- 1Department of Radiology, Ningbo Medical Treatment Center Lihuili Hospital, Ningbo University, Ningbo, China
- 2Department of Academic Research, Ningbo Kangning Hospital, Ningbo University, Ningbo, China
- 3Key Laboratory of Addiction Research of Zhejiang Province, Ningbo, China
Brain resting-state functional connectivity (rsFC) has been widely analyzed in substance use disorders (SUDs), including methamphetamine (MA) dependence. Most of these studies utilized Pearson correlation analysis to assess rsFC, which cannot determine whether two brain regions are connected by direct or indirect pathways. Moreover, few studies have reported the application of rsFC-based graph theory in MA dependence. We evaluated alterations in Tikhonov regularization-based rsFC and rsFC-based topological attributes in 46 MA-dependent patients, as well as the correlations between topological attributes and clinical variables. Moreover, the topological attributes selected by least absolute shrinkage and selection operator (LASSO) were used to construct a support vector machine (SVM)-based classifier for MA dependence. The MA group presented a subnetwork with increased rsFC, indicating overactivation of the reward circuit that makes patients very sensitive to drug-related visual cues, and a subnetwork with decreased rsFC suggesting aberrant synchronized spontaneous activity in subregions within the orbitofrontal cortex (OFC) system. The MA group demonstrated a significantly decreased area under the curve (AUC) for the clustering coefficient (Cp) (Pperm < 0.001), shortest path length (Lp) (Pperm = 0.007), modularity (Pperm = 0.006), and small-worldness (σ, Pperm = 0.004), as well as an increased AUC for global efficiency (E.glob) (Pperm = 0.009), network strength (Sp) (Pperm = 0.009), and small-worldness (ω, Pperm < 0.001), implying a shift toward random networks. MA-related increased nodal efficiency (E.nodal) and altered betweenness centrality were also discovered in several brain regions. The AUC for ω was significantly positively associated with psychiatric symptoms. An SVM classifier trained by 36 features selected by LASSO from all topological attributes achieved excellent performance, cross-validated prediction area under the receiver operating characteristics curve, accuracy, sensitivity, specificity, and kappa of 99.03 ± 1.79, 94.00 ± 5.78, 93.46 ± 8.82, 94.52 ± 8.11, and 87.99 ± 11.57%, respectively (Pperm < 0.001), indicating that rsFC-based topological attributes can provide promising features for constructing a high-efficacy classifier for MA dependence.
Introduction
According to the World Drug Report 20211 and Annual Report on Drug Control in China in 2021,2 approximately 3.59 million people worldwide use drugs each year, and methamphetamine (MA) is the most abused drug in China and one of the most abused across the world. Long-term use of MA causes molecular alterations in the dopamine system, contributing to nerve terminal impairment in the central nervous system and leading to damaged motor skills, rapid cognitive decline, increased anxiety, psychotic disorders, violent behavior, hallucinations, delusions, and depression (Rusyniak, 2013).
In recent years, functional connectivity (FC) has gained visibility as a salient tool for assessing functional brain organization and as an important biomarker for neurological disorders (Pervaiz et al., 2020). The resting-state-based functional connectome has been extensively applied to help reveal neurological mechanisms, as well as applied for diagnosis and prediction of treatment outcomes, in patients with substance use disorders (SUDs), such as cannabis (Ramaekers et al., 2022), heroin (Sun et al., 2018), cocaine (Yip et al., 2019), and MA dependence (Kohno et al., 2016; Ipser et al., 2018; Malina et al., 2021). Kohno et al. (2016) analyzed resting-state FC (rsFC) between brain networks and reported that acute exposure to MA increased connectivity between both the thalamus and cerebellum to sensorimotor areas and the middle temporal gyrus and decreased connectivity between the sensorimotor and middle temporal gyrus networks. In terms of chronic MA dependence, Ipser et al. (2018) found an increased correlation between anterior and posterior default mode networks (DMN), which became less apparent with increasing duration of abstinence from MA. By combining 18F-fallypride positron emission tomography with rsFC studies in MA-dependent patients, Kohno et al. (2016) concluded that ventral striatal D2-type receptor signaling may affect the system-level activity within the mesocorticolimbic system, providing a functional link that may help explain high impulsivity in patients.
