
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 27 February 2020
Sec. Clinical Nutrition
Volume 7 - 2020 | https://doi.org/10.3389/fnut.2020.00005
This article is part of the Research Topic Ageing-Related Symptoms, Kampo Medicine and Treatment View all 16 articles
 Kanako Miyano1
Kanako Miyano1 Kaori Ohshima1,2
Kaori Ohshima1,2 Nozomi Suzuki1,3
Nozomi Suzuki1,3 Saho Furuya1,3
Saho Furuya1,3 Yuki Yoshida1,4
Yuki Yoshida1,4 Miki Nonaka1
Miki Nonaka1 Yoshikazu Higami4
Yoshikazu Higami4 Kazumi Yoshizawa2
Kazumi Yoshizawa2 Hideaki Fujii3
Hideaki Fujii3 Yasuhito Uezono1,5,6*
Yasuhito Uezono1,5,6*Cancer cachexia is highly prevalent in patients with progressive cancer and is characterized by decreased food consumption, and body weight. Japanese herbal medicine Ninjinyoeito (NYT), composed of 12 herbal crude drugs, is prescribed in Asian countries to improve several symptoms such as anorexia and fatigue, which are commonly observed in patients with cancer cachexia. However, the action mechanisms of NYT in improving anorexia or fatigue in patients with cancer are not clear. Therefore, in the present study, we examined the effects of NYT on the activities of several G-protein-coupled receptors (GPCRs), which activate hyperphagia signaling in the central nervous system, using an in vitro assay with the CellKey™ system, which detects the activation of GPCRs as a change in intracellular impedance (ΔZ). NYT increased the ΔZ of human embryonic kidney 293 (HEK293) cells expressing orexin 1 receptor (OX1R) and those expressing neuropeptide Y1 receptor (NPY1R) in a dose-dependent manner. On the contrary, NYT did not significantly increase the ΔZ of HEK293A cells expressing growth hormone secretagogue receptor (GHSR) and those expressing NPY5R. The selective OX1R antagonist SB674042 significantly decreased the NYT-induced increase in ΔZ in OX1R-expressing cells. Contrarily, the selective NPY1R antagonist BIBO3340 failed to inhibit the NPY-induced increase in ΔZ in NPY1R-expressing cells. Additionally, we prepared modified NYT excluding each one of the 12 herbal crude drugs in NYT and investigated the effects on the activity of OX1R. Among the 12 modified NYT formulations, the one without citrus unshiu peel failed to activate OX1R. A screening of each of the 12 herbal crude drugs showed that citrus unshiu peel significantly activated OX1R, which was significantly suppressed by SB674042. These finding suggest that NYT and citrus unshiu peel could increase food intake via activation of orexigenic OX1R-expressing neurons in the hypothalamus. This study provides scientific evidence to support the potential of NYT for cancer patients with anorexia.
Cancer cachexia, which is characterized by a decrease in body weight and food consumption, occurs in 80% of patients with progressive cancer, causing at least 20% of cancer-related deaths (1–3). This syndrome not only decreases the quality of life (QOL) but also attenuates the efficacy of chemotherapy (4–7). Studies suggest that cancer cachexia is caused by complicated interrelation among several mediators in the hypothalamus, such as hormones (e.g., leptin and ghrelin), and neuropeptides (e.g., neuropeptide Y and orexin), which regulate food intake (8–10). However, the mechanisms underlying this syndrome are not fully understood, and appropriate therapies for the treatment of cancer cachexia have not been established. The current treatment options for cancer cachexia are far from being satisfactory because of the lack of effective drugs currently available (6).
Ninjinyoeito (NYT), a traditional Japanese kampo medicine that contains extracted ingredients of 12 herbal crude drugs, is approved by Japan's Ministry of Health, Labor, and Welfare as a prescribed medicine in clinical practice. Since the 16th century, NYT has been prescribed in Japan and other Asian countries to ameliorate diseases and improve several symptoms such as anorexia and fatigue (11). In addition, several studies have shown that some herbal crude drugs of NYT improved appetite in a cancer cachexia model of animals or patients with cancer (12–16). However, the action mechanisms of NYT in improving anorexia and/or fatigue in cancer cachexia–anorexia syndrome are not clear.
Therefore, in the present study, we examined the effects of NYT on the activities of several G-protein-coupled receptors (GPCRs), which activate hyperphagia signaling in the central nervous system (CNS). With respect to GPCR-activating hyperphagia signaling, we focused on activated appetite-stimulating receptors, such as growth hormone secretagogue receptor 1a (GHSR), neuropeptide Y1 receptor (NPY1R), neuropeptide Y5 receptor (NPY5R), and orexin 1 receptor (OX1R) (17–32). First, we analyzed the effects of NYT on the activities of these receptors. Second, we identified active medicinal herbs contained in NYT by screening both modified NYT excluding 1 of the 12 herbal crude drugs and each of the 12 herbal crude drugs contained in NYT.
The following reagents and medium were used in the present study: poly-D-lysine and bovine serum albumin (BSA) (Sigma-Aldrich, St. Louis, MO, USA); fetal bovine serum (FBS), Dulbecco's modified Eagle's medium (DMEM), and geneticin (Gibco, Carlsbad, CA, USA); penicillin/streptomycin and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (Nacalai Tesque, Kyoto, Japan); and DMEM (Fujifilm Wako Pure Chemical, Osaka, Japan).
