- 1Biofortis, Inc., Addison, IL, United States
- 2Nutrisystem, Inc., Fort Washington, PA, United States
Objective: To examine changes in weight and related outcomes in response to a commercial weight loss program compared to a self-directed diet in adults with overweight or obesity.
Design: Participants were randomly assigned [stratified by body mass index (BMI) and age] to a commercial weight loss program (n = 38) or to a self-directed Dietary Approaches to Stop Hypertension (DASH) diet (n = 40) for a 16-week period. Daily energy intake goals were 1,500 kcal/d for men and 1,200 kcal/d for women, except for the first week of the commercial program (1,000 kcal/d). This study was registered at http://ClinicalTrials.gov (NCT03017443).
Participants: Primarily Caucasian (71%) women (n = 61) and men (n = 17) from the greater metropolitan area of the city of Chicago, IL, USA. with a mean baseline BMI of 34.4 kg/m2, body weight of 95.7 kg, and age of 50.4 years.
Results: Data = mean (95% CI). At week 16, the commercial program group lost significantly more body weight [−5.9 (−7.5, −4.3) kg vs. −1.8 (−2.9, −0.8) kg; or −6.4 vs. −1.8% of initial body weight, respectively], fat mass [−4.4 (−5.7, −3.1) kg vs. −1.2 (−2.1, −0.4) kg] and total body circumference (chest + waist + hip + upper arm + thigh) [−16.9 (−21.5, −12.3) cm vs. −5.8 (−9.0, −2.6) cm] (p < 0.01 for all). Additionally, more participants in the commercial program group lost a clinically meaningful amount of weight, defined as ≥5% of initial body weight, at week 16 (58% vs. 13%, p < 0.001).
Conclusion: The commercial program resulted in greater weight loss and improvements in body composition/anthropometric parameters compared to a self-directed DASH diet over a 16-week period. Some important limitations were that no objective measurements of dietary intake or physical activity were collected to potentially ascertain the independent or combined effects of these components on weight loss (or lack thereof). Additionally, future research is warranted in order to understand the effects of this program, and similar programs, on longer term changes in body weight, in particular weight loss maintenance, as weight regain is common following the cessation of a structured weight loss intervention.
Introduction
Factors contributing to weight gain are complex and obesity remains a multifaceted public health problem (1, 2). A reduction of ≥5% of initial body weight is often recommended as a primary step in managing or preventing comorbidities associated with obesity; however, recent advances in the understanding of the etiology of obesity, along with development of novel pharmaceutical and surgical treatment options, have yet to markedly impact obesity prevalence in the US population. Improving diet and physical activity remain the foundation of most obesity interventions.
In this context, the role of commercially available weight loss programs has received increased attention by the scientific community (3, 4). One approach shown to be useful in supporting weight loss is provision of pre-portioned foods and beverages (i.e., meal replacements or portion and calorie controlled foods) (5–8), especially compared to more conventional dietary advice, such as self-selected diets based on general concepts such as variety and moderation (9). Some commercially available weight loss programs utilize this approach, although data from randomized clinical trials documenting the degree of weight loss achievable with these programs are needed.
The primary objective of this study was to determine changes in body weight achievable in apparently healthy men and women with overweight or obesity following a commercial weight loss program, consisting of a nutritionally balanced calorie restricted diet via provision of portion-controlled foods and shakes, along with phone support from trained weight loss counselors, compared to a self-directed diet over a 16-week period. Key secondary objectives included determining changes in body composition, body circumference measures, and self-reported health-related quality of life, including sleep.
Materials and Methods
Study Design
This was a randomized, parallel group study conducted from April 2015 (first participant screened) to October 2015 (last participant completed). The intervention period was 16 weeks in duration, with clinic visits at baseline (week 0) and weeks 1, 2, 3, 4, 8, 12, and 16. Participants from the greater metropolitan area of the city of Chicago, IL, USA, that initially qualified via telephone screening and subsequently met all entry criteria at the screening visit (week 1) were randomized to one of the study arms. A statistician who was blinded to intervention assignment during data analysis generated a randomization list for intervention sequence using SAS PROC PLAN with a 1:1 allocation across groups, stratified by two body mass index (BMI) categories (25.00–29.99; 30.00–44.99 kg/m2) and three age categories (18–35; 36–54; 55–70 years). Numbered, opaque, and sealed envelopes concealing the allocation sequence were opened sequentially by a clinic staff member only after a participant was confirmed eligible for the study and the randomization number/test sequence was recorded with the subject’s source documentation. Blinding of study staff (with the exception of the statistician) or participants was not possible given the nature of the study interventions.
This study was conducted at Biofortis, Inc., Addison, IL, USA, in accordance with the recommendations of Good Clinical Practice Guidelines, the Declaration of Helsinki (10), and the United States 21 Code of Federal Regulations. The protocol was approved by an accredited Institutional Review Board (IntegReview IRB, Austin, TX, USA). All participants provided signed informed consent and authorization for disclosure of protected health information before any study specific procedures were carried out. At the time the study was initiated, the need to prospectively register as a clinical trial in a public database was not given proper consideration. Therefore, this study was registered post hoc at ClinicalTrials.gov at https://clinicaltrials.gov/ct2/show/NCT03017443?term=NCT03017443&rank=1 with the unique identifier NCT03017443.
Study Participants
Men and non-pregnant, non-lactating women, 18–70 years old, each with a BMI 25.00–44.99 kg/m2 and in good general health on the basis of medical history and routine laboratory tests at screening were initially eligible for the study. Users of tobacco products were allowed, although these participants were required to maintain habitual use throughout the duration of the study. Premenopausal female participants were also required to have a regular menstrual cycle (ranging from 21 to 35 days) and were required to be willing to use a medically approved form of contraception throughout the study.
