- 1Department of Physiology and Cell Biology, College of Medicine, University of South Alabama, Mobile, AL, United States
- 2Department of Neurology, Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research, Intellectual and Developmental Disabilities Research Center, University of California, Los Angeles, Los Angeles, CA, United States
- 3Department of Health, Kinesiology, and Sport, University of South Alabama, Mobile, AL, United States
- 4Department of Psychology, University of South Alabama, Mobile, AL, United States
- 5Department of Physician Assistant Studies, University of South Alabama, Mobile, AL, United States
- 6College of Medicine, University of South Alabama, Mobile, AL, United States
- 7Department of Internal Medicine, College of Medicine, University of South Alabama, Mobile, AL, United States
Alzheimer’s disease and related dementias (ADRD) are an expanding worldwide crisis. In the absence of scientific breakthroughs, the global prevalence of ADRD will continue to increase as more people are living longer. Racial or ethnic minority groups have an increased risk and incidence of ADRD and have often been neglected by the scientific research community. There is mounting evidence that vascular insults in the brain can initiate a series of biological events leading to neurodegeneration, cognitive impairment, and ADRD. We are a group of researchers interested in developing and expanding ADRD research, with an emphasis on vascular contributions to dementia, to serve our local diverse community. Toward this goal, the primary objective of this review was to investigate and better understand health disparities in Alabama and the contributions of the social determinants of health to those disparities, particularly in the context of vascular dysfunction in ADRD. Here, we explain the neurovascular dysfunction associated with Alzheimer’s disease (AD) as well as the intrinsic and extrinsic risk factors contributing to dysfunction of the neurovascular unit (NVU). Next, we ascertain ethnoregional health disparities of individuals living in Alabama, as well as relevant vascular risk factors linked to AD. We also discuss current pharmaceutical and non-pharmaceutical treatment options for neurovascular dysfunction, mild cognitive impairment (MCI) and AD, including relevant studies and ongoing clinical trials. Overall, individuals in Alabama are adversely affected by social and structural determinants of health leading to health disparities, driven by rurality, ethnic minority status, and lower socioeconomic status (SES). In general, these communities have limited access to healthcare and healthy food and other amenities resulting in decreased opportunities for early diagnosis of and pharmaceutical treatments for ADRD. Although this review is focused on the current state of health disparities of ADRD patients in Alabama, future studies must include diversity of race, ethnicity, and region to best be able to treat all individuals affected by ADRD.
Introduction
ADRD remain a global health crisis for all affected individuals including patients with ADRD, individuals at risk, and caregivers such as friends and family. For over a century, ADRD research has made significant efforts to cure, treat, or prevent ADRD. However, there is a clear lack of research focusing on regional, racial, and ethnic disparities in ADRD, particularly in Southern, rural and poor states like Alabama. It is estimated that over 94,000 people in Alabama have AD (Alzheimer’s Association, 2022) and AD is the sixth leading cause of death of people in Alabama (Alabama, 2021). African Americans are more likely to develop AD than White Americans (Blum et al., 2018; Alzheimer’s Association, 2022). According to the US census as of 2021, African Americans account for 13.4% of the American population, but in Alabama 26.8% of the over 5 million people are African American (Census, 2022), presenting a greater risk and prevalence of ADRD in Alabama. In addition, more than a million Alabamians live in rural areas, where there are even more significant health disparities (Rural Health Information Hub, 2022). This combination of high-risk populations presents an urgent need for research focusing on ADRD in Alabama and other regions with similar demographics.
The neuropathologies found in the post-mortem brains of ADRD patients are complex and multifactorial. A number of amyloid isotypes accumulate in the brains of these patients including amyloid-β (Aβ) plaques, neurofibrillary tau tangles (NFTs), Lewy body α-synuclein pathologies, and transactive response DNA and RNA binding protein 43 kDa aggregates (Nelson et al., 2019; Robinson et al., 2021; Uemura et al., 2022). These neuropathologies are often associated with neurovascular abnormalities including large macroscopic, lacunar and microscopic infarcts, hemorrhages, and vessel pathologies including cerebral amyloid angiopathy, intracranial atherosclerosis and arteriolosclerosis. For example, in the Religious Order Study and Rush Memory and Aging Project cohort, ∼87% of probable AD diagnosed subjects had abnormal vascular neuropathologies. Approximately 74% of these subjects also had traditional AD and/or other neurodegenerative neuropathologies (Kapasi et al., 2017). This work and others described below support that neurovascular dysfunction occurs more often than not in ADRD.
Neurovascular dysfunction in ADRD can be partially attributed to cardiovascular deficits (Shabir et al., 2018). Neurovascular uncoupling, an early event in AD, leads to dysregulation of cerebral blood flow (CBF) and the NVU and is a major contributor to AD progression (Iadecola, 2004, 2013; Zlokovic, 2011; Montagne et al., 2015; Sweeney et al., 2015, 2019; Zhao et al., 2015; Arvanitakis et al., 2016; Iturria-Medina et al., 2016; Nelson et al., 2016; Kisler et al., 2017a; Nation et al., 2019). Blood-brain barrier (BBB) breakdown as well as pericyte injury and loss are also hallmark findings in AD, leading to chronic neuroinflammation, gliosis, Aβ deposition, and tau hyperphosphorylation (Collins-Praino and Corrigan, 2017; Perea et al., 2018). The aforementioned clinical findings in AD may be partially mitigated by education and awareness of several extrinsic vascular risk factors linked to AD. The most common genetic risk factor for AD is apolipoprotein ε4 (APOE4) carriage (Mahley et al., 2006). Studies have suggested that APOE4 mice (Bell et al., 2012) and humans (Montagne et al., 2020a) have increased BBB breakdown that corresponds with cognitive decline. Furthermore, APOE functions to transport lipids (e.g., cholesterol) in the bloodstream (Di Battista et al., 2016). Previous studies indicate that APOE4 carriage disrupts brain cells’ ability to metabolize lipids (Dupuy et al., 2001; Huang and Mahley, 2014). Metabolism deficiencies may be the cause of gut dysbiosis seen in AD cases, which contributes to increased proinflammatory cytokine levels and systemic inflammation (Al Bander et al., 2020). Furthermore, systemic infections, such as pneumonia, lead to peripheral generation of amyloids (e.g., Aβ and tau) and incident dementia that may ultimately contribute to ADRD (Nelson, 2022). Stress and anxiety also contribute to AD progression through consequences in subsequent behavioral and physiological changes (Dimsdale, 2008). Diet and lifestyle differences have also been identified as a risk factor for AD. Use of alcohol and recreational drugs is linked to ADRD diagnosis and cognitive impairment. These identified risk factors not only contribute to AD progression, but related cardiovascular and pulmonary diseases as well. For this reason, cardiovascular and pulmonary diseases have been linked to dementia via disruption of the BBB and neuroinflammation. However, it is important to note that vascular risk factors that occur in midlife may be temporally uncoupled with cognitive dysfunction, suggesting that aging is a contributing factor to neurovascular dysfunction in ADRD.
Although there is no cure for ADRD, several medications may be prescribed to temporarily alleviate symptoms through decreasing hypertension, protecting neurovasculature, or correcting gut dysbiosis (Ahmed, 2005; Stirban et al., 2006; Raj et al., 2018; Williamson et al., 2019; Nasrallah et al., 2021). Non-pharmaceutical treatments may also be viable options to slow cognitive decline in AD patients, also commonly through the correction of gut dysbiosis. Physical activity has been shown to decrease the risk of AD and slow cognitive decline, even in APOE4 carriers (Allard et al., 2017). However, the South has the highest prevalence of physical inactivity in the United States (Centers for Disease Control and Prevention, 2021). Treatment of mood disorders has also been shown to protect vascular health through prevention of adopting unhealthy behaviors such as smoking and physical inactivity (Abed et al., 2014). Other non-pharmaceutical and alternative treatments for AD such as hormone replacement therapy and oxygen therapy have also been suggested to treat patients with AD, but their efficacies are still unclear (Smith et al., 2010; Harch and Fogarty, 2018; Guo et al., 2020; Song et al., 2020; Shapira et al., 2021; Somaa, 2021).
Every individual has a unique social determinants of health profile and many individuals living in Alabama suffer from inequities in health and health care. Social determinants of health are defined as, “the conditions in the environments where people are born, live, learn, work, play, worship, and age that affect a wide range of health, functioning, and quality-of-life outcomes and risks” (Healthy People, 2022). Health disparities are usually manifested in groups that are disadvantaged. Individuals and groups may be disadvantaged due to their race or ethnicity, sex, sexual identity, age, disability, SES, cognitive abilities, and geography (Foundation Health Measures Archive, 2020). In most of the US and in Alabama specifically, SES and race/ethnicity are the areas of disadvantage that have the greatest impact on health disparities (Arrieta et al., 2008).
Although there are numerous potential treatments to slow cognitive decline and vascular dysfunction progression in AD, many social and structural determinants of health in Alabama make it difficult to access these treatments or even diagnose ADRD. Many regions within Alabama suffer from low SES creating the challenge to adopt healthy eating and exercise habits that might prevent or delay onset of ADRD. Diagnosis of dementia tends to be earlier in individuals with high SES, when interventions may have an impact, than people with low SES (Cha et al., 2021; Petersen et al., 2021). Many areas in Alabama, both urban and rural, have limited access to healthcare and healthy food, making it more difficult to initiate crucial lifestyle changes to slow AD progression. The large rural population of Alabama further adds to the increased prevalence of AD. Taken together, it is clear that this region presents numerous risk factors of ADRD as well as substantial barriers preventing the mitigation of these risks. Reports are desperately needed to highlight the urgency of developing preventative strategies targeted at ADRD, especially in states with an ethnic and racial makeup like Alabama. Low SES and health disparities exist in global communities contributing to ADRD worldwide. Therefore, in this review, we present the current social and structural determinants of health that lead to health disparities contributing to neurovascular dysfunction and ADRD in Alabama and beyond.
Neurovascular Dysfunction in Alzheimer’s Disease and Dementia
Neurovascular Uncoupling and Cerebral Blood Flow Reductions
The brain is metabolically active and requires 20% of the body’s oxygen and glucose for proper functioning (Iadecola, 2013). The NVU consists of vascular cells (e.g., endothelial cells, pericytes, and vascular smooth muscle cells), glia (e.g., astrocytes, microglia, and oligodendrocytes), and neurons (Nelson et al., 2016; Sweeney et al., 2019) (Figure 1). Neurovascular coupling refers to the communication and connection between the vasculature and brain cells. The NVU regulates CBF through neurovascular coupling to assure that brain energy needs are met (Kisler et al., 2017a). There is growing appreciation and strong evidence that neurovascular uncoupling, CBF reductions and dysregulation, and breakdown of the BBB, including the loss of pericytes, are early events in the AD pathophysiological cascade (Iadecola, 2004, 2013; Zlokovic, 2011; Montagne et al., 2015, 2020a; Sweeney et al., 2015, 2019; Arvanitakis et al., 2016; Iturria-Medina et al., 2016; Nelson et al., 2016; Kisler et al., 2017a) (Figure 1). In a clinical study, African Americans were found to have lower intracranial arterial blood flow than White people which was associated with higher fasting glucose and triglyceride levels (Clark et al., 2019).
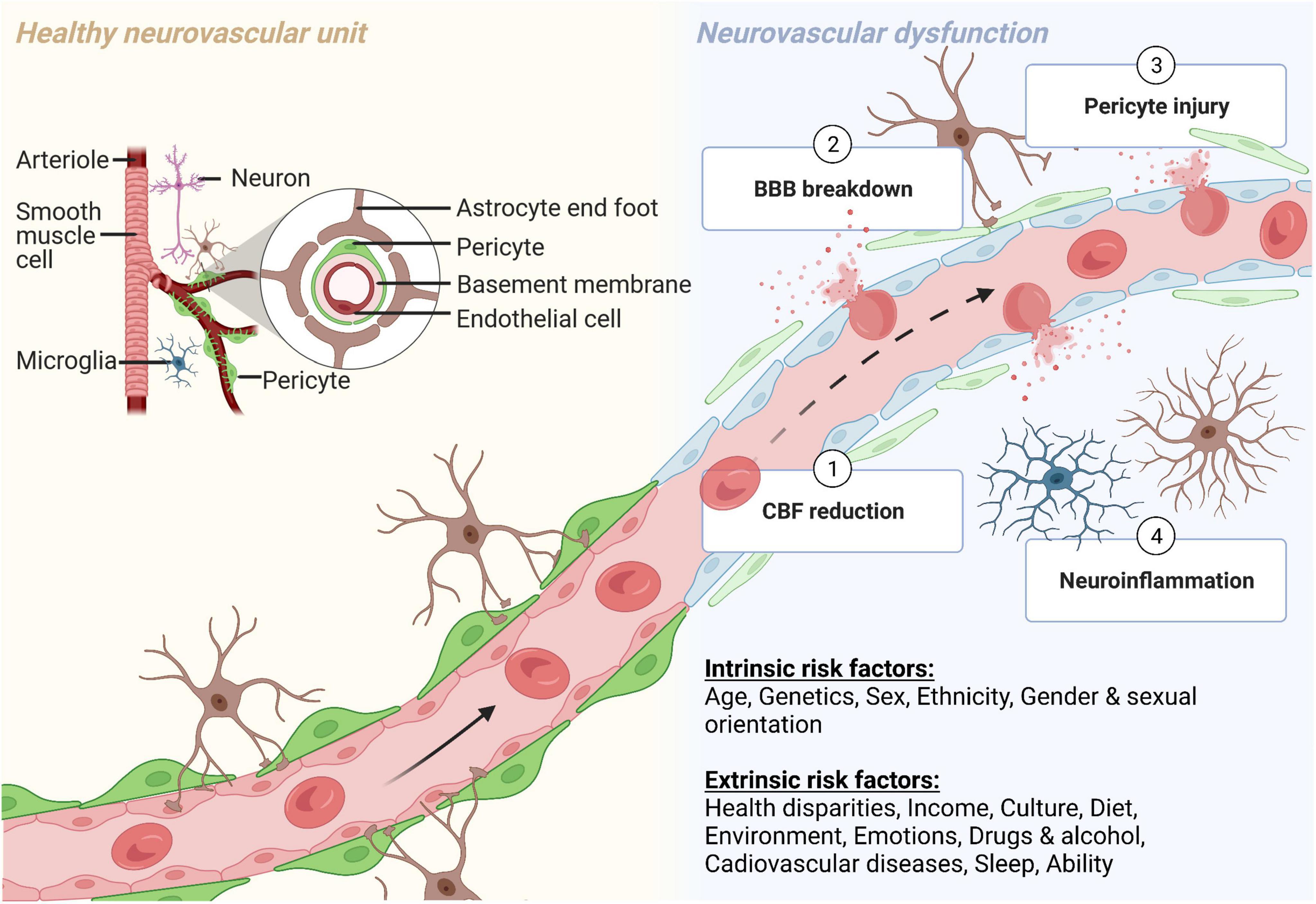
Figure 1. The neurovascular unit (NVU) during normal physiological and pathological conditions. The NVU is comprised of many cell types. The blood-brain barrier (BBB) is formed by endothelial cells, which at the capillary level, are supported by pericytes. Astrocytic endfeet provide additional support to the BBB. Other cellular components of the NVU include microglia and neurons (yellow, left side). (1) Cerebral blood flow (CBF) reductions, (2) BBB breakdown, (3) pericyte injury, and (4) neuroinflammation all contribute to neurovascular dysfunction in many neurodegenerative diseases and disorders, including Alzheimer’s disease (blue, right side). Both intrinsic and extrinsic factors can contribute to neurovascular dysfunction. Created with BioRender.com.
Blood-Brain Barrier Breakdown
The BBB has been referred to as the gatekeeper of the brain. Increased adherens and tight junctions in the BBB prevent uninhibited entry of blood-derived products, toxins, and molecules from entering the brain (Zlokovic, 2011; Zhao et al., 2015; Nelson et al., 2016). Unlike peripheral vessels, the BBB has a controlled specialized substrate-specific transport system (Zlokovic, 2011; Zhao et al., 2015; Nelson et al., 2016).
BBB breakdown in AD has been detected by immunohistochemistry (Salloway et al., 2002; Bailey et al., 2004; Wu et al., 2005; Baloyannis and Baloyannis, 2012; Sengillo et al., 2013; Halliday et al., 2016), fluid biomarker assessment of albumin quotient, plasminogen and fibrinogen (Craig-Schapiro et al., 2011; Montagne et al., 2015; Sweeney et al., 2015), and magnetic resonance imaging neuroimaging sequences of perivascular hemosiderin deposits/microbleeds (Goos et al., 2009; Brundel et al., 2012; Uetani et al., 2013; Heringa et al., 2014; Olazarán et al., 2014; Yates et al., 2014; Zonneveld et al., 2014; Shams et al., 2015; Poliakova et al., 2016). More recent studies have used dynamic contrast enhanced magnetic resonance imaging to quantify BBB permeability and found increased BBB breakdown in individuals with normal aging (Montagne et al., 2015; Verheggen et al., 2020), MCI (Montagne et al., 2015, 2020a; Nation et al., 2019; Rensma et al., 2020), and early AD (van de Haar et al., 2016, 2017a,b).
Pericyte Injury and Loss
With endothelial cells being the gatekeepers of the brain, pericytes function as the padlock, providing a second layer of protection to ensure the gate regulated by the BBB is fortified. Pericytes are critical support cells of the BBB and have other key functions including angiogenesis, clearance of toxic metabolites (Ma et al., 2018), and regulating capillary hemodynamic responses (Kisler et al., 2017a,b; Nelson et al., 2020). Pericyte loss has been demonstrated using electron microscopy of AD cortex (Farkas and Luiten, 2001; Baloyannis and Baloyannis, 2012) and by decreased levels of pericyte marker platelet derived growth factor receptor β (PDGFRβ) in the precuneus and underlying white matter (Miners et al., 2018). Through immunohistochemical analysis, it was shown that pericyte number and coverage of brain capillaries were reduced in the AD cortex and hippocampus when compared to control brains (Sengillo et al., 2013), and this loss was accelerated in APOE4 carriers (Halliday et al., 2016). Pericyte injury marker soluble PDGFRβ has also been found to be elevated in cerebrospinal fluid (CSF) in MCI and early AD (Montagne et al., 2015, 2020a; Miners et al., 2019; Nation et al., 2019; Wang et al., 2022). No studies to date, that we could identify, have assessed BBB breakdown or pericyte injury in diverse populations with MCI or ADRD.
Neuroinflammation
Neuroinflammation may be triggered by injury, infection, stress, or aging (DiSabato and Sheridan, 2021), and short-term neuroinflammation generally leads to improved patient outcome (Hopper et al., 2012). However, neuroinflammation can be prolonged and may become detrimental when the stimulus persists, leading to neuronal dysfunction, injury, or deficit (Streit et al., 2004; Delgado et al., 2021). Specific to AD, neuroinflammation has been correlated with increased levels of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin (IL)-6 (Strauss et al., 1992; Chang et al., 2017) in both the brain and blood. Another study comparing the release and presence of microvessel-associated cytokines between AD and control brain microvessels found increased levels of IL-1β, IL-6, and TNF-α in AD brains (Grammas and Ovase, 2001). IL-1β has been shown to activate certain kinases that promote tau hyperphosphorylation (Collins-Praino and Corrigan, 2017). Proinflammatory cytokines have also been shown to increase Aβ production via upregulation of beta-secretase 1 (BACE1), the key enzyme that initiates production of Aβ (Vassar, 2004).
Neuroinflammation can be beneficial or detrimental and is mediated by astrocytes and microglia (Fakhoury, 2018). Microglia are the primary immune cells of the central nervous system (CNS), act as resident macrophages (DiSabato and Sheridan, 2021) and are important for synapse integrity and learning and memory (Wolf et al., 2017). Microglia are activated by Aβ plaques as well as hyperphosphorylated tau (Perea et al., 2018). Although microglia initially provide neuroprotection through clearance and degradation of Aβ, prolonged activation leads to hypersecretion of proinflammatory cytokines (Shastri et al., 2013) and pronounced proliferation of microglia and inflammatory markers (Varnum and Ikezu, 2012). Preclinical studies from AD models suggest specialized proresolving mediator (SPM) deficiencies and dysregulation due to imbalance between proinflammatory cytokines and SPM or SPM synthesis interruption (Ponce et al., 2022). Microglia also communicate with astrocytes, specialized glial cells that are the most abundant cells in the CNS (Shastri et al., 2013). IL-1β, a cytokine that functions in astrocyte proliferation and astrogliosis, has been shown to be present in 30x as many glial cells in AD brains compared to age-matched controls (Griffin et al., 1989; Grammas and Ovase, 2001).
Aβ in both its plaque and soluble form activates the pathway of microglia priming, leading to release of reactive oxygen species. Microglial priming in AD patients is exceptionally detrimental because the cytokines and chemokines released during this process fuels a positive feedback mechanism or neuroinflammation inducing astrogliosis, Aβ deposition, as well as further release of proinflammatory cytokines (Ho et al., 2005; Delgado et al., 2021). Further studies regarding neuroinflammation should include various races and ethnicities to reveal potential variance in neuroinflammatory patterns in diverse populations.
Vascular Risk Factors Linked to Alzheimer’s Disease
Race and Ethnicity
An analysis of 2014 Medicare and US Census Bureau data found that ADRD were most prevalent in Black Americans and Hispanic adults over the age of 65 years. The rates were 14.7% for African Americans, followed by 12.9% for Hispanic adults, 11.3% for non-Hispanic White people, 10.5% for American Indian and Alaska Natives, and 10.1% for Asian and Pacific Islanders (Matthews et al., 2019).
A study focusing on plasma metabolites in AD subjects across multiple races and ethnicities showed that amino acid metabolism was altered in African Americans, non-Hispanic White people, and Caribbean Hispanics adults. Fatty acid metabolism was altered in African Americans and non-Hispanic White people. African Americans also had altered glycolytic metabolism (Vardarajan et al., 2020). Neurodegenerative disease panels from a study on AD in African Americans revealed that levels of soluble receptor for advanced glycation endproducts (sRAGE) were significantly elevated in African Americans (Ferguson et al., 2021). sRAGE is a receptor on the luminal side of the BBB involved in transport of Aβ into the brain and increased sRAGE levels are correlated with increased Aβ accumulation (Nelson et al., 2016). The same study that identified elevated sRAGE also found higher levels of innate immunity regulator nuclear factor kappa B and its inhibitor, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor-alpha, in African American AD male patients compared to white people and African American females, with a 116% increase in Fas-associated death domain protein levels for African Americans, indicating increased apoptosis (Ferguson et al., 2020). In a separate study, it was found that levels of IL-1β, monokine induced by gamma, and TNF-related apoptosis-inducing ligand were increased and levels of IL-8 and IL-3 were decreased in African Americans, independent of gender, further explaining increased incidence of apoptosis (Ferguson et al., 2021). In addition, CSF IL-9 levels were increased in African Americans with AD but not in white people with AD (Wharton et al., 2019).
The specific reasons for these differences across races and ethnicities remain unclear. Contributing to this knowledge gap is the lack of multi-ethnic studies in clinical research including studies of ADRD. There has been work to understand the barriers to enrolling participants from underrepresented groups into clinical research projects (George et al., 2014). Non-Hispanic White people were found to be the most willing to participate in ADRD research studies, while Hispanic adults, non-Hispanic Asians, and non-Hispanic Black people were 44, 46, and 64%, respectively, less willing to participate in AD prevention trials (Salazar et al., 2020). African Americans’ participation in research studies should be increased in order to better understand ADRD and other diseases that disproportionately impact them. Among African Americans, there is a long-standing mistrust in the health care system and a significant contributor to that mistrust is rooted in Alabama. The U.S. Public Health Service Syphilis Study at Tuskegee, 1932–1972 is a well-known unethical 40-year clinical study that continues to be a major barrier to the participation of Black people in research (Freimuth et al., 2001). Mistrust stems from this and other historical events and is a known barrier to research participation reinforced by discriminatory events and flaws in the healthcare system (Scharff et al., 2010). Therefore, current research in race- and ethnicity-dependent vascular risk factors for AD is also limited by small sample sizes. In addition, most multi-racial and ethnicity studies in ADRD have focused on comparing non-Hispanic White people to Black people, while inclusion of other races and ethnicities is less common. Further multi-racial studies investigating the mechanisms mediating ethnic differences in AD pathology are needed. Encouraging community engagement and involvement early in the design and development of a research project helps to build trust while also allowing the participants to share the importance of research with other members of the surrounding community (Crook et al., 2019).