NYT extract powder (lot no. 15112017), the base powder of NYT without excipients, was obtained from Kracie Pharma, Ltd. (Tokyo, Japan), as an aqueous extract of the following 12 medicinal herbs (percentage): atractylodes rhizome (12.9), Japanese angelica root (12.9), poria sclerotium (12.9), Rehmannia root (12.9), ginseng (9.7), cinnamon bark (8.1), citrus unshiu peel (6.5), peony root (6.5), polygala root (6.5), astragalus root (4.8), Glycyrrhiza (3.2), and schisandra fruit (3.2). NYT formulations excluding each one of the 12 herbal crude drugs were also obtained from Kracie Pharma, Ltd. The dried powdered extract of NYT and its crude drugs were suspended in sterile water at 100 mg/ml concentration, diluted 100-fold with Hanks' balanced salt solution (in mM: 1.3 CaCl2·2H2O, 0.81 MgSO4, 5.4 KCl, 0.44 KH2PO4, 4.2 NaHCO3, 136.9 NaCl, 0.34 Na2HPO4, and 5.6 D-glucose) containing 20 mM HEPES and 0.1% BSA, and filtered through a 0.2 μm membrane (KURABO Industry Ltd., Osaka, Japan). The solution was used to treat cells at final concentrations of 3, 10, 30, and 100 μg/ml. All other reagents were of the highest purity available from commercial sources.
GHSR-expressing cells were cultured as described previously (33). The expression vector C-terminal FLAG-tagged human GHS-R1a was transfected into human embryonic kidney 293A (HEK293A) cells using PEI Max (Polysciences, Inc., Warrington, PA, USA). For human NPY1R and NPY5R clones, we synthesized the combined fragment of the NruI restriction site, human EF1 promoter (34), N-terminal cleavable hemagglutinin secretion signal (MKTIIALSYIFCLVFA) (35), and NheI site and inserted it into the pIRESpuro3 expression vector (Takara, Shiga, Japan) using its recognition site (pEF1-IRESpuro). Subsequently, we amplified the cDNA encoding hNPY1R (NM_000909.6) ORF and hNPY5R (NM_001317091.1) ORF with the primers tagged with the NheI (N′) and BamHI (C′) sites from a full-length cDNA clone (Genscript, Piscataway, NJ, USA), and it was transferred into the pEF1-IRESpuro vector. The expression constructs were transfected into HEK293T cells according to the manufacturers' instructions, and 48 h after transfection, cells stably expressing either NPY1R or NPY5R were selected. The human OX1R clone (GenBank accession: AB463762; Kazusa DNA Research Institute, Chiba, Japan) was amplified according to the manufacturer's instructions. HEK293 cells (American Type Culture Collection, Manassas, VA, USA) stably expressing OX1R were generated through transfection of plasmids using ScreenFect™ (Fujifilm Wako Pure Chemical) and selected based on the OX1R activity measured using the CellKey™ assay. Ethical approval of the experimental procedures was obtained from National Cancer Center Research Institute (approval no. B85M1-13).
All cells were cultured at 37°C in a humidified atmosphere of 95% air and 5% CO2. GHSR-expressing HEK293A cells and OX1R-expressing HEK293 cells were maintained in DMEM (Gibco or Fujifilm Wako Pure Chemical) supplemented with 10% FBS, penicillin (100 U/ml), streptomycin (100 mg/ml), and geneticin (800 μg/ml). HEK293T cells expressing NPY1R or NPY5R were maintained in DMEM (Gibco) supplemented with sodium pyruvate (1 mM), 10% FBS, penicillin (100 U/ml), and streptomycin (100 mg/ml).
The assay with the CellKey™ system was conducted as described previously (36–40). Briefly, the cells were cultured at a density of 4.0 × 104 (GHSR-expressing HEK293A), 6.0 × 104 (NPY1R-expressing HEK293T), 5.0 × 104 (NPY5R-expressing HEK293T), and 6.0 × 104 cells/well (OX1R-expressing HEK293) in CellKey™ 96-well microplates. After incubating at 37°C for 24 h, the cells were washed with Hanks' balanced salt solution containing 20 mM HEPES and 0.1% BSA, and allowed to equilibrate in the assay buffer for 30 min before the assay. The CellKey™ instrument applies small voltages to the electrodes every 10 s and measures impedance of the cell layer. In this study, we recorded at 5 min baseline, added drugs, and measured changes in impedance (ΔZ) for 25 min. The rate of change in impedance is expressed as the difference of the minimum impedance and maximum impedance after drug injection as previously reported (39).
The data are presented as mean ± S.E.M. The statistical analyses were performed using the one-way analysis of variance (ANOVA) followed by Bonferroni's multiple comparison test (GraphPad Prism 8, GraphPad Software, San Diego, CA, USA). The results with a probability value p < 0.05 were considered statistically significant.
We examined the effects of NYT on the activation of GHSR, NPY1R, NPY5R, and OX1R using the CellKey™ system. As shown in Figures 1A,C, NYT (3–100 μg/ml) did not significantly change the ΔZ of GHSR-expressing HEK293A cells and NPY5R-expressing HEK293T cells. On the contrary, the GHSR agonist ghrelin (10−7 M) and NPYR agonist neuropeptide Y (NPY, 10−6 M) increased the ΔZ of these cells, respectively, in GHSR-expressing cells: control vs. ghrelin (10−7 M) (% of control, mean ± S.E.M.), 100 ± 7.81 vs. 2,227.5 ± 288.4; in NPY5R-expressing cells: control vs. NPY (10−6 M), 100 ± 10.24 vs. 172.8 ± 9.68. NYT significantly increased the ΔZ of cells expressing NPY1R or OX1R in a dose-dependent manner (Figures 1B–D). However, the NYT-induced increase in ΔZ of NPY1R-expressing HEK293T cells was not significantly attenuated by BIBO3340 (BIBO, 10−5 or 10−4 M), at concentrations that completely inhibited the increase in ΔZ induced by NPY (10−8 M) (Figures 2A,B). Contrarily, SB676042 (SB, 10−6 or 10−5 M) significantly suppressed the NYT-induced increase in ΔZ of OX1R-expressing HEK293 cells (Figure 2C). Pretreatment with SB676042 (SB, 10−6, or 10−5 M) significantly inhibited the increase in ΔZ induced by the OX1R agonist orexin A (OXA) in a dose-dependent manner (Figure 2D).
