- 1Oak Ridge Institute for Science and Education Supporting the Military Nutrition Division of the US Army Research Institute of Environmental Medicine, Natick, MA, United States
- 2Military Nutrition Division of the US Army Research Institute of Environmental Medicine, Natick, MA, United States
- 3Military Performance Division of the US Army Research Institute of Environmental Medicine, Natick, MA, United States
Objective: This study characterized habitual dietary protein intake in healthy young adults entering military service and explored whether diet protein density is associated with diet quality and micronutrient intake.
Methods: An FFQ was used to estimate habitual dietary intake and calculate HEI scores in 276 males [mean(SD), age:21.1y(3.8)] and 254 females [age:21.2y(3.7)]. Multivariate-adjusted MANCOVA and ANCOVA models were used to identify associations between protein density quartiles and HEI scores and micronutrient intake. Higher HEI components scores for sodium, refined grains, and empty calories indicate lower intake; higher scores for all other components indicate higher intakes.
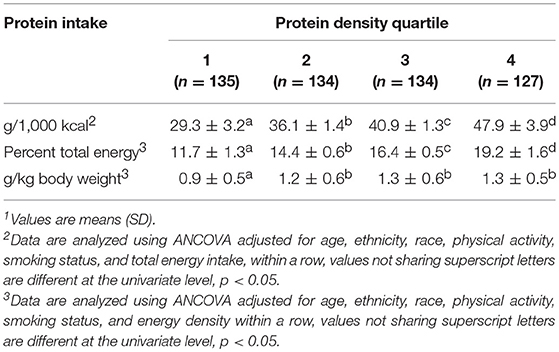
Results: Mean(SD) energy-adjusted protein intakes were 29.3(3.2), 36.0(1.4), 40.8(1.3), and 47.9(3.9) g/1,000 kcal for protein density quartiles 1–4, respectively. For males, empty calorie scores as well as dark green and orange vegetable scores were higher in quartiles 3 and 4 than 1 and 2 (all, p < 0.05). Scores for total vegetable, dairy, and total protein foods were lower in quartile 1 vs. quartiles 2, 3, and 4 (all, p < 0.05). Sodium scores decreased as quartiles increased (p < 0.001). Total HEI, fruit, whole grains, seafood and plant protein, fatty acids, and refined grain scores did not differ. For females, total HEI, vegetable, and total protein foods scores were higher in quartiles 3 and 4 than 1 and 2 (all, p < 0.05). Empty calorie scores increased as quartile increased (p < 0.05). Dairy scores were higher in quartiles 2, 3, and 4 than 1 (p < 0.05). Whole fruit scores were lowest in quartile 1 (p < 0.05). Whole grain as well as seafood and plant protein scores were higher in quartile 4 vs. 1 (both, p < 0.05). Sodium scores decreased as quartile increased (p < 0.001). Fatty acids scores did not differ. For males and females, micronutrient intakes progressively increased across quartiles with the exception of calcium and vitamin C, (all, p < 0.05). Intakes remained nearly the same when controlled for fruit and vegetable intake.
Conclusion: These cross-sectional data suggest that habitually consuming a higher protein density diet is associated with better scores for some, but not all, diet quality components in males, better overall diet quality scores in females, and greater intakes of micronutrients in both male and female healthy, young adults entering military service.
Introduction
Dietary protein recommendations are established as the minimum amount of dietary protein intake necessary to maintain nitrogen balance (1). However, accumulating evidence demonstrates that protein intakes above the Recommended Dietary Allowance (RDA; 0.8 g • kg−1 • d−1 for healthy adults) are metabolically advantageous and may reduce chronic disease risk (2), enhance satiety (3), and body composition during weight loss (4) and exercise training (5). Protein-containing foods are comprised of more than their constituent amino acids; they also contain a high ratio of micronutrients to energy and are therefore nutrient-dense (1). As a result, consuming a higher protein-dense diet, defined as consuming more energy from protein-containing whole foods without increasing total energy intake, may enhance diet quality and improve micronutrient intake (1, 6).
Diet quality is considered a primary modifiable risk factor associated with preventable health complications and chronic disease development (7, 8). Ensuring dietary micronutrient intakes meet minimum requirements is one strategy to optimize diet quality. Optimizing diet quality is particularly important for populations whose health and physical performance are critical to occupational success and resilience to injury and stressors associated with unaccustomed physical training, including healthy young adults entering initial military training (9–11). The Dietary Guidelines for Americans 2015–2020 recently identified several micronutrients that are often underconsumed and are therefore considered shortfall nutrients (12). These include potassium, choline, magnesium, calcium, vitamins A, D, E, and C (12), which serve critical roles in bone health, blood pressure regulation, cancer, and cardiovascular disease prevention (13–16). Suboptimal intakes of iron, folate, zinc, and vitamins B1, B2, B3, and B12 may diminish physical performance and limit beneficial adaptations to physical training (5, 17, 18). Nutrient-dense, protein-containing whole-foods are excellent sources of the aforementioned micronutrients (19, 20). It is also possible that increasing the protein density of the diet may be related to better overall food choices that contribute to better diet quality and micronutrient intake. In contrast it is conceivable that consuming more protein-containing foods may negatively impact diet quality if these foods are higher in saturated-fat and sodium (i.e., processed and non-lean meats). However, whether consuming higher amounts of total energy as protein-containing foods improves or diminishes diet quality and micronutrient intakes is not well-described (1).