Genes
Autosomal-dominant AD (ADAD) is a form of AD caused by mutations in PSEN1, PSEN2, and APP. ADAD accounts for a small percentage of all AD cases and has an early age of onset (<65 years of age). The presentation and symptoms of ADAD are very similar to those seen in the more common sporadic cases of AD (Bateman et al., 2011). While sporadic AD is not primarily a genetic disease, several genes significantly increase the risk for AD development. The most common genetic risk factor of sporadic AD is APOE4 (Mahley et al., 2006). In addition, mutations in PICALM, CLU, and SORL1 have been implicated in AD (Zhao et al., 2015; Nelson et al., 2016; Sweeney et al., 2019). Interestingly, all the above-mentioned genes have been implicated in neurovascular dysfunction, as recently reviewed (Zhao et al., 2015; Nelson et al., 2016; Sweeney et al., 2019). Also, ABCA7 and TREM2 have been implicated in AD and are linked to innate immunity (Sweeney et al., 2019).
Although many genome-wide association studies (GWAS) have identified genetic risk factors in AD (Lambert et al., 2013; Naj et al., 2014; Kunkle et al., 2019; Wightman et al., 2021), these studies predominantly included Europeans or Americans of European descent. Moreover, individuals from several US states were included, but none specifically from Alabama. Recently, GWAS have been done to evaluate AD risk genes in African American populations (Logue et al., 2011, 2018; Kunkle et al., 2021), showing that African Americans and white people have variation in the top genetic risk factors for AD. Specifically, the DRD2 Taq A1 allele reduces the D2 receptors in the brain, causing impaired cognitive function in older age, creating a magnified risk of AD (Blum et al., 2018). This A1 allele is more prevalent in African American populations. Kunkle et al., 2021, identified 8 novel loci (TRANK1, FABP2, LARP1B, TSRM, ARAP1, STARD10, SPHK1, and SERPINB13) as being significant risk factors for African Americans. This study only identified TREM2 and C2DAP as being significant in both African American and White populations. ABCA7 mutations are more prevalent in African American populations and could be a greater risk factor than APOE4 (Stepler et al., 2022). When ATP Binding Cassette Subfamily A Member 7 (ABCA7) dysfunctions, more Aβ is produced and not cleared properly from the brain by microglia (Aikawa et al., 2018). Precision medicine is one mechanism to lessen the impact of the social determinants of health and other contributors to health disparities in the future. Understanding the genetic—environment interaction is critical if that future is to be realized. Therefore, studies seeking to identify genes conferring increased risk, likelihood of response to therapy, and prognosis for ADRD must include more diverse populations with regards to ethnicity, gender, and geography.
Diet and Lifestyle
The Centers for Disease Control and Prevention (CDC) has recently reported that over 35% of the adult population in the US was obese in 2021, with the highest obesity rates in the Southern states (Mississippi, Louisiana, and Alabama) (CDC, 2021c). In Alabama, non-Hispanic Black adults (46.2%) encounter obesity-related health disparities more than non-Hispanic White adults (34.3%) (CDC, 2021c) with high-energy food intake and physical inactivity as main factors contributing to this problem. The 2021 county health rankings revealed that 29% of adults in Alabama had no leisure-time physical activity (Alabama, 2022). In addition, several areas in Alabama suffer from low income and limited accessibility to healthy foods (USDA, 2022). It has previously been shown that energy-dense food consumption triggers severe health conditions such as metabolic syndrome, cardiovascular disorder, neurovascular dysfunction, memory deficits, as well as AD (Thériault et al., 2016; Ting et al., 2017; Moreno-Fernández et al., 2018; Lasker et al., 2019; Bracko et al., 2020). Studies in animals fed with short-term or long-term enriched-energy diet increased the permeability of the BBB as demonstrated by an elevation of extravascular immunoglobulin G deposits and albumin content in the hippocampus (de Aquino et al., 2018; Yamamoto et al., 2019; de Paula et al., 2021). Animals fed with a high-calorie diet exhibited attenuation of tight junction proteins claudin 5 and occludin, alleviation of collagen type IV, augmented fenestration of endothelial cells, and astrogliosis (de Aquino et al., 2018; Yamamoto et al., 2019). In mice, consumption of high-salt diets were found to reduce the brain endothelial cells’ capacity to generate nitric oxide, leading to cognitive and neurovascular dysfunction (Faraco et al., 2018). These negative effects not only disrupt the structure of the NVU, but also reduce CBF, which plays a crucial role in neurovascular coupling processes (Faraco et al., 2018; Nielsen et al., 2020). Notably, both in vivo and clinical experiments have shown that a disruption of the BBB and neurovascular uncoupling potentially diminish cognitive performance, resulting in the development of AD and dementia (Lourenço et al., 2017; Nielsen et al., 2020; Lin et al., 2021). For these reasons, the modification of structural and social determinants of health (e.g., improved access to food, safer neighborhoods for exercise) may allow improvements in dietary behaviors and the enhancement of physical activities which are beneficial ways to decrease risk of obesity, vascular dysfunction, and AD in Alabama and other states.
Emotions, Stress, and Anxiety
Stress has been identified as a vascular risk factor and can contribute to negative vascular outcomes through influencing behavior and physiology. A systematic review found stress to be related to behavioral factors that are known to lead to poor vascular health outcomes (Dimsdale, 2008). Stress influenced the adoption of unhealthy lifestyle habits, including smoking, eating a poor-quality diet, disordered eating, and living a sedentary lifestyle (Dimsdale, 2008). Increased levels of stress hormones, such as cortisol, have been found to impair cerebrovascular function (Münzel et al., 2018). Chronic mental stress was found to be a risk factor for metabolic syndrome and for the development of hypertension (Esler et al., 2008). Stress facilitates the progression of AD and exacerbates symptom severity (Justice, 2018). Additionally, AD patients exhibit increased levels of stress hormones (Csernansky et al., 2006). Stress is a psychological factor that impacts behavioral and physiological functioning, consequentially impacting vascular health and increasing the risk of AD.
Anxiety is an independent cardiovascular disease risk factor (Shen et al., 2008). Additionally, it has been linked to adverse cardiovascular outcomes such as autonomic dysfunction, inflammation, and endothelial dysfunction (Celano et al., 2016). A meta-analysis of 46 studies found anxiety to be associated with several vascular events such as a 71% higher risk of stroke, a 41% higher risk of cardiovascular mortality and coronary heart disease, and a 35% higher risk of heart failure (Emdin et al., 2016). In a study examining neuronal activity and blood supply in individuals with generalized anxiety disorder (GAD), patients with GAD displayed a decrease in neurovascular coupling and alteration in CBF (Chen et al., 2021).
Allostatic load is the cumulative burden of chronic stress and life events (Guidi et al., 2021). Black Americans have a greater allostatic load than White Americans, which was shown to be associated with poorer physical and mental health outcomes (Guidi et al., 2021). Living with the negative impacts of racism is a significant stressor on Black Americans and other minorities. A study using multinomial logistic regression to quantify experiences of racism and subjective cognitive function found that Black women subjected to institutional racism had worsened subjective cognitive function that was in part mediated by depression and insomnia (Coogan et al., 2020). Another study found that higher perceived stress in older Black Americans was associated with faster declines in global cognition, especially episodic memory and visuospatial ability (Turner et al., 2017). Stressful life events were reported more frequently in Black Americans than White people and were associated with age-related cognitive decline (Zuelsdorff et al., 2020). Stress in Black Americans has been linked to elevated Aβ and tau CSF biomarkers (Garrett et al., 2019; Trammell et al., 2020). Modifying environmental (e.g., larger living space), sociocultural (e.g., larger social network size), behavioral (e.g., more purpose in life), and biological (e.g., higher global cognition) levels was associated with a lower odds of having higher levels of perceived stress (Glover et al., 2021). This suggests that improving social determinants of health, including those affecting Black Alabamians, may help alleviate stress-associated cognitive decline and ADRD.
Drugs and Alcohol
There is a positive association between heavy drinking (as defined by the World Health Organization) and diagnosis with dementia or cognitive impairment (Schwarzinger et al., 2018). A clinical review in 2018 described that chronic heavy alcohol consumers had an increased risk of dementia and cognitive impairment (Topiwala and Ebmeier, 2018). For example patients with chronic alcoholism were discussed to have a frontal lobe more vulnerable to dementia (Topiwala and Ebmeier, 2018). It is already known that frontal cortex and hippocampus size and function are significantly altered with alcoholism while white matter recovery is seen in cases upon abstinence from alcohol (Alvarez et al., 1989; Bengoechea and Gonzalo, 1991; Kril and Halliday, 1999). Although alcohol has been identified as a risk factor for AD, a statistically significant association between light use of alcohol and lower risk of cognitive decline and dementia has also been found (Yan et al., 2021). However, studies presented are limited by obvious ethical obstacles and inherent biases. That is, alcohol use is always self-reported, studies often lack controls for confounding variables, and there are few studies that examine interactions between other risk factors of AD such as drug use and genetics.
Smoking tobacco has been identified as a modifiable risk factor for AD (Etgen et al., 2011). In 2021, it was reported that approximately 18.5% of adults in Alabama smoked cigarettes (Explore Smoking in Alabama, 2021 Annual Report), whereas the national prevalence is only 12.5%. A longitudinal study suggested that smokers should be encouraged to quit because continual smokers have an increased risk of overall dementia (Choi et al., 2018). The same study also showed that long-term quitters as well as individuals who have never smoked had a decreased risk of developing AD as well as vascular dementia when compared to continual smokers (Choi et al., 2018). It is hypothesized that a combination of smoking-induced oxidative stress and the brain’s high susceptibility to oxidative stress contributes to neuroinflammation via release of proinflammatory cytokines and gliosis (Voloboueva and Giffard, 2011). Oxidative stress is known to be a consequence of Aβ- or tau-based neuropathies (Praticò et al., 2002; Sutherland et al., 2013). However, an in vitro study reported that oxidative stress stimulates BACE1 transcription which subsequently promotes Aβ production (Tamagno et al., 2008).
Certain prescription drug use has also been identified as a risk factor for AD. Benzodiazepines have been shown to induce memory deficit states through targeting of gamma-Aminobutyric acid (GABA), and it is proposed that the α5 subunit of GABA is most affected as it controls cognitive functions (Ettcheto et al., 2019).
Recreational use of marijuana has also been identified as a risk factor for AD, although related studies present mixed results. For instance, it has been demonstrated that marijuana users exhibited a lower average cerebral perfusion and decreased right hippocampal perfusion (Amen et al., 2017). However, the active ingredient in marijuana (i.e., THC) is a competitive inhibitor of acetylcholinesterase, a critical region involved in the formation of Aβ (Eubanks et al., 2006). Therefore, it is shown that low doses of marijuana may have therapeutic effects on AD (Cao et al., 2014).
Sex, Gender, and Sexuality
Two-thirds of the Americans affected by AD are women (Alzheimer’s Association, 2022). Originally, this was attributed to women’s longer lifespan since AD development increases with age (Mielke et al., 2014). However, further research suggests that there may be other factors contributing to this sex difference than solely longevity (Snyder et al., 2016). One study examining APOE4-by-sex interaction as a risk of converting from healthy aging to MCI/AD suggested that the effect of APOE4 carriage was stronger in women (Altmann et al., 2014). The same study also showed more elevated Aβ in the CSF of women compared to men (Altmann et al., 2014). There is still an evident gap in knowledge regarding sex differences in AD onset and progression and more studies should focus on gender- and sex-based disparities of ADRD risk factors.
Approximately 3.1% of adults in Alabama identify as lesbian, gay, bisexual, transgender, queer, or questioning (LGBTQ) (Williams Institute, 2022). Individuals in the LGBTQ community experience various health disparities and exhibit a greater risk for dementia than their straight, cisgender counterparts. For example, researchers found increased reports of subjective cognitive decline in LGBTQ individuals compared to straight, cisgender participants (Flatt et al., 2021). Human immunodeficiency virus, acquired immunodeficiency syndrome (HIV/AIDS) is most prevalent within the LGBTQ community, and is also linked to AD (Davis, 2013). BBB breakdown plays a major role in HIV-associated dementia by allowing HIV-1-infected monocyte-macrophages to traverse the BBB and enter the brain (Strazza et al., 2011; Sweeney et al., 2019). Once HIV accesses the brain, it negatively influences cognitive function by eventually leading to Aβ plaque deposition and/or NFTs (Canet et al., 2018).
LGBTQ individuals are less likely to seek needed medical care due to lifetime experiences of discrimination and victimization (Quinn et al., 2015). LGBTQ Americans are also less likely to develop support systems through marriage or having children and are twice as likely to live alone (Zelle and Arms, 2015; Goldsen et al., 2017). This lack of support may have negative effects on mental health and lead to increased stress, a known vascular risk factor for AD (Caruso et al., 2018). One notable research study conducted by multiple universities is Research Inclusion Supports Equity (RISE). The RISE study functions to ensure that LGBTQ adults are properly represented in ADRD research.1 More studies must bridge the gap between healthcare and research of LGBTQ individuals and the heterosexual population by including LGBTQ demographics in health-related research.
Pathological Diseases
Systemic Inflammation and Gut Dysbiosis
Systemic inflammation is an important mechanism linked to vascular impairment and AD (Takeda et al., 2013; Elwood et al., 2017). Previous studies have demonstrated a high level of proinflammatory markers in the periphery in AD subjects (Cattaneo et al., 2017). Multiple studies have revealed the association between peripheral inflammation and various diseases including gut dysbiosis, metabolic disease, diabetes, and cardiovascular disease (Cox et al., 2015; García et al., 2017; Brandsma et al., 2019; Al Bander et al., 2020).
Gut dysbiosis is an imbalance of intestinal microbial composition and is related to dietary alteration or pathological conditions, including inflammation and oxidative stress (Proctor et al., 2017; Dumitrescu et al., 2018; Al Bander et al., 2020). A reduction of Bacteroidetes and an enhancement of Actinobacteria was detected in AD patients (Zhuang et al., 2018). However, an increase of Bacteroidetes along with the reduction of Firmicutes and Bifidobacterium was also measured in fecal samples of AD participants (Vogt et al., 2017). All changes of gut components related to the production of proinflammatory markers in the blood subsequently caused systemic inflammation (Al Bander et al., 2020). In addition, the mentioned differences of gut microbiomes is linked to the deterioration of intestinal integrity, which leads to the leakage of lipopolysaccharides as well as gram-negative bacteria into blood circulation (Thiennimitr et al., 2018; Salguero et al., 2019; Marizzoni et al., 2020). These microbiome differences are also associated with changes of short-chain fatty acids levels in blood, which contribute to peripheral inflammation (Marizzoni et al., 2020). Systemic inflammation or gut dysbiosis increases vascular permeability as demonstrated by an elevation of albumin in brain parenchyma (Takeda et al., 2013) and attenuates the level of tight junction protein expression in brain endothelial cells (Hu et al., 2020; Wen et al., 2020). These effects caused by systemic inflammation and gut dysbiosis ultimately contribute to cognitive decline in ADRD.
Gut microbiota alteration and/or systemic infection may disproportionately affect ethnic minorities, though the influence of race and ethnicity on gut composition remains to be fully discovered. One study observed variation in the phyla of microbiota, including Actinobacteria, Firmicutes, Proteobacteria, Bacteroidetes, and Verrucomicrobia across ethnicities (Brooks et al., 2018). There was also significant variation of gut microbiota across ethnicities as determined by Shannon’s alpha diversity index from the American Gut Project which ranked Hispanic adults greater than white people, followed by Asians, then African Americans (Brooks et al., 2018). Furthermore, the relationship of patients with infectious diseases, such as ulcerative colitis, and ethnic disparities was shown in a previous study (Castaneda et al., 2017). Taken together, ethnicity and race are factors contributing to gut dysbiosis and inflammation in US population as well as in Alabama. Nevertheless, the link between racial and ethnic disparities and gut dysbiosis as it is associated with neurovascular impairment and cognitive decline in AD should be further studied.
Metabolic Syndrome and Vascular Dysfunction
Metabolic syndrome is a group of metabolic risk factors characterized by a large waist circumference, abnormality of lipid profiles (e.g., high triglycerides and low high-density lipoprotein cholesterol levels), hypertension, and hyperglycemia (NHLBI, 2022). Being overweight or obese as a consequence of poor diet is the main cause of metabolic syndrome (NHLBI, 2022). Previous studies have demonstrated that a high caloric intake not only leads to hyperlipidemia but also triggers insulin resistance, both of which are linked to pre-diabetes and type 2 diabetes (Lozano et al., 2016; Saiyasit et al., 2020; Kumar et al., 2021). The national diabetes report from the CDC indicated that approximately 88 million adults in the United States had pre-diabetes and 26.8 million were diagnosed with diabetes (CDC, 2022c). In Alabama, 14.8% of adults currently have diabetes, which was shown to affect more African Americans (18.1%) than White people (14.1%) Americans in 2020 (Explore Diabetes in Alabama, 2021 Annual Report). Additionally, Alabamians with less education (Figure 2A) and low income (Figure 2B) were more susceptible to diabetes (Explore Diabetes in Alabama, 2021 Annual Report). Several studies have shown an association between diet-induced obesity or diabetes with vascular dysfunction (Chang et al., 2014; de Paula et al., 2021; Li et al., 2021). Excessive caloric consumption stimulates peripheral immune cells, induces the generation of proinflammatory cytokines and increases oxidative stress, which have all been linked to BBB dysfunction (Salameh et al., 2019). In a recent study in obese Zucker rats, researchers observed BBB disruption (e.g., increased aquaporin-4 and reduced glucose transporter 1) in the frontal cortex and hippocampus, which was associated with cognitive impairment (Tomassoni et al., 2020). BBB breakdown was also found in a streptozotocin-induced diabetes model that had reduced levels of tight junction proteins (e.g., occludin, and zonula occludens-1), and increased protein levels of cell adhesion molecules (e.g., intercellular adhesion molecule 1, and vascular adhesion protein 1) (Aggarwal et al., 2018). Importantly, BBB leakage leads to the activation of glial cells, resulting in the release of proinflammatory cytokines in the brain, causing neuroinflammation and cognitive decline (de Aquino et al., 2018; Salameh et al., 2019; de Paula et al., 2021). Notably, a large amount of Aβ accumulation was detected in the brain of a high-fat diet-induced obese model (Busquets et al., 2017). All the aforementioned studies support the theory that high-caloric consumption causes metabolic disturbance and disrupts neurovascular function, which enhances the risk of ADRD. As mentioned above, many Alabamians struggle with obesity (Figure 2C) and diabetes (Figure 2D), prompted by a high-calorie diet and/or reduced access to healthy food (Figure 2E). For these reasons, metabolic disturbances in Alabama may contribute to the increased risk for neurovascular dysfunction and ultimately progression of AD.
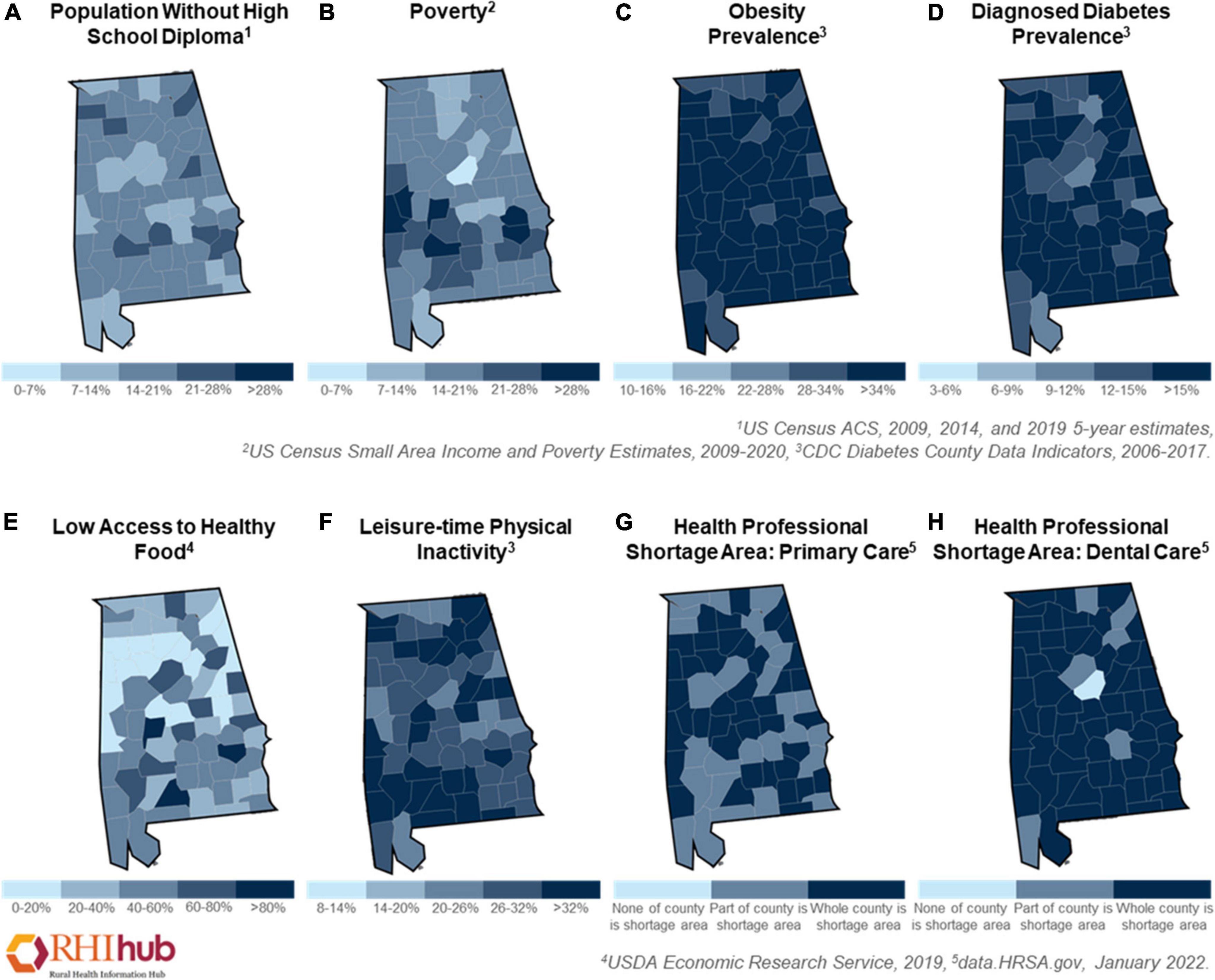
Figure 2. Health disparities in Alabama. The disparities prevalence of Alabamians in 2006–2022 as shown by (A) population without school diploma, (B) poverty, (C) the prevalence of obesity, (D) the prevalence of diagnosed diabetes, (E) low access to healthy food, (F) leisure-time physical inactivity, (G) health professional shortage area in primary care, and (H) health professional shortage area in dental care. These figures were generated using Rural Health Information Hub (RHIhub, www.ruralhealthinfo.org).
Cardiovascular Disease
Another important factor for neurovascular disruption is cardiovascular impairment (Aires et al., 2020; Vanherle et al., 2020). Based on the data in 2018, heart disease (mostly coronary heart disease) is the most common cause of death in the US, as well as in the state of Alabama (Alabama, 2021; Heart Disease and Stroke Statistics, 2021 Update). In 2020, the CDC reported the number of people in Alabama who died with heart disease: 14,739 per 100,000 individuals (Stats of the States Heart Disease Mortality, 2021). Cholesterol deposition inside the arterial wall, also known as atherosclerosis, is a vascular disease that underlies major ischemic events such as myocardial infarction and stroke (Atherosclerosis, 2022). It has been shown that there is a relationship between a score of vascular risk factors and AD risk factors in middle aged adults (Lockhart et al., 2021). A lower score of cardiovascular risk factors, aging, and incidence of dementia (CAIDE) was found in White individuals when compared with African Americans, resulting in a decreased risk for AD (Lockhart et al., 2021). A higher CAIDE score is also linked to increased Aβ deposition (Lockhart et al., 2021). Therefore, the impact of the social determinants of health in ethnic minority communities, particularly African Americans, leads to higher risk of vascular disease and AD.