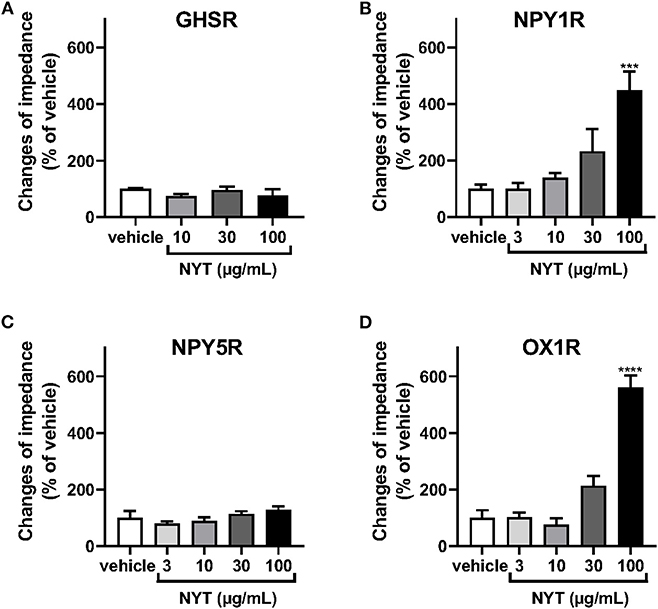
Figure 1. Effects of Ninjinyoeito (NYT) on impedance changes in cells expressing several G-protein-coupled receptors, which activate hyperphagia signaling in the central nervous system using the CellKey™ assay. The cells stably expressing growth hormone secretagogue receptor 1a (GHSR) (A, n = 6–8), neuropeptide Y1 receptor (NPY1R) (B, n = 6), neuropeptide Y5 receptor (NPY5R) (C, n = 6), or orexin 1 receptor (OX1R) (D, n = 6) were treated with NYT (3–100 μg/kg) or its vehicle (control). The rate of change in impedance was measured using the CellKey™ system and expressed as the difference of the minimum impedance and maximum impedance after drug injection. The data are expressed as mean ± S.E.M. *** and **** indicate p < 0.001 and p < 0.0001, respectively, compared with the control; Bonferroni's multiple comparison test following one-way ANOVA.
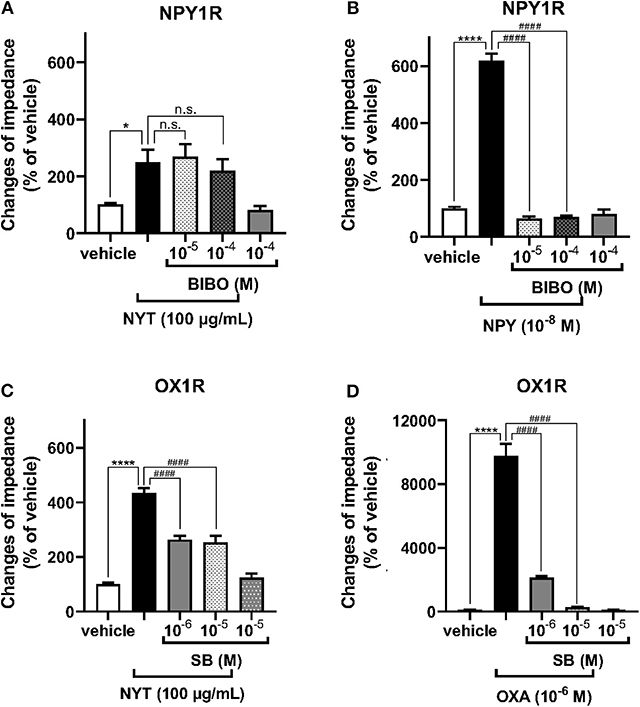
Figure 2. Effects of the antagonists of NPY1R and OX1R on the NYT-induced increase in impedance of cells expressing NPY1R or OX1R. The cells expressing NPY1R (A,B) were pretreated with or without BIBO3340 (BIBO, 10−5 or 10−4 M), a selective NPY1R antagonist, for 30 min and then treated with NYT (A, 100 μg/ml) or NPY (B, 10−8 M), respectively. After treatment with the vehicle or selective OX1R antagonist SB676042 (SB, 10−6 or 10−5 M) for 30 min, OX1R-expressing HEK293 cells were treated with NYT (C) or orexin A (D, OXA). The rate of change in impedance is expressed as the difference of the minimum and maximum impedance after drug injection. The data are expressed as mean ± S.E.M. (n = 6–12). *p < 0.05, and ****p < 0.0001, respectively, compared with the vehicle (control); ####p < 0.0001, compared with NYT or each selective agonist; Bonferroni's multiple comparison test following one-way ANOVA.
To clarify the medical herbal crude drugs involved in NYT-induced OX1R activation, we investigated the effects of modified NYT excluding 1 of the 12 herbal drugs composing NYT on the increase in ΔZ of OX1R-expressing HEK293 cells. As shown in Figure 3, only the modified NYT without citrus unshiu peel (100 μg/ml) failed to significantly increase the ΔZ of OX1R-expressing cells; compared with the NPY-induced increase in ΔZ, the responses were significantly low (Figure 3).