To date, there is no single widely accepted standard for estimating FC. A full (Pearson) correlation analysis between brain regions of interest (ROIs) or voxels is the most commonly used method to analyze FC; however, this approach cannot distinguish whether two brain regions are connected through direct or indirect pathways (Pervaiz et al., 2020). To alleviate this drawback, Pervaiz et al. (2020) defined partial correlations as the correlations between the time series of two network nodes after adjusting for the time series of all other network nodes. This method, however, becomes problematic when there are not considerably more timepoints than nodes. The Tikhonov regularization, one of the approaches based on regularization, also referred to as L2 ridge regression involving a regularization term Γ = αI, was implemented to address these problems. As an efficient and stable method, Tikhonov regularization has been shown to increase predictive power compared to simple partial correlations and hence outperformed other connectivity estimation techniques in terms of both qualitative and quantitative evaluation (Pervaiz et al., 2020).
As rsFC analyses focus only on relationships between brain regions, graph theory offers a powerful mathematical framework for modeling the human brain as a complex network or graph whose topological architectures can be quantitatively characterized (Xia and He, 2017). This technique has been applied to analyze human brain structural and functional networks in the context of SUDs (Sjoerds et al., 2017; Sun et al., 2017; Pandria et al., 2018). However, few studies have reported the application of rsFC-based graph theory in the context of MA dependence. By comparing 17 MA-dependent individuals with normal controls, Mansoory et al. (2017) reported disrupted global topological graph properties under several network sparsity thresholds (up to 9 out of 41 thresholds). To avoid issues caused by evaluating topological features dependent on an arbitrarily chosen threshold, some studies integrated the curve of the graph indices over a range of thresholds, i.e., the area under the curve (AUC) for each topological attribute, before conducting statistical analysis of network topology attributes. The integrated AUC metric has been used in previous brain network studies and is sensitive for detecting topological alterations in brain disorders (Zhang J. et al., 2011; Zhu et al., 2016; Vriend et al., 2018). In the present study, we hypothesized that MA dependence disrupts rsFC and rsFC-based topological organization of intrinsic functional brain networks. To verify our hypothesis, we collected Tikhonov regularization-based rsFC data from MA-dependent patients and healthy control (HC) subjects and analyzed the AUC for topological attributes of their resting-state functional networks using graph theoretical approaches. Between-group differences and relationships with clinical variables were investigated with univariate methods.
An important emerging trend in the analysis of SUDs brain imaging data is the application of supervised machine-learning techniques on complex, high-dimensional datasets to make predictions at the individual level with high accuracy, making them potential clinically actionable diagnostic/prognostic tools (Barenholtz et al., 2020). To date, the most commonly used machine-learning algorithm in the classification of SUDs [e.g., cannabinoid (Celik et al., 2020), nicotine (Wetherill et al., 2019), cocaine (Mete et al., 2016), and heroin (Zhang Y. et al., 2011) dependence] is the support vector machine (SVM) (Noble, 2006), which can help achieve a good separation of a sample into two groups by determining the hyperplane separating the multidimensional (multivariate) feature spaces of the two classes. Yan et al. (2021) constructed an SVM with rsFC network topological attributes from MA-dependent patients and achieved a classification accuracy of 73.2%. Li et al. (2019) trained an SVM on arterial spin labeling (ASL) data, a variant form of functional magnetic resonance imaging (fMRI), to classify MA-dependent subjects with an accuracy of 89%. By exploiting AUC data from rsFC network topological metrics derived from Tikhonov regularization-based rsFC data, the present study also aimed to develop an SVM that could improve the classification efficacy of MA-dependent individuals.
Materials and methods
Participants
Forty-six right-handed, male MA-dependent patients were recruited from the voluntary detoxification ward of Key Laboratory of Addiction Research of Zhejiang Province, which unit has now been incorporated into Ningbo Kangning Hospital since 2020. Forty-six age- and education-matched, right-handed, healthy male subjects were recruited as HCs from local communities. All patients were diagnosed by one expertized psychiatrist. Two expertized psychiatrists interviewed all subjects and collected their clinical data.
The inclusion criteria for MA dependence were (a) meeting the Diagnostic and Statistical Manual of Mental Disorders, Fourth edition, Text revision (DSM-IV-TR) criteria for current MA dependence. All patients received an MRI scan within 4–7 days after the last use of MA. (b) Patients had no current or history of dependence on other drugs of abuse (except nicotine). The exclusion criteria included (a) having a history of psychiatric illness, neurological disorder, or major chronic medical illnesses before MA use and (b) having metallic or electronic devices or implants.