Notably, the correlation of atherosclerosis, cardiovascular disease, and APOE4 was reported (Bennet et al., 2007; Granér et al., 2008; Duong et al., 2021). APOE4 expression was highly correlated to neurovascular impairment, stroke, and AD (Montagne et al., 2020b; Pendlebury et al., 2020). In addition, mice with targeted replacement of ApoE with human APOE4 displayed a reduction of resting CBF and a lower density of brain vascular structures such as endothelial cells and pericytes (Koizumi et al., 2018). APOE4 carriers exhibited degradation of BBB structures via several pathways, including pericyte degeneration, reduction in low density lipoprotein receptor-related protein 1 expression, increased proinflammatory cytokines, and elevated apoptosis. APOE4 carriers also exhibited synaptic dysfunction, hyperphosphorylated tau, and increased Aβ levels (Halliday et al., 2016; Zhao et al., 2020). Furthermore, an association of increased BBB permeability and elevated levels of the pericyte injury marker, soluble PDGFRβ, in CSF and cognitive impairment was found in APOE4 carrier participants (Montagne et al., 2020a). Therefore, cardiovascular disease not only causes systemic pathologies, but can also progress into cerebrovascular dysfunction and eventually AD, including in individuals living in Alabama.
Pulmonary Diseases
Human lungs are constantly exposed to airborne microbes, pollutants, and small particulates, all of which can directly impact their function, and secondarily the health of the brain. Specific to the lung-brain axis, emerging evidence indicates that damage to the lung can lead to cognitive impairment (Ely et al., 2004). The intricate lung-brain link has been implicated in lung damage associated with mechanical ventilation, bacterial and viral pneumonia, and air pollution.
Clinical studies have shown that many patients in the intensive care unit (ICU) suffer from the rapid onset of delirium during their ICU stay, which may transition into prolonged cognitive sequelae even after the patients recover from the critical illness and are released from the ICU (Girard et al., 2010). In fact, a hospital stay itself can harm cognition, and in the ICU settings, animal studies have implicated that mechanical ventilation can increase peripheral inflammation that results in neuroinflammation and impairs brain function (Reade and Finfer, 2014; Lahiri et al., 2019). While the mechanisms underlying clinical delirium and/or the consequent impairment to the brain remain unclear, cognitive impairment is more rampant in infection-induced pneumonia patients, including patients that contract either community- or hospital-acquired pneumonia infections (Karhu et al., 2011; Girard et al., 2018). Although the infection-induced peripheral inflammatory response may be a common mechanism for several types of infection, emerging evidence has indicated that additional mechanisms may underlie the lung-brain axis.
It was recently discovered that lung capillary endothelium produces and releases amyloids into the surrounding milieu (Ochoa et al., 2012; Morrow et al., 2016). These amyloids include Aβ variants that possess antimicrobial activity against invading microbes (Voth et al., 2020). However, when exposed to virulent clinical bacterial strains, including bacteria such as Pseudomonas aeruginosa, Klebsiella pneumoniae, and Staphylococcus aureus, the amyloids produced by lung endothelium become cytotoxic. In addition to Aβ, lung endothelium also produces several tau isoforms that are also released upon bacterial infection (Balczon et al., 2017; Choi et al., 2021).
Intriguingly, virulent bacteria-elicited lung endothelial Aβ and tau gain tropism toward the brain (i.e., neurotropic), are neurotoxic to brain cells, and may cause Aβ and tau aggregation (Choi et al., 2021). Thus, a bacterial lung infection that elicits the release of endothelial Aβ and tau may induce incident dementia indirectly by triggering the senile plaques and/or NFT pathways (Lin et al., 2018; Balczon et al., 2019, 2021; Scott et al., 2020).
Chlamydia pneumoniae bacterium, on the other hand, has been found to invade the brain and trigger brain impairment. Indeed, along the same line of findings, viruses and viral particles (e.g., herpes viruses) found entangled in the senile plaques of AD brains, likely due to the antimicrobial activity of Aβ, have been suspected to be the cause of AD (Kumar et al., 2016; Eimer et al., 2018; Abbott, 2020). Whether these microbes could be the smoking guns that trigger AD is still under heavy debate, but it is not hard to imagine that the presence of neurotropic viruses in the brain and the consequent neuroinflammation would directly impact brain function. Studies from the recent COVID-19 pandemic show that the SARS-CoV-2 virus could also damage the brain (Kotfis et al., 2020; Mao et al., 2020). However, it appears that the virus may do so both via the lung-brain axis and via the olfactory transneuronal retrograde pathways (Barthold et al., 1990; Perlman et al., 1990; St-Jean et al., 2004). Altogether, these findings support the concept that pathogenic microorganisms cause infections generating an innate immune response and have fueled the formation of the peripheral amyloid hypothesis to cognitive impairment and AD (Nelson, 2022).
The lung-brain axis could further expand to include chemical pollutants and particulate matters < 2.5 μm (PM2.5). In addition to the risk of developing asthma, cardiovascular disease, lung disease, and premature death, emerging evidence has implicated that greater exposure to airborne pollutants is associated with an increased risk of dementia (Ehlenbach et al., 2010; Corrales-Medina et al., 2015; Peters et al., 2019). Alabama ranks 33rd among US states for air quality with a value of 7.8. This value correlates to an average exposure to PM2.5 (Explore Air Pollution in Alabama, 2021 Annual Report). This line of research has implicated that oxidative stress induced by breathing in harmful particles results in chronic respiratory and systemic inflammation, which impairs BBB integrity and triggers neuroinflammation (Moulton and Yang, 2012). There is evidence in North America, that areas with low SES have higher concentrated air pollutants (Hajat et al., 2015). Individuals with low income also tend to live nearer to sources of pollution, increasing their exposure (O’Neill et al., 2003).
Potential Preventions and Treatments
Pharmaceutical Treatments
There are a few drugs approved by the Food and Drug Administration (FDA) for AD patient use; these include acetylcholinesterase inhibitors (e.g., rivastigmine and donepezil), a N-methyl-d-aspartate receptor agonist (e.g., memantine), and a monoclonal antibody to Aβ (e.g., aducanumab). There are no FDA approved drugs for the treatment of neurovascular dysfunction that occurs in vascular cognitive impairment and dementia. However, there are several candidates in the pipeline which we describe below and summarize in Figure 3.
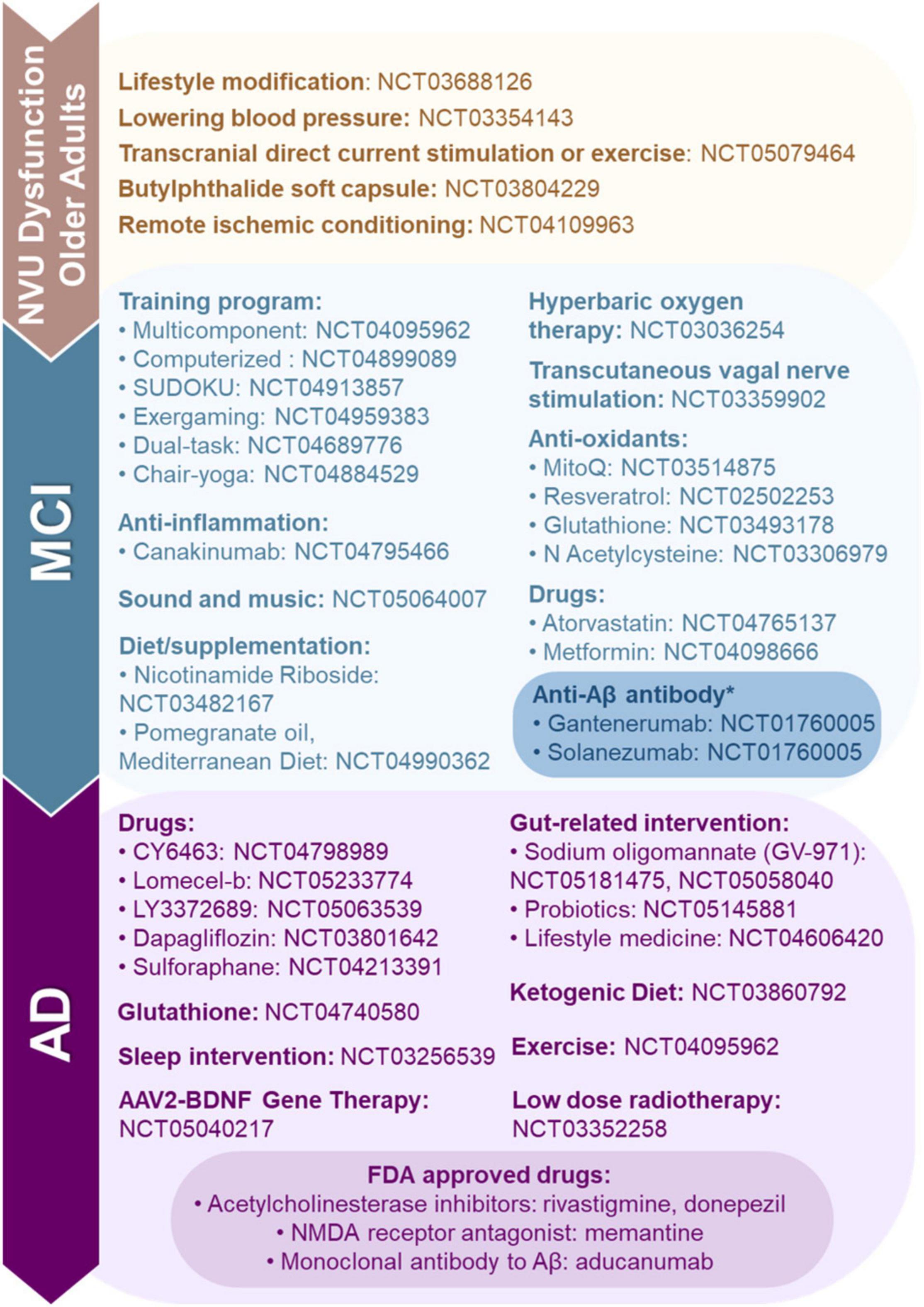
Figure 3. Food and Drug Administration (FDA) approved, clinical trials and preclinical studies of neurovascular unit (NVU) dysfunction, mild-cognitive impairment (MCI) and Alzheimer’s disease (AD). Here, we highlight FDA approved drugs for AD, and ongoing preclinical studies and clinical trials targeting NVU dysfunction, MCI, and AD. *Clinical trials for anti-Aβ antibodies Gantenerumab and Solanezumab are being conducted in Alabama.
Currently, we are aware of only one interventional ADRD-related clinical trial recruiting in Alabama (NCT01760005). This study is investigating the use of anti-Aβ antibodies (e.g., Gantenerumab and Solanezumab) in patients with ADAD. These drugs have been shown to bind to Aβ aggregates and improve downstream biomarkers, but no cognitive benefits have been observed in early trials (Salloway et al., 2021).
Efforts have been made to assess the vascular and cognitive benefits of currently approved drugs. For example, lowering blood pressure was shown to reduce the risk of cognitive impairment in the Systolic Blood Pressure Intervention Trial—Memory and Cognition in Decreased Hypertension cohort (Williamson et al., 2019; Nasrallah et al., 2021) and other cohorts (Ding et al., 2020; Peters et al., 2020). The beneficial effect of anti-hypertensive classes of drugs on cognitive impairment have yielded conflicting results (De Oliveira et al., 2016; Ding et al., 2020; Peters et al., 2020).
Lomecel-b is made from medicinal signaling cells that have been isolated from bone marrow in adult donors and was recently tested in a Phase I clinical trial for AD (Brody et al., 2022). This allogenic drug functions by multimodal mechanisms of action and was able to increase anti-inflammatory (e.g., IL-10, IL-12, sIL-2Rα) and pro-vascular (e.g., VEGF, IL-4, IL-6) biomarkers in patient serum (Oliva et al., 2021).
CY6463 is a soluble guanyl cyclase stimulator that is meant to normalize the nitric oxide-cGMP signaling pathway. Deficiency in this pathway has been associated with neurovascular dysfunction (Garthwaite, 2019). This study suggests that there may be additive effects when CY6463 is administered with donepezil (Correia et al., 2021).
AD patients exhibit a decline in thiamine diphosphate-dependent enzymes, which are involved in glucose metabolism in the brain (Gibson et al., 2020). Benfotiamine is a synthetic thiamine precursor that acts on metabolic pathways, oxidative stress, and inflammation by activating transketolase which reduces advanced glycation end products production (Ahmed, 2005; Raj et al., 2018). In a Phase IIa study, Benfotiamine was determined to be safe for AD patients and lessened cognitive decline, especially in APOE4 non-carriers (Gibson et al., 2020).
Gut microbiota play an important role in Th1/M1 microglia-predominated neuroinflammation in AD progression (Xiao et al., 2021). Sodium oligomannate (GV-971) effectively remodels gut microbiota and reduces Th1-related inflammation in the brain (Wang et al., 2020; Xiao et al., 2021). Furthermore, it was shown to improve cognitive function in AD patients in a Phase III trial (Wang X. et al., 2019; Wang et al., 2020; Xiao et al., 2021). GV-971 has been approved in China for treatment of mild to moderate AD to improve cognitive function (Syed, 2020). It has been hypothesized that the benefit of GV-971 might be due to antimicrobial and antiviral activities, specifically against herpes simplex virus type 1 (Itzhaki, 2020). Future studies should not only assess cognitive function but also examine the neurovascular benefits of GV-971 in MCI and AD patients.
Non-pharmaceutical and Alternative Treatments
Diet and Supplements
While there is no standard treatment for ADRD, altering one’s diet and taking supplements may be beneficial against the cognitive decline and pathological severity of AD. Currently, multiple studies support the hypothesis that modulation of gut dysbiosis reduces negative pathologies and memory loss, delaying the severity of AD both in experimental studies and in clinical trials. Probiotic supplementations in Aβ1–42-induced AD rats showed improved learning and synaptic plasticity (Rezaeiasl et al., 2019). Furthermore, probiotic supplementation along with moderate-intensity interval training in AD rats enhanced the mRNA expression of hippocampal choline acetyltransferase and brain-derived neurotrophic factor (BDNF), which are essential for synaptic function (Shamsipour et al., 2021). Another experiment demonstrated that co-treatment of probiotics and selenium for 12 weeks decreased the abnormalities of metabolic profiles, reduced circulating inflammatory markers, increased antioxidant activity, and enhanced the cognitive score in AD participants (Tamtaji et al., 2019). A recent study also revealed the benefits of gut modulation on mental and stress adjustment not only in AD patients, but also in healthy older adults (Kim et al., 2021). Diet is a part of culture and may be challenging to change for individuals in the South. However, taking supplements may be an easier change to implement.
Supplementation with choline, a nutrient that plays a key role in cholinergic system and synaptic processes, reduced Aβ plaques and neuroinflammation in the hippocampus, decreased the activation of microglia, augmented synaptic proteins, and improved spatial memory in AD mice (Velazquez et al., 2019; Wang Y. et al., 2019). Furthermore, AD mice (e.g., J20) supplemented with lactoferrin, a multifunctional protein that acts as an antioxidant or anti-inflammatory, improved Aβ clearance as demonstrated by increased brain levels of ApoE and Abca1 (Abdelhamid et al., 2020). Lactoferrin supplementation not only led to a reduction of Aβ but also lowered BACE1 levels (Abdelhamid et al., 2020). Administration of ɤ-glutamylcysteine not only increased the brain antioxidant activity and anti-inflammatory expression, but also reduced lipid peroxidation, Aβ deposition, and inflammatory markers, leading to cognitive improvements in APP/PS1 mice (Liu Y. et al., 2021). Vitamin D was also shown to improve working memory at early and late stages in 5XFAD transgenic mice (Morello et al., 2018). Resveratrol, a plant-derived phytoalexin, has been shown to regulate BBB permeability and neurovascular function (Shin et al., 2015; Wei et al., 2015). Several studies showed that resveratrol administration attenuated neurovascular dysfunction (Wei et al., 2015) by enhancing antioxidants and reducing cognitive loss (Zhao et al., 2012). While the results of these experimental studies are promising, the benefits of these supplements should be tested in future clinical studies including diverse populations and regions.
A clinical study reported the benefits of dietary Omega-3 fatty acids, specifically docosahexaenoic acid and eicosapentaenoic acid, on cognition in MCI subjects (Chiu et al., 2008). Interestingly, a long-term modified ketogenic diet, a common diet for subjects with impaired brain energy metabolism, improved cognition in AD patients (Ota et al., 2019; Phillips et al., 2021).
In addition, the Mediterranean-Dietary Approach to Systolic Hypertension (DASH) diet intervention for neurodegenerative delay (MIND), which is a diet enriched with high antioxidants, has been shown to delay cognitive dysfunction associated with aging (Morris et al., 2015; de la Rubia Ortí et al., 2018; Liu X. et al., 2021). Dietary supplementation serves as a feasible and effective method to delay or protect against the progression of cognitive loss and AD. However, further studies should be done to include the effects of dietary supplementation in AD populations with diverse racial, ethnic and regional backgrounds.
Exercise
Individuals with higher levels of physical activity present with decreased cognitive decline and reduced risk of AD (Kramer et al., 2005, 2006; Rovio et al., 2005; Rolland et al., 2008; Lautenschlager et al., 2010; Barnes, 2015; Allard et al., 2017; Gallaway et al., 2017; Stephen et al., 2017; Barnes and Corkery, 2018; Rabin et al., 2018, 2019; Coelho-Junior et al., 2020; De la Rosa et al., 2020; Pasek et al., 2020; Sinha et al., 2020). It has been estimated that an increase of 25% in physically active adults would prevent > 230,000 cases of AD in the US (Barnes and Yaffe, 2011). This is largely based on evidence that demonstrates exercise promotes Aβ turnover (Baker et al., 2010; Liang et al., 2010; Rabin et al., 2018, 2019), the synthesis and release of neurotrophins (Coelho et al., 2013, 2014), and cerebral (Burdette et al., 2010; Bliss et al., 2020) and peripheral blood flow (Scicchitano et al., 2019; O’Brien et al., 2020; Pasek et al., 2020), while also eliciting a positive systemic inflammatory effect (Jensen et al., 2019; De la Rosa et al., 2020). Thus, there is growing support that, at minimum, exercise has the ability to delay the onset of AD and related vascular conditions such as small-vessel-type ischemic stroke and cardiovascular disease (Ngandu et al., 2015; Gallaway et al., 2017; Barnes and Corkery, 2018; Nystoriak and Bhatnagar, 2018; Wardlaw et al., 2019; Alty et al., 2020; Pasek et al., 2020), which African Americans experience at a disproportionately higher prevalence (Soden et al., 2018; Benjamin et al., 2019; El Husseini et al., 2020). While the benefits of regular exercise are known, many adults are not meeting the recommended amount (Benjamin et al., 2019; Centers for Disease Control and Prevention, 2021). For instance, the South has the highest prevalence of physical inactivity compared to other US regions (Centers for Disease Control and Prevention, 2021). Alabama is the 4th least active state (Figure 2F), and African Americans residing in Alabama self-reported physical inactivity at a prevalence of 34.3% (Centers for Disease Control and Prevention, 2021). Perhaps as a direct consequence, Alabama exhibits the 2nd highest AD mortality rate (CDC, 2022e: 50.8). Interventions are typically designed to circumvent these outcomes by including exercise prescriptions consisting of aerobic and/or resistance exercise, while also fostering exercise adherence. It has been shown that aerobic exercise increased serum BDNF in African Americans with MCI, but this improvement was only seen in non-APOE4 carriers (Allard et al., 2017). This suggested that genotype is an important factor when examining the efficacy of exercise interventions aimed at ADRD risk reduction. This has garnered additional support via a report that aerobic exercise in older African Americans provided no improvement in a hippocampus-related assessment of generalization following rule learning in a high-genetic risk group (Sinha et al., 2020). Interestingly, however, it has been stated that APOE4 status influences the associations between exercise and ADRD risk such that exercise has a greater ability to protect among APOE4 carriers (Rovio et al., 2005; Kaufman et al., 2021). Taken together (Rovio et al., 2005; Allard et al., 2017; Sinha et al., 2020), exercise is still beneficial for genetically at-risk individuals, but the most efficacious exercise prescription has yet to be elucidated. Notably, there is currently limited understanding concerning the ability of resistance exercise to improve vascular health with the goal of reducing ADRD risk (Gallaway et al., 2017; Barnes and Corkery, 2018; Landrigan et al., 2020), especially within the African American population (Shin and Doraiswamy, 2016). However, a recent meta-analysis (Coelho-Junior et al., 2020) suggested that resistance training likely improves cognition, but there was no available data regarding the impact of genotype or race/ethnicity. Therefore, this critical gap presents a promising future direction with opportunities for high impact discoveries.
Diet and exercise along with cognitive training and monitoring vascular risk factors may maintain or improve cognitive function in older adults who may have increased risk of developing cognitive decline or dementia. This is supported by results from the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) study (Ngandu et al., 2015). The World-Wide FINGER network2 is working to replicate results with larger and more diverse populations, including the US POINTER Study (NCT03688126).
Emotional Wellbeing
The treatment of mood disorders with psychotherapy may serve as a protective factor for vascular health. Several studies have shown that mood disorders predict vascular dysfunction (Frasure-Smith and Lespérance, 2008; Fiedorowicz et al., 2011). Mood disorders such as anxiety and depression may increase the chance of adopting behaviors such as smoking, sedentary lifestyle, and issues with medication adherence (Abed et al., 2014). Depression has been found to impact vascular health (Valkanova and Ebmeier, 2013). Depressed individuals presenting with simultaneous symptoms of anxious distress appear to be at an even higher risk compared to other depressed individuals (Rowan et al., 2005; Almas et al., 2015). Patients with established cardiovascular disease and mental health comorbidities struggle with medication adherence and adopting healthy lifestyle recommendations as well as experience greater disease progression (Ryder and Cohen, 2021). Psychotherapy can potentially serve as a preventative measure for vascular dysfunction through improving behaviors linked to protective factors of vascular dysfunction. Psychotherapy has been effective in improving vascular outcomes for patients with hypertension through adherence to treatment (Ma et al., 2014). Meta-analyses of randomized control trials supported that mental health treatments led to improvements in depression and anxiety, in addition to reductions in coronary heart disease events and cardiovascular mortality (Rutledge et al., 2013; Richards et al., 2018). To prevent the negative cardiovascular consequences of mood disorders, appropriate mental health screening should be conducted by providers (Ryder and Cohen, 2021). The treatment of mood disorders has an overall positive impact on vascular health and functioning.
Non-pharmaceutical interventions for AD have been found to be beneficial preventative measures. Social support can serve as a protective factor for AD. In a study that examined positive and negative effects of social support on the development of AD, positive social support from children was associated with reduced risk of developing dementia (Khondoker et al., 2017). Children may be in a unique position to be impactful members of an elder’s psychological health and should be incorporated into their support system. In a study that examined the relationship between individual forms of social support with early AD vulnerability and cognitive functioning, social support in the form of supportive listening was associated with greater cognitive resilience (Salinas et al., 2021). Social support is one non-pharmaceutical, early intervention that may lead to better outcomes for individuals with ADRD.
Hormone Replacement
Midlife aging is a critical time for preventing and delaying neurodegeneration (Mishra et al., 2022). Studies have shown that deprivation of estrogen as well as testosterone relate to cognitive decline in rats, with obesity accelerating the process (Pratchayasakul et al., 2015; Chunchai et al., 2019). The loss of control effect that estrogen has on brain glucose metabolism during the menopausal transition in women, accelerated by APOE4, creates a bioenergetic crisis leading to neurodegeneration (Rahman et al., 2019; Mishra et al., 2022). The sex disparities seen in AD patients could be explained by the neuroprotective nature of estrogen in females and the changes in estrogen expression that occur at or around the time of menopause (Snyder et al., 2016; Rahman et al., 2019). Estrogen replacement therapy has potential as a treatment for women with AD but more studies are needed to determine the effects of the stages of the menopausal transition and ultimately if the therapy would be beneficial (Smith et al., 2010; Guo et al., 2020; Song et al., 2020).