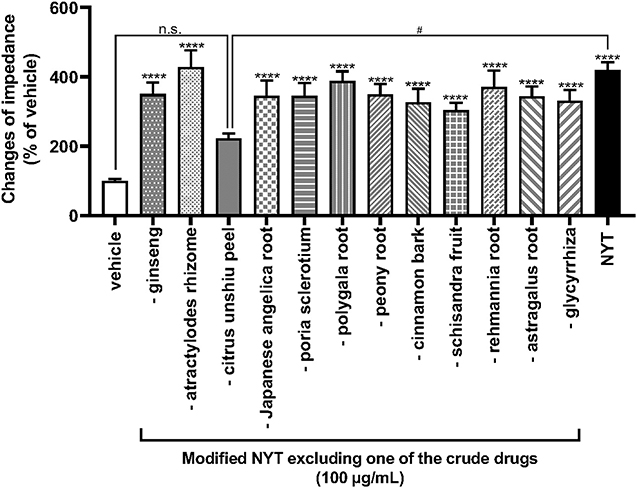
Figure 3. Effects of modified NYT excluding each one of the 12 herbal crude drugs on the increase in impedance of OX1R-expressing HEK293 cells. HEK293 cells stably expressing OX1R were treated with modified NYT excluding 1 herbal crude drug (100 μg/ml) from 12 ingredients of NYT or its vehicle (control). The rate of change in impedance is expressed as the difference of the minimum and maximum impedance after drug injection. The data are expressed as mean ± S.E.M. (n = 6). ****p < 0.0001, compared with the control; #p < 0.05, compared with the NYT; Bonferroni's multiple comparison test following one-way ANOVA.
We further examined the effects of each of the medical herbal crude drugs composing NYT on the activity of OX1R. NYT contains 12 medicinal herbs in the following ratio: atractylodes rhizome (4), Japanese angelica root (4), poria sclerotium (4), Rehmannia root (4), ginseng (3), cinnamon bark (2.5), citrus unshiu peel (2), peony root (2), polygala root (2), astragalus root (1.5), Glycyrrhiza (1), and schisandra fruit (1). Thus, the major components of the herbal drugs were atractylodes rhizome, Japanese angelica root, poria sclerotium, and Rehmannia root, each accounting for 13% of NYT. We therefore analyzed the effects of 20 μg/ml of the 12 individual medicinal herbs (20% of the volume of NYT) on the activity of OX1R to reveal the medicinal herbs in NYT that activate OX1R. As shown in Figure 4A, only citrus unshiu peel (20 μg/ml) significantly increased the ΔZ (Figure 4A), and the responses were suppressed by pretreatment with SB676042 (SB, 10−6 and 10−5 M) in a dose-dependent manner (Figure 4B).
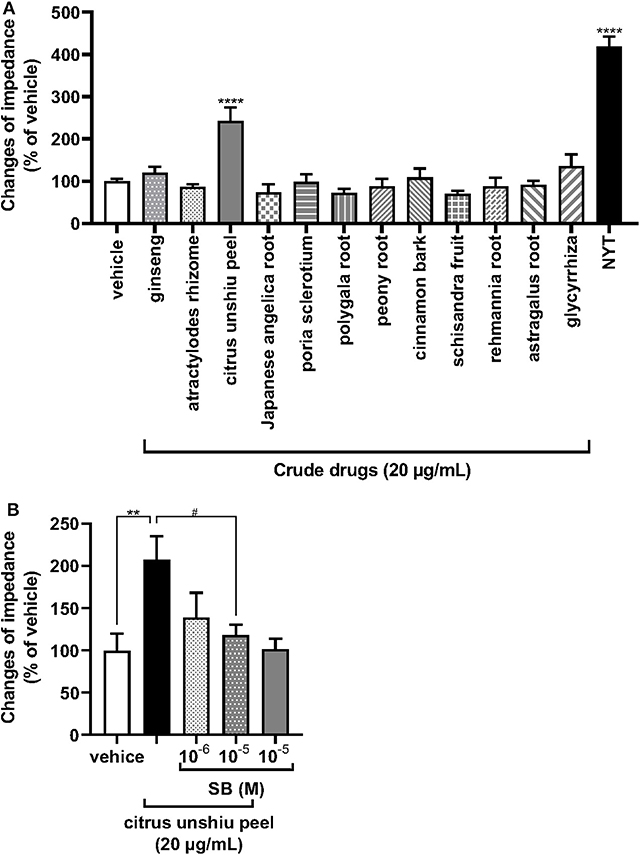
Figure 4. Effects of each herbal crude drug containing NYT on the increase in impedance of OX1R-expressing HEK293 cells. (A) HEK293 cells stably expressing OX1R were treated with each herbal crude drug alone contained in NYT (20 μg/ml) or its vehicle (control) (n = 6–12). (B) The cells expressing OX1R were pretreated with or without the selective OX1R antagonist SB676042 (SB, 10−6 or 10−5 M) for 30 min and then treated with citrus unshiu peel (20 μg/ml) (n = 5–6). The rate of change in impedance is expressed as the difference of the minimum and maximum impedance after drug injection. The data are expressed as mean ± S.E.M (n = 6). ** < 0.001, and ****p < 0.0001, respectively, compared with the vehicle (control); #p < 0.05, compared with citrus unshiu peel; Bonferroni's multiple comparison test following one-way ANOVA.
The present study, to the best of our knowledge, for the first time, revealed that NYT activated OX1R but not GHSR, NPY1R, and NPY5R, which are receptors of various hypothalamic peptides regulating feeding behavior. Moreover, we found that citrus unshiu peel in NYT induced the activation of OX1R. These data suggest that citrus unshiu peel in NYT is an activator of feeding behavior.