This cross-sectional study characterized habitual dietary protein intake in healthy young adults entering military service and explored whether the protein density of the diet was associated with diet quality, as indicated by Healthy Eating Index (HEI) scores and micronutrient intake. We hypothesized that when controlling for energy density and total energy intake, diet quality, and micronutrient intakes would be greater in those consuming higher quantities of dietary protein.
Methods
This research was carried out in accordance with US Army Regulation 70–25 and the provisions of Title 32 Code of Federal Regulations Part 219 Protection of Human Subjects. This research was approved by the Institutional Review Board at the US Army Research Institute of Environmental Medicine.
Participants
The study sample included 890 healthy adults (ages 17–42 y) entering initial military training. Data collection occurred over four study iterations which took place as follows: February, 2010 at Fort Jackson, SC (n = 223); June 2012 and February 2013 at Fort Sill, OK (n = 492); and April 2015 at Fort Jackson, SC (n = 175). All data were collected as part of primary studies designed to assess the effects of calcium and vitamin D supplementation on bone health (11, 21, 22). All participants provided informed, written consent.
Dietary Intake
The 3-month 2005 Block Food Frequency Questionnaire (FFQ) was used to assess dietary intake prior to initial military training accession. This semi-quantitative FFQ captures usual intakes of food groups and nutrients for 3 months prior to administration using a food item list (23). Respondents select the frequency (i.e., never to every day) and quantity of foods they consumed from the food item list, and are asked to specify if foods were modified or standard items (i.e., low-fat vs. full-fat foods). The FFQ is commonly used to assess dietary intakes and is validated for use in the general US population and has been used to assess dietary intake in military populations (24–27). Charts of photographed foods denoting portion sizes were provided to assist in portion size estimation, and registered Dietitians were available to answer participant questions regarding the FFQ. Questionnaires were analyzed by Nutrition Quest (Berkeley, CA, USA) using the US Department of Agriculture Food and Nutrient Database for Studies version 1.0. Analysis included computation macro- and micronutrient intakes in addition to HEI total and component scores. Participants were excluded from analyses if they had missing data or indicated implausible energy intakes (males < 800 or > 5,000 kcal/d; females < 300 or > 4,500 kcal/d) (8, 9). Those that reported consuming supplements at least once per week were also excluded. Demographic information was collected through self-report. Dietary intakes from 276 males [mean (SD), age: 21.1 y (3.8), body mass index: 25.8 kg/m2 (3.7)] and 254 females [age: 21.2 y (3.7), BMI: 24.2 kg/m2 (2.9)] were included in the final statistical analyses.
Healthy Eating Index Components
HEI is a diet quality measure that reflects conformance to the Dietary Guidelines for Americans (28). HEI total scores are the composite of 12 component scores and range from 0 to 100; with 100 denoting perfect compliance with the Dietary Guidelines for Americans. HEI component scores are categorized as indicators of adequacy and moderation. Adequacy components included: total fruit, whole fruit, total vegetables, dark green and orange vegetables and legumes (DGOVL), dairy, total protein foods, seafood and plant protein, whole grains, and fatty acids. Higher adequacy component scores indicate higher consumption. Moderation components included: sodium, refined grains, and empty calories (i.e., calories from solid fats, alcohol, and added sugars). Higher moderation component scores indicate lower consumption of these categories. Collectively, higher total and component scores suggest better dietary quality vs. lower scores. HEI 2010 was used for the current analyses to maintain consistency within the data set since a majority of the data had been analyzed by Nutrition Quest (Berkeley, CA, USA) prior to the release of HEI 2015.
Statistical Analyses
A multivariate-adjusted ANCOVA model was used to identify associations between quartiles of protein intake and means of energy adjusted protein intake as well as means of relative protein intake. A multivariate-adjusted ANCOVA model was used to identify associations between quartiles of protein intake and total HEI score. Multivariate-adjusted MANCOVA models were used to identify associations between quartiles of protein intake and HEI component scores (i.e., total fruit, whole fruit, total vegetables, dark green and orange vegetables and legumes (DGOVL), dairy, total protein foods, seafood and plant protein, whole grains, fatty acids, sodium, refined grains, and empty calories) as well as micronutrients of interest. A sex-by-protein density quartile interaction was detected for the Total HEI and HEI component scores. Therefore, these data was analyzed for each sex separately (i.e., Model 1). Model 1 was adjusted for study iteration, age, ethnicity, race, physical activity, smoking status, energy density (kcal/g of food consumed) and total energy intake. No sex-by-protein density quartile interaction was detected for micronutrient intakes. Thus, sex was added to Model 2 as a covariate. For Model 3, fruit and vegetable intakes were added to Model 2 as covariates. Race was categorized as white, black, or other. Ethnicity was categorized as Hispanic or non-Hispanic. Habitual physical activity was categorized as yes (i.e., at least one time per week) or no (i.e., never or rarely). Smoking habits were categorized as yes (i.e., current smoker) or no (i.e., non-smoker or former smoker). Data were analyzed using the Statistical Package for the Social Sciences (version 24.0; IBM SPSS). A Bonferroni correction was applied to correct for multiple comparisons. All results are presented as mean (SD) as appropriate. Adherence to model assumptions was verified and statistical significance was set at p < 0.05.
