
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr., 14 February 2018
Sec. Plant Nutrition
Volume 5 - 2018 | https://doi.org/10.3389/fnut.2018.00012
This article is part of the Research TopicImproving the Nutritional Content and Quality of Crops: Promises, Achievements, and Future ChallengesView all 29 articles
Biofortification is an upcoming, promising, cost-effective, and sustainable technique of delivering micronutrients to a population that has limited access to diverse diets and other micronutrient interventions. Unfortunately, major food crops are poor sources of micronutrients required for normal human growth. The manuscript deals in all aspects of crop biofortification which includes—breeding, agronomy, and genetic modification. It tries to summarize all the biofortification research that has been conducted on different crops. Success stories of biofortification include lysine and tryptophan rich quality protein maize (World food prize 2000), Vitamin A rich orange sweet potato (World food prize 2016); generated by crop breeding, oleic acid, and stearidonic acid soybean enrichment; through genetic transformation and selenium, iodine, and zinc supplementation. The biofortified food crops, especially cereals, legumes, vegetables, and fruits, are providing sufficient levels of micronutrients to targeted populations. Although a greater emphasis is being laid on transgenic research, the success rate and acceptability of breeding is much higher. Besides the challenges biofortified crops hold a bright future to address the malnutrition challenge.
“Biofortification” or “biological fortification” refers to nutritionally enhanced food crops with increased bioavailability to the human population that are developed and grown using modern biotechnology techniques, conventional plant breeding, and agronomic practices. The United Nations Food and Agriculture Organization has estimated that around 792.5 million people across the world are malnourished, out of which 780 million people live in developing countries (1). Apart from this, around two billion people across the world suffer from another type of hunger known as “hidden hunger,” which is caused by an inadequate intake of essential micronutrients in the daily diet (2, 3) despite increased food crop production (4). Besides this overnutrition is growing matter of concern.
So far, our agricultural system has not been designed to promote human health; instead, it only focuses on increasing grain yield and crop productivity. This approach has resulted in a rapid rise in micronutrient deficiency in food grains, thereby increasing micronutrient malnutrition among consumers. Now agriculture is undergoing a shift from producing more quantity of food crops to producing nutrient-rich food crops in sufficient quantities. This will help in fighting “hidden hunger” or “micronutrient malnutrition” especially in poor and developing countries, where diets are dominated by micronutrient-poor staple food crops (5).
Traditionally, vitamins and minerals have been provided to the masses through nutrient supplementation programs, but it falls short of the goals set by the international health organizations as the supplementation programs rely on external funding that is not guaranteed to be available from year to year. Other limitations are purchasing power of poor people, their access to markets and health-care systems, and lack of awareness regarding the long-term health benefits of these nutrient supplements (6, 7). Hence, biofortification of different crop varieties offers a sustainable and long-term solution in providing micronutrients-rich crops to people. Furthermore, biofortified crops with increased bioavailable concentrations of essential micronutrients are deployed to consumers through traditional practices used by agriculture and food trade which therefore provides a feasible way of reaching undernourished and low income group families with limited access to diverse diets, supplements, and fortified foods. From an economic viewpoint, biofortification is a one-time investment and offers a cost-effective, long-term, and sustainable approach in fighting hidden hunger because once the biofortified crops are developed; there are no costs of buying the fortificants and adding them to the food supply during processing (8–14). Furthermore, in the next few decades, a major population increase might take place in the developing world and with the changing climatic conditions; achieving food security will pose a greater challenge (15, 16). Thus, organizations such as the World Health Organization and the Consultative Group on International Agricultural Research (CGIAR) have included the development of nutritionally enhanced high-yielding biofortified crops as one of their main goals (17).
Humans require around 40 known nutrients in adequate amounts to live healthy and productive lives (Table 1). The mineral elements—sodium, potassium, calcium, magnesium, phosphorous, chlorine, and sulfur—are classified as essential nutrients that are required in small amounts in the body. The other class of essential nutrients required in very small amounts in the human body are termed as micronutrients—namely iron, zinc, copper, manganese, iodine, selenium, molybdenum, cobalt, nickel, and vitamin A (18). Collectively, these nutrients play crucial roles in humans and dictate our physical and mental development (19). Many micronutrients act as cofactors for the functioning of various enzymes in the human body and thereby regulate important functions and metabolic processes in our body (20). For humans, agricultural products are the primary source of nutrients, especially for those living in developing countries (21–23). However, the diet of the population based on cereals such as rice, wheat, cassava, and maize contain insufficient amounts of several nutrients such as vitamin A, iron, zinc, calcium, manganese, copper, iodine, or selenium with respect to meeting daily requirements. These nutrient deficient agricultural products cannot support healthy lives and can result in poor health, sickness, increased morbidity and disability, impaired development, stunted mental and physical growth, diminished livelihoods, and reduced national socioeconomic development (24–29). Childhood stunting prevalent in many developing countries is associated with micronutrient malnutrition in children starting from fetal development to 4 years of age (25). Micronutrient deficiencies affect about 38% of pregnant women and 43% of pre-school children worldwide. More than 30% of the world’s population has been reported to be anemic (30) and suffering from hidden hunger. The prevalence of anemia is more in developing countries compared with developed countries. Africa and South-East Asian countries are most affected (Figures 1A,B). Estimates have indicated that approximately half of this is attributed to iron deficiency (31). Hence, micronutrient malnutrition is the major challenge in many developing countries. Another important point of consideration is uneven distribution of the nutrients among different plant parts (32). For example, the iron content is high in rice leaves, but low in polished rice grain. Apart from under nutrition, growing incidence of overnutrition leading to problems of overweight and in particular, high rate of diabetes is a matter of concern. Consequently, biofortification is also directed toward enhancing the contents of desired micronutrients in the edible portion of crop plants. Nutritional targets for biofortification include elevated mineral content, improved vitamin content, increased essential amino acid levels, better fatty acid composition, and heightened antioxidant levels in crops (12). Biofortification of crop plants can provide enough calories to meet the energy needs along with providing all the essential nutrients needed for sound health. Furthermore, biofortifying the crops which are consumed by the poor population of the world can significantly improve the amount of nutrients consumed by this target population (33).
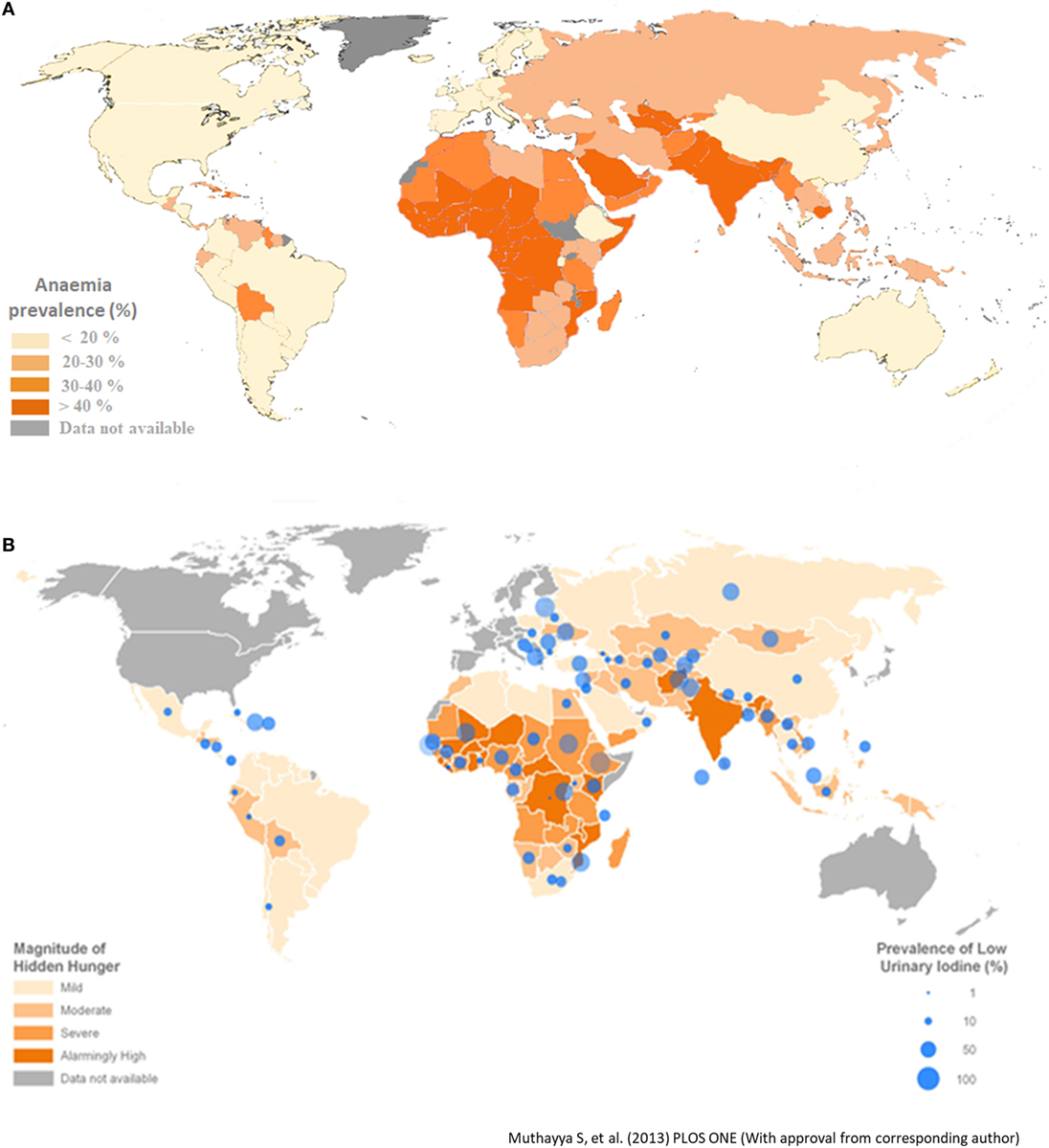
Figure 1. (A) Prevalence of anemia in different parts of the world. Developing countries in Africa and Asia have high prevalence of anemia [Data from Stevens et al. (30)]. (B) Global map representing hidden hunger index and low urinary iodine concentration (3).
Producing nutritious and safe foods, sufficiently and sustainably, is the ultimate goal of biofortification (34). Biofortification of essential micronutrients into crop plants can be achieved through three main approaches, namely transgenic, conventional, and agronomic, involving the use of biotechnology, crop breeding, and fertilization strategies, respectively. Most of the crops targeted by transgenic, conventional breeding, and agronomical approaches include staple crops like rice, wheat, maize, sorghum, lupine, common bean, potato, sweet potato, and tomato (Figure 2). Cassava, cauliflower, and banana have been biofortified by both transgenic and breeding approaches while barley, soybean, lettuce, carrot, canola, and mustard have been biofortified with transgene and agronomic approaches. Higher numbers of crops have been targeted by transgenic means, while the practical utilization of biofortification is higher by breeding methods (Figures 3A,B). Cereals being staple crop have been targeted by all three approaches. Same is the case of legumes and vegetables. Interestingly, oil seed biofortification has been achieved through transgenic means, because limited availability of genetic diversity for the targeted component, low heritability, and linkage drag in the targeted crop (Figure 3B). Biofortification by breeding has been achieved in crops and specified components when genetic diversity is available in the utilizable form in the primary, secondary, or tertiary gene pool of the targeted crop. When genetic diversity is unavailable, genetic transformation is the better option. Transgenic-based approach has advantages that a useful gene once discovered, can be utilized for targeting multiple crops (Figure 4). Some important genes like phytoene synthase (PSY), carotene desaturase, nicotinamide synthase, and ferritin have been utilized in multiple events including multiple crops. In this manuscript, we have compiled the data from research to release on different food crops that are being targeted by the different approaches of biofortification.

Figure 2. Biofortified crops generated by different approaches: transgenic, agronomic, and breeding. Staple cereals, most common vegetables, beans, and fruits have been targeted by all three approaches. Some crops have been targeted by only one or two approaches depending on its significance and prevalence in the daily human diet.
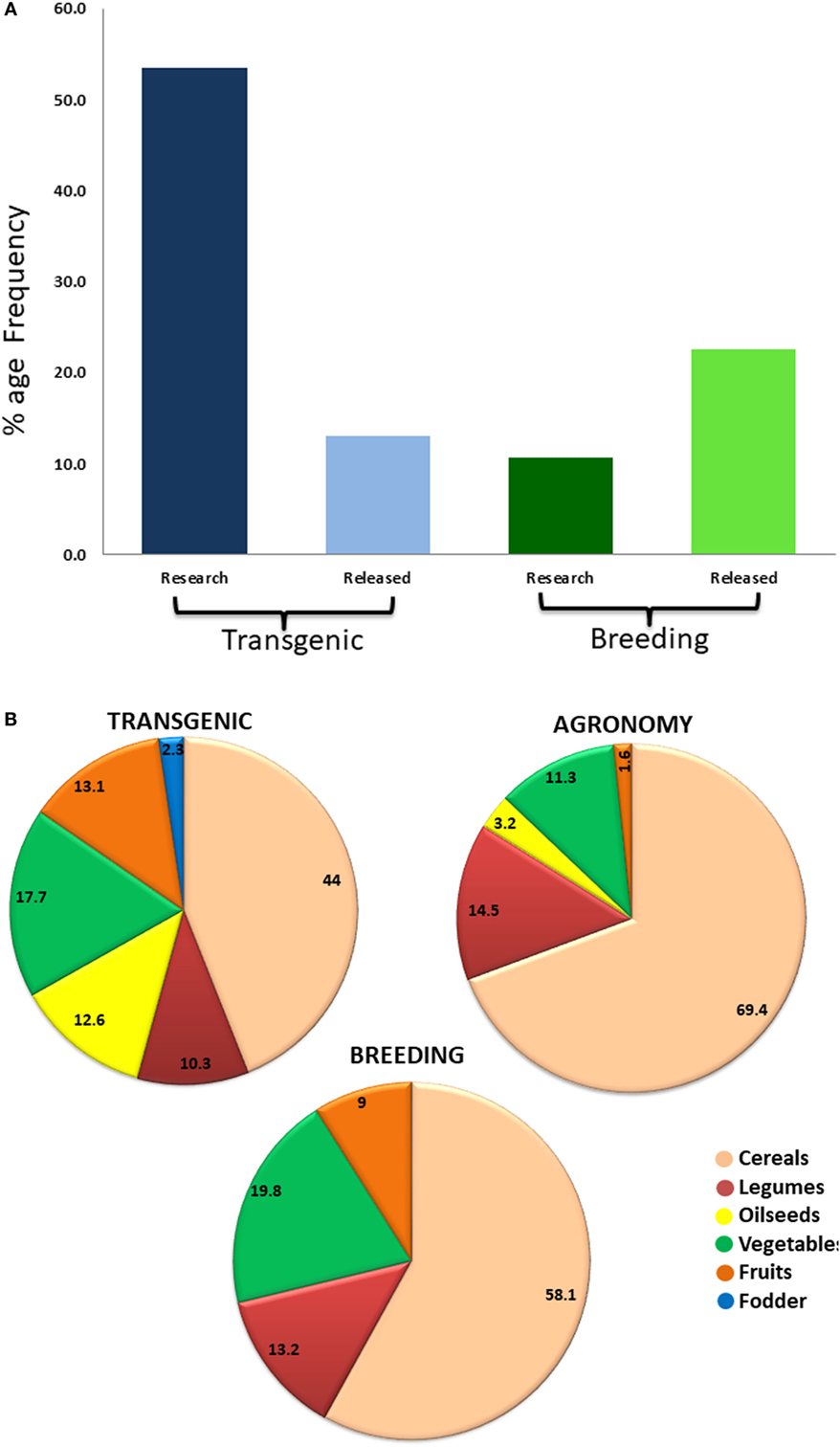
Figure 3. Representation of reported biofortified crops by transgenic, agronomic, and breeding means. (A) Comparison of transgenic and breeding approaches of biofortification in terms of relative research and release of commercial crops. While higher emphasis is being laid on transgenic-based biofortification, success rate in terms of cultivar release is higher for breeding-based approach. (B) Percentage of different crops biofortified by different approaches. Cereals have been biofortified in largest number by all three biofortification approaches. Legumes and vegetables have also been targeted by all the approaches in almost equal percentage. Transgenic approach covers highest number of crops. Oilseed crops have been mainly targeted by transgenic approaches due to limited genetic variability.
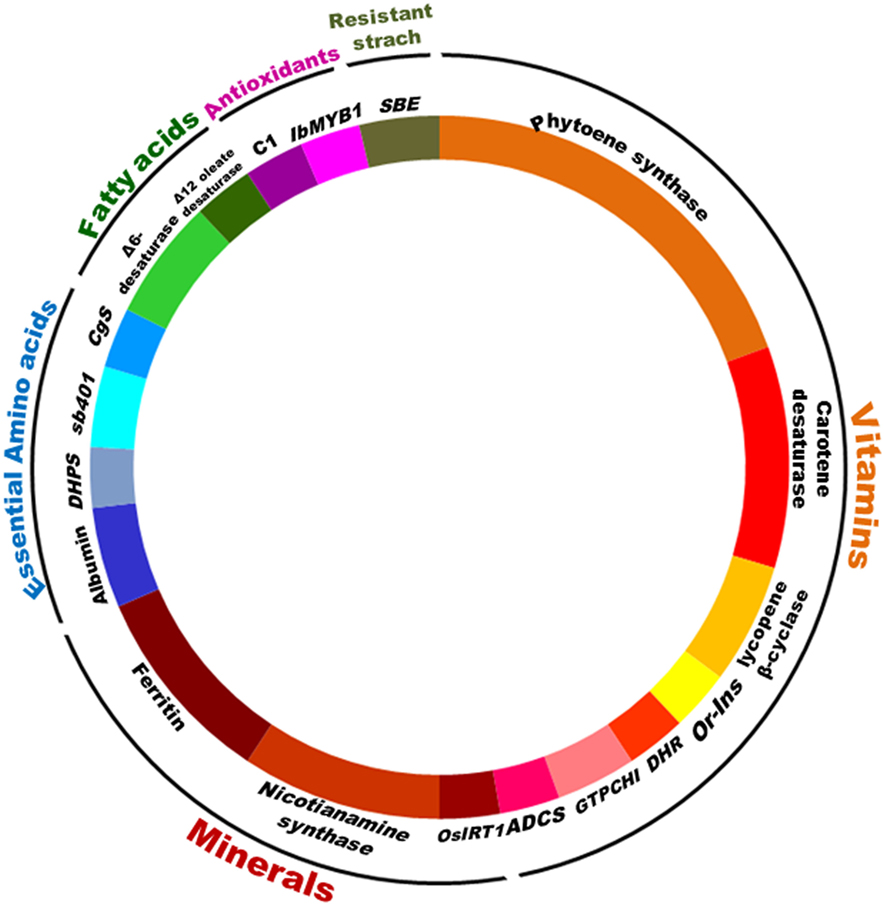
Figure 4. Utilization of different genes for biofortification by transgenic means. Large numbers of genes have been utilized for crop biofortification. Transgenic-based approach has advantages that a useful gene once discovered, can be utilized for targeting multiple crops. Some important genes like phytoene synthase, carotene desaturase, nicotinamide synthase, and ferritin have been utilized in multiple events including multiple crops.
Transgenic approach can be a valid alternative for the development of biofortified crops when there is a limited or no genetic variation in nutrient content among plant varieties (32, 35). It relies on the access to the unlimited genetic pool for the transfer and expression of desirable genes from one plant species to another which is independent of their evolutionary and taxonomic status. Furthermore, when a particular micronutrient does not naturally exist in crops, transgenic approaches remain the only feasible option to fortify these crop with the particular nutrient (7). The ability to identify and characterize gene function and then utilize these genes to engineer plant metabolism has been a key for the development of transgenic crops (36). Furthermore, pathways from bacteria and other organisms can also be introduced into crops to exploit alternative pathways for metabolic engineering (37).
Transgenic approaches can also be used for the simultaneous incorporation of genes involved in the enhancement of micronutrient concentration, their bioavailability, and reduction in the concentration of antinutrients which limit the bioavailability of nutrients in plants. In addition, genetic modifications can be targeted to redistribute micronutrients between tissues, enhance the micronutrient concentration in the edible portions of commercial crops, increasing the efficiency of biochemical pathways in edible tissues, or even the reconstruction of selected pathways (38–40). Development of transgenically biofortified crops initially involves substantial amount of time, efforts, and investment during research and development stage, but in a long run, it is a cost-effective and sustainable approach, unlike nutrition-based organizational and agronomic biofortification programs (14, 19). Furthermore, genetic engineering has no taxonomic constraints and even synthetic genes can be constructed and used. Transgenic crops with enhanced micronutrient contents hold a potential to reduce micronutrient malnutrition among its consumers, especially poor people in developing countries (12). Numerous crops have been genetically modified to enhance their micronutrient contents. Among micronutrients, vitamins, minerals, essential amino acids, and essential fatty acids have been targeted by the use of various genes from different sources to enhance the food crop nutritional level (Table 2). It has been found that PSY, carotene desaturase, and lycopene β-cyclase for vitamins, ferritin and nicotinamine synthase for minerals, albumin for essential amino acids, and Δ6 desaturase for essential fatty acids have been widely reported as targets for biofortification (Figure 4). Successful examples of transgenic method are high lysine maize, high unsaturated fatty acid soybean, high provitamin A and iron rich cassava, and high provitamin A Golden rice. Reports are available for biofortified cereals, legumes, vegetables, oilseeds, fruits, and fodder crops.