Oxygen Therapy
As stated above, there are currently limited options available to improve the prognosis of AD, and thus, researchers continue to attempt to understand the efficacy of various off-label treatments such as hyperbaric oxygen therapy (HBOT), notably in trials aimed at promoting neuroplasticity and improving neurocognitive function in humans (Harch and Fogarty, 2018; Xu et al., 2019; Chen et al., 2020; Gottfried et al., 2021; Marcinkowska et al., 2021; Shapira et al., 2021; Somaa, 2021). The effects of this specific treatment have also been investigated via various animal models to reveal HBOT has the ability to reduce neuroinflammation (Shapira et al., 2021), reduce hippocampal neuronal apoptosis (Zhao et al., 2017), and promote neuro- (Zhang et al., 2010) and angiogenesis (Yang et al., 2017). Accordingly, there is rationale to examine HBOT implementation due to the synergistic effects of vascular disease and AD; HBOT might delay or prevent vascular disease and AD via the alleviation of both cerebrovascular occlusion and the resulting cerebral hypoperfusion (Zhang and Le, 2010; Harch and Fogarty, 2018; Xu et al., 2019; You et al., 2019; Gottfried et al., 2021; Shapira et al., 2021; Somaa, 2021). Typical administration of HBOT consists of inhaling 97–100% oxygen under a pressure greater than 1 atmospheric absolute (ATA) (Tibbles and Edelsberg, 1996; Carson et al., 2005), which, in theory, increases the concentration of oxygen dissolved in the plasma as well as arterial saturation to subsequently improve tissue hypoxia (Xu et al., 2019; Fischer and Barak, 2020). In practice, it has been shown that 60 sessions of HBOT (5 d⋅wk–1, 90 min of exposure, 100% O2 at 2 ATA) induces angiogenesis as demarcated by increases in cerebral perfusion and velocity of blood flow (Hu et al., 2014; Tal et al., 2017). These vascular function/structure benefits have fostered investigations of HBOT pertaining to improved cognitive outcomes (Tal et al., 2017; Zhao et al., 2017; Xu et al., 2019; Gottfried et al., 2021; Marcinkowska et al., 2021; Shapira et al., 2021; Somaa, 2021). For instance, following a similar HBOT exposure prescription (Tal et al., 2017), six elderly patients with significant memory loss exhibited increases in CBF in multiple brain areas as well as improved global cognitive scores (memory, attention, and processing speed were most ameliorated) (Shapira et al., 2021). Additionally, in a large sample of patients diagnosed with vascular dementia, it was revealed that HBOT (5 d⋅wk–1, 60 min of exposure, 100% O2 at 2 ATA) plus 5 mg⋅d–1 of donepezil hydrochloride resulted in higher mini-mental state examination (MMSE) scores, a commonly used cognitive impairment screening test, post treatment than the control (5 mg⋅d–1 of donepezil hydrochloride) group (19.8% vs. 9.7%, respectively) (Xu et al., 2019). Further, within this investigation (Xu et al., 2019), it was reported that humanin was increased to a greater extent in HBOT condition compared to the control group (17.4% vs. 13.2%), and of interest, the humanin levels were positively correlated (r = 0.409) with the MMSE scores. Overall, the understanding of HBOT to alleviate symptoms of AD via vascular improvements remains in its infancy, but the currently available data is undoubtedly promising, and warrants continued investigation. Future work remains particularly needed to uncover potential race/ethnicity and genetic based differences in responses as well as the efficacy of combining HBOT with other suspected AD preventative care strategies such as diet and exercise.
Health Disparities for Prevention
Rural Health Disparities
Some rural Americans, particularly individuals living in the rural South, face greater health disparities compared to both their urban counterparts as well as individuals in other rural areas in the United States (Murray et al., 2006; Miller and Vasan, 2021). The rural mortality penalty is a term used to describe the increased mortality rate observed in rural areas in certain parts of the United States compared to non-rural populations. Southern rural areas, particularly Appalachia, the Mississippi Delta Region, and the Alabama Black Belt have the lowest life expectancies in the country, and this trend has persisted for the last five decades (Singh and Siahpush, 2014; James et al., 2018). While mortality rates are higher in all areas in the South, mortality rates in rural areas in the East South Central Region (e.g., Kentucky, Tennessee, Mississippi, and Alabama) have the highest rates of mortality (Murray et al., 2006; James et al., 2018). Additionally, lower SES and higher rates of poverty correlate to poorer health outcomes. Non-metropolitan areas in Alabama have a poverty rate of 20.6%, which further increases disparities in access to quality healthcare in this population (Rural Health Information Hub, 2022), as shown in Figure 2G. These higher mortality and poverty rates in these underserved Southern rural communities are due to long-standing structural and social barriers to good health, education, jobs, and income/wealth. These communities have greater barriers to accessing quality health care as individuals have to travel longer distance to physicians’ offices, do not have access to public transportation, are more likely to be uninsured, and there are fewer physicians per capita (Miller and Vasan, 2021). The rural South has the fewest number of physicians relative to population, and according to data collected from the Health Resources and Services Administration, in non-metropolitan regions in Alabama, there are only nine physicians per ten thousand people (Miller and Vasan, 2021; Rural Health Information Hub, 2022). The prevalence of health professional shortage in primary care (Figure 2G) and dentistry (Figure 2H). Furthermore, the University of Alabama at Birmingham Alzheimer’s Disease Center is the only academic center for the specialized care of patients with AD in the state. A lack of support and resources for both patients and caregivers in addition to rural Americans having less frequent interactions with healthcare providers all likely contribute to this population receiving a diagnosis of AD at later stages of cognitive decline than their urban counterparts (Rahman et al., 2021). These observations are why it is vital to address the disparities in care and resources present in rural communities, particularly in states like Alabama, where over one million people reside in rural areas of the state (Rural Health Information Hub, 2022).
Socioeconomic Status and Environment
Although age is a major factor in the development of vascular dysfunction, dementia, and AD, it does not affect the older populations equally, especially those individuals of disadvantaged backgrounds, due to socioeconomic and environmental contributors. SES, the social ranking of an individual or group that is often measured by the combination of education, income, and employment, frequently reveals discrepancies and inequities regarding resource access, ultimately leading to health disparities (Riley, 2012; American Psychological Association, 2021).
Components of SES have been identified as modifiable risk factors for the development of dementia and dementia-related mortality. For example, when comparing individuals with higher SES to those with lower SES, higher SES people should anticipate to live a much longer amount of time without dementia (Cha et al., 2021). In addition, lower SES with low income and financial stress is associated with increased risk of dementia in older persons, and the correlations are similar to those found in older adults with lower education in the United States (Samuel et al., 2020). Also, caregivers who share a home with AD patients with severe neuropsychiatric symptoms (e.g., aggression and anxiety) while living in lower SES areas had more caregiver stress, further showing that different physical and social environmental factors have distinct effects on the likelihood of AD (Alhasan et al., 2021).
Collectively, the aforementioned examples provide evidence that people of lower SES will suffer major consequences of healthcare outcomes and caregiver expenses. Thus, earlier detection and intervention strategies should be developed to steer preventative and risk-reduction measures for vascular dysfunction, dementia, and AD.
Accessibility to Healthcare and Quality Food
The incidence of AD in Alabama is compounded by poor health predispositions and barriers to receiving necessary medical care. In 2019, the Rural Health Information Hub reported that 47.5% of metropolitan Alabama residents and 31.7% of non-metropolitan Alabama residents had low access to healthy food. Low-access areas were defined as having at least 500 people, or 33% of the population, living more than 1 mile (urban areas) or 10 miles (rural areas) from the nearest supermarket (Rural Health Information Hub, 2022). Additionally, in 2018, there were 7 Primary Care Physicians (PCPs) in metropolitan areas of Alabama per 10,000 people with only 5 PCPs per 10,000 in non-metropolitan areas (Rural Health Information Hub, 2022). The prevalence of healthcare disparities and low accessibility to healthy food in Alabamians is shown in Figure 2. A 2015 report by the Alzheimer’s Association further revealed that 51.0% of adults in Alabama aged 45 and over that are experiencing Subjective Cognitive Decline have not talked to a health care provider (ADPH, 2021).
In the South, including in Alabama, we like our food fried (e.g., chicken, okra, and almost anything else), buttery, salty, and/or sweet. Just writing that sentence can make a Southerner hungry. Detaching culture from food is challenging but if there is more awareness about the impact of diet on cardiovascular health and brain health, perhaps we can reduce the risk of ADRD in Alabama. However, saying one should eat better and exercise more is much easier said than done. It requires creating healthy communities (Crook et al., 2019). Healthy communities are walkable, safe, supportive, intergenerational, loving, and have access to healthy food and other essentials. These safe communities are non-violent, supportive, and invite social interaction and physical activity. These healthy communities are structured on the health equity principle of meeting people where they are and they are not judgmental.
Beyond Alabama
The aforementioned ADRD risk factors are concentrated in Alabama; however, other regions exhibit many of these risk factors and health disparities as well and should be highlighted. As previously stated, African Americans make up 26.8% of the population in Alabama (Census, 2022). However, the proportion of Black participants and other ethnic minorities in the United States population is growing (Tamir, 2021). Furthermore, the population of minority children and youth grew from 33% (1990) to 38% (2000) to 43% (2008) (Johnson and Lichter, 2010). Therefore, ethnicity- and race-related health disparities must be addressed not only in Alabama but across the United States as well.
Lifestyle-related risk factors for ADRD are also present outside of Alabama. For example, only 8.4% of adults in West Virginia met fruit intake recommendations, compared to the American average of 12.3% (Lee, 2022). Obesity rates also vary between regions of the United States with the highest obesity prevalence in the Midwest (34.1%) and South (34.1%) (CDC, 2021c). Alcohol binge drinking prevalence among adults was highest in Montana, Wyoming, Colorado, North Dakota, South Dakota, Nebraska, Minnesota, Iowa, Missouri, Wisconsin, Illinois, Michigan, Connecticut, Massachusetts, Maine, and Hawaii in 2018 (CDC, 2022b). Cigarette use was highest among adults in West Virginia, Kentucky, Louisiana, Ohio, Mississippi, Alabama, Tennessee, Missouri, Indiana, Oklahoma, Michigan, and North Carolina in 2019 (CDC, 2021b). The LGBTQ student population in 2019 was greater than 18% in New York, Connecticut, Alabama, New Mexico, South Carolina, Florida, Nevada, and Vermont (CDC, 2021a), suggesting that there will be a shift in sexuality among the American adult population in the future. In addition, California, Nevada, Texas, Mississippi, Alabama, Georgia, Florida, South Carolina, North Carolina, Maryland, New Jersey, and Massachusetts had the highest rates of HIV diagnosis among adults and adolescents in 2019 (CDC, 2022d). It will be important to understand the association between these risk factors and ADRD so future ADRD research should include lifestyle-related risk factors, gender and sexuality as variables.
Pathological risk factors for ADRD are present in certain ethnic groups that were not highlighted previously in this review due to lower relevance to Alabama. For example, Filipino Americans are known to have a high risk for cardiovascular disease, hypertension, type 2 diabetes, and metabolic syndrome at lower body mass index levels, likely due to diet and genetics (Abesamis et al., 2016). Despite a higher prevalence rate in non-Hispanic White people, among APOE4 carriers, Asians had a significantly steeper memory decline when compared to White people (Makkar et al., 2020). Asian-Indians are also known to have the highest coronary artery disease rates (Ardeshna et al., 2018). As previously stated in this review, cardiovascular disease is a known ADRD risk factor leading to impairment of the NVU (Vanherle et al., 2020). Pulmonary diseases, a known risk factor for ADRD, exist at high prevalence in states other than Alabama. Patients with chronic obstructive pulmonary disease (COPD) were found to have a higher risk for dementia (Liao et al., 2015). In 2019, the mortality rate due to COPD ranges from 49.9 to 61.4 deaths per 100,000 people not only in Alabama, but Wyoming, Oklahoma, Arkansas, Mississippi, Tennessee, Kentucky, Indiana, and West Virginia as well (CDC, 2022a).
Cardiovascular and lifestyle risk factors for ADRD are also present in countries other than the United States. For example, Asians including Indonesians (Malays and Chinese ancestry), Singaporean Chinese, Malays and Indians, and Hong Kong Chinese have 3–5% higher body fat than White people at any given body mass index (Deurenberg et al., 2002). A study performed in India showed that South Asians are at high risk for obesity-associated cardiovascular disorders thought to be due to low adipokine production, lower lean body mass, and ethno-specific SES factors (Prasad et al., 2011). In Italy, high cholesterol was found in over 40% of the subject population and abnormal low density lipoprotein values were observed in about 30% (Sofi et al., 2005). In Sub-Saharan Africa, rates of overweight and obesity are rising in children and adolescents due to lifestyle and social determinants of health such as physical inactivity, unhealthy diets, SES, and gender (Choukem et al., 2020). The same study also found many cardiovascular risk factors for ADRD such as metabolic syndrome, hypertension, dyslipidemia, diabetes, and glucose intolerance in the 86,637 children and adolescents in the study (Choukem et al., 2020). A nationwide study in France between 2008 and 2013 showed that 38.9% of early onset dementia cases were alcohol-related and 17.6% of cases had an additional alcohol use disorder (Schwarzinger et al., 2018).
Health disparities due to differences in SES can also be seen globally. A study estimating the global, regional, and national burden of stroke showed that age-standardized low-income groups had several times higher stroke incidence than high-income groups (Feigin et al., 2021). A Danish study also revealed societal inequalities, indicating that those of higher SES appear to be diagnosed with dementia earlier (Petersen et al., 2021). Environmental factors such as air quality, toxic heavy metals, and trace elements have also been shown to be risk factors for ADRD (Killin et al., 2016). Bangladesh (76.9 μg/mL) was found to have the worst air pollution in 2020, followed by Chad (75.9 μg/mL) (IQAIR, 2022) based on particulate matter up to 2.5 μm in diameter. A study focusing on the reasons for Finland’s high dementia mortality rate found that environmental factors such as climate leads to production of neurotoxins that contribute to dementia pathophysiology (Eiser, 2017). The same study also found that trace elements such as mercury is also found in Finnish bodies of water, reducing the quantity and effectiveness of glutathione’s ability to protect against neurotoxins (Eiser, 2017).
Conclusion
Black, Hispanic and Native Americans have a higher incidence of NVU intrinsic and extrinsic risk factors and consequentially ADRD when compared to White Americans (Alzheimer’s Association, 2022). Black Alabamians, in particular, have a higher prevalence of AD risk factors than other Alabama residents, highlighting tangible health disparities that warrant immediate action. These racial, ethnic, and geographic differences, when compounded with additional disparities in the social determinants of health, further increase the risk for early death for Black Americans in Alabama. In this review, we identified vascular risk factors linked to AD as well as pharmaceutical and non-pharmaceutical treatments and prevention practices to slow or prevent neurovascular dysfunction and cognitive decline as demonstrated in Figure 3. However, individuals living in many regions in Alabama face barriers to implementing these lifestyle changes due to a number of factors such as low SES, the southern rural health penalty, food deserts, and decreased access to quality health care. Consequently, individuals living in Alabama, and similar states, are more prone to adopt unhealthy diets and habits including sedentary lifestyles as well as drug and alcohol use. Ultimately, the combined effect of these inequities lead to a higher risk of NVU dysfunction in Alabama. Community engaged investigators will have to give intentional attention to the mistrust for the health care system within AL’s Black American community. Such engagement by investigators and community members will foster broader community involvement in research that is relevant to underrepresented communities. There is a clear and dire need for future ADRD studies to include more diverse populations with a specific focus on the individuals affected by the disparities outlined in this review.
Author Contributions
NS and AN conceptualized the initial review outline. NS, E-AB, SC, MT, NH, RR, HB, CF, ML, BH, JK, and AN wrote the first draft of the manuscript. NS, E-AB, SC, MT, HB, FT, EC, JK, and AN reviewed and edited the manuscript. All authors read and approved the final draft of the manuscript.
Funding
The research of ML was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (R01HL140182). The research of JK along with CF, BH, and AN was partially funded by the University of South Alabama via the 2021 Research and Scholarly Development Grant. The research of AN was supported by the National Institute on Aging of the National Institutes of Health (R00AG058780) and AlzOut (https://alzout.org/).
Author Disclaimer
The views and opinions expressed in this manuscript represent those of the author and do not necessarily reflect those of the National Institutes of Health, or AlzOut.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Abbott, A. (2020). Are infections seeding some cases of Alzheimer’s disease? Nature 587, 22–25. doi: 10.1038/d41586-020-03084-9
Abdelhamid, M., Jung, C.-G., Zhou, C., Abdullah, M., Nakano, M., Wakabayashi, H., et al. (2020). Dietary lactoferrin supplementation prevents memory impairment and reduces amyloid-β generation in j20 mice. J. Alzheimers Dis. 74, 245–259. doi: 10.3233/JAD-191181
Abed, M. A., Kloub, M. I., and Moser, D. K. (2014). Anxiety and adverse health outcomes among cardiac patients: a biobehavioral model. J. Cardiovasc. Nurs. 29, 354–363. doi: 10.1097/JCN.0b013e318292b235
Abesamis, C. J., Fruh, S., Hall, H., Lemley, T., and Zlomke, K. R. (2016). Cardiovascular health of filipinos in the united states: a review of the literature. J. Transcult. Nurs. 27, 518–528. doi: 10.1177/1043659615597040
ADPH (2021). Related Programs | Alabama Department of Public Health (ADPH). Available online at: https://www.alabamapublichealth.gov/brfss/related-programs.html [Accessed December 17, 2021].
Aggarwal, A., Singh, I., and Sandhir, R. (2018). Protective effect of S-nitrosoglutathione administration against hyperglycemia induced disruption of blood brain barrier is mediated by modulation of tight junction proteins and cell adhesion molecules. Neurochem. Int. 118, 205–216. doi: 10.1016/j.neuint.2018.05.009
Ahmed, N. (2005). Advanced glycation endproducts–role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 67, 3–21.
Aikawa, T., Holm, M.-L., and Kanekiyo, T. (2018). ABCA7 and pathogenic pathways of alzheimer’s disease. Brain Sci. 8:E27. doi: 10.3390/brainsci8020027
Aires, A., Andrade, A., Azevedo, E., Gomes, F., Araújo, J. P., and Castro, P. (2020). Neurovascular coupling impairment in heart failure with reduction ejection fraction. Brain Sci. 10:714. doi: 10.3390/brainsci10100714
Al Bander, Z., Nitert, M. D., Mousa, A., and Naderpoor, N. (2020). The Gut Microbiota and Inflammation: an overview. Int. J. Environ. Res. Public Health 17:E7618. doi: 10.3390/ijerph17207618
Alabama (2021). Available online at: https://www.cdc.gov/nchs/pressroom/states/alabama/al.htm [Accessed January 28, 2022].
Alabama (2022). Compare Counties in Alabama County Health Rankings & Roadmaps. Available online at: https://www.countyhealthrankings.org/app/alabama/2021/measure/factors/11/description [Accessed January 6, 2022].
Alhasan, D. M., Hirsch, J. A., Jackson, C. L., Miller, M. C., Cai, B., and Lohman, M. C. (2021). Neighborhood characteristics and the mental health of caregivers cohabiting with care recipients diagnosed with Alzheimer’s disease. Int. J. Environ. Res. Public Health 18:913. doi: 10.3390/ijerph18030913
Allard, J. S., Ntekim, O., Johnson, S. P., Ngwa, J. S., Bond, V., Pinder, D., et al. (2017). APOEε4 impacts up-regulation of brain-derived neurotrophic factor after a six-month stretch and aerobic exercise intervention in mild cognitively impaired elderly african americans: a pilot study. Exp. Gerontol. 87, 129–136. doi: 10.1016/j.exger.2016.11.001
Almas, A., Forsell, Y., Iqbal, R., Janszky, I., and Moller, J. (2015). Severity of depression, anxious distress and the risk of cardiovascular disease in a swedish population-based cohort. PLoS One 10:e0140742. doi: 10.1371/journal.pone.0140742
Altmann, A., Tian, L., Henderson, V. W., Greicius, M. D., and Alzheimer’s Disease Neuroimaging Initiative, and Investigators (2014). Sex modifies the APOE-related risk of developing Alzheimer disease. Ann. Neurol. 75, 563–573. doi: 10.1002/ana.24135
Alty, J., Farrow, M., and Lawler, K. (2020). Exercise and dementia prevention. Pract. Neurol. 20, 234–240. doi: 10.1136/practneurol-2019-002335
Alvarez, I., Gonzalo, L. M., and Llor, J. (1989). Effects of chronic alcoholism on the amygdaloid complex. A study in human and rats. Histol. Histopathol. 4, 183–192.
Alzheimer’s Association (2022). 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 17, 327–406. doi: 10.1002/alz.12638
Amen, D. G., Darmal, B., Raji, C. A., Bao, W., Jorandby, L., Meysami, S., et al. (2017). Discriminative properties of hippocampal hypoperfusion in marijuana users compared to healthy controls: implications for marijuana administration in Alzheimer’s Dementia. J. Alzheimers Dis. 56, 261–273. doi: 10.3233/JAD-160833
American Psychological Association (2021). Socioeconomic Status. Available online at: https://www.apa.org/topics/socioeconomic-status (accessed June 8, 2022).
Ardeshna, D. R., Bob-Manuel, T., Nanda, A., Sharma, A., Skelton, W. P., Skelton, M., et al. (2018). Asian-Indians: a review of coronary artery disease in this understudied cohort in the United States. Ann. Transl. Med. 6:12. doi: 10.21037/atm.2017.10.18
Arrieta, M., White, H. L., and Crook, E. D. (2008). Using zip code-level mortality data as a local health status indicator in Mobile, Alabama. Am. J. Med. Sci. 335, 271–274. doi: 10.1097/maj.0b013e31816a49c0
Arvanitakis, Z., Capuano, A. W., Leurgans, S. E., Bennett, D. A., and Schneider, J. A. (2016). Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol. 15, 934–943. doi: 10.1016/S1474-4422(16)30029-1
Atherosclerosis (2022). Atherosclerosis | NHLBI, NIH. Available online at: https://www.nhlbi.nih.gov/health-topics/atherosclerosis [Accessed January 28, 2022].
Bailey, T. L., Rivara, C. B., Rocher, A. B., and Hof, P. R. (2004). The nature and effects of cortical microvascular pathology in aging and Alzheimer’s disease. Neurol. Res. 26, 573–578. doi: 10.1179/016164104225016272
Baker, L. D., Frank, L. L., Foster-Schubert, K., Green, P. S., Wilkinson, C. W., McTiernan, A., et al. (2010). Effects of aerobic exercise on mild cognitive impairment. Arch. Neurol. 67, 71–79. doi: 10.1001/archneurol.2009.307
Balczon, R., Lin, M. T., Lee, J. Y., Abbasi, A., Renema, P., Voth, S. B., et al. (2021). Pneumonia initiates a tauopathy. FASEB J. 35:e21807. doi: 10.1096/fj.202100718R
Balczon, R., Morrow, K. A., Zhou, C., Edmonds, B., Alexeyev, M., Pittet, J.-F., et al. (2017). Pseudomonas aeruginosa infection liberates transmissible, cytotoxic prion amyloids. FASEB J. 31, 2785–2796. doi: 10.1096/fj.201601042RR
Balczon, R., Pittet, J.-F., Wagener, B. M., Moser, S. A., Voth, S., Vorhees, C. V., et al. (2019). Infection-induced endothelial amyloids impair memory. FASEB J. 33, 10300–10314. doi: 10.1096/fj.201900322R
Baloyannis, S. J., and Baloyannis, I. S. (2012). The vascular factor in Alzheimer’s disease: a study in Golgi technique and electron microscopy. J. Neurol. Sci. 322, 117–121. doi: 10.1016/j.jns.2012.07.010
Barnes, D. E., and Yaffe, K. (2011). The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 10, 819–828. doi: 10.1016/S1474-4422(11)70072-2
Barnes, J. N. (2015). Exercise, cognitive function, and aging. Adv. Physiol. Educ. 39, 55–62. doi: 10.1152/advan.00101.2014
Barnes, J. N., and Corkery, A. T. (2018). Exercise improves vascular function, but does this translate to the brain? Brain Plast. 4, 65–79. doi: 10.3233/BPL-180075
Barthold, S. W., de Souza, M. S., and Smith, A. L. (1990). Susceptibility of laboratory mice to intranasal and contact infection with coronaviruses of other species. Lab. Anim. Sci. 40, 481–485.