Results
Protein Density Quartiles
Mean ± SD energy-adjusted protein intakes increased across protein density quartiles [29.3 (3.2), 36.1 (1.4), 40.9 (1.3), and 47.9 (3.9) g/1,000 kcal, respectively; all, p < 0.05; Table 1)]. Protein intakes expressed as percent of total energy intake increased across protein density quartiles (11.7 (1.3), 14.4 (0.6), 16.4 (0.5), and 19.2 (1.6) percent, respectively; all, p < 0.05). Relative protein intakes across protein density quartiles were lower in quartile 1 [0.9 (0.5)] than quartiles 2, 3, and 4 (1.2 (0.6), 1.3 (0.6), and 1.3 (0.5) g • kg−1 • d−1, respectively; all, p < 0.05.
HEI Total and Component Scores
For males empty calorie and DGOVL component scores were higher in protein density quartiles 3 and 4 than 1 and 2 (all, p < 0.05; Table 2). Component scores for total vegetable, dairy, and total protein food consumption were lower in protein density quartile 1 compared to quartiles 2, 3, and 4 (all, p < 0.05). Sodium component scores decreased progressively as protein density quartile increased (p < 0.001). Total HEI, total fruit, whole fruit, whole grains, seafood and plant protein, fatty acids, and refined grain scores did not differ across protein density quartiles.
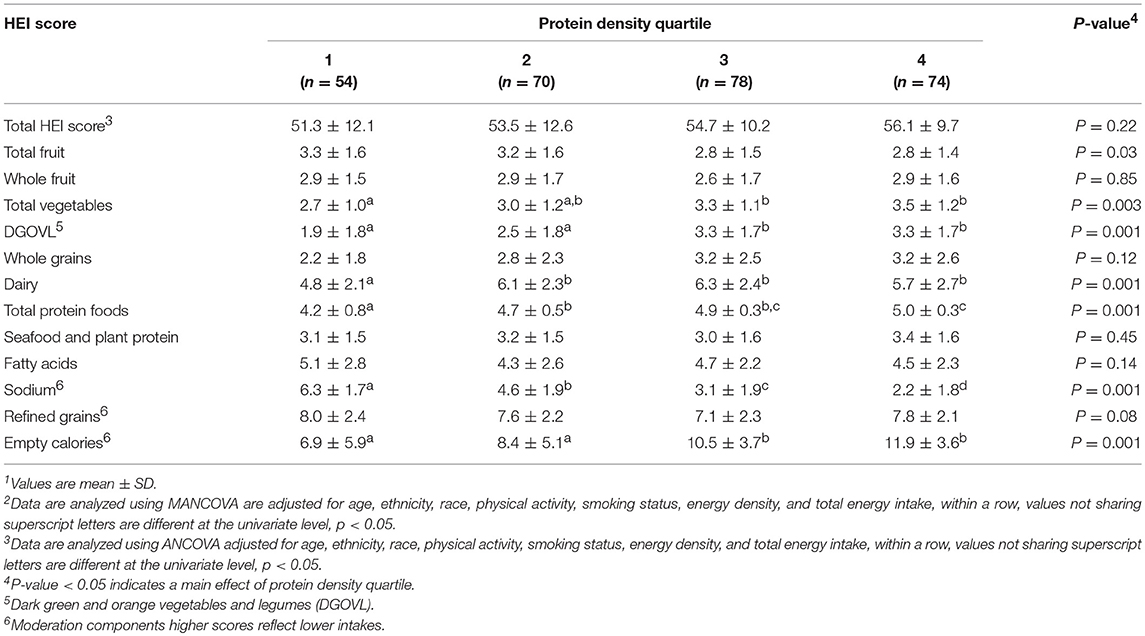
Table 2. Healthy Eating Index (HEI) scores based on habitual protein intake in healthy young males1, 2.
For females total HEI, total vegetables, DGOVL, and total protein foods were higher in protein density quartiles 3 and 4 than quartiles 1 and 2 (all, p < 0.05; Table 3). Empty calorie component scores increased as protein density quartile increased (p < 0.05). Dairy component scores were higher in quartiles 2, 3, and 4 than quartile 1 (p < 0.05). Refined grain component scores were lowest in protein density quartile 2 and whole fruit scores were lowest in quartile 1 (both, p < 0.05). Seafood and plant protein as well as whole grain consumption were only higher in protein density quartile 4 compared to quartile 1 (both, p < 0.05). Sodium component scores decreased progressively as protein density quartile increased (p < 0.001). Fatty acids component scores did not differ across protein density quartiles.
