- 1Cedars-Sinai Medical Center, Los Angeles, CA, USA
- 2Clinical Dynamix, Wilmington, NC, USA
- 3University of South Alabama, Mobile, AL, USA
The sphincter of Oddi (SO) is a smooth muscle valve regulating the flow of biliary and pancreatic secretions into the duodenum, initially described in 1887 by the Italian anatomist, Ruggero Oddi. SO dysfunction (SOD) is a broad term referring to numerous biliary, pancreatic, and hepatic disorders resulting from spasms, strictures, and relaxation of this valve at inappropriate times. This review brings attention to various factors that may increase the risk of SOD, including but not limited to: cholecystectomy, opiates, and alcohol. Lack of proper recognition and treatment of SOD may be associated with clinical events, including pancreatitis and biliary symptoms with hepatic enzyme elevation. Pharmacologic and non-pharmacologic approaches are discussed to help recognize, prevent, and treat SOD. Future studies are needed to assess the treatment benefit of agents such as calcium-channel blockers, glyceryl trinitrate, or tricyclic antidepressants in patients with SOD.
Historical Notes and Purpose of the Review
In 1941, Williamson wrote a letter to the British Medical Journal, concerned that “a fair number of people without gallbladders who are potential air raid causalities and to whom … morphine will probably be administered … (would), far from relieving their pain and shock … increase it” (1). The letter was in response to a publication by Smyth, who noted that selected drugs dilate the sphincter of Oddi (SO) while, “contrary to what might be expected,” morphine, codeine, and hydromorphone produced “spasm of the sphincter” (2). This formed the basis for the syndrome later termed SO dysfunction (SOD). The purpose of this paper is to review SO anatomy and physiology, SOD (including subtypes), the effect of cholecystectomy on the SO, and the impact of exogenous compounds on the SO, and to provide an overview of the diagnosis and management of SOD.
Structure and Function of the SO
The SO is a muscular structure surrounding the confluence of the distal common bile duct (CBD) and the pancreatic duct (PD) into the ampulla of Vater (Figure 1) (3, 4). The sphincter structure with overlying mucosa protruding into the duodenum is called the papilla of Vater. At least three functions of the SO have been identified: (1) regulation of bile flow into the duodenum; (2) prevention of duodenal reflux; and (3) regulation of gallbladder filling by diverting bile into the gallbladder with SO closure.
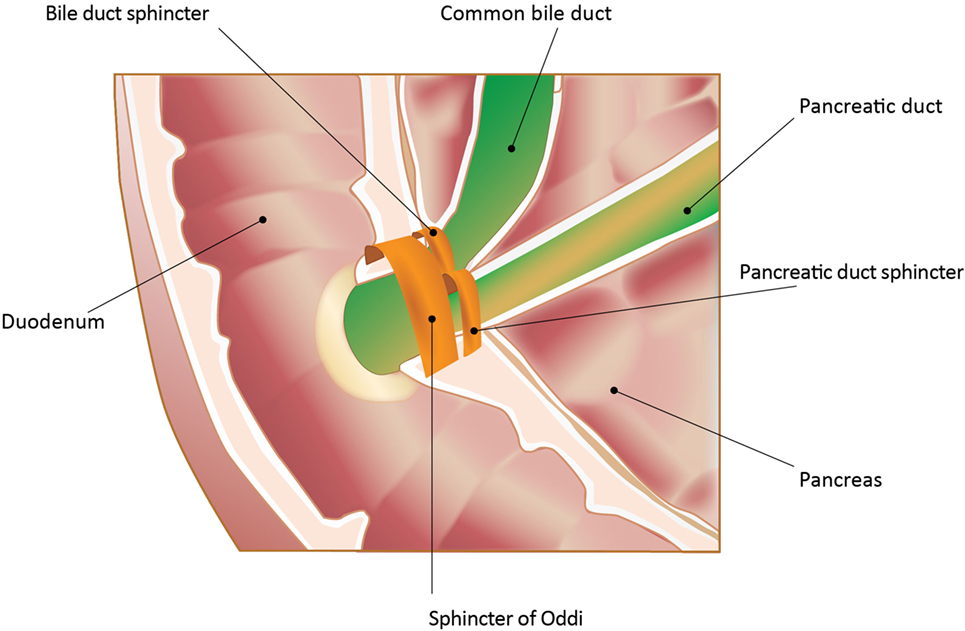
Figure 1. Sphincter of Oddi (SO) and its anatomic relationships. In the majority of patients, pancreatic and biliary secretions enter the duodenum through the SO. The sphincter structure with overlying mucosa protruding into the duodenum is called the papilla of Vater. The image is an endoscopic photograph of the duodenum at this entry site. In addition, there are individual sphincters for both the common bile duct and the pancreatic duct proximal to the SO. All the sphincters are neurohumorally regulated. Adapted from Gorelick et al. (5). Used with permission. Copyright, American Gastroenterological Association Institute, Bethesda, MD, USA.
The physiology of the SO has been studied in both animals and humans. While varying species have been studied, dog, cat, and pig physiology are most similar to humans. It is critical to consider species studied when analyzing pharmacological data (see Medications and the Risk of SOD). In humans, basal pressure of the SO is 10 mmHg. Superimposed anterograde phasic contractions, initiated at the junction of the CBD and the SO and progressing into the duodenum, occur in response to physiologic and exogenous stimuli and result in evacuation of contents already present within the SO into the duodenum. During contraction, no additional flow from the CBD into the SO occurs. The SO then relaxes, allowing passive refilling of bile into the SO segment. Once filled, another wave of phasic contractions begins (6, 7). When basal pressure increases, resistance to flow increases, resulting in gallbladder filling and prevention of flow into the duodenum. When basal pressure decreases below CBD and PD pressures, flow into the duodenum occurs.
Sphincter of Oddi motility patterns have been defined for both the inter-digestive period and the digestive period. During the inter-digestive period, the SO has a cyclical activity pattern throughout all phases and the frequency increases prior to phase 3 duodenal activity, prompting discharge of bile and pancreatic secretions into the duodenum (8–11). Motility during the digestive period involves both neural and hormonal inputs. In this period, there are gallbladder contractions, stimulation of pancreatic secretion, and SO relaxation, leading to high rates of bile and pancreatic secretion into the duodenum. Of note, during the digestive period, there are different phases of the meal, each providing input. For example, up to 30–40% of gallbladder emptying and 25% of pancreatic secretion occurs during the cephalic phase via vagal inputs, while another 10–20% of the response occurs during the gastric phase via vagovagal pathways. However, the gallbladder empties most of its remaining contents and the pancreas up to 50% of its total secretion during the intestinal phase, induced by the release of cholecystokinin (CCK) and secretin from the duodenum and proximal jejunum. Duodenal CCK contracts the gallbladder, relaxes the SO, and causes pancreatic exocrine digestive enzyme secretion via direct actions on CCK receptors and indirectly through cholinergic neurons. This is supported by the observation that atropine pretreatment blocks gallbladder contraction and pancreatic secretion induced by physiological concentrations of CCK and by a protein-fatty meal (12–15).
