
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Syst. Neurosci., 21 March 2023
Volume 17 - 2023 | https://doi.org/10.3389/fnsys.2023.1045396
This article is part of the Research TopicThe Role of Brain Oscillatory Activity in Human Sensorimotor Control and Learning: Bridging Theory and PracticeView all 6 articles
Introduction: Like alpha rhythm, the somatosensory mu rhythm is suppressed in the presence of somatosensory inputs by implying cortical excitation. Sensorimotor rhythm (SMR) can be classified into two oscillatory frequency components: mu rhythm (8–13 Hz) and beta rhythm (14–25 Hz). The suppressed/enhanced SMR is a neural correlate of cortical activation related to efferent and afferent movement information. Therefore, it would be necessary to understand cortical information processing in diverse movement situations for clinical applications.
Methods: In this work, the EEG of 10 healthy volunteers was recorded while fingers were moved passively under different kinetic and kinematic conditions for proprioceptive stimulation. For the kinetics aspect, afferent brain activity (no simultaneous volition) was compared under two conditions of finger extension: (1) generated by an orthosis and (2) generated by the orthosis simultaneously combined and assisted with functional electrical stimulation (FES) applied at the forearm muscles related to finger extension. For the kinematic aspect, the finger extension was divided into two phases: (1) dynamic extension and (2) static extension (holding the extended position).
Results: In the kinematic aspect, both mu and beta rhythms were more suppressed during a dynamic than a static condition. However, only the mu rhythm showed a significant difference between kinetic conditions (with and without FES) affected by attention to proprioception after transitioning from dynamic to static state, but the beta rhythm was not.
Discussion: Our results indicate that mu rhythm was influenced considerably by muscle kinetics during finger movement produced by external devices, which has relevant implications for the design of neuromodulation and neurorehabilitation interventions.
Electroencephalography (EEG) and magnetoencephalography (MEG) acquire brain activity’s electrical and magnetic fields, respectively. They can be categorized by activated cortical topography and oscillatory frequency components responding to specific intrinsic and extrinsic causes. The alpha rhythm is one of the most well-known oscillations among them. It is prominently found over the occipital cortex in the absence of visual stimulus in closed eyes, but its amplitude is suppressed while visual input is present. Similar phenomena were observed during the somatosensory stimulation, known as mu rhythm. Both mu and alpha rhythms share common characteristics, such as reflecting the idle state and tuning to be ready for the upcoming input (Kuhlman, 1978). However, they are considered spatially and functionally independent because visual stimuli do not significantly influence the mu rhythm, and somatosensory stimuli do not considerably affect the alpha rhythm either (Kuhlman, 1978). While the mu rhythm is suppressed in response to the sensorimotor tasks, the blood-oxygen-level-dependent (BOLD) signal in functional magnetic resonance imaging (fMRI) increased (Ritter et al., 2009). Also, the gamma rhythm in EEG (Ball et al., 2008; Wagner et al., 2019) is enhanced, and neuron spike rates (Miller et al., 2007; Klimesch, 2011) in invasive recordings increased during the sensorimotor tasks. Its inverse correlation with fMRI, EEG (oscillatory activity in other frequency bands), and neural recordings provided evidence that the suppressed mu rhythm implies cortical excitability, and the enhanced mu rhythm infers cortical inhibition. The enhanced/suppressed features of mu- and beta-rhythm are used for clinical application to detect the motor intention to control the external devices for assistive or motor rehabilitation devices. However, the neural mechanisms and origins of the enhanced/suppressed SMR have not been clearly explained in terms of psychological and physiological conditions. The level of enhancement/suppression varies within and between subjects; even no meaningful changes were detected in some people (Guger et al., 2003). The importance of the sensory role has not been emphasized in SMR research and clinical application. The SMR in response to different proprioceptive stimulation has not been widely studied.
Despite inconsistency in the terms and range of its frequency bands in the literature, sensorimotor rhythm (SMR) can be classified into two oscillatory frequency components: mu band and beta band (Hari and Salmelin, 1997; Jones et al., 2009). The beta band (14–25 Hz with a central frequency of around 20 Hz) had been regarded as nothing more than the harmonic rhythms of the mu band (8–13 Hz with a central frequency of around 10 Hz). Still, more evidence of functional and topographical differences has been uncovered. The lower frequency component is localized in the post-central somatosensory cortex, and the higher frequency component is located in the pre-central motor cortex (Pfurtscheller et al., 1994; Salmelin and Hari, 1994; Salmelin et al., 1995; Hari and Salmelin, 1997). In addition, the correlation with EMG and the time of recovery-to-baseline after events were different from each other (Stancák and Pfurtscheller, 1995). Therefore, they are hereafter referred to as mu and beta, respectively, to emphasize the separate frequency components of the oscillation. Both rhythms have been used to study sensorimotor processing (Birbaumer et al., 1990; Neuper et al., 2006; Buzsáki, 2009; Ramos-Murguialday and Birbaumer, 2015), and their suppression and enhancement in power are commonly known as event-related desynchronization (ERD) and event-related synchronization (ERS) (Pfurtscheller et al., 1997, 1998; Houdayer et al., 2006).
The somatosensory information plays a role beyond just providing sensory input. In a recent ECoG study, the primary somatosensory cortex (S1) was activated before the motor cortex activation in cued finger movements, which signifies that sensory information is involved in movement anticipation (Sun et al., 2015). Recent brain-machine interface (BMI) studies successfully decoded upper limb movements from S1 in paralyzed and amputated patients (Wang et al., 2013; Kikkert et al., 2016; Degenhart et al., 2018; Ramsey, 2019; Vidaurre et al., 2019b). The sensory evoked potential (SEP) confirms the arrival to the cortex, proving the intact sensory pathway, as an evoked response in short-lasting stimulation (e.g., tactile stimuli). However, the realistic somatosensory stimuli last a few seconds when related to multi-joint movement as a non-phase-locked response. Therefore, the somatosensory mu and beta ERD/ERS may be more appropriate than SEP in assessing cortical activation in response to movement.
When it comes to brain-body interactions, SMR is correlated with the kinematics (speed, velocity, and acceleration) and kinetics (muscles and force) of limb movement (Yuan et al., 2010; Bourguignon et al., 2019; Branco et al., 2019). In cortico-kinematic coherence (CKC) studies, S1 activity was highly correlated with hand kinematics, reflecting that the cortical processing was driven by movement rhythmicity (Bourguignon et al., 2015). However, one has to be careful regarding the methods used (Antelis et al., 2013). In line with the CKC studies, the mu and beta ERD during dynamic conditions were greater than those during static conditions (Nakayashiki et al., 2014). Different kinetic conditions of muscle and forces also influence the amplitude of the SMR, even at the head movements level (Bibián et al., 2021). Corticomuscular coherence (CMC) studies showed that beta rhythm was correlated with electromyography (EMG) activity, mainly reflecting efferent information (Chakarov et al., 2009; Bourguignon et al., 2019; Vidaurre et al., 2019a; Kenville et al., 2020).
Recently, interventions using multiple body actuating devices, such as the neurofeedback training in control of robotic devices and FES (Grimm and Gharabaghi, 2016; Liu et al., 2017), have attracted interest. Rehabilitative robotic devices could provide more prolonged, more intense, and controlled periods of practice, often combined with other strategies that suit individuals’ needs (Hogan et al., 2006; Mazzoleni et al., 2017; Weber and Stein, 2018). Peripheral electrical stimulation, for instance, neuromuscular stimulation and functional electrical stimulation (FES), can also work on muscle atrophy, muscle tone, and motor neuron activation resulting in motor recovery in patients with paralysis after stroke (Sheffler and Chae, 2007; Knutson et al., 2015; Moon et al., 2017; Yang et al., 2019). The mu and beta rhythm suppression between active and passive conditions has been reported in lower limb rehabilitation. More suppression of mu and beta rhythm has been shown in active robot-assisted walking than in passive robot-assisted walking in cortical activation (Wagner et al., 2012). Robots allow control of individual and well-defined joint kinematics for coordinated functional movements. On the other hand, FES has not reached fine control of complex coordinated movements, particularly with surface electrodes (Koutsou et al., 2016; Shin and Hu, 2018). However, only FES can produce muscle contraction mimicking a natural voluntary contraction-induced movement because the agonist muscles contract, and the antagonist muscles are stretched during the passive movement. Therefore, robots combined with FES should lead to higher cortical excitation than executing the identical passive movement without concomitant FES. Furthermore, this integrated tool could recruit more receptors derived from muscle contractions in addition to passive proprioception, activating the reticular system more, which could produce more sensorimotor neural network excitation, and thus result in an interesting tool to provide neurofeedback in BMI-based motor rehabilitation paradigms. The brain somatosensory mu and beta ERD/ERS response during passive movement induced by FES and robot movements independently has been investigated (Crone et al., 1998; Müller et al., 2003; Müller-putz et al., 2007; Taylor, 2008; Cho et al., 2011; Shaikhouni et al., 2013; Vidaurre et al., 2016, 2019b,2021; Tu-Chan et al., 2017; Corbet et al., 2018; Hishinuma et al., 2019). However, when combining FES and robotic actuators to produce a sequence of passive movement, brain oscillatory response needs further investigation before being combined in a rehabilitation system.
Somatosensory cortical activation is essential in motor learning and rehabilitation, and multiple studies reported that sensory inputs affect the plasticity of sensorimotor systems in healthy humans and patients with brain injuries (Sanes and Donoghue, 2000; Rosenkranz and Rothwell, 2006; Edwards et al., 2019). However, the afferent contribution has not drawn attention as much as the efferent information during sensorimotor integration. As discussed, the mu and beta ERD/ERS (or suppression/enhancement) is neural correlates of cortical excitability supported by BOLD, fMRI, gamma EEG, and neuronal spikes. Therefore, it would be necessary to know how the cortex responds to somatosensory stimulation according to various stimulus patterns and types, which will provide cortical processing in response to different stimulation and valuable information in the design of neurofeedback devices to optimize afferent information in sensorimotor integration. In the present study, we hypothesized:(1) SMR in response to proprioceptive stimulation is suppressed/enhanced according to kinematic conditions and (2) more SMR suppression is observed in the somatosensory cortex in passive mechanical movement + FES than passive mechanical movement alone. For the kinematic aspect, the finger extension was divided into two phases: (1) dynamic extension and (2) static extension (holding a position). For the kinetics aspect, afferent brain activity (no simultaneous volition) was compared under two conditions of finger extension: (1) generated by a robotic hand orthosis and (2) generated by the orthosis simultaneously combined and assisted with FES.