Bateman, R. J., Aisen, P. S., De Strooper, B., Fox, N. C., Lemere, C. A., Ringman, J. M., et al. (2011). Autosomal-dominant Alzheimer’s disease: a review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res. Ther. 3:1. doi: 10.1186/alzrt59
Bell, R. D., Winkler, E. A., Singh, I., Sagare, A. P., Deane, R., Wu, Z., et al. (2012). Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 485, 512–516. doi: 10.1038/nature11087
Bengoechea, O., and Gonzalo, L. M. (1991). Effects of alcoholization on the rat hippocampus. Neurosci. Lett. 123, 112–114. doi: 10.1016/0304-3940(91)90170-x
Benjamin, E. J., Muntner, P., Alonso, A., Bittencourt, M. S., Callaway, C. W., Carson, A. P., et al. (2019). Heart disease and stroke statistics—2019 update: a report from the american heart association. Circulation 139, e56–e528. doi: 10.1161/CIR.0000000000000659
Bennet, A. M., Di Angelantonio, E., Ye, Z., Wensley, F., Dahlin, A., Ahlbom, A., et al. (2007). Association of apolipoprotein e genotypes with lipid levels and coronary risk. JAMA 298, 1300–1311. doi: 10.1001/jama.298.11.1300
Bliss, E. S., Wong, R. H., Howe, P. R., and Mills, D. E. (2020). Benefits of exercise training on cerebrovascular and cognitive function in ageing. J. Cereb. Blood Flow Metab. 41, 447–470. doi: 10.1177/0271678X20957807
Blum, K., Badgaiyan, R. D., Dunston, G. M., Baron, D., Modestino, E. J., McLaughlin, T., et al. (2018). The DRD2 Taq1A A1 allele may magnify the risk of alzheimer’s in aging african-americans. Mol. Neurobiol. 55, 5526–5536. doi: 10.1007/s12035-017-0758-1
Bracko, O., Vinarcsik, L. K., Cruz Hernández, J. C., Ruiz-Uribe, N. E., Haft-Javaherian, M., Falkenhain, K., et al. (2020). High fat diet worsens Alzheimer’s disease-related behavioral abnormalities and neuropathology in APP/PS1 mice, but not by synergistically decreasing cerebral blood flow. Sci. Rep. 10:9884. doi: 10.1038/s41598-020-65908-y
Brandsma, E., Kloosterhuis, N. J., Koster, M., Dekker, D. C., Gijbels, M. J. J., van der Velden, S., et al. (2019). A proinflammatory gut microbiota increases systemic inflammation and accelerates atherosclerosis. Circ. Res. 124, 94–100. doi: 10.1161/CIRCRESAHA.118.313234
Brody, M., Agronin, M., Herskowitz, B. J., Bookheimer, S. Y., Small, G. W., Hitchinson, B., et al. (2022). Results and insights from a phase I clinical trial of Lomecel-B for Alzheimer’s disease. Alzheimers Dement. doi: 10.1002/alz.12651
Brooks, A. W., Priya, S., Blekhman, R., and Bordenstein, S. R. (2018). Gut microbiota diversity across ethnicities in the United States. PLoS Biol. 16:e2006842. doi: 10.1371/journal.pbio.2006842
Brundel, M., Heringa, S. M., de Bresser, J., Koek, H. L., Zwanenburg, J. J. M., Jaap Kappelle, L., et al. (2012). High prevalence of cerebral microbleeds at 7Tesla MRI in patients with early Alzheimer’s disease. J. Alzheimers Dis. 31, 259–263. doi: 10.3233/JAD-2012-120364
Burdette, J. H., Laurienti, P. J., Espeland, M. A., Morgan, A., Telesford, Q., Vechlekar, C. D., et al. (2010). Using network science to evaluate exercise-associated brain changes in older adults. Front. Aging Neurosci. 2:23. doi: 10.3389/fnagi.2010.00023
Busquets, O., Ettcheto, M., Pallàs, M., Beas-Zarate, C., Verdaguer, E., Auladell, C., et al. (2017). Long-term exposition to a high fat diet favors the appearance of β-amyloid depositions in the brain of C57BL/6J mice. A potential model of sporadic Alzheimer’s disease. Mech. Ageing Dev. 162, 38–45. doi: 10.1016/j.mad.2016.11.002
Canet, G., Dias, C., Gabelle, A., Simonin, Y., Gosselet, F., Marchi, N., et al. (2018). HIV neuroinfection and Alzheimer’s Disease: similarities and potential links? Front. Cell Neurosci. 12:307. doi: 10.3389/fncel.2018.00307
Cao, C., Li, Y., Liu, H., Bai, G., Mayl, J., Lin, X., et al. (2014). The potential therapeutic effects of THC on Alzheimer’s disease. J. Alzheimers Dis. 42, 973–984. doi: 10.3233/JAD-140093
Carson, S., McDonagh, M., Russman, B., and Helfand, M. (2005). Hyperbaric oxygen therapy for stroke: a systematic review of the evidence. Clin. Rehabil. 19, 819–833. doi: 10.1191/0269215505cr907oa
Caruso, A., Nicoletti, F., Mango, D., Saidi, A., Orlando, R., and Scaccianoce, S. (2018). Stress as risk factor for Alzheimer’s disease. Pharmacol. Res. 132, 130–134. doi: 10.1016/j.phrs.2018.04.017
Castaneda, G., Liu, B., Torres, S., Bhuket, T., and Wong, R. J. (2017). Race/Ethnicity-specific disparities in the severity of disease at presentation in adults with ulcerative colitis: a cross-sectional study. Dig. Dis. Sci. 62, 2876–2881. doi: 10.1007/s10620-017-4733-5
Cattaneo, A., Cattane, N., Galluzzi, S., Provasi, S., Lopizzo, N., Festari, C., et al. (2017). Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 49, 60–68. doi: 10.1016/j.neurobiolaging.2016.08.019
CDC (2021b). Map of Cigarette Use Among Adults | STATE System | CDC (2021). Available online at: https://www.cdc.gov/statesystem/cigaretteuseadult.html [Accessed May 23, 2022].
CDC (2021a). 2019 Number And Percentage Of Students, By Sexual Identity | Results | YRBSS | Data | Adolescent and School Health. Available online at: https://www.cdc.gov/healthyyouth/data/yrbs/2019_tables/students_by_sexual_identity.htm [Accessed May 23, 2022].
CDC (2021c). New Adult Obesity Maps. Centers for Disease Control and Prevention. Available online at: https://www.cdc.gov/obesity/data/prevalence-maps.html [Accessed January 6, 2022].
CDC (2022c). Incidence of Newly Diagnosed Diabetes | Diabetes | CDC (2022). Available online at: https://www.cdc.gov/diabetes/data/statistics-report/newly-diagnosed-diabetes.html [Accessed January 28, 2022].
CDC (2022e). Stats of the States – Alzheimer’s Disease Mortality CDC (2022). Available online at: https://www.cdc.gov/nchs/pressroom/sosmap/alzheimers_mortality/alzheimers_disease.htm (accessed June 8, 2022).
CDC (2022b). Data on Excessive Drinking | CDC. Available online at: https://www.cdc.gov/alcohol/data-stats.htm [Accessed May 23, 2022].
CDC (2022d). National Profile | Volume 32 | HIV Surveillance | Reports | Resource Library | HIV/AIDS | CDC. Available online at: https://www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-32/content/national-profile.html#Diagnoses [Accessed May 23, 2022].
CDC (2022a). CDC – Data and Statistics – Chronic Obstructive Pulmonary Disease (COPD). Available online at: https://www.cdc.gov/copd/data.html [Accessed May 23, 2022].
Celano, C. M., Daunis, D. J., Lokko, H. N., Campbell, K. A., and Huffman, J. C. (2016). Anxiety disorders and cardiovascular disease. Curr. Psychiatry Rep. 18:101. doi: 10.1007/s11920-016-0739-5
Census (2022). U.S. Census Bureau QuickFacts. Alabama Available online at: https://www.census.gov/quickfacts/AL [Accessed March 18, 2022].
Centers for Disease Control and Prevention (2021). Adult Physical Inactivity Prevalence Maps by Race/Ethnicity. Available online at: https://www.cdc.gov/physicalactivity/data/inactivity-prevalence-maps/index.html#black [Accessed December 14, 2021].
Cha, H., Farina, M. P., and Hayward, M. D. (2021). Socioeconomic status across the life course and dementia-status life expectancy among older Americans. SSM Popul. Health 15:100921. doi: 10.1016/j.ssmph.2021.100921
Chang, H.-C., Tai, Y.-T., Cherng, Y.-G., Lin, J.-W., Liu, S.-H., Chen, T.-L., et al. (2014). Resveratrol attenuates high-fat diet-induced disruption of the blood-brain barrier and protects brain neurons from apoptotic insults. J. Agric. Food Chem. 62, 3466–3475. doi: 10.1021/jf403286w
Chang, R., Yee, K.-L., and Sumbria, R. K. (2017). Tumor necrosis factor α Inhibition for Alzheimer’s Disease. J. Cent. Nerv. Syst. Dis. 9:1179573517709278. doi: 10.1177/1179573517709278
Chen, J., Zhang, F., Zhao, L., Cheng, C., Zhong, R., Dong, C., et al. (2020). Hyperbaric oxygen ameliorates cognitive impairment in patients with Alzheimer’s disease and amnestic mild cognitive impairment. Alzheimers Dement. (N. Y.) 6:e12030. doi: 10.1002/trc2.12030
Chen, Y., Cui, Q., Sheng, W., Tang, Q., Lu, F., Pang, Y., et al. (2021). Anomalous neurovascular coupling in patients with generalized anxiety disorder evaluated by combining cerebral blood flow and functional connectivity strength. Prog. Neuropsychopharmacol. Biol. Psychiatry 111:110379. doi: 10.1016/j.pnpbp.2021.110379
Chiu, C.-C., Su, K.-P., Cheng, T.-C., Liu, H.-C., Chang, C.-J., Dewey, M. E., et al. (2008). The effects of omega-3 fatty acids monotherapy in Alzheimer’s disease and mild cognitive impairment: a preliminary randomized double-blind placebo-controlled study. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 1538–1544. doi: 10.1016/j.pnpbp.2008.05.015
Choi, C.-S., Gwin, M., Voth, S., Kolb, C., Zhou, C., Nelson, A. R., et al. (2021). Cytotoxic tau released from lung microvascular endothelial cells upon infection with P. aeruginosa promotes neuronal tauopathy. J. Biol. Chem. 298:101482. doi: 10.1016/j.jbc.2021.101482
Choi, D., Choi, S., and Park, S. M. (2018). Effect of smoking cessation on the risk of dementia: a longitudinal study. Ann. Clin. Transl. Neurol. 5, 1192–1199. doi: 10.1002/acn3.633
Choukem, S.-P., Tochie, J. N., Sibetcheu, A. T., Nansseu, J. R., and Hamilton-Shield, J. P. (2020). Overweight/obesity and associated cardiovascular risk factors in sub-Saharan African children and adolescents: a scoping review. Int. J. Pediatr. Endocrinol. 2020:6. doi: 10.1186/s13633-020-0076-7
Chunchai, T., Apaijai, N., Keawtep, P., Mantor, D., Arinno, A., Pratchayasakul, W., et al. (2019). Testosterone deprivation intensifies cognitive decline in obese male rats via glial hyperactivity, increased oxidative stress, and apoptosis in both hippocampus and cortex. Acta Physiol. (Oxf) 226:e13229. doi: 10.1111/apha.13229
Clark, L. R., Norton, D., Berman, S. E., Johnson, S. C., Bendlin, B. B., Wieben, O., et al. (2019). Association of cardiovascular and alzheimer’s disease risk factors with intracranial arterial blood flow in whites and african americans. J. Alzheimers Dis. 72, 919–929. doi: 10.3233/JAD-190645
Coelho, F. G., de, M., Gobbi, S., Andreatto, C. A. A., Corazza, D. I., Pedroso, R. V., et al. (2013). Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (BDNF): a systematic review of experimental studies in the elderly. Arch. Gerontol. Geriatr. 56, 10–15. doi: 10.1016/j.archger.2012.06.003
Coelho, F. G., de, M., Vital, T. M., Stein, A. M., Arantes, F. J., Rueda, A. V., et al. (2014). Acute aerobic exercise increases brain-derived neurotrophic factor levels in elderly with Alzheimer’s Disease. J. Alzheimers Dis. 39, 401–408. doi: 10.3233/JAD-131073
Coelho-Junior, H., Marzetti, E., Calvani, R., Picca, A., Arai, H., and Uchida, M. (2020). Resistance training improves cognitive function in older adults with different cognitive status: a systematic review and meta-analysis. Aging Ment. Health 26, 213–224. doi: 10.1080/13607863.2020.1857691
Collins-Praino, L. E., and Corrigan, F. (2017). Does neuroinflammation drive the relationship between tau hyperphosphorylation and dementia development following traumatic brain injury? Brain Behav. Immunity 60, 369–382. doi: 10.1016/j.bbi.2016.09.027
Coogan, P., Schon, K., Li, S., Cozier, Y., Bethea, T., and Rosenberg, L. (2020). Experiences of racism and subjective cognitive function in African American women. Alzheimers Dement. (Amst) 12:e12067. doi: 10.1002/dad2.12067
Corrales-Medina, V. F., Alvarez, K. N., Weissfeld, L. A., Angus, D. C., Chirinos, J. A., Chang, C.-C. H., et al. (2015). Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA 313, 264–274. doi: 10.1001/jama.2014.18229
Correia, S. S., Iyengar, R. R., Germano, P., Tang, K., Bernier, S. G., Schwartzkopf, C. D., et al. (2021). The CNS-penetrant soluble guanylate cyclase stimulator CY6463 reveals its therapeutic potential in neurodegenerative diseases. Front. Pharmacol. 12:656561. doi: 10.3389/fphar.2021.656561
Cox, A. J., West, N. P., and Cripps, A. W. (2015). Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 3, 207–215. doi: 10.1016/S2213-8587(14)70134-2
Craig-Schapiro, R., Kuhn, M., Xiong, C., Pickering, E. H., Liu, J., Misko, T. P., et al. (2011). Multiplexed immunoassay panel identifies novel CSF biomarkers for Alzheimer’s disease diagnosis and prognosis. PLoS One 6:e18850. doi: 10.1371/journal.pone.0018850
Crook, E. D., Pierre, K., and Arrieta, M. A. (2019). Identifying and overcoming roadblocks that limit the translation of research findings to the achievement of health equity. J. Health Care Poor Underserved 30, 43–51. doi: 10.1353/hpu.2019.0114
Csernansky, J. G., Dong, H., Fagan, A. M., Wang, L., Xiong, C., Holtzman, D. M., et al. (2006). Plasma cortisol and progression of dementia in DAT subjects. Am. J. Psychiatry 163, 2164–2169. doi: 10.1176/appi.ajp.163.12.2164
Davis, J. A. (2013). HIV and the LGBT community: a medical update. J. Gay Lesbian Ment. Health 17, 64–79. doi: 10.1080/19359705.2013.737738
de Aquino, C. C., Leitão, R. A., Oliveira Alves, L. A., Coelho-Santos, V., Guerrant, R. L., Ribeiro, C. F., et al. (2018). Effect of hypoproteic and high-fat diets on hippocampal blood-brain barrier permeability and oxidative stress. Front. Nutr. 5:131. doi: 10.3389/fnut.2018.00131
De la Rosa, A., Olaso-Gonzalez, G., Arc-Chagnaud, C., Millan, F., Salvador-Pascual, A., García-Lucerga, C., et al. (2020). Physical exercise in the prevention and treatment of Alzheimer’s disease. J. Sport Health Sci. 9, 394–404. doi: 10.1016/j.jshs.2020.01.004
de la Rubia Ortí, J. E., García-Pardo, M. P., Drehmer, E., Sancho Cantus, D., Julián Rochina, M., Aguilar, M. A., et al. (2018). Improvement of main cognitive functions in patients with alzheimer’s disease after treatment with coconut oil enriched mediterranean diet: a pilot study. J. Alzheimers Dis. 65, 577–587. doi: 10.3233/JAD-180184
De Oliveira, F. F., Chen, E. S., Smith, M. C., and Bertolucci, P. H. F. (2016). Associations of blood pressure with functional and cognitive changes in patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 41, 314–323. doi: 10.1159/000447585
de Paula, G. C., Brunetta, H. S., Engel, D. F., Gaspar, J. M., Velloso, L. A., Engblom, D., et al. (2021). Hippocampal function is impaired by a short-term high-fat diet in mice: increased blood-brain barrier permeability and neuroinflammation as triggering events. Front. Neurosci. 15:734158. doi: 10.3389/fnins.2021.734158
Delgado, A., Cholevas, C., and Theoharides, T. C. (2021). Neuroinflammation in Alzheimer’s disease and beneficial action of luteolin. BioFactors 47, 207–217. doi: 10.1002/biof.1714
Deurenberg, P., Deurenberg-Yap, M., and Guricci, S. (2002). Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes. Rev. 3, 141–146. doi: 10.1046/j.1467-789x.2002.00065.x
Di Battista, A. M., Heinsinger, N. M., and Rebeck, G. W. (2016). Alzheimer’s Disease genetic risk factor APOE-ε4 also affects normal brain function. Curr. Alzheimer Res. 13, 1200–1207. doi: 10.2174/1567205013666160401115127
Dimsdale, J. E. (2008). Psychological stress and cardiovascular disease. J. Am. Coll. Cardiol. 51, 1237–1246. doi: 10.1016/j.jacc.2007.12.024
Ding, J., Davis-Plourde, K. L., Sedaghat, S., Tully, P. J., Wang, W., Phillips, C., et al. (2020). Antihypertensive medications and risk for incident dementia and Alzheimer’s disease: a meta-analysis of individual participant data from prospective cohort studies. Lancet Neurol. 19, 61–70. doi: 10.1016/S1474-4422(19)30393-X
DiSabato, D. J., and Sheridan, J. F. (2021). Stress, inflammation and depression: a new possible molecular pathway. Brain Behav. Immun. 91, 6–7. doi: 10.1016/j.bbi.2020.10.011
Dumitrescu, L., Popescu-Olaru, I., Cozma, L., Tulbã, D., Hinescu, M. E., Ceafalan, L. C., et al. (2018). Oxidative stress and the microbiota-gut-brain axis. Oxid. Med. Cell Longev. 2018:2406594. doi: 10.1155/2018/2406594
Duong, M. T., Nasrallah, I. M., Wolk, D. A., Chang, C. C. Y., and Chang, T.-Y. (2021). Cholesterol, atherosclerosis, and APOE in vascular contributions to cognitive impairment and dementia (VCID): potential mechanisms and therapy. Front. Aging Neurosci. 13:647990. doi: 10.3389/fnagi.2021.647990
Dupuy, A. M., Mas, E., Ritchie, K., Descomps, B., Badiou, S., Cristol, J. P., et al. (2001). The relationship between apolipoprotein E4 and lipid metabolism is impaired in Alzheimer’s disease. Gerontology 47, 213–218. doi: 10.1159/000052801
Ehlenbach, W. J., Hough, C. L., Crane, P. K., Haneuse, S. J. P. A., Carson, S. S., Curtis, J. R., et al. (2010). Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA 303, 763–770. doi: 10.1001/jama.2010.167
Eimer, W. A., Vijaya Kumar, D. K., Navalpur Shanmugam, N. K., Rodriguez, A. S., Mitchell, T., Washicosky, K. J., et al. (2018). Alzheimer’s disease-associated β-Amyloid Is rapidly seeded by herpesviridae to protect against brain infection. Neuron 99, 56–63.e3. doi: 10.1016/j.neuron.2018.06.030
Eiser, A. R. (2017). Why does Finland have the highest dementia mortality rate? Environmental factors may be generalizable. Brain Res. 1671, 14–17. doi: 10.1016/j.brainres.2017.06.032
El Husseini, N., Bushnell, C., Brown, C. M., Attix, D., Rost, N. S., Samsa, G. P., et al. (2020). Vascular cellular adhesion molecule-1 (VCAM-1) and memory impairment in african-americans after small vessel-type stroke. J. Stroke Cerebrovasc. Dis. 29:104646. doi: 10.1016/j.jstrokecerebrovasdis.2020.104646
Elwood, E., Lim, Z., Naveed, H., and Galea, I. (2017). The effect of systemic inflammation on human brain barrier function. Brain Behav. Immun 62, 35–40. doi: 10.1016/j.bbi.2016.10.020
Ely, E. W., Shintani, A., Truman, B., Speroff, T., Gordon, S. M., Harrell, F. E., et al. (2004). Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 291, 1753–1762. doi: 10.1001/jama.291.14.1753
Emdin, C. A., Odutayo, A., Wong, C. X., Tran, J., Hsiao, A. J., and Hunn, B. H. M. (2016). Meta-analysis of anxiety as a risk factor for cardiovascular disease. Am. J. Cardiol. 118, 511–519. doi: 10.1016/j.amjcard.2016.05.041
Esler, M., Eikelis, N., Schlaich, M., Lambert, G., Alvarenga, M., Dawood, T., et al. (2008). Chronic mental stress is a cause of essential hypertension: presence of biological markers of stress. Clin. Exp. Pharmacol. Physiol. 35, 498–502. doi: 10.1111/j.1440-1681.2008.04904.x
Etgen, T., Sander, D., Bickel, H., and Förstl, H. (2011). Mild cognitive impairment and dementia: the importance of modifiable risk factors. Dtsch. Arztebl. Int. 108, 743–750. doi: 10.3238/arztebl.2011.0743
Ettcheto, M., Olloquequi, J., Sánchez-López, E., Busquets, O., Cano, A., Manzine, P. R., et al. (2019). Benzodiazepines and related drugs as a risk factor in alzheimer’s disease dementia. Front. Aging Neurosci. 11:344. doi: 10.3389/fnagi.2019.00344
Eubanks, L. M., Rogers, C. J., Beuscher, A. E., Koob, G. F., Olson, A. J., Dickerson, T. J., et al. (2006). A molecular link between the active component of marijuana and Alzheimer’s disease pathology. Mol. Pharm. 3, 773–777. doi: 10.1021/mp060066m
Explore Air Pollution in Alabama (2021). Annual Report America’s Health Rankings. Available online at: https://www.americashealthrankings.org/explore/annual/measure/air/state/AL [Accessed May 25, 2022].
Explore Diabetes in Alabama (2021). Annual Report America’s Health Rankings. Available online at: https://www.americashealthrankings.org/explore/annual/measure/Diabetes/state/AL [Accessed January 28, 2022].
Explore Smoking in Alabama (2021). Annual Report America’s Health Rankings. Available online at: https://www.americashealthrankings.org/explore/annual/measure/Smoking/state/AL [Accessed March 29, 2022].
