. The Fresh Food Farmacy initiative recognizes and addresses food insecurity in local communities and promotes health through providing public education on diabetes, consultations with pharmacists and dietitians, and distribution of two free meals to families for five days (7). The Fresh Food Farmacy combats malnutrition and metabolic disorders by encouraging community members to adopt healthy behaviors and educating community members about the importance of nutrition and wellness (7).
In this work, we outline the deleterious effects of deficiencies of both macronutrients and micronutrients on the regression and recovery of neurological, cardiovascular, and immunological disorders. We summarize the assessment tools for nutrition in clinical settings. We also demonstrate how Geisinger, a large and integrated healthcare system with over one million active patients, has implemented a unified pipeline targeting malnutrition to improve care and outcome. Finally, we discuss how leveraging modeling and machine learning can help identify the most vulnerable population to better tailor care while optimizing resource utilization.
Malnutrition and Neurological Disorders
The central nervous system and peripheral nervous system require sufficient nutritional resources to function optimally as major control centers of the body. Compensatory mechanisms during disease onset and progression rely heavily on macromolecules, coenzymes, and cofactors. Therefore, balanced nutrients are essential for appropriate recovery responses and disease regression. It is estimated that nearly 20% of patients with cerebrovascular accidents suffer from malnutrition (8). Malnutrition in stroke patients is directly associated with increased length of hospitalization and decreased rehabilitation improvements (9, 10). Mechanistically, these dietary protein-deficient patients exhibit altered genomic expression, leading to decreased hippocampal fiber plasticity and poor recovery (11). Studies on protein malnutrition have demonstrated increased hippocampal expression of trkB & GAP-43 protein, indicating increased stress response (11). In addition to macronutrients, deficiencies in micronutrients, including vitamins, are implicated in a variety of disorders and delayed recovery mechanisms (12). Vitamin B12 which is essential in the conversion of homocysteine to methionine via methionine synthase for DNA and RNA synthesis (13), produces a large number of biological agents, including the myelin sheath. It has been shown that deficiencies in Vitamin B12 are associated with higher latencies in visual evoked potentials (VEP) in patients with multiple sclerosis (14). Additionally, deficiencies of Vitamin B1, which is an essential cofactor in many cellular enzymes involved in glucose metabolism, results in energy inadequacy, and presents with similar symptomatology as Alzheimer (15). Identifying patients at higher risk of malnutrition will help design care path that is most tailored to the individual needs. This encompasses both dietary considerations with medications, increasing the chances of successful recovery and reducing the likelihood of certain neurological conditions while improving the health and well-being of patients.
Malnutrition and Cardiovascular Disorders
Adequate intake of nutrition is important for blood vessel integrity, proper cardiac function, and sufficient myocardial mass (16). Vitamin B12 deficiency can lead to a fatal accumulation of unmetabolized homocysteine substrates, resulting in detrimental peripheral vasculature effects (17). These effects include loss of peripheral endothelium integrity, continuous vasoconstriction, and chronic inflammation (17, 18). In addition to these peripheral effects, micronutrient deficiency is also implicated in abnormal heart function and heart disease (19). Vitamin D deficiency has been shown to result in arterial stiffening, left ventricular hypertrophy, and hyperlipidemia (19).
Nutritional macromolecules can impact the electrophysiological properties of the heart (for example, calcium and sodium, which assist in the regulation of blood pressure and contractile properties of cardiac tissue, respectively), leading to the development of atrial fibrillation. Paradoxically, atrial fibrillation is associated with both underweight and overweight patients (20). In the case of malnutrition, the endotoxic neutralization properties of cholesterol and lipoproteins are reduced (21, 22). Additionally, loss of muscle mass is associated with increased levels of TNF-alpha, seen in atrial fibrillation (23). In the case of overnutrition, the pericardial adipose cells secrete adipokines, which alter the arrhythmogenic properties of the heart and decreases release of atrial natriuretic peptides (24, 25). (Figure 1) provides an overview of the link between specific nutritional deficiency and its cardiovascular outcome.
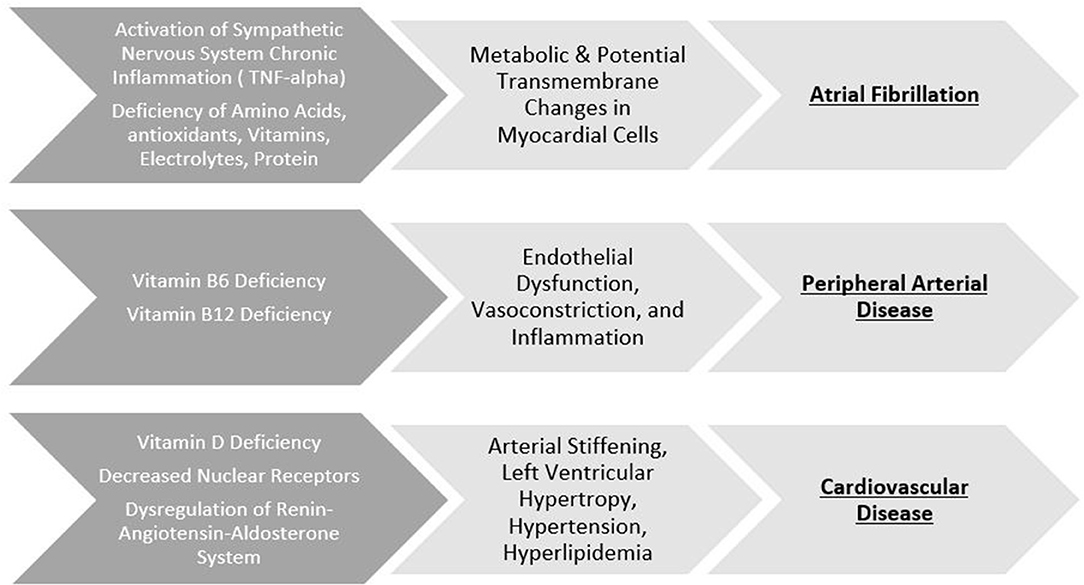
Figure 1. Specific nutritional deficiency and its cardiovascular outcome. Top row: Macronutrient and micronutrient deficiency induce various pathological changes to electrical properties leading to the development of atrial fibrillation. Middle row: B-vitamin deficiency disrupt the integrity of endothelial properties leading to the development of peripheral artery disease. Bottom row: Deficiencies of Vitamin D can result in pathological changes to the heart leading to cardiovascular disease.