NPY is a 36-amino-acid neuropeptide and abundantly distributed in the arcuate nucleus of the hypothalamus, which integrates signals for energy homeostasis (18). NPYR is classified into five subtypes (NPY1, NPY2, NPY3, NPY5, and NPY6), and NPY1R and NPY5R play roles in appetite control (30). The present results showed that NYT induced an increase of ΔZ in HEK293T cells expressing NPY1R but not NPY5R (Figure 1). However, the selective NPY1R antagonist BIBO3340 did not significantly decrease NPY1R-induced responses (Figure 2A). Recently, it has been reported that NPYR forms heterodimers with other GPCRs (41, 42). In some cases, the responses of GPCR heterodimers were not notably inhibited by the antagonist of each GPCR monomer (43). Overall, our present data suggest that NYT might activate NPY1R/endogenous unidentified GPCRs expressed in HEK293 cells as heterodimers.
Orexin (orexin A and orexin B), one of the neuropeptides, was initially recognized as a regulator of feeding behavior, because of its exclusive production in the lateral hypothalamic area (LHA), a region known as the feeding center (17, 20, 29, 31). The orexin receptor is classified into two subtypes (OX1R and OX2R), and OX1R is mainly involved in the increase in food intake (20, 22, 31). In this study, NYT-induced OX1R activities were significantly attenuated by SB676042 (Figure 2C), suggesting that NYT could be an agonist of OX1R. Modified NYT without citrus unshiu peel (100 μg/ml) failed to increase OX1R activities in the cells (Figure 3), and the increase was completely suppressed by SB676042 (Figure 4). However, the level of increase in ΔZ induced by NYT or citrus unshiu peel [NYT (% of control): 433.3 ± 19.5, citrus unshiu peel: 207.4 ± 28.0] was considerably smaller than that caused by OXA (% of control: 9,781.5 ± 252.9). Theoretically, kampo medicine comprises several medical herbal crude drugs; thus, it is considered to exert multiple actions (11, 44–51). Furthermore, each action of kampo medicine is considered milder than that of a drug composed of only one component, such as western drugs. Thus, kampo medicines are known to cause fewer adverse effects (52). Taken together, these results suggest that NYT might have other actions, besides the activation of OX1R, to improve appetite and fatigue.
In the present study, modified NYT excluding citrus unshiu peel (100 μg/ml) did not significantly activate OX1R (Figure 3). In addition, the OX1R activities were induced by citrus unshiu peel alone, and this was suppressed by SB676042 (Figure 4). These data suggest that citrus unshiu peel is an agonist of OX1R. In an aqueous extract mixture of NYT, the main ingredients of citrus unshiu peel were hesperidine, nobiletin, tangeretin, heptamethoxyflavone, naringin, and synephrine (53–55). Some studies on blood pharmacokinetics indicated that these ingredients are absorbed into the blood in humans after oral administration (56, 57). In addition, some reports have shown permeation of polymethoxyflavones and nobiletin into the brain using animal models (58, 59). Therefore, these data suggest that ingredients derived from citrus unshiu peel could pass the blood–brain barrier (BBB), and reach the OX1R on neurons. The facts suggest that these ingredients in citrus unshiu peel could pass the BBB and act as agonists of OX1R in neurons. However, further studies are required, and we will elucidate the ingredients that induce the activation of OX1R in the future.
Several studies have shown that cancer cachexia induced a decrease in food intake in accordance with the changes in orexigenic/anorexigenic neuropeptides such as orexin. This suggests that modulating orexigenic/anorexigenic neuropeptide expression is important for patients with cancer to improve cachexia (10, 18). In this study, we revealed that NYT and citrus unshiu peel activated OX1R using an in vitro assay. Kim et al. have shown that citrus unshiu peel extract alleviates cancer-induced weight loss in mice bearing CT-26 adenocarcinoma (60). Although further studies using cancer cachexia–anorexia model animals are required, these previous and our present data suggest that NYT might improve cancer cachexia–anorexia via the activation of OX1R.
Ghrelin, a 28-amino-acid peptide, is mainly secreted from X/A-like cells in the stomach as an orexigenic peptide (19, 28, 32). The ghrelin receptor GHSR is primarily located in NPY and agouti-related protein (AGRP) containing neurons of the hypothalamus–pituitary unit (21–28). The plasma ghrelin levels increase in response to prolonged fasting and rapidly decrease after feeding, suggesting that peripheral ghrelin is significantly important for appetite regulation (26). We previously reported that rikkunshito (RKT), a Japanese herbal kampo medicine, improved appetite as assessed using a visual analog scale (VAS) in a randomized phase II study (61). In addition, we previously revealed the mechanism through which RKT ameliorated anorexia in cancer cachexia model rats (32, 62). It has been demonstrated that RKT alleviated ghrelin resistance by enhancement of ghrelin signaling (32). We also previously reported that atractylodin, an ingredient in Atractylodes lancea rhizome, an herbal drug composing RKT, enhanced ghrelin-induced GHSR activation via an increase in the ghrelin/GHSR-binding activity (33). However, NYT does not contain Atractylodes lancea rhizome, and the present study showed that NYT neither activated GHSR (Figure 1A) nor enhanced the ghrelin-induced GHSR activation (Supplementary Figure 1). These data suggest that although both RKT and NYT are involved in the improvement of anorexia, they may ameliorate cancer cachexia–related anorexia via different mechanisms.
In conclusion, the present results suggest that NYT and its ingredient citrus unshiu peel activated OX1R. Although further studies using animal models with cancer cachexia–anorexia are needed, these data suggest that NYT might improve cancer cachexia–anorexia partially via activation of OX1R. This study provides scientific evidence supporting the use of NYT in patients with cancer cachexia–anorexia.
All datasets generated for this study are included in the article/Supplementary Material.