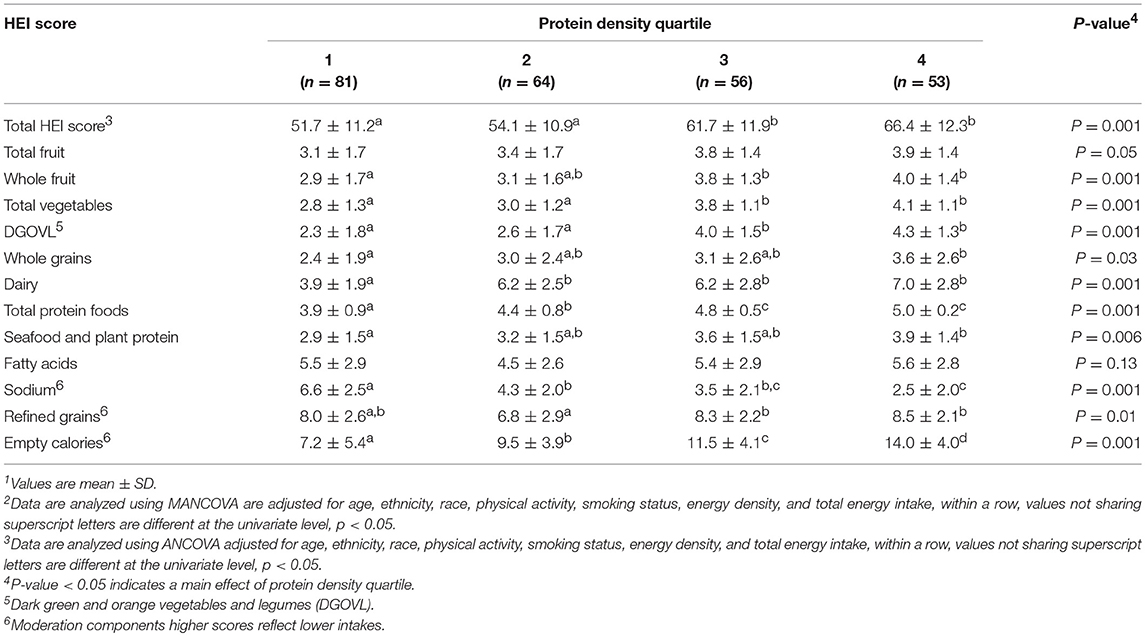
Table 3. Healthy Eating Index (HEI) scores based on habitual protein intake in healthy young females1, 2.
Micronutrient Intakes
Composite micronutrient intake differed (p < 0.001) by protein density quartile in both model 2 and 3. For model 2, individual micronutrient intakes, except calcium and vitamin C, progressively increased (all, p < 0.05) with increasing protein density quartiles (Table 4). Calcium intakes in protein density quartile 1 were lower than quartiles 2, 3, and 4 (p < 0.001). Vitamin C intakes were not different across protein density quartiles. Model 3 indicated that independent of fruit and vegetable intake, all micronutrients progressively increased across protein density quartiles except vitamins A, E, C, and folate (all, p < 0.05) (Table 5). Vitamin A and C intakes in protein density quartile 1 were lower than quartiles 2, 3, and 4 (all, p < 0.001). Vitamin E intakes were not different across protein density quartiles. There was not a protein density quartile-by-sex interaction indicated by model 3, (p = 0.13).
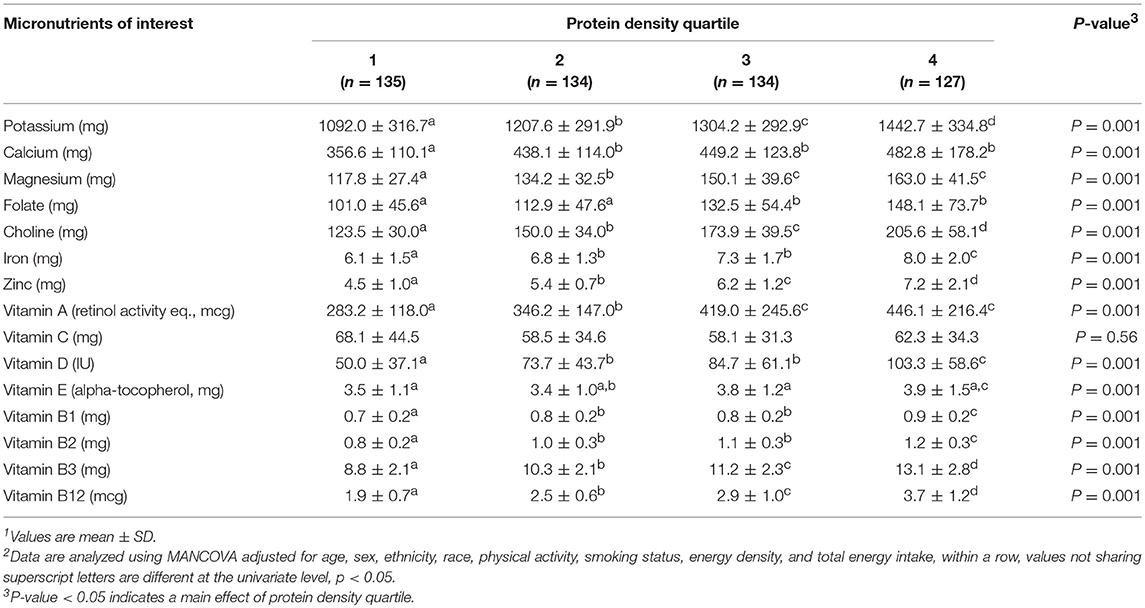
Table 4. Estimated daily micronutrient intakes across protein density quartile in healthy young adults1, 2.