Malnutrition and the Immune- Mediated Disorders
Malnutrition is associated with immune dysfunction, characterized by chronic inflammation and infections (26). Immunological responses, including activation of the immune system and secretion of inflammatory mediators, are heightened during malnutrition and can result in derangements of metabolic processes and hormone regulation (27–29). Malnutrition, in the context of inflammatory bowel disease, is bidirectional in nature. In patients with inflammatory bowel disease, nutritional deficiencies impact the cytokine profiles, which contribute to the loss of epithelial integrity and impairment in the ion transport systems (30). In turn, these changes during malnutrition and inflammation lead to exacerbation of malnutrition (30). Similarly, patients afflicted with chronic pulmonary obstructive disorder exhibit inflammatory mediators and hormones that play a role in enhanced energy utilization and decreased physical activity (31).
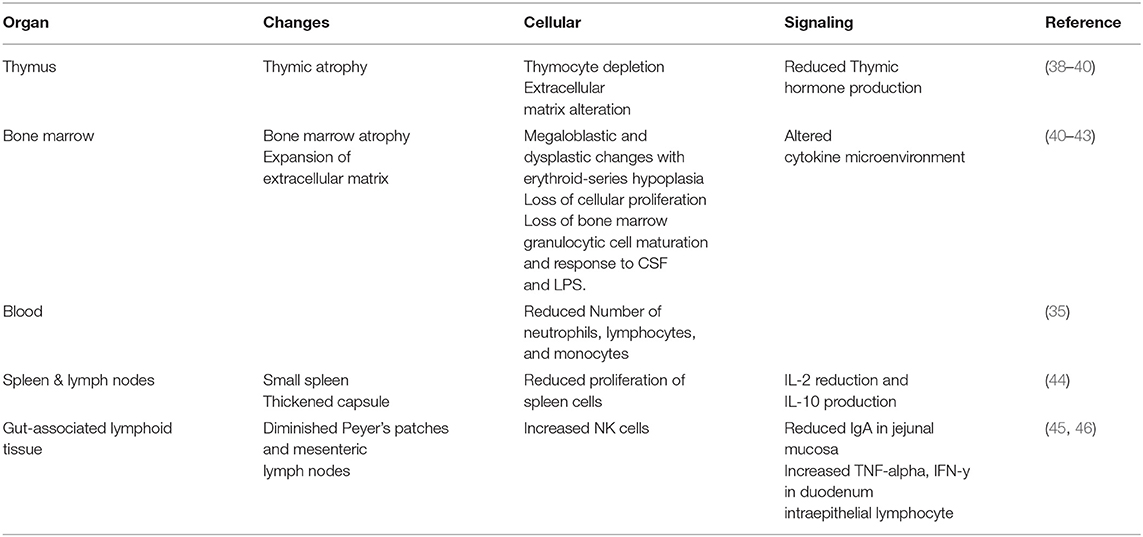
Patients with nutritional deficiencies have depressed immune systems and are at a higher risk for infections. Specifically, malnutrition impacts the function of hematopoietic and lymphoid organs, including bone marrow, thymus, and spleen (32). When there is a lack of protein and zinc sufficiency, the thymus can undergo atrophy and result in the death of CD4 and CD8 T cells (33, 34). Further, changes in cytokine profiles contributes to immunological alterations and immune system dysfunction. Adipose-derived from bone marrow (35) and dying spleen cells (36) secrete mediators that can alter appropriate immune responses and immune organ function. Blood collected from children with malnutrition showed decreased number of dendritic cells (37). Refer to (Table 1) for a complete list of immunological impact from malnutrition.
Malnutrition Assessment Tools and Models in Clinical Settings
Malnutrition assessment tools are utilized in clinical settings to identify specific nutritional deficiencies in patients. Assessment tools enable clinicians to gain a better understanding of the patient's condition and can promote targeted treatment strategies. American Society for Parenteral & Enteral Nutrition (ASPEN) is an example of a general approach to nutrition assessment. This toolbox considers family and medical history in addition to clinical diagnosis to determine the presence of inflammation and malnutrition. Clinical signs of fever, hypothermia, tachycardia, edema and weight gain/loss are used to recognize malnutrition. The ASPEN guidelines emphasize laboratory parameters consisting of serum albumin, C-reactive protein, white cell count, negative nitrogen balance for an objective confirmation. Further, strength tests, including handgrip dynamometer and physical performances (including timed gait, chair stands, and stair tests) are employed for assessments in the elderly (47). In addition to ASPEN, Short Nutritional Assessment Questionnaire (SNAQ) and Nutrition Risk Screening (NRS) are quick and general tools for early detection of malnutrition patients (48, 49). These tools consider biometrics, including weight, body-mass-index, food intake, and age (48, 49).