Ten healthy female volunteers aged between 22 and 38 years (nine right-handed, one left-handed) participated in the study. They were sitting upright in a comfortable chair facing a computer screen located 1 meter from the chair (Figure 1A). They were instructed to keep their gaze in the middle of the black computer screen and to remain still and relaxed during the measurement. A plastic panel was placed above the wrist as a visual blockade to prevent participants from seeing their hands being passively moved, and earplugs were used to prevent participants from hearing the different noises produced by using only the robot or the robot and FES combined. The two experimental conditions (robot only or ORTHOSIS and robot and FES combined or ORTHOFES) were randomly applied to their right hand in addition to one rest condition (REST) that was used as a reference or control.
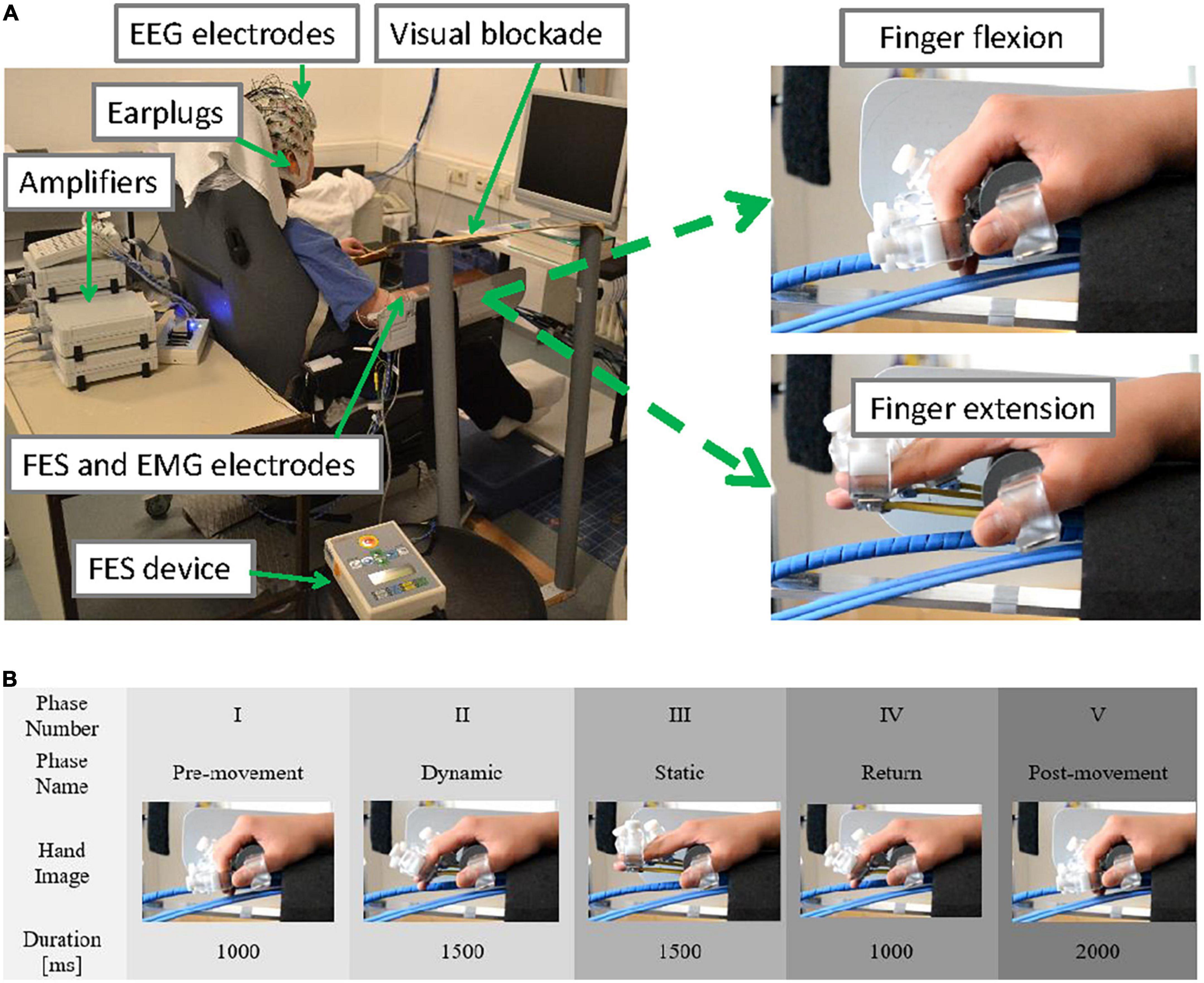
Figure 1. (A) Experimental setup (upper panel). The participant was sitting in a comfortable chair with the EEG cap on, the EMG electrodes placed over the forearm and upper arm muscles while the right hand was strapped to a robotic hand orthosis, and a pair of FES electrodes were attached to forearm muscles (left). A visual blockade (to prevent the user from receiving visual feedback from her right-hand movement) and earplugs were used. The maximal range of passive finger flexion (top right) and finger extension (bottom right) are shown. (B) Time course of one trial (lower panel). No stimulus was provided during the pre-movement period (phase I at –1,000 ms). No cue was presented. The fingers were extended according to the experimental conditions (ORTHOSIS or ORTHOFES) to reach a full finger extension (phase II at 0 ms). During the finger stretched period, the finger position was maintained (phase III at 1,500 ms). During the return period (phase IV at 3,000 ms), the fingers were returned to the starting position by the orthosis alone in both conditions (ORTHOSIS and ORTHOFES). No stimulus was delivered during the Post-movement period (phase V at 4,000 ms).
In the robot condition (ORTHOSIS), each finger was extended individually using a custom-made hand orthosis using 4 DC-Motors M-28 (Kaehlig Antriebstechnik GmbH, Hannover, Germany) with a worm gearhead for each finger. The motor drove a Bowden cable via cogwheel and cog rail. A finger holder was mounted on the other side of each Bowden cable (details on the robotic orthosis’ construction can be found in Ramos-Murguialday et al. (2012, 2013). The maximum range of finger extension was determined at a position each participant felt comfortable. During the measurements (see Figure 1B), the participant’s fingers were extended by the orthosis to their predefined position (dynamic phase II) and maintained (static phase III). Then, the fingers were flexed automatically by the orthosis for all participants returning to the start position for the subsequent trial in the return phase IV.
In the orthosis and FES condition (ORTHOFES), two FES unipolar electrodes were placed over the extensor digitorum communis (EDC) muscle for the finger extension following anatomical landmarks. Before the measurement, the FES (UNAFET 8, UNA Systems, Belgrade, Serbia) parameters were configured for a finger extension. We adjusted the stimulation intensity with fixed 30 Hz and 300 μs pulses until fingers were extended at the same speed and in the same range of motion in ORTHOSIS. Both orthosis and FES concurrently induced the finger extension. The kinematics of the finger extension was identical to ORTHOSIS because the fingers were always in finger holders of the orthosis, limiting the movement (see Figure 1B). The fingers were extended by the orthosis and FES together after onset (dynamic phase II for 1.5 s) and were maintained in an extended position during the static period (phase III for 1.5 s). While fingers were being flexed in return phase IV for 1 s, FES was off, and the movement was produced by the orthosis alone (same as in the ORTHOSIS) for the subsequent trial.
No cue and random inter-trial intervals between 5 and 9 s were presented to minimize the subject’s anticipatory activity and allow the central nervous system (CNS) to return to baseline levels. The whole right arm was fixed to and positioned in an apparatus, which guaranteed negligible kinematic differences between conditions. Each condition was repeated 70 times. This study was approved by the ethics committee of the University of Tübingen, Medical Faculty.
The EEG data were acquired using a BrainAmp (Brainproducts GmbH, Munich, Germany) with a sampling rate of 1,000 Hz. 64 EEG electrodes were referenced to the nasion and grounded anteriorly to Fz. The data were first filtered using a band-pass filter (2–45 Hz). Then, an independent component analysis-based method was used to detect and eliminate eye blinks and movement artifacts, and neck, cranial and facial EMG-related artifacts using Fieldtrip (Oostenveld et al., 2011). Afterward, data were spatially filtered using a short Laplacian (McFarland et al., 1997). In order to control for undesired peripheral muscle activity that could introduce confounds in the EEG processing, we recorded EMG in the moved and non-moved limbs. EMG data were collected with four bipolar Ag/AgCl electrodes and placed on muscles on both arms; one close to the external epicondyle on the extensor digitorum (forearm extension), the other on the flexor carpi radialis (forearm flexion), further on the external head of the biceps (upper arm flexion) and the last one placed on the external head of the triceps (upper arm extension). Then, they were processed using a high-pass filter at 10 Hz to detect unwanted upper limb movements. A trial was rejected and marked as “EMG contaminated” when it contained significant EMG activity irrelevant to the experimental design: any muscle activity in REST or ORHTOSIS; on the left arm and the right upper arm in ORTHOFES. In addition, trials were rejected if EMG activity was higher than three standard deviations (SD) from the baseline mean longer than 200 ms following (Ramos-Murguialday et al., 2012, 2013).