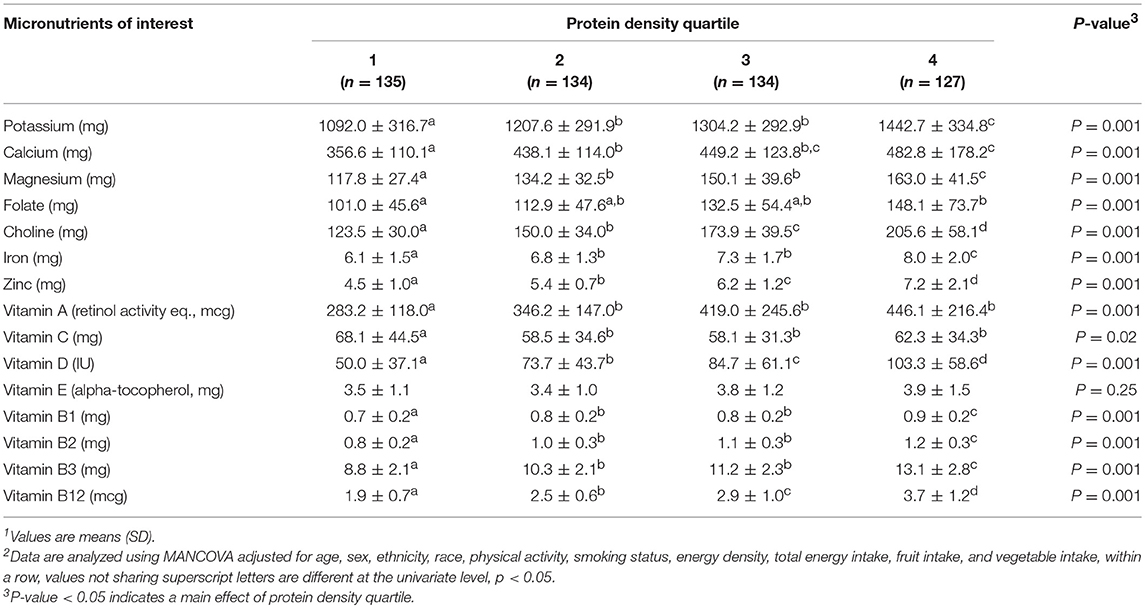
Table 5. Estimated daily micronutrient intakes across protein density quartile when adjusted for fruit and vegetable intake in healthy young adults1, 2.
Discussion
This cross-sectional study assessed whether consuming greater amounts of dietary protein, resulting in higher diet protein density, was associated with diet quality and dietary micronutrient intakes in healthy young adults. We demonstrate that habitually consuming high protein density diets, independent of energy density and total energy intake, was associated with better scores for select diet quality components in males, better overall diet quality scores in females, and greater micronutrient intakes for both males and females. We also demonstrate that the relationship between protein density and intake of several micronutrients was independent of fruit and vegetable intake. These associative data suggest that consuming a high proportion of total energy derived from protein-containing whole foods supports healthy dietary intake patterns that align with current nutrition guidelines for Americans.
To our knowledge, only one other study (29) has addressed whether consuming greater amounts of protein-containing foods was associated with diet quality in healthy adults. In that study total protein intake was negatively associated with diet quality in males but positively associated with diet quality in females. In addition, animal-based protein intake was negatively associated with diet quality in males, whereas animal-based protein intake was positively associated with diet quality in females. Regardless of sex, plant-based protein intake was positively associated with better diet quality. Similarly, we demonstrate that consuming a higher protein-dense diet was associated with consuming more protein from seafood and plants than lower protein-dense diets, but only in females. It is possible that, in this population, males may consume more of their protein from animal sources, whereas in females there may be a larger contribution from plant-based sources. The remaining divergent associations between diet quality, protein quantity, and intakes of animal- and plant-based protein foods (i.e., without including seafood proteins) in the Camilleri study are difficult to reconcile with our findings, which may be largely a function of the diet scoring methodologies used. HEI estimates dietary conformance to the Dietary Guidelines for Americans, whereas the PANDiet index used by Camilleri et al. estimates the probability that usual dietary intakes meet French and or European Union nutritional recommendations (28, 29). Nevertheless, the data from Camilleri et al. highlight an important analytical limitation of the HEI, which does not provide specific examination of all protein food sources or the characteristics of these food items (i.e., lean meats or low-fat dairy). As such, the potential for animal- and plant-based protein foods to be differentially related to diet quality in healthy young adults entering military service cannot be discerned. We were also unable to identify which food sources were responsible for the increasing sodium intakes across protein density quartile. It is possible that the individuals in the highest protein density quartile consumed more processed protein-containing foods, which are typically higher in sodium (30). Lastly, at the time of data analysis, the HEI 2010 was the current method for assessing diet quality and conformance to the Dietary Guidelines for Americans. Future analyses in similar cross-sectional studies using the HEI 2015 would allow for differentiation of added sugars and saturated fats that is unattainable within the empty calories component score of the HEI 2010.
The Dietary Guidelines for Americans recommend a shift toward consuming nutrient-dense foods at the expense of limiting empty calorie intake (i.e., energy from solid fats, added sugars, and alcohol) (12). In the current study, we demonstrate that those in the highest dietary protein density quartile also habitually consumed more total vegetables, including nutrient-dense dark greens, orange vegetables, and legumes, more whole grains (females only), more dairy, and less empty calories. The apparent protein-related increases in dietary nutrient-density and diet quality were not a function of simply consuming more food (i.e., total energy), but rather a combination of consuming more nutrient-dense foods, and fewer nutrient-poor foods that contain empty calories. These findings are comparable to those derived from NHANES 2003–2004, which suggest that those consuming greater amounts of nutrient-dense foods, not protein per se, limited empty calorie intake (31). These findings may be particularly beneficial in the context of healthy weight management and are corroborated by data from other prospective, cross-sectional studies demonstrating lower central adiposity and body mass index in American adults habitually consuming more protein, independent of total energy intake (32, 33).