As certain patient populations require a more tailored approach to malnutrition assessments, there are different toolboxes geared toward these specific patients. For example, Mini-Nutritional Assessment Short Form (MNA-SF) examines the cognitive problems of dementia and depression in the elderly (50, 51). Patients in the intensive care unit require different nutritional assessments, which considers the severity of their illness. The Nutrition Risk in the Critically Ill (NUTRIC) and the modified Nutrition Risk in the Critically Ill (mNUTRIC) both utilize Acute Physiology, Age, Chronic Health Evaluation II (APACHEII) parameter which determines the severity of disease in patients admitted to the intensive care units (52). Both assessment tools calculate Sequential Organ Failure Assessment (SOFA) score which tracks the patient's organ function in the intensive care unit (53). In addition to these specific tools, Generated-Subjective Global Assessment (PG-SGA) is an example of an assessment tool specific to oncology patients that utilizes patient symptoms for nutrition calculation. Symptomology in cancer patients, including vertigo and nausea, negatively impact the nutritional status and improvements in these symptoms will enhance the patient's overall nutrition status (54). Refer to (Table 2) for a complete listing of general and specific nutritional assessment tools and their applications.
Geisinger's Implementation of ASPEN
ASPEN promotes nutritional care through nutrition screening, assessment, and intervention (66). Individuals who are at risk for malnutrition or are malnourished are first identified (67). Next, an assessment is performed to examine the specific malnutrition issues present so that appropriate dietary changes can be made (67). By providing nutrition support, ASPEN enables health professionals to utilize evidence-based clinical guidelines to determine malnutrition patient cases. Specifically, Geisinger implements ASPEN recommendations for nutrition support therapy in the following ways. If a patient requires nutrition support, ASPEN prefers enteral feeding over parenteral feeding (68). This is due to the fact that enteral feeding has reduced infectious morbidity (69) and fewer septic complications (70). ASPEN recommends that nutrition support therapy should be started within 24–48 h to preserve gut integrity (71). Additionally, Geisinger recognizes that malnutrition can have clinical indications, including sepsis, hyperglycemia, and obesity (72). For individuals with sepsis, low dose feedings are recommended rather than full caloric feedings (73). While ASPEN recommends that nearly 25% of nutrient intake should be protein (74), no recommendations are available for vitamins and minerals (75). ASPEN recommends that 50–60% of nutritional requirements should be glucose and 10–30% of nutritional requirements should be fat (75). Geisinger has implemented ASPEN guidelines since 2016 and has been closely following these clinical guidelines to ensure quality care for hospitalized patients with nutritional deficiencies with plans to expand to outpatient settings. Geisinger's records indicate that ~1 out of every 10 hospitalized patient has some form of malnutrition in central Pennsylvania where the participating hospitals are located. Hospitals within Geisinger document malnutrition via best practice alert (BPA). The process begins when a patient is screened for nutritional risk by a nurse and, if indicated, is then assessed by a licensed dietitian. The dietitian performs a thorough nutritional assessment and determines the degree of malnutrition, including mild, moderate, or severe, along with its clinical presentations, including fluid accumulation and loss of muscle, fat, and weight. The detailed assessment information obtained by the dietitian populates the physician note and is easily accessible to the physician. Next, the physician reviewing the patient's chart will become aware of this status, which will be addressed either during the same or future encounters depending on the patient's specific needs and overall well-being. The physician must document their encounter with the patient and document how the plan of care has been developed to treat malnutrition. Over the past year Geisinger has been working toward improving their malnutrition documentation process, by using Epic technology to incorporate SmartLinks in the SmartTexts of provider notes. These improved documentation systems have allowed Geisinger to meet the requirements set forth by Centers for Medicare and Medicaid Services, including classifying, characterizing, and planning care for patients with malnutrition. The goal is to recognize a patient who is at risk for or has malnutrition and investigate specific nutritional deficiencies and promote well-being.
We have collected clinical data from the various departments across five Geisinger hospitals in central Pennsylvania. As shown in (Figure 2), the percentage of BPA that was followed by healthcare providers differed by the department. Our data shows that BPA followed through in neurology and cardiology departments fluctuated whereas BPA followed through in cancer and immunology departments remained consistent between 2017 and 2019. Furthermore, at its inception in July 2016, the percentage of physicians addressing the nutritional needs of the patients was very low (between 5 and 25%); however, once the representation of the alert in the system was refined through usability assessment, the utilization of this resource significantly improved (25–85%). These findings show that cases involving malnutrition in cancer and immunology departments are more actively and consistently addressed. Finally, the average resource utilization (acting based on the results of the assessment tool) remained stable between 60 and 80% from 2017 to 2019.