After preprocessing, the spectral power of each EEG channel in experimental conditions (ORTHOSIS and ORTHOFES) was analyzed to detect the significance of within-subject and within-conditions (see Figure 2 and Supplementary material). The event-related spectral perturbation was then calculated using Morlet transforms (Daubechies, 1996) with 3 cycles at the lowest frequencies, 23.04 at the highest frequencies, a time window of 1,114 ms, and a 30 ms overlap. A 300 ms time window from −350 to −50 ms before the passive movement onset as a baseline for event-related spectra perturbation analysis. Forty-three linear-spaced frequencies were produced from 3 to 45 Hz and 200-time points. The brain activity in the experimental conditions was compared to the REST condition in a pairwise manner in the time-frequency domain to mask out the non-experimental as baseline condition: (ORTHOSIS vs. REST) and (ORTHOFES vs. REST). The spectral differences from REST were estimated using the EEGLAB toolbox.1 A null hypothesis distribution (p = 0.01) was calculated by accumulating surrogate data (200 bootstrap replications), shuffling the single-trial spectral DIFF estimates using a two-tailed bootstrap significance probability level implemented in the EEGLAB bootstrap method (Sivaganesan, 1994; Burgess and Gruzelier, 1999). Both sides of the surrogate distribution obtained for every frequency and time bin from the spectral DIFF were used for significance tests. Although we know this method is not corrected for multiple comparisons, we used it to orient further analysis reducing dimensionality in frequency band and electrodes (Ramos-Murguialday and Birbaumer, 2015). We observed significant band power changes in each subject when comparing ORTHOFES and ORTHOSIS to REST (see Supplementary material for more details).
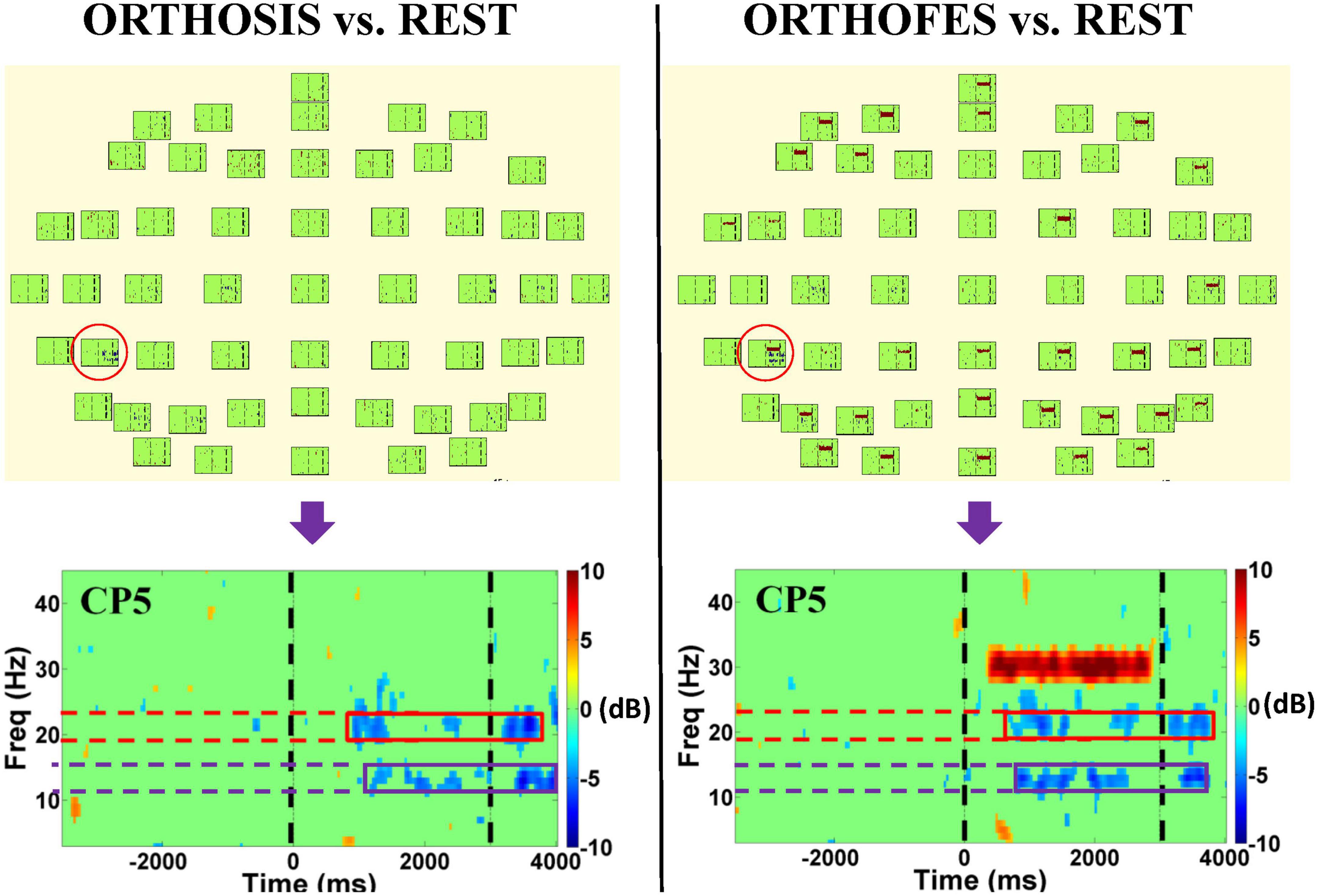
Figure 2. EEG spectral power bootstrap analysis during passive right-hand movement in one representative participant. The left and right panels show the differences in the time-frequency domain when ORTHOSIS and ORTHOFES conditions were compared to REST, respectively. CP5 was selected as a representative electrode (red circle) after visual inspection due to its more salient power suppression compared to other electrodes. The figures at the bottom show the enlarged plot of the selected electrode. The purple and red boxes indicate the frequency bands in which we found more significant power changes when using the bootstrap analysis. The green areas indicate no significant power differences compared to REST conditions. The color represents the power suppression, power enhancement, or no differences compared to the REST condition: blue being power suppression in dB, red being power enhancement in dB, and green being not significantly different (P < 0.01) from REST. The two black dashed vertical lines indicate the beginning (0 ms) and end of the passive movement during a trial (3,000 ms). The high spectral power in the range of 30 Hz was observed in the right panel during the trial period due to FES (stimulation frequency: 30 Hz) artifact, which was not found in ORTHOSIS (left panel).
EEG subject-specific frequency bands were visually selected based on the bootstrap analysis of each participant, as most statistically differential changes with a total significant period is longer than 600 ms between two conditions were detected in the time-frequency domain from movement-related afferent signals. Therefore, the manual selection of the frequency band was used to observe the profile of power reduction/enhancement over the entire trial rather than instantaneous power changes. Next, the preprocessed EEG signals were band-pass filtered according to these individual frequency bands and then squared. Finally, the proportional power decrease or increase to the activity during a baseline period (from −350 to −50 ms) at each electrode was averaged according to:
with Experimental and Baseline in Equation 1 denoting the activity of each electrode during the experimental condition (ORTHOSIS and ORTHOFES) and baseline period, respectively.
The relative power calculated from Equation 1 was used to compare power dynamics differences between ORTHOSIS and ORTHOFES. A two-sided Monte-Carlo permutation test at the 5% significance level was applied to the mean band power of all the participants’ trial-based data to test the hypothesis that the band power decrease (ERD) during ORTHOFES is significantly different compared to the one during ORTHOSIS. We used 1,000 repetitions of Monte-Carlo procedures to find the time points with the significant mean difference between two conditions, and p-values were corrected by the False Discovery Rate (FDR) described by Benjamini and Hochberg (1995) for multiple comparisons in time series. We also analyzed the power of the sensorimotor frequency band at electrode C3, which is commonly used to study motor-related EEG oscillations during upper limb movement. Even though each subject may have slightly different frequency bands for motor commands (mu rhythm ERD) in neurofeedback tasks, we analyzed 8–13 Hz to observe the influence of the induced movement on EEG oscillations over the electrode C3.
The data from two participants were not included in the group analysis due to no changes in the entire frequency band (n = 1) and the noisy EEG (n = 1), of which the spectral power maps are seen in Supplementary Figures 18, 19. After conservative trial rejections based on EMG and EEG artifacts, the number of clean trials available for REST, ORTHOSIS, and ORTHOFES across participants were 35.4 ± 9.6 (mean ± SD), 36.6 ± 9.1, and 39.0 ± 9.0 respectively, being EMG artifacts the primary source of trial elimination in line with previous work (Ramos-Murguialday et al., 2013; Ramos-Murguialday and Birbaumer, 2015; López-Larraz et al., 2018; Ray et al., 2020).
CP5 in seven subjects and P5 in one subject presented the most salient expected power changes in the contralateral hemisphere (see Figures 2, 3). Two common discrete frequency bands typically analyzed were individualized and used for further analyses: the mu (11.3 ± 2.6 Hz) and the beta (21.8 ± 2.4 Hz), as shown in Figure 4. The significant differences between ORTHOSIS and ORTHOFES were observed in time windows during movement in the mu (see Figure 5A). In the dynamic period (phase II, 900–1,100 ms), ORTHOFES produced a faster mu suppression, which induces significantly more cortical excitation (i.e., more ERD) than ORTHOSIS during this specific time window. However, both conditions reach similar maximum levels of mu suppression. In the static period (phase III, 2,000–2,400 ms), the movement stopped after reaching the predefined range of motion. In beta rhythm, no other significant differences were observed between ORTHOFES and ORTHOSIS.