Participants who reported a weight change of ≥4.5 kg or used prescription medications for weight-reducing purposes within 6 months of screening, or who used weight loss supplements or other commercially available products/programs with the intent to lose weight within 1 month of screening, were excluded from the study. Participants with clinically significant abnormal routine laboratory test results or uncontrolled hypertension (resting systolic blood pressure ≥160 mmHg and/or a diastolic blood pressure ≥100 mm Hg) at screening were excluded. Additional exclusion criteria included the following: medical history indicating clinically relevant cardiac, renal, hepatic, endocrine (including diabetes mellitus at screening), pulmonary, biliary, gastrointestinal, pancreatic, or neurologic disorders; cancer in the past 2 years; known sensitivity to any of the ingredients in the study foods; a history of weight-reducing surgery; a history of eating disorders, extreme dietary habits, or alcohol abuse; use of thyroid hormones (except stable dose replacement therapy for at least 2 months prior to screening); or systemic corticosteroid use within 4 weeks of screening.
Participants were recruited via e-mail notifications to a database of individuals that had previously expressed interest in participating in research studies, via newspaper and social media/Internet-based advertisements, and by word of mouth.
Dietary Interventions
Commercial Weight Loss Program
The commercial weight loss program utilized a two-phase approach. Participants followed a 1,000 kcal/day diet during the first week of the study, consisting of prepackaged portion-controlled foods and shakes, and were allowed to add non-starchy vegetables and no-calorie beverages within program guidelines during this first week. After the first week, daily energy intake targets were increased to 1,500 kcal/day (men) or 1,200 kcal/day (women) and participants were provided 7 breakfasts, 6 lunches, 6 dinners, and 7 (women) or 14 (men) snacks/desserts per week consisting of prepackaged portion-controlled foods. Participants were instructed to prepare one lunch and one dinner within program guidelines on their own each week. Prepackaged portion-controlled foods accounted for ~60% of daily energy intake, with recommended grocery food additions making up the balance. Written recommendations were provided to allow participants to self-select appropriate foods for these eating occasions that fit within program guidelines (~50% kcal/day from carbohydrate, ~25% kcal/day from protein, ~25% kcal/day from fat), and food intake was self-monitored through the use of a tracker (similar to a food checklist) consistent with the commercially available program. Participants also had access to support from weight loss counselors by phone at any time throughout the study period.
Self-Directed Diet
The control group was designed to mimic typical dietary advice that might be received in a primary care setting. Participants randomized to the self-directed diet received a limited intervention that included provision of publicly available information consistent with the Dietary Approaches to Stop Hypertension (DASH) diet (11), with instructions to utilize these dietary recommendations to consume a reduced calorie diet for weight loss. DASH is a well-balanced dietary pattern for the general public, encompassing fruits and vegetables, whole grains, legumes/nuts, and lean sources of protein such as fish and poultry, with limited consumption of added sugars, added fats, and red meat (12, 13). Daily energy intake targets were the same as the commercial weight loss program group (1,500 kcal/day for men and 1,200 kcal/day for women) with the exception of the first week of that program (1,000 kcal/day). To encourage study completion, participants randomized to the DASH group received vouchers to obtain 4 weeks of foods associated with the commercial weight loss program at the end of the study.
Anthropometric and Blood Pressure Measurements
Body weight, body circumference (chest, waist, hip, upper arm, and thigh), and blood pressure measurements were obtained at baseline (week 0) and at weeks 1, 2, 3, 4, 8, 12, and 16 in the morning following an overnight fast (9–14 h). Height was obtained at screening without shoes to the nearest 0.1 cm using a wall mounted stadiometer (Seca model 2161814009, Hamburg, Germany). Body weight was measured using a digital floor scale (Health-O-Meter Professional model 349KLX, Boca Raton, FL) with all participants in a gown, without shoes, and after emptying their bladder/bowels. A stretch-resistant anthropometric tape (Gulick II model #67020, Gays Mills, WI, USA) with an indicator buckle to denote proper amount of tension applied to the tape was used for body circumference measurements. An average of two measures was recorded for each body site, unless the two measures differed by more than 1.0 cm, then a third measure was taken and the average of all three measures was used. Waist circumference was measured on a horizontal plane at the level of the iliac crest at the end of a normal expiration. Hip circumference was measured on a horizontal plane at the widest portion of the buttocks. Chest circumference was measured on a horizontal plan at the level of the nipples at the end of a normal expiration with the arms down at the subject’s sides. Upper arm (dominant arm) circumference was obtained at the measured midpoint between the shoulder and the elbow, with the arm hanging down the side of the body in a relaxed position. Thigh circumference was measured using the dominant leg at the widest portion of the thigh, which was typically the midpoint between the lower buttocks and the back of the knee.
Seated, resting blood pressure was obtained after the participant had been seated for at least 5 min. Participants refrained from smoking cigarettes (if applicable) or ingesting caffeine during the 30 min preceding the measurement. Blood pressure was measured using an automatic blood pressure device (Welch Allyn 300 Series, Skaneateles Falls, NY, USA).
Body Composition
Total and regional fat mass and fat-free mass (lean mass and bone) were quantified by dual energy X-ray absorptiometry (DXA; GE Lunar Prodigy, enCORE software version 16, Madison, WI, USA) at baseline (week 0) and at weeks 4, 8, and 16. Total fat mass precision as reported by the manufacturer was <1.0%. A urine pregnancy test was performed on all women <60 years of age at screening and weeks 4, 8, and 16 prior to receiving a DXA scan.
Laboratory Methods
Fasting blood samples were collected at screening for analysis of serum lipoprotein lipids, a comprehensive metabolic panel, and a complete blood count with automated differential performed by Elmhurst Memorial Hospital (EMH) Reference Laboratory (Elmhurst, IL, USA) according to their standard validated procedures, including the Standardization Program of the Centers for Disease Control and Prevention and the National Heart, Lung and Blood Institute for lipid measurements (14). Lipoprotein lipid assessments (milligrams per deciliter) included total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), non-HDL-C (calculated as TC minus HDL-C), triglycerides, and the TC/HDL-C ratio. The LDL-C concentration in milligrams per deciliter was calculated according to the Friedewald equation as: LDL-C = TC − HDL-C − TG/5 (15).
