- 1Actinide Thermodynamics Department, Institute of Resource Ecology, Helmholtz-Zentrum Dresden-Rossendorf e.V., Dresden, Germany
- 2Department Repository Research, Gesellschaft für Anlagen- und Reaktorsicherheit (GRS) gGmbH, Braunschweig, Germany
A Corrigendum on
The solubility of oxygen in water and saline solutions
by Bok F, Moog HC and Brendler V (2023). Front. Nucl. Eng. 2:1158109. doi: 10.3389/fnuen.2023.1158109
In the published article, there was an error in Table 5. In line 3 [without table head, O2(aq)] in the Tmin/Tmax column the stated temperature range is wrong. Since no temperature coefficients for
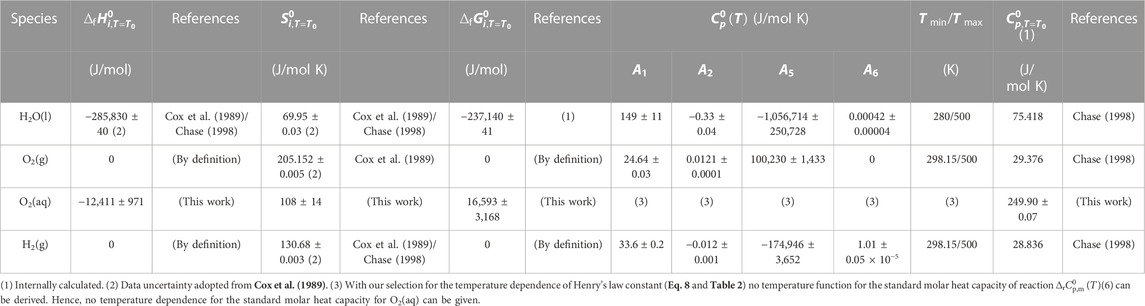
TABLE 5. Standard formation data for H2O(l), O2(g), H2(g), and O2(aq). Temperature parameters for
In the published article, there was an error in Table 9. In line 7 [without table head, O2(aq)-OH−] in the last column (X2) it has to be 3.934 ± 0.882, not 3.6934 ± 0.882. In line 15 [without table head, O2(aq)-K+-SO42−] in the second column (X0) it has to be −0.1618 ± 0.078 not −0.1575 ± 0.0688 and in the third column (X1) it has to be 164.7 ± 102 not 183.5 ± 70.3. The corrected Table 9 and its caption *Parameters for the temperature dependency function of the binary and ternary interaction coefficients (λ, ζ). The uncertainty information refers to one standard deviation.* appears below.
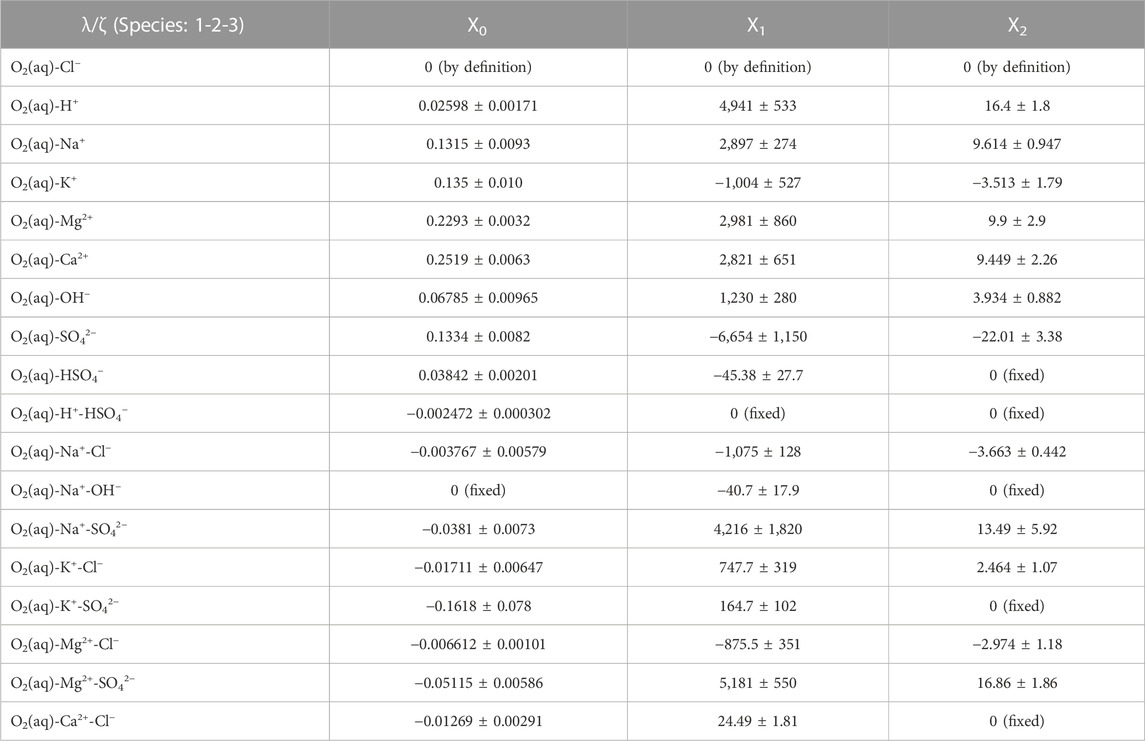
TABLE 9. Parameters for the temperature dependency function of the binary and ternary interaction coefficients (λ, ζ). The uncertainty information refers to one standard deviation.
The authors apologize for these errors and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: oxygen solubility, electrolyte solutions, water, Pitzer ion-interaction approach, EH equation
Citation: Bok F, Moog HC and Brendler V (2023) Corrigendum: The solubility of oxygen in water and saline solutions. Front. Nucl. Eng. 2:1292254. doi: 10.3389/fnuen.2023.1292254
Received: 11 September 2023; Accepted: 02 October 2023;
Published: 17 October 2023.
Edited and reviewed by:
Taishi Kobayashi, Kyoto University, JapanCopyright © 2023 Bok, Moog and Brendler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: F. Bok, Zi5ib2tAaHpkci5kZQ==
 F. Bok
F. Bok H. C. Moog2
H. C. Moog2 V. Brendler
V. Brendler