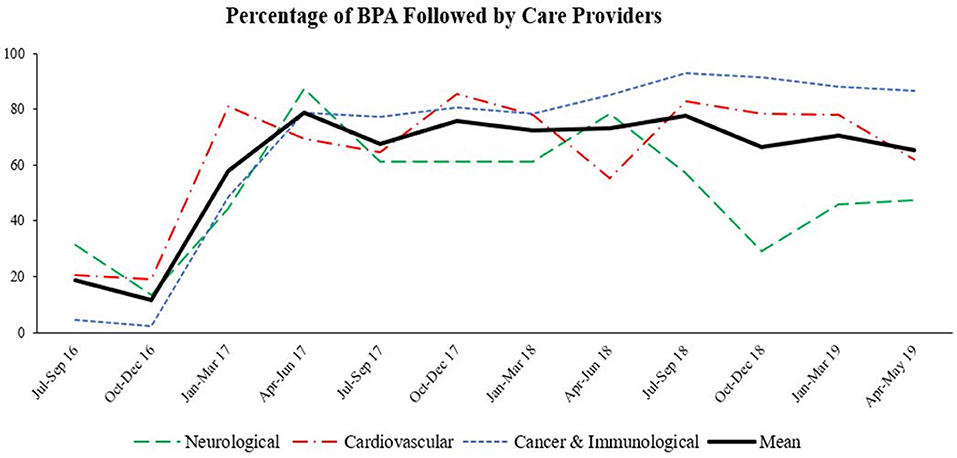
Figure 2. Percentage of BPA followed by care providers. Inpatient cases of malnutrition were marked as best practice alert (BPA). Percentage of BPA that was targeted for further in-depth nutritional assessment by healthcare providers in different departments among five Geisinger hospitals between July 2016–May 2019 was determined.
Technological advances could also aid in the fight against malnutrition. For instance, individualized recommendations about meal plans could be one of the ways to promote healthier food-consumption behaviors (76). In addition, modeling algorithms can be designed as assistive technologies for the pediatrician to help them better detect at-risk patients and (a) advise parents or guardian regarding nutrition enhancement (77), or (b) use healthcare resources including breastfeeding and vaccinations on the most vulnerable population more effectively (78).
Modeling-Enabled Nutritional Assessment Strategies
The nutritional status of patients can be determined by performing laboratory testing, as we have outlined above. The use of these laboratory tests can enhance healthcare delivery by providing a quick and more efficient method of detecting nutritional deficiencies and prompting physicians to begin the process of in-depth assessment and intervention. However, these methodologies often fall short in identifying a larger patient population as well as following up on these patients. Artificial intelligence (AI) offers a scalable solution to these shortcomings when addressing malnutrition. Algorithms based on AI consist of various modeling strategies, including supervised learning, unsupervised learning, deep learning, and cognitive learning. While supervised learning is useful for classification and regression (79), unsupervised learning is useful for identifying patterns in data (80). Deep learning models account for parameters when making predictions from datasets whereas cognitive learning can perform pattern recognition and language processing from datasets (79). Finally, various modeling strategies, including AI, or statistical modeling approaches, such as metamodeling based sensitivity analysis (81, 82), can be designed to pull rich longitudinal clinical parameters to mine and find patterns that could lead to actionable predictions. In a review article by Verma and colleagues, authors describe the challenges in personalized nutrition and health and use of different modeling strategies in this field (83). These innovative modeling strategies provide efficient ways for healthcare systems to implement decision support algorithms to assist their care providers for a wide range of applications, including identification of at-risk patients for malnutrition.
AI could be designed to enhance the quality of care through early diagnosis, effective and personalized care plans, and risk identification and mitigation in some patients (79). Studies on integration of technology have shown that algorithms can be developed to recommend individualized meal plans for the elderly (76) as well as provide advice on nutritional enhancement and healthcare resources for children (77, 78). Further, well designed and validated algorithms can collect and mine immense amounts of longitudinal patient data from electronic health records, including body mass index, blood pressure, and body composition to predict potential clinical outcomes and recommendations for patients affected by obesity or malnutrition (84). Machine learning algorithms can be developed by integrating multi-dimensional data, including dietary intake, physical activity, blood parameters, and gut microbiota, to make personalized predictions on glycemic responses after meals (85). These machine learning methods could help promote health on a more personalized and targeted level for patients suffering from malnutrition.
Conclusion
Nutrition impacts patients' general health, occurrence of diseases, and hospital systems at large. Although malnutrition screening tools exist, healthcare systems are not using these resources optimally and systematically. Modeling strategies, including the metamodel sensitivity analysis or machine learning-based approaches, can help identify a larger population of patients at risk for malnutrition. Our preliminary finding from one healthcare system shows the value of engaging physicians and care providers; however, more studies are warranted before malnutrition assessment tools could become truly transformative for already complex healthcare systems.
Author Contributions
VA and RZ designed the architecture of the study. VaS, ViS, and VA performed the searches. MS, VaS, and ViS assisted with the data collection and data analysis for the case study. DW, AK, RZ, RH, and JB-R provided critical feedback and contributed to the editing of various sections of the study. VaS, ViS, and VA wrote the manuscript.
Funding
VA is partly supported by the National Institute of Health (NIH) grant No. R56HL116832 sub-awarded to Geisinger, as well as funds from the Defense Threat Reduction Agency (DTRA) grant No. HDTRA1-18-1-0008 to JB-R and RH, and sub-awarded to VA (Sub-PI, Geisinger, sub-award No. 450557-19D03). RZ is partly supported by Geisinger Health Plan Quality. The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. US Department of Agriculture. Dietary Guidelines for Americans. (2018). Available online at: https://www.fns.usda.gov/cnpp/dietary-guidelines-americans.