KM and YU: conceptualization and writing—review and editing. KM, KO, and YY: methodology: MN: validation. KO, NS, and SF: investigation. KM, KO, and NS: data curation. KM: writing—original draft preparation. YH, KY, and HF: supervision. YU: project administration and funding acquisition.
This work was supported by a grant from Kracie Pharma, Ltd. This funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
YU received grant support from Kracie Pharma, Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Editage (www.editage.jp) for English language editing.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2020.00005/full#supplementary-material
1. Laviano A, Meguid MM, Inui A, Muscaritoli M, Rossi-Fanelli F. Therapy insight: cancer anorexia-cachexia syndrome–when all you can eat is yourself. Nat Clin Pract Oncol. (2005) 2:158–65. doi: 10.1038/ncponc0112
2. Muscaritoli M, Bossola M, Aversa Z, Bellantone R, Rossi Fanelli F. Prevention and treatment of cancer cachexia: new insights into an old problem. Eur J Cancer. (2006) 42:31–41. doi: 10.1016/j.ejca.2005.07.026
3. Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. (2008) 27:793–9. doi: 10.1016/j.clnu.2008.06.013
4. Theologides A. Cancer cachexia. Cancer. (1979) 43:2004–12. doi: 10.1002/1097-0142(197905)43:5+<2004::AID-CNCR2820430708>3.0.CO;2-%23
6. Topkan E, Yavuz AA, Ozyilkan O. Cancer cachexia: pathophysiologic aspects and treatment options. Asian Pac J Cancer Prev. (2007) 8:445–51.
7. Sudo Y, Otsuka H, Miyakawa R, Goto A, Kashiwase Y, Terawaki K, et al. Differential metabolic responses to adipose atrophy associated with cancer cachexia and caloric restriction in rats and the effect of rikkunshito in cancer cachexia. Int J Mol Sci. (2018) 19:3852. doi: 10.3390/ijms19123852
8. Hanada T, Toshinai K, Kajimura N, Nara-Ashizawa N, Tsukada T, Hayashi Y, et al. Anti-cachectic effect of ghrelin in nude mice bearing human melanoma cells. Biochem Biophys Res Commun. (2003) 301:275–9. doi: 10.1016/S0006-291X(02)03028-0
9. Neary NM, Small CJ, Wren AM, Lee JL, Druce MR, Palmieri C, et al. Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. J Clin Endocrinol Metab. (2004) 89:2832–6. doi: 10.1210/jc.2003-031768
10. Ramos EJ, Suzuki S, Marks D, Inui A, Asakawa A, Meguid MM. Cancer anorexia-cachexia syndrome: cytokines and neuropeptides. Curr Opin Clin Nutr Metab Care. (2004) 7:427–34. doi: 10.1097/01.mco.0000134363.53782.cb
11. Miyano K, Nonaka M, Uzu M, Ohshima K, Uezono Y. Multifunctional actions of ninjinyoeito, a Japanese kampo medicine: accumulated scientific evidence based on experiments with cells and animal models, and clinical studies. Front Nutr. (2018) 5:93. doi: 10.3389/fnut.2018.00093
12. Liu Y, Jia Z, Dong L, Wang R, Qiu G. A randomized pilot study of atractylenolide I on gastric cancer cachexia patients. Evid Based Complement Alternat Med. (2008) 5:337–44. doi: 10.1093/ecam/nem031
13. Lobina C, Carai MA, Loi B, Gessa GL, Riva A, Cabri W, et al. Protective effect of Panax ginseng in cisplatin-induced cachexia in rats. Future Oncol. (2014) 10:1203–14. doi: 10.2217/fon.13.276
15. Tahaghoghi-Hajghorbani S, Ebrahimzadeh MA, Rafiei A, Golpour M, Hosseini-Khah Z, Akhtari J. Improvement of chemotherapy through reducing of cachexia by using Citrus unshiu peel extract. J Ethnopharmacol. (2019) 242:111929. doi: 10.1016/j.jep.2019.111929
16. Bae T, Jang J, Lee H, Song J, Chae S, Park M, et al. Paeonia lactiflora root extract suppresses cancer cachexia by down-regulating muscular NF-kappaB signalling and muscle-specific E3 ubiquitin ligases in cancer-bearing mice. J. Ethnopharmacol. (2020) 246:112222. doi: 10.1016/j.jep.2019.112222
17. Edwards CM, Abusnana S, Sunter D, Murphy KG, Ghatei MA, Bloom SR. The effect of the orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin. J Endocrinol. (1999) 160:R7–12. doi: 10.1677/joe.0.160r007