The same inclusion and exclusion criteria were applied for the HCs but without a history of drug abuse or dependence, other than nicotine.
The psychiatric symptoms of MA-dependent patients were rated using the Hamilton Anxiety Rating Scale (HAMA) (Hamilton, 1969) and Brief Psychiatric Rating Scale (BPRS) (Overall and Gorham, 1962). The BPRS covers five factors: anxiety-depression, lack of vitality, activity, hostility-suspicion, and thinking disorder.
This study was approved by the Institutional Review Board of Ningbo Medical Center Lihuili Hospital, Ningbo University, Zhejiang, China. Written informed consent was obtained from all subjects or their relatives.
Magnetic resonance imaging data acquisition
All MRI data were acquired using a 3.0-T clinical MR image unit (Discovery MR750, GE Healthcare, Milwaukee, WI, USA) with an eight−channel head coil. Conventional axial T2−weighted images had previously been obtained to rule out cerebral infarction or other evident lesions. The structural MRI data were collected using a sagittal T1-weighted three-dimensional sequence [repetition time (TR), 7.4 ms; echo time (TE), 3.2 ms; inversion time, 450 ms; flip angle, 12°; field of view, 25.6 mm × 25.6 mm; matrix, 256 × 256; and voxel size = 1 mm × 1 mm × 1 mm].
Resting-state fMRI (RS-fMRI) data were acquired for 6 min and 40 s using a T2*-weighted gradient-echo planar imaging (EPI) sequence (TR = 2,000 ms; TE = 30 ms; field of view = 24 mm × 24 mm; matrix size = 64 × 64; voxel size = 3.75 mm × 3.75 mm × 4 mm; flip angle = 90°; 38 transverse slices; and 200 phases). All subjects were placed in the supine position with foam padding between their head and the head coil to minimize head motion and instructed to keep their eyes closed and not fall asleep.
Preprocessing of resting-state-functional magnetic resonance imaging data
The RS-fMRI data were preprocessed using Data Processing Assistant for Resting-State fMRI (DPARSF) toolbox, a convenient plug-in software within DPABI (v6.1_220101) software (Yan et al., 2016). For RS-fMRI data preprocessing, the first four volumes were discarded to avoid signal instability. Slice timing was conducted to compensate for systematic slice-dependent time shifts, and realignment to the first volume was performed to correct for head movement artifacts. The realigned images were spatially transformed to a standard Montreal Neurological Institute template using Advanced Normalization Tools (ANTs) (Avants et al., 2009) (v2.1.0) and resampled to a voxel size of 2 mm × 2 mm × 2 mm. Then, the cerebrospinal fluid signal, white matter signal and 24 head motion parameters were regressed out to produce a residual blood oxygen level-dependent (BOLD) signal. Afterward, the images were smoothed with a 6-mm full-width at half-maximum (FWHM) kernel and bandpass filtered (0.01–0.08 Hz) to reduce the low-frequency drift and physiological high-frequency noise, including the breath and heartbeats. The RS-fMRI data for each subject were checked for head motion. In accordance with the criteria that the translation and rotation of head motion in any direction were not more than 2 mm or 2°, no subjects were disqualified.
Graph construction and calculation of topological attributes
In brain rsFC networks, graphs are composed of nodes, i.e., brain regions and edges between pairs of nodes. All the graphs in this study were undirected and weighted.
In the present study, a brainnetome atlas was utilized to parcellate the brain into 246 ROIs (105 cortical regions and 18 subcortical regions per hemisphere) as network nodes (Fan et al., 2016). DPABINet, a module within DPABI, was used to quantify the Tikhonov regularization-based correlation, i.e., edge, between the averaged time series of the BOLD signals for each pair of nodes, and then a 246 × 246 connectivity matrix was generated, which was subsequently converted to a normal distribution using Fisher’s r-to-z transformation. To exclude possible effects of spurious correlations between network nodes, a sparsity threshold (i.e., the ratio of the number of existing edges divided by the maximum possible number of edges in a network) was applied to individual connectivity matrices to retain only high correlations. As there is currently no definitive way to select a single threshold, each connectivity matrix was empirically thresholded using a wide range of sparsity thresholds [0.05, 0.4] (interval = 0.01) to generate sparse and weighted networks.