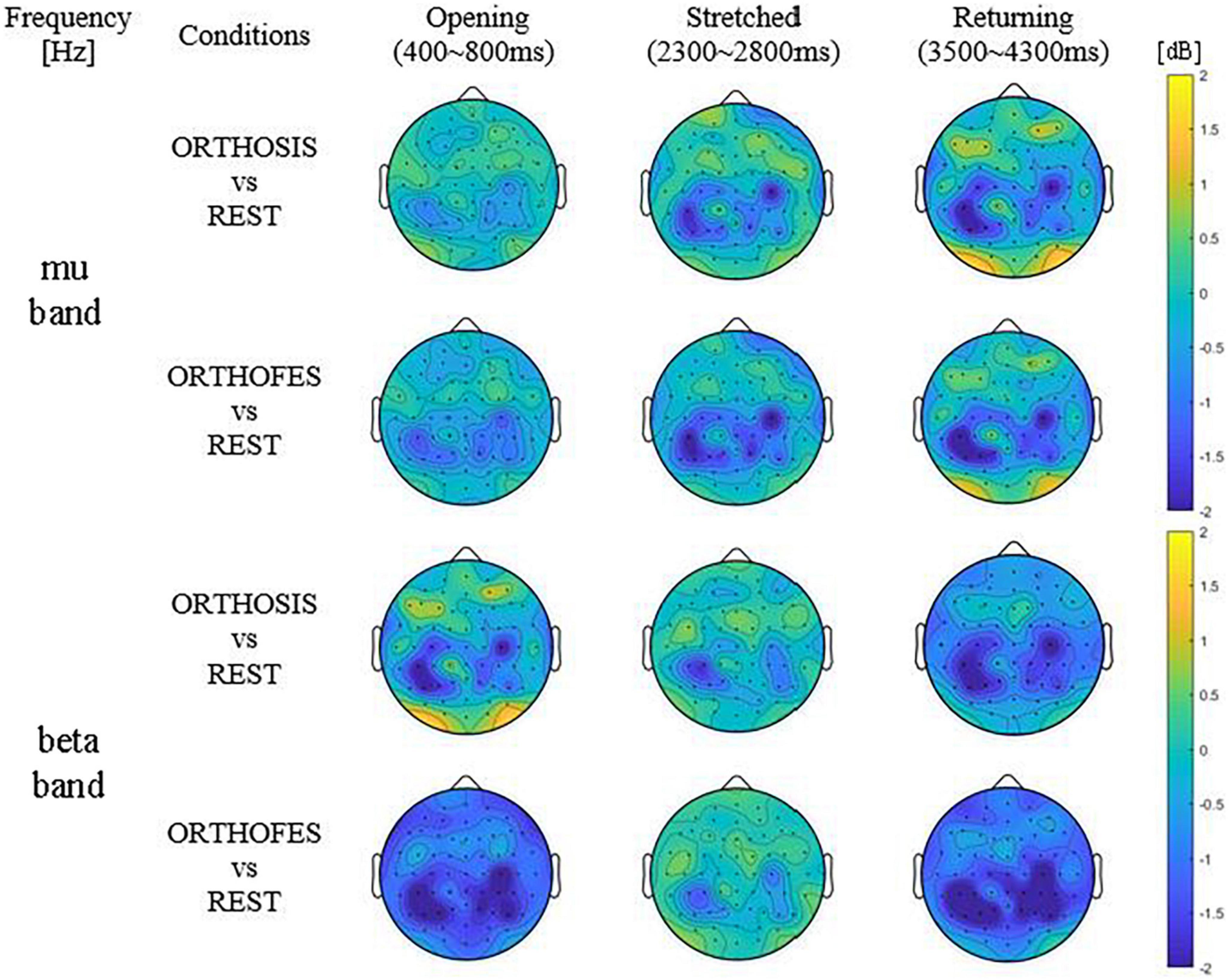
Figure 3. Averaged differential power values of all included participants’ data. Topography presenting the power changes in ORTHOSIS and ORTHOFES averaged (N = 8). The frequency band of each subject was selected from the above information (Table 1). The power changes in ORTHOSIS and ORTHOFES were compared to REST. The pronounced power reduction was observed in contralateral S1 and ipsilateral M1 and S1—the color bars fixed scale (–2 to 2 dB) for comparisons between conditions and phases. (S1 = primary somatosensory cortex, M1 = primary motor cortex). Yellow indicates ERS (power increase in dB), and blue shows ERD (power decrease in dB) compared with REST, as expressed in the color bar.
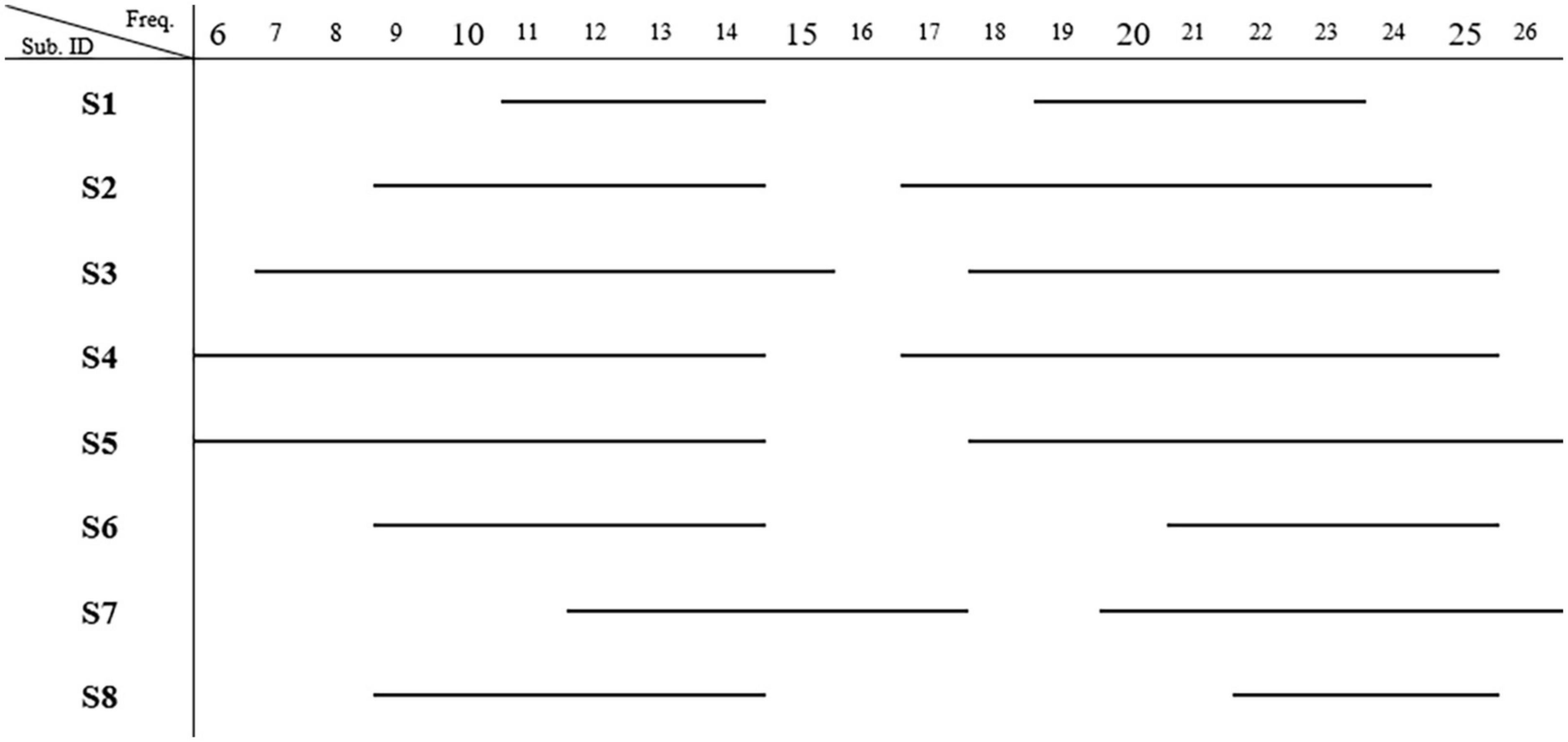
Figure 4. Most representative frequency bands. The frequencies present significant band power changes in each subject. Two frequency bands showing significant power changes (p < 0.01) were observed (N = 8) after bootstrap analysis. The mu band was 11.3 ± 2.6 (mean ± SD) Hz, and the beta band was 21.8 ± 2.4 Hz.
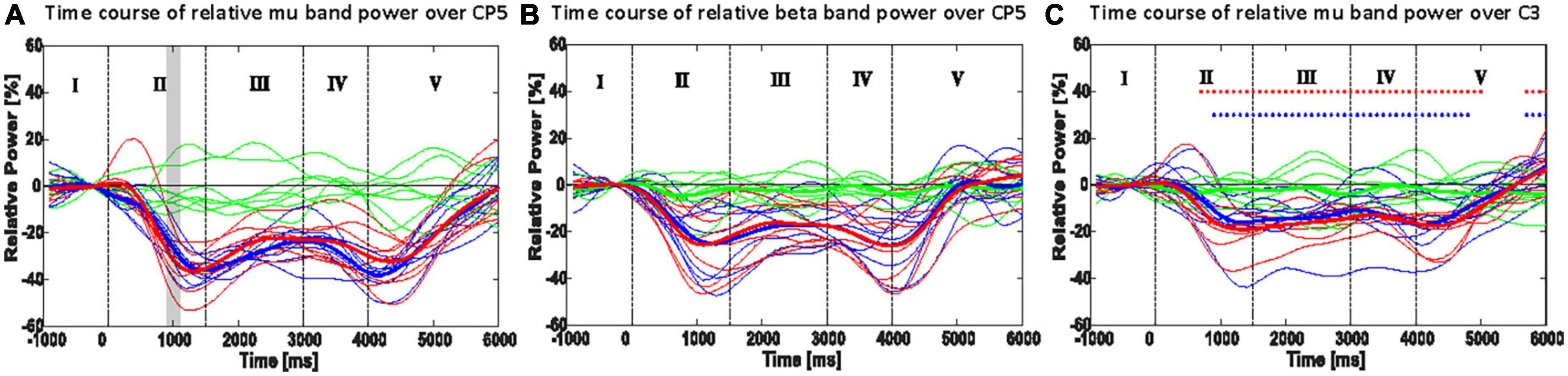
Figure 5. Time course of the most responsive electrode in power (ERD/ERS): CP5 or P5 of mu rhythm (A) and beta rhythm (B); C3 in mu (8–13 Hz) power (C). Thin lines show the mean band power of a single participant, and thick lines indicate the mean of the thin lines. Shaded areas in panels (A,B) show the 100 ms time bins, which resulted in a significant difference between the ORTHOFES and ORTHOSIS conditions using a two-sided Monte Carlo permutation test (α = 0.05). Red asterisks (*) in panel (C) indicate the 100 ms time bin, which has a significant difference between REST and ORTHOFES of grand averages (thick lines) with a two-sided Monte-Carlo permutation test (α = 0.05). Blue asterisk (*) indicates the same between REST and Orthosis. Green, red, and blue lines represent REST, ORTHOFES, and ORTHOSIS, respectively. I: pre-movement phase, II: opening phase, III: stretched phase, IV: returning phase, and V: post-movement phase.