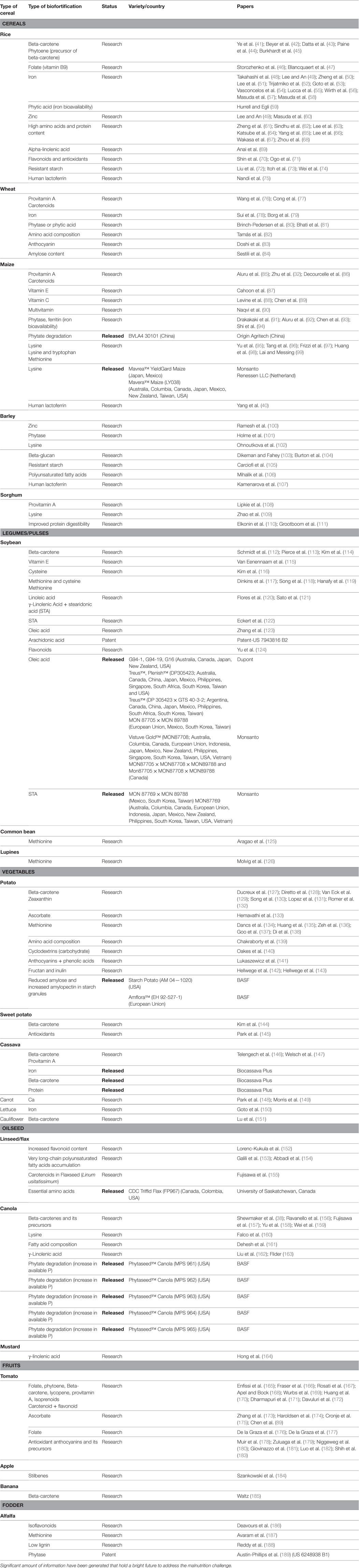
Table 2. Tabulation of crops, nutrients, research status, and concerned publications on biofortification by transgenic means.
Rice has been targeted to address the global challenge of undernutrition. Vitamin deficiency is one of the major challenges that affect underprivileged population due to poor affordability. Golden Rice was an important breakthrough in this direction as an effective source of provitamin A (beta-carotene) with a significant potential to reduce disease burden by expressing genes encoding PSY and carotene desaturase (41–45). The level of beta-carotene precursor, i.e., phytoene, has been enhanced up to 23-fold by targeting gene encoding carotene desaturase (45). Folic acid (vitamin B9) is important for normal pregnancy and anemia (190). Rice has been genetically modified to increase folate content (up to 150-fold) by overexpressing genes encoding Arabidopsis GTP-cyclohydrolase I (GTPCHI) and aminodeoxychorismate synthase [ADCS (46, 47)]. The 100 g of modified rice was found to be sufficient to meet daily folate requirements of an adult individual.
Rice has also been targeted to address the global challenge of iron deficiency anemia. Multiple reports have indicated an increase in iron content in rice by expressing genes encoding, nicotianamine aminotransferase (48), iron transporter OsIRT1 (49), nicotianamine synthase 1 (OsNAS1) and 2 (OsNAS2) (50–52, 191), soybean ferritin (52–54), and common bean ferritin (55). Iron biofortified rice was also synthesized by introducing multiple genes involved in iron nutrition (56–58). In addition to enhanced iron content, improvement in iron bioavailability was also achieved by reducing antinutrient compounds in rice such as phytic acid (59). Similarly, zinc content was also elevated in GM rice by overexpressing OsIRT1 (49) and mugineic acid synthesis genes from barley [HvNAS1, HvNAS1, HvNAAT-A, HvNAAT-B, IDS3 (60)].
Improvement in quality protein has been addressed by targeting essential amino acid content in rice by expressing seed-specific genes of bean β-phaseolin (61), pea legumin (62); Sesame 2S Albumin (63); soybean glycinin (64); bacterial aspartate kinase, dihydrodipicolinate synthase (DHPS) (65); maize DHPS (66); rice anthranilate synthase α-subunit (67); and E. coli aspartate aminotransferase (68). Rice has also been targeted for seed oil quality by increasing amount of polyunsaturated fatty acid that can help in the reduction of bad cholesterol levels in the body and improve human nutrition (192). An essential fatty acid α-linolenic acid has been enhanced in rice by expressing soybean omega-3 fatty acid desaturase (FAD3) gene [GmFAD3 (69)]. Flavonoids are associated with antioxidant activity and its content in rice has been enhanced by expressing maize C1 and R-S regulatory genes [Myb-type and basic helix-loop-helix-type transcription factors (70)]; and phenylalanine ammonia lyase and chalcone synthase (CHS) genes (71). To address the challenge of overnutrition and obesity, the content of less digestible and resistant amylose starch has been enhanced by expression of antisense waxy genes (72, 73) and antisense RNA inhibition of starch-branching enzymes (SBE) (74). Besides introducing micronutrients, expression of functional human milk protein (lactoferrin) in rice grains has opened the possibility for creating a value-added cereal-based ingredients that can be introduced into infant formula and baby food (75, 193).
Wheat is one of the most widely grown staple food crops in the world. Researchers have tried to address the challenges of most deficient nutrients like vitamin A, iron, and quality proteins through wheat. The provitamin A content of wheat has been enhanced by expressing bacterial PSY and carotene desaturase genes [CrtB, CrtI (76, 77)]. The iron content in wheat has been enhanced by expression of ferritin gene from soybean (78) and wheat [TaFer1-A (79)]. To increase iron bioavailability phytase activity was increased by the expression of the phytochrome gene [phyA (80)] and phytic acid content has been decreased by silencing of wheat ABCC13 transporter (81). Protein content, especially essential amino acids lysine, methionine, cysteine, and tyrosine contents of wheat grains were enhanced using Amaranthus albumin gene [ama1 (82)]. Wheat has also been targeted to improve the antioxidant activity by expressing maize regulatory genes (C1, B-peru) involved in anthocyanin production (83). To address the challenge of overnutrition and obesity, the content of less digestible and resistant amylose starch has been enhanced by silencing gene encoding SBE [SBEIIa (84)].
Maize is one of the important staple crops in developing countries, and it has been addressed for vitamins, minerals, quality protein, and antinutrient components by means of genetic engineering. Maize endosperm has been enriched with provitamin A (carotenoids) by expressing bacterial crtB (85) and multiple (5) carotenogenic genes (86, 194). Vitamin E and its analog are potent antioxidants with implications over human health and many research groups are emphasizing on biofortification of these components in maize crop. Tocotrienol and tocopherol content in maize has been increased by overexpression of homogentisic acid geranylgeranyl transferase [HGGT (87)]. Vitamin C (l-ascorbic acid) a water-soluble antioxidant play roles in cardiovascular function, immune cell development, and iron utilization (88). Its level in corn has been enhanced nearly 100-fold times by recycling oxidized ascorbic acid to reduced form by the expression of dehydroascorbate reductase [DHAR (89)]. On the other hand, Naqvi et al. (90) developed multivitamin corn containing 169-fold the normal amount of beta-carotene, double the normal amount of folate and 6-fold the normal amount of ascorbate by engineering three distinct metabolic pathways.
Bioavailability of micronutrients is hindered by antinutrient components. Bioavailability of iron has been increased by expressing soybean ferritin and Aspergillus phytase (91), soybean ferritin (92), Aspergillus niger phyA2 (93), and silencing the expression of ATP-binding cassette transporter and multidrug resistance-associated protein (94). As a practical example, BVLA4 30101 variety released by Origin Agritech in China has been biofortified for phytate degradation.
The major maize seed storage proteins, zeins have poor nutritional quality due to lower content of essential amino acids lysine and tryptophan. In maize essential amino acid content has been targeted with significant achievement. Lysine content in maize has been increased by expression of sb401 from potato (95, 96), single bifunctional expression/silencing transgene cassette (97). Both lysine and tryptophan content have been increased in maize by antisense dsRNA targeting alpha-zeins [both 19- and 22-kDa (98)]. Importance of lysine content in maize is evident from maize varieties rich in lysine viz., Mavrea™YieldGard Maize that has been released by Monsanto in Japan and Mexico; Mavera™ Maize (LY038) by Renessen LLC (Netherland) in Australia, Columbia, Canada, Japan, Mexico, New Zealand, Taiwan, USA The amino acid methionine is a common protein building block that is also important in other cellular processes. Its content has been increased in maize by modifying cis-acting site for Dzs10 (99). Amino acid balance of maize has also been improved by expressing milk protein α-lactalbumin (40).
Barley being a model cereal crop has been targeted to improve its micronutrient content. Its zinc content has been improved by overexpression of zinc transporters (100). To increase the bioavailability of iron and zinc, phytase activity has been increased in barely seeds by expression of phytase gene [HvPAPhy_a (101)]. Essential amino acid lysine has been enhanced in barley by expressing DHPS gene [dapA (102)]. β glucans are dietary fibers and are believed to dramatically reduce the risk of contracting serious human diseases such as cardiovascular disease and type II diabetes (103). Its content has been increased in barley by overexpression of cellulose synthase-like gene [HvCslF (104)]. Resistant starch (amylose only) barley has been produced by the RNAi approach by suppressing all genes coding for SBE [SBE I, SBE IIa, SBE IIb (105)]. Content of health promoting polyunsaturated fatty acids, γ-linolenic acid, and stearidonic acid (STA) has been improved in barley by expressing Δ6-desaturase [D6D (106)]. Barley has been targeted to express human lactoferrin gene [HLF (107)]. Apart from this several medicinally and industrially important bioactives including enzymes and antibiotics have been expressed in barley.
Sorghum is one of the most important staple foods for millions of poor rural people. It has an ability to grow well in harsh environments. It has been targeted to improve provitamin A (beta-carotene) by expressing Homo188-A (108). Content of essential amino acid lysine has been improved in sorghum by the introduction of a high lysine protein [HT12 (109)]. One of the issues with sorghum consumption is that its grains are less digestible than the other major staple crops. Its seed storage proteins, γ-kafirin, is resistant to protease digestion. Digestibility index of transgenic sorghum has been increased by RNAi silencing of the γ-kafirin (110) and combined suppression involving three genes [γ-kafirin-1, γ-kafirin-2, and α-kafirin A1 (111)].
Soybean is a global source of vegetable oil and high-quality protein. The soybean has been targeted to increase provitamin A (beta-carotene), a monounsaturated ω-9 fatty acid (oleic acid) and seed protein contents by expressing bacterial PSY gene (112). In a different approach provitamin A (Canthaxanthin) was enhanced by expressing bacterial PSY [crtB, crtW, bkt1 (113)]. Kim et al. (114) has demonstrated the production of a high provitamin A (beta-carotene) soybean through overexpression of PSY and carotene desaturase. Another important nutrient vitamin E activity in barley has been enhanced with increased content of δ-tocopherol and decreased γ-tocopherol by coexpressing 2-methyl-6-phytyl benzoquinol methyltransferase genes [At-VTE3; At-VTE4 (115)]. Soybeans contain approximately 40% protein, but they are deficient in one or more of the essential amino acids, especially the sulfur-containing amino acids, cysteine and methionine. The cysteine content of soybean seeds has been increased through overexpression of the sulfur assimilatory enzyme, O-acetylserine sulfhydrylase (116). Similarly, Dinkins et al. (117) increased methionine and cysteine content in soybean by overexpressing the maize zein protein. The methionine content of soybean has been increased by expressing cystathionine γ-synthase (118, 119). Soybean is rich in healthy oil and has approximately 20% oil content. But 7–10% of the oil contains unstable fatty acid α-linolenic acids that contribute to reduced soybean seed oil quality. It results in the formation of undesirable trans-fatty acid as a result of hydrogenation (195). To enhance the agronomic value of soybean seed oil by reducing the levels of α-linolenic acids (18:3), siRNA-mediated gene silencing-based approach has been utilized for silencing of ω-3 FAD3 (120). In another experiment γ-linolenic acid (GLA) and STA (ω-3 fatty acids) content in soybean oil has been increased by expression of Δ6-desaturase gene that is responsible for the conversion of linoleic acid and α-linolenic acid to GLA and STA (121). Similarly, STA content has been increased by simultaneous expression of Δ6 desaturase and Δ15 desaturase (122). Antisense RNA technology has been used to reduce the amount of linoleic acid and palmitic acid and increase the amount of oleic acid by inhibition of expression of Δ12 oleate desaturase [GmFAD2-1b (123)] that converts oleic acid into linoleic acid. Soybean seeds are low in isoflavone content. Consumption of isoflavone is associated with human health benefits such as decreased risk of heart disease, reduced menopausal symptoms, and reduced risk of some hormone-related cancers (196). Isoflavone content has been enhanced in soybean seeds by the combination of maize C1 and R transcription factor-driven gene activation and suppression of a competing pathway (124).
Importance of improvement in ω-3 fatty acid content in soybean is evident from the fact that a large number of cultivars with improved oleic, linoleic, and STA have been released by private companies. Transgenic soybean varieties rich in oleic acid viz., G94-1, G94-19, G168 have been released in Australia, Canada, Japan, New Zealand, USA; and Treus™, Plenish™ (DP305423) in Australia, Canada, China, European Union, Japan, Mexico, New Zealand, Philippines, Singapore, South Africa, South Korea, Taiwan, USA; and Treus™ (DP 305423 × GTS 40-3-2) in Argentina, Canada, China, Japan, Mexico, Philippines, South Africa, South Korea, Taiwan by Dupont. The transgenic varieties of soybean rich in oleic acid were released by Monsanto, viz., Vistive Gold™ (MON87705) in Australia, Columbia, Canada, European Union, Indonesia, Japan, Mexico, New Zealand, Philippines, Singapore, South Korea, Taiwan, USA, Vietnam; MON87705 × MON87708 × MON89788 and MON 87705 × MON 87708 × MON 89788 in Canada. The soybean variety rich in oleic acid and linoleic acid was released in the European Union, Mexico, South Korea, and Taiwan. The other varieties rich in STA viz., MON 87769 × MON 89788 were released in Mexico, South Korea, Taiwan and MON87769 released in Australia, Columbia, Canada, European Union, Indonesia, Japan, Mexico, New Zealand, Philippines, South Korea, Taiwan, USA, Vietnam by Monsanto company.
The common bean is among the most important grain legumes used for human consumption. However, although beans are rich in some essential amino acids, e.g., lysine, threonine, valine, isoleucine, and leucine, their nutritional value is limited because of the small amounts of the essential amino acid methionine and cysteine. Common bean methionine content has been increased by the expression of methionine-rich storage albumin from Brazil nut (125).
Lupine is the major grain legume. The lupine seed protein, in common with the protein of most other grain legumes, is deficient in the sulfur-containing amino acids methionine and cysteine. Its methionine content has been increased by the expression of sunflower seed albumin gene (126).
Potato is the world’s fourth most important source of calories, and it’s any nutritional enhancement is of great significance. In potato tuber, provitamin A (carotenoid forms) have been increased by incorporating PSY gene (127) and by simultaneous incorporation of three genes: PSY, phytoene desaturase, and lycopene β-cyclase (128). Beta-carotene content in tubers has been also enhanced by using RNAi to silence the beta-carotene hydroxylase gene (bch), which converts beta-carotene to zeaxanthin (129) and by regulation of beta-carotene synthesis through expression of lycopene β-cyclase [StLCYb (130)]. In another experiment, it has been observed that incorporation of Or gene from orange cauliflower mutant leads to increase in carotenoids along with three additional metabolite intermediates phytoene, phytofluene, and z-carotene (131). Zeaxanthin which is another form of carotenoid has been also increased by expressing zeaxanthin epoxidase genes in transgenic potato tuber (132).
The potato has been also targeted for enhancement of vitamin C (ascorbic acid) by overexpressing strawberry GalUR (133). Potato tubers are very poor in essential amino acid, methionine, which has been targeted for its enhancement by coexpressing cystathionine γ-synthase (CgSΔ90) and methionine-rich storage protein (134). Similarly, silencing of StMGL1 (135) and antisense inhibition of threonine synthase (136) led to increase in methionine to isoleucine ratio and methionine content (up to 239-folds) in potato tubers. Methionine content has been also enhanced by overexpressing the gene encoding the seed storage protein from Perilla [PrLeg polypeptide (137)] and cystathionine γ-synthase (CgS) genes (138). Transgenic potatoes expressing Amaranth albumin (ama1) result in an increase in total protein content in tubers along with the significant increase in the concentration of several essential amino acids including methionine (139).
High value carbohydrate rich potato tubers has been synthesized by expressing cyclodextrin glycosyltransferases (CGT) gene, which results in the production of multipurpose dietary fiber cyclodextrins from starch (140). Potato tubers have been also focused upon to increase the phenolic acid, and anthocyanins contents by the single-gene overexpression or by simultaneous expression of CHS, chalcone isomerase (CHI), and dihydroflavonol reductase (141). It has been also targeted to improve the content of dietary fiber fructan and inulin (142, 143). Transgenic potato varieties engineered for starch quality, which has reduced amylose and increased amylopectin in starch granules were released by BASF viz., Starch Potato (AM 04—1020) in the USA and Amflora™ (EH 92-527-1) in the European Union. Transgenic potato varieties that limit formation of the reducing sugars through starch degradation have been released in Canada and USA by J. R. Simplot Co.
Sweet potato is an alternative source of bioenergy and natural antioxidants. It is rich in various phytochemicals, anthocyanins, vitamin C, carbohydrates, potassium, and dietary fiber (197). Its nutrition properties have been further enhanced by increasing the contents of carotene, lutein, and total carotenoids by overexpressing orange IbOr-Ins gene in white fleshed sweet potato (144). The antioxidant capacity of orange-fleshed sweet potato cultivar has been increased by overexpression of IbMYB1 a key regulator of anthocyanin biosynthesis in the storage roots (145).
Cassava is an important staple food crop for millions of poor people worldwide as it is tolerant to different stresses. However, cassava is deficient in several important nutrients like provitamin A, vitamin E, iron, and zinc. Cassava biofortification of provitamin A, iron, and zinc has been carried out to reduce their deficiency among the undernourished communities. Telengech et al. (146) as a part of the BioCassava Plus project developed transgenic cassava that expresses beta-carotene in roots using nptII, crtB, and DXS. Similarly, Welsch et al. (147) showed that the cassava plants overexpressing a PSY transgene produced yellow-fleshed, high-carotenoid roots. Different transgenic cassava varieties biofortified for enhanced levels of iron, beta-carotene, and zinc are under development and field trials in the Biocassava Plus Program targeted at African countries.
Carrots are one of the most popular vegetables and contain high levels of beta-carotene and vitamins and minerals; however, like many vegetables, these are poor in calcium content (198). Bioavailable calcium content in transgenic carrot has been increased by expressing the Arabidopsis H+/Ca2+ transporter [CAX1 (148, 149)].
Lettuce is one of the most popular leafy vegetables all around the world. Compared to spinach, the iron content of lettuce is low. The lettuce has been improved for iron content, yield, and growth rate by expressing a soybean ferritin gene (150).
Cauliflower is a popular vegetable in several parts of the world. It is rich in antioxidant phytonutrients. Its nutritional value has been further enhanced by increasing beta-carotene content in mutant orange cauliflower by the insertion of a copia-like LTR retrotransponson in the Or (151).
Linseed edible oil is in demand as a nutritional supplement. Linseed or flax seeds are the richest source of polyunsaturated fatty acids, but linseed oil is highly susceptible to auto-oxidation, which generates toxic derivatives. Genetically modified flax plants with increased antioxidant potential, stable, and healthy oil production has been generated by suppressing CHS gene that resulted in hydrolyzable tannin accumulation (152). Very long-chain unsaturated fatty acids (VLCPUFA) are important fatty acids with limited supply due to decrease in marine resources such as fish oils. It can be compensated by implementation of VLCPUFA biosynthesis into oilseed crops (153). VLCPUFA such as arachidonic acid (C20:4 n-6), eicosapentenoic acid (EPA C20:5 n-3), and docosahexenoic acid (DHA C22:5 n-3) are considered to be nutritionally beneficial because of their function as cholesterol-lowering agents (199). Researchers have intended to enhance the accumulation of Δ6 desaturated C18 fatty acids and C20 polyunsaturated fatty acids, including arachidonic and eicosapentaenoic acid by seed-specific expression of cDNAs encoding fatty acyl-desaturases and elongases in linseed (154). Enrichment of carotenoids in flaxseed has been done by the introduction of PSY gene [crtB (155)]. Transgenic linseed rich in essential amino acids viz., CDC Triffid Flax (FP967) has been released by University of Saskatchewan, in Colombia, USA, and Canada.