Individuals in the highest dietary protein density quartile also consumed more non-protein, nutrient-rich foods. This could suggest that the relationship between protein density of the diet and diet quality was mediated by those non-protein foods and general eating habits rather than solely the protein density of the diet. However, when controlling for fruit and vegetable intake, micronutrient intakes across protein density quartiles remained nearly the same. This suggests that food sources of protein within a protein-dense diet are related to nutrient intakes. However, we did not examine whether individuals who consumed a diet with a higher protein density were doing so as part of a strategy to eat an overall healthier diet. Thus, future prospective studies are required to determine causation and identify which food sources of protein may drive this relationship. Additionally, relative protein intakes in the current study were above the RDA, but within the Acceptable Macronutrient Distribution Range for protein (10–35% of total calories) (34). These findings align with our previous observations that protein intakes are generally higher than the RDA in similar military populations (35) and free-living Americans (36).
The potential impact of inadequate micronutrient intake cannot be delineated in the current study, although the biological functions of micronutrients suggest suboptimal intakes may hinder physiologic adaptations and performance during strenuous, unaccustomed physical training (9). Specifically, suboptimal intakes of vitamin D and iron have been recognized to have detrimental effects on health and performance in those entering the military (9–11, 37). Intakes of these nutrients progressively increased as protein density quartile increased suggesting consuming more protein may be beneficial. While the effects of folate and vitamin E on performance are not well-studied, in general suboptimal intakes of folate and vitamin E raise concern as folate is vital for cellular synthesis, growth, and repair (38) and vitamin E is a key antioxidant and contributes to anti-inflammatory processes (39). Similarly, low magnesium intake would suggest a potential greater risk of inefficient energy metabolism and suboptimal neuro-muscular function (40). Although it would be helpful to understand how the estimated micronutrient intakes across protein density quartiles compares to recommended intakes, we cannot directly compare micronutrient intake adequacies to the DRIs due to limitations of the FFQ (41, 42). For example, FFQs rely on single time-point data collection to estimate food intake. Multiple days of direct dietary intake assessment are required when determining adequacy of nutrient intakes (41, 42). However, these data do suggest that increasing the protein density of the diet does seem to relate to better overall diet quality, in this population of healthy young people.
While we were not able to directly address the effects of dietary protein on muscle and performance in the current study, it is reasonable to speculate that higher quality, higher protein diet patterns positively influence skeletal muscle mass, adaptations to exercise, and physical performance. Higher protein intakes offset protein catabolism and support nitrogen balance in individuals exposed to aerobic exercise training (43, 44). Dietary protein, and its constituent amino acids, are also a primary determinant of skeletal muscle protein turnover. Thus, dietary patterns that support routine high-quality protein ingestion, particularly following exercise, should promote beneficial adaptations to training, and facilitate repair and remodeling of existing muscle protein, and accretion of new muscle protein mass (45). The well-established effects of dietary protein on muscle integrity would support shifting dietary patterns in favor of protein dense foods and such a shift would not reduce diet quality since consuming a higher protein density diet appears possible without displacing other nutrient rich, non-protein foods that contribute an overall healthy diet.
Conclusion
This study demonstrated that habitually consuming more protein resulting in a diet with a higher protein density is associated with better scores for some, but not all, diet quality components in males, better overall diet quality scores in females, and greater intakes of micronutrients in both young male and female adults prior to reporting for military service.
Data Availability
The ethics approval given by the Institutional Review Board at the US Army Research Institute of Environmental Medicine was given on the provision that the data would not be shared with researchers upon request.
Ethics Statement
This research was approved by the Institutional Review Board at the US Army Research Institute of Environmental Medicine. Investigators adhered to US Army Regulation 70-25 and the research was conducted in adherence with the provisions of Title 32 Code of Federal Regulations Part 219 Protection of Human Subjects. The consent procedure used was an informed, written consent.
Disclosure
The views and assertions expressed herein are those of the authors and do not reflect the official policy of the Department of Army, Department of Defense, or the US Government. Any citations of commercial organizations and trade names in this report do not constitute an official Department of the Army endorsement of approval of the products or services of these organizations.
Author Contributions
JG and SP had primary responsibility for the final content and wrote the manuscript. JG and JK analyzed the data. JG, PK, LL, EG-S, JM, and SP designed the research and approved the final paper.
Funding
This project was funded by US Army Medical Research and Materiel Command. The study sponsor had no role in study design, collection, analysis, and interpretation of data; writing the report, nor the decision to submit the report for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge the Soldier volunteers that participated in this study and the command staff at Fort Jackson, SC, and Fort Sill, OK who provided access to potential volunteers. We also thank Susan McGraw for assisting with data management and Katelyn Guerriere assisting for with study coordination.
Abbreviations
DGOVL, Dark green and orange vegetables and legumes; FFQ, food frequency questionnaire; HEI, Healthy Eating Index; RDA, recommended dietary allowance.
References
1. Phillips SM, Fulgoni VL III, Heaney RP, Nicklas TA, Slavin JL, Weaver CM. Commonly consumed protein foods contribute to nutrient intake, diet quality, and nutrient adequacy. Am J Clin Nutr. (2015) 101:1346S−2S. doi: 10.3945/ajcn.114.084079
2. Rodriguez NR. Introduction to Protein Summit 2.0: continued exploration of the impact of high-quality protein on optimal health. Am J Clin Nutr. (2015). 101:1317S−9S. doi: 10.3945/ajcn.114.083980
3. Leidy HJ, Clifton PM, Astrup A, Wycherley TP, Westerterp-Plantenga MS, Luscombe-Marsh ND, et al. The role of protein in weight loss and maintenance. Am J Clin Nutr. (2015) 101:1320S−9S. doi: 10.3945/ajcn.114.084038
4. Carbone JW, McClung JP, Pasiakos SM. Recent advances in the characterization of skeletal muscle and whole-body protein responses to dietary protein and exercise during negative energy balance. Adv Nutr. (2018) 10:70–9. doi: 10.1093/advances/nmy087
5. Thomas DT, Erdman KA, Burke LM. American college of sports medicine joint position statement. Nutrition and athletic performance. Med Sci Sports Exerc. (2016) 48:543–68. doi: 10.1249/MSS.0000000000000852