2. Corkins MR, Guenter P, DiMaria-Ghalili RA, Jensen GL, Malone A, Miller S, et al. Malnutrition diagnoses in hospitalized patients: United States, 2010. JPEN J Parenter Enteral Nutr. (2014) 38:186–95. doi: 10.1177/0148607113512154
3. Agarwal E, Miller M, Yaxley A, Isenring E. Malnutrition in the elderly: a narrative review. Maturitas. (2013) 76:296–302. doi: 10.1016/j.maturitas.2013.07.013
4. Barker LA, Gout BS, Crowe TC. Hospital malnutrition: prevalence, identification and impact on patients and the healthcare system. Int J Environ Res Public Health. (2011) 8:514–27. doi: 10.3390/ijerph8020514
5. White JV, Guenter P, Jensen G, Malone A, Schofield M, Academy of N, et al. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. (2012) 112:730–8. doi: 10.1016/j.jand.2012.03.012
6. American Dietetic Association Evidence Analysis Library. Available online at: http://www.adaevidencelibrary.com/conclusion.cfm?conclusion_statement_id=251313&highlight=prealbumin&home=1
7. The, Fresh Food Farmacy - Our Purpose,. Available online at: https://www.geisinger.org/freshfoodfarmacy/our-purpose.
8. Sanchez-Moreno C, Jimenez-Escrig A, Martin A. Stroke: roles of B vitamins, homocysteine and antioxidants. Nutr Res Rev. (2009) 22:49–67. doi: 10.1017/S0954422409990023
9. Corrigan ML, Escuro AA, Celestin J, Kirby DF. Nutrition in the stroke patient. Nutr Clin Pract. (2011) 26:242–52. doi: 10.1177/0884533611405795
10. Mould J. Nurses 'must' control of the nutritional needs of stroke patients. Br J Nurs. (2009) 18:1410–4. doi: 10.12968/bjon.2009.18.22.45572
11. Prosser-Loose EJ, Verge VM, Cayabyab FS, Paterson PG. Protein-energy malnutrition alters hippocampal plasticity-associated protein expression following global ischemia in the gerbil. Curr Neurovasc Res. (2010) 7:341–60. doi: 10.2174/156720210793180792
12. Bousselamti A, El Hasbaoui B, Echahdi H, Krouile Y. Psychomotor regression due to vitamin B12 deficiency. Pan Afr Med J. (2018) 30:152. doi: 10.11604/pamj.2018.30.152.12046
13. Kennedy DO. B vitamins and the brain: mechanisms. Dose and efficacy–A review. Nutrients. (2016) 8:68. doi: 10.3390/nu8020068
14. Kocer B, Engur S, Ak F, Yilmaz M. Serum vitamin B12, folate, and homocysteine levels and their association with clinical and electrophysiological parameters in multiple sclerosis. J Clin Neurosci. (2009) 16:399–403. doi: 10.1016/j.jocn.2008.05.015
15. Blass JP, Gleason P, Brush D, DiPonte P, Thaler H. Thiamine and Alzheimer's disease. A pilot study. Arch Neurol. (1988) 45:833–5. doi: 10.1001/archneur.1988.00520320019008
17. Di Minno MN, Tremoli E, Coppola A, Lupoli R, Di Minno G. Homocysteine and arterial thrombosis: Challenge and opportunity. Thromb Haemost. (2010) 103:942–61. doi: 10.1160/TH09-06-0393
18. Dionisio N, Jardin I, Salido GM, Rosado JA. Homocysteine, intracellular signaling and thrombotic disorders. Curr Med Chem. (2010) 17:3109–19. doi: 10.2174/092986710791959783
19. Al Mheid I, Quyyumi AA. Vitamin D and cardiovascular disease: controversy unresolved. J Am Coll Cardiol. (2017) 70:89–100. doi: 10.1016/j.jacc.2017.05.031
20. Anaszewicz M, Budzynski J. Clinical significance of nutritional status in patients with atrial fibrillation: An overview of current evidence. J Cardiol. (2017) 69:719–30. doi: 10.1016/j.jjcc.2016.06.014
21. Velavan P, Huan Loh P, Clark A, Cleland JG. The cholesterol paradox in heart failure. Congest Heart Fail. (2007) 13:336–41. doi: 10.1111/j.1527-5299.2007.07211.x
22. Rauchhaus M, Coats AJ, Anker SD. The endotoxin-lipoprotein hypothesis. Lancet. (2000) 356:930–3. doi: 10.1016/S0140-6736(00)02690-8
23. Wu N, Xu B, Xiang Y, Wu L, Zhang Y, Ma X, et al. Association of inflammatory factors with occurrence and recurrence of atrial fibrillation: a meta-analysis. Int J Cardiol. (2013) 169:62–72. doi: 10.1016/j.ijcard.2013.08.078
24. Al-Rawahi M, Proietti R, Thanassoulis G. Pericardial fat and atrial fibrillation: epidemiology, mechanisms and interventions. Int J Cardiol. (2015) 195:98–103. doi: 10.1016/j.ijcard.2015.05.129
25. Wozakowska-Kaplon B, Opolski G, Herman Z, Kosior D. Natriuretic peptides in patients with atrial fibrillation. Cardiol J. (2008) 15:525–9. Available online at: https://journals.viamedica.pl/cardiology_journal/article/view/21551
26. Rytter MJ, Kolte L, Briend A, Friis H, Christensen VB. The immune system in children with malnutrition–a systematic review. PLoS ONE. (2014) 9:e105017. doi: 10.1371/journal.pone.0105017
27. Yu J, Ordiz MI, Stauber J, Shaikh N, Trehan I, Barnell E, et al. Environmental enteric dysfunction includes a broad spectrum of inflammatory responses and epithelial repair processes. Cell Mol Gastroenterol Hepatol. (2016) 2:158–74 e1. doi: 10.1016/j.jcmgh.2015.12.002
28. Ramsay G, Cantrell D. Environmental and metabolic sensors that control T cell biology. Front Immunol. (2015) 6:99. doi: 10.3389/fimmu.2015.00099
29. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. (2012) 149:274–93. doi: 10.1016/j.cell.2012.03.017
30. Ghishan FK, Kiela PR. Epithelial transport in inflammatory bowel diseases. Inflamm Bowel Dis. (2014) 20:1099–109. doi: 10.1097/MIB.0000000000000029
31. Breyer MK, Rutten EP, Locantore NW, Watkins ML, Miller BE, Wouters EF, et al. Dysregulated adipokine metabolism in chronic obstructive pulmonary disease. Eur J Clin Invest. (2012) 42:983–91. doi: 10.1111/j.1365-2362.2012.02686.x
32. Ibrahim MK, Zambruni M, Melby CL, Melby PC. Impact of Childhood Malnutrition on Host Defense and Infection. Clin Microbiol Rev. (2017) 30:919–71. doi: 10.1128/CMR.00119-16
33. Ortiz R, Cortes L, Cortes E, Medina H. Malnutrition alters the rates of apoptosis in splenocytes and thymocyte subpopulations of rats. Clin Exp Immunol. (2009) 155:96–106. doi: 10.1111/j.1365-2249.2008.03796.x
34. Mitsumori K, Takegawa K, Shimo T, Onodera H, Yasuhara K, Takahashi M. Morphometric and immunohistochemical studies on atrophic changes in lympho-hematopoietic organs of rats treated with piperonyl butoxide or subjected to dietary restriction. Arch Toxicol. (1996) 70:809–14. doi: 10.1007/s002040050343
35. Cunha MC, Lima Fda S, Vinolo MA, Hastreiter A, Curi R, Borelli P, et al. Protein malnutrition induces bone marrow mesenchymal stem cells commitment to adipogenic differentiation leading to hematopoietic failure. PLoS ONE. (2013) 8:e58872. doi: 10.1371/journal.pone.0058872
36. Hughes SM, Amadi B, Mwiya M, Nkamba H, Tomkins A, Goldblatt D. Dendritic cell anergy results from endotoxemia in severe malnutrition. J Immunol. (2009) 183:2818–26. doi: 10.4049/jimmunol.0803518
37. Najera O, Gonzalez C, Toledo G, Lopez L, Ortiz R. Flow cytometry study of lymphocyte subsets in malnourished and well-nourished children with bacterial infections. Clin Diagn Lab Immunol. (2004) 11:577–80. doi: 10.1128/CDLI.11.3.577-580.2004
38. Lyra JS, Madi K, Maeda CT, Savino W. Thymic extracellular matrix in human malnutrition. J Pathol. (1993) 171:231–6. doi: 10.1002/path.1711710312
39. Barone KS, O'Brien PC, Stevenson JR. Characterization and mechanisms of thymic atrophy in protein-malnourished mice: role of corticosterone. Cell Immunol. (1993) 148:226–33. doi: 10.1006/cimm.1993.1105
40. Savino W. The thymus gland is a target in malnutrition. Eur J Clin Nutr. (2002) 56:S46–9. doi: 10.1038/sj.ejcn.1601485
41. Ozkale M, Sipahi T. Hematologic and bone marrow changes in children with protein-energy malnutrition. Pediatr Hematol Oncol. (2014) 31:349–58. doi: 10.3109/08880018.2013.813098
42. Xavier JG, Favero ME, Vinolo MA, Rogero MM, Dagli ML, Arana-Chavez VE, et al. Protein-energy malnutrition alters histological and ultrastructural characteristics of the bone marrow and decreases haematopoiesis in adult mice. Histol Histopathol. (2007) 22:651–60. doi: 10.14670/HH-22.651
43. Fock RA, Blatt SL, Beutler B, Pereira J, Tsujita M, de Barros FE, et al. Study of lymphocyte subpopulations in bone marrow in a model of protein-energy malnutrition. Nutrition. (2010) 26:1021–8. doi: 10.1016/j.nut.2009.08.026
44. Mello AS, de Oliveira DC, Bizzarro B, Sa-Nunes A, Hastreiter AA, Beltran JS, et al. Protein malnutrition alters spleen cell proliferation and IL-2 and IL-10 production by affecting the STAT-1 and STAT-3 balance. Inflammation. (2014) 37:2125–38. doi: 10.1007/s10753-014-9947-5
45. Green F, Heyworth B. Immunoglobulin-containing cells in jejunal mucosa of children with protein-energy malnutrition and gastroenteritis. Arch Dis Child. (1980) 55:380–3. doi: 10.1136/adc.55.5.380
46. Flo J, Elias F, Benedetti R, Massouh E. Reversible effects on B and T cells of the gut-associated lymphoid tissues in rats malnourished during suckling: impaired induction of the immune response to intra-Peyer patches immunization with cholera toxin. Clin Immunol Immunopathol. (1996) 80:147–54. doi: 10.1006/clin.1996.0108
47. Jensen GL, Hsiao PY, Wheeler D. Adult nutrition assessment tutorial. JPEN J Parenter Enteral Nutr. (2012) 36:267–74. doi: 10.1177/0148607112440284
48. Bolayir B, Arik G, Yesil Y, Kuyumcu ME, Varan HD, Kara O, et al. Validation of nutritional risk screening-2002 in a hospitalized adult population. Nutr Clin Pract. (2019) 34:297–303. doi: 10.1002/ncp.10082
49. Kruizenga HM, Seidell JC, de Vet HC, Wierdsma NJ, van Bokhorst-de van der Schueren MA. Development and validation of a hospital screening tool for malnutrition: the short nutritional assessment questionnaire (SNAQ). Clin Nutr. (2005) 24:75–82. doi: 10.1016/j.clnu.2004.07.015
50. Kaiser MJ, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T, et al. Validation of the mini nutritional assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. (2009) 13:782–8. doi: 10.1007/s12603-009-0214-7