Canola is an important oilseed crop for millions of people around the world. Canola produces edible oil lower in saturated fat and higher in omega-3 fatty acids. To further enhance its health benefits its carotenoid content (mainly alpha and beta-carotenes) has been increased by overexpressing bacterial PSY [crtB (37)]. Higher β-carotenoid content has been achieved by simultaneous expression of PSY, phytoene desaturase, and lycopene cyclase genes (155) and simultaneous expression of seven bacterial genes; idi, crtE, crtB, crtI, crtY, crtW, and crtZ (157). Higher beta-carotene content along with high xanthophylls and lutein contents have been achieved by RNAi silencing of lycopene ε-cyclase [ε-CYC (158)] and DET1 (159). Essential amino acid lysine has been increased in canola by expression of aspartokinase (AK) and dihydrodipicolinic acid synthase (DHDPS) genes (160). Increase in level of two fatty acids viz., caprylate (8:0) and caprate (10:0) in canola seed oil accompanied by a preferential decrease in the levels of linoleate (18:2) and linolenate (18:3) has been achieved by overexpression of thioesterase gene [Ch FatB2 (161)]. Canola normally does not have any Δ6 desaturase activity and thus lack GLA. In order to produce GLA more economically and to make it more readily available transgenic lines rich in GLA has been developed by expression of Δ12 or Δ6 desaturases genes (162, 163). Phytic acid is known as a food inhibitor, which chelates micronutrient and prevents its bioavailability, as human and other monogastic animals lack the phytase enzyme in their digestive track. Transgenic canola varieties viz., Phytaseed™ Canola (MPS 961-965) engineered for phytase degradation to enhance the availability of phosphorus in canola has been produced and released by BASF in USA.
Mustard is an economically significant crop and extensively cultivated for oil throughout the world. It has been targeted for improving the nutritionally important unsaturated fatty acids. This has been achieved by the expression of the enzyme Δ6 FAD3 that led to the production of gamma linoleic acid in the transgenic mustard (164).
Tomato is one of the most popular fruits, consumed by billions around the world and is an important source of vitamin C, micronutrients, and other phytonutrients. It derives its color from isopernoid lycopene. Isoprenoids are one of the largest classes of natural products with several thousand compounds. In higher plants, isoprenoids have essential roles in membrane structure (sterols), free radical scavenging (carotenoids and tocopherols), redox chemistry (plastoquinone, ubiquinone), defense mechanisms (phytoalexins), and growth regulation (gibberellins, cytokinins, brassinosteroids, and abscisic acid) (200). Several attempts have been made to increase the isoprenoid content in tomato. The sterol content was elevated in tomato by expression of 3-hydroxymethylglutaryl CoA [hmgr-1 (165)]. Tomato phytoene and beta-carotene content has been enhanced by expression of 1-deoxy-d-xylulose-5-phosphate synthase [dxs (165)]. Higher contents of lycopene, beta-carotene, and lutein have also been achieved in tomato by the expression of PSY gene [crtB (166)]. Double biofortification of carotenoid and flavonoid contents have also been achieved by RNAi technology by suppressing photomorphogenesis regulatory gene [DET1 (172)]. The beta-carotene content has also been increased by overexpression of lycopene beta-cyclase gene [beta-Lcy (167–169)]. Higher contents beta-carotene as well as its hydroxylation product xanthophylls (beta-cryptoxanthin and zeaxanthin) has been obtained by simultaneous expression of beta-Lcy and beta-carotene hydroxylase [b-Chy (171)]. Total carotenoid and high value astaxanthin content (hydroxylation product of a beta-carotene) have been enhanced in tomato by expression of beta-carotene ketolase and hydroxylase (170). The tomato has been targeted to improve its vitamin C (ascorbic acid) content by overexpressing GDP-mannose 3′,5′-epimerase [SlGME1, SlGME2 (173)], DHAR (174), and coexpression of three genes GDP-mannose pyrophosphorylase, arabinono-1,4-lactone oxidase, and myo-inositol oxygenase 2 (88, 175). Another important nutrient folic acid has been targeted by overexpression of GTPCHI (176) and aminodeoxychorismate synthase (177).
Tomato has also been selected to increase antioxidant anthocyanins by expression of CHI (178), transcriptional activators AtMYB75 (179), and expression of two transcription factors, Delila and Rosea1 (201). Other antioxidants like chlorogenic acid have been targeted by gene silencing of HQT (180), trans-resveratrol by expression of stilbene synthase (181), polyphenolic antioxidants by expression of AtMYB12 (182), and genistin by overexpression of isoflavone synthase (IFS) gene (183). Anthocynin rich blue transgenic tomato has been developed by Norfolk plant sciences.
Apple has long been recognized as a great source of antioxidants. Apple has been bioengineered with a stilbene synthase gene from the grapevine (Vitis vinifera L.) thereby leading to synthesis of resveratrol in transgenic apple, thereby, expanding the antioxidant capacity (184).
The banana, a fourth most important food crop of the developing countries, has been predominantly targeted for beta-carotene. This has been achieved by developing transgenic banana (Super Banana) by expressing PSY gene (PSY2a) of Asupina banana, which is naturally high in beta-carotene (185).
Alfalfa is as an important feed legume crop in many countries. Attempts have been made to improve its nutritional status through enhancement of isoflavonoids, essential amino acids, and improve its digestibility. Isoflavonoids are a predominantly legume-specific subclass of flavonoid secondary metabolites. Transgenic alfalfa has been generated by constitutively expressing IFS that is correlated with its increased isoflavonid composition (186). Alfalfa suffers from a limited level of the sulfur-containing amino acids, methionine, and cysteine. Its methionine content has been increased by the expression of cystathionine γ-synthase [AtCgS (187)]. Improvement in the digestibility of forages has also been an area of interest as it correlates with animal performance. By targeting three specific cytochrome P450 enzymes for antisense downregulation, transgenic alfalfa lines have been generated with low lignin content (188). Alfalfa has also been engineered to increase phytase activity, and thereby enabling its use in animal feeds, including livestock, poultry, and fish feed (189).
Biofortification through agronomic methods requires physical application of nutrients to temporarily improve the nutritional and health status of crops and consumption of such crops improves the human nutritional status (202). In comparison with inorganic forms of minerals, the organic ones are more available for a man, as they can be absorbed more easily; and are less excreted (203) and their toxicity symptoms are less intensive (DRI 2000). It generally relies on the application of mineral fertilizers and/or increase in their solubilization and/or mobilization from the soil in the edible parts of plants. Macrominerals like nitrogen, phosphorus, and potassium (NPK) make an important contribution to the attainment of higher crop yields (204). Through the application of NPK-containing fertilizers, agricultural productivity increased in many countries of the world in the late 1960s and resulted in Green Revolution and saved them from starvation. In the current scenario, these fertilizers are important and necessary to improve crop yield and save the human population from starvation as low-input agriculture cannot feed the current seven billion world population (205). Microminerals iron, zinc, copper, manganese, I, Se, Mo, Co, and Ni are found in varying degrees in the edible portion of certain plants and are usually absorbed from the soil. Improvement of the soil micronutrient status by their application as fertilizers can contribute to decrease in micronutrient deficiency in humans (206). When crops are grown in soils, where mineral elements become immediately unavailable in the soil and/or not readily translocated to edible tissues targeted application of soluble inorganic fertilizers to the roots or to the leaves are practiced. Agronomic biofortification is simple and inexpensive, but needs special attention in terms of source of nutrient, application method and effects on the environment. These should be applied regularly in every crop season and thus are less cost-effective in some cases. Use of mineral fertilizers is evidently feasible in the developed world, as exemplified by the success of Se fertilization of crops in Finland (207), zinc fertilization in Turkey (208), and I fertilization in irrigation water in China (209).
In addition to fertilizers, plant growth-promoting soil microorganisms can be used to enhance the nutrient mobility from soil to edible parts of plants and improve their nutritional status. Soil microorganisms like different species of genera Bacillus, Pseudomonas, Rhizobium, Azotobacter, etc. can also be utilized to increase the phytoavailability of mineral elements (210, 211). The N2-fixing bacteria play important role in increasing crop productivity in nitrogen limited conditions (212). Many crops are associated with mycorrhizal fungi that can release organic acids, siderophores, and enzymes capable of degrading organic compounds and increasing mineral concentrations in edible produce (210, 213). Different crops have been targeted through agronomical biofortification to improve the human nutritional status (Table 3).
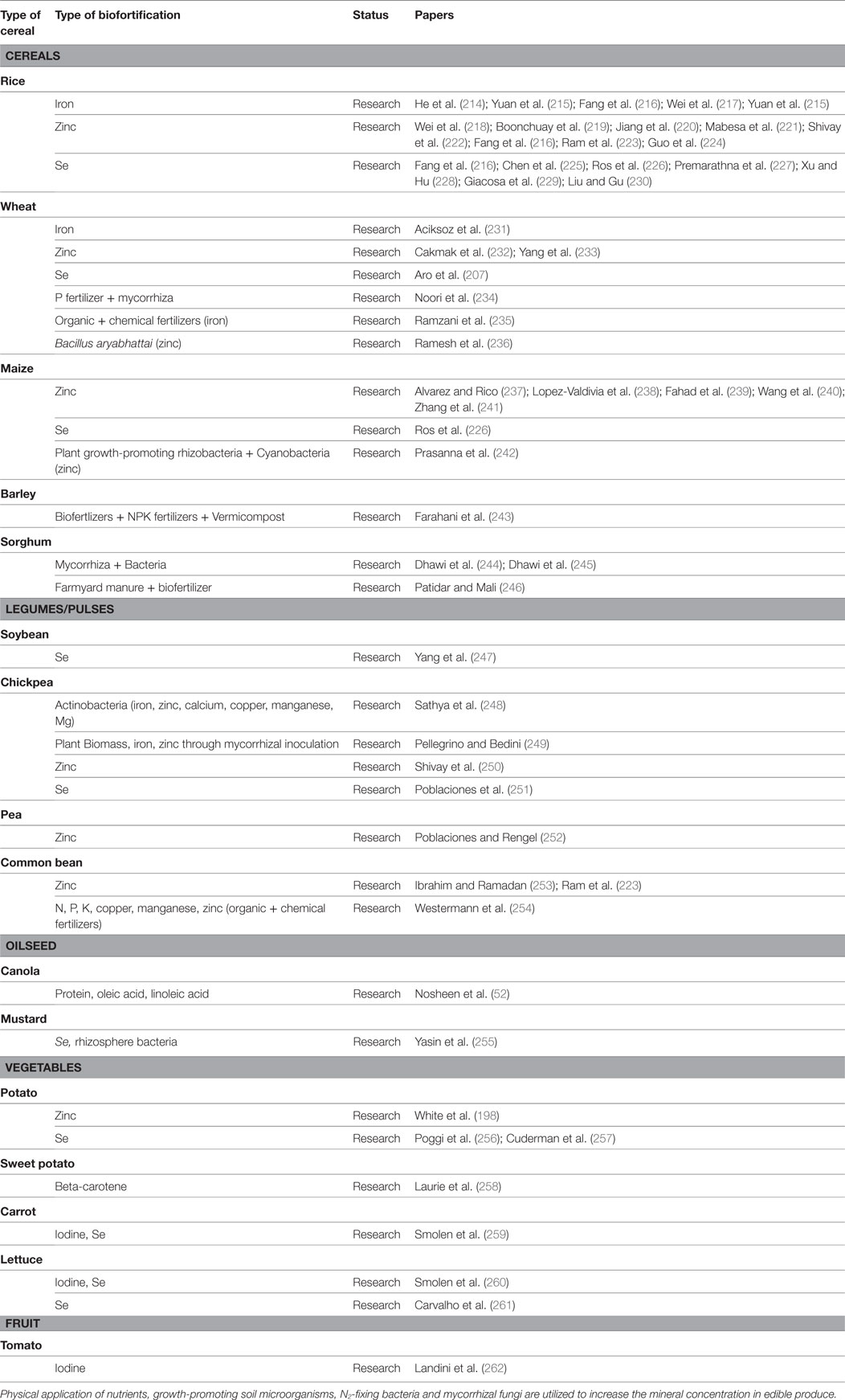
Table 3. Tabulation of crops, nutrients, research status, and concerned publications on biofortification through agronomic approaches.
Micronutrient biofortification through agronomical practices is an alternative strategy to reduce the iron and zinc deficiency in rice grain. Biofortification of rice plants by foliar spray of iron was an effective way to promote iron concentration in rice grains (214–216). Similarly, fortifying germinating rice plantlets with ferrous sulfate lead to increase iron concentration in germinated brown rice [up to 15.6 times the control (215)]. Foliar application of zinc has been reported as an effective agronomic practice to promote rice grain zinc concentration and zinc bioavailability (216, 218–223). On the other hand, application of zinc to soil as fertilizer in addition to a foliar spray proves to be an important strategy to increase the grain zinc content of rice grown in soils with low background levels of zinc (224). Selenium, which is an essential trace element for human health and proved to be a potent antioxidant, has been also increased by the application of selenate as a foliar spray or as fertilizer in rice (216, 225–230).
Agronomic biofortification has been very efficiently utilized in wheat grain quality improvement. Inclusion of iron in foliar urea fertilizers has been positively correlated with high iron accumulation (231). Application of foliar zinc has reduced human zinc deficiency in regions with potentially zinc-deficient soil and also improved its bioavailability by reducing antinutrient factors like phytic acid (233). Due to significant effects of zinc fertilizers on grain yield, the total amount of zinc-containing NPK fertilizers increased from 0 in 1994 to a record level of 400,000 t per annum in 10–15 years in Turkey. Use of zinc-containing fertilizers increased zinc concentration in grain, and obviously contributed to human nutrition and health in Turkey, especially in rural areas, where wheat provided more than 50% of the daily calorie intake (206). Agronomic biofortification of Se in wheat has been adopted with success in Finland (207). Compound fertilizers supplemented with Se were utilized since 1984, and it resulted in an increase in human serum selenium. Apart from chemical and organic fertilizers, researchers have also investigated the role of biofertilizers in promoting the yield of grains. Mycorrhizal fungi along with fertilizers are extensively being used for biofortification (234). Iron biofortification of wheat grains has been accomplished through integrated use of organic and chemical fertilizers and zinc biofortification by using Bacillus aryabhattai (235, 236).
Among micronutrients, zinc is required for obtaining nutrient-enriched grain and optimum yield in maize. For achieving this, various zinc fertilizer treatments and foliar applications have been carried out in maize crop (237, 239–241). Plant growth-promoting rhizobacteria have led to nutrient enrichment in the plants and have been included in agronomic approaches to develop effective biofortification strategies for the staple crops. One of the effective examples is the maize crop with increased zinc content (242). The Selenium (Se) importance in human and animal health has been known worldwide, and it has also been increased by applying fertilization as an effective agronomic biofortification strategy (226).
The micronutrient profile of barley has been improved by the application of various organic and inorganic biofertilizers. The concentration of zinc and iron in grains has been enhanced by the application of biofertilizers along with inorganic fertilizers and vermicompost (243).
Sorghum is cultivated worldwide for grain and fodder. This crop often suffers from the challenge of growing in nutrient poor and contaminated soil. Its nutrient profile has been promoted by the application of fertilizers (both organic and inorganic) that have an additive effect on the yield. Researchers have intended to improve the nutrient uptake and alter the metabolic profile of sorghum by using the combination of plant growth-promoting bacteria and arbuscular mycorrhizal fungi (AMF) (244, 245). Also, the inoculation of Azospirillum alone and in combination with phosphate-solubilizing bacteria increased sorghum grain yield and protein content by improving the status of phosphorous and nitrogen in the soil (246).
Selenium-enriched soybean has been produced by the foliar application of selenium complex salts as fertilizers (247).
Chickpea has been targeted for the mineral deficiencies, especially the mineral iron, zinc, calcium, copper, manganese, and Mg by using plant growth-promoting actinobacteria (248). Chickpea biofortification for iron and zinc has been addressed by using AMF (249). Similarly, zinc and Se have been fortified in chickpea by foliar spray of respective minerals (250, 251).
Field peas are the second largest legume crop worldwide, also known for their high protein content and its enrichment for zinc has been obtained with foliar zinc applications alone or in combination with soil zinc applications (252).
A common bean is an herbaceous annual plant grown for edible dry grain. Beans are a good vehicle for zinc biofortification and have been enriched with zinc by the application of foliar zinc fertilizer (223, 253). Furthermore, it has been studied that administration of organic and chemical fertilizers stimulated the uptake of N, P, K, copper, manganese, and zinc in common bean (254).
Canola supplemented with plant growth-promoting rhizobacteria viz. Azospirillum brasilense, Azotobacter vinelandii along with chemical fertilizers resulted in increased protein, oleic acid, and linoleic acid content in the seed which indicated that rhizobacteria are highly effective in improving yield and nutritive value of canola oil (263).
Mustard has been targeted for Se enhancement. Plant uptake of Se as selenate has been enhanced by rhizosphere bacteria from a seleniferous area (255).
Field experiments were undertaken to increase zinc concentrations in potato tubers (both flesh and skin of tubers) using foliar zinc fertilizers, which significantly increased tuber zinc concentrations. It was also found that zinc oxide and zinc sulfate were more effective than zinc nitrate as foliar fertilizers for increasing tuber zinc concentrations while maintaining yields (264). Increase in Se content of potato tubers has been reported after foliar application of selenium, selenite, and selenate to potato (256, 257). Foliar application of selenium with humic acids was proven to be a good way to increase the selenium content of potatoes (256).
Increase in beta-carotene in orange-fleshed sweet potato has been observed with irrigation and chemical fertilizer treatments (258).
Carrot leaves and storage roots have been supplemented with I and Se by application of both as fertilizers. It has been reported that consumption of 100 g fresh weight of carrots fertilized with I and Se (KICNa2SeO3, KIO3CNa2SeO3) can supply 100% of the recommended daily allowance (259).
Lettuce I and Se biofortification have been achieved by the application of KIO3 and Na2SeO4 as foliar spray and nutrient medium (260). Lettuce Se biofortification in the leaves has been carried out with good results after soil agronomic biofortification with an inorganic form of selenium (261).
Studies have concluded that a tomato is an excellent crop for iodine biofortification programs when treated with iron fertilizers (262).
Biofortification through conventional breeding in the most accepted method of biofortification. It offers a sustainable, cost-effective alternative to transgenic- and agronomic-based strategies. Sufficient genotypic variation in the trait of interest is necessary for conventional breeding to be feasible. Breeding programs can utilize this variation to improve the levels of minerals and vitamins in crops. In conventional plant breeding, parent lines with high nutrients are crossed with recipient line with desirable agronomic traits over several generations to produce plants with desired nutrient and agronomic traits. However, breeding strategies have to sometimes rely on the limited genetic variation present in the gene pool. In some cases, this can be overcome by crossing to distant relatives and thus moving the trait slowly into the commercial cultivars. Alternatively, new traits can be introduced directly into commercial varieties by mutagenesis.
Because this approach is likely to be the most expedient method to improve plants, several international organizations have initiated programs to improve the nutritional content of crops through breeding programs. The Health grain Project (2005–2010) involving 44 partners from 15 countries and over £10 million was carried out in the European Union to develop health promoting and safe cereal foods and ingredients of high eating quality. It has since developed into the Healthgrain forum with a wide range of participants from academia and industry. More than 100 publications have reported bioactive compounds in whole-grain cereals, genetic variation, heritability, and effect on reducing risks of many lifestyle-related diseases (265–267). The CGIAR along with the International Center for Tropical Agriculture (CIAT) and the International Food Policy Research Institute have launched the HarvestPlus program to breed biofortified staple food crops. HarvestPlus is investing heavily to boost three key nutrients-vitamin A, iron, and zinc and is targeting the staple crops, wheat, rice, maize, cassava, pearl millet, beans, and sweet potato in Asia and Africa (268). It is directed to produce staple food crops with enhanced levels of bioavailable essential minerals and vitamins that will have measurable impact on improving the micronutrient status of target populations, primarily resource-poor people in the developing world. The Biocassava Plus program had been initiated to improve the nutrition status of cassava crop. Due to better acceptability, large numbers of crops have been targeted for biofortification through crop breeding (Table 4).
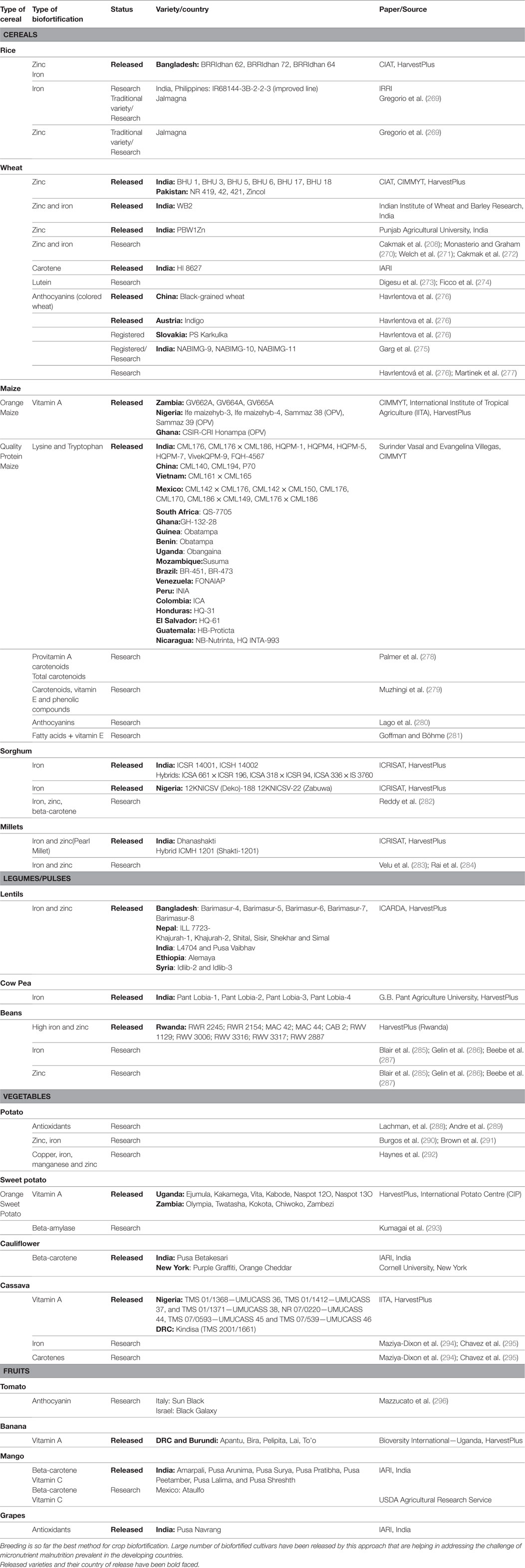
Table 4. Tabulation of crops, nutrients, research status, and concerned publications on biofortification through breeding.