7. World Health Organization. Preventing Chronic Diseases: A Vital Investment. Geneva: World Health Organization (2005).
8. Sotos-Prieto M, Bhupathiraju SN, Mattei J, Fung TT, Li Y, Pan A, et al. Association of changes in diet quality with total and cause-specific mortality. N Engl J Med. (2017) 377:143–53. doi: 10.1056/NEJMoa1613502
9. McClung JP, Karl JP, Cable SJ, Williams KW, Nindl BC, Young AJ, et al. Randomized, double-blind, placebo-controlled trial of iron supplementation in female soldiers during military training: effects on iron status, physical performance, and mood. Am J Clin Nutr. (2009) 90:124–31. doi: 10.3945/ajcn.2009.27774
10. Karl JP, Lieberman HR, Cable SJ, Williams KW, Young AJ, McClung JP. Randomized, double-blind, placebo-controlled trial of an iron-fortified food product in female soldiers during military training: relations between iron status, serum hepcidin, and inflammation. Am J Clin Nutr. (2010) 92:93–100. doi: 10.3945/ajcn.2010.29185
11. Gaffney-Stomberg E, Lutz LJ, Rood JC, Cable SJ, Pasiakos SM, Young AJ, et al. Calcium and vitamin D supplementation maintains parathyroid hormone and improves bone density during initial military training: a randomized, double-blind, placebo controlled trial. Bone. (2014) 68:46–56. doi: 10.1016/j.bone.2014.08.002
12. U.S. Department of Health and Human Services, U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th ed. (2015). Available online at: http://health.gov/dietaryguidelines/2015/guidelines/ (accessed October 19, 2019).
13. Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. (1996) 334:1150–5. doi: 10.1056/NEJM199605023341802
14. Zeisel SH, Da Costa K-A. Choline: an essential nutrient for public health. Nutr Rev. (2009) 67:615–23. doi: 10.1111/j.1753-4887.2009.00246.x
16. Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev. (2015) 95:1–46. doi: 10.1152/physrev.00012.2014
17. Lukaski HC. Vitamin and mineral status: effects on physical performance. Nutrition. (2004) 20:632–44. doi: 10.1016/j.nut.2004.04.001
18. McClung JP, Gaffney-Stomberg E. Optimizing performance, health, and well-being: nutritional factors. Mil Med. (2016) 181(Suppl. 1):86–91. doi: 10.7205/milmed-d-15-00202
19. O'Neil CE, Keast DR, Fulgoni VL, Nicklas TA. Food sources of energy and nutrients among adults in the US: NHANES 2003–2006. Nutrients. (2012) 4:2097–120. doi: 10.3390/nu4122097
20. Huth PJ, Fulgoni VL, Keast DR, Park K, Auestad N. Major food sources of calories, added sugars, and saturated fat and their contribution to essential nutrient intakes in the U.S. diet: data from the national health and nutrition examination survey (2003–2006). Nutr J. (2013) 12:116. doi: 10.1186/1475-2891-12-116
21. Lutz LJ, Karl JP, Rood JC, Cable SJ, Williams KW, Young AJ, et al. Vitamin D status, dietary intake, and bone turnover in female soldiers during military training: a longitudinal study. J Int Soc Sports Nutr. (2012) 9:38. doi: 10.1186/1550-2783-9-38
22. Hughes JM, Gaffney-Stomberg E, Guerriere KI, Taylor KM, Popp KL, Xu C, et al. Changes in tibial bone microarchitecture in female recruits in response to 8 weeks of US Army Basic Combat Training. Bone. (2018) 113:9–16. doi: 10.1016/j.bone.2018.04.021
23. Block G, Subar AF. Estimates of nutrient intake from a food frequency questionnaire: the 1987 National Health Interview Survey. J Am Diet Assoc. (1992) 92:969–77.
24. Lutz LJ, Gaffney-Stomberg E, Scisco JL, Cable SJ, Karl JP, Young AJ, et al. Assessment of dietary intake using the healthy eating index during military training. US Army Med Dep J. (2013) 91, 91–8.