Fakhoury, M. (2018). Microglia and astrocytes in alzheimer’s disease: implications for therapy. Curr. Neuropharmacol. 16, 508–518. doi: 10.2174/1570159X15666170720095240
Faraco, G., Brea, D., Garcia-Bonilla, L., Wang, G., Racchumi, G., Chang, H., et al. (2018). Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response. Nat. Neurosci. 21, 240–249. doi: 10.1038/s41593-017-0059-z
Farkas, E., and Luiten, P. G. (2001). Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog. Neurobiol. 64, 575–611. doi: 10.1016/s0301-0082(00)00068-x
Feigin, V. L., Stark, B. A., Johnson, C. O., Roth, G. A., Bisignano, C., Abady, G. G., et al. (2021). Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 20, 795–820. doi: 10.1016/S1474-4422(21)00252-0
Ferguson, S. A., Panos, J. J., Sloper, D., Varma, V., and Sarkar, S. (2021). Alzheimer’s disease: a step closer to understanding type 3 diabetes in African Americans. Metab. Brain Dis. 36, 1803–1816. doi: 10.1007/s11011-021-00754-z
Ferguson, S. A., Varma, V., Sloper, D., Panos, J. J., and Sarkar, S. (2020). Increased inflammation in BA21 brain tissue from African Americans with Alzheimer’s disease. Metab. Brain Dis. 35, 121–133. doi: 10.1007/s11011-019-00512-2
Fiedorowicz, J. G., He, J., and Merikangas, K. R. (2011). The association between mood and anxiety disorders with vascular diseases and risk factors in a nationally representative sample. J. Psychosom. Res. 70, 145–154. doi: 10.1016/j.jpsychores.2010.07.010
Fischer, I., and Barak, B. (2020). Molecular and therapeutic aspects of hyperbaric oxygen therapy in neurological conditions. Biomolecules 10:1247. doi: 10.3390/biom10091247
Flatt, J. D., Cicero, E. C., Lambrou, N. H., Wharton, W., Anderson, J. G., Bouldin, E. D., et al. (2021). Subjective cognitive decline higher among sexual and gender minorities in the United States, 2015-2018. Alzheimers Dement. (N. Y.) 7:e12197. doi: 10.1002/trc2.12197
Foundation Health Measures Archive (2020). Foundation Health Measures Archive | Healthy People 2020. Available online at: https://www.healthypeople.gov/2020/About-Healthy-People/Foundation-Health-Measures2020/About-Healthy-People/Foundation-Health-Measures/Archive [Accessed April 5, 2022].
Frasure-Smith, N., and Lespérance, F. (2008). Depression and anxiety as predictors of 2-year cardiac events in patients with stable coronary artery disease. Arch. Gen. Psychiatry 65, 62–71. doi: 10.1001/archgenpsychiatry.2007.4
Freimuth, V. S., Quinn, S. C., Thomas, S. B., Cole, G., Zook, E., and Duncan, T. (2001). African americans’ views on research and the tuskegee syphilis study. Soc. Sci. Med. 52, 797–808. doi: 10.1016/s0277-9536(00)00178-7
Gallaway, P. J., Miyake, H., Buchowski, M. S., Shimada, M., Yoshitake, Y., Kim, A. S., et al. (2017). Physical activity: a viable way to reduce the risks of mild cognitive impairment, alzheimer’s disease, and vascular dementia in older adults. Brain Sci. 7:22. doi: 10.3390/brainsci7020022
García, N., Zazueta, C., and Aguilera-Aguirre, L. (2017). Oxidative stress and inflammation in cardiovascular disease. Oxid. Med. Cell Longev. 2017:5853238. doi: 10.1155/2017/5853238
Garrett, S. L., McDaniel, D., Obideen, M., Trammell, A. R., Shaw, L. M., Goldstein, F. C., et al. (2019). Racial disparity in cerebrospinal fluid amyloid and tau biomarkers and associated cutoffs for mild cognitive impairment. JAMA Netw. Open 2:e1917363. doi: 10.1001/jamanetworkopen.2019.17363
Garthwaite, J. (2019). NO as a multimodal transmitter in the brain: discovery and current status. Br. J. Pharmacol. 176, 197–211. doi: 10.1111/bph.14532
George, S., Duran, N., and Norris, K. (2014). A systematic review of barriers and facilitators to minority research participation among African americans, latinos, asian americans, and pacific islanders. Am. J. Public Health 104, e16–e31. doi: 10.2105/AJPH.2013.301706
Gibson, G. E., Luchsinger, J. A., Cirio, R., Chen, H., Franchino-Elder, J., Hirsch, J. A., et al. (2020). Benfotiamine and cognitive decline in alzheimer’s disease: results of a randomized placebo-controlled phase iia clinical trial. J. Alzheimers Dis. 78, 989–1010. doi: 10.3233/JAD-200896
Girard, T. D., Jackson, J. C., Pandharipande, P. P., Pun, B. T., Thompson, J. L., Shintani, A. K., et al. (2010). Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit. Care Med. 38, 1513–1520. doi: 10.1097/CCM.0b013e3181e47be1
Girard, T. D., Self, W. H., Edwards, K. M., Grijalva, C. G., Zhu, Y., Williams, D. J., et al. (2018). Long-term cognitive impairment after hospitalization for community-acquired pneumonia: a prospective cohort study. J. Gen. Intern. Med. 33, 929–935. doi: 10.1007/s11606-017-4301-x
Glover, C. M., Capuano, A. W., Wilson, R. S., Bennett, D. A., and Barnes, L. L. (2021). Correlates of perceived stress among community-dwelling older African Americans. PLoS One 16:e0260749. doi: 10.1371/journal.pone.0260749
Goldsen, J., Bryan, A. E. B., Kim, H.-J., Muraco, A., Jen, S., and Fredriksen-Goldsen, K. I. (2017). Who Says i do: the changing context of marriage and health and quality of life for LGBT older adults. Gerontologist 57, S50–S62. doi: 10.1093/geront/gnw174
Goos, J. D. C., Kester, M. I., Barkhof, F., Klein, M., Blankenstein, M. A., Scheltens, P., et al. (2009). Patients with Alzheimer disease with multiple microbleeds: relation with cerebrospinal fluid biomarkers and cognition. Stroke 40, 3455–3460. doi: 10.1161/STROKEAHA.109.558197
Gottfried, I., Schottlender, N., and Ashery, U. (2021). Hyperbaric oxygen treatment—from mechanisms to cognitive improvement. Biomolecules 11:1520. doi: 10.3390/biom11101520
Grammas, P., and Ovase, R. (2001). Inflammatory factors are elevated in brain microvessels in Alzheimer’s disease. Neurobiol. Aging 22, 837–842. doi: 10.1016/S0197-4580(01)00276-7
Granér, M., Kahri, J., Varpula, M., Salonen, R. M., Nyyssönen, K., Jauhiainen, M., et al. (2008). Apolipoprotein E polymorphism is associated with both carotid and coronary atherosclerosis in patients with coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 18, 271–277. doi: 10.1016/j.numecd.2007.01.003
Griffin, W. S., Stanley, L. C., Ling, C., White, L., MacLeod, V., Perrot, L. J., et al. (1989). Brain interleukin 1 and S-100 immunoreactivity are elevated in down syndrome and alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 86, 7611–7615. doi: 10.1073/pnas.86.19.7611
Guidi, J., Lucente, M., Sonino, N., and Fava, G. A. (2021). Allostatic load and its impact on health: a systematic review. PPS 90, 11–27. doi: 10.1159/000510696
Guo, H., Liu, M., Zhang, L., Wang, L., Hou, W., Ma, Y., et al. (2020). The critical period for neuroprotection by estrogen replacement therapy and the potential underlying mechanisms. Curr. Neuropharmacol. 18, 485–500. doi: 10.2174/1570159X18666200123165652
Hajat, A., Hsia, C., and O’Neill, M. S. (2015). Socioeconomic disparities and air pollution exposure: a global review. Curr. Environ. Health Rep. 2, 440–450. doi: 10.1007/s40572-015-0069-5
Halliday, M. R., Rege, S. V., Ma, Q., Zhao, Z., Miller, C. A., Winkler, E. A., et al. (2016). Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J. Cereb. Blood Flow Metab. 36, 216–227. doi: 10.1038/jcbfm.2015.44
Harch, P. G., and Fogarty, E. F. (2018). Hyperbaric oxygen therapy for Alzheimer’s dementia with positron emission tomography imaging: a case report. Med. Gas Res. 8, 181–184. doi: 10.4103/2045-9912.248271
Healthy People (2022). Social Determinants of Health – Healthy People 2030 | Health.Gov. Available online at: https://health.gov/healthypeople/objectives-and-data/social-determinants-health [Accessed April 5, 2022].
Heart Disease and Stroke Statistics (2021). Update professional.Heart.Org. Available online at: https://professional.heart.org/en/science-news/heart-disease-and-stroke-statistics-2021-update [Accessed January 28, 2022].
Heringa, S. M., Reijmer, Y. D., Leemans, A., Koek, H. L., Kappelle, L. J., Biessels, G. J., et al. (2014). Multiple microbleeds are related to cerebral network disruptions in patients with early Alzheimer’s disease. J. Alzheimers Dis. 38, 211–221. doi: 10.3233/JAD-130542
Ho, G. J., Drego, R., Hakimian, E., and Masliah, E. (2005). Mechanisms of cell signaling and inflammation in Alzheimer’s disease. Curr. Drug Targets Inflamm. Allergy 4, 247–256. doi: 10.2174/1568010053586237
Hopper, A. T., Campbell, B. M., Kao, H., Pintchovski, S. A., and Staal, R. G. W. (2012). “Chapter four – recent developments in targeting neuroinflammation in disease,” in Annual Reports in Medicinal Chemistry Annual Reports in Medicinal Chemistry, ed. M. C. Desai (Cambridge, MA: Academic Press), 37–53. doi: 10.1016/B978-0-12-396492-2.00004-7
Hu, L., Zhu, S., Peng, X., Li, K., Peng, W., Zhong, Y., et al. (2020). High salt elicits brain inflammation and cognitive dysfunction, accompanied by alternations in the gut microbiota and decreased SCFA production. J. Alzheimers Dis. 77, 629–640. doi: 10.3233/JAD-200035
Hu, Q., Liang, X., Chen, D., Chen, Y., Doycheva, D., Tang, J., et al. (2014). Delayed hyperbaric oxygen therapy promotes neurogenesis through reactive oxygen species/hypoxia-inducible factor-1α/β-catenin pathway in middle cerebral artery occlusion rats. Stroke 45, 1807–1814. doi: 10.1161/STROKEAHA.114.005116
Huang, Y., and Mahley, R. W. (2014). Apolipoprotein E: structure and function in lipid metabolism, neurobiology, and Alzheimer’s diseases. Neurobiol. Dis. 72, 3–12. doi: 10.1016/j.nbd.2014.08.025
Iadecola, C. (2004). Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 5, 347–360. doi: 10.1038/nrn1387
Iadecola, C. (2013). The pathobiology of vascular dementia. Neuron 80, 844–866. doi: 10.1016/j.neuron.2013.10.008
IQAIR (2022). World’s Most Polluted Countries in 2021 - PM2.5 Ranking | IQAir. Available online at: https://www.iqair.com/world-most-polluted-countries [Accessed May 23, 2022].
Iturria-Medina, Y., Sotero, R. C., Toussaint, P. J., Mateos-Pérez, J. M., Evans, A. C., and Alzheimer’s Disease Neuroimaging Initiative (2016). Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat. Commun. 7:11934. doi: 10.1038/ncomms11934
Itzhaki, R. F. (2020). Hypothesis: does the apparent protective action of green valley’s drug GV971 against cognitive decline result from antiviral action against herpes simplex virus type 1 in brain? J. Alzheimers Dis. 76, 85–87. doi: 10.3233/JAD-200210
James, W., Cossman, J., and Wolf, J. (2018). Persistence of death in the United States: The remarkably different mortality patterns between America’s Heartland and Dixieland. Demogr. Res. 39, 897–910. doi: 10.4054/DemRes.2018.39.33
Jensen, C. S., Bahl, J. M., Østergaard, L. B., Høgh, P., Wermuth, L., Heslegrave, A., et al. (2019). Exercise as a potential modulator of inflammation in patients with Alzheimer’s disease measured in cerebrospinal fluid and plasma. Exp. Gerontol. 121, 91–98. doi: 10.1016/j.exger.2019.04.003
Johnson, K. M., and Lichter, D. T. (2010). Growing diversity among america’s children and youth: spatial and temporal dimensions. Popul. Dev. Rev. 36, 151–176. doi: 10.1111/j.1728-4457.2010.00322.x
Justice, N. J. (2018). The relationship between stress and Alzheimer’s disease. Neurobiol. Stress 8, 127–133. doi: 10.1016/j.ynstr.2018.04.002
Kapasi, A., DeCarli, C., and Schneider, J. A. (2017). Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 134, 171–186. doi: 10.1007/s00401-017-1717-7
Karhu, J., Ala-Kokko, T. I., Ylipalosaari, P., Ohtonen, P., Laurila, J. J., and Syrjälä, H. (2011). Hospital and long-term outcomes of ICU-treated severe community- and hospital-acquired, and ventilator-associated pneumonia patients. Acta Anaesthesiol. Scand. 55, 1254–1260. doi: 10.1111/j.1399-6576.2011.02535.x
Kaufman, C. S., Honea, R. A., Pleen, J., Lepping, R. J., Watts, A., Morris, J. K., et al. (2021). Aerobic exercise improves hippocampal blood flow for hypertensive Apolipoprotein E4 carriers. J. Cereb. Blood Flow Metab. 41, 2026–2037. doi: 10.1177/0271678X21990342
Khondoker, M., Rafnsson, S. B., Morris, S., Orrell, M., and Steptoe, A. (2017). Positive and negative experiences of social support and risk of dementia in later life: an investigation using the english longitudinal study of ageing. J. Alzheimers Dis. 58, 99–108. doi: 10.3233/JAD-161160
Killin, L. O. J., Starr, J. M., Shiue, I. J., and Russ, T. C. (2016). Environmental risk factors for dementia: a systematic review. BMC Geriatr. 16:175. doi: 10.1186/s12877-016-0342-y
Kim, C.-S., Cha, L., Sim, M., Jung, S., Chun, W. Y., Baik, H. W., et al. (2021). Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: a randomized, double-blind, placebo-controlled, multicenter trial. J. Gerontol. A Biol. Sci. Med. Sci. 76, 32–40. doi: 10.1093/gerona/glaa090
Kisler, K., Nelson, A. R., Montagne, A., and Zlokovic, B. V. (2017a). Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 18, 419–434. doi: 10.1038/nrn.2017.48
Kisler, K., Nelson, A. R., Rege, S. V., Ramanathan, A., Wang, Y., Ahuja, A., et al. (2017b). Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat. Neurosci. 20, 406–416. doi: 10.1038/nn.4489
Koizumi, K., Hattori, Y., Ahn, S. J., Buendia, I., Ciacciarelli, A., Uekawa, K., et al. (2018). Apoε4 disrupts neurovascular regulation and undermines white matter integrity and cognitive function. Nat. Commun. 9:3816. doi: 10.1038/s41467-018-06301-2
Kotfis, K., Williams Roberson, S., Wilson, J. E., Dabrowski, W., Pun, B. T., and Ely, E. W. (2020). COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit. Care 24:176. doi: 10.1186/s13054-020-02882-x
Kramer, A. F., Colcombe, S. J., McAuley, E., Scalf, P. E., and Erickson, K. I. (2005). Fitness, aging and neurocognitive function. Neurobiol. Aging 26, 124–127.
Kramer, A. F., Erickson, K. I., and Colcombe, S. J. (2006). Exercise, cognition, and the aging brain. J. Appl. Physiol. (1985) 101, 1237–1242. doi: 10.1152/japplphysiol.00500.2006
Kril, J. J., and Halliday, G. M. (1999). Brain shrinkage in alcoholics: a decade on and what have we learned? Prog. Neurobiol. 58, 381–387. doi: 10.1016/s0301-0082(98)00091-4
Kumar, A., Ren, Y., Sundaram, K., Mu, J., Sriwastva, M. K., Dryden, G. W., et al. (2021). miR-375 prevents high-fat diet-induced insulin resistance and obesity by targeting the aryl hydrocarbon receptor and bacterial tryptophanase (tnaA) gene. Theranostics 11, 4061–4077. doi: 10.7150/thno.52558
Kumar, D. K. V., Choi, S. H., Washicosky, K. J., Eimer, W. A., Tucker, S., Ghofrani, J., et al. (2016). Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci. Transl. Med. 8:340ra72. doi: 10.1126/scitranslmed.aaf1059
Kunkle, B. W., Grenier-Boley, B., Sims, R., Bis, J. C., Damotte, V., Naj, A. C., et al. (2019). Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 51, 414–430. doi: 10.1038/s41588-019-0358-2
Kunkle, B. W., Schmidt, M., Klein, H.-U., Naj, A. C., Hamilton-Nelson, K. L., Larson, E. B., et al. (2021). Novel alzheimer disease risk loci and pathways in african american individuals using the african genome resources panel: a meta-analysis. JAMA Neurol. 78, 102–113. doi: 10.1001/jamaneurol.2020.3536
Lahiri, S., Regis, G. C., Koronyo, Y., Fuchs, D.-T., Sheyn, J., Kim, E. H., et al. (2019). Acute neuropathological consequences of short-term mechanical ventilation in wild-type and Alzheimer’s disease mice. Crit. Care 23:63. doi: 10.1186/s13054-019-2356-2
Lambert, J. C., Ibrahim-Verbaas, C. A., Harold, D., Naj, A. C., Sims, R., Bellenguez, C., et al. (2013). Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 45, 1452–1458. doi: 10.1038/ng.2802
Landrigan, J.-F., Bell, T., Crowe, M., Clay, O., and Mirman, D. (2020). Lifting cognition: a meta-analysis of effects of resistance exercise on cognition. Psychol. Res. 84, 1167–1183. doi: 10.1007/s00426-019-01145-x
Lasker, S., Rahman, M. M., Parvez, F., Zamila, M., Miah, P., Nahar, K., et al. (2019). High-fat diet-induced metabolic syndrome and oxidative stress in obese rats are ameliorated by yogurt supplementation. Sci. Rep. 9:20026. doi: 10.1038/s41598-019-56538-0
Lautenschlager, N. T., Cox, K., and Kurz, A. F. (2010). Physical activity and mild cognitive impairment and Alzheimer’s Disease. Curr. Neurol. Neurosci. Rep. 10, 352–358. doi: 10.1007/s11910-010-0121-7
Lee, S. H. (2022). Adults meeting fruit and vegetable intake recommendations — united states, 2019. MMWR Morb. Mortal. Wkly. Rep. 71, 1–9. doi: 10.15585/mmwr.mm7101a1
Li, C., Shi, L., Wang, Y., Peng, C., Wu, L., Zhang, Y., et al. (2021). High-fat diet exacerbates lead-induced blood-brain barrier disruption by disrupting tight junction integrity. Environ. Toxicol. 36, 1412–1421. doi: 10.1002/tox.23137
Liang, K. Y., Mintun, M. A., Fagan, A. M., Goate, A. M., Bugg, J. M., Holtzman, D. M., et al. (2010). Exercise and Alzheimer’s Disease biomarkers in cognitively normal older adults. Ann. Neurol. 68, 311–318. doi: 10.1002/ana.22096
Liao, K.-M., Ho, C.-H., Ko, S.-C., and Li, C.-Y. (2015). Increased risk of dementia in patients with chronic obstructive pulmonary disease. Medicine (Baltimore) 94:e930. doi: 10.1097/MD.0000000000000930
Lin, M. T., Balczon, R., Pittet, J.-F., Wagener, B. M., Moser, S. A., Morrow, K. A., et al. (2018). Nosocomial pneumonia elicits an endothelial proteinopathy: evidence for a source of neurotoxic amyloids in critically ill patients. Am. J. Respir. Crit. Care Med. 198, 1575–1578. doi: 10.1164/rccm.201801-0060LE
Lin, Z., Sur, S., Liu, P., Li, Y., Jiang, D., Hou, X., et al. (2021). Blood-brain barrier breakdown in relationship to alzheimer and vascular disease. Ann. Neurol. 90, 227–238. doi: 10.1002/ana.26134
Liu, X., Morris, M. C., Dhana, K., Ventrelle, J., Johnson, K., Bishop, L., et al. (2021). Mediterranean-DASH intervention for neurodegenerative delay (MIND) study: rationale, design and baseline characteristics of a randomized control trial of the MIND diet on cognitive decline. Contemp. Clin. Trials 102:106270. doi: 10.1016/j.cct.2021.106270
Liu, Y., Chen, Z., Li, B., Yao, H., Zarka, M., Welch, J., et al. (2021). Supplementation with γ-glutamylcysteine (γ-GC) lessens oxidative stress, brain inflammation and amyloid pathology and improves spatial memory in a murine model of AD. Neurochem. Int. 144:104931. doi: 10.1016/j.neuint.2020.104931
Lockhart, S. N., Schaich, C. L., Craft, S., Sachs, B. C., Rapp, S. R., Jung, Y., et al. (2021). Associations among vascular risk factors, neuroimaging biomarkers, and cognition: preliminary analyses from the multi-ethnic study of atherosclerosis (MESA). Alzheimers Dement. 18, 551–560. doi: 10.1002/alz.12429
Logue, M. W., Lancour, D., Farrell, J., Simkina, I., Fallin, M. D., Lunetta, K. L., et al. (2018). Targeted sequencing of alzheimer disease genes in african americans implicates novel risk variants. Front. Neurosci. 12:592. doi: 10.3389/fnins.2018.00592
Logue, M. W., Schu, M., Vardarajan, B. N., Buros, J., Green, R. C., Go, R. C. P., et al. (2011). A comprehensive genetic association study of Alzheimer disease in African Americans. Arch. Neurol. 68, 1569–1579. doi: 10.1001/archneurol.2011.646
Lourenço, C. F., Ledo, A., Barbosa, R. M., and Laranjinha, J. (2017). Neurovascular uncoupling in the triple transgenic model of Alzheimer’s disease: impaired cerebral blood flow response to neuronal-derived nitric oxide signaling. Exp. Neurol. 291, 36–43. doi: 10.1016/j.expneurol.2017.01.013
Lozano, I., Van der Werf, R., Bietiger, W., Seyfritz, E., Peronet, C., Pinget, M., et al. (2016). High-fructose and high-fat diet-induced disorders in rats: impact on diabetes risk, hepatic and vascular complications. Nutr. Metab. (Lond.) 13:15. doi: 10.1186/s12986-016-0074-1
Ma, C., Zhou, Y., Zhou, W., and Huang, C. (2014). Evaluation of the effect of motivational interviewing counselling on hypertension care. Patient Educ. Couns. 95, 231–237. doi: 10.1016/j.pec.2014.01.011
Ma, Q., Zhao, Z., Sagare, A. P., Wu, Y., Wang, M., Owens, N. C., et al. (2018). Blood-brain barrier-associated pericytes internalize and clear aggregated amyloid-β42 by LRP1-dependent apolipoprotein E isoform-specific mechanism. Mol. Neurodegener. 13:57. doi: 10.1186/s13024-018-0286-0
Mahley, R. W., Weisgraber, K. H., and Huang, Y. (2006). Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 103, 5644–5651. doi: 10.1073/pnas.0600549103
Makkar, S. R., Lipnicki, D. M., Crawford, J. D., Kochan, N. A., Castro-Costa, E., Lima-Costa, M. F., et al. (2020). APOE ε4 and the influence of sex, age, vascular risk factors, and ethnicity on cognitive decline. J. Gerontol. A Biol. Sci. Med. Sci. 75, 1863–1873. doi: 10.1093/gerona/glaa116
Mao, L., Jin, H., Wang, M., Hu, Y., Chen, S., He, Q., et al. (2020). Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in wuhan, china. JAMA Neurol. 77, 683–690. doi: 10.1001/jamaneurol.2020.1127
Marcinkowska, A. B., Mankowska, N. D., Kot, J., and Winklewski, P. J. (2021). Impact of hyperbaric oxygen therapy on cognitive functions: a systematic review. Neuropsychol. Rev. 32, 99–126. doi: 10.1007/s11065-021-09500-9
Marizzoni, M., Cattaneo, A., Mirabelli, P., Festari, C., Lopizzo, N., Nicolosi, V., et al. (2020). Short-chain fatty acids and lipopolysaccharide as mediators between gut dysbiosis and amyloid pathology in Alzheimer’s Disease. J. Alzheimers Dis. 78, 683–697. doi: 10.3233/JAD-200306
Matthews, K. A., Xu, W., Gaglioti, A. H., Holt, J. B., Croft, J. B., Mack, D., et al. (2019). Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015-2060) in adults aged =65 years. Alzheimers Dement. 15, 17–24. doi: 10.1016/j.jalz.2018.06.3063
Mielke, M. M., Vemuri, P., and Rocca, W. A. (2014). Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin. Epidemiol. 6, 37–48. doi: 10.2147/CLEP.S37929
Miller, C. E., and Vasan, R. S. (2021). The southern rural health and mortality penalty: a review of regional health inequities in the United States. Soc. Sci. Med. 268:113443. doi: 10.1016/j.socscimed.2020.113443
Miners, J. S., Kehoe, P. G., Love, S., Zetterberg, H., and Blennow, K. (2019). CSF evidence of pericyte damage in Alzheimer’s disease is associated with markers of blood-brain barrier dysfunction and disease pathology. Alzheimers Res. Ther. 11:81. doi: 10.1186/s13195-019-0534-8