Hormones and Neurotransmitters Involved in SO Function
The most important hormone involved in SO function is CCK. CCK released from the enteroendocrine cells in response to a meal exerts direct hormonal effects as well as indirect effects by interacting with neural pathways, leading to gallbladder contraction and pancreatic enzyme secretion. CCK decreases SO basal pressures and inhibits phasic contractions, thereby promoting anterograde flow (16, 17). Vasoactive intestinal polypeptide and nitric oxide, present in the intrinsic neurons of the SO, are involved in the relaxation response to CCK as well as the relaxation observed in the cephalic phase of the meal (18, 19).
Motilin, somatostatin, and octreotide hormonally influence SO function. Motilin, secreted by the M cells within the duodenum and jejunum, induces contraction of the smooth muscle of the gallbladder and stimulates bile secretion (20–22). Somatostatin, present in endocrine cells throughout the gastrointestinal tract, likely exerts inhibitory effects on both gallbladder contraction and relaxation of the SO (23), supported by the observation that 50 μg intravenous octreotide, an agent that mimics somatostatin, causes a significant increase in SO basal pressure and frequency of phasic contractions (24).
What is SOD?
Sphincter of Oddi dysfunction is a clinical syndrome caused by SO dyskinesia (functional) or anatomic (mechanical) obstruction associated with abdominal pain and elevation of liver or pancreatic enzymes, CBD or PD dilation, or pancreatitis (25). Of note, the term SO dyskinesia more specifically denotes motility disorders of the SO, while SOD includes both mechanical obstruction and SO dyskinesia. In this context, the term biliary dyskinesia has historically been used as a general term that included both SO dyskinesia and gallbladder dyskinesia (26). Since the availability of scintigraphy, a functional gallbladder disorder (i.e., gallbladder dyskinesia/dysfunction) is now recognized as a discrete entity and should be distinguished from SO dyskinesia (27–29). The various forms of primary SOD are considered functional gastrointestinal disorders and may occur in adults or children of any age, but SOD is most commonly encountered in females aged 20–50 years (30–32). The estimated prevalence of SOD is 1.5% in the general population and may be as high as 72% in patients with idiopathic recurrent pancreatitis based on small cohort studies (30, 32–35). However, its true prevalence is difficult to determine due to the lack of definitive biomarkers or diagnostic criteria as well as the multitude of secondary causes of SOD, such as fibrosis of the sphincteric channel (papillary stenosis and sclerosing papillitis) or obstructive carcinoma.
Clinical Presentations of SOD
Sphincter of Oddi dysfunction can involve the biliary sphincter, the pancreatic sphincter, or both (25). Biliary SOD typically presents with recurrent biliary pain, characterized as disabling epigastric or right upper quadrant pain lasting 30 min to several hours with or without hepatic enzyme elevation. It may radiate to the back, shoulder, or scapula and may be accompanied by nausea and vomiting, mimicking a gallbladder attack. Pain is not consistently postprandial and is not relieved by postural changes, antacids, or bowel movements.
Pancreatic SOD is thought to be responsible for a portion of patients with recurrent episodes of acute pancreatitis. Patients will have mid-abdominal, pancreatic pain, radiating to the back, associated with elevations in serum amylase and lipase. Symptoms involving the pancreatic sphincter are frequently exacerbated by food intake. No other causes for pancreatitis are usually found in these patients, and they may be classified as having idiopathic acute recurrent pancreatitis (IARP) (36, 37). Toouli and colleagues compared the SO manometric pressures in 28 patients with IARP with those of 10 controls and found that more than 57% of patients with IARP had elevated SO pressures (38). However, the true incidence of pancreatitis caused by SOD is unknown. When both the pancreas and biliary sphincters are involved, the abdominal pain may be more diffuse and both hepatic and pancreatic enzyme elevation can occur.
Diagnosis of SOD
The diagnosis of SOD is challenging, but history, physical exam, relevant labs, and imaging studies are critical. Some view SOD as a structural abnormality while others view it as a functional disorder. A classification system for SOD as a structural abnormality was established in 1988. Initially known as the Hogan–Geenan SOD classification and later modified as the Milwaukee classification, it classifies SOD patients into types 1, 2, and 3 based on clinical presentation as well as laboratory and/or imaging abnormalities (see Table 1 for biliary SOD and Table 2 for pancreatic SOD) (26, 39). Table 3 lists the Rome III criteria for SOD as a functional disorder (25). These criteria were meant to make the diagnostic evaluation more applicable to clinical practice and, whenever possible, avoid invasive procedures by emphasizing non-invasive imaging of CBD diameter. Earlier studies showed higher rates of depression, obsessive compulsive disorders, and anxiety in patients with type 3 SOD when compared with controls (40). Conversely, a randomized, controlled trial of SOD type 3 patients showed that psychosocial disability in patients with severe symptoms may not be different than in the general population (41).

Table 1. Milwaukee classification for biliary SOD (39).
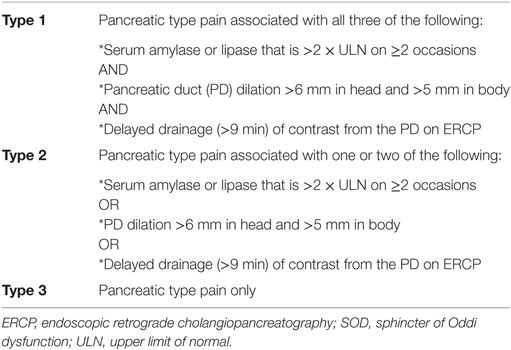
Table 2. Milwaukee classification for pancreatic SOD (39).
Other Evaluations for SOD
In the past, non-invasive testing to diagnose SOD included quantitative hepatobiliary scintigraphy to assess biliary flow (42–44), endoscopic ultrasound, or magnetic resonance cholangiopancreatography with secretin injection (45). However, these tests are neither sensitive nor specific for SOD.
Manometry of the SO is considered the gold-standard test to diagnose SOD (46, 47). During SO manometry, a catheter is inserted into the duodenum and calibrated to 0 mmHg. Next, the catheter is inserted into the CBD and/or PD for 30 s; basal pressures ≥40 mmHg indicate SOD (48). Prior to the procedure, patients should avoid agents that inhibit SO function, such as anticholinergics, nitrates, and calcium-channel blockers, and those that stimulate it, such as opiates and cholinergics. There are limitations to SO manometry: first, it requires skilled endoscopists with special equipment not readily available at most institutions; second, it is associated with up to 30% increased risk of iatrogenic pancreatitis (49); and finally, isolated point in time pressure measurements obtained during SO manometry may not reflect the dynamic nature of the SO, leading to difficulties in applying the results. Therefore, the use of SO manometry as a gold-standard test remains controversial. Furthermore, isolated basal pressures cannot differentiate between SO motor disturbances and anatomical stenosis. Of note, manometry is not confirmatory in 13–40% of patients ultimately diagnosed with type 1 SOD (50–53).