25. Lutz LJ, Gaffney-Stomberg E, Williams KW, McGraw SM, Niro PJ, Karl JP, et al. Adherence to the dietary guidelines for americans is associated with psychological resilience in young adults: a cross-sectional study. J Acad Nutr Diet. (2017) 117:396–403. doi: 10.1016/j.jand.2016.09.018
26. Lutz LJ, Gaffney-Stomberg E, Karl JP, Hughes JM, Guerriere KI, McClung JP. Dietary intake in relation to military dietary reference values during army basic combat training; a multi-center, cross-sectional study. Mil Med. (2018a) 184:usy153. doi: 10.1093/milmed/usy153
27. Lutz LJ, Nakayama AT, Karl JP, McClung JP, Gaffney-Stomberg E. Serum and erythrocyte biomarkers of nutrient status correlate with short-term ?-carotene, ?-carotene, folate, and vegetable intakes estimated by food frequency questionnaire in military recruits. J Am Coll Nutr. (2018b) 38:171–8. doi: 10.1080/07315724.2018.1490215
28. Guenther PM, Casavale KO, Reedy J, Kirkpatrick SI, Hiza HA, Kuczynski KJ, et al. Update of the healthy eating index: HEI-2010. J Acad Nutr Diet. (2013) 113:569–80. doi: 10.1016/j.jand.2012.12.016
29. Camilleri GM, Verger EO, Huneau JF, Carpentier F, Dubuisson C, Mariotti F. Plant and animal protein intakes are differently associated with nutrient adequacy of the diet of French adults. J Nutr. (2013) 143:1466–73. doi: 10.3945/jn.113.177113
30. Poti JM, Mendez MA, Ng SW, Popkin BM. Is the degree of food processing and convenience linked with the nutritional quality of foods purchased by US households? Am J Clin Nutr. (2015) 101:1251–62. doi: 10.3945/ajcn.114.100925
31. Britten P, Cleveland LE, Koegel KL, Kuczynski KJ, Nickols-Richardson SM. Impact of typical rather than nutrient-dense food choices in the US Department of Agriculture Food Patterns. J Acad Nutr Diet. (2012) 112:1560–9. doi: 10.1016/j.jand.2012.06.360
32. Pasiakos SM, Lieberman HR, Fulgoni IIIVL. Higher-protein diets are associated with higher HDL cholesterol and lower BMI and waist circumference in US adults. J Nutr. (2015) 145:605–14. doi: 10.3945/jn.114.205203
33. Berryman CE, Agarwal S, Lieberman HR, Fulgoni VL III, Pasiakos SM. Diets higher in animal and plant protein are associated with lower adiposity and do not impair kidney function in US adults. Am J Clin Nutr. (2016) 104:743–49. doi: 10.3945/ajcn.116.133819
34. Trumbo P, Schlicker S, Yates AA, Poos M. Dietary reference intakes for energy, carbohdrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Acad Nutr Diet. (2002) 102:1621–30. doi: 10.1016/S0002-8223(02)90346-9
35. Pasiakos SM, Karl JP, Lutz LJ, Murphy NE, Margolis LM, Rood JC, et al. Cardiometabolic risk in US Army recruits and the effects of basic combat training. PLoS ONE. (2012) 7:e31222. doi: 10.1371/journal.pone.0031222
36. Berryman CE, Lieberman HR, Fulgoni VL, Pasiakos SM. Protein intake trends and conformity with the Dietary Reference Intakes in the United States: analysis of the National Health and Nutrition Examination Survey, 2001–2014. Am J Clin Nutr. (2018) 108:405–13. doi: 10.1093/ajcn/nqy088
37. Richards T, Wright C. British Army recruits with low serum vitamin D take longer to recover from stress fractures. J R Army Med Corps. (2018) jramc-2018–000983. doi: 10.1136/jramc-2018-000983
38. Woolf K, Manore MM. B-vitamins and exercise: does exercise alter requirements? Int J Sport Nutr Exerc Metab. (2006) 16:453–84. doi: 10.1123/ijsnem.16.5.453
39. Traber MG. Vitamin E inadequacy in humans: causes and consequences. Adv Nutr. (2014) 5:503–14. doi: 10.3945/an.114.006254
40. Zhang Y, Xun P, Wang R, Mao L, He K. Can magnesium enhance exercise performance? Nutrients. (2017) 9:946. doi: 10.3390/nu9090946
41. Otten JJ, Hellwig JP, Meyers LD. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, DC: National Academies Press (2006).
42. Subar AF, Freedman LS, Tooze JA, Kirkpatrick SI, Boushey C, Neuhouser ML, et al. Addressing current criticism regarding the value of self-report dietary data. J Nutr. (2015) 145:2639–45. doi: 10.3945/jn.115.219634
43. Moore DR, Camera DM, Areta JL, Hawley JA. Beyond muscle hypertrophy: why dietary protein is important for endurance athletes. Appl Physiol Nutr Metab. (2014) 39:987–97. doi: 10.1139/apnm-2013-0591
44. Kato H, Suzuki K, Bannai M, Moore DR. Protein requirements are elevated in endurance athletes after exercise as determined by the indicator amino acid oxidation method. PLoS ONE. (2016) 11:e0157406. doi: 10.1371/journal.pone.0157406
Keywords: protein, diet quality, micronutrients, shortfall nutrients, healthy eating index
Citation: Gwin JA, Karl JP, Lutz LJ, Gaffney-Stomberg E, McClung JP and Pasiakos SM (2019) Higher Protein Density Diets Are Associated With Greater Diet Quality and Micronutrient Intake in Healthy Young Adults. Front. Nutr. 6:59. doi: 10.3389/fnut.2019.00059
Received: 26 February 2019; Accepted: 15 April 2019;
Published: 07 May 2019.
Edited by:
Daniel Moore, University of Toronto, CanadaReviewed by:
Ida Aliisa Heikura, Australian Catholic University, AustraliaCaoileann Healy Murphy, University College Dublin, Ireland
Copyright © 2019 Gwin, Karl, Lutz, Gaffney-Stomberg, McClung and Pasiakos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefan M. Pasiakos, c3RlZmFuLm0ucGFzaWFrb3MuY2l2QG1haWwubWls
 Jess A. Gwin
Jess A. Gwin J. Philip Karl
J. Philip Karl Laura J. Lutz3
Laura J. Lutz3 Erin Gaffney-Stomberg
Erin Gaffney-Stomberg Stefan M. Pasiakos
Stefan M. Pasiakos