A number of topological attributes of the rsFC networks were calculated for each subject under every sparsity threshold using the brainGraph package3 in R (v4.1.0) for Linux (R Core Team, 2018). The global-level attributes included clustering coefficient (Cp), shortest path length (Lp), network strength (Sp), local efficiency (E.loc), global efficiency (E.glob), modularity and small-worldness [i.e., σ (Humphries and Gurney, 2008) and ω (Telesford et al., 2011)], and the local-level attributes included nodal efficiency (E.nodal) and betweenness centrality (btwn.cent). A network was regarded as a small world when σ > 1 and when ω was within the range [−0.5 to 0.5]. Furthermore, we calculated the AUC for each topological attribute, which provides a summarized scalar for topological characterization of brain networks independent of single threshold selection.
Statistical analysis
Comparative analyses
All comparisons in the present study were performed using general linear models (GLMs) with permutation tests. The statistical significance level was set at Pperm < 0.05. A network-based statistic (NBS) method was employed to determine intergroup differences in connectionwise connectivity strength (Zalesky et al., 2010). First, a GLM was specified for each element of the 246 × 246 connectivity matrix. A 246 × 246 matrix of t-statistics associated with the contrast of interest was thresholded by an initial P value threshold (P < 0.001). A graph was then created from this matrix, and the largest connected component was recorded. Next, the data were permuted 5,000 times according to the Freedman–Lane procedure in which each subject was randomly assigned to one of the subject groups. The same GLM was again specified at every matrix entry, a t-statistic matrix was calculated for the permuted dataset, and the associated P values were thresholded (P < 0.001). Finally, the largest connected component was recorded for the resultant graph, which was repeated for each permutation. The null distribution of the largest connected component sizes was used to calculate a Pperm value associated with the connected components of the observed data.
In terms of detecting intergroup differences in the AUC of topological attributes, the AUC data were permuted. The same GLM was tested on the permuted data, and the maximum statistic was recorded, building a null distribution. This procedure was repeated 10,000 times for global-level measures and 5,000 times for local-level measures. The null distribution of the maximum statistics and the observed statistics was compared to estimate a Pperm value for each attribute.
The topological attributes with significant intergroup differences were examined for Pearson’s partial correlations with clinical parameters (duration of MA use, age at first MA use, HAMA score, and BPRS and its five factor scores) controlling for age, education, and Fagerstrőm test for nicotine dependence (FTND) score. The correlations were adjusted for multiple comparisons using Bonferroni correction.
Support vector machine-based classification
Least absolute shrinkage and selection operator (Zhang et al., 2021), a regularization and variable selection algorithm implemented in the glmnet package4 in R, was used to select an optimal subset of features from all global and nodal topological attributes with 10 repeats of fivefold cross-validation. The features selected were used to construct a linear SVM using the caret package5 in R. The procedure is similar to that detailed in our previous work (Li et al., 2019). In brief, a fivefold cross-validation framework was applied to evaluate the performance of the classifier. Before every cross-validation, the training dataset was scaled to between 0 and 1, and the acquired parameters were used to scale the test dataset. As the fivefold split is random, we repeated the fivefold cross-validation 100 times. The only parameter, C, which controls the trade-off between the margin width and the misclassification penalty, was set to the default value (C = 1).
Accuracy, sensitivity, specificity, and kappa were calculated to quantify the cross-validated predictive performance of these classifiers. Specifically, accuracy is related to the proportion of subjects who were correctly classified into the MA-dependence or HC groups, and sensitivity and specificity are related to the proportion of individuals in the MA-dependence and HC groups correctly classified. Kappa is similar to accuracy, except that it is normalized at the baseline of random chance on the dataset. The performance of the classifier was also assessed using receiver operating characteristic (ROC) curves from the results of the cross-validation data (Li et al., 2019). The area under the ROC curve (AUC) represents the classification power of a classifier, with larger AUC values indicating better classification power.
A one-tailed permutation test was utilized to evaluate the probability of obtaining cross-validation accuracy values higher than those achieved by chance. All subjects were randomly relabeled, and fivefold cross-validation classification was performed. This procedure was repeated 5,000 times, and the number of times the accuracy for the permuted labels was higher than that derived for the real labels was recorded. A Pperm value for the classification was then calculated by dividing this number by 5,000.