51. Jeong DH, Hong SB, Lim CM, Koh Y, Seo J, Kim Y, et al. Comparison of accuracy of NUTRIC and modified NUTRIC scores in predicting 28-day mortality in patients with sepsis: a single center retrospective study. Nutrients. (2018) 10:7. doi: 10.3390/nu10070911
52. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. (1985) 13:818–29. doi: 10.1097/00003246-198510000-00009
53. Jones AE, Trzeciak S, Kline JA. The sequential organ failure assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit Care Med. (2009) 37:1649–54. doi: 10.1097/CCM.0b013e31819def97
54. Bauer J, Capra S, Ferguson M. Use of the scored patient-generated subjective global assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr. (2002) 56:779–85. doi: 10.1038/sj.ejcn.1601412
55. Miyata S, Tanaka M, Ihaku D. Usefulness of the malnutrition screening tool in patients with pulmonary tuberculosis. Nutrition. (2012) 28:271–4. doi: 10.1016/j.nut.2011.07.013
56. Isenring EA, Bauer JD, Banks M, Gaskill D. The malnutrition screening tool is a useful tool for identifying malnutrition risk in residential aged care. J Hum Nutr Diet. (2009) 22:545–50. doi: 10.1111/j.1365-277X.2009.01008.x
57. Wang T, Shen J. Usefulness of simplified nutritional appetite questionnaire (SNAQ) in appetite assessment in elder patients with liver cirrhosis. J Nutr Health Aging. (2018) 22:911–5. doi: 10.1007/s12603-018-1086-5
58. Ozbilgin S, Hanc V, Omur D, Ozbilgin M, Tosun M, Yurtlu S, et al. Morbidity and mortality predictivity of nutritional assessment tools in the postoperative care unit. Medicine. (2016) 95:e5038. doi: 10.1097/MD.0000000000005038
59. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: european consensus on definition and diagnosis: report of the european working group on sarcopenia in older people. Age Ageing. (2010) 39:412–23. doi: 10.1093/ageing/afq034
60. National Alliance for Infusion T, The American Society for P, Enteral Nutrition Public Policy C, Board of D. Disease-related malnutrition and enteral nutrition therapy: a significant problem with a cost-effective solution. Nutr Clin Pract. (2010) 25:548–54. doi: 10.1177/0884533610378524
61. Jensen GL, Bistrian B, Roubenoff R, Heimburger DC. Malnutrition syndromes: a conundrum vs continuum. JPEN J Parenter Enteral Nutr. (2009) 33:710–6. doi: 10.1177/0148607109344724
62. Jensen GL, Mirtallo J, Compher C, Dhaliwal R, Forbes A, Grijalba RF, et al. Adult starvation and disease-related malnutrition: a proposal for etiology-based diagnosis in the clinical practice setting from the International Consensus Guideline Committee. JPEN J Parenter Enteral Nutr. (2010) 34:156–9. doi: 10.1177/0148607110361910
63. American Dietetic Association Evidence Analysis Library. Available online at: http://www.adaevidencelibrary.com/conclusion.cfm?conclusion_statement_id=251263&highlight=albumin&home=1
64. Skipper A, Ferguson M, Thompson K, Castellanos VH, Porcari J. Nutrition screening tools: an analysis of the evidence. JPEN J Parenter Enteral Nutr. (2012) 36:292–8. doi: 10.1177/0148607111414023
65. Soeters PB, Schols AM. Advances in understanding and assessing malnutrition. Curr Opin Clin Nutr Metab Care. (2009) 12:487–94. doi: 10.1097/MCO.0b013e32832da243
66. Mueller C, Compher C, Ellen DM, American Society for P, Enteral Nutrition Board of D, et al. Nutrition screening, assessment, and intervention in adults. JPEN J Parenter Enteral Nutr. (2011) 35:16–24. doi: 10.1177/0148607110389335
67. American Society for Parenteral Enteral Nutrition (A,.S.P.E.N.), Board of Directors Clinical Practice Committee. American Society for Parenteral Enteral Nutrition. (2010). Available online at: http://www.nutritioncare.org/Library.aspx
68. McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically Ill patient: society of critical care medicine (SCCM) and american society for parenteral and enteral nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. (2009) 33:277–316. doi: 10.1177/0148607109335234
69. Kudsk KA, Croce MA, Fabian TC, Minard G, Tolley EA, Poret HA, et al. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. (1992) 215:503–11; discussion 11–3. doi: 10.1097/00000658-199205000-00013
70. Moore FA, Feliciano DV, Andrassy RJ, McArdle AH, Booth FV, Morgenstein-Wagner TB, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg. (1992) 216:172–83. doi: 10.1097/00000658-199208000-00008
71. McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically Ill patient: society of critical care medicine (SCCM) and american society for parenteral and enteral nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. (2016) 40:159–211. doi: 10.1177/0148607115621863
72. Wooley JA, Frankenfield D. Energy. In: Gottschlich MM, editor. The A.S.P.E.N. Nutrition Support Core Curriculum: A Case-Based Approach - The Adult Patient. (2007). p. 19–32.
73. Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. (2008) 36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41
74. Martindale RG, McClave SA, Vanek VW, McCarthy M, Roberts P, Taylor B, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: society of critical care medicine and american society for parenteral and enteral nutrition: executive summary. Crit Care Med. (2009) 37:1757–61. doi: 10.1097/CCM.0b013e3181a40116
75. Cresci GA, Gottschlich MM, Mayes T, Mueller C. Trauma, surgery, and burns. In: Gottschlich MM, editor. The A.S.P.E.N. Nutrition Support Core Curriculum: A Case-Based Approach - The Adult Patient. (2007). p. 455–76.
76. Ãberg J. Dealing with malnutrition: a meal planning system for elderly. In: AAAI Spring Symposium: Argumentation for Consumers of Healthcare. (2006). Available online at: https://www.aaai.org/Papers/Symposia/Spring/2006/SS-06-01/SS06-01-001.pdf
77. Xu Dezhi GUG. Rule based classification to detect malnutrition in children. Int J Comp Sci Eng. (2011) 3:423–9. Available online at: http://www.enggjournals.com/ijcse/doc/IJCSE11-03-01-115.pdf
78. Khare SK, Gupta D, Jyotishi A. Investigation of nutritional status of children based on machine learning techniques usingindian demographic and health survey data. Procedia Computer Science. (2017) 2017:338–49. doi: 10.1016/j.procs.2017.09.087
79. Noorbakhsh-Sabet N, Zand R, Zhang Y, Abedi V. Artificial Intelligence Transforms the Future of Health Care. Am J Med. (2019) 132:795–801. doi: 10.1016/j.amjmed.2019.01.017
80. Deo RC. Machine Learning in medicine. Circulation. (2015) 132:1920–30. doi: 10.1161/CIRCULATIONAHA.115.001593
81. Chen X, Wang W, Xie G, Hontecillas R, Verma M, Leber A, et al. Multi-resolution sensitivity analysis of model of immune response to helicobacter pylori infection via spatio-temporal metamodeling. Front App Math Stat. (2019). 5:4. doi: 10.3389/fams.2019.00004
82. Verma M, Bassaganya-Riera J, Leber A, Tubau-Juni N, Hoops S, Abedi V, et al. High-resolution computational modeling of immune responses in the gut. Gigascience. (2019) 8:62. doi: 10.1093/gigascience/giz062
83. Verma M, Hontecillas R, Tubau-Juni N, Abedi V, Bassaganya-Riera J. Challenges in personalized nutrition and health. Front Nutr. (2018) 5:117. doi: 10.3389/fnut.2018.00117
84. DeGregory KW, Kuiper P, DeSilvio T, Pleuss JD, Miller R, Roginski JW, et al. A review of machine learning in obesity. Obes Rev. (2018) 19:668–85. doi: 10.1111/obr.12667
Keywords: malnutrition, nutrition assessment tools, ASPEN, artificial intelligence, machine learning
Citation: Sharma V, Sharma V, Khan A, Wassmer DJ, Schoenholtz MD, Hontecillas R, Bassaganya-Riera J, Zand R and Abedi V (2020) Malnutrition, Health and the Role of Machine Learning in Clinical Setting. Front. Nutr. 7:44. doi: 10.3389/fnut.2020.00044
Received: 22 October 2019; Accepted: 23 March 2020;
Published: 15 April 2020.
Edited by:
Jia Sun, Jiangnan University, ChinaReviewed by:
Manfred Eggersdorfer, DSM Nutritional Produts, ChinaMarjolein Meijerink, Netherlands Organisation for Applied Scientific Research (TNO), Netherlands
Copyright © 2020 Sharma, Sharma, Khan, Wassmer, Schoenholtz, Hontecillas, Bassaganya-Riera, Zand and Abedi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vida Abedi, dmFiZWRpQGdlaXNpbmdlci5lZHU=; dmlkYWFiZWRpQGdtYWlsLmNvbQ==
 Vaibhav Sharma1
Vaibhav Sharma1 Vishakha Sharma
Vishakha Sharma Ayesha Khan
Ayesha Khan David J. Wassmer
David J. Wassmer Raquel Hontecillas
Raquel Hontecillas Josep Bassaganya-Riera
Josep Bassaganya-Riera Ramin Zand
Ramin Zand Vida Abedi
Vida Abedi