Non-Pharmacologic Risk Factors for SOD
Certain populations, such as patients who have undergone cholecystectomy (32), are predisposed to SOD (31). In subjects with an intact gallbladder, CCK inhibits SO phasic wave activity, but 6 months after cholecystectomy, CCK fails to inhibit this activity (54). Sherman reported that 10–20% of postcholecystectomy patients experience biliary colic and a review of patients with postcholecystectomy pain found that 9–51% met diagnostic criteria for SOD after cholecystectomy (31). Overall, ~1.5% of patients develop SOD after cholecystectomy (25, 55).
It is postulated that the gallbladder acts as a backflow reservoir for bile to dampen sudden increases in pressure resulting from physiologic or extra-physiologic ductal obstruction (56–59). Luman and colleagues demonstrated that patients with postcholecystectomy syndrome had elevated basal SO pressure, retrograde phasic wave contraction, and an increase in phasic wave frequency greater than seven contractions per minute (tachyoddia) (60). It is unclear if postcholecystectomy patients are susceptible to developing SOD because of elevated pressures, altered SO motility, or both.
Sphincter of Oddi dysfunction has also been linked to agenesis of the gallbladder (61), preoperative cholelithiasis (25), gallstone lithotripsy (62), liver transplantation (63), and alcoholism (64). Delayed emptying of the biliary tract related to hypothyroidism suggests another risk factor for SOD (65, 66). Additionally, subjects with hypothyroidism have an increased risk of gallstones, thought secondary to the absence of thyroxine’s relaxing effect on the SO (67).
Patients with irritable bowel syndrome (IBS) may be at an increased risk of SOD. Evans and colleagues reported that patients with IBS who undergo cholecystectomy are more likely to demonstrate a blunted response to sphincter-relaxing properties of CCK compared with postcholecystectomy patients without IBS (68). The prevalence and incidence of SOD among IBS patients is unclear because of the difficulty in diagnosing SOD in IBS patients due to overlap of symptoms (69).
Sphincter of Oddi dyskinesia occurs more frequently in women than in men, and animal models offer some insight. In the prairie dog, CCK increases SO phasic wave frequency in both sexes, but amplitude increases were significantly greater in females than in males (70). Further support for gender differences was shown by estrogen suppression of SO wave frequency in prairie dogs, while progesterone’s effect on the SO is unclear (71).
Medications and the Risk of SOD
Exogenous agents play an additive role in populations at risk for SOD. Opiates are known to alter flow through the SO. In the absence of a gallbladder, morphine, meperidine, and pentazocine increase biliary pressure in opossums (56). Behar and Biancani found that leucine and methionine-enkephalin caused an initial contraction followed by a prolonged relaxation of the cat SO, suggesting that endogenous delta opioid agonism is involved in increasing flow through the sphincter (72, 73). Importantly, the mu antagonist naloxone had no effect on morphine-induced increases in basal SO pressure but did diminish morphine’s effect on SO phasic wave frequency and amplitude. The naloxone inhibitory effect suggests that the mu opioid receptor is involved, while the absence of naloxone antagonism on SO basal pressure may be non-mu opioid receptor mediated.
Thus, morphine increases the amplitude and frequency of the phasic wave (via mu opioid receptors) as well as basal pressure (via non-mu opioid receptors) of the SO (74, 75). These effects have also been demonstrated with fentanyl (76) and codeine (77). Morphine shows limited effect on the SO in patients prior to cholecystectomy, whereas it caused a notable rise in basal sphincter pressure postoperatively (59). Since morphine can precipitate abdominal pain not related to the SO, Holtzer and Hulst proposed a “morphine-enzyme-pain” provocation test: pain accompanied by at least a doubling of the alanine aminotransferase 8 h after administration indicated SO dyskinesia (75). The magnitude of transaminase elevation associated with morphine has been reported as high as 65 times above normal in patients without a gallbladder (78). Mousavi and colleagues demonstrated that chronic opiates induce SOD compared with case controls (79), and several authors have documented asymptomatic, dilated CBDs in patients addicted to opiates (80–82).
The effects of tramadol, buprenorphine, pentazocine, and pethidine have been evaluated with SO manometry. Pentazocine increased the duration of sphincter contraction and ductal pressure while tramadol, buprenorphine, and pethidine did not (83, 84). Meperidine was shown to increase the pancreatic component, biliary component, and SO phasic frequency and to decrease phasic duration in 3/47 patients studied with manometry (85). Eluxadoline, a mixed opioid receptor modulator with mu and kappa opioid receptor agonist effects and delta opioid receptor antagonist effects that was recently approved by the FDA for IBS with diarrhea, was linked to a small number of non-serious cases of SO spasm and pancreatitis in the phase 3 studies of this medication (86). Among 1,619 patients exposed to eluxadoline in these trials, 8 (0.49%) developed SO spasm and 5 (0.31%) developed pancreatitis, 1 case of which was attributed to SO spasm. Importantly, all cases of eluxadoline-associated SO spasm occurred in patients who did not have gallbladders and were more common with 100 mg twice daily (BID) compared with 75 mg BID. One case of pancreatitis was associated with biliary sludge while the other three were associated with heavy alcohol use.
Opiates have been reported to incite pancreatitis, and their effects on the SO represent the most likely etiology (87). The mu antagonist naloxone reduces the severity of pancreatitis induced by intraductal injection of trypsin–bile mixture in dogs. In the opossum, Chen and colleagues induced pancreatitis when they combined simulated SOD (by PD ligation mimicking the opiate effect) with pharmacologically stimulated pancreatic secretion (88). In humans, drug rechallenge (89, 90) with heroin (91, 92), codeine (93–95), tapentadol,1 and loperamide (96–99) have established a link with these drugs and acute pancreatitis. Loperamide inhibits the normal contractile response of the gallbladder to CCK (100) and, in patients with short bowel syndrome, reduces pancreatic and biliary output (101). The mechanism of opiate-induced pancreatitis is assumed to be related to their action on the SO; some suggest that loperamide’s effect may be different as it is structurally more related to haloperidol (102) than to morphine.