Quality of Life Assessments
Questionnaires designed to assess aspects of quality of life and sleep were administered at baseline and at weeks 4, 8, and 16. The Impact of Weight on Quality of Life-Lite questionnaire is a validated 31-item self-report measure used to assess obesity-specific quality of life domains, including physical function, self-esteem, sexual life, public distress, and work “over the past week” (16). Responses to questions within each domain were rated on a 5-point scale as “always true” (scored as 5), “usually true” (scored as 4), “sometimes true” (scored as 3), “rarely true” (scored as 2), or “never true” (scored as 1). Scores for each domain were obtained by summing responses to each individual question within that domain, and a total score was obtained by summing the domain scores. Higher scores indicate poorer quality of life.
The SF-36 health survey (17, 18) was used to assess more general aspects of quality of life, including physical functioning, role limitations caused by physical health problems, role limitations caused by emotional problems, social functioning, emotional well-being, energy/fatigue, pain, and general health perceptions “over the previous 4 weeks.” A higher score defines a more favorable health state.
The Leeds Sleep Evaluation Questionnaire (LSEQ), a validated 10-item self-report measure, was used to assess changes in sleep quality “compared to usual” over the course of the intervention pertaining to four aspects of sleep: Getting to Sleep, Quality of Sleep, Awakening from Sleep, and Behavior following Wakefulness (19). Responses to each question were measured on a visual analog scale using a 10-cm line with two extreme states defined at the ends of the line (e.g., “more difficult than usual”/“easier than usual”). Scores were averaged to provide a single score for each domain. A higher score indicates more favorable sleep outcomes.
Statistical Analyses
A written statistical analysis plan was developed prior to the last subject completing the study before any data were analyzed. Statistical analyses were performed with SAS (SAS Institute, Cary, NC, USA, version 9.3). The current data was part of a four-arm parallel trial where three different commercial weight loss programs were each compared to the self-directed DASH diet control (20). Therefore, the original sample size calculation assumed a nominal α = 0.017 (one-sided) to account for up to three primary comparisons (each commercial weight loss program vs. control). A sample size of at least 35 per group was determined to provide 80% power to detect an effect size (d) of 0.72 for change in body weight between the groups. Additional participants were randomized to account for possible attrition. Participants were stratified across groups by age (18–35; 36–54; 55–70 years) and BMI (25.00–29.99; 30.00–44.99 kg/m2), and the total study sample included 70–80% women representing the typical profile of the commercial weight loss program consumer.
An intent-to-treat (ITT) analysis with last observation carried forward (LOCF) was used. Data at baseline are reported as mean ± SD and change from baseline data are reported as mean (95% confidence interval). Repeated measures analysis of covariance was used to assess differences between groups in the primary outcome variable (body weight) and continuous secondary outcome variables (body composition, body circumference parameters, and questionnaire data) at each post-randomization visit. Initial models contained terms for intervention, sex, stratification factors (BMI and age categories), and phase of menstrual cycle at start of intervention (follicular, luteal, or N/A), with baseline measures as covariates. Models were reduced using a backward selection method until only significant terms and/or intervention and baseline measure remained in the model (described for each parameter in footnotes of the results tables). Normality of residuals was investigated for each continuous outcome variable and if the normality assumption was rejected at the 1% level with the Shapiro–Wilk test, rank transformation was performed. Within each group, the paired t-test was used to determine if changes from baseline to each post-randomization visit in outcome variables were statistically significant. Safety assessments included an evaluation of post-randomization intervention-emergent adverse events.
Results
Participants
The disposition of participants throughout the study is shown in Supplementary Material. A total of 78 participants were randomized to the DASH diet (n = 40) or the commercial weight loss program (n = 38). Two participants randomized to the DASH diet discontinued the study at the baseline visit prior to beginning the assigned dietary intervention on the following day, so no LOCF imputation was applied for these individuals. Participants included predominantly Caucasian (70.6%) women (n = 61) and men (n = 17) with overweight and obesity, with a mean baseline age of 50.4 years, body weight of 95.7 kg, and BMI of 34.4 kg/m2. Additional demographic characteristics are presented in Table 1.
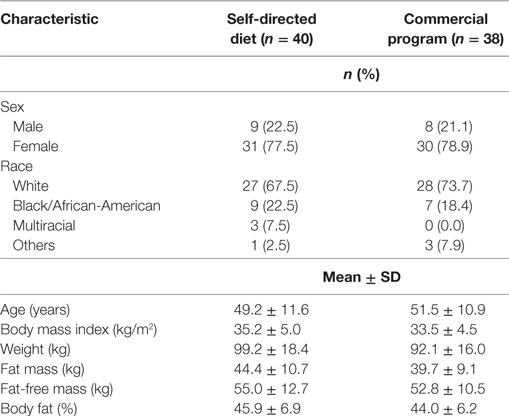
Table 1. Demographic characteristics at baseline of participants in a randomized trial allocated to a commercial weight loss program or a self-directed Dietary Approaches to Stop Hypertension diet.
Body Weight
Changes in body weight at each time point over the 16-week study period are presented in Figure 1. Both groups lost a statistically significant amount of body weight from baseline to week 16, with the commercial weight loss program group losing more weight on average than the DASH group at every timepoint, about three times the weight loss of the DASH diet (p < 0.001 for all). Additionally, more participants in the commercial weight loss program group lost ≥5% of initial body weight by the end of the study (57.9% vs. 13.2%, p < 0.001).
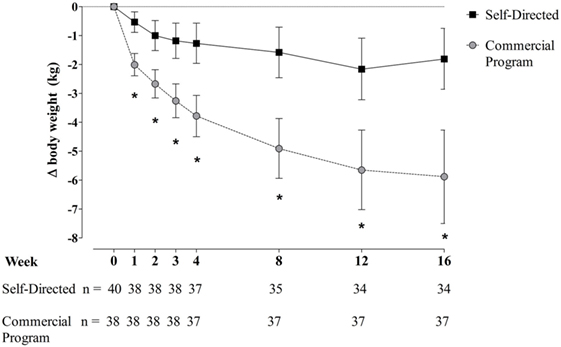
Figure 1. Change in body weight in men and women with overweight/obesity randomized to a commercial weight loss program or a self-directed Dietary Approaches to Stop Hypertension diet for a 16-week study period. Change from baseline data presented as mean ± 95% CI for the intent-to-treat sample population. Missing data were imputed using the method of last observation carried forward (LOCF). Two participants randomized to the self-directed diet discontinued the study at the baseline visit prior to beginning the assigned dietary intervention on the following day, so no LOCF imputation was applied for these individuals. Repeated measures analysis of covariance was used to assess differences between groups. Initial models contained terms for intervention, sex, stratification factors (body mass index and age categories), and phase of menstrual cycle at start of intervention (follicular, luteal, or N/A), with baseline measures as covariates. Models were reduced using a backward selection method until only significant terms and/or intervention and baseline measure remained in the model.