Results
Demographics and clinical data
The subject demographic features are displayed in Table 1. The subjects in the MA group had similar ages (34.43 ± 7.19), years of education (13.2 ± 4.2), and FTND scores (6.01 ± 2.78) to those in the HC group (age, 34.37 ± 10.31; education, 13.5 ± 3.7 years; and FTND, 5.98 ± 2.57). With respect to the MA group, the mean duration of self-reported MA use was 51.80 ± 36.99 months. The daily dosage of MA use was 0.45 ± 0.53 g. The age of the first MA use was 29.72 ± 7.20 years. The time between last MA use and MRI scan was 5.67 ± 1.08 days.
Network-based statistic analyses
In the MA group, when compared with the HC group, the NBS analysis revealed one subnetwork with 10 nodes and 10 edges with an increased correlation (Pperm = 0.002) and another subnetwork with 12 nodes and 14 edges with a decreased correlation (Pperm < 0.001; Figure 1). For the 1st subnetwork, most increased connectivity existed between the orbitofrontal gyri (OFG) and inferior temporal cortices. Each decreased connectivity in the 2nd subnetwork had at least one node in the OFG, and half of these connections were present between subregions within the OFG. Based on the seven networks defined by Yeo et al. (2011), the brain regions that comprised the two subnetworks could generally be categorized into the following two groups of networks: ➀ the frontoparietal (FP) network, limbic network, visual network, ventral attention (VA) network, and subcortical gray matter (SCGM) network; ➁ the DMN, FP network, limbic network, and SCGM network.
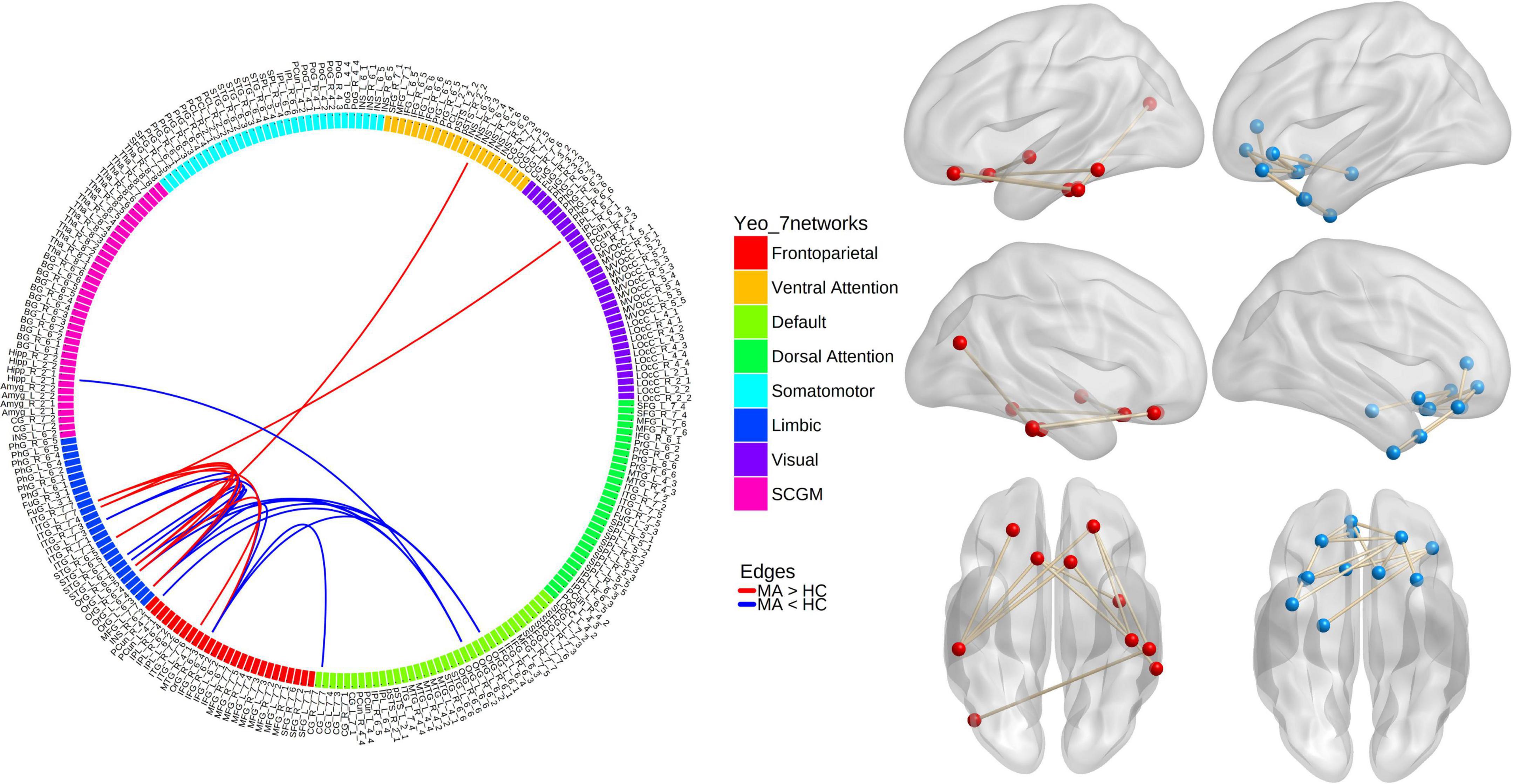
Figure 1. Results from network-based analyses of resting-state functional connectivity (rsFC) differences between methamphetamine (MA)-dependent patients and healthy controls (HCs). Red lines/balls: edges/nodes with increased rsFC; blue lines/balls: edges/nodes with decreased rsFC.
Graph theory analysis
Compared with the HC group, the MA group demonstrated significantly decreased AUC for Cp (Pperm < 0.001), Lp (Pperm = 0.007), modularity (Pperm = 0.006), and small-worldness (σ, Pperm = 0.004), as well as increased AUC for E.glob (Pperm = 0.009), Sp (Pperm = 0.009), and small-worldness (ω, Pperm < 0.001), of the rsFC network (Figure 2). It should be noted that the closer ω is to 0, the more a network is considered small world. In the present study, the ω at every network sparsity threshold for each subject was smaller than zero, and the increased AUC for ω is suggestive of impaired small-worldness.