18. Inui A. Cancer anorexia-cachexia syndrome: are neuropeptides the key? Cancer Res. (1999) 59:4493–501.
19. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. (1999) 402:656–60. doi: 10.1038/45230
20. Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, et al. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept. (2000) 96:45–51. doi: 10.1016/S0167-0115(00)00199-3
21. Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Central effect of ghrelin, an endogenous growth hormone secretagogue, on hypothalamic peptide gene expression. Endocrinology. (2000) 141:4797–800. doi: 10.1210/endo.141.12.7920
22. Yamada H, Okumura T, Motomura W, Kobayashi Y, Kohgo Y. Inhibition of food intake by central injection of anti-orexin antibody in fasted rats. Biochem Biophys Res Commun. (2000) 267:527–31. doi: 10.1006/bbrc.1999.1998
23. Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. (2001) 120:337–45. doi: 10.1053/gast.2001.22158
24. Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, et al. A role for ghrelin in the central regulation of feeding. Nature. (2001) 409:194–8. doi: 10.1038/35051587
25. Shintani M, Ogawa Y, Ebihara K, Aizawa-Abe M, Miyanaga F, Takaya K, et al. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. (2001) 50:227–32. doi: 10.2337/diabetes.50.2.227
26. Tschop M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, et al. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest. (2001) 24:Rc19–21. doi: 10.1007/BF03351037
27. Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. (2001) 86:5992. doi: 10.1210/jcem.86.12.8111
28. Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. (2002) 123:1120–8. doi: 10.1053/gast.2002.35954
29. Haynes AC, Chapman H, Taylor C, Moore GB, Cawthorne MA, Tadayyon M, et al. Anorectic, thermogenic and anti-obesity activity of a selective orexin-1 receptor antagonist in ob/ob mice. Regul Pept. (2002) 104:153–9. doi: 10.1016/S0167-0115(01)00358-5
30. Lin S, Boey D, Herzog H. NPY and Y receptors: lessons from transgenic and knockout models. Neuropeptides. (2004) 38:189–200. doi: 10.1016/j.npep.2004.05.005
31. Sakurai T. Roles of orexins in the regulation of body weight homeostasis. Obes Res Clin Pract. (2014) 8:e414–20. doi: 10.1016/j.orcp.2013.12.001
32. Terawaki K, Kashiwase Y, Sawada Y, Hashimoto H, Yoshimura M, Ohbuchi K, et al. Development of ghrelin resistance in a cancer cachexia rat model using human gastric cancer-derived 85As2 cells and the palliative effects of the Kampo medicine rikkunshito on the model. PLoS ONE. (2017) 12:e0173113. doi: 10.1371/journal.pone.0173113
33. Fujitsuka N, Asakawa A, Uezono Y, Minami K, Yamaguchi T, Niijima A, et al. Potentiation of ghrelin signaling attenuates cancer anorexia-cachexia and prolongs survival. Transl Psychiatry. (2011) 1:e23. doi: 10.1038/tp.2011.25
34. Mizushima S, Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. (1990) 18:5322. doi: 10.1093/nar/18.17.5322
35. Quitterer U, Pohl A, Langer A, Koller S, Abdalla S. A cleavable signal peptide enhances cell surface delivery and heterodimerization of Cerulean-tagged angiotensin II AT1 and bradykinin B2 receptor. Biochem Biophys Res Commun. (2011) 409:544–9. doi: 10.1016/j.bbrc.2011.05.041
36. Miyano K, Sudo Y, Yokoyama A, Hisaoka-Nakashima K, Morioka N, Takebayashi M, et al. History of the G protein-coupled receptor (GPCR) assays from traditional to a state-of-the-art biosensor assay. J Pharmacol Sci. (2014) 126:302–9. doi: 10.1254/jphs.14R13CP
37. Hisaoka-Nakashima K, Miyano K, Matsumoto C, Kajitani N, Abe H, Okada-Tsuchioka M, et al. Tricyclic antidepressant amitriptyline-induced glial cell line-derived neurotrophic factor production involves pertussis toxin-sensitive galphai/o activation in astroglial cells. J Biol Chem. (2015) 290:13678–91. doi: 10.1074/jbc.M114.622415
38. Kajitani N, Miyano K, Okada-Tsuchioka M, Abe H, Itagaki K, Hisaoka-Nakashima K, et al. Identification of lysophosphatidic acid receptor 1 in astroglial cells as a target for glial cell line-derived neurotrophic factor expression induced by antidepressants. J Biol Chem. (2016) 291:27364–70. doi: 10.1074/jbc.M116.753871
39. Meguro Y, Miyano K, Hirayama S, Yoshida Y, Ishibashi N, Ogino T, et al. Neuropeptide oxytocin enhances mu opioid receptor signaling as a positive allosteric modulator. J Pharmacol Sci. (2018) 137:67–75. doi: 10.1016/j.jphs.2018.04.002
40. Manabe S, Miyano K, Fujii Y, Ohshima K, Yoshida Y, Nonaka M, et al. Possible biased analgesic of hydromorphone through the G protein-over beta-arrestin-mediated pathway: cAMP, CellKey, and receptor internalization analyses. J Pharmacol Sci. (2019) 140:171–7. doi: 10.1016/j.jphs.2019.06.005
41. Parker MS, Sah R, Balasubramaniam A, Park EA, Sallee FR, Parker SL. Dimers of G-protein coupled receptors as versatile storage and response units. Int J Mol Sci. (2014) 15:4856–77. doi: 10.3390/ijms15034856
42. Kilpatrick LE, Humphrys LJ, Holliday ND. A G protein-coupled receptor dimer imaging assay reveals selectively modified pharmacology of neuropeptide Y Y1/Y5 receptor heterodimers. Mol Pharmacol. (2015) 87:718–32. doi: 10.1124/mol.114.095356
43. Hubner H, Schellhorn T, Gienger M, Schaab C, Kaindl J, Leeb L, et al. Structure-guided development of heterodimer-selective GPCR ligands. Nat Commun. (2016) 7:12298. doi: 10.1038/ncomms12298