Body Composition
Weight loss with the commercial weight loss program was accompanied by larger reductions in total fat mass, and fat mass expressed as a percent of body weight (body fat%), compared to the DASH diet at every timepoint (Table 2). The commercial weight loss program group also experienced greater changes in regional body composition, including larger changes in android and abdominal visceral fat mass by week 16 (Table 2). Even though the DASH group lost less body weight, on average, than the commercial program group, approximately two-thirds of weight loss at week 16 was body fat.
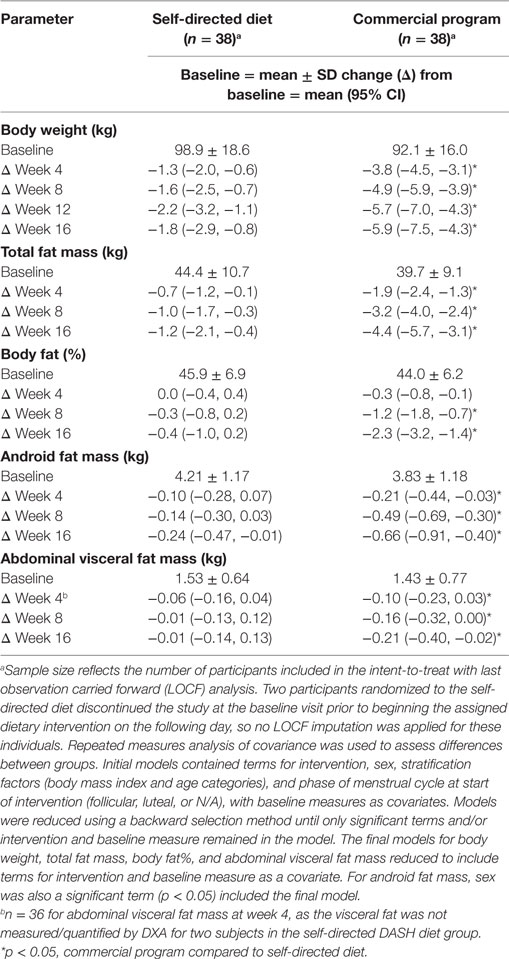
Table 2. Changes in body weight and selected body composition parameters in men and women with overweight/obesity randomized to a commercial weight loss program or a self-directed Dietary Approaches to Stop Hypertension diet each for a 16-week study period.
Body Circumference Measurements
Changes in body circumference parameters at each 4-week time point over the course of the study are presented in Table 3. While both groups experienced reductions in body circumference measurements, changes within the commercial weight loss program group were significantly greater (p < 0.05).
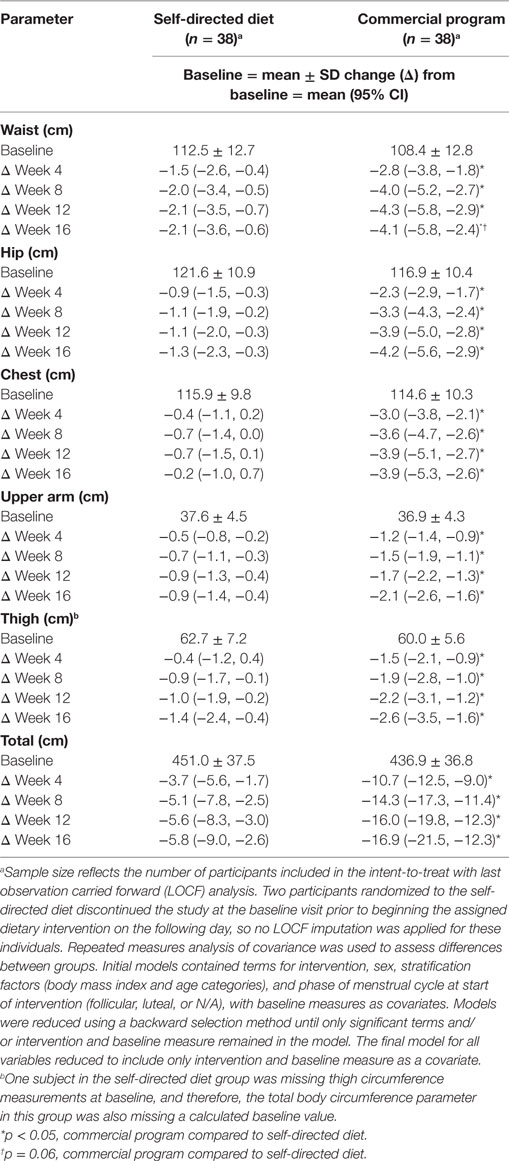
Table 3. Changes in body circumference parameters in men and women with overweight/obesity randomized to a commercial weight loss program or a self-directed Dietary Approaches to Stop Hypertension diet for a 16-week study period.
Quality of Life and Sleep Quality
The total IWQOL score improved in both groups over the 16-week study period, but to a greater extent in the commercial weight loss program group compared to the DASH diet group at week 16 (Table 4) indicating improvements in weight associated quality of life in general for both groups. The individual domains generally followed the same pattern of improvement with weight loss in the commercial weight loss program group by the end of the study, with changes in physical function and self-esteem significantly different from the DASH diet.