Rice is greatly emphasized for micronutrient enhancement. It is one of the most consumed staple food crop and its biofortification can have a significant effect on malnutrition challenge. The milled rice is poor source of minerals. Different old rice varieties with high iron and zinc content in grain have been screened and the higher mineral trait has been combined with improved agronomic traits by breeding methods. The world’s first zinc enriched rice varieties developed by HarvestPlus were released in 2013 by the Bangladesh Rice Research Institute (BRRIdhan 62, BRRIdhan 72, and BRRIdhan 64), which is claimed to contain 20–22 ppm zinc in brown rice. In India and Philippines, an improved line (IR68144-3B-2-2-3) was identified in a cross between a high-yielding variety (IR72) and a tall, traditional variety (Zawa Bonday) with a high concentration of grain iron [about 21 ppm in brown rice (269)]. Similarly, Jalmagna, a traditional variety which had almost double the iron concentration of common rice variety and zinc concentration, nearly 40% more than that of common rice variety has been identified for further breeding programs to improve iron and zinc concentration (269).
Wheat as a staple crop is the first and foremost target for biofortification. Wide variation in grain iron and zinc concentrations in wheat and its closely related wild species has been observed that it can be exploited for improvement of modern elite cultivars (270, 272, 297). Utilizing this variation HarvestPlus has released several varieties of wheat with 4–10 ppm higher zinc content. Six varieties of high zinc wheat (BHU 1, BHU 3, BHU 5, BHU 6, BHU 7, and BHU 18) were released in India in 2014 followed by the release of four varieties in Pakistan in 2015 (NR 419, 42, 421, and Zincol). Two varieties BHU 1 and BHU 6 have high yield, disease resistance in addition to high zinc. Recently, variety with high zinc (PBW1Zn) has been released by Punjab Agricultural University, India. Another variety with high zinc and iron content (WB2) has been developed and released by Indian Institute of Wheat and Barley Research, India. Apart from releasing cultivars, several researchers have reported an increase in the zinc and iron content of wheat by plant breeding (208, 270–272). Provitamin A has been another important nutrient targeted for biofortification through breeding. High provitamin A durum wheat variety (HI 8627) has been released by the Indian Agricultural Research Institute (IARI), India in 2005. Several new cultivars have been released after that with the improved beta-carotene content. Yellow pigment content (YPC; carotenoids mainly xanthophyll lutein) in durum wheat is an important quality trait and an antioxidant. A large number of recent durum wheat varieties released in different countries in the past decade show significantly higher YPC than the old varieties released before the 1970s [(273, 274) and others]. Improvement of antioxidant properties contributed by anthocyanins had also been an area of significant research in wheat. Colored wheat (black, blue, and purple) trait has been used in several breeding programs in different countries. Black-grained wheat cultivar has been released in China after more than 20 years running effort in breeding and has been reported to be high in protein content and selenium (298). The purple wheat cultivar Indigo has been released in Austria in 2006 (299). The purple wheat cultivar PS Karkulka has been registered in Slovakia in 2014. Purple, blue, and black white lines have been developed and registered in India in 2017 (275). The importance of colored wheat can be adjudged from the patent on functional foods from colored wheat in China (CN102217664 B). Apart from this several researchers have worked on different aspects of colored wheat [reviewed in Ref. (276, 277)].
Maize is a cash crop grown for animal feed, industrial purposes (source of sugar, oil, starch, and ethanol) and for use for human consumption. The vast genetic diversity of maize has been the basis for the breeding programs that have generated much of the higher yielding maize used worldwide. Scientists have discovered varieties that have naturally high levels of provitamin A. HarvestPlus is using these lines to breed high-yielding varieties of biofortified maize with higher levels of provitamin A to combat vitamin A deficiency. The provitamin A maize is one of the significant achievements in the field of biofortification. Biofortified orange maize varieties have been grown commercially in Zambia (GV662A, GV664A, and GV665A), Nigeria {Ife maizehyb-3, Ife maizehyb-4, Sammaz 38 (OPV), Sammaz 39 (OPV)} and Ghana {CSIR-CRI Honampa (OPV)} since 2013 (300). Malawi, Zimbabwe (ZS242) and Tanzania have also released biofortified orange maize recently (301). As a positive effect an increase in pupillary response was observed among Zambian children consuming vitamin A biofortified maize (301). Breeders have evaluated antioxidants like tocochromanols, oryzanol, and phenolic compounds in proVA biofortified maize (279). Another significant achievement in the field of maize biofortification is quality protein maize (QPM). Maize breeders have developed QPM with high essential amino acids lysine and tryptophan by incorporating opaque-2 (o2) mutant gene from naturally occurring maize into the maize cultivars. International Maize and Wheat Improvement Center (CIMMYT) has released such hybrid varieties in India (CML176, CML176 × CML186, HQPM4, HQPM-7, VivekQPM-9, HQPM-5, HQPM-1, FQH-4567), China (CML140, CML194, P70), Vietnam (CML161 × CML165), Mexico (CML142 × CML176, CML142 × CML150, CML176, CML170, CML186 × CML149, CML176 × CML186), South Africa (QS-7705), Ghana (GH-132-28), Guinea (Obatampa), Uganda (Obangaina), Benin (Obangaina), Mozambique (Susuma), Brazil (BR-451, BR-473), Venezuela (FONAIAP), Peru (INIA), Colombia (ICA), Honduras (HQ-31), El Salvador (HQ-61), Guatemala (HB-Proticta), and Nicaragua (NB-Nutrinta, HQ INTA-993). For QPM maize breeders, Surinder Vasal and Evangelina Villegas won 2000 world food prize. Maize has also been inbred by recurrent selection scheme, to increase the carotenoids (278) alone or in combination of vitamin E and phenolics (279) and antioxidant power (280). Attempts have been made to increase its vitamin E content (281).
The prospects of breeding for micronutrients and beta-carotene rich sorghums have been discussed by Reddy et al. (282). Sorghum varieties have been screened for high minerals, protein (302), lutein, zeaxanthin, and beta-carotene contents (303). Sorghum germplasm has shown large variability and genetic heritability for iron and zinc content (304). Biofortified iron rich sorghum lines (ICSR 14001, ICSH 14002) and hybrids (ICSA 661 × ICSR 196, ICSA 318 × ICSR 94, ICSA 336 × IS 3760) have been bred by ICRISAT and released in India.
New nutritionally high (Fe) sorghum varieties (12KNICSV-22 and 12KNICSV-188) have been released in Nigeria that may boost the malnourished populations, especially children in Nigeria. One of the new varieties (12KNICSV-188) has iron content three times higher than typically grown sorghum. These new varieties involved crossing local Nigerian germplasm with improved lines from ICRISAT (Mali).
Pearl millet is the cheapest source of iron and zinc (305) and large variation has been seen in its germplasm for these micronutrients (283). In India, biofortified (iron and zinc) pearl millet variety “Dhanashakti” and a hybrid ICMH 1201 (Shakti-1201) has been released by ICRISAT, HarvestPlus in 2014. Besides that, two varieties, ICMH 1202 (Nirmal-7) and ICMH 1301, are currently undergoing advanced farm trials. Various well-adapted commercial varieties, their progenies, and hybrids containing high content of iron and zinc in grain have been reported (283, 284).
Lentil, a key pulse in many dryland countries and has easy to cook properties. It has been directed by ICARDA, HarvestPlus for biofortification of iron and zinc with the help of breeding process using genetic diversity stored in gene banks. Research findings have shown that there is a positive correlation of iron and zinc synthesis with protein synthesis, therefore lentil varieties with higher iron, zinc, and protein content can be developed together [ICARDA, HarvestPlus (306)]. High iron and zinc lentil varieties, five in Bangladesh (Barimasur-4, Barimasur-5, Barimasur-6, Barimasur-7, and Barimasur-8), seven in Nepal (ILL 7723, Khajurah-1, Khajurah-2, Shital, Sisir Shekhar, Simal), two in India (L4704, Pusa Vaibhav), one in Ethiopia (Alemaya), and two in Syria (Idlib-2, Idlib-3) has been released by ICARDA, HarvestPlus biofortification program till date. Lentil varieties have been screened for variation in Se content (307).
Cow pea which is also known as poor man meat, rich in protein content has been biofortified for iron content by means of breeding methods. Pant Lobia-1 (2008), Pant Lobia-2 (2010), Pant Lobia-3 (2013), and Pant Lobia-4 (2014) varieties with increased iron content have been released by GB Pant University, Pantnagar, India in collaboration to HarvestPlus.
Studies till date suggest that the iron content of the common bean (P. vulgaris) could be increased by 60–80%, while zinc content would be more modest, perhaps around 50%. High heritability has been observed in iron and zinc content in common bean (285, 287, 308). Genes associated with zinc accumulation have been identified in navy bean (286). HarvestPlus is working in this direction and promoting iron biofortified beans in several developing countries. They have released 10 Fe-biofortified common bean varieties in Rwanda (RWR 2245, RWR 2154, MAC 42, MAC 44, CAB 2, RWV 1129, RWV 3006, RWV 3316, RWV 3317, and RWV 2887). HarvestPlus also released ten biofortified iron bean varieties in the Democratic Republic of Congo, i.e., COD MLB 001, COD MLB 032, HM 21-7, RWR 2245, PVA 1438, COD MLV 059, VCB 81013, Nain de Kyondo, Cuarentino, Namulenga.
Potato tubers are the richest sources of antioxidants in human diet. The natural variation of cultivated potato germplasm containing red and purple pigment could possibly represent the contribution of the potatoes to the portion of antioxidants in human nutrition. Therefore, effort of breeders focuses on the breeding of such variants (288). Furthermore, vast genetic variation for micronutrients (291) exists in potato that can be exploited for breeding to further increase iron and zinc levels in human diets (290). A genetically diverse sample of potato cultivars native to the Andes of South America has been obtained from a collection of nearly 1,000 genotypes and evaluated as a source of antioxidants and minerals (copper, iron, manganese, and zinc) (289, 292). International potato center (CIP) and HarvestPlus have developed high iron and zinc advanced breeding material after crossing diploid Andean landrace potatoes with high zinc and iron with disease resistant tetraploid clones. The main target countries for biofortified potato are Rwanda and Ethiopia. National Institute for Agrarian Innovation’s (INIA) Potato Program has developed the INIA 321 Kawsay variety in Peru that has a high content of iron and zinc.
Developing countries are growing 95% of the world’s sweet potato crop, where malnutrition is the biggest problem. The sweet potato has been targeted for improvement in vitamin A. HarvestPlus and International Potato Centre (CIP) have developed and released several varieties of orange sweet potato with high vitamin A. Six varieties have been released in Uganda (Ejumula, Kakamega, Vita, Kabode, Naspot 12O, and Naspot 13O) and three in Zambia (Twatasha, Kokota, and Chiwoko). Zambia Agriculture Research Institute has successfully completed the development of 15 new varieties of vitamin A fortified sweet potatoes. The HarvestPlus orange sweet potato consumption had a significant effect on household food and nutritional security in Sub Saharan Africa, and for this contribution; they have been recently honored with World Food Prize-2016. Furthermore, researchers have identified several sweet potato genotypes that completely lack or have only traces of β-amylase in their storage roots. Such verities could facilitate the breeding of sweet potato for low β-amylase content which can be potentially used for processing and as a staple food (293).
Brassica oleracea including cauliflower gene pool has been screened for genetic variation of zinc concentration and sufficient natural variation has been identified (309). The provitamin A (beta-carotene) rich orange colored cauliflower variety (Pusa BetaKesari; 800–1,000 μg/100g) has been released by the Indian Agricultural Research Institute (IARI). Now numbers of colored cauliflower verities are known at world level, having orange and purple color rich in beta-carotene and anthocyanin, respectively. Colored cauliflower varieties, Purple Graffiti and Orange Cheddar, have been developed by Cornell University, USA.
Cassava is a staple vegetable root crop in developing countries, especially in Africa, Latin America, and the Caribbean. In the African continent, it has been targeted for alleviation in provitamin A (beta-carotene) by HarvestPlus in collaboration with International Institute of Tropical Agriculture. Under these collaborations, they have released six vitamin A fortified varieties in Nigeria (2011; TMS 01/1368—UMUCASS 36, TMS 01/1412—UMUCASS 37 and 2014; TMS 01/1371—UMUCASS 38 and NR 07/0220—UMUCASS 44, TMS 07/0593—UMUCASS 45, and TMS 07/539—UMUCASS 46) and one in DRC-Democratic Republic of Congo [Kindisa (TMS 2001/1661)]. Cassava also has a wide range of genotype differences for total carotene, proteins, and minerals (iron and zinc) which has led to the development of improved nutritive value cassava crop (294, 295).
Tomato is a highly valuable crop and an important source of vitamin A and C. Genetically diverse wild population of tomato has been investigated intensively for specific traits and exploited in tomato breeding (310). Anthocyanin biofortified tomato “Sun Black” with deep purple fruit pigmentation due to high anthocyanin content in the peel has been developed by conventional breeding approach (296). Another variety “Black Galaxy” generated by similar approach has been reported from Israel.
Breeding banana is difficult and expensive, as commercial varieties are sterile triploids (3×) and also a high degree of cross incompatibility can exist among the fertile groups. For combating this problem, large scale screening of several banana germplasm for the identification of high levels of provitamin A has been carried out in the Democratic Republic of Congo (DRC) and Burundi by Biodiversity International (BI) in collaboration with HarvestPlus. In this program, they released five varieties (Apantu, Bira, Pelipita, Lai, and To’o) rich in provitamin A in Eastern DRC and Burundi.
Mango offers a natural source of beta-carotene, vitamin C, and valuable antioxidants but their nutrient levels vary with mango variety. It has been observed that most of the mango varieties provide more than recommended daily value of vitamin C and beta-carotene. Mango also contains a variety of phenolics like ellagic acid, gallotannin, and mangiferin (311). The Mexican-grown Ataulfo variety ranked highest in both vitamin C (ascorbic acid) and beta-carotene (USDA’s Agricultural Research Service). In India, IARI introduced many varieties with enhanced nutritional and agronomical important characters.
Grapes have high mineral content, including high vitamins C and K, and are a natural source of antioxidants and other polyphenols, and offer a variety of additional health benefits. Phenolic compounds and antioxidant properties of different grape cultivars grown in China have been assessed (312). The Indian Agricultural Institute has released an improved variety, i.e., Pusa Navrang which contains higher amount of total soluble solids (carbohydrates, organic acids, proteins, fats, and minerals) and antioxidants.
Application of fertilizers fortified with micronutrients is the simplest method among all biofortification methods. But the success of agronomical biofortification is highly variable due to the differences in mineral mobility, mineral accumulation among plant species, soil compositions in the specific geographical location of each crop (313). For example, a study involving diverse rice genotypes indicated that, in the phosphate deficient soils due to reduction in the root biomass, differences in the phosphate uptake among the genotypes were as high as 20-fold (314). Soil composition analysis has indicated that almost 1/2 of the agricultural soils of India, 1/3 of China, 14 Mha of Turkey, 8 Mha of Australia are zinc deficient (315). Agronomic biofortification is less cost-effective and labor intensive as it demands continuous inputs, through the application of micronutrient to the soil or plant regularly. Furthermore, it is not always possible to target the micronutrient into edible plant parts like seed or fruit and can sometimes result in the accumulation of desired nutrients in the leaves or other non-edible portions of plants; therefore, this technique is only successful in certain minerals and specific plant species. For instance, higher zinc efficiency in cereals grown in zinc deficient soils in Turkey was associated with higher uptake of zinc from the soil, but not with increased accumulation of zinc in the grain (208). Furthermore, mineral bioavailability hindered by antinutrient compound like phytic acid is another major challenge (316). In addition, the biggest of all constraints is that the fertilizers accumulation in soil and water poses adverse environmental effects (317).
The design of conventional plant breeding programs to improve micronutrient content has proved to be successful and is a sustainable and cost-effective solution in the long run; however, there are limitations with respect to the amount of genetic variability for the micronutrients in the plant gene pool and the time needed to generate cultivars with the desired trait(s). In some cases, this can be overcome by crossing to distant relatives and thus introgressing traits into commercial cultivars, but in many occasions, it would be impossible to breed for a specific trait using conventional means, and the timescale and effort involved may be quite unrealistic, e.g., improving Se concentration in wheat grains (318) and improvement of oleic, linoleic, and linolenic fatty acid content in soybean (319). In general, improvement in oil quality has been targeted with better results with transgenic-based approach (Figure 3B) due to limited variability, heritability, and linkage drag.
Transgenic crops overcome the limitation of restricted genetic variation among plants as in the case of conventional breeding but the major limitation of this method is its low acceptance among masses. It is very important that the biofortified crops be readily adapted by farmers and community in significant enough numbers to improve the general nutritional health of a given community (320). Another limitation is that different countries have adopted different regulatory processes for the acceptance and commercialization of these transgenic crops. Regrettably, the current political and economic landscape is not receptive to this technology (321). Furthermore, these regulatory processes are very expensive and time consuming (322). Let us take the example of Bt Brinjal. It has been initially developed by Mahyco, an Indian seed company. Unfortunately, it was not released in Indian because some of the scientists, farmers, and anti-GMO activists, raised concerns and a moratorium on its release was imposed, until further tests were conducted. However, four varieties of Bt Brinjal were given approval for commercial release in Bangladesh in 2013–2014. Although the research efforts devoted to the transgenic-based approach are quite higher compared with breeding based, its success rate in terms of cultivar release in very low (Figure 3A) due to time required from target trait and gene identification, modification, expression, and assessment of agronomical traits to understanding the possible effect on other life forms. For example, after 8 years project, the scientific details of the Golden rice were first published in Science in 2000 (41), and since then different groups, including International Rice Research Institute scientists are working on it, but Golden Rice is still not ready for farmers due to issues with its yield. Its dissemination is also being held back due to inability to get approval from Governments.
The postharvest processing of each crop must be considered to optimize biofortification strategies. For example, the seeds of many cereals are often consumed after milling or polishing. Although the concentrations of some essential mineral elements, such as Se and S, are highest in the embryo, others, such as iron, zinc, and copper, are highest in the bran (269, 317). Milling or polishing cereal seeds can, therefore, remove large quantities of minerals from the diet; the extent of these losses is genotype dependent (269). In addition, the presence of certain antinutrients in crops reduces the bioavailability of certain nutrients in crops. For examples, antinutrients like phytate, tannins, oxalate, fiber, and hemaglutinins reduce the bioavailability of minerals in human gut (20, 101). Furthermore, in the context of global environmental change, approaches for improving food production, improvements in a crop’s ability to maintain yields with lower water supply and quality will be critical. In addition, numerous genes are involved in controlling the amount of a mineral element that is absorbed by roots, translocated to shoot, remobilized from vegetative tissues, and deposited in edible portions of seeds and grains in forms that are utilizable in persons consuming the crop (323, 324). Considerations must also include the micronutrient concentrations in the edible portions of crops, and the amount of nutrients that can be absorbed by the consumer, after processing and cooking (325).
It is well established that biofortification is a promising, cost-effective, agricultural strategy for improving the nutritional status of malnourished populations throughout the world. Biofortification strategies based on crop breeding, targeted genetic manipulation, and/or the application of mineral fertilizers hold great potential for addressing mineral malnutrition in humans. The generation of biofortified food crops with improved nutrient contents such as increases in iron, zinc, Se, and provitamin A content are providing sufficient levels of these and other such micronutrients that are frequently lacking in the diets of the developing and developed world. International initiatives, such as the HarvestPlus program and national initiatives, are acting as pillars to achieve these targets. These efforts have delivered crops with the potential to increase both the amounts and bioavailability of essential mineral elements in human diets, especially in staple cereal crops like wheat, maize, cassava, beans, sweet potatoes, and millets. But biofortification of crops is a challenging endeavor. To achieve this, collaboration between plant breeders, nutrition scientists, genetic engineers, and molecular biologists is essential. Traditional breeding approaches are finding widespread and easy acceptance and have been used to enhance the nutritional qualities of foods. Although a greater emphasis is being laid on transgenic means success rates of breeding based approaches are much higher as transgenically fortified crop plants have to face hurdles due to acceptance constraints among consumers and different expensive and time consuming regulatory approval processes, adopted by different countries. Besides these challenges, biofortified crops hold a very bright future as these have the potential to remove micronutrient malnutrition among billions of poor people, especially in the developing countries.