Therapy for SOD
Certain exogenous agents relax the SO, reducing its pressure and resistance. This includes calcium-channel blockers (103, 104), tricyclic antidepressants (105), Botox®, glyceryl trinitrate (GTN), and somatostatin. Nifedipine has been shown to reverse opiate-induced effects on the SO (106) and improve pain associated with SOD in a short-term study (103). Injection of Botox® into the SO via sclerotherapy needle reduced sphincter pressure by 50% for 4 months and was followed by 50% improvement in bile flow in two patients with postcholecystectomy pain and elevated SO pressures (107). GTN has been used to assist removal of lodged CBD stones without endoscopic papillary dilatation or endoscopic papillotomy (108) and decreased both basal SO pressure as well as the amplitude and frequency of SO phasic wave contractions in a non-randomized, controlled clinical trial (108). Intravenous somatostatin was shown to reduce mean SO (109) basal pressures in patients with acute alcoholic pancreatitis (64).
One prospective study of patients with biliary SOD (defined by clinical and laboratory data) evaluated the combination of a low-dose tricyclic antidepressants, nifedipine, and GTN. If there was no improvement after 3–6 months, patients were offered biliary sphincterotomy. Fifty-one percent of the patients (76% with type 3) had symptomatic resolution or improvement on medical therapy alone, 12% had symptomatic improvement or resolution with sphincterotomy, and 10% had improvement with both medical therapy and sphincterotomy (110). Although promising, opiates were allowed during this study, confounding the determination of symptom improvement due solely to interventions.
The most commonly used non-pharmacologic treatment for SOD has been endoscopic sphincterotomy for patients with types 1 and 2 biliary SOD and pancreatic SOD. Pain relief has been shown in 90% of patients with type 1 biliary SOD and 70% of patients with type 2. However, it is not effective, and may be harmful, in patients with type 3. The evaluating predictors and interventions in sphincter of Oddi dysfunction trial was a landmark study for treatment of type 3. This was a multicenter, sham-controlled, randomized trial in patients with pain after cholecystectomy, without abnormalities on imaging or laboratory studies, and no prior SO treatment. Participants underwent sphincterotomy or sham sphincterotomy for abdominal pain. The investigators concluded that performing a sphincterotomy in patients with type 3 SOD was ineffective (111). As a result, endoscopists are shifting away from performing sphincterotomy in these patients.
There are limited studies evaluating the role of PD stenting and sphincterotomy in patients with pancreatic SOD. Jacob and colleagues found a significant reduction in the incidence of recurrent acute pancreatitis in those who were stented (112). Coté and colleagues evaluated the role of endoscopic dual (biliary and pancreatic) sphincterotomy vs. biliary sphincterotomy alone in patients with IARP and found that both types of sphincterotomies had similar effects in preventing recurrence of acute pancreatitis (113). Another study found no difference in preventing recurrent pancreatitis when dual sphincterotomy was compared with either pancreatic or biliary sphincterotomy (114).
Conclusion
Sphincter of Oddi dysfunction denotes impaired fluid flow through the SO, either by a fixed stenosis or disordered muscular control (dyskinesia). Gallbladder function appears to play a critical role in SO mechanics, and patients without a gallbladder are more likely to experience SOD. Other potential contributing factors include female gender, hypothyroidism, IBS, prior pancreatitis, and exogenous medications. The data supporting a link between opiates and SOD are clear and reproducible, and the resulting clinical syndromes, especially in postcholecystectomy patients, include abdominal pain with sudden, yet reversible, elevations in liver enzymes as well as acute pancreatitis. Different opiate agents appear to have varying effects on SO basal and phasic contractions. While exogenous mu opioid agonists negatively affect flow through the SO, endogenous enkephalins (possibly delta agonists) may improve flow through the sphincter.
While there is some evidence that nifedipine, Botox®, and GTN may improve flow through the SO and mitigate SOD symptoms, the efficacy of these agents remains to be proven in sufficiently large, randomized, and controlled trials. Endoscopic sphincterotomy remains the treatment of choice for select patients confidently diagnosed with SOD. However, increased awareness by caregivers of risk factors for SOD provides opportunities for diagnosis and intervention, including avoidance of potential precipitating agents, especially in the absence of a gallbladder.
Author Contributions
EA, SP, and PC were involved in the initial drafting of the manuscript. EA, SL, PC, BC, and SP were involved in the drafting and revision of the manuscript. All authors approved the final draft of this manuscript for submission. SP is the guarantor of the article.
Conflict of Interest Statement
PC serves as a scientific consultant for Allergan plc. BC has served as an advisor, consultant, or speaker for Actavis, Inc., a subsidiary of Allergan plc, Salix Pharmaceuticals, Takeda Pharmaceuticals, Prometheus Laboratories, IM HealthScience, and Ironwood Pharmaceuticals. SP serves as a member of the VIBERZI Response Team for Allergan plc, monitoring use and adverse responses, and is the Specialty Chief Editor for the Gastrointestinal Sciences section of Frontiers. EA and SL have no relevant disclosures to report.
Acknowledgments
Writing and finalization of the manuscript content was performed exclusively by the authors. PC has previously acted as a consultant to Allergan plc and requested assistance in finalization of the reference list, formatting the manuscript, and support of submission. Allergan has a contract with Complete HealthVizion, Inc., Chicago, IL, USA for publication activities and paid for Complete HealthVizion to provide this logistical assistance to the authors.
Funding
This work was supported by NIH grants P50 AA011999, P01 DK098108, and NCI/DCP NWU2014-04-0; Department of Veterans Affairs; and Department of Defense PR140717P2.
Footnote
References
1. Williamson JB. Effect of morphine after cholecystectomy. Br Med J (1941) 1:215. doi:10.1136/bmj.1.4179.215-a
2. Smyth MJ. Exploration of the common bile duct for stone. Drainage with T-tube and cholangiography. Br Med J (1941) 1:111–26. doi:10.1136/bmj.1.4177.111
3. Eichhorn EP Jr, Boyden EA. The choledochoduodenal junction in the dog; a restudy of Oddi’s sphincter. Am J Anat (1955) 97:431–59. doi:10.1002/aja.1000970305
4. Leung WD, Sherman S. Endoscopic approach to the patient with motility disorders of the bile duct and sphincter of Oddi. Gastrointest Endosc Clin N Am (2013) 23:405–34. doi:10.1016/j.giec.2012.12.006
5. Gorelick F, Pandol SJ, Topazian M. Pancreatic Physiology, Pathophysiology, Acute and Chronic Pancreatitis. Bethesda, MD: Gastrointestinal Teaching Project, American Gastroenterological Association Institute (2003).
6. Carr-Locke DL, Gregg JA. Endoscopic manometry of pancreatic and biliary sphincter zones in man. Basal results in healthy volunteers. Dig Dis Sci (1981) 26:7–15. doi:10.1007/BF01307970
7. Csendes A, Kruse A, Funch-Jensen P, Oster MJ, Ornsholt J, Amdrup E. Pressure measurements in the biliary and pancreatic duct systems in controls and in patients with gallstones, previous cholecystectomy, or common bile duct stones. Gastroenterology (1979) 77:1203–10.