The ERD/ERS changes of mu rhythm (8–13 Hz) are shown in Figure 5C for the right-hand movement. When each condition was compared to REST, both conditions presented significantly larger cortical excitation (larger ERD) since the pre-movement phase (baseline). In phase V, we observed a significant difference due to the time needed for the oscillatory brain activity to return to baseline.
Using a bootstrap analysis to identify the most salient power changes allowed us to determine the most representative electrode over the sensorimotor cortex to focus our analysis. Sensors CP5 or P5 of the contralateral hemisphere presented the most salient power changes in mu and beta bands when passively extending and flexing the fingers using a robotic orthosis alone or combined with FES. Focusing on these representative electrodes allowed us to reduce the problem’s dimensionality and run a subsequent permutation analysis to compare ERD/ERS changes during the movement sequence between conditions on mu and beta bands independently. The suppression of visual and auditory stimuli, the cue presentation protocol, and the control of the movement trajectory (range of motion) left haptic and proprioceptive receptors as the only movement-related generators of afferent activity we could measure with EEG. Our results describe the evolution of the somatosensory mu rhythm recorded with EEG during a finger extension/flexion passive movement sequence, i.e., moved by external devices with no subject’s intention. Furthermore, our experimental protocol allowed us to isolate the influence of muscle spindle recruitment in the sensory mu rhythm evolution during the movement sequence.
Our results align with previous literature and confirmed similar mu rhythm evolutions during passive-movement-related brain oscillatory activity (Pfurtscheller and Lopes da Silva, 1999; Alegre et al., 2004; Ramos-Murguialday and Birbaumer, 2015). In both mu and beta rhythms, ERD peaks (local minima in power) were produced only during or immediately after dynamic movement phases (phases II and V). During the movement sequence static period (phase III), the ERD was sustained but decreased continuously until the following dynamic condition (return period, phase IV) began. Even though the ERD decreased during the static condition of phase III, the power decrease or ERD was constantly significantly larger than during rest (baseline), suggesting cognitive processing sustains the ERD. We speculate that the observed cortical activity is produced because the brain processes the position as part of a passive movement sequence, similar to what has been found in active movement sequences (Alegre et al., 2002, 2004), which showed that ERD/ERS is related to the whole motor process, and not to each sub-movement or subsequence. However, as far as we know, our data demonstrate this effect for the first time during pure passive movement only. Our observation is reinforced by the incremental ERD decrease initiated during the static phase, which was very different from the ERD decrease observed in post-movement phase V (end of the movement sequence). The change in ERD at the end of the movement sequence was faster (shorter latency returning to baseline level or steeper return to baseline level). Another plausible explanation could be that the observed cortical activity is produced because the subjects were cognitively paying attention to their proprioceptive signals (visual and auditory feedback was blocked) or as a result of motor control theory, feedforward control based on sensory input forecasting (Sun et al., 2015; Branco et al., 2017).
Between 900 and 1,100 ms after stimuli onset during passive finger extension (phase II), we observed a significantly larger mu ERD with FES than without FES, reflecting a significantly faster ERD increase when FES and orthosis were combined to produce the passive movement. However, the maximum ERD (i.e., peak) was not significantly different between conditions. This expected result confirms that the induced passive movement via both electrical stimulation and orthosis stimulated more receptors, causing a faster but not a larger ERD on the sensory cortex. Based on previous work (Stancák et al., 2003; Insausti-Delgado et al., 2021), we expected a more prominent ERD peak for the ORTHOFES condition. Therefore, the combination of different receptors might not increase the amplitude but the modulation speed of the ERD. Our experimental design isolated artificial electrically induced muscle contraction as the only difference between conditions. The firing rate of GTOs is muscle-force-dependent (Paillard and Brouchon, 1968; Purves et al., 2004), and that of the muscle spindles depends on muscle length or velocity (Chye et al., 2010). Thus the effect may come from a more considerable afferent inflow to cortical structures due to the extra firing of skin mechanoreceptors, mainly GTO, and muscle spindles excited by the FES-induced muscle contraction.
Another explanation of the faster but more considerable decrease in mu-band power during ORTHOFES, albeit unlikely, could be due to greater cognitive attention during FES than during orthosis during the first second after onset. Multiple studies reported that attention modulated somatosensory mu rhythm (Babiloni et al., 2004, 2008; Jones et al., 2010; Anderson and Ding, 2011; Wiesman and Wilson, 2020). None of the participants in the present study had experienced electrical stimulation in their bodies before. Therefore, the FES-related arousal might have occurred only at the beginning of stimulation, the dynamic phase, or the experiment. Still, we did not see any difference between trials at the beginning and end of the experimental session in the ERD that could explain this option.
Interestingly, during the static condition (phase III), the mu ERD decrease was different in both conditions in the mu band only, contradicting previous work indicating that EEG beta activity is related to the afferent activity (Alegre et al., 2002). Our results demonstrate that changes in afferent activity are reflected mainly in the mu band and primarily in the ERD’s modulation speed. The ERD decreased at the beginning of the static phase. In both conditions, there was no movement and no change in receptors excitation compared to the previous movement sequence phase. In the ORTHOFES condition, the extensor muscle stimulation induced an isometric passive contraction, which could produce more afferent stimulation than in the ORTHOSIS condition (equivalent to resting in terms of movement). Indeed, the ORTHOFES condition during the static phase could be compared to a muscle contraction against resistance, which usually induces extra inhibition of antagonists’ muscles when the movement is actively performed (Katz et al., 1991; Perlmutter et al., 1998). Intuitively, one would assume that if ERD is produced by sensory receptors excitation during passive movement, the ORTHOFES condition should present a larger ERD or at least a slower decrease of ERD, but this was not the case.
As opposed to the conventional understanding of contralateral S1 activation in sensory stimulation, the electrode over M1 in the ipsilateral hemisphere showed a significant mu ERD in response to the proprioceptive information caused by the passive movement. The role of the ipsilateral M1 activation has not been elucidated yet. However, this result agrees with the previous study, which investigated the interferences of afferent feedback in the mu rhythm modulation (Hommelsen et al., 2017). Furthermore, a recent motor task study showed that ipsilateral M1 ERD was related to fine hand and finger movements and the ability to maintain a steady level of contraction (Porcaro et al., 2021). Therefore, the ERD in ipsilateral M1 might have been caused to maintain a steady hand and finger posture during the trials in the present study.
In the neurofeedback system based on mu rhythm, subjects’ movement intention is usually classified against rest (or idle state) in the absence of any sensorimotor feedback (except for visual or auditory feedback (Birbaumer et al., 1999; Sellers and Donchin, 2006; Takahashi et al., 2008; Furdea et al., 2009; Ramos-Murguialday et al., 2012) or the presence of vibrotactile or proprioceptive stimuli (Pfurtscheller et al., 1998; Klimesch, 1999; Cassim et al., 2000; Chatterjee et al., 2007; Ramos et al., 2009). One of the challenges in asynchronous feedback devices (Ramos-Murguialday et al., 2012; Vidaurre et al., 2016) is the insufficient understanding of the effects of sensory information inflow from the brain-controlled peripheral stimulation (Cho et al., 2011; Hoffmann et al., 2011; Vidaurre et al., 2013). The previous study showed that the afferent information can cause false positives by the classifier in detecting motor commands, particularly in the presence of sensory information induced incorrectly or accidentally without motor intentions (Hommelsen et al., 2017). Therefore, the afferent feedback should be considered in the design of the closed-loop rehabilitation system, such as using spatial filters for only motor-related mu-rhythm. In addition, more attention should be paid to investigating the simultaneous efferent and afferent components for a contingent link between brain signals and feedback devices (e.g., exoskeleton or FES).
Even though no significant difference between the two conditions was found in the second dynamic period (phase IV), the suppression in mu rhythm induced during ORTHOSIS tends to be faster and larger compared to the ORTHOFES condition. This trend could reflect a net increase in afferent activity change in the cortex, as during ORTHOFES, muscle contraction occurred during the previous static phase. We demonstrate that the net increase in afference cortical activity is larger in the transition between posture and movement during ORTHOSIS than during the ORTHOFES condition. This trend indicates that the afference produced by the contribution of electrical stimulation (afference related to muscle contraction and mechanoreceptors) has a significantly lower influence on alpha ERD than the one evoked simply by proprioception. Therefore, the movement of the limb (the receptors related to it) is the principal component of the afferent activity. The trend has important implications for neurorehabilitation based on Brain-Machine-interfaces. It highlights proprioceptive feedback as probably the best option to induce cortical changes based on afferent signals to close the loop between the brain and movement. We are aware that the number of subjects studied in the here presented work is a limitation, but we compensated for this with our conservative artifact rejection and processing methods. However, further studies with a higher number of subjects are necessary to confirm this tendency.