MG and NG built the layout of the article, collected literature, and wrote the article. SS and PK collected literature and helped in manuscript writing. AK and VC edited it. PA assisted in reference management.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by the National Agri-Food Biotechnology Institute Core grant for improvement of nutrition and processing quality, for which the authors are deeply indebted.
1. McGuire S. FAO, IFAD, and WFP. The state of food insecurity in the world 2015: meeting the 2015 international hunger targets: taking stock of uneven progress. Rome: FAO. Adv Nutr (2015) 6(5):623–4. doi:10.3945/an.115.009936
2. Hodge J. Hidden hunger: approaches to tackling micronutrient deficiencies. In: Gillespie S, Hodge J, Yosef S, Pandya-Lorch R, editors. Nourishing Millions: Stories of Change in Nutrition. Washington: International Food Policy Research Institute (IFPRI) (2016). p. 35–43.
3. Muthayya A, Rah JH, Sugimoto JD, Roos FF, Kraemer K, Black RE. The global hidden hunger indices and maps: an advocacy tool for action. PLoS One (2013) 8(6):e67860. doi:10.1371/journal.pone.0067860
5. Khush GS, Lee S, Cho JI, Jeon JS. Biofortification of crops for reducing malnutrition. Plant Biotechnol Rep (2012) 6:195–202. doi:10.1007/s11816-012-0216-5
6. Gilani GS, Nasim A. Impact of foods nutritionally enhanced through biotechnology in alleviating malnutrition in developing countries. J AOAC Int (2007) 90(5):1440–4.
7. Perez-Massot E, Banakar R, Gomez-Galera S, Zorrilla-Lopez U, Sanahuja G, Arjo G, et al. The contribution of transgenic plants to better health through improved nutrition: opportunities and constraints. Genes Nutr (2013) 8(1):29–41. doi:10.1007/s12263-012-0315-5
8. Bouis HE. Economics of enhanced micronutrient density in food staples. Field Crops Res (1999) 60:165–73. doi:10.1016/S0378-4290(98)00138-5
9. Nestel P, Bouis HE, Meenakshi JV, Pfeiffer W. Biofortification of staple food crops. J Nutr (2006) 136:1064–7. doi:10.1093/jn/136.4.1064
10. Pfeiffer WH, McClafferty B. HarvestPlus: breeding crops for better nutrition. Crop Sci (2007) 47:S88–100. doi:10.2135/cropsci2007.09.0020IPBS
11. Qaim M, Stein AJ, Meenakshi JV. Economics of biofortification. Agric Econ (2007) 37:119–33. doi:10.1111/j.1574-0862.2007.00239.x
12. Hirschi KD. Nutrient biofortification of food crops. Annu Rev Nutr (2009) 29:401–21. doi:10.1146/annurev-nutr-080508-141143
13. Meenakshi JV, Johnson NL, Manyong VM, DeGroote H, Javelosa J, Yanggen DR, et al. How cost-effective is biofortification in combating micronutrient malnutrition? An ex ante assessment. World Dev (2010) 38(1):64–75. doi:10.1016/j.worlddev.2009.03.014
14. Hefferon KL. Can biofortified crops help attain food security? Curr Mol Biol Rep (2016) 2(4):180–5. doi:10.1007/s40610-016-0048-0
15. Bazuin S, Azadi H, Witlox F. Application of GM crops in Sub-Saharan Africa: lessons learned from green revolution. Biotechnol Adv (2011) 29:908–12. doi:10.1016/j.biotechadv.2011.07.011
16. Das JK, Kumar R, Salam RA, Bhutta ZA. Systematic review of zinc fortification trials. Ann Nutr Metab (2013) 62(1):44–56. doi:10.1159/000348262
17. Bouis HE. Enrichment of food staples through plant breeding: a new strategy for fighting micronutrient malnutrition. Nutrition (2000) 16:701–4. doi:10.1016/S0899-9007(00)00266-5
18. Prashanth L, Kattapagari KK, Chitturi RT, Baddam VR, Prasad LK. A review on role of essential trace elements in health and disease. J NTR Univ Health Sci (2015) 4:75–85. doi:10.4103/2277-8632.158577
19. White J, Broadley MR. Biofortifying crops with essential mineral elements. Trends Plant Sci (2005) 10:586–93. doi:10.1016/j.tplants.2005.10.001
20. Welch RM, Graham RD. Breeding for micronutrients in staple food crops from a human nutrition perspective. J Exp Bot (2004) 55:353–64. doi:10.1093/jxb/erh064
21. Graham RD, Welch RM, Bouis HE. Addressing micronutrient malnutrition through enhancing the nutritional quality of staple foods: principles, perspectives and knowledge gaps. Adv Agron (2001) 70:77–142. doi:10.1016/S0065-2113(01)70004-1
23. Schneeman BO. Linking agricultural production and human nutrition. J Sci Food Agri (2001) 81:3–9. doi:10.1002/1097-0010(20010101)81:1<3::AID-JSFA743>3.0.CO;2-Q
24. Chizuru N, Ricardo U, Shiriki K, Prakash S. The joint WHO/FAO expert consultation on diet, nutrition and the prevention of chronic diseases: process, product and policy implications. Public Health Nutr (2003) 7(1a):245–50. doi:10.1079/PHN2003592
25. Branca F, Ferrari M. Impact of micronutrient deficiencies on growth: the stunting syndrome. Ann Nutr Metab (2002) 46:8–17. doi:10.1159/000066397
26. Golden MHN. The nature of nutritional deficiencies in relation to growth failure and poverty. Acta Paediatr Scand (1991) 374:95–110. doi:10.1111/j.1651-2227.1991.tb12012.x
27. Grantham-McGregor SM, Ani CC. The role of micronutrients in psychomotor sad cognitive development. Br Med Bull (1999) 55:511–27. doi:10.1258/0007142991902583
28. Ramakrishnan U, Manjrekar R, Rivera J, Gonzales-Cossio T, Martorell R. Micronutrients and pregnancy outcome: a review of the literature. Nutr Res (1999) 19:103–59. doi:10.1016/S0271-5317(98)00178-X
29. Caballero B. Global patterns of child health: the role of nutrition. Ann Nutr Metab (2002) 46:3–7. doi:10.1159/000066400
30. Stevens GA, Finucane MM, De-Regil L, Paciorek CJ, Flaxman SR, Branca F, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health (2013) 1(1):e16–25. doi:10.1016/S2214-109X(13)70001-9
31. Brotanek JM, Halterman JS, Auinger P, Flores G, Weitzman M. Iron deficiency, prolonged bottle-feeding, and racial/ethnic disparities in young children. Arch Pediatr Adolesc Med (2005) 159:1038–42. doi:10.1001/archpedi.159.11.1038
32. Zhu C, Naqvi S, Gomez-Galera S, Pelacho AM, Capell T, Christou P. Transgenic strategies for the nutritional enhancement of plants. Trends Plant Sci (2007) 12:548–55. doi:10.1016/j.tplants.2007.09.007
33. Welch RM, Graham RD. A new paradigm for world agriculture: meeting human needs-productive, sustainable, nutritious. Field Crops Res (1999) 60:1–10. doi:10.1016/S0378-4290(98)00129-4
34. Saltzman A, Birol E, Bouis HE, Boy E, De Moura FF, Islam Y, et al. Biofortification: progress toward a more nourishing future. Glob Food Secur (2014) 2(1):9–17. doi:10.1016/j.gfs.2012.12.003
35. Brinch-Pedersen H, Borg S, Tauris B, Holm PB. Molecular genetic approaches to increasing mineral availability and vitamin content of cereals. J Cereal Sci (2007) 46:308–26. doi:10.1016/j.jcs.2007.02.004
36. Christou P, Twyman RM. The potential of genetically enhanced plants to address food insecurity. Nutr Res Rev (2004) 17:23–42. doi:10.1079/NRR200373
37. Newell-McGloughlin M. Nutritionally improved agricultural crops. Plant Physiol (2008) 147:939–53. doi:10.1104/pp.108.121947
38. Shewmaker CK, Sheehu JA, Daley M, Colburn S, Ke DY. Seed-specific overexpression of phytoene synthase: increase in carotenoids and metabolic effects. Plant J (1999) 20:41–412. doi:10.1046/j.1365-313x.1999.00611.x
39. Agrawal PK, Kohli A, Twyman RM, Christou P. Transformation of plants with multiple cassettes generates simple transgene integration patterns and high expression levels. Mol Breed (2005) 16:247–60. doi:10.1007/s11032-005-0239-5
40. Yang SH, Moran DL, Jia HW, Bicar EH, Lee M, Scott MP. Expression of a synthetic porcine alpha-lactalbumin gene in the kernels of transgenic maize. Transgenic Res (2002) 11:11–20. doi:10.1023/A:1013996129125
41. Ye X, Al-Babili S, Kloti A, Zhang J, Lucca P, Beyer P, et al. Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoids-free) rice endosperm. Science (2000) 287:303–5. doi:10.1126/science.287.5451.303
42. Beyer P, Al-Babili S, Ye X, Lucca P, Schaub P, Welsch R, et al. Golden rice: introducing the β-carotene biosynthesis pathway into rice endosperm by genetic engineering to defeat vitamin A deficiency. J Nutr (2002) 132(3):506S–10S. doi:10.1093/jn/132.3.506S
43. Datta K, Baisakh N, Oliva N, Torrizo L, Abrigo E, Tan J, et al. Bioengineered ‘golden’ Indica rice cultivars with beta-carotene metabolism in the endosperm with hygromycin and mannose selection systems. Plant Biotechnol J (2003) 1:81–90. doi:10.1046/j.1467-7652.2003.00015.x
44. Paine JA, Shipton CA, Chaggar S, Howells RM, Kennedy MJ, Vernon G, et al. Improving the nutritional value of golden rice through increased pro-vitamin A content. Nat Biotechnol (2005) 23:482–7. doi:10.1038/nbt1082
45. Burkhardt PK, Beyer P, Wuenn J, Kloeti A, Armstrong GA, Schledz M, et al. Transgenic rice (Oryza sativa) endosperm expressing daffodil (Narcissus pseudonarcissus) phytoene synthase accumulates phytoene, a key intermediate of provitamin A biosynthesis. Plant J (1997) 11:1071–8. doi:10.1046/j.1365-313X.1997.11051071.x
46. Storozhenko S, De Brouwer V, Volckaert M, Navarrete O, Blancquaert D, Zhang GF, et al. Folate fortification of rice by metabolic engineering. Nat Biotechnol (2007) 25(11):1277–9. doi:10.1038/nbt1351
47. Blancquaert D, Van daele J, Strobbe S, Kiekens F, Storozhenko S, De Steur H, et al. Improving folate (vitamin B9) stability in biofortified rice through metabolic engineering. Nat Biotechnol (2015) 33:1076–8. doi:10.1038/nbt.3358
48. Takahashi M, Nakanishi H, Kawasaki S, Nishizawa NK, Mori S. Enhanced tolerance of rice to low iron availability in alkaline soils using barley nicotianamine aminotransferase genes. Nat Biotechnol (2001) 19:466–9. doi:10.1038/88143
49. Lee S, An G. Over-expression of OsIRT1 leads to increased iron and zinc accumulations in rice. Plant Cell Environ (2009) 32:408–16. doi:10.1111/j.1365-3040.2009.01935.x
50. Zheng L, Cheng Z, Ai C, Jiang X, Bei X, Zheng Y, et al. Nicotianamine, a novel enhancer of rice iron bioavailability to humans. PLoS One (2010) 5(4):e10190. doi:10.1371/journal.pone.0010190
51. Lee S, Kim YS, Jeon US, Kim YK, Schjoerring JK, An G. Activation of rice nicotinamine synthase 2 (OsNAS2) enhances iron availability for biofortification. Mol Cell (2012) 33:269–75. doi:10.1007/s10059-012-2231-3
52. Trijatmiko K, Duenas C, Tsakirpaloglou N, Torrizo L, Arines FM, Adeva C, et al. Biofortified indica rice attains iron and zinc nutrition dietary targets in the field. Sci Rep (2016) 6:19792. doi:10.1038/srep19792
53. Goto F, Yoshihara T, Shigemoto N, Toki S, Takaiwa F. Iron fortification of rice seed by the soybean ferritin gene. Nat Biotechnol (1999) 17:282–6. doi:10.1038/7029
54. Vasconcelos M, Datta K, Oliva N, Khalekuzzaman M, Torrizo L, Krishnan S, et al. Enhanced iron and zinc accumulation in transgenic rice with the ferritin gene. Plant Sci (2003) 164:371–8. doi:10.1016/S0168-9452(02)00421-1
55. Lucca P, Hurrell R, Potrykus I. Fighting iron deficiency anemia with iron-rich rice. J Am Coll Nutr (2002) 21:184S–90S. doi:10.1080/07315724.2002.10719264
56. Wirth J, Poletti S, Aeschlimann B, Yakandawala N, Drosse B, Osorio S, et al. Rice endosperm iron biofortification by targeted and synergistic action of nicotianamine synthase and ferritin. Plant Biotechnol J (2009) 7:631–44. doi:10.1111/j.1467-7652.2009.00430.x
57. Masuda H, Ishimaru Y, Aung MS, Kobayashi T, Kakei Y, Takahashi M, et al. Iron biofortification in rice by the introduction of multiple genes involved in iron nutrition. Sci Rep (2012) 2:534. doi:10.1038/srep00543
58. Masuda H, Kobayashi T, Ishimaru Y, Takahashi M, Aung MS, Nakanishi H, et al. Iron-biofortification in rice by the introduction of three barley genes participated in mugineic acid biosynthesis with soybean ferritin gene. Front Plant Sci (2013) 4:132. doi:10.3389/fpls.2013.00132
59. Hurrell R, Egli I. Iron bioavailability and dietary reference values. Am J Clin Nutr (2010) 91:1461S–7S. doi:10.3945/ajcn.2010.28674F
60. Masuda H, Suzuki M, Morikawa KC, Kobayashi T, Nakanishi H, Takahashi M, et al. Increase in iron and zinc concentrations in rice grains via the introduction of barley genes involved in phytosiderophore synthesis. Rice (2008) 1:100–8. doi:10.1007/s12284-008-9007-6
61. Zheng A, Sumi K, Tanaka K, Murai N. The bean seed storage protein β-phaseolin is synthesized, processed and accumulated in the vacuolar type-II protein bodies of transgenic rice endosperm. Plant Physiol (1995) 109:777–86. doi:10.1104/pp.109.3.777
62. Sindhu AS, Zheng Z, Murai N. The pea seed storage protein legumin was synthesized, processed, and accumulated stably in transgenic rice endosperm. Plant Sci (1997) 130:189–96. doi:10.1016/S0168-9452(97)00219-7
63. Lee TTT, Wang MMC, Hou RCW, Chen LJ, Su RC, Wang CS, et al. Enhanced methionine and cysteine levels in transgenic rice seeds by the accumulation of sesame 2S albumin. Biosci Biotechnol Biochem (2003) 67:1699–705. doi:10.1271/bbb.67.1699
64. Katsube T, Kurisaka N, Ogawa M, Maruyama N, Ohtsuka R, Utsumi S, et al. Accumulation of soybean glycinin and its assembly with the glutelins in rice. Plant Physiol (1999) 120:1063–73. doi:10.1104/pp.120.4.1063
65. Yang QQ, Zhang CQ, Chan ML, Zhao DS, Chen JZ, Wang Q, et al. Biofortification of rice with the essential amino acid lysine: molecular characterization, nutritional evaluation, and field performance. J Exp Bot (2016) 67(14):4285–96. doi:10.1093/jxb/erw209
66. Lee SI, Kim HU, Lee YH, Suh SC, Lim YP, Lee HY, et al. Constitutive and seed-specific expression of a maize lysine-feedback-insensitive dihydrodipicolinate synthase gene leads to increased free lysine levels in rice seeds. Mol Breed (2001) 8:75–84. doi:10.1023/A:1011977219926
67. Wakasa K, Hasegawa H, Nemoto H, Matsuda F, Miyazawa H, Tozawa Y, et al. High-level tryptophan accumulation in seeds of transgenic rice and its limited effects on agronomic traits and seed metabolite profile. J Exp Bot (2006) 57:3069–78. doi:10.1093/jxb/erl068
68. Zhou Y, Cai H, Xiao J, Li X, Zhang Q, Lian X. Over-expression of aspartate aminotransferase genes in rice resulted in altered nitrogen metabolism and increased amino acid content in seeds. Theor Appl Genet (2009) 118:1381–90. doi:10.1007/s00122-009-0988-3
69. Anai T, Koga M, Tanaka H, Kinoshita T, Rahman SM, Takagi Y. Improvement of rice (Oryza sativa L.) seed oil quality through introduction of a soybean microsomal omega-3 fatty acid desaturase gene. Plant Cell Rep (2003) 21(10):988–92. doi:10.1007/s00299-003-0609-6
70. Shin YM, Park HJ, Yim SD, Baek NI, Lee CH, An G, et al. Transgenic rice lines expressing maize C1 and R-S regulatory genes produce various flavonoids in the endosperm. Plant Biotechnol J (2006) 4:303–15. doi:10.1111/j.1467-7652.2006.00182.x
71. Ogo Y, Ozawa K, Ishimaru T, Murayama T, Takaiwa F. Transgenic rice seed synthesizing diverse flavonoids at high levels: a new platform for flavonoid production with associated health benefits. Plant Biotechnol J (2013) 11:734–46. doi:10.1111/pbi.12064
72. Liu Q, Wang Z, Chen X, Cai X, Tang S, Yu H, et al. Stable inheritance of the antisense waxy gene in transgenic rice with reduced amylose level and improved quality. Transgenic Res (2003) 12(1):71–82. doi:10.1023/A:1022148824018
73. Itoh K, Ozaki H, Okada K, Hori H, Takeda Y, Mitsui T. Introduction of Wx transgene into rice wx mutants leads to both high- and low-amylose rice. Plant Cell Physiol (2003) 44(5):473–80. doi:10.1093/pcp/pcg068
74. Wei C, Zhang J, Chen Y, Zhou W, Xu B, Wang Y, et al. Physicochemical properties and development of wheat large and small starch granules during endosperm development. Acta Physiol Plant (2010) 32:905–16. doi:10.1007/s11738-010-0478-x
75. Nandi S, Suzuki YA, Huang J, Yalda D, Pham P, Wu L, et al. Expression of human lactoferrin in transgenic rice grains for the application in infant formula. Plant Sci (2002) 163:713–22. doi:10.1016/S0168-9452(02)00165-6
76. Wang C, Zeng J, Li Y, Hu W, Chen L, Miao Y, et al. Enrichment of provitamin A content in wheat (Triticum aestivum L.) by introduction of the bacterial carotenoid biosynthetic genes CrtB and CrtI. J Exp Bot (2014) 65(9):2545–56. doi:10.1093/jxb/eru138
77. Cong L, Wang C, Chen L, Liu H, Yang G, He G. Expression of phytoene synthase1 and carotene desaturase crtI genes result in an increase in the total carotenoids content in transgenic elite wheat (Triticum aestivum L.). J Agric Food Chem (2009) 57(18):8652–60. doi:10.1021/jf9012218
78. Xiaoyan S, Yan Z, Shubin W. Improvement Fe content of wheat (Triticum aestivum) grain by soybean ferritin expression cassette without vector backbone sequence. J Agric Biotechnol (2012) 20:766–73.