44. Kono T, Kaneko A, Matsumoto C, Miyagi C, Ohbuchi K, Mizuhara Y, et al. Multitargeted effects of hangeshashinto for treatment of chemotherapy-induced oral mucositis on inducible prostaglandin E2 production in human oral keratinocytes. Integr Cancer Ther. (2014) 13:435–45. doi: 10.1177/1534735413520035
45. Fukamachi H, Matsumoto C, Omiya Y, Arimoto T, Morisaki H, Kataoka H, et al. Effects of Hangeshashinto on growth of oral microorganisms. Evid Based Complement Alternat Med. (2015) 2015:512947. doi: 10.1155/2015/512947
46. Matsumoto C, Sekine-Suzuki E, Nyui M, Ueno M, Nakanishi I, Omiya Y, et al. Analysis of the antioxidative function of the radioprotective Japanese traditional (Kampo) medicine, hangeshashinto, in an aqueous phase. J Radiat Res. (2015) 56:669–77. doi: 10.1093/jrr/rrv023
47. Hiroshima Y, Bando M, Inagaki Y, Kido R, Kataoka M, Nagata T, et al. Effect of Hangeshashinto on calprotectin expression in human oral epithelial cells. Odontology. (2016) 104:152–62. doi: 10.1007/s10266-015-0196-3
48. Hitomi S, Ono K, Yamaguchi K, Terawaki K, Imai R, Kubota K, et al. The traditional Japanese medicine hangeshashinto alleviates oral ulcer-induced pain in a rat model. Arch Oral Biol. (2016) 66:30–7. doi: 10.1016/j.archoralbio.2016.02.002
49. Hitomi S, Ono K, Terawaki K, Matsumoto C, Mizuno K, Yamaguchi K, et al. [6]-gingerol and [6]-shogaol, active ingredients of the traditional Japanese medicine hangeshashinto, relief oral ulcerative mucositis-induced pain via action on Na(+) channels. Pharmacol Res. (2017) 117:288–302. doi: 10.1016/j.phrs.2016.12.026
50. Miyashita T, Kono T, Matsui D, Yamazaki Y, Sadatomi D, Fujitsuka N, et al. Preventive effect of oral hangeshashinto (TJ-14) on the development of reflux-induced esophageal cancer. Surgery. (2018) 164:49–55. doi: 10.1016/j.surg.2018.02.003
51. Hitomi S, Ujihara I, Ono K. Pain mechanism of oral ulcerative mucositis and the therapeutic traditional herbal medicine hangeshashinto. J Oral Biosci. (2019) 61:12–5. doi: 10.1016/j.job.2019.01.004
52. Tatsumi L, Suzuki T, Yamada K, Mimura M, Uchida H. Kampo, A Japanese traditional medicinal system for psychiatric conditions: a narrative review. Pharmacopsychiatry. (2019) 52:251–60. doi: 10.1055/a-0637-9760
53. Zhang C, Lu Y, Tao L, Tao X, Su X, Wei D. Tyrosinase inhibitory effects and inhibition mechanisms of nobiletin and hesperidin from citrus peel crude extracts. J Enzyme Inhib Med Chem. (2007) 22:83–90. doi: 10.1080/14756360600953876
54. Matsumoto T, Nishikawa T, Furukawa A, Itano S, Tamura Y, Hasei T, et al. Antimutagenic effects of polymethoxy flavonoids of Citrus unshiu. Nat Prod Commun. (2017) 12:23–6. doi: 10.1177/1934578X1701200108
55. Kim JJ, Kim K, Jung YR, Bian Y, Ngo T, Bae ON, et al. Co-existence of hypertensive and anti-hypertensive constituents, synephrine, and nobiletin in Citrus unshiu Peel. Molecules. (2019) 24:1197. doi: 10.3390/molecules24071197
56. Kanaze FI, Bounartzi MI, Georgarakis M, Niopas I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur J Clin Nutr. (2007) 61:472–7. doi: 10.1038/sj.ejcn.1602543
57. Kitagawa H, Munekage M, Matsumoto T, Sadakane C, Fukutake M, Aoki K, et al. Pharmacokinetic profiles of active ingredients and its metabolites derived from rikkunshito, a ghrelin enhancer, in healthy Japanese volunteers: a cross-over, randomized study. PLoS ONE. (2015) 10:e0133159. doi: 10.1371/journal.pone.0133159
58. Singh SP, Wahajuddin Tewari D, Patel K, Jain GK. Permeability determination and pharmacokinetic study of nobiletin in rat plasma and brain by validated high-performance liquid chromatography method. Fitoterapia. (2011) 82:1206–14. doi: 10.1016/j.fitote.2011.08.010
59. Okuyama S, Miyazaki K, Yamada R, Amakura Y, Yoshimura M, Sawamoto A, et al. Permeation of Polymethoxyflavones into the mouse brain and their effect on MK-801-induced locomotive hyperactivity. Int J Mol Sci. (2017) 18:489. doi: 10.3390/ijms18030489
60. Kim A, Im M, Gu MJ, Ma JY. Citrus unshiu peel extract alleviates cancer-induced weight loss in mice bearing CT-26 adenocarcinoma. Sci Rep. (2016) 6:24214. doi: 10.1038/srep24214
61. Inoue T, Takagi H, Owada Y, Watanabe Y, Yamaura T, Fukuhara M, et al. The efficacy of the Kampo medicine rikkunshito for chemotherapy-induced anorexia (RICH trial): study protocol for a randomized controlled trial. Trials. (2017) 18:485. doi: 10.1186/s13063-017-2227-6
Keywords: anorexia, citrus unshiu peel, kampo medicine, ninjinyoeito, orexin 1 receptor
Citation: Miyano K, Ohshima K, Suzuki N, Furuya S, Yoshida Y, Nonaka M, Higami Y, Yoshizawa K, Fujii H and Uezono Y (2020) Japanese Herbal Medicine Ninjinyoeito Mediates Its Orexigenic Properties Partially by Activating Orexin 1 Receptors. Front. Nutr. 7:5. doi: 10.3389/fnut.2020.00005
Received: 15 November 2019; Accepted: 10 January 2020;
Published: 27 February 2020.
Edited by:
Sergueï O. Fetissov, Université de Rouen, FranceReviewed by:
Koji Ataka, Kagoshima University, JapanCopyright © 2020 Miyano, Ohshima, Suzuki, Furuya, Yoshida, Nonaka, Higami, Yoshizawa, Fujii and Uezono. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yasuhito Uezono, eXVlem9ub0BuY2MuZ28uanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.