It is known that beta ERS (or beta rebound) is induced after movement, reflecting the processing of sensorimotor information in the previous phase for an inhibitory rebound after excitation (Pfurtscheller et al., 1998; Cassim et al., 2000; Alegre et al., 2002). Different movements of the same limb could be classified by decoding EEG beta rebound in a post-movement period (Pfurtscheller et al., 1998). In our results, the post-movement beta-band rebound was not different between conditions in any of the bands analyzed, which might not reflect the difference in afferent information processing between conditions during the entire movement sequence but only during the last movement sub-sequence or phase, which was identical (phase V in Figure 5B). Unfortunately, our experimental protocol does not allow us to study the sensory post-processing difference between conditions. FES was applied during hand opening (phase II) and hand-stretched (phase III), but the hand was closed only by the orthosis in both ORTHOSIS and ORTHOFES conditions. During the closing phase, the afferent differences between conditions might have been washed out because it takes about a few seconds for the ERD to return to the baseline (Cassim et al., 2000).
Our results indicate that mu rhythm was influenced considerably by muscle kinematics during finger flexion/extension produced by external devices, which has relevant implications for the design of neuromodulation and neurorehabilitation. Besides, the ERD decrease during the static condition (as part of a movement sequence) represents cognitive processing sustaining the ERD (as there is no movement at all). As far as we know, this is the first time this effect has been demonstrated during pure passive movement only. Furthermore, we showed that the combination of different movement afferent receptors might not increase the amplitude, but the modulation speed of the ERD and that proprioception is the principal component of the afferent activity during the passive movement.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Prof. Dr. med. D. Luft, Ethik-Kommission an der Medizinischen Fakultät der Eberhard-Karls-Universität und am Universitätsklinikum Tübingen. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
WC contributed to the research design and implementation, data acquisition, analysis of the results, and the manuscript. AR-M supervised the whole process of data acquisition, analysis, and manuscript revision. NB managed the entire project and reviewed the manuscript. JA participated in the scientific input and contributed to the manuscript revision. CV aided in interpreting the results and revising the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by Daegu Gyeongbuk Institute of Science and Technology (DGIST), the Ministry of Education, Science and Technology of Republic of Korea, the Fortüne-Program of the University of Tübingen (2422-0-0), Eurostars Project E! 113928 SubliminalHomeRehab, the BMBF (Bundesministerium für Bildung und Forschung) (FKZ:01QE2023), and “Brain2Move” project (GFA:ID08, Diputación Foral de Gipuzkoa).
We thank Jürgen Dax for his technical support in the development of FES triggering system.
WC was employed by g.tec Medical Engineering GmbH.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnsys.2023.1045396/full#supplementary-material
Alegre, M., de Gurtubay, I. G., Labarga, A., Iriarte, J., Malanda, A., and Artieda, J. (2004). Alpha and beta oscillatory activity during a sequence of two movements. Clin. Neurophysiol. 115, 124–130. doi: 10.1016/S1388-2457(03)00311-0
Alegre, M., Labarga, A., Gurtubay, I. G., Iriarte, J., Malanda, A., and Artieda, J. (2002). Beta electroencephalograph changes during passive movements: Sensory afferences contribute to beta event-related desynchronization in humans. Neurosci. Lett. 331, 29–32. doi: 10.1016/s0304-3940(02)00825-x
Anderson, K. L., and Ding, M. (2011). Attentional modulation of the somatosensory mu rhythm. Neuroscience 180, 165–180. doi: 10.1016/j.neuroscience.2011.02.004
Antelis, J. M., Montesano, L., Ramos-Murguialday, A., Birbaumer, N., and Minguez, J. (2013). On the usage of linear regression models to reconstruct limb kinematics from low frequency EEG signals. PLoS One 8:e61976. doi: 10.1371/journal.pone.0061976
Babiloni, C., Brancucci, A., Arendt-Nielsen, L., Babiloni, F., Capotosto, P., Carducci, F., et al. (2004). Attentional processes and cognitive performance during expectancy of painful galvanic stimulations: A high-resolution EEG study. Behav. Brain Res. 152, 137–147. doi: 10.1016/j.bbr.2003.10.004
Babiloni, C., Capotosto, P., Brancucci, A., del Percio, C., Petrini, L., Buttiglione, M., et al. (2008). Cortical alpha rhythms are related to the anticipation of sensorimotor interaction between painful stimuli and movements: A high-resolution EEG study. J. Pain 9, 902–911. doi: 10.1016/j.jpain.2008.05.007
Ball, T., Demandt, E., Mutschler, I., Neitzel, E., Mehring, C., Vogt, K., et al. (2008). Movement related activity in the high gamma range of the human EEG. Neuroimage 41, 302–310. doi: 10.1016/j.neuroimage.2008.02.032
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Methodol. 57, 289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
Bibián, C., Irastorza-Landa, N., Schönauer, M., Birbaumer, N., López-Larraz, E., and Ramos-Murguialday, A. (2021). On the extraction of purely motor EEG neural correlates during an upper limb visuomotor task. Cereb. Cortex 32, 4243–4254. doi: 10.1093/cercor/bhab479
Birbaumer, N., Elbert, T., Canavan, A. G., and Rockstroh, B. (1990). Slow potentials of the cerebral cortex and behavior. Physiol. Rev. 70, 1–41.
Birbaumer, N., Ghanayim, N., Hinterberger, T., Iversen, I., Kotchoubey, B., Kübler, A., et al. (1999). A spelling device for the paralysed. Nature 398, 297–298. doi: 10.1038/18581
Bourguignon, M., Jousmäki, V., Dalal, S. S., Jerbi, K., and de Tiège, X. (2019). Coupling between human brain activity and body movements: Insights from non-invasive electromagnetic recordings. Neuroimage 203:116177. doi: 10.1016/j.neuroimage.2019.116177
Bourguignon, M., Piitulainen, H., de Tiège, X., Jousmäki, V., and Hari, R. (2015). Corticokinematic coherence mainly reflects movement-induced proprioceptive feedback. Neuroimage 106, 382–390. doi: 10.1016/j.neuroimage.2014.11.026
Branco, M. P., de Boer, L. M., Ramsey, N. F., and Vansteensel, M. J. (2019). Encoding of kinetic and kinematic movement parameters in the sensorimotor cortex: A Brain-Computer Interface perspective. Eur. J. Neurosci. 50, 2755–2772. doi: 10.1111/ejn.14342
Branco, M. P., Freudenburg, Z. V., Aarnoutse, E. J., Bleichner, M. G., Vansteensel, M. J., and Ramsey, N. F. (2017). Decoding hand gestures from primary somatosensory cortex using high-density ECoG. Neuroimage 147, 130–142. doi: 10.1016/j.neuroimage.2016.12.004
Burgess, A. P., and Gruzelier, J. H. (1999). “Methodological advances in the analysis of event-related desynchronization data: Reliability and robust analysis,” in Handbook of electroencephalography and clinical neurophysiology, revised series, Vol. 6, eds G. Pfurtscheller and F. H. Lopes da Silva (Amsterdam: Elsevier), 139–158.
Buzsáki, G. (2009). Rhythms of the Brain. Oxford: Oxford University Press, doi: 10.1093/acprof:oso/9780195301069.001.0001
Cassim, F., Szurhaj, W., Sediri, H., Devos, D., Bourriez, J., Poirot, I., et al. (2000). Brief and sustained movements: Differences in event-related (de)synchronization (ERD/ERS) patterns. Clin. Neurophysiol. 111, 2032–2039. doi: 10.1016/s1388-2457(00)00455-7
Chakarov, V., Naranjo, J. R., Schulte-Mönting, J., Omlor, W., Huethe, F., and Kristeva, R. (2009). Beta-range EEG-EMG coherence with isometric compensation for increasing modulated low-level forces. J. Neurophysiol. 102, 1115–1120. doi: 10.1152/jn.91095.2008
Chatterjee, A., Aggarwal, V., Ramos, A., Acharya, S., and Thakor, N. V. (2007). A brain-computer interface with vibrotactile biofeedback for haptic information. J. Neuroeng. Rehabil. 4:40. doi: 10.1186/1743-0003-4-40
Cho, W., Vidaurre, C., Hoffmann, U., Birbaumer, N., and Ramos-Murguialday, A. (2011). “Afferent and efferent activity control in the design of brain computer interfaces for motor rehabilitation,” in Engineering in medicine and biology society, EMBC, 2011 annual international conference of the IEEE, (Piscataway, NJ: IEEE), 7310–7315. doi: 10.1109/IEMBS.2011.6091705
Chye, L., Nosaka, K., Murray, L., Edwards, D., and Thickbroom, G. (2010). Corticomotor excitability of wrist flexor and extensor muscles during active and passive movement. Hum. Mov. Sci. 29, 494–501. doi: 10.1016/j.humov.2010.03.003
Corbet, T., Iturrate, I., Pereira, M., Perdikis, S., Millán, J., and del, R. (2018). Sensory threshold neuromuscular electrical stimulation fosters motor imagery performance. Neuroimage 176, 268–276. doi: 10.1016/j.neuroimage.2018.04.005
Crone, N. E., Miglioretti, D. L., Gordon, B., Sieracki, J. M., Wilson, M. T., Uematsu, S., et al. (1998). Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event-related desynchronization. Brain 121, 2271–2299.