79. Borg S, Brinch-Pedersen H, Tauris B, Madsen LH, Darbani B, Noeparvar S, et al. Wheat ferritins: improving the iron content of the wheat grain. J Cereal Sci (2012) 56:204–13. doi:10.1016/j.jcs.2012.03.005
80. Brinch-Pederson H, Olesen A, Rasmussen SK, Holm PB. Generation of transgenic wheat (Triticum aestivum L.) for constitutive accumulation of an Aspergillus phytase. Mol Breed (2000) 6:195–206. doi:10.1023/A:1009690730620
81. Bhati KK, Alok A, Kumar A, Kaur J, Tiwari S, Pandey AK. Silencing of ABCC13 transporter in wheat reveals its involvement in grain development, phytic acid accumulation and lateral root formation. J Exp Bot (2016) 67(14):4379–89. doi:10.1093/jxb/erw224
82. Tamas C, Kisgyorgy BN, Rakszegi M, Wilkinson MD, Yang MS, Lang L, et al. Transgenic approach to improve wheat (Triticum aestivum L.) nutritional quality. Plant Cell Rep (2009) 28(7):1085–94. doi:10.1007/s00299-009-0716-0
83. Doshi KM, Eudes F, Laroche A, Gaudet D. Transient embryo specific expression of anthocyanin in wheat. In Vitro Cell Dev Biol Plant (2006) 42:432–8. doi:10.1079/IVP2006778
84. Sestili F, Janni M, Doherty A, Botticella E, D’Ovidio R, Masci S, et al. Increasing the amylose content of durum wheat through silencing of the SBEIIa genes. BMC Plant Biol (2010) 10:144. doi:10.1186/1471-2229-10-144
85. Aluru M, Xu Y, Guo R, Wang Z, Li S, White W, et al. Generation of transgenic maize with enhanced provitamin A content. J Exp Bot (2008) 59(13):3551–62. doi:10.1093/jxb/ern212
86. Decourcelle M, Perez-Fons L, Baulande S, Steiger S, Couvelard L, Hem S, et al. Combined transcript, proteome, and metabolite analysis of transgenic maize seeds engineered for enhanced carotenoid synthesis reveals pleotropic effects in core metabolism. J Exp Bot (2015) 66(11):3141–50. doi:10.1093/jxb/erv120
87. Cahoon EB, Hall SE, Ripp KG, Ganzke TS, Hitz WD, Coughlan SJ. Metabolic redesign of vitamin E biosynthesis in plants for tocotrienol production and increased antioxidant content. Nat Biotechnol (2003) 21:1082–7. doi:10.1038/nbt853
88. Levine M, Dhariwal KR, Welch RW, Wang Y, Park JB. Determination of optimal vitamin C requirements in humans. Am J Clin Nutr (1995) 62:1347S–56S. doi:10.1093/ajcn/62.6.1347S
89. Chen Z, Young TE, Ling J, Chang SC, Gallie DR. Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc Natl Acad Sci U S A (2003) 100:3525–30. doi:10.1073/pnas.0635176100
90. Naqvi S, Zhu C, Farre G, Ramessar K, Bassie L, Breitenbach J, et al. Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proc Natl Acad Sci U S A (2009) 106(19):7762–7. doi:10.1073/pnas.0901412106
91. Drakakaki G, Marcel S, Glahn RP, Lund EK, Pariagh S, Fischer R, et al. Endosperm-specific co-expression of recombinant soybean ferritin and Aspergillus phytase in maize results in significant increases in the levels of bioavailable iron. Plant Mol Biol (2005) 59(6):869–80. doi:10.1007/s11103-005-1537-3
92. Aluru MR, Rodermel SR, Reddy MB. Genetic modification of low phytic acid 1-1 maize to enhance iron content and bioavailability. J Agric Food Chem (2011) 59(24):12954–62. doi:10.1021/jf203485a
93. Chen R, Xue G, Chen P, Yao B, Yang W, Ma Q, et al. Transgenic maize plants expressing a fungal phytase gene. Transgenic Res (2008) 17(4):633–43. doi:10.1007/s11248-007-9138-3
94. Shi J, Wang H, Schellin K, Li B, Faller M, Stoop JM, et al. Embryo-specific silencing of a transporter reduces phytic acid content of maize and soybean seeds. Nat Biotechnol (2007) 25(8):930–7. doi:10.1038/nbt1322
95. Yu J, Peng P, Zhang X, Zhao Q, Zhu D, Sun X, et al. Seed-specific expression of the lysine-rich protein gene sb401 significantly increases both lysine and total protein content in maize seeds. Food Nutr Bull (2005) 26(4):427–31. doi:10.1177/15648265050264S311
96. Tang M, He X, Luo Y, Ma L, Tang X, Huang K. Nutritional assessment of transgenic lysine-rich maize compared with conventional quality protein maize. J Sci Food Agric (2013) 93:1049–54. doi:10.1002/jsfa.5845
97. Frizzi A, Huang S, Gilbertson LA, Armstrong TA, Luethy MH, Malvar TM. Modifying lysine biosynthesis and catabolism in corn with a single bifunctional expression/silencing transgene cassette. Plant Biotechnol J (2008) 6(1):13–21. doi:10.1111/j.1467-7652.2007.00290.x
98. Huang S, Frizzi A, Florida CA, Kruger DE, Luethy MH. High lysine and high tryptophan transgenic maize resulting from the reduction of both 19- and 22-kD alpha-zeins. Plant Mol Biol (2006) 61(3):525–35. doi:10.1007/s11103-006-0027-6
99. Lai JS, Messing J. Increasing maize seed methionine by mRNA stability. Plant J (2002) 30:395–402. doi:10.1046/j.1365-313X.2001.01285.x
100. Ramesh SA, Choimes S, Schachtman DP. Over-expression of an Arabidopsis zinc transporter in Hordeum vulgare increases short-term zinc uptake after zinc deprivation and seed zinc content. Plant Mol Biol (2004) 54(3):373–85. doi:10.1023/B:PLAN.0000036370.70912.34
101. Holme IB, Dionisio G, Brinch-Pedersen H, Wendt T, Madsen CK, Vincze E, et al. Cisgenic barley with improved phytase activity. Plant Biotechnol J (2012) 10(2):237–47. doi:10.1111/j.1467-7652.2011.00660.x
102. Ohnoutkova L, Zitka O, Mrizova K, Vaskova J, Galuszka P, Cernei N, et al. Electrophoretic and chromatographic evaluation of transgenic barley expressing a bacterial dihydrodipicolinate synthase. Electrophoresis (2012) 33(15):2365–73. doi:10.1002/elps.201200033
103. Dikeman CL, Fahey GC. Viscosity as related to dietary fiber: a review. Crit Rev Food Sci Nutr (2006) 46:649–63. doi:10.1080/10408390500511862
104. Burton RA, Collins HM, Kibble NA, Smith JA, Shirley NJ, Jobling SA, et al. Over-expression of specific HvCslF cellulose synthase-like genes in transgenic barley increases the levels of cell wall (1,3;1,4)-β-d-glucans and alters their fine structure. Plant Biotechnol J (2011) 9(2):117–35. doi:10.1111/j.1467-7652.2010.00532.x
105. Carciofi M, Blennow A, Jensen SL, Shaik SS, Henriksen A, Buleon A, et al. Concerted suppression of all starch branching enzyme genes in barley produces amylose-only starch granules. BMC Plant Biol (2012) 12(1):223. doi:10.1186/1471-2229-12-223
106. Mihalik D, Gubisova M, Klempova T, Certik M, Ondreickova K, Hudcovicova M, et al. Transgenic barley producing essential polyunsaturated fatty acids. Biol Plant (2014) 58(2):348–54. doi:10.1007/s10535-014-0406-9
107. Kamenarova K, Gecheff K, Stoyanova M, Muhovski Y, Anzai H, Atanassov A, et al. Production of recombinant human lactoferin in transgenic barley. Biotechnol Biotech Eq (2007) 21(1):18–27. doi:10.1080/13102818.2007.10817407
108. Lipkie TE, De Moura FF, Zhao Z-Y, Albertsen MC, Che P, Glassman K, et al. Bioaccessibility of carotenoids from transgenic provitamin A biofortified Sorghum. J Agric Food Chem (2013) 61(24):5764–71. doi:10.1021/jf305361s
109. Zhao ZY, Glassman K, Sewalt V, Wang N, Miller M, Chang S, et al. Nutritionally Improved Transgenic Sorghum. InPlant Biotechnology 2002 and Beyond. Netherlands: Springer (2003). p. 413–6.
110. Elkonin LA, ItalianskayaI JV, Domanina VN, Selivanov NY, Rakitin AL, Ravi NV. Transgenic Sorghum with improved digestibility of storage proteins obtained by Agrobacterium-mediated transformation. Russ J Plant Physiol (2016) 63:678–89. doi:10.1134/S1021443716050046
111. Grootboom AW, Mkhonza NL, Mbambo Z, O’Kennedy MM, Da Silva LS, Taylor J, et al. Co-suppression of synthesis of major α-kafirin sub-class together with γ-kafirin-1 and γ-kafirin-2 required for substantially improved protein digestibility in transgenic Sorghum. Plant Cell Rep (2014) 33(3):521–37. doi:10.1007/s00299-013-1556-5
112. Schmidt MA, Parrott WA, Hildebrand DF, Berg RH, Cooksey A, Pendarvis K, et al. Transgenic soya bean seeds accumulating β-carotene exhibit the collateral enhancements of oleate and protein content traits. Plant Biotechnol J (2015) 13(4):590–600. doi:10.1111/pbi.12286
113. Pierce EC, LaFayette PR, Ortega MA, Joyce BL, Kopsell DA, Parrott WA. Ketocarotenoid production in soybean seeds through metabolic engineering. PLoS One (2015) 10(9):e0138196. doi:10.1371/journal.pone.0138196
114. Kim MJ, Kim JK, Kim HJ, Pak JH, Lee JH, Kim DH, et al. Genetic modification of the soybean to enhance the β-carotene content through seed-specific expression. PLoS One (2012) 7(10):e48287. doi:10.1371/journal.pone.0048287
115. Van Eenennaam AL, Lincoln K, Durrett TP, Valentin HE, Shewmaker CK, Thorne GM, et al. Engineering vitamin E content: from Arabidopsis mutant to soy oil. Plant Cell (2003) 15:3007–19. doi:10.1105/tpc.015875
116. Kim WS, Chronis D, Juergens M, Schroeder AC, Hyun SW, Jez J, et al. Transgenic soybean plants overexpressing O-acetylserine sulfhydrylase accumulate enhanced levels of cysteine and Bowman-Birk protease inhibitor in seeds. Planta (2012) 235(1):13–23. doi:10.1007/s00425-011-1487-8
117. Dinkins RD, Reddy MSS, Meurer CA, Yan B, Trick H, Thibaud-Nissen F, et al. Increased sulfur amino acids in soybean plants overexpressing the maize 15 kDa zein protein. In Vitro Cell Dev Biol Plant (2001) 37:742–7. doi:10.1007/s11627-001-0123-x
118. Song S, Hou W, Godo I, Wu C, Yu Y, Matityahu I, et al. Soybean seeds expressing feedback-insensitive cystathionine γ-synthase exhibit a higher content of methionine. J Exp Bot (2013) 64(7):1917–26. doi:10.1093/jxb/ert053
119. Hanafy MS, Rahman SM, Nakamoto Y, Fujiwara T, Naito S, Wakasa K, et al. Differential response of methionine metabolism in two grain legumes, soybean and azuki bean, expressing a mutated form of Arabidopsis cystathionine γ-synthase. J Plant Physiol (2013) 170(3):338–45. doi:10.1016/j.jplph.2012.10.018
120. Flores T, Karpova O, Su X, Zeng P, Bilyeu K, Sleper DA, et al. Silencing of GmFAD3 gene by siRNA leads to low alpha-linolenic acids (18:3) of fad3-mutant phenotype in soybean [Glycine max (Merr.)]. Transgenic Res (2008) 17(5):839–50. doi:10.1007/s11248-008-9167-6
121. Sato S, Xing A, Ye X, Schweiger B, Kinney A, Graef G, et al. Production of γ-linolenic acid and stearidonic acid in seeds of marker-free transgenic soybean. Crop Sci (2004) 44(2):646–52. doi:10.2135/cropsci2004.0646
122. Eckert H, La Vallee B, Schweiger BJ, Kinney AJ, Cahoon EB, Clemente T. Co-expression of the borage Δ6 desaturase and the Arabidopsis delta 15 desaturase results in high accumulation of stearidonic acid in the seeds of transgenic soybean. Planta (2006) 224(5):1050–7. doi:10.1007/s00425-006-0291-3
123. Zhang L, Yang XD, Zhang YY, Yang J, Qi GX, Guo DQ, et al. Changes in oleic acid content of transgenic soybeans by antisense RNA mediated posttranscriptional gene silencing. Int J Genomics (2014) 2014:8. doi:10.1155/2014/921950
124. Yu O, Shi J, Hession AO, Maxwell CA, McGonigle B, Odell JT. Metabolic engineering to increase isoflavone biosynthesis in soybean seed. Phytochemistry (2003) 63:753–63. doi:10.1016/S0031-9422(03)00345-5
125. Aragao FJL, Barros LMG, De Sousa MV, Grossi de Sa MF, Almeida ERP, Gander ES, et al. Expression of a methionine-rich storage albumin from the Brazil nut (Bertholletia excelsa H.B.K., Lecythidaceae) in transgenic bean plants (Phaseolus vulgaris L., Fabaceae). Genet Mol Biol (1999) 22(3):445–9. doi:10.1590/S1415-47571999000300026
126. Molvig L, Tabe LM, Eggum BO, Moore AE, Craig S, Spencer D, et al. Enhanced methionine levels and increased nutritive value of seeds of transgenic lupins (Lupinus angustifolius L.) expressing a sunflower seed albumin gene. Proc Natl Acad Sci U S A (1997) 94(16):8393–8. doi:10.1073/pnas.94.16.8393
127. Ducreux LJM, Morris WL, Hedley PE, Shepherd T, Davies HV, Millam S, et al. Metabolic engineering of high carotenoid potato tubers containing enhanced levels of β-carotene and lutein. J Exp Bot (2004) 56:81–9. doi:10.1093/jxb/eri016
128. Diretto G, Tavazza R, Welsch R, Pizzichini D, Mourgues F, Papacchioli V, et al. Metabolic engineering of potato tuber carotenoids through tuber-specific silencing of lycopene epsilon cyclase. BMC Plant Biol (2006) 6:13. doi:10.1186/1471-2229-6-13
129. Van Eck J, Conlin B, Garvin DF, Mason H, Navarre DA, Brown CR. Enhancing beta-carotene content in potato by rnai-mediated silencing of the beta-carotene hydroxylase gene. Am J Potato Res (2007) 84:331–42. doi:10.1007/BF02986245
130. Song XY, Zhu WJ, Tang RM, Cai JH, Chen M, Yang Q. Over-expression of StLCYb increases β-carotene accumulation in potato tubers. Plant Biotechnol Rep (2016) 10(2):95–104. doi:10.1007/s11816-016-0390-y
131. Lopez AB, Van Eck J, Conlin BJ, Paolillo DJ, O’Neill J, Li L. Effect of the cauliflower or transgene on carotenoid accumulation and chromoplast formation in transgenic potato tubers. J Exp Bot (2008) 59(2):213–23. doi:10.1093/jxb/erm299
132. Romer S, Lubeck J, Kauder F, Steiger S, Adomat C, Sandmann G. Genetic engineering of a zeaxanthin-rich potato by antisense inactivation and co-suppression of carotenoid epoxidation. Metab Eng (2002) 4(4):263–72. doi:10.1006/mben.2002.0234
133. Upadhyaya CP, Young KE, Akula N, Soon Kim H, Heung JJ, Oh O, et al. Over-expression of strawberry d-galacturonic acid reductase in potato leads to accumulation of vitamin C with enhanced abiotic stress tolerance. Plant Sci (2009) 177(6):659–67. doi:10.1016/j.plantsci.2009.08.004
134. Dancs G, Kondrak M, Banfalvi Z. The effects of enhanced methionine synthesis on amino acid and anthocyanin content of potato tubers. BMC Plant Biol (2008) 8:65. doi:10.1186/1471-2229-8-65
135. Huang T, Joshi V, Jander G. The catabolic enzyme methionine gamma-lyase limits methionine accumulation in potato tubers. Plant Biotechnol J (2014) 12(7):883–93. doi:10.1111/pbi.12191
136. Zeh M, Casazza AP, Kreft O, Roessner U, Bieberich K, Willmitzer L, et al. Antisense inhibition of threonine synthase leads to high methionine content in transgenic potato plants. Plant Physiol (2001) 127:792–802. doi:10.1104/pp.010438
137. Goo YM, Kim TW, Lee MK, Lee SW. Accumulation of PrLeg, a Perilla legumin protein in potato tuber results in enhanced level of sulphur-containing amino acids. C R Biol (2013) 336(9):433–9. doi:10.1016/j.crvi.2013.09.002
138. Di R, Kim J, Martin MN, Leustek T, Jhoo J, Ho CT, et al. Enhancement of the primary flavor compound methional in potato by increasing the level of soluble methionine. J Agric Food Chem (2003) 51(19):5695–702. doi:10.1021/jf030148c
139. Chakraborty S, Chakraborty N, Agrawal L, Ghosh S, Narula K, Shekhar S, et al. Next-generation protein-rich potato expressing the seed protein gene AmA1 is a result of proteome rebalancing in transgenic tuber. Proc Natl Acad Sci U S A (2010) 107(41):17533–8. doi:10.1073/pnas.1006265107
140. Oakes JV, Shewmaker CK, Stalker DM. Production of cyclodextrins, a novel carbohydrate, in the tubers of transgenic potato plants. Biotechnology (N Y) (1991) 9(10):982–6. doi:10.1038/nbt1091-982
141. Lukaszewicz M, Matysiak-Kata I, Skala J, Fecka I, Cisowski W, Szopa J. Antioxidant capacity manipulation in transgenic potato tuber by changes in phenolic compounds content. J Agric Food Chem (2004) 52:1526–33. doi:10.1021/jf034482k
142. Hellwege EM, Gritscher D, Willmitzer L, Heyer AG. Transgenic potato tubers accumulate high levels of 1-kestose and nystose: functional identification of a sucrose 1-fructosyltransferase of artichoke (Cynara scolymus) blossom discs. Plant J (1997) 12:1057–65. doi:10.1046/j.1365-313X.1997.12051057.x
143. Hellwege EM, Czapla S, Jahnke A, Willmitzer L, Heyer AG. Transgenic potato (Solanum tuberosum) tubers synthesize the full spectrum of inulin molecules naturally occurring in globe artichoke (Cynara scolymus) roots. Proc Natl Acad Sci U S A (2000) 97:8699–704. doi:10.1073/pnas.150043797
144. Kim SH, Kim YH, Ahn YO, Ahn MJ, Jeong JC, Lee HS, et al. Downregulation of the lycopene ε-cyclase gene increases carotenoid synthesis via the β-branch-specific pathway and enhances salt-stress tolerance in sweetpotato transgenic calli. Physiol Plant (2013) 147(4):432–42. doi:10.1111/j.1399-3054.2012.01688.x
145. Park SC, Kim YH, Kim SH, Jeong YJ, Kim CY, Lee JS, et al. Overexpression of the IbMYB1 gene in an orange-fleshed sweet potato cultivar produces a dual-pigmented transgenic sweet potato with improved antioxidant activity. Physiol Plant (2015) 153(4):525–37. doi:10.1111/ppl.12281
146. Telengech PK, Maling’a JN, Nyende AB, Gichuki ST, Wanjala BW. Gene expression of beta carotene genes in transgenic biofortified cassava. 3 Biotech (2015) 5(4):465–72. doi:10.1007/s13205-014-0243-8
147. Welsch R, Arango J, Bar C, Salazar B, Al-Babili S, Beltran J, et al. Provitamin A accumulation in cassava (Manihot esculenta) roots driven by a single nucleotide polymorphism in a phytoene synthase gene. Plant Cell (2010) 22:3348–56. doi:10.1105/tpc.110.077560
148. Park S, Kim C-K, Pike LM, Smith RH, Hirschi KD. Increased calcium in carrots by expression of an Arabidopsis H+/Ca2+ transporter. Mol Breed (2004) 14:275–82. doi:10.1023/B:MOLB.0000047773.20175.ae
149. Morris J, Hawthorne KM, Hotze T, Abrams SA, Hirschi KD. Nutritional impact of elevated calcium transporter activity in carrots. Proc Natl Acad Sci U S A (2008) 105:1431–5. doi:10.1073/pnas.0709005105
150. Goto F, Yoshihara T, Saiki H. Iron accumulation and enhanced growth in transgenic lettuce plants expressing the iron-binding protein ferritin. Theor Appl Genet (2000) 100:658–64. doi:10.1007/s001220051336
151. Lu S, Van Eck J, Zhou X, Lopez AB, O’Halloran DM, Cosman KM, et al. The cauliflower or gene encodes a DnaJ cysteine-rich domain-containing protein that mediates high levels of β-carotene accumulation. Plant Cell (2006) 18:3594–605. doi:10.1105/tpc.106.046417
152. Lorenc-Kukula K, Wrobel-Kwiatkowska M, Starzycki M, Szopa J. Engineering flax with increased flavonoid content and thus Fusarium resistance. Physiol Mol Plant Pathol (2007) 70:38–48. doi:10.1016/j.pmpp.2007.05.005
153. Galili G, Galili S, Lewinsohn E, Tadmor Y. Genetic, molecular and genomic approaches to improve the value of plant foods and feeds. Crit Rev Plant Sci (2002) 21:167–204. doi:10.1080/0735-260291044232
154. Abbadi A, Domergue F, Bauer J, Napier JA, Welti R, Zähringer U, et al. Biosynthesis of very-long-chain polyunsaturated fatty acids in transgenic oilseeds: constraints on their accumulation. Plant Cell (2004) 16:2734–48. doi:10.1105/tpc.104.026070
155. Fujisawa M, Watanabe M, Choi SK, Teramoto M, Ohyama K, Misawa N. Enrichment of carotenoids in flaxseed (Linumu sitatissimum) by metabolic engineering with introduction of bacterial phytoene synthase gene crtB. J Biosci Bioeng (2008) 105(6):636–41. doi:10.1263/jbb.105.636
156. Ravanello MP, Ke D, Alvarez J, Huang B, Shewmaker CK. Coordinate expression of multiple bacterial carotenoid genes in canola leading to altered carotenoid production. Metab Eng (2003) 5(4):255–63. doi:10.1016/j.ymben.2003.08.001