8. Hauge CW, Mark JB. Common bile duct motility and sphincter mechanism. I. Pressure measurements with multiple-lumen catheter in dogs. Ann Surg (1965) 162:1028–38. doi:10.1097/00000658-196512000-00009
9. Hedner P, Rorsman G. On the mechanism of action for the effect of cholecystokinin on the choledochoduodenal junction in the cat. Acta Physiol Scand (1969) 76:248–54. doi:10.1111/j.1748-1716.1969.tb04467.x
10. LaMorte WW, Gaca JM, Wise WE, Birkett DH, Williams LF Jr. Choledochal sphincter relaxation in response to histamine in the primate. J Surg Res (1980) 28:373–8. doi:10.1016/0022-4804(80)90098-0
11. Ono K. The discharge of bile into the duodenum and electrical activities of the muscle of Oddi and duodenum. Nihon Heikatsukin Gakkai Zasshi (1970) 6:123–8.
12. Pandol SJ, Raybould HE, Yee HF. Integrative responses of the gastrointestinal tract and liver to a meal. 4th ed. In: Yamada T, editor. Textbook of Gastroenterology. (Vol. 1), Oxford, UK: Wiley-Blackwell (2009). p. 3–14.
14. Pandol SJ. Pancreatic secretion. In: Feldman M, Friedman L, Brandt L, editors. Gastrointestinal and Liver Disease. Philadelphia, PA: Saunders Elsevier (2015). p. 934–43.
15. Pandol SJ. Normal Pancreatic Function. San Rafael, CA: Pancreapedia: Exocrine Pancreas Knowledge Base (2015).
16. Behar J, Biancani P. Effect of cholecystokinin and the octapeptide of cholecystokinin on the feline sphincter of Oddi and gallbladder. Mechanisms of action. J Clin Invest (1980) 66:1231–9. doi:10.1172/JCI109974
17. Toouli J, Hogan WJ, Geenen JE, Dodds WJ, Arndorfer RC. Action of cholecystokinin-octapeptide on sphincter of Oddi basal pressure and phasic wave activity in humans. Surgery (1982) 92:497–503.
18. Pálvölgyi A, Sári R, Németh J, Szabolcs A, Nagy I, Hegyi P, et al. Interplay between nitric oxide and VIP in CCK-8-induced phasic contractile activity in the rabbit sphincter of Oddi. World J Gastroenterol (2005) 11:3264–6. doi:10.3748/wjg.v11.i21.3264
19. Zhang ZH, Qin CK, Wu SD, Xu J, Cui XP, Wang ZY, et al. Roles of sphincter of Oddi motility and serum vasoactive intestinal peptide, gastrin and cholecystokinin octapeptide. World J Gastroenterol (2014) 20:4730–6. doi:10.3748/wjg.v20.i16.4730
20. Behar J, Biancani P. Effects and mechanisms of action of motilin on the cat sphincter of Oddi. Gastroenterology (1988) 95:1099–105. doi:10.1016/0016-5085(88)90188-6
21. Muller EL, Grace PA, Conter RL, Roslyn JJ, Pitt HA. Influence of motilin and cholecystokinin on sphincter of Oddi and duodenal mobility. Am J Physiol (1987) 253:G679–83.
22. Saccone GT, Liu YF, Thune A, Harvey JR, Baker RA, Toouli J. Erythromycin and motilin stimulate sphincter of Oddi motility and inhibit trans-sphincteric flow in the Australian possum. Naunyn Schmiedebergs Arch Pharmacol (1992) 346:701–6. doi:10.1007/BF00168745
23. Ito T, Igarashi H, Jensen RT. Pancreatic neuroendocrine tumors: clinical features, diagnosis and medical treatment: advances. Best Pract Res Clin Gastroenterol (2012) 26:737–53. doi:10.1016/j.bpg.2012.12.003
24. Binmoeller KF, Dumas R, Harris AG, Delmont JP. Effect of somatostatin analog octreotide on human sphincter of Oddi. Dig Dis Sci (1992) 37:773–7. doi:10.1007/BF01296438
25. Behar J, Corazziari E, Guelrud M, Hogan W, Sherman S, Toouli J. Functional gallbladder and sphincter of Oddi disorders. Gastroenterology (2006) 130:1498–509. doi:10.1053/j.gastro.2005.11.063
26. Hogan WJ, Geenen JE. Biliary dyskinesia. Endoscopy (1988) 20(Suppl 1):179–83. doi:10.1055/s-2007-1018172
27. Adams DB. Biliary dyskinesia: does it exist? If so, how do we diagnose it? Is laparoscopic cholecystectomy effective or a sham operation? J Gastrointest Surg (2013) 17:1550–2. doi:10.1007/s11605-013-2267-5
28. Bielefeldt K, Saligram S, Zickmund SL, Dudekula A, Olyaee M, Yadav D. Cholecystectomy for biliary dyskinesia: how did we get there? Dig Dis Sci (2014) 59:2850–63. doi:10.1007/s10620-014-3342-9
29. Veenstra BR, Deal RA, Redondo RE, Daly SC, Najman J, Myers JA, et al. Long-term efficacy of laparoscopic cholecystectomy for the treatment of biliary dyskinesia. Am J Surg (2014) 207:366–70. doi:10.1016/j.amjsurg.2013.09.012
30. George J, Baillie J. Biliary and gallbladder dyskinesia. Curr Treat Options Gastroenterol (2007) 10:322–7. doi:10.1007/s11938-007-0075-2