Daubechies, I. (1996). Where do wavelets come from? A personal point of view. Proc. IEEE 84, 510–513. doi: 10.1109/5.488696
Degenhart, A. D., Hiremath, S., Yang, Y., Foldes, S., Collinger, J. L., Boninger, M., et al. (2018). Remapping cortical modulation for electrocorticographic brain-computer interfaces: A somatotopy-based approach in individuals with upper-limb paralysis. J Neural Eng. 15:026021. doi: 10.1088/1741-2552/aa9bfb.Remapping
Edwards, L. L., King, E. M., Buetefisch, C. M., and Borich, M. R. (2019). Putting the “sensory” into sensorimotor control: The role of sensorimotor integration in goal-directed hand movements after stroke. Front. Integr. Neurosci. 13:16. doi: 10.3389/fnint.2019.00016
Furdea, A., Halder, S., Krusienski, D. J., Bross, D., Nijboer, F., Birbaumer, N., et al. (2009). An auditory oddball (P300) spelling system for brain-computer interfaces. Psychophysiology 46, 617–625. doi: 10.1111/j.1469-8986.2008.00783.x
Grimm, F., and Gharabaghi, A. (2016). Closed-loop neuroprosthesis for reach-to-grasp assistance: Combining adaptive multi-channel neuromuscular stimulation with a multi-joint arm exoskeleton. Front. Neurosci. 10:284. doi: 10.3389/fnins.2016.00284
Guger, C., Edlinger, G., Harkam, W., Niedermayer, I., and Pfurtscheller, G. (2003). How many people are able to operate an EEG-based brain-computer interface (BCI)? IEEE Trans. Neural Syst. Rehabil. Eng. 11, 145–147. doi: 10.1109/TNSRE.2003.814481
Hari, R., and Salmelin, R. (1997). Human cortical oscillations: A neuromagnetic view through the skull. Trends Neurosci. 20, 44–49. doi: 10.1016/S0166-2236(96)10065-5
Hishinuma, A. K., Gulati, T., Burish, M. J., and Ganguly, K. (2019). Large-scale changes in cortical dynamics triggered by repetitive somatosensory electrical stimulation. J. Neuroeng. Rehabil. 16:59. doi: 10.1186/s12984-019-0520-1
Hoffmann, U., Cho, W., Ramos-Murguialday, A., and Keller, T. (2011). “Detection and removal of stimulation artifacts in electroencephalogram recordings,” in 2011 Annual international conference of the IEEE engineering in medicine and biology society, (Piscataway, NJ: IEEE), 7159–7162. doi: 10.1109/IEMBS.2011.6091809
Hogan, N., Krebs, H. I., Rohrer, B., Palazzolo, J. J., Dipietro, L., Fasoli, S. E., et al. (2006). Motions or muscles? Some behavioral factors underlying robotic assistance of motor recovery. J. Rehabil. Res. Dev. 43, 605–618. doi: 10.1682/JRRD.2005.06.0103
Hommelsen, M., Schneiders, M., Schuld, C., Keyl, P., and Rüpp, R. (2017). Sensory feedback interferes with mu rhythm based detection of motor commands from electroencephalographic signals. Front. Hum. Neurosci. 11:523. doi: 10.3389/fnhum.2017.00523
Houdayer, E., Labyt, E., Cassim, F., Bourriez, J. L., and Derambure, P. (2006). Relationship between event-related beta synchronization and afferent inputs: Analysis of finger movement and peripheral nerve stimulations. Clin. Neurophysiol. 117, 628–636. doi: 10.1016/j.clinph.2005.12.001
Insausti-Delgado, A., López-Larraz, E., Omedes, J., and Ramos-Murguialday, A. (2021). Intensity and dose of neuromuscular electrical stimulation influence sensorimotor cortical excitability. Front. Neurosci. 14:593360. doi: 10.3389/fnins.2020.593360
Jones, S. R., Kerr, C. E., Wan, Q., Pritchett, D. L., Hämäläinen, M., and Moore, C. I. (2010). Cued spatial attention drives functionally relevant modulation of the mu rhythm in primary somatosensory cortex. J. Neurosci. 30, 13760–13765. doi: 10.1523/JNEUROSCI.2969-10.2010
Jones, S. R., Pritchett, D. L., Sikora, M. A., Stufflebeam, S. M., Hämäläinen, M., and Moore, C. I. (2009). Quantitative analysis and biophysically realistic neural modeling of the MEG mu rhythm: Rhythmogenesis and modulation of sensory-evoked responses. J. Neurophysiol. 102, 3554–3572. doi: 10.1152/jn.00535.2009
Katz, R., Penicaud, A., and Rossit, A. (1991). Reciprocal Ia inhibition between elbow flexors and extensors in the human. J. Physiol. 437, 269–286. doi: 10.1113/jphysiol.1991.sp018595
Kenville, R., Maudrich, T., Vidaurre, C., Maudrich, D., Villringer, A., Nikulin, V. V., et al. (2020). Corticomuscular interactions during different movement periods in a multi-joint compound movement. Sci. Rep. 10:5021. doi: 10.1038/s41598-020-61909-z
Kikkert, S., Kolasinski, J., Jbabdi, S., Tracey, I., Beckmann, C. F., Berg, H. J., et al. (2016). Revealing the neural fingerprints of a missing hand. Elife 5:e15292. doi: 10.7554/eLife.15292
Klimesch, W. (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 29, 169–195. doi: 10.1016/s0165-0173(98)00056-3
Klimesch, W. (2011). Evoked alpha and early access to the knowledge system: The P1 inhibition timing hypothesis. Brain Res. 1408, 52–71. doi: 10.1016/j.brainres.2011.06.003
Knutson, J. S., Fu, M. J., Sheffler, L. R., and Chae, J. (2015). Neuromuscular electrical stimulation for motor restoration in hemiplegia. Phys. Med. Rehabil. Clin. N. Am. 26, 729–745. doi: 10.1016/j.pmr.2015.06.002
Koutsou, A. D., Moreno, J. C., del Ama, A. J., Rocon, E., and Pons, J. L. (2016). Advances in selective activation of muscles for non-invasive motor neuroprostheses. J. Neuroeng. Rehabil. 13:56. doi: 10.1186/s12984-016-0165-2
Kuhlman, W. N. (1978). Functional topography of the human mu rhythm. Electroencephalogr. Clin. Neurophysiol. 44, 83–93. doi: 10.1016/0013-4694(78)90107-4
Liu, D., Chen, W., Lee, K., Chavarriaga, R., Bouri, M., Pei, Z., et al. (2017). Brain-actuated gait trainer with visual and proprioceptive feedback. J. Neural Eng. 14:56017. doi: 10.1088/1741-2552/aa7df9
López-Larraz, E., Figueiredo, T. C., Insausti-Delgado, A., Ziemann, U., Birbaumer, N., and Ramos-Murguialday, A. (2018). Event-related desynchronization during movement attempt and execution in severely paralyzed stroke patients: An artifact removal relevance analysis. Neuroimage Clin. 20, 972–986. doi: 10.1016/j.nicl.2018.09.035
Mazzoleni, S., Duret, C., Grosmaire, A. G., and Battini, E. (2017). Combining upper limb robotic rehabilitation with other therapeutic approaches after stroke: Current status, rationale, and challenges. Biomed. Res. Int. 2017:8905637. doi: 10.1155/2017/8905637
McFarland, D. J., McCane, L. M., David, S. V., and Wolpaw, J. R. (1997). Spatial filter selection for EEG-based communication. Electroencephalogr. Clin. Neurophysiol. 103, 386–394.
Miller, K. J., Leuthardt, E. C., Schalk, G., Rao, R. P. N., Anderson, N. R., Moran, D. W., et al. (2007). Spectral changes in cortical surface potentials during motor movement. J. Neurosci. 27, 2424–2432. doi: 10.1523/JNEUROSCI.3886-06.2007
Moon, S. H., Choi, J. H., and Park, S. E. (2017). The effects of functional electrical stimulation on muscle tone and stiffness of stroke patients. J. Phys. Ther. Sci. 29, 238–241. doi: 10.1589/jpts.29.238
Müller, G. R., Neuper, C., Rupp, R., Keinrath, C., Gerner, H. J., and Pfurtscheller, G. (2003). Event-related beta EEG changes during wrist movements induced by functional electrical stimulation of forearm muscles in man. Neurosci. Lett. 340, 143–147. doi: 10.1016/S0304-3940(03)00019-3
Müller-putz, G. R., Zimmermann, D., Graimann, B., Nestinger, K., Korisek, G., and Pfurtscheller, G. (2007). Event-related beta EEG-changes during passive and attempted foot movements in paraplegic patients. Brain Res. 1137, 84–91. doi: 10.1016/j.brainres.2006.12.052
Nakayashiki, K., Saeki, M., Takata, Y., Hayashi, Y., and Kondo, T. (2014). Modulation of event-related desynchronization during kinematic and kinetic hand movements. J. Neuroeng. Rehabil. 11:90. doi: 10.1186/1743-0003-11-90
Neuper, C., Wörtz, M., and Pfurtscheller, G. (2006). ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog. Brain Res. 159, 211–222. doi: 10.1016/S0079-6123(06)59014-4
Oostenveld, R., Fries, P., Maris, E., and Schoffelen, J. M. (2011). FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011:156869. doi: 10.1155/2011/156869
Paillard, J., and Brouchon, M. (1968). “Active and passive movements in the calibration of position sense,” in The neuropsychology of spatially oriented behavior, ed. S. Freedman (Chicago, IL: Dorsey Press), 37–55.
Perlmutter, S. I., Maier, M. A., and Fetz, E. E. (1998). Activity of spinal interneurons and their effects on forearm muscles during voluntary wrist movements in the monkey. J. Neurophysiol. 80, 2475–2494. doi: 10.1152/jn.1998.80.5.2475
Pfurtscheller, G., and Lopes da Silva, F. H. (1999). Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 110, 1842–1857. doi: 10.1016/s1388-2457(99)00141-8
Pfurtscheller, G., Neuper, C., Flotzinger, D., and Pregenzer, M. (1997). EEG-based discrimination between imagination of right and left hand movement. Electroencephalogr. Clin. Neurophysiol. 103, 642–651.