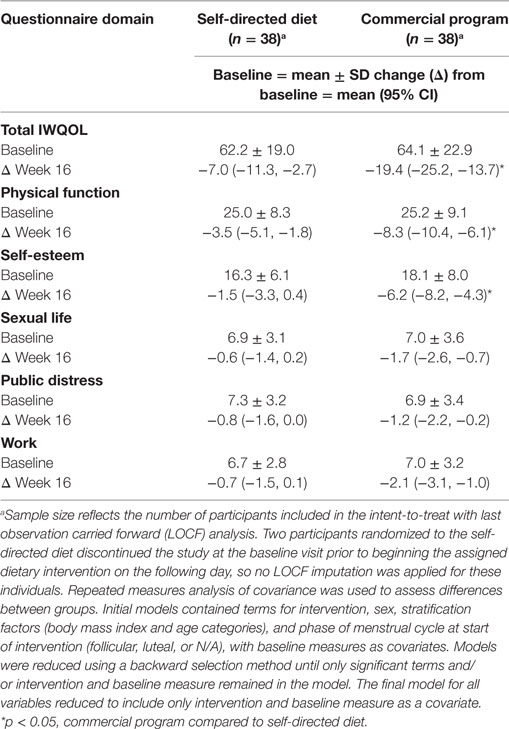
Table 4. Responses to the Impact of Weight on Quality of Life-Lite questionnaire in men and women with overweight/obesity randomized to a commercial weight loss program or a self-directed Dietary Approaches to Stop Hypertension diet for a 16-week study period.
Findings associated with the SF-36 questionnaire were, in general, variable both within and between groups, indicating the SF-36 might not have been a sensitive indicator of quality of life in this study sample (data not presented). Similarly, findings from the LSEQ were inconclusive as there were no significant differences between groups in sleep outcomes assessed.
Blood Pressure
Both groups experienced reductions in systolic and diastolic blood pressure, as shown in Table 5, but there were no statistically significant differences in blood pressure changes between groups at any time point during the 16-week study period.
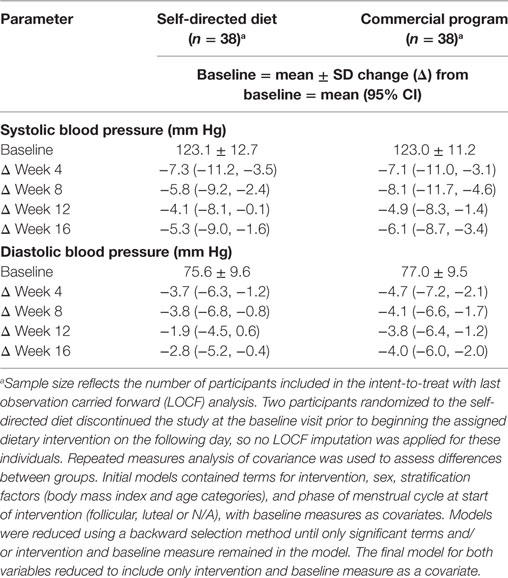
Table 5. Changes in blood pressure parameters in men and women with overweight/obesity randomized to a commercial weight loss program or a self-directed Dietary Approaches to Stop Hypertension diet for a 16-week study period.
Adverse Events
There were 15 adverse events reported in the self-directed DASH diet group, and 17 adverse events reported in the commercial program group; however, no adverse were reported as serious or severe, and no adverse events were deemed by the study physicians as related to either dietary intervention.
Discussion
In this 16-week randomized parallel group study, adults with overweight and obesity who followed a comprehensive commercially available weight loss program experienced significantly greater reductions in body weight, body fat, and body circumference parameters compared to a self-directed diet modeled after the DASH dietary pattern with caloric restriction. These results suggest commercially available weight loss programs could be a reasonable option to consider, especially in a primary care setting where health-care practitioners may lack requisite nutrition training and/or time to engage with individuals seeking strategies for weight management.
The data from the present study are generally consistent with previous research showing that provision of portioned controlled foods as a dietary intervention for weight loss is an effective approach compared to conventional diets (5–8, 21–23). For example, a 2003 meta-analysis of six randomized controlled trials found that 3-month weight loss in participants consuming a partial meal replacement diet exceeded that of participants consuming a standard reduced calorie diet by 2.5–3.0 kg (~7% change from baseline) (5). These data, along with other studies, have been considered in the Academy of Nutrition and Dietetics (AND) Evidence Analysis Library (24) on research into Single Serving Portion Sized Meals (SSPSM) for weight management, which concluded that there is good evidence supporting the use of SSPSM for weight loss in adults. Furthermore, the 2009 AND position statement on weight management indicates strong evidence for the use of portion-controlled meal replacements for weight management (25).
In more recent studies of comparable design and duration to the present intervention, significant reductions in weight, body fat, and/or anthropometric parameters have been observed in participants following structured diets or commercially available programs incorporating portion-controlled foods compared to various control diets (22, 23). Participants following a commercially available program consisting of a reduced calorie diet achieved through the provision of portion-controlled foods, and delivered in a manner similar to the current study, lost more weight (−7.5 vs. −3.8 kg) than participants following a food-based energy-restricted diet at 26 weeks (22). These results are generally in-line with weight loss observed over a shorter time period (16 weeks) in the present study (−5.9 vs. −1.8 kg, commercial weight loss program vs. self-directed DASH diet). Another study published by Rock et al. (23) found that consumption of portion-controlled prepackaged lunch and dinner frozen entrees for 12 weeks, in conjunction with a prescribed reduced-energy diet and behavioral counseling, resulted in larger changes in weight compared to a standard self-selected diet in adults with overweight/obesity. However, it should also be mentioned that not all studies of similar duration and design have shown added benefits of portion-controlled foods relative to conventional energy-restricted diets on weight or related outcomes, but rather comparative effects or no statistically significant differences from conventional energy-restricted diets (26, 27).