Miners, J. S., Schulz, I., and Love, S. (2018). Differing associations between Aβ accumulation, hypoperfusion, blood-brain barrier dysfunction and loss of PDGFRB pericyte marker in the precuneus and parietal white matter in Alzheimer’s disease. J. Cereb. Blood Flow Metab. 38, 103–115. doi: 10.1177/0271678X17690761
Mishra, A., Wang, Y., Yin, F., Vitali, F., Rodgers, K. E., Soto, M., et al. (2022). A tale of two systems: lessons learned from female mid-life aging with implications for alzheimer’s prevention & treatment. Ageing Res. Rev. 74:101542. doi: 10.1016/j.arr.2021.101542
Montagne, A., Barnes, S. R., Sweeney, M. D., Halliday, M. R., Sagare, A. P., Zhao, Z., et al. (2015). Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302. doi: 10.1016/j.neuron.2014.12.032
Montagne, A., Nation, D. A., Sagare, A. P., Barisano, G., Sweeney, M. D., Chakhoyan, A., et al. (2020a). APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature 581, 71–76. doi: 10.1038/s41586-020-2247-3
Montagne, A., Nation, D. A., and Zlokovic, B. V. (2020b). APOE4 accelerates development of dementia after stroke: is there a role for cerebrovascular dysfunction? Stroke 51, 699–700. doi: 10.1161/STROKEAHA.119.028814
Morello, M., Landel, V., Lacassagne, E., Baranger, K., Annweiler, C., Féron, F., et al. (2018). Vitamin D Improves Neurogenesis and Cognition in a Mouse Model of Alzheimer’s Disease. Mol Neurobiol 55, 6463–6479. doi: 10.1007/s12035-017-0839-1
Moreno-Fernández, S., Garcés-Rimón, M., Vera, G., Astier, J., Landrier, J. F., and Miguel, M. (2018). High fat/high glucose diet induces metabolic syndrome in an experimental rat model. Nutrients 10:E1502. doi: 10.3390/nu10101502
Morris, M. C., Tangney, C. C., Wang, Y., Sacks, F. M., Barnes, L. L., Bennett, D. A., et al. (2015). MIND diet slows cognitive decline with aging. Alzheimers Dement. 11, 1015–1022. doi: 10.1016/j.jalz.2015.04.011
Morrow, K. A., Ochoa, C. D., Balczon, R., Zhou, C., Cauthen, L., Alexeyev, M., et al. (2016). Pseudomonas aeruginosa exoenzymes U and Y induce a transmissible endothelial proteinopathy. Am. J. Physiol. Lung Cell Mol. Physiol. 310, L337–L353. doi: 10.1152/ajplung.00103.2015
Moulton, P. V., and Yang, W. (2012). Air pollution, oxidative stress, and Alzheimer’s disease. J. Environ. Public Health 2012:472751. doi: 10.1155/2012/472751
Münzel, T., Schmidt, F. P., Steven, S., Herzog, J., Daiber, A., and Sørensen, M. (2018). Environmental noise and the cardiovascular system. J. Am. Coll. Cardiol. 71, 688–697. doi: 10.1016/j.jacc.2017.12.015
Murray, C. J. L., Kulkarni, S. C., Michaud, C., Tomijima, N., Bulzacchelli, M. T., Iandiorio, T. J., et al. (2006). Eight americas: investigating mortality disparities across races, counties, and race-counties in the united states. PLoS Med. 3:e260. doi: 10.1371/journal.pmed.0030260
Naj, A. C., Jun, G., Reitz, C., Kunkle, B. W., Perry, W., Park, Y. S., et al. (2014). Effects of multiple genetic loci on age at onset in late-onset Alzheimer disease: a genome-wide association study. JAMA Neurol. 71, 1394–1404. doi: 10.1001/jamaneurol.2014.1491
Nasrallah, I. M., Gaussoin, S. A., Pomponio, R., Dolui, S., Erus, G., Wright, C. B., et al. (2021). Association of intensive vs standard blood pressure control with magnetic resonance imaging biomarkers of alzheimer disease: secondary analysis of the SPRINT MIND randomized trial. JAMA Neurol. 78, E1–E10. doi: 10.1001/jamaneurol.2021.0178
Nation, D. A., Sweeney, M. D., Montagne, A., Sagare, A. P., D’Orazio, L. M., Pachicano, M., et al. (2019). Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 25, 270–276. doi: 10.1038/s41591-018-0297-y
Nelson, A. R. (2022). Peripheral pathways to neurovascular unit dysfunction, cognitive impairment, and Alzheimer’s Disease. Front. Aging Neurosci. 14:858429. doi: 10.3389/fnagi.2022.858429
Nelson, A. R., Sagare, M. A., Wang, Y., Kisler, K., Zhao, Z., and Zlokovic, B. V. (2020). Channelrhodopsin excitation contracts brain pericytes and reduces blood flow in the aging mouse brain in vivo. Front. Aging Neurosci. 12:108. doi: 10.3389/fnagi.2020.00108
Nelson, A. R., Sweeney, M. D., Sagare, A. P., and Zlokovic, B. V. (2016). Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim. Biophys. Acta 1862, 887–900. doi: 10.1016/j.bbadis.2015.12.016
Nelson, P. T., Dickson, D. W., Trojanowski, J. Q., Jack, C. R., Boyle, P. A., Arfanakis, K., et al. (2019). Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain 142, 1503–1527. doi: 10.1093/brain/awz099
Ngandu, T., Lehtisalo, J., Solomon, A., Levälahti, E., Ahtiluoto, S., Antikainen, R., et al. (2015). A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 385, 2255–2263. doi: 10.1016/S0140-6736(15)60461-5
NHLBI (2022). Metabolic Syndrome | NHLBI, NIH. Available online at: https://www.nhlbi.nih.gov/health-topics/metabolic-syndrome [Accessed January 28, 2022]
Nielsen, R. B., Parbo, P., Ismail, R., Dalby, R., Tietze, A., Brændgaard, H., et al. (2020). Impaired perfusion and capillary dysfunction in prodromal Alzheimer’s disease. Alzheimers Dement. (Amst.) 12:e12032. doi: 10.1002/dad2.12032
Nystoriak, M. A., and Bhatnagar, A. (2018). Cardiovascular effects and benefits of exercise. Front. Cardiovasc. Med. 5:135. doi: 10.3389/fcvm.2018.00135
O’Brien, M. W., Johns, J. A., Robinson, S. A., Bungay, A., Mekary, S., and Kimmerly, D. S. (2020). Impact of high-intensity interval training, moderate-intensity continuous training, and resistance training on endothelial function in older adults. Med. Sci. Sports Exerc. 52, 1057–1067. doi: 10.1249/MSS.0000000000002226
O’Neill, M. S., Jerrett, M., Kawachi, I., Levy, J. I., Cohen, A. J., Gouveia, N., et al. (2003). Health, wealth, and air pollution: advancing theory and methods. Environ. Health Perspect. 111, 1861–1870. doi: 10.1289/ehp.6334
Ochoa, C. D., Alexeyev, M., Pastukh, V., Balczon, R., and Stevens, T. (2012). Pseudomonas aeruginosa exotoxin Y is a promiscuous cyclase that increases endothelial tau phosphorylation and permeability. J. Biol. Chem. 287, 25407–25418. doi: 10.1074/jbc.M111.301440
Olazarán, J., Ramos, A., Boyano, I., Alfayate, E., Valentí, M., Rábano, A., et al. (2014). Pattern of and risk factors for brain microbleeds in neurodegenerative dementia. Am. J. Alzheimers Dis. Other Dement. 29, 263–269. doi: 10.1177/1533317513517043
Oliva, A. A. Jr., Brody, M., Agronin, M., Herskowitz, B., Bookheimer, S. Y., Hitchinson, B., et al. (2021). Safety and efficacy of Lomecel-B in patients with mild Alzheimer’s disease: Results of a double-blinded, randomized, placebo-controlled phase 1 clinical trial. Alzheimers Dement. 17:e057581. doi: 10.1002/alz.057581
Ota, M., Matsuo, J., Ishida, I., Takano, H., Yokoi, Y., Hori, H., et al. (2019). Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer’s disease. Neurosci. Lett. 690, 232–236. doi: 10.1016/j.neulet.2018.10.048
Pasek, J., Stanek, A., and Cieślar, G. (2020). The role of physical activity in prevention and treatment of peripheral vascular disorders. Acta Angiol. 26, 19–27. doi: 10.5603/AA.2020.0004
Pendlebury, S. T., Poole, D., Burgess, A., Duerden, J., Rothwell, P. M., and Oxford Vascular</snm>, et al. (2020). APOE-ε4 genotype and dementia before and after transient ischemic attack and stroke: population-based cohort study. Stroke 51, 751–758. doi: 10.1161/STROKEAHA.119.026927
Perea, J. R., Llorens-Martín, M., Ávila, J., and Bolós, M. (2018). The Role of Microglia in the Spread of Tau: Relevance for Tauopathies. Frontiers in Cellular Neuroscience 12. Available online at: https://www.frontiersin.org/article/10.3389/fncel.2018.00172 [Accessed March 18, 2022].
Perlman, S., Evans, G., and Afifi, A. (1990). Effect of olfactory bulb ablation on spread of a neurotropic coronavirus into the mouse brain. J. Exp. Med. 172, 1127–1132. doi: 10.1084/jem.172.4.1127
Peters, R., Ee, N., Peters, J., Booth, A., Mudway, I., and Anstey, K. J. (2019). Air pollution and dementia: a systematic review. J. Alzheimers Dis. 70, S145–S163. doi: 10.3233/JAD-180631
Peters, R., Yasar, S., Anderson, C. S., Andrews, S., Antikainen, R., Arima, H., et al. (2020). An investigation of antihypertensive class, dementia, and cognitive decline: a meta-analysis. Neurology 94, 1–15. doi: 10.1212/WNL.0000000000008732
Petersen, J. D., Wehberg, S., Packness, A., Svensson, N. H., Hyldig, N., Raunsgaard, S., et al. (2021). Association of socioeconomic status with dementia diagnosis among older adults in denmark. JAMA Netw. Open 4:e2110432. doi: 10.1001/jamanetworkopen.2021.10432
Phillips, M. C. L., Deprez, L. M., Mortimer, G. M. N., Murtagh, D. K. J., McCoy, S., Mylchreest, R., et al. (2021). Randomized crossover trial of a modified ketogenic diet in Alzheimer’s disease. Alzheimers Res. Ther. 13:51. doi: 10.1186/s13195-021-00783-x
Poliakova, T., Levin, O., Arablinskiy, A., Vasenina, E., and Zerr, I. (2016). Cerebral microbleeds in early Alzheimer’s disease. J. Neurol. 263, 1961–1968.
Ponce, J., Ulu, A., Hanson, C., Cameron-Smith, E., Bertoni, J., Wuebker, J., et al. (2022). Role of specialized pro-resolving mediators in reducing neuroinflammation in neurodegenerative disorders. Front. Aging Neurosci. 14:780811. doi: 10.3389/fnagi.2022.780811
Prasad, D. S., Kabir, Z., Dash, A. K., and Das, B. C. (2011). Abdominal obesity, an independent cardiovascular risk factor in Indian subcontinent: a clinico epidemiological evidence summary. J. Cardiovasc. Dis. Res. 2, 199–205. doi: 10.4103/0975-3583.89803
Pratchayasakul, W., Sa-Nguanmoo, P., Sivasinprasasn, S., Pintana, H., Tawinvisan, R., Sripetchwandee, J., et al. (2015). Obesity accelerates cognitive decline by aggravating mitochondrial dysfunction, insulin resistance and synaptic dysfunction under estrogen-deprived conditions. Horm. Behav. 72, 68–77. doi: 10.1016/j.yhbeh.2015.04.023
Praticò, D., Clark, C. M., Liun, F., Rokach, J., Lee, V. Y.-M., and Trojanowski, J. Q. (2002). Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Arch. Neurol. 59, 972–976. doi: 10.1001/archneur.59.6.972
Proctor, C., Thiennimitr, P., Chattipakorn, N., and Chattipakorn, S. C. (2017). Diet, gut microbiota and cognition. Metab. Brain Dis. 32, 1–17. doi: 10.1007/s11011-016-9917-8
Quinn, G. P., Sutton, S. K., Winfield, B., Breen, S., Canales, J., Shetty, G., et al. (2015). Lesbian, gay, bisexual, transgender, queer/questioning (LGBTQ) perceptions and health care experiences. J. Gay Lesbian Soc. Serv. 27, 246–261. doi: 10.1080/10538720.2015.1022273
Rabin, J. S., Klein, H., Kirn, D. R., Schultz, A. P., Yang, H.-S., Hampton, O., et al. (2019). Associations of physical activity and β-amyloid with longitudinal cognition and neurodegeneration in clinically normal older adults. JAMA Neurol. 76, 1203–1210. doi: 10.1001/jamaneurol.2019.1879
Rabin, J. S., Schultz, A. P., Hedden, T., Viswanathan, A., Marshall, G. A., Kilpatrick, E., et al. (2018). Interactive associations of vascular risk and β-amyloid burden with cognitive decline in clinically normal elderly individuals: findings from the harvard aging brain study. JAMA Neurol. 75, 1124–1131. doi: 10.1001/jamaneurol.2018.1123
Rahman, A., Jackson, H., Hristov, H., Isaacson, R. S., Saif, N., Shetty, T., et al. (2019). Sex and gender driven modifiers of alzheimer’s: the role for estrogenic control across age, race, medical, and lifestyle risks. Front. Aging Neurosci. 11:315. doi: 10.3389/fnagi.2019.00315
Rahman, M., White, E. M., Mills, C., Thomas, K. S., and Jutkowitz, E. (2021). Rural-urban differences in diagnostic incidence and prevalence of Alzheimer’s disease and related dementias. Alzheimers Dement. 17, 1213–1230. doi: 10.1002/alz.12285
Raj, V., Ojha, S., Howarth, F. C., Belur, P. D., and Subramanya, S. B. (2018). Therapeutic potential of benfotiamine and its molecular targets. Eur. Rev. Med. Pharmacol. Sci. 22, 3261–3273. doi: 10.26355/eurrev_201805_15089
Reade, M. C., and Finfer, S. (2014). Sedation and delirium in the intensive care unit. N. Engl. J. Med. 370, 444–454. doi: 10.1056/NEJMra1208705
Rensma, S. P., van Sloten, T. T., Houben, A. J. H. M., Köhler, S., van Boxtel, M. P. J., Berendschot, T. T. J. M., et al. (2020). Microvascular dysfunction is associated with worse cognitive performance: the maastricht study. Hypertension 75, 237–245. doi: 10.1161/HYPERTENSIONAHA.119.13023
Rezaeiasl, Z., Salami, M., and Sepehri, G. (2019). The effects of probiotic lactobacillus and bifidobacterium strains on memory and learning behavior, long-term potentiation (LTP), and some biochemical parameters in β-amyloid-induced rat’s model of Alzheimer’s Disease. Prev. Nutr. Food. Sci. 24, 265–273. doi: 10.3746/pnf.2019.24.3.265
Richards, S. H., Anderson, L., Jenkinson, C. E., Whalley, B., Rees, K., Davies, P., et al. (2018). Psychological interventions for coronary heart disease: cochrane systematic review and meta-analysis. Eur. J. Prev. Cardiol. 25, 247–259. doi: 10.1177/2047487317739978
Riley, W. J. (2012). Health disparities: gaps in access, quality and affordability of medical care. Trans. Am. Clin. Climatol. Assoc. 123, 167–172; discussion 172–174.
Robinson, J. L., Richardson, H., Xie, S. X., Suh, E., Van Deerlin, V. M., Alfaro, B., et al. (2021). The development and convergence of co-pathologies in Alzheimer’s disease. Brain 144, 953–962. doi: 10.1093/brain/awaa438
Rolland, Y., Abellan van Kan, G., and Vellas, B. (2008). Physical activity and alzheimer’s disease: from prevention to therapeutic perspectives. J. Am. Med. Dir. Assoc. 9, 390–405. doi: 10.1016/j.jamda.2008.02.007
Rovio, S., Kåreholt, I., Helkala, E.-L., Viitanen, M., Winblad, B., Tuomilehto, J., et al. (2005). Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 4, 705–711. doi: 10.1016/S1474-4422(05)70198-8
Rowan, P. J., Haas, D., Campbell, J. A., Maclean, D. R., and Davidson, K. W. (2005). Depressive symptoms have an independent, gradient risk for coronary heart disease incidence in a random, population-based sample. Ann. Epidemiol. 15, 316–320. doi: 10.1016/j.annepidem.2004.08.006
Rural Health Information Hub (2022). Rural Data Explorer – Rural Health Information Hub. Available online at: https://www.ruralhealthinfo.org/data-explorer [Accessed January 24, 2022].
Rutledge, T., Redwine, L. S., Linke, S. E., and Mills, P. J. (2013). A meta-analysis of mental health treatments and cardiac rehabilitation for improving clinical outcomes and depression among patients with coronary heart disease. Psychosom. Med. 75, 335–349. doi: 10.1097/PSY.0b013e318291d798
Ryder, A. L., and Cohen, B. E. (2021). Evidence for depression and anxiety as risk factors for heart disease and stroke: implications for primary care. Fam. Pract. 38, 365–367. doi: 10.1093/fampra/cmab031
Saiyasit, N., Chunchai, T., Prus, D., Suparan, K., Pittayapong, P., Apaijai, N., et al. (2020). Gut dysbiosis develops before metabolic disturbance and cognitive decline in high-fat diet-induced obese condition. Nutrition 69:110576. doi: 10.1016/j.nut.2019.110576
Salameh, T. S., Mortell, W. G., Logsdon, A. F., Butterfield, D. A., and Banks, W. A. (2019). Disruption of the hippocampal and hypothalamic blood-brain barrier in a diet-induced obese model of type II diabetes: prevention and treatment by the mitochondrial carbonic anhydrase inhibitor, topiramate. Fluids Barriers CNS 16:1. doi: 10.1186/s12987-018-0121-6
Salazar, C. R., Hoang, D., Gillen, D. L., and Grill, J. D. (2020). Racial and ethnic differences in older adults’ willingness to be contacted about Alzheimer’s disease research participation. Alzheimers Dement. (N. Y.) 6:e12023. doi: 10.1002/trc2.12023
Salguero, M. V., Al-Obaide, M. A. I., Singh, R., Siepmann, T., and Vasylyeva, T. L. (2019). Dysbiosis of Gram-negative gut microbiota and the associated serum lipopolysaccharide exacerbates inflammation in type 2 diabetic patients with chronic kidney disease. Exp. Ther. Med. 18, 3461–3469. doi: 10.3892/etm.2019.7943
Salinas, J., O’Donnell, A., Kojis, D. J., Pase, M. P., DeCarli, C., Rentz, D. M., et al. (2021). Association of social support with brain volume and cognition. JAMA Netw. Open 4:e2121122. doi: 10.1001/jamanetworkopen.2021.21122
Salloway, S., Farlow, M., McDade, E., Clifford, D. B., Wang, G., Llibre-Guerra, J. J., et al. (2021). A trial of gantenerumab or solanezumab in dominantly inherited Alzheimer’s disease. Nat. Med. 27, 1187–1196. doi: 10.1038/s41591-021-01369-8
Salloway, S., Gur, T., Berzin, T., Tavares, R., Zipser, B., Correia, S., et al. (2002). Effect of APOE genotype on microvascular basement membrane in Alzheimer’s disease. J. Neurol. Sci. 203–204, 183–187. doi: 10.1016/s0022-510x(02)00288-5
Samuel, L. J., Szanton, S. L., Wolff, J. L., Ornstein, K. A., Parker, L. J., and Gitlin, L. N. (2020). Socioeconomic disparities in six-year incident dementia in a nationally representative cohort of U.S. older adults: an examination of financial resources. BMC Geriatr. 20:156. doi: 10.1186/s12877-020-01553-4
Scharff, D. P., Mathews, K. J., Jackson, P., Hoffsuemmer, J., Martin, E., and Edwards, D. (2010). More than tuskegee: understanding mistrust about research participation. J. Health Care Poor Underserved 21, 879–897. doi: 10.1353/hpu.0.0323
Schwarzinger, M., Pollock, B. G., Hasan, O. S. M., Dufouil, C., Rehm, J., and QalyDays Study Group (2018). Contribution of alcohol use disorders to the burden of dementia in France 2008-13: a nationwide retrospective cohort study. Lancet Public Health 3, e124–e132. doi: 10.1016/S2468-2667(18)30022-7
Scicchitano, P., Cortese, F., Gesualdo, M., Palo, M. D., Massari, F., Giordano, P., et al. (2019). The role of endothelial dysfunction and oxidative stress in cerebrovascular diseases. Free Radic. Res. 53, 579–595. doi: 10.1080/10715762.2019.1620939
Scott, A. M., Jager, A. C., Gwin, M., Voth, S., Balczon, R., Stevens, T., et al. (2020). Pneumonia-induced endothelial amyloids reduce dendritic spine density in brain neurons. Sci. Rep. 10:9327. doi: 10.1038/s41598-020-66321-1
Sengillo, J. D., Winkler, E. A., Walker, C. T., Sullivan, J. S., Johnson, M., and Zlokovic, B. V. (2013). Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer’s disease. Brain Pathol. 23, 303–310. doi: 10.1111/bpa.12004
Shabir, O., Berwick, J., and Francis, S. E. (2018). Neurovascular dysfunction in vascular dementia, Alzheimer’s and atherosclerosis. BMC Neurosci. 19:62. doi: 10.1186/s12868-018-0465-5
Shams, S., Martola, J., Granberg, T., Li, X., Shams, M., Fereshtehnejad, S. M., et al. (2015). Cerebral microbleeds: different prevalence, topography, and risk factors depending on dementia diagnosis—the Karolinska Imaging Dementia Study. AJNR Am. J. Neuroradiol. 36, 661–666. doi: 10.3174/ajnr.A4176
Shamsipour, S., Sharifi, G., and Taghian, F. (2021). Impact of interval training with probiotic (L. plantarum / Bifidobacterium bifidum) on passive avoidance test, ChAT and BDNF in the hippocampus of rats with Alzheimer’s disease. Neurosci. Lett. 756:135949. doi: 10.1016/j.neulet.2021.135949
Shapira, R., Gdalyahu, A., Gottfried, I., Sasson, E., Hadanny, A., Efrati, S., et al. (2021). Hyperbaric oxygen therapy alleviates vascular dysfunction and amyloid burden in an Alzheimer’s disease mouse model and in elderly patients. Aging (Albany N.Y.) 13, 20935–20961. doi: 10.18632/aging.203485
Shastri, A., Bonifati, D. M., and Kishore, U. (2013). Innate immunity and neuroinflammation. Med. Inflamm. 2013:342931. doi: 10.1155/2013/342931
Shen, B.-J., Avivi, Y. E., Todaro, J. F., Spiro, A., Laurenceau, J.-P., Ward, K. D., et al. (2008). Anxiety characteristics independently and prospectively predict myocardial infarction in men the unique contribution of anxiety among psychologic factors. J. Am. Coll. Cardiol. 51, 113–119. doi: 10.1016/j.jacc.2007.09.033
Shin, J. A., Oh, S., Ahn, J.-H., and Park, E.-M. (2015). Estrogen receptor-mediated resveratrol actions on blood-brain barrier of ovariectomized mice. Neurobiol. Aging 36, 993–1006. doi: 10.1016/j.neurobiolaging.2014.09.024
Shin, J., and Doraiswamy, P. M. (2016). Underrepresentation of african-americans in Alzheimer’s trials: a call for affirmative action. Front. Aging. Neurosci. 8:123. doi: 10.3389/fnagi.2016.00123
Singh, G. K., and Siahpush, M. (2014). Widening rural-urban disparities in life expectancy, U.S., 1969-2009. Am. J. Prev. Med. 46, e19–e29. doi: 10.1016/j.amepre.2013.10.017
Sinha, N., Berg, C. N., Shaw, A., and Gluck, M. A. (2020). ABCA7 genotype moderates the effect of aerobic exercise intervention on generalization of prior learning in healthy older african americans. J. Alzheimer Dis. 74, 309–318. doi: 10.3233/JAD-190723
Smith, C. C., Vedder, L. C., Nelson, A. R., Bredemann, T. M., and McMahon, L. L. (2010). Duration of estrogen deprivation, not chronological age, prevents estrogen’s ability to enhance hippocampal synaptic physiology. Proc. Natl. Acad. Sci. U.S.A. 107, 19543–19548. doi: 10.1073/pnas.1009307107
Snyder, H. M., Asthana, S., Bain, L., Brinton, R., Craft, S., Dubal, D. B., et al. (2016). Sex biology contributions to vulnerability to Alzheimer’s disease: A think tank convened by the Women’s Alzheimer’s Research Initiative. Alzheimers Dement. 12, 1186–1196. doi: 10.1016/j.jalz.2016.08.004
Soden, P. A., Zettervall, S. L., Deery, S. E., Hughes, K., Stoner, M. C., Goodney, P. P., et al. (2018). Black patients present with more severe vascular disease and a greater burden of risk factors than white patients at time of major vascular intervention. J. Vasc. Surg. 67, 549–556.e3. doi: 10.1016/j.jvs.2017.06.089
Sofi, F., Vecchio, S., Giuliani, G., Martinelli, F., Marcucci, R., Gori, A. M., et al. (2005). Dietary habits, lifestyle and cardiovascular risk factors in a clinically healthy Italian population: the “Florence” diet is not Mediterranean. Eur. J. Clin. Nutr. 59, 584–591. doi: 10.1038/sj.ejcn.1602112
Somaa, F. (2021). A review of the application of hyperbaric oxygen therapy in Alzheimer’s Disease. J. Alzheimers Dis. 81, 1361–1367. doi: 10.3233/JAD-210157
Song, Y.-J., Li, S.-R., Li, X.-W., Chen, X., Wei, Z.-X., Liu, Q.-S., et al. (2020). The effect of estrogen replacement therapy on Alzheimer’s Disease and parkinson’s disease in postmenopausal women: a meta-analysis. Front. Neurosci. 14:157. doi: 10.3389/fnins.2020.00157
Stats of the States Heart Disease Mortality (2021). Available online at: https://www.cdc.gov/nchs/pressroom/sosmap/heart_disease_mortality/heart_disease.htm [Accessed January 17, 2022].