Pfurtscheller, G., Pregenzer, M., and Neuper, C. (1994). Visualization of sensorimotor areas involved in preparation for hand movement based on classification of μ and central β rhythms in single EEG trials in man. Neurosci. Lett. 181, 43–46. doi: 10.1016/0304-3940(94)90556-8
Pfurtscheller, G., Zalaudek, K., and Neuper, C. (1998). Event-related beta synchronization after wrist, finger and thumb movement. Electroencephalogr. Clin. Neurophysiol. 109, 154–160. doi: 10.1016/s0924-980x(97)00070-2
Porcaro, C., Mayhew, S. D., and Bagshaw, A. P. (2021). Role of the ipsilateral primary motor cortex in the visuo-motor network during fine contractions and accurate performance. Int. J. Neural Syst. 31:2150011. doi: 10.1142/S0129065721500118
Purves, D., Augustine, G. J., Fitzpatrick, D., Hall, W. C., LaMantia, A. S., McNamara, J. O., et al. (2004). Neuroscience, 3rd Edn. Sunderland, MA: Sinauer Associates Inc., doi: 10.1111/j.1365-2486.2006.01288.x
Ramos, A., Halder, S., and Birbaumer, N. (2009). “Proprioceptive feedback in BCI,” in 2009 4th international IEEE/EMBS conference on neural engineering, NER ’, (Piscataway, NJ: IEEE), 279–282. doi: 10.1109/NER.2009.5109287
Ramos-Murguialday, A., and Birbaumer, N. (2015). Brain oscillatory signatures of motor tasks. J. Neurophysiol. 113, 3663–3682. doi: 10.1152/jn.00467.2013
Ramos-Murguialday, A., Broetz, D., Rea, M., Läer, L., Yilmaz, O., Brasil, F. L., et al. (2013). Brain-machine interface in chronic stroke rehabilitation: A controlled study. Ann. Neurol. 74, 100–108. doi: 10.1002/ana.23879
Ramos-Murguialday, A., Schürholz, M., Caggiano, V., Wildgruber, M., Caria, A., Hammer, E. M., et al. (2012). Proprioceptive feedback and brain computer interface (BCI) based neuroprostheses. PLoS One 7:e47048. doi: 10.1371/journal.pone.0047048
Ramsey, N. F. (2019). Preservation of hand movement representation in the sensorimotor areas of amputees. Brain 140, 3166–3178. doi: 10.1093/brain/awx274.Preservation
Ray, A. M., Figueiredo, T. D. C., López-Larraz, E., Birbaumer, N., and Ramos-Murguialday, A. (2020). Brain oscillatory activity as a biomarker of motor recovery in chronic stroke. Hum. Brain Mapp. 41, 1296–1308. doi: 10.1002/hbm.24876
Ritter, P., Moosmann, M., and Villringer, A. (2009). Rolandic alpha and beta EEG rhythms’ strengths are inversely related to fMRI-BOLD signal in primary somatosensory and motor cortex. Hum. Brain Mapp. 30, 1168–1187. doi: 10.1002/hbm.20585
Rosenkranz, K., and Rothwell, J. C. (2006). Differences between the effects of three plasticity inducing protocols on the organization of the human motor cortex. Eur. J. Neurosci. 23, 822–829. doi: 10.1111/j.1460-9568.2006.04605.x
Salmelin, R., and Hari, R. (1994). Characterization of spontaneous MEG rhythms in healthy adults. Electroencephalogr. Clin. Neurophysiol. 91, 237–248. doi: 10.1016/0013-4694(94)90187-2
Salmelin, R., Hämäläinen, M., Kajola, M., and Hari, R. (1995). Functional segregation of movement-related rhythmic activity in the human brain. Neuroimage 2, 237–243. doi: 10.1006/nimg.1995.1031
Sanes, J. N., and Donoghue, J. P. (2000). Plasticity and primary motor cortex. Annu. Rev. Neurosci. 23, 393–415. doi: 10.1146/annurev.neuro.23.1.393
Sellers, E. W., and Donchin, E. (2006). A P300-based brain-computer interface: Initial tests by ALS patients. Clin. Neurophysiol. 117, 538–548. doi: 10.1016/j.clinph.2005.06.027
Shaikhouni, A., Donoghue, J. P., and Hochberg, L. R. (2013). Somatosensory responses in a human motor cortex. J. Neurophysiol. 109, 2192–2204. doi: 10.1152/jn.00368.2012
Sheffler, L. R., and Chae, J. (2007). Neuromuscular electrical stimulation in neurorehabilitation. Muscle Nerve 35, 562–590. doi: 10.1002/mus.20758
Shin, H., and Hu, X. (2018). “Flexibility of finger activation patterns elicited through non-invasive multi-electrode nerve stimulation,” in 2018 40th annual international conference of the IEEE engineering in medicine and biology society (EMBC), (Piscataway, NJ: IEEE), 1428–1431. doi: 10.1109/EMBC.2018.8512479
Sivaganesan, S. (1994). An Introduction to the Bootstrap (Bradley Efron and Robert J. Tibshirani). SIAM Rev. 36, 677–678. doi: 10.1137/1036171
Stancák, A., and Pfurtscheller, G. (1995). Desynchronization and recovery of β rhythms during brisk and slow self-paced finger movements in man. Neurosci. Lett. 196, 21–24. doi: 10.1016/0304-3940(95)11827-J
Stancák, A., Svoboda, J., Rachmanová, R., Vrána, J., Králík, J., and Tintera, J. (2003). Desynchronization of cortical rhythms following cutaneous stimulation: Effects of stimulus repetition and intensity, and of the size of corpus callosum. Clin. Neurophysiol. 114, 1936–1947. doi: 10.1016/S1388-2457(03)00201-3
Sun, H., Blakely, T. M., Darvas, F., Wander, J. D., Johnson, L. A., Su, D. K., et al. (2015). Sequential activation of premotor, primary somatosensory and primary motor areas in humans during cued finger movements. Clin. Neurophysiol. 126, 2150–2161. doi: 10.1016/j.clinph.2015.01.005
Takahashi, C. D., Der-Yeghiaian, L., Le, V., Motiwala, R. R., and Cramer, S. C. (2008). Robot-based hand motor therapy after stroke. Brain 131, 425–437. doi: 10.1093/brain/awm311
Taylor, D. M. (2008). “Functional electrical stimulation and rehabilitation applications of BCIs,” in Brain-computer interfaces, (Dordrecht: Springer), 81–94. doi: 10.1007/978-1-4020-8705-9_6
Tu-Chan, A. P., Natraj, N., Godlove, J., Abrams, G., and Ganguly, K. (2017). Effects of somatosensory electrical stimulation on motor function and cortical oscillations. J. Neuroeng. Rehabil. 14:113. doi: 10.1186/s12984-017-0323-1
Vidaurre, C., Jorajuría, T., Ramos-Murguialday, A., Müller, K. R., Gómez, M., and Nikulin, V. V. (2021). Improving motor imagery classification during induced motor perturbations. J. Neural Eng. 18:0460b1. doi: 10.1088/1741-2552/ac123f
Vidaurre, C., Klauer, C., Schauer, T., Ramos-Murguialday, A., and Müller, K. R. (2016). EEG-based BCI for the linear control of an upper-limb neuroprosthesis. Med. Eng. Phys. 38, 1195–1204. doi: 10.1016/j.medengphy.2016.06.010
Vidaurre, C., Pascual, J., Ramos-Murguialday, A., Lorenz, R., Blankertz, B., Birbaumer, N., et al. (2013). Neuromuscular electrical stimulation induced brain patterns to decode motor imagery. Clin. Neurophysiol. 124, 1824–1834. doi: 10.1016/j.clinph.2013.03.009
Vidaurre, C., Ramos Murguialday, A., Haufe, S., Gómez, M., Müller, K. R., and Nikulin, V. V. (2019b). Enhancing sensorimotor BCI performance with assistive afferent activity: An online evaluation. Neuroimage 199, 375–386. doi: 10.1016/j.neuroimage.2019.05.074
Vidaurre, C., Nolte, G., de Vries, I. E. J., Gómez, M., Boonstra, T. W., Müller, K. R., et al. (2019a). Canonical maximization of coherence: A novel tool for investigation of neuronal interactions between two datasets. Neuroimage 201:116009. doi: 10.1016/j.neuroimage.2019.116009
Wagner, J., Makeig, S., Hoopes, D., and Gola, M. (2019). Can oscillatory alpha-gamma phase-amplitude coupling be used to understand and enhance TMS effects? Front. Hum. Neurosci. 13:263. doi: 10.3389/fnhum.2019.00263
Wagner, J., Solis-Escalante, T., Grieshofer, P., Neuper, C., Müller-Putz, G., and Scherer, R. (2012). Level of participation in robotic-assisted treadmill walking modulates midline sensorimotor EEG rhythms in able-bodied subjects. Neuroimage 63, 1203–1211. doi: 10.1016/j.neuroimage.2012.08.019
Wang, W., Collinger, J. L., Degenhart, A. D., Tyler-Kabara, E. C., Schwartz, A. B., Moran, D. W., et al. (2013). An electrocorticographic brain interface in an individual with tetraplegia. PLoS One 8:e55344. doi: 10.1371/journal.pone.0055344
Weber, L. M., and Stein, J. (2018). The use of robots in stroke rehabilitation: A narrative review. NeuroRehabilitation 43, 99–110. doi: 10.3233/NRE-172408
Wiesman, A. I., and Wilson, T. W. (2020). Attention modulates the gating of primary somatosensory oscillations. Neuroimage 211:116610. doi: 10.1016/j.neuroimage.2020.116610
Yang, J. D., Liao, C., Huang, S. W., Tam, K. W., Liou, T. H., Lee, Y. H., et al. (2019). Effectiveness of electrical stimulation therapy in improving arm function after stroke: A systematic review and a meta-analysis of randomised controlled trials. Clin. Rehabil. 33, 1286–1297. doi: 10.1177/0269215519839165
Keywords: somatosensory mu rhythm, robot, functional electrical stimulation, kinematic, kinetic, neurorehabilitation
Citation: Cho W, Vidaurre C, An J, Birbaumer N and Ramos-Murguialday A (2023) Cortical processing during robot and functional electrical stimulation. Front. Syst. Neurosci. 17:1045396. doi: 10.3389/fnsys.2023.1045396
Received: 15 September 2022; Accepted: 28 February 2023;
Published: 21 March 2023.
Edited by:
Andrea Canessa, University of Genoa, ItalyReviewed by:
Scott A. Beardsley, Marquette University, United StatesCopyright © 2023 Cho, Vidaurre, An, Birbaumer and Ramos-Murguialday. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Woosang Cho, woosang.cho@student.uni-tuebingen.de
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.