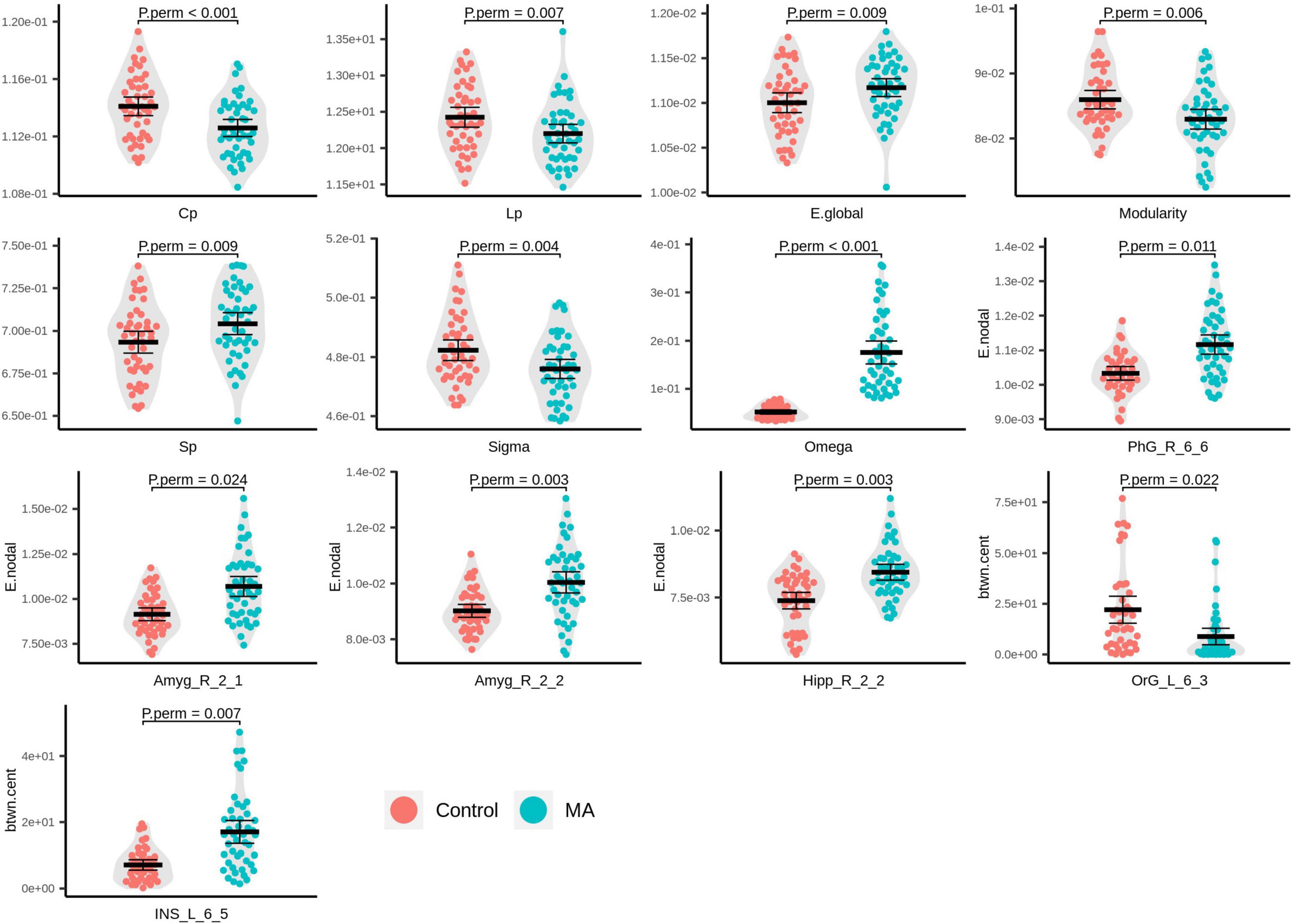
Figure 2. Beeswarm and violin plots of global/local topological attributes with evident inter-group differences. Red: healthy controls (HCs); green: methamphetamine (MA)-dependent group.
In the MA group, significantly increased E.nodal was detected in the right parahippocampal gyrus (PhG_R_6_6, Pperm = 0.011), right amygdala (Amyg_R_2_1, Pperm = 0.024; Amyg_R_2_2, Pperm = 0.003), and right caudal hippocampus (Hipp_R_2_2, Pperm = 0.003; Figure 2). With respect to btwn.cent values, the MA group manifested higher levels in the left dorsal granular insula (INS_L_6_5, Pperm = 0.007) and lower levels in the medial part of the left mOFG (OrG_L_6_3, Pperm = 0.022; Figure 2).
ω was the only topological attribute that presented significant associations with clinical variables: anxiety (r = 0.484, Pcorrected = 0.006), BPRS scores (r = 0.617, Pcorrected < 0.001), lack of vitality scores (r = 0.581, Pcorrected < 0.001), activity scores (r = 0.693, Pcorrected < 0.001), and hostility-suspicion scores (r = 0.548, Pcorrected = 0.001; Figure 3).
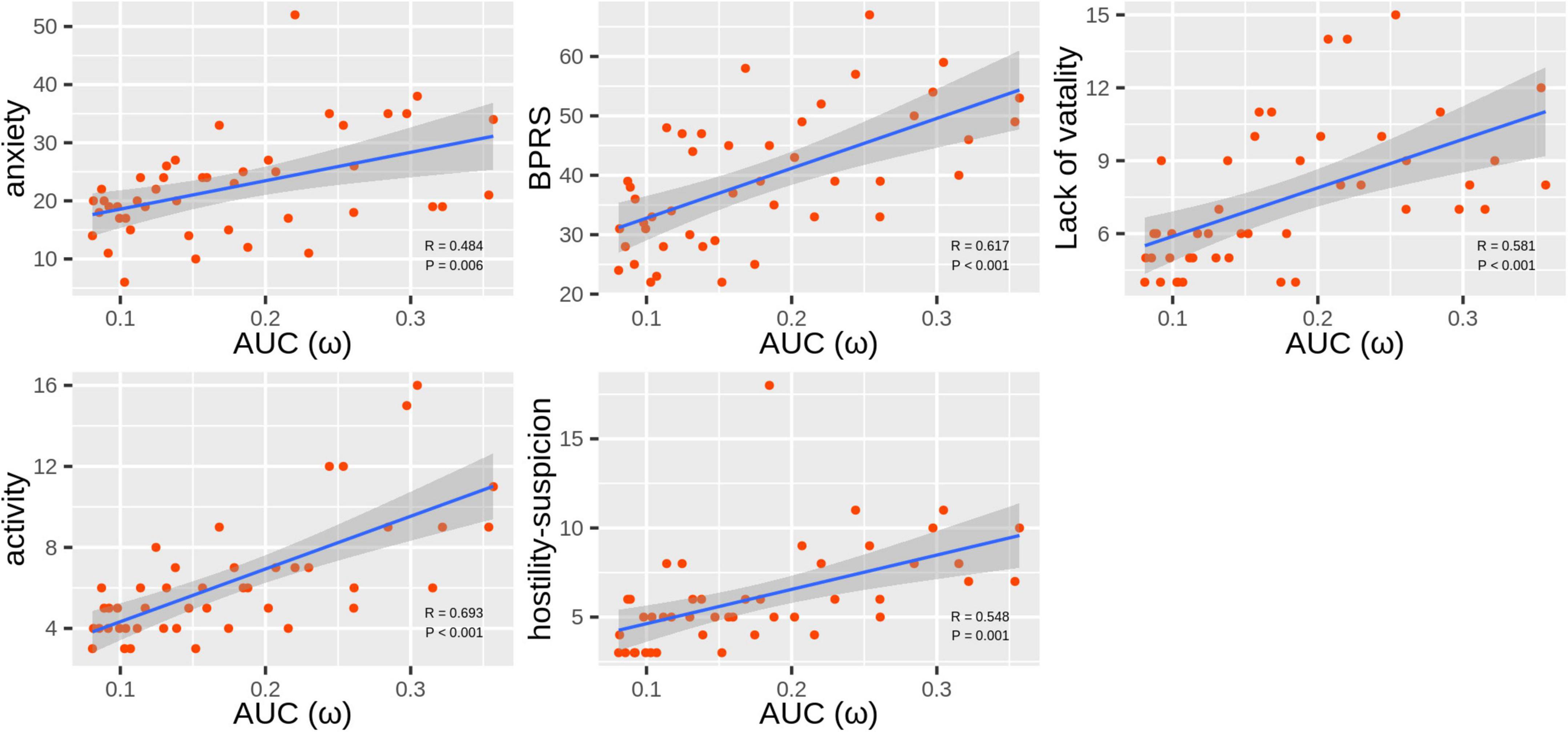