31. Sherman S, Lehman GA. Sphincter of Oddi dysfunction: diagnosis and treatment. JOP (2001) 2:382–400.
33. Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci (1993) 38:1569–80. doi:10.1007/BF01303162
34. Eversman D, Fogel EL, Rusche M, Sherman S, Lehman GA. Frequency of abnormal pancreatic and biliary sphincter manometry compared with clinical suspicion of sphincter of Oddi dysfunction. Gastrointest Endosc (1999) 50:637–41. doi:10.1016/S0016-5107(99)80011-X
35. Kaw M, Brodmerkel GJ Jr. ERCP, biliary crystal analysis, and sphincter of Oddi manometry in idiopathic recurrent pancreatitis. Gastrointest Endosc (2002) 55:157–62. doi:10.1067/mge.2002.118944
36. Coyle WJ, Pineau BC, Tarnasky PR, Knapple WL, Aabakken L, Hoffman BJ, et al. Evaluation of unexplained acute and acute recurrent pancreatitis using endoscopic retrograde cholangiopancreatography, sphincter of Oddi manometry and endoscopic ultrasound. Endoscopy (2002) 34:617–23. doi:10.1055/s-2002-33245
37. Geenen JE, Nash JA. The role of sphincter of Oddi manometry and biliary microscopy in evaluating idiopathic recurrent pancreatitis. Endoscopy (1998) 30:A237–41. doi:10.1055/s-2007-1001447
38. Toouli J, Roberts-Thomson IC, Dent J, Lee J. Sphincter of Oddi motility disorders in patients with idiopathic recurrent pancreatitis. Br J Surg (1985) 72:859–63. doi:10.1002/bjs.1800721104
39. Silverman WB, Slivka A, Rabinovitz M, Wilson J. Hybrid classification of sphincter of Oddi dysfunction based on simplified Milwaukee criteria: effect of marginal serum liver and pancreas test elevations. Dig Dis Sci (2001) 46:278–81. doi:10.1023/A:1005692530034
40. Desautels SG, Slivka A, Hutson WR, Chun A, Mitrani C, DiLorenzo C, et al. Postcholecystectomy pain syndrome: pathophysiology of abdominal pain in sphincter of Oddi type III. Gastroenterology (1999) 116:900–5. doi:10.1016/S0016-5085(99)70073-9
41. Brawman-Mintzer O, Durkalski V, Wu Q, Romagnuolo J, Fogel E, Tarnasky P, et al. Psychosocial characteristics and pain burden of patients with suspected sphincter of Oddi dysfunction in the EPISOD multicenter trial. Am J Gastroenterol (2014) 109:436–42. doi:10.1038/ajg.2013.467
42. Darweesh RM, Dodds WJ, Hogan WJ, Geenen JE, Collier BD, Shaker R, et al. Efficacy of quantitative hepatobiliary scintigraphy and fatty-meal sonography for evaluating patients with suspected partial common duct obstruction. Gastroenterology (1988) 94:779–86. doi:10.1016/0016-5085(88)90254-5
43. Roberts-Thomson IC, Toouli J, Blanchett W, Lichtenstein M, Andrews JT. Assessment of bile flow by radioscintigraphy in patients with biliary-type pain after cholecystectomy. Aust N Z J Med (1986) 16:788–93. doi:10.1111/j.1445-5994.1986.tb00038.x
44. Zeman RK, Burrell MI, Dobbins J, Jaffe MH, Choyke PL. Postcholecystectomy syndrome: evaluation using biliary scintigraphy and endoscopic retrograde cholangiopancreatography. Radiology (1985) 156:787–92. doi:10.1148/radiology.156.3.4023244
45. Bolondi L, Gaiani S, Gullo L, Labò G. Secretin administration induces a dilatation of main pancreatic duct. Dig Dis Sci (1984) 29:802–8. doi:10.1007/BF01318422
46. Smithline A, Hawes R, Lehman G. Sphincter of Oddi manometry: interobserver variability. Gastrointest Endosc (1993) 39:486–91. doi:10.1016/S0016-5107(93)70156-X
47. Thune A, Scicchitano J, Roberts-Thomson I, Toouli J. Reproducibility of endoscopic sphincter of Oddi manometry. Dig Dis Sci (1991) 36:1401–5. doi:10.1007/BF01296806
48. Toouli J. Sphincter of Oddi: function, dysfunction, and its management. J Gastroenterol Hepatol (2009) 24(Suppl 3):S57–62. doi:10.1111/j.1440-1746.2009.06072.x
49. Freeman ML, DiSario JA, Nelson DB, Fennerty MB, Lee JG, Bjorkman DJ, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc (2001) 54:425–34. doi:10.1067/mge.2001.117550
50. Meshkinpour H, Mollot M. Sphincter of Oddi dysfunction and unexplained abdominal pain: clinical and manometric study. Dig Dis Sci (1992) 37:257–61. doi:10.1007/BF01308180
51. Rolny P, Geenen JE, Hogan WJ. Post-cholecystectomy patients with “objective signs” of partial bile outflow obstruction: clinical characteristics, sphincter of Oddi manometry findings, and results of therapy. Gastrointest Endosc (1993) 39:778–81. doi:10.1016/S0016-5107(93)70264-3
52. Sherman S, Troiano FP, Hawes RH, O’Connor KW, Lehman GA. Frequency of abnormal sphincter of Oddi manometry compared with the clinical suspicion of sphincter of Oddi dysfunction. Am J Gastroenterol (1991) 86:586–90.
53. Toouli J, Roberts-Thomson IC, Kellow J, Dowsett J, Saccone GT, Evans P, et al. Manometry based randomised trial of endoscopic sphincterotomy for sphincter of Oddi dysfunction. Gut (2000) 46:98–102. doi:10.1136/gut.46.1.98
54. Devereaux BM, Sherman S, Lehman GA. Sphincter of Oddi (pancreatic) hypertension and recurrent pancreatitis. Curr Gastroenterol Rep (2002) 4:153–9. doi:10.1007/s11894-002-0053-8
55. Corazziari E, Shaffer EA, Hogan WJ, Sherman S, Toouli J. Functional disorders of the biliary tract and pancreas. Gut (1999) 45(Suppl 2):II48–54. doi:10.1136/gut.45.2008.ii48
56. Coelho JC, Senninger N, Runkel N, Herfarth C, Messmer K. Effect of analgesic drugs on the electromyographic activity of the gastrointestinal tract and sphincter of Oddi and on biliary pressure. Ann Surg (1986) 204:53–8. doi:10.1097/00000658-198607000-00007
57. Ginsberg AL. Very high levels of SGOT and LDH in patients with extrahepatic biliary tract obstruction. Am J Dig Dis (1970) 15:803–7. doi:10.1007/BF02236040
58. McGowan JM, Butsch WL, Walters W. Pressure in the common bile duct of man: its relation to pain following cholecystectomy. JAMA (1936) 106:2227–30. doi:10.1001/jama.1936.02770260021006
59. Tanaka M, Ikeda S, Nakayama F. Change in bile duct pressure responses after cholecystectomy: loss of gallbladder as a pressure reservoir. Gastroenterology (1984) 87:1154–9.