It is noteworthy that the amount of weight loss observed in the present study was significantly different from the self-directed DASH diet by the first week and remained so throughout the course of the trial. However, it should be noted that the lower calorie intake level provided during the first week of the commercial program (1,000 kcal/d) was not the same as the 1,200 kcal/d (women) to 1,500 kcal/d (men) intake goal recommended during the first week of the self-directed diet. The lower calorie level during the first week of the commercial program was designed to promote an early larger initial weight loss to encourage compliance and continued weight loss with this program, and to understand how this compares to a more usual care approach to weight loss. This approach was deemed relevant, as large initial weight loss is meaningful from a clinical perspective, as previous research has shown that initial weight loss during the first few weeks of intervention is associated with better long-term weight loss outcomes (28–31). Additionally, analysis of pooled data from over 2,000 participants that took part in multiple phase 3 studies of a novel obesity pharmacotherapy demonstrated that participants who met or exceeded the threshold for clinically meaningful weight loss (≥5% initial body weight) at week 16 were more likely to maintain that amount of weight loss at 1 year (32). However, the present study did not examine longer term changes in body weight or composition, so additional research is needed to determine if the observed 16-week findings could contribute to long-term weight loss or maintenance of weight loss.
Other noteworthy findings from the present study include improvements in some self-reported weight associated quality of life parameters in response to the commercial weight loss program relative to the self-directed DASH diet. The inverse relationship between excess body weight or high BMI and poor health-related quality of life, and more specifically the prospective observation of improvements in specific aspects of quality of life with weight loss, has previously been shown (33, 34).
The present study had some limitations. It is recognized that there is no single best diet or program for all individuals seeking weight loss (35), and it should be acknowledged that the results of the present study were observed in predominantly Caucasian females with overweight and obesity. Therefore, the findings may not be generalizable to other specific segments of the population; however, the demographics of the study participants represent a population that reflects the average consumer of commercial programs to support weight loss, and therefore, the study results may have external validity. Additionally, in many dietary intervention studies, blinding is difficult or not possible and therefore participants and research staff were not fully blinded to intervention assignments. This is a potential limitation of the study design; however, the study biostatistician was blinded to randomization assignment prior to data analysis in an attempt to limit potential bias.
The choice of the control diet is also an important consideration when assessing strengths and limitations, and in this case, the choice to use the DASH diet deserves some discussion. While the DASH diet was originally designed to examine effects on blood pressure (12), investigators have noted weight loss in participants following the DASH diet with or without prescribed energy restriction in hypertension studies (36). Various forms of this diet have also been used in studies as a dietary intervention for weight loss (37). A systematic review and meta-analysis of 13 studies found that mean weight reduction with the DASH diet as the primary intervention was approximately 1.4 kg for interventions ranging 8–24 weeks in duration (37), which is similar to mean weight loss observed with the self-directed DASH diet in the present study by week 16 (−1.8 kg). Additionally, the DASH diet has been promoted in the popular press as an effective strategy for weight management (38, 39). Interestingly, although participants following the self-directed DASH diet experienced only modest weight loss on average, this group demonstrated statistically significant improvements in systolic and diastolic blood pressure, which is one indication the participants were adhering to the DASH dietary guidelines. Overall, the intent of using the DASH diet was to provide a well-studied, structured diet previously shown to promote weight loss that could be used to replicate usual care in a general clinic setting, wherein patients are typically provided with written materials or similar resources to support weight loss efforts with limited or no specific counseling or nutrition education provided by a health-care practitioner.
Additional limitations include no objective assessments of dietary intake or physical activity to potentially ascertain the independent or combined effects of these components on weight loss (or lack thereof), so results are attributable to the entirety of the commercial program (i.e., nutrition support and provision of foods). However, it should be noted that self-reported measures of dietary intake tend to underestimate true energy intakes, while self-reported measures of physical activity tend to overestimate true activity levels (references). Objectively measuring either variable is challenging to do with reasonable accuracy and precision in a cost-effective manner over a longer time frame in free-living individuals (references). That said physical activity levels could have been different among subjects within and between groups, which likely contributed to some of the variability in weight loss. Also, the study was conducted from April to October of the same calendar year, so potential seasonal differences (i.e., spring/fall vs. summer) in physical activity were not considered in our analyses and could be a limitation of the study. Lastly, there was a differential attrition rate between the two study groups, with the self-directed DASH diet having more drop-outs (n = 8; 15%) compared to the commercial program (n = 1; 3%). The higher rate of attrition in the self-directed DASH diet arm may suggest some individuals may not be able to follow a diet that does not provide pre-measured and prepared foods directly to participants; however, this is one reason for studying a self-directed diet vs. a commercial program, as the latter would be hypothesized to promote better compliance given the structured nature of the program with provision of foods.
One strength of the study was the use of DXA to quantify changes in body composition in addition to only examining changes in body weight. Another possible strength of the study was the low attrition rate, which resulted in limited missing data that may provide a more accurate representation of expected results when individuals fully complete a dietary program. The low attrition rate could have been due to several factors. Participants randomized to the DASH diet who completed the study were provided with a voucher to obtain 4 weeks of access to the commercial weight loss program, including foods, at no-cost to encourage study completion, which may have contributed to a lower attrition rate than observed in other weight loss studies. Of note, 4 weeks of access to the commercial program and provision of foods was the most the IRB would allow for these participants because the IRB deemed this a “bonus” form of compensation. The original intent was to provide the full 16 weeks to these participants. It is important to also consider that the typical consumer of commercial weight loss programs pays out of pocket and therefore may experience different outcomes compared to research participants who received all study foods and program support at no cost. The economics of weight management is a complicated issue and is beyond the scope of this study; however, it should be acknowledged that the potential impact of the cost of the program on weight outcomes in the present trial was not examined. The costs of a commercial weight loss program relative to those incurred with a self-directed advice based approach need to be carefully considered in the overall context of each individual’s unique weight management circumstances. Last, the structured dietary program, which consisted primarily of the provision of pre-portioned foods and shakes along with access to weight loss counselors (via phone at the discretion of participants) and other supporting print materials, may have supported overall compliance compared to that typically observed with usual care or conventional self-directed diets (40); however, compliance was not directly assessed in this study.