157. Fujisawa M, Takita E, Harada H, Sakurai N, Suzuki H, Ohyama K, et al. Pathway engineering of Brassica napus seeds using multiple key enzyme genes involved in ketocarotenoid formation. J Exp Bot (2009) 60(4):1319–32. doi:10.1093/jxb/erp006
158. Yu B, Lydiate DJ, Young LW, Schafer UA, Hannoufa A. Enhancing the carotenoid content of Brassica napus seeds by downregulating Lycopene epsilon cyclase. Transgenic Res (2008) 17(4):573–85. doi:10.1007/s11248-007-9131-x
159. Wei S, Li X, Gruber MY, Li R, Zhou R, Zebarjadi A, et al. RNAi-mediated suppression of DET1 alters the levels of carotenoids and sinapate esters in seeds of Brassica napus. J Agric Food Chem (2009) 57(12):5326–33. doi:10.1021/jf803983w
160. Falco SC, Guida T, Locke M, Mauvais J, Sanders C, Ward RT, et al. Transgenic canola and soybean seeds with increased lysine. Nat Biotechnol (1995) 13:577–82. doi:10.1038/nbt0695-577
161. Dehesh K, Jones A, Knutzon DS, Voelker TA. Production of high levels of 8:0 and 10:0 fatty acids in transgenic canola by overexpression of Ch FatB2, a thioesterase cDNA from Cuphea hookeriana. Plant J (1996) 9:167–72. doi:10.1046/j.1365-313X.1996.09020167.x
162. Liu JW, DeMichele S, Bergana M, Bobik E, Hastilow C, Chuang LT, et al. Characterization of oil exhibiting high γ-linolenic acid from a genetically transformed canola strain. J Am Oil Chem Soc (2001) 78(5):489–93. doi:10.1007/s11746-001-0291-2
164. Hong H, Datla N, Reed DW, Covello PS, MacKenzie SL, Qiu X. High-level production of γ-linolenic acid in Brassica juncea using a Δ6 desaturase from pythium irregular. Plant Physiol (2002) 129(1):354–62. doi:10.1104/pp.001495
165. Enfissi E, Fraser PD, Lois LM, Boronat A, Schuch W, Bramley PM. Metabolic engineering of the mevalonate and nonmevalonate isopentenyl diphosphate-forming pathways for the production of health-promoting isoprenoids in tomato. Plant Biotechnol J (2005) 3:17–27. doi:10.1111/j.1467-7652.2004.00091.x
166. Fraser PD, Enfissi EM, Halket JM, Truesdale MR, Yu D, Gerrish C, et al. Manipulation of phytoene levels in tomato fruit: effects on isoprenoids, plastids, and intermediary metabolism. Plant Cell (2007) 19(10):3194–211. doi:10.1105/tpc.106.049817
167. Rosati C, Aquilani R, Dharmapuri S, Pallara P, Marusic C, Tavazza R, et al. Metabolic engineering of β-carotene and lycopene content in tomato fruit. Plant J (2000) 24:413–9. doi:10.1046/j.1365-313x.2000.00880.x
168. Apel W, Bock R. Enhancement of carotenoid biosynthesis in transplastomic tomatoes by induced lycopene-to-provitamin A conversion. Plant Physiol (2009) 151(1):59–66. doi:10.1104/pp.109.140533
169. Wurbs D, Rup S, Bock R. Contained metabolic engineering in tomatoes by expression of carotenoid biosynthesis genes from the plastid genome. Plant J (2007) 49(2):276–88. doi:10.1111/j.1365-313X.2006.02960.x
170. Huang JC, Zhong YJ, Liu J, Sandmann G, Chen F. Metabolic engineering of tomato for high-yield production of astaxanthin. Metab Eng (2013) 17:59–67. doi:10.1016/j.ymben.2013.02.005
171. Dharmapuri S, Rosati C, Pallara P, Aquilani R, Bouvier F, Camara B, et al. Metabolic engineering of xanthophyll content in tomato fruits. FEBS Lett (2002) 519(1–3):30–4. doi:10.1016/S0014-5793(02)02699-6
172. Davuluri GR, Van Tuinen A, Fraser PD, Manfredonia A, Newman R, Burgess D, et al. Fruit-specific RNAi-mediated suppression of DET1 enhances carotenoid and flavonoid content in tomatoes. Nat Biotechnol (2005) 23:890–5. doi:10.1038/nbt1108
173. Zhang C, Liu J, Zhang Y, Cai X, Gong P, Zhang J, et al. Overexpression of SlGMEs leads to ascorbate accumulation with enhanced oxidative stress, cold, and salt tolerance in tomato. Plant Cell Rep (2011) 30:389–98. doi:10.1007/s00299-010-0939-0
174. Haroldsen VM, Chi-Ham CL, Kulkarni S, Lorence A, Bennet AB. Constitutively expressed DHAR and MDHAR influence fruit, but not foliar ascorbate levels in tomato. Plant Physiol Biochem (2011) 49:1244–9. doi:10.1016/j.plaphy.2011.08.003
175. Cronje C, George GM, Fernie AR, Bekker J, Kossmann J, Bauer R. Manipulation of L-ascorbic acid biosynthesis pathways in Solanum lycopersicum elevated GDP-mannose pyrophosphorylase activity enhances L-ascorbate levels in red fruit. Planta (2012) 235:553–64. doi:10.1007/s00425-011-1525-6
176. De la Graza RD, Quinlivan LP, Klaus MJS, Basset GJC, Gregory JF III, Hanson AD. Folate biofortification in tomatoes by engineering the pteridine branch of folate synthesis. Proc Natl Acad Sci U S A (2004) 101(38):13720–5. doi:10.1073/pnas.0404208101
177. De la Graza RD, Gregory JF III, Hanson AD. Folate biofortification of tomato fruit. Proc Natl Acad Sci U S A (2007) 104(10):4218–22. doi:10.1073/pnas.0700409104
178. Muir SR, Collins GJ, Robinson S, Hughes S, Bovy A, Ric De Vo CH, et al. Overexpression of petunia chalcone isomerase in tomato results in fruit containing increased levels of flavonols. Nature (2001) 19:470–4. doi:10.1038/88150
179. Zuluaga DL, Gonzali S, Loreti E, Pucciariello C, Degl’Innocenti E, Guidi L, et al. Arabidopsis thaliana MYB75/PAP1 transcription factor induces anthocyanin production in transgenic tomato plants. Funct Plant Biol (2008) 35(7):606–18. doi:10.1071/FP08021
180. Niggeweg R, Michael AJ, Martin C. Engineering plants with increased levels of the antioxidant chlorogenic acid. Nat Biotechnol (2004) 22:746–54. doi:10.1038/nbt966
181. Giovinazzo G, D’Amico L, Paradiso A, Bollini R, Sparvoli F, DeGara L. Antioxidant metabolite profiles in tomato fruit constitutively expressing the grapevine stilbene synthase gene. Plant Biotechnol J (2005) 3(1):57–69. doi:10.1111/j.1467-7652.2004.00099.x
182. Luo J, Butelli E, Hill L, Parr A, Niggeweg R, Bailey P, et al. AtMYB12 regulates caffeoyl quinic acid and flavonol synthesis in tomato: expression in fruit results in very high levels of both types of polyphenol. Plant J (2008) 56(2):316–26. doi:10.1111/j.1365-313X.2008.03597.x
183. Shih CH, Chen Y, Wang M, Chu IK, Lo C. Accumulation of isoflavone genistin in transgenic tomato plants overexpressing a soybean isoflavone synthase gene. J Agric Food Chem (2008) 56(14):5655–61. doi:10.1021/jf800423u
184. Szankowski I, Briviba K, Fleschhut J, Schönherr J, Jacobsen HJ, Kiesecker H. Transformation of apple (Malus domestica Borkh.) with the stilbene synthase gene from grapevine (Vitis vinifera L.) and a PGIP gene from kiwi (Actinidia deliciosa). Plant Cell Rep (2003) 22:141–9. doi:10.1007/s00299-003-0668-8
185. Waltz E. Vitamin A super banana in human trials. Nat Biotechnol (2014) 32:857. doi:10.1038/nbt0914-857
186. Deavours BE, Dixon RA. Metabolic engineering of isoflavonoid biosynthesis in alfalfa. Plant Physiol (2005) 138(4):2245–59. doi:10.1104/pp.105.062539
187. Avaram T, Badani H, Galili S, Aamir R. Enhanced levels of methionine and cysteine in transgenic alfalfa (Medicago sativa L.) plants over-expressing the Arabidopsis cystathionine γ-synthase gene. Plant Biotechnol J (2005) 3:71–9. doi:10.1111/j.1467-7652.2004.00102.x
188. Reddy MS, Chen F, Shadle G, Jackson L, Aljoe H, Dixon RA. Targeted down-regulation of cytochrome P450 enzymes for forage quality improvement in alfalfa (Medicago sativa L.). Proc Natl Acad Sci U S A (2005) 102:16573–8. doi:10.1073/pnas.0505749102
189. Austin-Phillips S, Koegel RG, Straub RJ, Cook M. Animal Feed Compositions Containing Phytase Derived from Transgenic Alfalfa and Methods of Use Thereof. United States patent US 6248938 (2001).
190. Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, Garcia FA, et al. Folic acid supplementation for the prevention of neural tube defects US preventive services task force recommendation statement. JAMA (2017) 317(2):183–9. doi:10.1001/jama.2016.19438
191. Lee S, Jeon US, Lee SJ, Kim YK, Persson DP, Husted S, et al. Iron fortification of rice seeds through activation of the nicotianamine synthase gene. Proc Natl Acad Sci U S A (2009) 106:22014–9. doi:10.1073/pnas.0910950106
192. Crawford M, Galli C, Visioli F, Renaud S, Simopoulos AP, Spector AA. Role of plant-derived omega-3 fatty acids in human nutrition. Ann Nutr Metab (2000) 44:263–5. doi:10.1159/000046694
193. Lee JH, Kim IG, Kim HS, Shin KS, Suh SC, Kweon SJ, et al. Development of transgenic rice lines expressing the human lactoferrin gene. J Plant Biotechnol (2010) 37(4):556–61. doi:10.5010/JPB.2010.37.4.556
194. Zhu C, Naqvi S, Breitenbach J, Sandmann G, Christou P, Capell T. Combinatorial genetic transformation generates a library of metabolic phenotypes for the carotenoid pathway in maize. Proc Natl Acad Sci U S A (2008) 105(47):18232–7. doi:10.1073/pnas.0809737105
195. Dutton HJ, Lancaster CJ, Evans CD, Cowan JC. The flavor problem of soybean oil. VIII. Linolenic acid. J Am Oil Chem Soc (1951) 28:115–8. doi:10.1007/BF02612206
196. Watanabe S, Uesugi S, Kikuchi Y. Isoflavones for prevention of cancer, cardiovascular diseases, gynecological problems and possible immune potentiation. Biomed Pharmacother (2002) 56:302–12. doi:10.1016/S0753-3322(02)00182-8
197. Teow CC, Truong VD, McFeeters RF, Thompson RL, Pecota KV, Yencho GC. Antioxidant activities, phenolic and b-carotene contents of sweet potato genotypes with varying flesh colours. Food Chem (2007) 103:829–38. doi:10.1016/j.foodchem.2006.09.033
198. Miller GD, Jarvis JK, McBean LD. The importance of meeting calcium needs with food. J Am Coll Nutr (2001) 20:168–85. doi:10.1080/07315724.2001.10719029
199. Newton IS. Long-chain polyunsaturated fatty acids—the new frontier in nutrition. Lipid Technol (1998) 10:77–81.
200. Harborne JB. Recent advances in the ecological chemistry of plant terpenoids. Ecol Chem Biochem Plant Terpenoids (1991) 6:399–426.
201. Maligeppagol M, Chandra GS, Navale PM, Deepa H, Rajeev PR, Asokan R, et al. Anthocyanin enrichment of tomato (Solanum lycopersicum L.) fruit by metabolic engineering. Curr Sci (2013) 105(1):72–80.
202. Cakmak I, Kutman UB. Agronomic biofortification of cereals with zinc: a review. Eur J Soil Sci (2017) 69:172–80. doi:10.1111/ejss.12437
203. Daniels LA. Selenium metabolism and bioavailability. Biol Trace Elem Res (1996) 54(3):185–99. doi:10.1007/BF02784430
204. Erisman JW, Sutton MA, Galloway JN, Klimont Z, Winiwarter W. How a century of ammonia synthesis changed the world. Nat Geo Sci (2008) 1:636–9. doi:10.1038/ngeo325
205. Graham RD, Welch RM, Saunders DA, Ortiz-Monasterio I, Bouis HE, Bonierbale M, et al. Nutritious subsistence food systems. Adv Agron (2007) 92:1–74. doi:10.2134/agronj2005.0222
206. Cakmak I. Enrichment of cereal grains with zinc: agronomic or genetic biofortification. Plant Soil (2008) 302:1–17. doi:10.1007/s11104-008-9584-6
207. Aro A, Alfthan G, Varo P. Effects of supplementation of fertilizers on human selenium status in Finland. Analyst (1995) 120:841–3. doi:10.1039/an9952000841
208. Cakmak I, Kalaycı M, Ekiz H, Braun HJ, Kılınç Y, Yılmaz A. Zinc deficiency as a practical problem in plant and human nutrition in Turkey: a NATO-science for stability project. Field Crops Res (1999) 60:175–88. doi:10.1016/S0378-4290(98)00139-7
209. Jiang XM, Cao XY, Jiang JY, Ma T, James DW, Rakeman MA, et al. Dynamics of environmental supplementation of iodine: four years’ experience in iodination of irrigation water in Hotien, Xinjiang, China. Arch Environ Health (1997) 52(6):399–408. doi:10.1080/00039899709602218
210. Rengel Z, Batten GD, Crowley DE. Agronomic approaches for improving the micronutrient density in edible portions of field crops. Field Crops Res (1999) 60:27–40. doi:10.1016/S0378-4290(98)00131-2
212. Hardarson G, Broughton WJ. Maximising the use of biological nitrogen fixation in agriculture. Ann Bot (2004) 93(4):477. doi:10.1093/aob/mch065
213. Cavagnaro TR. The role of arbuscular mycorrhizas in improving plant zinc nutrition under low soil zinc concentrations: a review. Plant Soil (2008) 304:315–25. doi:10.1007/s11104-008-9559-7
214. He W, Shohag MJ, Wei Y, Feng Y, Yang X. Iron concentration, bioavailability, and nutritional quality of polished rice affected by different forms of foliar iron fertilizer. Food Chem (2013) 141(4):4122–6. doi:10.1016/j.foodchem.2013.07.005
215. Yuan L, Wu L, Yang C, Quin LV. Effects of iron and zinc foliar applications on rice plants and their grain accumulation and grain nutritional quality. J Sci Food Agric (2013) 93(2):254–61. doi:10.1002/jsfa.5749
216. Fang Y, Wang L, Xin Z, Zhao L, An X, Hu Q. Effect of foliar application of zinc, selenium, and iron fertilizers on nutrients concentration and yield of rice grain in China. J Agric Food Chem (2008) 6(56):2079–84. doi:10.1021/jf800150z
217. Wei Y, Shohag MJ, Yang X, Yibin Z. Effects of foliar iron application on iron concentration in polished rice grain and its bioavailability. J Agric Food Chem (2012) 60(45):11433–9. doi:10.1021/jf3036462
218. Wei Y, Shohag MJ, Yang X. Biofortification and bioavailability of rice grain zinc as affected by different forms of foliar zinc fertilization. PLoS One (2012) 7(9):e45428. doi:10.1371/journal.pone.0045428
219. Boonchuay P, Cakmak I, Rerkasem B, Prom-U-Thai C. Effect of different foliar zinc application at different growth stages on seed zinc concentration and its impact on seedling vigor in rice. Soil Sci Plant Nutr (2013) 59(2):180–8. doi:10.1080/00380768.2013.763382
220. Jiang W, Struik PC, Keulen HV, Zhao M, Jin LN, Stomph TJ. Does increased zinc uptake enhance grain zinc mass concentration in rice? Ann Appl Biol (2008) 153(1):135–47. doi:10.1111/j.1744-7348.2008.00243.x
221. Mabesa RL, Impa SM, Grewal D, Beebout SEJ. Contrasting grain-Zn response of biofortification rice (Oryza sativa L.) breeding lines to foliar Zn application. Field Crops Res (2013) 149:223–33. doi:10.1016/j.fcr.2013.05.012
222. Shivay YS, Kumar D, Prasad R, Ahlawat IPS. Relative yield and zinc uptake by rice from zinc sulphate and zinc oxide coatings onto urea. Nutr Cycl Agroecosys (2008) 80(2):181–8. doi:10.1007/s10705-007-9131-5
223. Ram H, Rashid A, Zhang W, Duarte AP, Phattarakul N, Simunji S, et al. Biofortification of wheat, rice and common bean by applying foliar zinc fertilizer along with pesticides in seven countries. Plant Soil (2016) 1(403):389–401. doi:10.1007/s11104-016-2815-3
224. Guo JX, Feng XM, Hu XY, Tian GL. Effects of soil zinc availability, nitrogen fertilizer rate and zinc fertilizer application method on zinc biofortification of rice. J Agric Sci (2016) 154(4):584–97. doi:10.1017/S0021859615000441
225. Chen L, Yang F, Xu J, Hu Y, Hu Q, Zhang Y, et al. Determination of selenium concentration of rice in china and effect of fertilization of selenite and selenate on selenium content of rice. J Agric Food Chem (2002) 50(18):5128–30. doi:10.1021/jf0201374
226. Ros GH, VanRotterdm AMD, Bussink DW, Bindraban PS. Selenium fertilization strategies for bio-fortification of food: an agro-ecosystem approach. Plant Soil (2016) 404:99–112. doi:10.1007/s11104-016-2830-4
227. Premarathna L, McLaughlin MJ, Kirby JK, Hettiarachchi GM, Stacey S, Chittleborough DJ. Selenate-enriched urea granules are a highly effective fertilizer for selenium biofortification of paddy rice grain. J Agric Food Chem (2012) 60(23):6037–44. doi:10.1021/jf3005788
228. Xu J, Hu Q. Effect of foliar application of selenium on the antioxidant activity of aqueous and ethanolic extracts of selenium-enriched rice. J Agric Food Chem (2004) 52(6):1759–63. doi:10.1021/jf0349836
229. Giacosa A, Faliva MA, Perna S, Minoia C, Ronchi A, Rondanelli M. Selenium fortification of an Italian rice cultivar via foliar fertilization with sodium selenate and its effects on human serum selenium levels and on erythrocyte glutathione peroxidase activity. Nutrients (2014) 6(3):1251–61. doi:10.3390/nu6031251
230. Liu K, Gu Z. Selenium accumulation in different brown rice cultivars and its distribution in fractions. J Agric Food Chem (2009) 57(2):695–700. doi:10.1021/jf802948k
231. Aciksoz SB, Yazici A, Ozturk L, Cakmak I. Biofortification of wheat with iron through soil and foliar application of nitrogen and iron fertilizers. Plant Soil (2011) 349(1):215–25. doi:10.1007/s11104-011-0863-2
232. Cakmak I. Biofortification of cereals with zinc and iron through fertilization strategy. In 19th World Congress of Soil Science, Soil Solutions for a Changing World (2010) Vol. 5. p. 1–6.
233. Yang XW, Tian XH, Lu XC, Cao YX, Chen ZH. Impacts of phosphorus and zinc levels on phosphorus and zinc nutrition and phytic acid concentration in wheat (Triticum aestivum L.). J Sci Food Agric (2011) 91(13):2322–8. doi:10.1002/jsfa.4459
234. Nooria M, Adibiana M, Sobhkhizia A, Eyidozehib K. Effect of phosphorus fertilizer and mycorrhiza on protein percent, dry weight, weight of 1000 grain in wheat. Int J Plant Anim Environ Sci (2014) 4(2):561–4.
235. Ramzani PMA, Khalid M, Naveed M, Ahmad R, Shahid M. Iron biofortification of wheat grains through integrated use of organic and chemical fertilizers in pH affected calcareous soil. Plant Physiol Biochem (2016) 104:284–93. doi:10.1016/j.plaphy.2016.04.053
236. Ramesh A, Sharma SK, Sharma MP, Yadav N, Joshi OP. Inoculation of zinc solubilizing Bacillus aryabhattai strains for improved growth, mobilization and biofortification of zinc in soybean and wheat cultivated in vertisols of central India. Appl Soil Ecol (2014) 73:87–96. doi:10.1016/j.apsoil.2013.08.009
237. Alvarez JM, Rico MI. Effects of zinc complexes on the distribution of zinc in calcareous soil and zinc uptake by maize. J Agric Food Chem (2003) 51(19):5760–7. doi:10.1021/jf030092m
238. Lopez-Valdivia LM, Fernandez MD, Obrador A, Alvarez JM. Zinc transformations in acidic soil and zinc efficiency on maize by adding six organic zinc complexes. J Agric Food Chem (2002) 50(6):1455–60.
239. Fahad S, Hussain S, Saud S, Hassan S, Shan D, Chen Y, et al. Grain cadmium and zinc concentrations in maize influenced by genotypic variations and zinc fertilization. Clean Soil Air Water (2015) 43(10):1433–40. doi:10.1002/clen.201400376
240. Wang J, Mao H, Zhao H, Huang D, Wang Z. Different increases in maize and wheat grain zinc concentrations caused by soil and foliar applications of zinc in Loess plateau, China. Field Crops Res (2012) 135:89–96. doi:10.1016/j.fcr.2012.07.010
241. Zhang YQ, Pang LL, Yan P, Liu DY, Zhang W, Yost R, et al. Zinc fertilizer placement affects zinc content in maize plant. Plant Soil (2013) 372:81–92. doi:10.1007/s11104-013-1904-9
242. Prasanna R, Bidyarani N, Babu S, Hossain F, Shivay YS, Nain L. Cyanobacterial inoculation elicits plant defence response and enhanced Zn mobilization in maize hybrids. Cogent Food Agric (2015) 1(1):998507. doi:10.1080/23311932.2014.998507
243. Maleki FS, Chaichi MR, Mazaheri D, Tavakkol AR, Savaghebi G. Barley grain mineral analysis as affected by different fertilizing systems and by drought stress. J Agric Sci Tec (2011) 13:315–26.