60. Luman W, Williams AJK, Pryde A, Smith GD, Nixon SJ, Heading RC, et al. Influence of cholecystectomy on sphincter of Oddi motility. Gut (1997) 41:371–4. doi:10.1136/gut.41.3.371
61. Peloponissios N, Gillet M, Cavin R, Halkic N. Agenesis of the gallbladder: a dangerously misdiagnosed malformation. World J Gastroenterol (2005) 11:6228–31. doi:10.3748/wjg.v11.i39.6228
62. Wehrmann T, Lembcke B, Caspary WF, Seifert H. Sphincter of Oddi dysfunction after successful gallstone lithotripsy (postlithotripsy syndrome): manometric data and results of endoscopic sphincterotomy. Dig Dis Sci (1999) 44:2244–50. doi:10.1023/A:1026652619959
63. Clavien PA, Camargo CA Jr, Baillie J, Fitz JG. Sphincter of Oddi dysfunction after liver transplantation. Dig Dis Sci (1995) 40:73–4. doi:10.1007/BF02063944
64. Lai KH, Lo GH, Cheng JS, Fu MT, Wang EM, Chan HH, et al. Effect of somatostatin on the sphincter of Oddi in patients with acute non-biliary pancreatitis. Gut (2001) 49:843–6. doi:10.1136/gut.49.6.843
65. Inkinen J, Sand J, Arvola P, Porsti I, Nordback I. Direct effect of thyroxine on pig sphincter of Oddi contractility. Dig Dis Sci (2001) 46:182–6. doi:10.1023/A:1005674211976
66. Laukkarinen J, Sand J, Saaristo R, Salmi J, Turjanmaa V, Vehkalahti P, et al. Is bile flow reduced in patients with hypothyroidism? Surgery (2003) 133:288–93. doi:10.1067/msy.2003.77
67. Völzke H, Robinson DM, John U. Association between thyroid function and gallstone disease. World J Gastroenterol (2005) 11:5530–4. doi:10.3748/wjg.v11.i35.5530
68. Evans PR, Dowsett JF, Bak YT, Chan YK, Kellow JE. Abnormal sphincter of Oddi response to cholecystokinin in postcholecystectomy syndrome patients with irritable bowel syndrome. The irritable sphincter. Dig Dis Sci (1995) 40:1149–56. doi:10.1007/BF02064214
69. Bistritz L, Bain VG. Sphincter of Oddi dysfunction: managing the patient with chronic biliary pain. World J Gastroenterol (2006) 12:3793–802. doi:10.3748/wjg.v12.i24.3793
70. Tierney S, Qian Z, Yung B, Lipsett PA, Pitt HA, Sostre S, et al. Gender influences sphincter of Oddi response to cholecystokinin in the prairie dog. Am J Physiol (1995) 269:G476–80.
71. Tierney S, Qian Z, Burrow C, Lipsett PA, Pitt HA, Lillemoe KD. Estrogen inhibits sphincter of Oddi motility. J Surg Res (1994) 57:69–73. doi:10.1006/jsre.1994.1112
72. Behar J, Biancani P. Neural control of the sphincter of Oddi. Physiologic role of enkephalins on the regulation of basal sphincter of Oddi motor activity in the cat. Gastroenterology (1984) 86:134–41.
73. Behar J. Physiology and pathophysiology of the biliary tract: the gallbladder and sphincter of Oddi – a review. ISRN Physiol (2013) 2013:837630. doi:10.1155/2013/837630
74. Helm JF, Venu RP, Geenen JE, Hogan WJ, Dodds WJ, Toouli J, et al. Effects of morphine on the human sphincter of Oddi. Gut (1988) 29:1402–7. doi:10.1136/gut.29.10.1402
75. Holtzer JD, Hulst SG. Confirmation of postcholecystectomy biliary dyskinesia by elevation of serum transaminases (GOT and GPT) after injection of morphine? Acta Med Scand (1973) 194:221–4. doi:10.1111/j.0954-6820.1973.tb19435.x
76. Jones RM, Detmer M, Hill AB, Bjoraker DG, Pandit U. Incidence of choledochoduodenal sphincter spasm during fentanyl-supplemented anesthesia. Anesth Analg (1981) 60:638–40. doi:10.1213/00000539-198109000-00005
77. Druart-Blazy A, Pariente A, Berthelemy P, Arotçarena R. The underestimated role of opiates in patients with suspected sphincter of Oddi dysfunction after cholecystectomy. Gastroenterol Clin Biol (2005) 29:1220–3. doi:10.1016/S0399-8320(05)82204-3
78. Mossberg SM, Bloom A, Berkowitz J, Ross G. Serum enzyme activities following morphine. A study of transaminase and alkaline phosphatase levels in normal persons and those with gallbladder disease. Arch Intern Med (1962) 109:429–37. doi:10.1001/archinte.1962.03620160055008
79. Mousavi S, Toussy J, Zahmatkesh M. Opium addiction as a new risk factor of sphincter of Oddi dysfunction. Med Sci Monit (2007) 13:CR528–31.
80. Chuah SY, Leong CK, Pang CW. Dilated common bile duct in opium addicts with and without biliary symptoms – implication for research in AIDS cholangiopathy. Singapore Med J (2003) 44:261–7.
81. Leopold SJ, Grady BP, Lindenburg CE, Prins M, Beuers U, Weegink CJ. Common bile duct dilatation in drug users with chronic hepatitis C is associated with current methadone use. J Addict Med (2014) 8:53–8. doi:10.1097/ADM.0000000000000006
82. Zahedi-Nejad N, Narouei S, Fahimy F. Common bile duct (CBD) diameter in opium-addicted men: comparison with non-addict controls. Pol J Radiol (2010) 75:20–4.
83. Staritz M, Poralla T, Manns M, Meyer zum Büschenfelde KH. Effect of modern analgesic drugs (tramadol, pentazocine, and buprenorphine) on the bile duct sphincter in man. Gut (1986) 27:567–9. doi:10.1136/gut.27.5.567
84. Wu SD, Zhang ZH, Jin JZ, Kong J, Wang W, Zhang Q, et al. Effects of narcotic analgesic drugs on human Oddi’s sphincter motility. World J Gastroenterol (2004) 10:2901–4. doi:10.3748/wjg.v10.i19.2901
85. Sherman S, Gottlieb K, Uzer MF, Smith MT, Khusro QE, Earle DT, et al. Effects of meperidine on the pancreatic and biliary sphincter. Gastrointest Endosc (1996) 44:239–42. doi:10.1016/S0016-5107(96)70158-X
86. Lembo AJ, Lacy BE, Zuckerman MJ, Schey R, Dove LS, Andrae DA, et al. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med (2016) 374:242–53. doi:10.1056/NEJMoa1505180
87. Shen J, Huang MK, Wu FL, Tang WH, Zao HY, Zhang H, et al. Effect of naloxone on the haemodynamics and the outcome of experimental acute pancreatitis in dogs. J Gastroenterol Hepatol (1992) 7:502–7. doi:10.1111/j.1440-1746.1992.tb01028.x
88. Chen JW, Thomas A, Woods CM, Schloithe AC, Toouli J, Saccone GT. Sphincter of Oddi dysfunction produces acute pancreatitis in the possum. Gut (2000) 47:539–45. doi:10.1136/gut.47.4.539
89. Badalov N, Baradarian R, Iswara K, Li J, Steinberg W, Tenner S. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroenterol Hepatol (2007) 5:648–61. doi:10.1016/j.cgh.2006.11.023
90. Trivedi CD, Pitchumoni CS. Drug-induced pancreatitis: an update. J Clin Gastroenterol (2005) 39:709–16. doi:10.1097/01.mcg.0000173929.60115.b4
91. Lankisch PG, Niederstadt H, Redlin-Kress E, Mahlke R, Brand A. Acute pancreatitis: induced by heroin intoxication? Pancreas (1993) 8:123–6. doi:10.1097/00006676-199301000-00022
92. Mitterhofer AP, Antonelli M, Genuini I, Bertazzoni G. High-dose intranasal snorted heroin: a new cause of pancreatitis. Pancreas (1998) 17:213–5. doi:10.1097/00006676-199808000-00018
93. Andersen V, Sonne J, Andersen M. Spontaneous reports on drug-induced pancreatitis in Denmark from 1968 to 1999. Eur J Clin Pharmacol (2001) 57:517–21. doi:10.1007/s002280100346
94. Hastier P, Buckley MJ, Peten EP, Demuth N, Dumas R, Demarquay JF, et al. A new source of drug-induced acute pancreatitis: codeine. Am J Gastroenterol (2000) 95:3295–8. doi:10.1111/j.1572-0241.2000.03213.x
95. Renkes P, Tréchot P. Acetaminophen-codeine combination induced acute pancreatitis. Pancreas (1998) 16:556–7. doi:10.1097/00006676-199805000-00016