In summary, a commercial weight loss program resulted in significantly greater weight loss, fat loss, and reductions in body circumference parameters compared to a self-directed DASH diet over a 16-week intervention period. This program appears to be one approach available for health-care professionals to consider in working with a population with overweight/obesity, although the long-term effects on weight loss and weight loss maintenance are unknown. As such, future research is warranted in order to understand the effects of this program, and similar programs, on longer term changes in body weight, in particular weight loss maintenance, since many diets and weight management programs have variable success over periods of time greater than the 16-week period used in the present study.
Ethics Statement
This study was conducted at Biofortis, Inc., Addison, IL, USA in accordance with the recommendations of Good Clinical Practice (GCP) Guidelines, the Declaration of Helsinki (2000), and the United States 21 Code of Federal Regulations. The protocol was approved by an accredited Institutional Review Board (IntegReview IRB; Austin, TX, USA). All participants provided signed informed consent and authorization for disclosure of protected health information before any study specific procedures were carried out.
Author Contributions
CC contributed to the study design, development of the statistical analysis plan, analysis and interpretation of data, and writing the manuscript. CM and MK contributed to the study design, development of the statistical analysis plan, and critically reviewed the manuscript but were not involved in any data collection or analysis. VK was involved in the acquisition of data and in revising the manuscript critically for important intellectual content. All authors listed have contributed substantially to the work as noted and agreed to submit the manuscript.
Conflict of Interest Statement
CM and MK are employees of Nutrisystem, Inc. CC and VK are employed by Biofortis, Inc., a contract research organization that conducted this study but did not directly receive funds from Nutrisystem.
Acknowledgments
The authors would like to acknowledge Margie Bell, MS, of Clindata Services (Fort Collins, CO, USA) for assistance in performing statistical analyses and Dr. Andrea Lawless, MD of Biofortis, Inc., as the study physician.
Funding
This study was funded by Nutrisystem Inc., Fort Washington, PA, USA.
Supplementary Material
The Supplementary Material for this article can be found online at http://www.frontiersin.org/article/10.3389/fnut.2017.00055/full#supplementary-material.
References
1. Chang L, Neu J. Early factors leading to later obesity: interactions of the microbiome, epigenome, and nutrition. Curr Probl Pediatr Adolesc Health Care (2015) 45(5):134–42. doi:10.1016/j.cppeds.2015.03.003
2. Huang T, Hu FB. Gene-environment interactions and obesity: recent developments and future directions. BMC Med Genomics (2015) 8(Suppl 1):S2. doi:10.1186/1755-8794-8-S1-S2
3. Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol (2014) 63(25 Pt B):2985–3023. doi:10.1016/j.jacc.2013.11.004
4. Gudzune KA, Doshi RS, Mehta AK, Chaudhry ZW, Jacobs DK, Vakil RM, et al. Efficacy of commercial weight-loss programs: an updated systematic review. Ann Intern Med (2015) 162(7):501–12. doi:10.7326/M14-2238
5. Heymsfield SB, van Mierlo CA, van der Knaap HC, Heo M, Frier HI. Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes Relat Metab Disord (2003) 27(5):537–49. doi:10.1038/sj.ijo.0802258
6. Hannum SM, Carson L, Evans EM, Canene KA, Petr EL, Bui L, et al. Use of portion-controlled entrees enhances weight loss in women. Obes Res (2004) 12(3):538–46. doi:10.1038/oby.2004.61
7. Hannum SM, Carson LA, Evans EM, Petr EL, Wharton CM, Bui L, et al. Use of packaged entrees as part of a weight-loss diet in overweight men: an 8-week randomized clinical trial. Diabetes Obes Metab (2006) 8(2):146–55. doi:10.1111/j.1463-1326.2005.00493.x
8. Cheskin LJ, Mitchell AM, Jhaveri AD, Mitola AH, Davis LM, Lewis RA, et al. Efficacy of meal replacements versus a standard food-based diet for weight loss in type 2 diabetes: a controlled clinical trial. Diabetes Educ (2008) 34(1):118–27. doi:10.1177/0145721707312463
9. Casazza K, Fontaine KR, Astrup A, Birch LL, Brown AW, Bohan Brown MM, et al. Myths, presumptions, and facts about obesity. N Engl J Med (2013) 368(5):446–54. doi:10.1056/NEJMsa1208051
10. National Heart, Lung, and Blood Institute (NHLBI) and North American Association for the Study of Obesity (NAASO). The Practical Guide: Identifcation, Evaluation and Treatment of Overweight and Obesity in Adults. National Institutes of Health (NIH) publication number 00-4084. Washington: US Department of Health and Human Services, Public Health Service (2000).
11. U. S. Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute. Your Guide to Lowering Your Blood Pressure With DASH (NIH Publication No. 06-5834). (2015). Available from: https://www.nhlbi.nih.gov/files/docs/public/heart/dash_brief.pdf
12. Sacks FM, Obarzanek E, Windhauser MM, Svetkey LP, Vollmer WM, McCullough M, et al. Rationale and design of the Dietary Approaches to Stop Hypertension trial (DASH). A multicenter controlled-feeding study of dietary patterns to lower blood pressure. Ann Epidemiol (1995) 5(2):108–18. doi:10.1016/1047-2797(95)00103-4
13. Sofer S, Stark AH, Madar Z. Nutrition targeting by food timing: time-related dietary approaches to combat obesity and metabolic syndrome. Adv Nutr (2015) 6(2):214–23. doi:10.3945/an.114.007518
14. Myers GL, Cooper GR, Winn CL, Smith SJ. The centers for disease control-national heart, lung and blood institute lipid standardization program. An approach to accurate and precise lipid measurements. Clin Lab Med (1989) 9(1):105–35.
15. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem (1972) 18(6):499–502.
16. Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res (2001) 9(2):102–11. doi:10.1038/oby.2001.13
17. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care (1992) 30(6):473–83. doi:10.1097/00005650-199206000-00002
18. Medical Outcomes Study. 36-Item Short Form Survey Scoring Instructions. RAND Health (2009). Available from: http://www.rand.org/health/surveys_tools/mos/mos_core_36item_scoring.html
19. Parrott AC, Hindmarch I. Factor analysis of a sleep evaluation questionnaire. Psychol Med (1978) 8(2):325–9. doi:10.1017/S0033291700014379
20. Cook CM, Kern HJ, Kaden VN, Kelley KM. Comparison of commercially-available portion controlled weight loss programs with a self-directed diet: a randomized clinical trial. FASEB J (2016) 30(1 Suppl):lb379.