244. Dhawi F, Datta R, Ramakrishna W. Mycorrhiza and PGPB modulate maize biomass, nutrient uptake and metabolic pathways in maize grown in mining-impacted soil. Plant Physiol Biochem (2015) 97:390–9. doi:10.1016/j.plaphy.2015.10.028
245. Dhawi F, Datta R, Ramakrishna W. Mycorrhiza and heavy metal resistant bacteria enhance growth, nutrient uptake and alter metabolic profile of Sorghum grown in marginal soil. Chemosphere (2016) 157:33–41. doi:10.1016/j.chemosphere.2016.04.112
246. Patidar M, Mali AL. Effect of farmyard manure, fertility levels and bio-fertilizers on growth, yield and quality of Sorghum (Sorghum bicolor). In J Agron (2004) 2(49):117–20.
247. Yang F, Chen L, Hu Q, Pan G. Effect of the application of selenium on selenium content of soybean and its products. Biol Trace Elem Res (2003) 93(1–3):249–56. doi:10.1385/BTER:93:1-3:249
248. Sathya A, Vijayabharati R, Srinivas V, Gopalakrishnan S. Plant growth-promoting action-bacteria on chickpea seed mineral density: an upcoming complementary tool for sustainable biofortification strategy. 3 Biotech (2013) 6(2):138. doi:10.1007/s13205-016-0458-y
249. Pellegrino E, Bedini S. Enhancing ecosystem services in sustainable agriculture: biofertilization and biofortification of chickpea (Cicer arietinum L.) by arbuscular mycorrhizal fungi. Soil Biol Biochem (2014) 68:429–39. doi:10.1016/j.soilbio.2013.09.030
250. Shivay YS, Prasad R, Pal M. Effects of source and method of zinc application on yield, zinc biofortification of grain, and Zn uptake and use efficiency in chickpea (Cicer arietinum L.). Commun Soil Sci Plant Anal (2015) 46(17):2191–200. doi:10.1080/00103624.2015.1069320
251. Poblaciones MJ, Rodrigo S, Santamaria O, Chen Y, McGrath SP. Selenium accumulation and speciation in biofortified chickpea (Cicer arietinum L.) under Mediterranean conditions. J Sci Food Agric (2014) 94(6):1101–6. doi:10.1002/jsfa.6372
252. Poblaciones MJ, Rengel Z. Soil and foliar zinc biofortification in field pea (Pisum sativum L.). Grain accumulation and bioavailability in raw and cooked grains. Food Chem (2016) 212:427–33. doi:10.1016/j.foodchem.2016.05.189
253. Ibrahim EA, Ramadan WA. Effect of zinc foliar spray alone and combined with humic acid or/and chitosan on growth, nutrient elements content and yield of dry bean (Phaseolus vulgaris L.) plants sown at different dates. Sci Hortic (2015) 184:101–15. doi:10.1016/j.scienta.2014.11.010
254. Westermann DT, Teran H, Munoz-Perea CG, Singh SP. Plant and seed nutrient uptake in common bean in seven organic and conventional production systems. Can J Plant Sci (2011) 91:1089–99. doi:10.4141/cjps10114
255. Yasin M, El Mehdawi AF, Jahn CE, Anwar A, Turner MF, Faisal M, et al. Seleniferous soils as a source for production of selenium-enriched foods and potential of bacteria to enhance plant selenium uptake. Plant Soil (2015) 386:385–94. doi:10.1007/s11104-014-2270-y
256. Poggi V, Arcioni A, Filippini P, Pifferi PG. Foliar application of selenite and selenate to potato (Solanum tuberosum): effect of a ligand agent on selenium content of tubers. J Agric Food Chem (2000) 48(10):4749–51. doi:10.1021/jf000368f
257. Cuderman P, Kreft I, Germ M, Kovacevic M, Stibilj V. Selenium species in selenium-enriched and drought-exposed potatoes. J Agric Food Chem (2008) 56(19):9114–20. doi:10.1021/jf8014969
258. Laurie SM, Faber M, Van Jaarsveld PJ, Laurie RN, Du Plooy CP, Modisane PC. β-Carotene yield and productivity of orange-fleshed sweet potato (Ipomoea batatas L. Lam.) as influenced by irrigation and fertilizer application treatments. Sci Hortic (2012) 142:180–4. doi:10.1016/j.scienta.2012.05.017
259. Smolen S, Skoczylas L, Ledwozyw-Smolen L, Rakoczy R, Kopec A, Piatkowska E, et al. Biofortification of carrot (Daucus carota L.) with iodine and selenium in a field experiment. Front Plant Sci (2016) 7:730. doi:10.3389/fpls.2016.00730
260. Smolen S, Kowalska L, Sady W. Assessment of biofortification with iodine and selenium of lettuce cultivated in the NFT hydroponic system. Sci Hortic (2014) 166:9–16. doi:10.1016/j.scienta.2013.11.011
261. Carvalho KM, Gallardo-Williams MT, Benson RF, Martin DF. Effects of selenium supplementation on four agricultural crops. J Agric Food Chem (2003) 51:704–9. doi:10.1021/jf0258555
262. Landini M, Gonzali S, Perata P. Iodine biofortification in tomato. J Plant Nutr Soil Sci (2011) 174(3):480–6. doi:10.1002/jpln.201000395
263. Nosheen A, Bano A, Ullah F. Nutritive value of canola (Brassica napus L.) as affected by plant growth promoting rhizobacteria. Eur J Lipid Sci Tech (2011) 113(11):1342–6. doi:10.1002/ejlt.201000549
264. White PJ, Thompson JA, Wright G, Rasmussen SK. Biofortifying Scottish potatoes with zinc. Plant Sci (2017) 411(1):151–65. doi:10.1007/s11104-016-2903-4
265. Fardart A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev (2010) 23(1):65–134. doi:10.1017/S0954422410000041
266. Tighe P, Duthie G, Vaughan N, Brittenden J, Simpson WG, Duthie S, et al. Effect of increased consumption of whole-grain foods on blood pressure and other cardiovascular risk markers in healthy middle-aged persons: a randomized controlled trial. Am J Clin Nutr (2010) 92(4):733–40. doi:10.3945/ajcn.2010.29417
267. Lafiandra D, Riccardi G, Shewry PR. Improving cereal grain carbohydrates for diet and health. J Cereal Sci (2014) 59:312–26. doi:10.1016/j.jcs.2014.01.001
268. Bouis HE, Welch RM. Biofortification—a sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crop Sci (2010) 50:S20–32. doi:10.2135/cropsci2009.09.0531
269. Gregorio GB, Senadhira D, Htut H, Graham RD. Breeding for trace mineral density in rice. Food Nutr Bull (2000) 21:382–6. doi:10.1177/156482650002100407
270. Monasterio I, Graham RD. Breeding for trace minerals in wheat. Food Nutr Bull (2000) 21(4):392–6. doi:10.1177/156482650002100409
271. Welch RM, House RA, Ortiz-Monasterio I, Cheng Z. Potential for improving bioavailable zinc in wheat grain (Triticum species) through plant breeding. J Agric Food Chem (2005) 53:2176–80. doi:10.1021/jf040238x
272. Cakmak I, Torun A, Millet E, Feldman M, Fahima T, Korol A, et al. Triticum dicoccoides: an important genetic resource for increasing zinc and iron concentration in modern cultivated wheat. Soil Sci Plant Nutr (2004) 50:1047–54. doi:10.1080/00380768.2004.10408573
273. Digesu AM, Platani C, Cattivelli L, Mangini G, Blanco A. Genetic variability in yellow pigment components in cultivated and wild tetraploid wheats. J Cereal Sci (2009) 50:210–8. doi:10.1016/j.jcs.2009.05.002
274. Ficco DB, Mastrangelo AM, Trono D, Borrelli GM, De Vita P, Fares C, et al. The colours of durum wheat: a review. Crop Pasture Sci (2014) 65(1):1–15. doi:10.1071/CP13293
275. Garg M, Chawla M, Chunduri V, Kumar R, Sharma S, Sharma NK, et al. Transfer of grain colors to elite wheat cultivars and their characterization. J Cereal Sci (2016) 71:138–44. doi:10.1016/j.jcs.2016.08.004
276. Havrlentova M, Psenakova I, Zofajova A, Ruckschloss L, Kraic J. Anthocyanins in wheat seed – a mini review. Nova Biotechnol Chim (2014) 13(1):1–12. doi:10.2478/nbec-2014-0001
277. Martinek P, Jirsa O, Vaculova K, Chrpova J, Watanabe N, Buresova V, et al. Use of wheat gene resources with different grain colour in breeding. Tagung Ver Pflanzenzüchter Saatgutkaufleute Osterreichs (2013–2014) 64(1):75–8.
278. Palmer AC, Healy K, Barffour MA, Siamusantu W, Chileshe J, Schulze KJ, et al. Provitamin A carotenoid-biofortified maize consumption increases pupillary responsiveness among Zambian children in a randomized controlled trial. J Nutr (2016) 146(12):2551–8. doi:10.3945/jn.116.239202
279. Muzhingi T, Palacios N, Miranda A, Cabrera ML, Yeum KJ, Tang G. Genetic variation of carotenoids, vitamin E and phenolic compounds in biofortified maize. J Sci Food Agric (2016) 97(3):793–801. doi:10.1002/jsfa.7798
280. Lago C, Cassani E, Zanzi C, Pilu R. Development and study of a maize cultivar rich in anthocyanins: coloured polenta, a new functional food. Plant Breed (2014) 133(2):210–7. doi:10.1111/pbr.12153
281. Goffman FD, Bohme T. Relationship between fatty acid profile and vitamin E content in maize hybrids (Zea mays L.). J Agric Food Chem (2001) 49(10):4990–4. doi:10.1021/jf010156y
282. Reddy BVS, Ramesh S, Longvah T. Prospects of breeding for micronutrients and β-carotene-dense sorghums. Int Sorghum Millets Newsl (2005) 46:10–4.
283. Velu G, Rai KN, Muralidharan V, Kulkarni VN, Longvah T, Raveendran TS. Prospects of breeding biofortified pearl millet with high grain iron and zinc content. Plant Breed (2007) 126:182–5. doi:10.1111/j.1439-0523.2007.01322.x
284. Rai KN, Govindraj M, Rao AS. Genetic enhancement of grain iron and zinc content in pearl millet. Crops Food (2012) 4(3):119–25. doi:10.1111/j.1757-837X.2012.00135.x
285. Blair MW, Astudillo C, Grusak MA, Graham R, Beebe SE. Inheritance of seed iron and zinc concentrations in common bean (Phaseolus vulgaris L.). Mol Breed (2009) 23(2):197–207. doi:10.1007/s11032-008-9225-z
286. Gelin JR, Forster S, Grafton KF, McClean P, Rojas-Cifuentes GA. Analysis of seed-zinc and other nutrients in a recombinant inbred population of navy bean (Phaseolus vulgaris L.). Crop Sci (2006) 47:1361–6. doi:10.2135/cropsci2006.08.0510
287. Beebe S, Gonzalez AV, Rengifo J. Research on trace minerals in the common bean. Food Nutr Bull (2000) 21:387–91. doi:10.1177/156482650002100408
288. Lachman J, Hamouz K. Red and purple coloured potatoes as a significant antioxidant source in human nutrition – a review. Plant Soil Environ (2005) 51:477–82.
289. Andre CM, Ghislain MP, Bertin O, Mouhssin M, Del Rosario H, Hoffmann L, et al. Andean potato cultivars (Solanum tuberosum L.) as a source of antioxidant and mineral micronutrients. J Agric Food Chem (2007) 55(2):366–78. doi:10.1021/jf062740i
290. Burgos G, Amoros W, Morote M, Stangoulis J, Bonierbale M. Fe and Zn concentration of native Andean potato cultivars from a human nutrition perspective. J Food Sci Agric (2007) 87:668–75. doi:10.1002/jsfa.2765
291. Brown CR, Haynes KG, Moore M, Pavek MJ, Hane DC, Love SL, et al. Stability and broad-sense heritability of mineral content in potato: iron. Am J Potato Res (2010) 87(4):390–6. doi:10.1007/s12230-010-9145-4
292. Haynes KG, Yencho GC, Clough ME, Henninger MR, Sterrett SB. Genetic variation for potato tuber micronutrient content and implications for biofortification of potatoes to reduce micronutrient malnutrition. Am J Potato Res (2012) 89:192–8. doi:10.1007/s12230-012-9242-7
293. Kumagai T, Umemura Y, Baba T, Iwanaga M. The inheritance of β-amylase null in storage roots of sweet potato, (Ipomoea batatas L.). Theor Appl Genet (1990) 79(3):369–76. doi:10.1007/BF01186081
294. Maziya-Dixon B, Kling JG, Menkir A, Dixon A. Genetic variation in total carotene, iron, and zinc contents of maize and cassava genotypes. Food Nutr Bull (2000) 21:419–22. doi:10.1177/156482650002100415
295. Chavez AL, Sanchez T, Jaramillo G, Bedoya JM, Echeverry J, Bolanos EA, et al. Variation of quality traits in cassava roots evaluated in landraces and improved clones. Euphytica (2005) 143(1–2):125–33. doi:10.1007/s10681-005-3057-2
296. Mazzucato A, Papa R, Bitocchi E, Mosconi P, Nanni R, Negri V, et al. Genetic diversity, structure and marker-trait associations in a collection of Italian tomato (Solanum lycopersicum L.) landraces. Theor Appl Genet (2008) 116(5):657–69. doi:10.1007/s00122-007-0699-6
297. Ortiz-Monasterio JI, Rojas NP, Meng E, Pixley K, Trethowan R, Pena RJ. Enhancing the mineral and vitamin content of wheat and maize through plant breeding. J Cereal Sci (2007) 46(3):293–307. doi:10.1016/j.jcs.2007.06.005
298. Li W, Beta T, Sun S, Corke H. Protein characteristics of Chinese black-grained wheat. Food Chem (2006) 98:463–72. doi:10.1016/j.foodchem.2005.06.020
299. Eticha F, Grausgruber H, Siebenhandl-ehn S, Berghofer E. Some agronomic and chemical traits of blue aleurone and purple pericarp wheat (Triticum L.). J Agric Sci Technol (2011) 1:48–58.
300. Pixley K, Palacios-Rojas N, Babu R, Mutale R, Surles R, Simpungwe E. Biofortification of maize with provitamin A carotenoids. In: Tanumihardjo SA, editor. Carotenoids and Human Health. New York: Springer Science (2013). p. 271–92.
301. CIMMYT. Biofortification to Fight “Hidden Hunger” in Zimbabwe. (2016). Available from: http://www.cimmyt.org/biofortification-to-fight-hidden-hunger-in-zimbabwe/
302. Waters BM, Pedersen JF. Sorghum germplasm profiling to assist breeding and gene identification for biofortification of grain mineral and protein concentrations. The Proceedings of the International Plant Nutrition Colloquium XVI. California (2009).
303. Fernandez MS, Kapran I, Souley S, Abdou M, Maiga IH, Acharya CB, et al. Collection and characterization of yellow endosperm fertilizers on human selenium status in Finland. Analyst (2009) 120:841–3. doi:10.1007/s10722-009-9417-3
304. Kumar AA, Reddy BVS, Ramaiah B. Biofortification for combating micronutrient malnutrition: identification of commercial Sorghum cultivars with high grain iron and zinc concentrations. Indian J Dryland Agric Dev (2013) 28(1):89–94.
305. Rao PP, Birthal PS, Reddy BVS, Rai KN, Ramesh S. Diagnostics of Sorghum and pearl millet grains-based nutrition in India. Int Sorghum Millets Newsl (2006) 47:93–6.
306. Sarker A, Agrawal SK. Combating Micronutrient Malnutrition with Biofortified Lentils. Amman Jordan the International Center for Agriculture Research in the Dry Areas. The International Center for Agriculture Research in the Dry Areas (2015).
307. Thavarajah D, Ruszkowski J, Vandenberg A. High potential for selenium biofortification of lentils (Lens culinaris L.). J Agric Food Chem (2008) 56(22):10747–53. doi:10.1021/jf802307h
308. Petry N, Boy E, Wirth JP, Hurrell RF. Review: the potential of the common bean (Phaseolus vulgaris) as a vehicle for iron biofortification. Nutrients (2015) 7(2):1144–73. doi:10.3390/nu7021144
309. Broadley M, Lochlainn S, Hammond J, Bowen H, Cakmak I, Eker S. Shoot zinc (Zn) concentration varies widely within Brassica oleracea L. and is affected by soil Zn and phosphorus (P) levels. J Hortic Sci (2010) 85(5):375–80. doi:10.1080/14620316.2010.11512683
310. Rick CM, Chetelat RT. Utilization of related wild species for tomato improvement. Acta Hortic (1995) 412:21–38. doi:10.17660/ActaHortic.1995.412.1
311. Lauricella M, Emanuele S, Calvaruso G, Giuliano M, D’Anneo A. Multifaceted health benefits of Mangifera indica L. (Mango): the inestimable value of orchards recently planted in Sicilian rural areas. Nutrients (2017) 9(5):525. doi:10.3390/nu9050525
312. Xu C, Zhang Y, Cao L, Lu J. Phenolic compounds and antioxidant properties of different grape cultivars grown in China. Food Chem (2010) 119:1557s–65s. doi:10.1016/j.foodchem.2009.09.042
313. Ismail AM, Heuer S, Thomson MJ, Wissuwa M. Genetic and genomic approaches to develop rice germplasm for problem soils. Plant Soil (2007) 65:547–70. doi:10.1007/s11103-007-9215-2
314. Wissuwa M, Ae N. Genotypic variation for tolerance to phosphorus deficiency in rice and the potential for its exploitation in rice improvement. Plant Breed (2001) 120:43–8. doi:10.1046/j.1439-0523.2001.00561.x
315. White JG, Zasoski RJ. Mapping soil micronutrients. Field Crops Res (1999) 60:11–26. doi:10.1016/S0378-4290(98)00130-0
316. Frossard E, Bucher M, Machler F, Mozafar A, Hurrell R. Potential for increasing the content and bioavailability of Fe, Zn and Ca in plants for human nutrition. J Sci Food Agric (2000) 80:861–79. doi:10.1002/(SICI)1097-0010(20000515)80:7<861::AID-JSFA601>3.0.CO;2-P
317. Waters BM, Sankaran RP. Moving micronutrients from the soil to the seeds: genes and physiological processes from a biofortification perspective. Plant Sci (2011) 180(4):562–74. doi:10.1016/j.plantsci.2010.12.003
318. Lyons G, Ortiz-Monasterio I, Stangoulis J, Graham R. Selenium concentration in wheat grain: is there sufficient genotypic variation to use in breeding? Plant Soil (2005) 269(1):369–80. doi:10.1007/s11104-004-0909-9
319. Oliva ML, Shannon JG, Sleper DA, Ellersieck MR, Cardinal AJ, Paris RL, et al. Stability of fatty acid profile in soybean genotypes with modified seed oil composition. Crop Sci (2006) 46:2069–75. doi:10.2135/cropsci2005.12.0474
320. Al-Babili S, Beyer P. Golden rice on the road-five years to go? Trends Plant Sci (2004) 10(12):565–73. doi:10.1016/j.tplants.2005.10.006
321. Inaba M, Macer D. Policy, regulation and attitudes towards agricultural biotechnology in Japan. J Int Biotechnol Laws (2004) 1(2):45–53. doi:10.1515/jibl.2004.1.2.45
322. Watanabe KN, Sassa Y, Suda E, Chen CH, Inaba M, Kikuchi A. Global political, economic, social and technological issues on transgenic crops—review. Plant Biotechnol J (2005) 22(5):515–22. doi:10.5511/plantbiotechnology.22.515
323. Welch RM. Effects of nutrient deficiencies on seed production and quality. Adv Plant Nutr (1986) 2:205–47.
324. Welch RM, Shuman L. Micronutrient nutrition of plants. Crit Rev Plant Sci (1995) 14(1):49–82. doi:10.1080/07352689509701922
Keywords: malnutrition, biofortification, transgenic, agronomic, breeding
Citation: Garg M, Sharma N, Sharma S, Kapoor P, Kumar A, Chunduri V and Arora P (2018) Biofortified Crops Generated by Breeding, Agronomy, and Transgenic Approaches Are Improving Lives of Millions of People around the World. Front. Nutr. 5:12. doi: 10.3389/fnut.2018.00012
Received: 09 August 2017; Accepted: 29 January 2018;
Published: 14 February 2018
Edited by:
Felipe Klein Ricachenevsky, Universidade Federal de Santa Maria, BrazilReviewed by:
Hannetz Roschzttardtz, Pontificia Universidad Católica de Chile, ChileCopyright: © 2018 Garg, Sharma, Sharma, Kapoor, Kumar, Chunduri and Arora. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Monika Garg, bW9uaWthZ2FyZ0BuYWJpLnJlcy5pbg==, bWdhcmcxMDBAeWFob28uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.