96. Epelde F, Boada L, Tost J. Pancreatitis caused by loperamide overdose. Ann Pharmacother (1996) 30:1339.
97. Howaizi M, Sbaï-Idrissi MS, Baillet P. [Loperamide-induced acute pancreatitis]. Gastroenterol Clin Biol (2000) 24:589–91.
98. Lee HM, Villa AF, Caudrelier S, Garnier R. Can loperamide cause acute pancreatitis? Pancreas (2011) 40:780–1. doi:10.1097/MPA.0b013e31821fa52f
99. Vidarsdottir H, Vidarsdottir H, Moller PH, Bjornsson ES. Loperamide-induced acute pancreatitis. Case Rep Gastrointest Med (2013) 2013:517414. doi:10.1155/2013/517414
100. Hopman WP, Rosenbusch G, Jansen JB, Lamers CB. Effect of increasing oral doses of loperamide on gallbladder motility in man. Br J Clin Pharmacol (1990) 29:55–60. doi:10.1111/j.1365-2125.1990.tb03602.x
101. Remington M, Fleming CR, Malagelada JR. Inhibition of postprandial pancreatic and biliary secretion by loperamide in patients with short bowel syndrome. Gut (1982) 23:98–101. doi:10.1136/gut.23.2.98
102. Koller EA, Cross JT, Doraiswamy PM, Malozowski SN. Pancreatitis associated with atypical antipsychotics: from the Food and Drug Administration’s MedWatch surveillance system and published reports. Pharmacotherapy (2003) 23:1123–30. doi:10.1592/phco.23.10.1123.32759
103. Khuroo MS, Zargar SA, Yattoo GN. Efficacy of nifedipine therapy in patients with sphincter of Oddi dysfunction: a prospective, double-blind, randomized, placebo-controlled, cross over trial. Br J Clin Pharmacol (1992) 33:477–85. doi:10.1111/j.1365-2125.1992.tb04074.x
104. Sand J, Nordback I, Koskinen M, Matikainen M, Lindholm TS. Nifedipine for suspected type II sphincter of Oddi dyskinesia. Am J Gastroenterol (1993) 88:530–5.
105. Nakeeb A. Sphincter of Oddi dysfunction: how is it diagnosed? How is it classified? How do we treat it medically, endoscopically, and surgically? J Gastrointest Surg (2013) 17:1557–8. doi:10.1007/s11605-013-2280-8
106. Santhosh S, Mittal BR, Arun S, Sood A, Bhattacharya A, Kochhar R. Quantitative cholescintigraphy with fatty meal in the diagnosis of sphincter of Oddi dysfunction and acalculous cholecystopathy. Indian J Gastroenterol (2012) 31:186–90. doi:10.1007/s12664-012-0241-x
107. Pasricha PJ, Miskovsky EP, Kalloo AN. Intrasphincteric injection of botulinum toxin for suspected sphincter of Oddi dysfunction. Gut (1994) 35:1319–21. doi:10.1136/gut.35.9.1319
108. Staritz M, Poralla T, Ewe K, Meyer zum Büschenfelde KH. Effect of glyceryl trinitrate on the sphincter of Oddi motility and baseline pressure. Gut (1985) 26:194–7. doi:10.1136/gut.26.2.194
109. Brandstätter G, Schinzel S, Wurzer H. Influence of spasmolytic analgesics on motility of sphincter of Oddi. Dig Dis Sci (1996) 41:1814–8. doi:10.1007/BF02088751
110. Kalaitzakis E, Ambrose T, Phillips-Hughes J, Collier J, Chapman RW. Management of patients with biliary sphincter of Oddi disorder without sphincter of Oddi manometry. BMC Gastroenterol (2010) 10:124. doi:10.1186/1471-230X-10-124
111. Cotton PB, Durkalski V, Romagnuolo J, Pauls Q, Fogel E, Tarnasky P, et al. Effect of endoscopic sphincterotomy for suspected sphincter of Oddi dysfunction on pain-related disability following cholecystectomy: the EPISOD randomized clinical trial. JAMA (2014) 311:2101–9. doi:10.1001/jama.2014.5220
112. Jacob L, Geenen JE, Catalano MF, Geenen DJ. Prevention of pancreatitis in patients with idiopathic recurrent pancreatitis: a prospective nonblinded randomized study using endoscopic stents. Endoscopy (2001) 33:559–62. doi:10.1055/s-2001-15314
113. Coté GA, Imperiale TF, Schmidt SE, Fogel E, Lehman G, McHenry L, et al. Similar efficacies of biliary, with or without pancreatic, sphincterotomy in treatment of idiopathic recurrent acute pancreatitis. Gastroenterology (2012) 143:1502–9.e1. doi:10.1053/j.gastro.2012.09.006
Keywords: pancreatitis, sphincter of Oddi, sphincter of Oddi dysfunction, functional biliary disorder, biliary colic, hepatic enzymes, lipase, amylase
Citation: Afghani E, Lo SK, Covington PS, Cash BD and Pandol SJ (2017) Sphincter of Oddi Function and Risk Factors for Dysfunction. Front. Nutr. 4:1. doi: 10.3389/fnut.2017.00001
Received: 21 October 2016; Accepted: 10 January 2017;
Published: 30 January 2017
Edited by:
Peter Hegyi, University of Szeged, HungaryReviewed by:
Hirohide Ohnishi, National Institute of Occupational Safety and Health, JapanEdwin Charles Thrower, Yale University, USA
Copyright: © 2017 Afghani, Lo, Covington, Cash and Pandol. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephen J. Pandol, stephen.pandol@cshs.org
 Elham Afghani
Elham Afghani Simon K. Lo1
Simon K. Lo1 Paul S. Covington
Paul S. Covington Brooks D. Cash
Brooks D. Cash Stephen J. Pandol
Stephen J. Pandol