21. Davis LM, Coleman C, Kiel J, Rampolla J, Hutchisen T, Ford L, et al. Efficacy of a meal replacement diet plan compared to a food-based diet plan after a period of weight loss and weight maintenance: a randomized controlled trial. Nutr J (2010) 9:11. doi:10.1186/1475-2891-9-11
22. Shikany JM, Thomas AS, Beasley TM, Lewis CE, Allison DB. Randomized controlled trial of the Medifast 5 & 1 Plan for weight loss. Int J Obes (Lond) (2013) 37(12):1571–8. doi:10.1038/ijo.2013.43
23. Rock CL, Flatt SW, Pakiz B, Barkai HS, Heath DD, Krumhar KC. Randomized clinical trial of portion-controlled prepackaged foods to promote weight loss. Obesity (Silver Spring) (2016) 24(6):1230–7. doi:10.1002/oby.21481
24. Evidence Analysis Library. In Overweight/Obese Adults (Aged 19+ Years), What Is the Effect of Consuming Single Serving Portion Sized Meals on Weight Loss? (2016). Available from: http://www.andeal.org/topic.cfm?menu=5311&cat=5386
25. Seagle HM, Strain GW, Makris A, Reeves RS, American Dietetic A. Position of the American Dietetic Association: weight management. J Am Diet Assoc (2009) 109(2):330–46. doi:10.1016/j.jada.2008.11.041
26. Noakes M, Foster PR, Keogh JB, Clifton PM. Meal replacements are as effective as structured weight-loss diets for treating obesity in adults with features of metabolic syndrome. J Nutr (2004) 134(8):1894–9.
27. Metzner CE, Folberth-Vogele A, Bitterlich N, Lemperle M, Schafer S, Alteheld B, et al. Effect of a conventional energy-restricted modified diet with or without meal replacement on weight loss and cardiometabolic risk profile in overweight women. Nutr Metab (Lond) (2011) 8(1):64. doi:10.1186/1743-7075-8-64
28. Wadden TA, Foster GD, Wang J, Pierson RN, Yang MU, Moreland K, et al. Clinical correlates of short- and long-term weight loss. Am J Clin Nutr (1992) 56(1 Suppl):271S–4S.
29. Dhurandhar NV, Blank RC, Schumacher D, Atkinson RL. Initial weight loss as a predictor of response to obesity drugs. Int J Obes Relat Metab Disord (1999) 23(12):1333–6. doi:10.1038/sj.ijo.0801111
30. Fabricatore AN, Wadden TA, Moore RH, Butryn ML, Heymsfield SB, Nguyen AM. Predictors of attrition and weight loss success: results from a randomized controlled trial. Behav Res Ther (2009) 47(8):685–91. doi:10.1016/j.brat.2009.05.004
31. Handjieva-Darlenska T, Handjiev S, Larsen TM, van Baak MA, Jebb S, Papadaki A, et al. Initial weight loss on an 800-kcal diet as a predictor of weight loss success after 8 weeks: the Diogenes study. Eur J Clin Nutr (2010) 64(9):994–9. doi:10.1038/ejcn.2010.110
32. Fujioka K, Plodkowski R, O’Neil PM, Gilder K, Walsh B, Greenway FL. The relationship between early weight loss and weight loss at 1 year with naltrexone ER/bupropion ER combination therapy. Int J Obes (Lond) (2016) 40(9):1369–75. doi:10.1038/ijo.2016.67
33. Warkentin LM, Das D, Majumdar SR, Johnson JA, Padwal RS. The effect of weight loss on health-related quality of life: systematic review and meta-analysis of randomized trials. Obes Rev (2014) 15(3):169–82. doi:10.1111/obr.12113
34. Kroes M, Osei-Assibey G, Baker-Searle R, Huang J. Impact of weight change on quality of life in adults with overweight/obesity in the United States: a systematic review. Curr Med Res Opin (2016) 32(3):485–508. doi:10.1185/03007995.2015.1128403
35. Matarese LE, Pories WJ. Adult weight loss diets: metabolic effects and outcomes. Nutr Clin Pract (2014) 29(6):759–67. doi:10.1177/0884533614550251
36. Saneei P, Salehi-Abargouei A, Esmaillzadeh A, Azadbakht L. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: a systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis (2014) 24(12):1253–61. doi:10.1016/j.numecd.2014.06.008
37. Soltani S, Shirani F, Chitsazi MJ, Salehi-Abargouei A. The effect of dietary approaches to stop hypertension (DASH) diet on weight and body composition in adults: a systematic review and meta-analysis of randomized controlled clinical trials. Obes Rev (2016) 17(5):442–54. doi:10.1111/obr.12391
39. U.S. News & World Report. Best Weight Loss Diets. (2016). Available from: http://health.usnews.com/best-diet/best-weight-loss-diets
Keywords: overweight, obesity, fat mass, body circumference, pre-portioned foods
Citation: Cook CM, McCormick CN, Knowles M and Kaden VN (2017) A Commercially Available Portion-Controlled Diet Program Is More Effective for Weight Loss than a Self-Directed Diet: Results from a Randomized Clinical Trial. Front. Nutr. 4:55. doi: 10.3389/fnut.2017.00055
Received: 26 July 2017; Accepted: 24 October 2017;
Published: 07 November 2017
Edited by:
Tuomas Kilpeläinen, University of Copenhagen, DenmarkReviewed by:
Arash Mirrahimi, University of Toronto, CanadaHassan S. Dashti, Massachusetts General Hospital, United States
Copyright: © 2017 Cook, McCormick, Knowles and Kaden. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chad M. Cook, Y2hhZC5jb29rJiN4MDAwNDA7bXhucy5jb20=
 Chad M. Cook
Chad M. Cook Courtney N. McCormick
Courtney N. McCormick Mandi Knowles
Mandi Knowles Valerie N. Kaden
Valerie N. Kaden