Stephen, R., Hongisto, K., Solomon, A., and Lönnroos, E. (2017). Physical activity and Alzheimer’s Disease: a systematic review. J. Gerontol. 72, 733–739. doi: 10.1093/gerona/glw251
Stepler, K. E., Gillyard, T. R., Reed, C. B., Avery, T. M., Davis, J. S., and Robinson, R. A. S. (2022). ABCA7, a genetic risk factor associated with alzheimer’s disease risk in african americans. J. Alzheimers Dis. 86, 5–19. doi: 10.3233/JAD-215306
Stirban, A., Negrean, M., Stratmann, B., Gawlowski, T., Horstmann, T., Götting, C., et al. (2006). Benfotiamine prevents macro- and microvascular endothelial dysfunction and oxidative stress following a meal rich in advanced glycation end products in individuals with type 2 diabetes. Diabetes Care 29, 2064–2071. doi: 10.2337/dc06-0531
St-Jean, J. R., Jacomy, H., Desforges, M., Vabret, A., Freymuth, F., and Talbot, P. J. (2004). Human respiratory coronavirus OC43: genetic stability and neuroinvasion. J. Virol. 78, 8824–8834. doi: 10.1128/JVI.78.16.8824-8834.2004
Strauss, S., Bauer, J., Ganter, U., Jonas, U., Berger, M., and Volk, B. (1992). Detection of interleukin-6 and alpha 2-macroglobulin immunoreactivity in cortex and hippocampus of Alzheimer’s disease patients. Lab. Invest. 66, 223–230.
Strazza, M., Pirrone, V., Wigdahl, B., and Nonnemacher, M. R. (2011). Breaking down the barrier: the effects of HIV-1 on the blood-brain barrier. Brain Res. 1399, 96–115. doi: 10.1016/j.brainres.2011.05.015
Streit, W. J., Mrak, R. E., and Griffin, W. S. T. (2004). Microglia and neuroinflammation: a pathological perspective. J. Neuroinflammation 1:14. doi: 10.1186/1742-2094-1-14
Sutherland, G. T., Chami, B., Youssef, P., and Witting, P. K. (2013). Oxidative stress in Alzheimer’s disease: primary villain or physiological by-product? Redox Rep. 18, 134–141. doi: 10.1179/1351000213Y.0000000052
Sweeney, M. D., Sagare, A. P., and Zlokovic, B. V. (2015). Cerebrospinal fluid biomarkers of neurovascular dysfunction in mild dementia and Alzheimer’s disease. J. Cereb. Blood Flow Metab. 35, 1055–1068. doi: 10.1038/jcbfm.2015.76
Sweeney, M. D., Zhao, Z., Montagne, A., Nelson, A. R., and Zlokovic, B. V. (2019). Blood-brain barrier: from physiology to disease and back. Physiol. Rev. 99, 21–78. doi: 10.1152/physrev.00050.2017
Syed, Y. Y. (2020). Sodium oligomannate: first approval. Drugs 80, 441–444. doi: 10.1007/s40265-020-01268-1
Takeda, S., Sato, N., Ikimura, K., Nishino, H., Rakugi, H., and Morishita, R. (2013). Increased blood-brain barrier vulnerability to systemic inflammation in an Alzheimer disease mouse model. Neurobiol. Aging 34, 2064–2070. doi: 10.1016/j.neurobiolaging.2013.02.010
Tal, S., Hadanny, A., Sasson, E., Suzin, G., and Efrati, S. (2017). Hyperbaric oxygen therapy can induce angiogenesis and regeneration of nerve fibers in traumatic brain injury patients. Front. Hum. Neurosci. 11:508. doi: 10.3389/fnhum.2017.00508
Tamagno, E., Guglielmotto, M., Aragno, M., Borghi, R., Autelli, R., Giliberto, L., et al. (2008). Oxidative stress activates a positive feedback between the gamma- and beta-secretase cleavages of the beta-amyloid precursor protein. J. Neurochem. 104, 683–695. doi: 10.1111/j.1471-4159.2007.05072.x
Tamir, C. (2021). The Growing Diversity of Black America. Pew Research Center’s Social & Demographic Trends Project. Available online at: https://www.pewresearch.org/social-trends/2021/03/25/the-growing-diversity-of-black-america/ [Accessed May 23, 2022].
Tamtaji, O. R., Heidari-Soureshjani, R., Mirhosseini, N., Kouchaki, E., Bahmani, F., Aghadavod, E., et al. (2019). Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: a randomized, double-blind, controlled trial. Clin. Nutr. 38, 2569–2575. doi: 10.1016/j.clnu.2018.11.034
Thériault, P., ElAli, A., and Rivest, S. (2016). High fat diet exacerbates Alzheimer’s disease-related pathology in APPswe/PS1 mice. Oncotarget 7, 67808–67827. doi: 10.18632/oncotarget.12179
Thiennimitr, P., Yasom, S., Tunapong, W., Chunchai, T., Wanchai, K., Pongchaidecha, A., et al. (2018). Lactobacillus paracasei HII01, xylooligosaccharides, and synbiotics reduce gut disturbance in obese rats. Nutrition 54, 40–47. doi: 10.1016/j.nut.2018.03.005
Tibbles, P. M., and Edelsberg, J. S. (1996). Hyperbaric-oxygen therapy. N. Engl. J. Med. 334, 1642–1648.
Ting, W.-J., Huang, C.-Y., Jiang, C.-H., Lin, Y.-M., Chung, L.-C., Shen, C.-Y., et al. (2017). Treatment with 17β-estradiol reduced body weight and the risk of cardiovascular disease in a high-fat diet-induced animal model of obesity. Int. J. Mol. Sci. 18:E629. doi: 10.3390/ijms18030629
Tomassoni, D., Martinelli, I., Moruzzi, M., Micioni Di Bonaventura, M. V., Cifani, C., Amenta, F., et al. (2020). Obesity and age-related changes in the brain of the zucker lepr fa/fa rats. Nutrients 12:E1356. doi: 10.3390/nu12051356
Topiwala, A., and Ebmeier, K. P. (2018). Effects of drinking on late-life brain and cognition. Evid. Based Ment. Health 21, 12–15. doi: 10.1136/eb-2017-102820
Trammell, A. R., McDaniel, D. J., Obideen, M., Okafor, M., Thomas, T. L., Goldstein, F. C., et al. (2020). Perceived stress is associated with alzheimer’s disease cerebrospinal fluid biomarkers in african americans with mild cognitive impairment. J. Alzheimers Dis. 77, 843–853. doi: 10.3233/JAD-200089
Turner, A. D., James, B. D., Capuano, A. W., Aggarwal, N. T., and Barnes, L. L. (2017). Perceived stress and cognitive decline in different cognitive domains in a cohort of older african americans. Am. J. Geriatr. Psychiatry 25, 25–34. doi: 10.1016/j.jagp.2016.10.003
Uemura, M. T., Robinson, J. L., Cousins, K. A. Q., Tropea, T. F., Kargilis, D. C., McBride, J. D., et al. (2022). Distinct characteristics of limbic-predominant age-related TDP-43 encephalopathy in Lewy body disease. Acta Neuropathol. 143, 15–31. doi: 10.1007/s00401-021-02383-3
Uetani, H., Hirai, T., Hashimoto, M., Ikeda, M., Kitajima, M., Sakamoto, F., et al. (2013). Prevalence and topography of small hypointense foci suggesting microbleeds on 3T susceptibility-weighted imaging in various types of dementia. AJNR Am. J. Neuroradiol. 34, 984–989. doi: 10.3174/ajnr.A3332
USDA (2022). The Food Access Research Atlas guide. Available online at: https://gisportal.ers.usda.gov/portal/apps/experiencebuilder/experience/?id=a53ebd7396cd4ac3a3ed09137676fd40 [Accessed January 7, 2022].
Valkanova, V., and Ebmeier, K. P. (2013). Vascular risk factors and depression in later life: a systematic review and meta-analysis. Biol. Psychiatry 73, 406–413. doi: 10.1016/j.biopsych.2012.10.028
van de Haar, H. J., Jansen, J. F. A., Jeukens, C. R. L. P. N., Burgmans, S., van Buchem, M. A., Muller, M., et al. (2017b). Subtle blood-brain barrier leakage rate and spatial extent: considerations for dynamic contrast-enhanced MRI. Med. Phys. 44, 4112–4125. doi: 10.1002/mp.12328
van de Haar, H. J., Burgmans, S., Jansen, J. F. A., van Osch, M. J. P., van Buchem, M. A., Muller, M., et al. (2017a). Blood-brain barrier leakage in patients with early alzheimer disease. Radiology 282:615. doi: 10.1148/radiol.2017164043
van de Haar, H. J., Jansen, J. F. A., van Osch, M. J. P., van Buchem, M. A., Muller, M., Wong, S. M., et al. (2016). Neurovascular unit impairment in early Alzheimer’s disease measured with magnetic resonance imaging. Neurobiol. Aging 45, 190–196. doi: 10.1016/j.neurobiolaging.2016.06.006
Vanherle, L., Matuskova, H., Don-Doncow, N., Uhl, F. E., and Meissner, A. (2020). Improving cerebrovascular function to increase neuronal recovery in neurodegeneration associated to cardiovascular disease. Front. Cell Dev. Biol. 8:53. doi: 10.3389/fcell.2020.00053
Vardarajan, B., Kalia, V., Manly, J., Brickman, A., Reyes-Dumeyer, D., Lantigua, R., et al. (2020). Differences in plasma metabolites related to Alzheimer’s disease, APOE ε4 status, and ethnicity. Alzheimers Dement. (N. Y.) 6:e12025. doi: 10.1002/trc2.12025
Varnum, M. M., and Ikezu, T. (2012). The classification of microglial activation phenotypes on neurodegeneration and regeneration in alzheimer’s disease brain. Arch. Immunol. Ther. Exp. 60, 251–266. doi: 10.1007/s00005-012-0181-2
Velazquez, R., Ferreira, E., Knowles, S., Fux, C., Rodin, A., Winslow, W., et al. (2019). Lifelong choline supplementation ameliorates Alzheimer’s disease pathology and associated cognitive deficits by attenuating microglia activation. Aging Cell 18:e13037. doi: 10.1111/acel.13037
Verheggen, I. C. M., de Jong, J. J. A., van Boxtel, M. P. J., Gronenschild, E. H. B. M., Palm, W. M., Postma, A. A., et al. (2020). Increase in blood-brain barrier leakage in healthy, older adults. Geroscience 42, 1183–1193. doi: 10.1007/s11357-020-00211-2
Vogt, N. M., Kerby, R. L., Dill-McFarland, K. A., Harding, S. J., Merluzzi, A. P., Johnson, S. C., et al. (2017). Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 7:13537. doi: 10.1038/s41598-017-13601-y
Voloboueva, L. A., and Giffard, R. G. (2011). Inflammation, mitochondria, and the inhibition of adult neurogenesis. J. Neurosci. Res. 89, 1989–1996. doi: 10.1002/jnr.22768
Voth, S., Gwin, M., Francis, C. M., Balczon, R., Frank, D. W., Pittet, J.-F., et al. (2020). Virulent Pseudomonas aeruginosa infection converts antimicrobial amyloids into cytotoxic prions. FASEB J. 34, 9156–9179. doi: 10.1096/fj.202000051RRR
Wang, J., Fan, D.-Y., Li, H.-Y., He, C.-Y., Shen, Y.-Y., Zeng, G.-H., et al. (2022). Dynamic changes of CSF sPDGFRβ during ageing and AD progression and associations with CSF ATN biomarkers. Mol. Neurodegener. 17:9. doi: 10.1186/s13024-021-00512-w
Wang, T., Kuang, W., Chen, W., Xu, W., Zhang, L., Li, Y., et al. (2020). A phase II randomized trial of sodium oligomannate in Alzheimer’s dementia. Alzheimers Res. Ther. 12:110. doi: 10.1186/s13195-020-00678-3
Wang, X., Sun, G., Feng, T., Zhang, J., Huang, X., Wang, T., et al. (2019). Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 29, 787–803. doi: 10.1038/s41422-019-0216-x
Wang, Y., Guan, X., Chen, X., Cai, Y., Ma, Y., Ma, J., et al. (2019). Choline supplementation ameliorates behavioral deficits and Alzheimer’s disease-like pathology in transgenic app/ps1 mice. Mol. Nutr. Food Res. 63:e1801407. doi: 10.1002/mnfr.201801407
Wardlaw, J. M., Smith, C., and Dichgans, M. (2019). Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 18, 684–696. doi: 10.1016/S1474-4422(19)30079-1
Wei, H., Wang, S., Zhen, L., Yang, Q., Wu, Z., Lei, X., et al. (2015). Resveratrol attenuates the blood-brain barrier dysfunction by regulation of the MMP-9/TIMP-1 balance after cerebral ischemia reperfusion in rats. J. Mol. Neurosci. 55, 872–879. doi: 10.1007/s12031-014-0441-1
Wen, J., Ding, Y., Wang, L., and Xiao, Y. (2020). Gut microbiome improves postoperative cognitive function by decreasing permeability of the blood-brain barrier in aged mice. Brain Res. Bull. 164, 249–256. doi: 10.1016/j.brainresbull.2020.08.017
Wharton, W., Kollhoff, A. L., Gangishetti, U., Verble, D. D., Upadhya, S., Zetterberg, H., et al. (2019). Interleukin 9 alterations linked to alzheimer disease in african americans. Ann. Neurol. 86, 407–418. doi: 10.1002/ana.25543
Wightman, D. P., Jansen, I. E., Savage, J. E., Shadrin, A. A., Bahrami, S., Holland, D., et al. (2021). A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nat. Genet. 53, 1276–1282. doi: 10.1038/s41588-021-00921-z
Williams Institute (2022). LGBT Data & Demographics – The Williams Institute. Available online at: https://williamsinstitute.law.ucla.edu/visualization/lgbt-stats/?topic=LGBT#density [Accessed March 31, 2022].
Williamson, J. D., Pajewski, N. M., Auchus, A. P., Bryan, R. N., Chelune, G., Cheung, A. K., et al. (2019). Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA 321, 553–561. doi: 10.1001/jama.2018.21442
Wolf, S. A., Boddeke, H. W. G. M., and Kettenmann, H. (2017). Microglia in physiology and disease. Annu. Rev. Physiol. 79, 619–643. doi: 10.1146/annurev-physiol-022516-034406
Wu, Z., Guo, H., Chow, N., Sallstrom, J., Bell, R. D., Deane, R., et al. (2005). Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat. Med. 11, 959–965. doi: 10.1038/nm1287
Xiao, S., Chan, P., Wang, T., Hong, Z., Wang, S., Kuang, W., et al. (2021). A 36-week multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3 clinical trial of sodium oligomannate for mild-to-moderate Alzheimer’s dementia. Alzheimers Res. Ther. 13:62. doi: 10.1186/s13195-021-00795-7
Xu, Y., Wang, Q., Qu, Z., Yang, J., Zhang, X., and Zhao, Y. (2019). Protective effect of hyperbaric oxygen therapy on cognitive function in patients with vascular dementia. Cell Transplant. 28, 1071–1075. doi: 10.1177/0963689719853540
Yamamoto, M., Guo, D.-H., Hernandez, C. M., and Stranahan, A. M. (2019). Endothelial Adora2a activation promotes blood-brain barrier breakdown and cognitive impairment in mice with diet-induced insulin resistance. J. Neurosci. 39, 4179–4192. doi: 10.1523/JNEUROSCI.2506-18.2019
Yan, Z., Yingjie, Z., Na, A., Qi, Q., Wei, L., Wenzheng, W., et al. (2021). The effects of light-to-moderate alcohol consumption on the cognitive function of community nondemented male elderly: a cohort study. Behav. Neurol. 2021:5681913. doi: 10.1155/2021/5681913
Yang, Y., Wei, H., Zhou, X., Zhang, F., and Wang, C. (2017). Hyperbaric oxygen promotes neural stem cell proliferation by activating vascular endothelial growth factor/extracellular signal-regulated kinase signaling after traumatic brain injury. Neuroreport 28, 1232–1238. doi: 10.1097/WNR.0000000000000901
Yates, P. A., Desmond, P. M., Phal, P. M., Steward, C., Szoeke, C., Salvado, O., et al. (2014). Incidence of cerebral microbleeds in preclinical Alzheimer disease. Neurology 82, 1266–1273. doi: 10.1212/WNL.0000000000000285
You, Q., Li, L., Xiong, S.-Q., Yan, Y.-F., Li, D., Yan, N.-N., et al. (2019). Meta-analysis on the efficacy and safety of hyperbaric oxygen as adjunctive therapy for vascular dementia. Front. Aging Neurosci. 11:86. doi: 10.3389/fnagi.2019.00086
Zelle, A., and Arms, T. (2015). Psychosocial effects of health disparities of lesbian, gay, bisexual, and transgender older adults. J. Psychosoc. Nurs. Ment. Health Serv. 53, 25–30. doi: 10.3928/02793695-20150623-04
Zhang, T., Yang, Q.-W., Wang, S.-N., Wang, J.-Z., Wang, Q., Wang, Y., et al. (2010). Hyperbaric oxygen therapy improves neurogenesis and brain blood supply in piriform cortex in rats with vascular dementia. Brain Inj. 24, 1350–1357. doi: 10.3109/02699052.2010.504525
Zhang, X., and Le, W. (2010). Pathological role of hypoxia in Alzheimer’s disease. Exp. Neurol. 223, 299–303. doi: 10.1016/j.expneurol.2009.07.033
Zhao, B., Pan, Y., Wang, Z., Xu, H., and Song, X. (2017). Hyperbaric oxygen pretreatment improves cognition and reduces hippocampal damage via p38 Mitogen-activated protein kinase in a rat model. Yonsei Med. J. 58, 131–138. doi: 10.3349/ymj.2017.58.1.131
Zhao, H., Niu, Q., Li, X., Liu, T., Xu, Y., Han, H., et al. (2012). Long-term resveratrol consumption protects ovariectomized rats chronically treated with d-galactose from developing memory decline without effects on the uterus. Brain Res. 1467, 67–80. doi: 10.1016/j.brainres.2012.05.040
Zhao, J., Fu, Y., Yamazaki, Y., Ren, Y., Davis, M. D., Liu, C.-C., et al. (2020). APOE4 exacerbates synapse loss and neurodegeneration in Alzheimer’s disease patient iPSC-derived cerebral organoids. Nat. Commun. 11:5540. doi: 10.1038/s41467-020-19264-0
Zhao, Z., Nelson, A. R., Betsholtz, C., and Zlokovic, B. V. (2015). Establishment and dysfunction of the blood-brain barrier. Cell 163, 1064–1078. doi: 10.1016/j.cell.2015.10.067
Zhuang, Z.-Q., Shen, L.-L., Li, W.-W., Fu, X., Zeng, F., Gui, L., et al. (2018). Gut microbiota is altered in patients with alzheimer’s disease. J. Alzheimers Dis. 63, 1337–1346. doi: 10.3233/JAD-180176
Zlokovic, B. V. (2011). Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 12, 723–738. doi: 10.1038/nrn3114
Zonneveld, H. I., Goos, J. D. C., Wattjes, M. P., Prins, N. D., Scheltens, P., van der Flier, W. M., et al. (2014). Prevalence of cortical superficial siderosis in a memory clinic population. Neurology 82, 698–704.
Keywords: neurovascular dysfunction, health disparities, Alzheimer’s disease, Alabama (United States), dementia
Citation: Saiyasit N, Butlig E-AR, Chaney SD, Traylor MK, Hawley NA, Randall RB, Bobinger HV, Frizell CA, Trimm F, Crook ED, Lin M, Hill BD, Keller JL and Nelson AR (2022) Neurovascular Dysfunction in Diverse Communities With Health Disparities—Contributions to Dementia and Alzheimer’s Disease. Front. Neurosci. 16:915405. doi: 10.3389/fnins.2022.915405
Received: 08 April 2022; Accepted: 31 May 2022;
Published: 29 June 2022.
Edited by:
Sumit Sarkar, National Center for Toxicological Research (FDA), United StatesReviewed by:
Costantino Iadecola, Cornell University, United StatesDana Niedowicz, University of Kentucky, United States
Copyright © 2022 Saiyasit, Butlig, Chaney, Traylor, Hawley, Randall, Bobinger, Frizell, Trimm, Crook, Lin, Hill, Keller and Nelson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amy R. Nelson, YXJuZWxzb25Ac291dGhhbGFiYW1hLmVkdQ==
†These authors have contributed equally to this work
 Napatsorn Saiyasit
Napatsorn Saiyasit Evan-Angelo R. Butlig
Evan-Angelo R. Butlig Samantha D. Chaney
Samantha D. Chaney Miranda K. Traylor
Miranda K. Traylor Nanako A. Hawley4
Nanako A. Hawley4 Ryleigh B. Randall
Ryleigh B. Randall Hanna V. Bobinger
Hanna V. Bobinger Carl A. Frizell
Carl A. Frizell Franklin Trimm
Franklin Trimm Mike Lin
Mike Lin Benjamin D. Hill
Benjamin D. Hill Joshua L. Keller
Joshua L. Keller Amy R. Nelson
Amy R. Nelson