Figure 3. Scatter plots of correlations between ω and Hamilton Anxiety Rating Scale (HAMA) and Brief Psychiatric Rating Scale (BPRS) scores in the methamphetamine (MA)-dependent patients.
Support vector machine-based classification
From all 499 global and local topological attributes, 36 features were selected by LASSO, including small-worldness ω, E.nodal for 15 brain regions (INS_L_6_6, OrG_L_6_3, Tha_R_8_8, SPL_L_5_1, OrG_R_6_3, IPL_R_6_1, MTG_R_4_2, Hipp_R_2_2, BG_L_6_6, LOcC_R_2_2, Tha_L_8_3, PhG_R_6_6, Hipp_L_2_1, ITG_R_7_5, and Amyg_R_2_2) and btwn.cent for 20 brain regions (CG_R_7_1, SFG_R_7_5, IFG_L_6_1, OrG_L_6_4, INS_L_6_6, STG_L_6_5, OrG_L_6_3, STG_L_6_1, Amyg_R_2_1, pSTS_L_2_1, LOcC_R_4_1, IPL_R_6_4, BG_L_6_6, BG_R_6_6, SFG_L_7_2, Tha_L_8_3, IFG_L_6_4, STG_L_6_3, Hipp_R_2_2, and INS_L_6_5).
An SVM trained by the selected features exhibited excellent performance, with a cross-validated prediction area under the ROC curve, accuracy, sensitivity, specificity, and kappa of 99.03 ± 1.79, 94.00 ± 5.78, 93.46 ± 8.82, 94.52 ± 8.11, and 87.99 ± 11.57%, respectively (Pperm < 0.001) (for the detailed weights of the 36 features in the SVM model, see Table 2 and Figure 4A; for the ROC for cross-validated prediction performance of classifiers trained on topological attributes selected using LASSO, see Figure 4B).
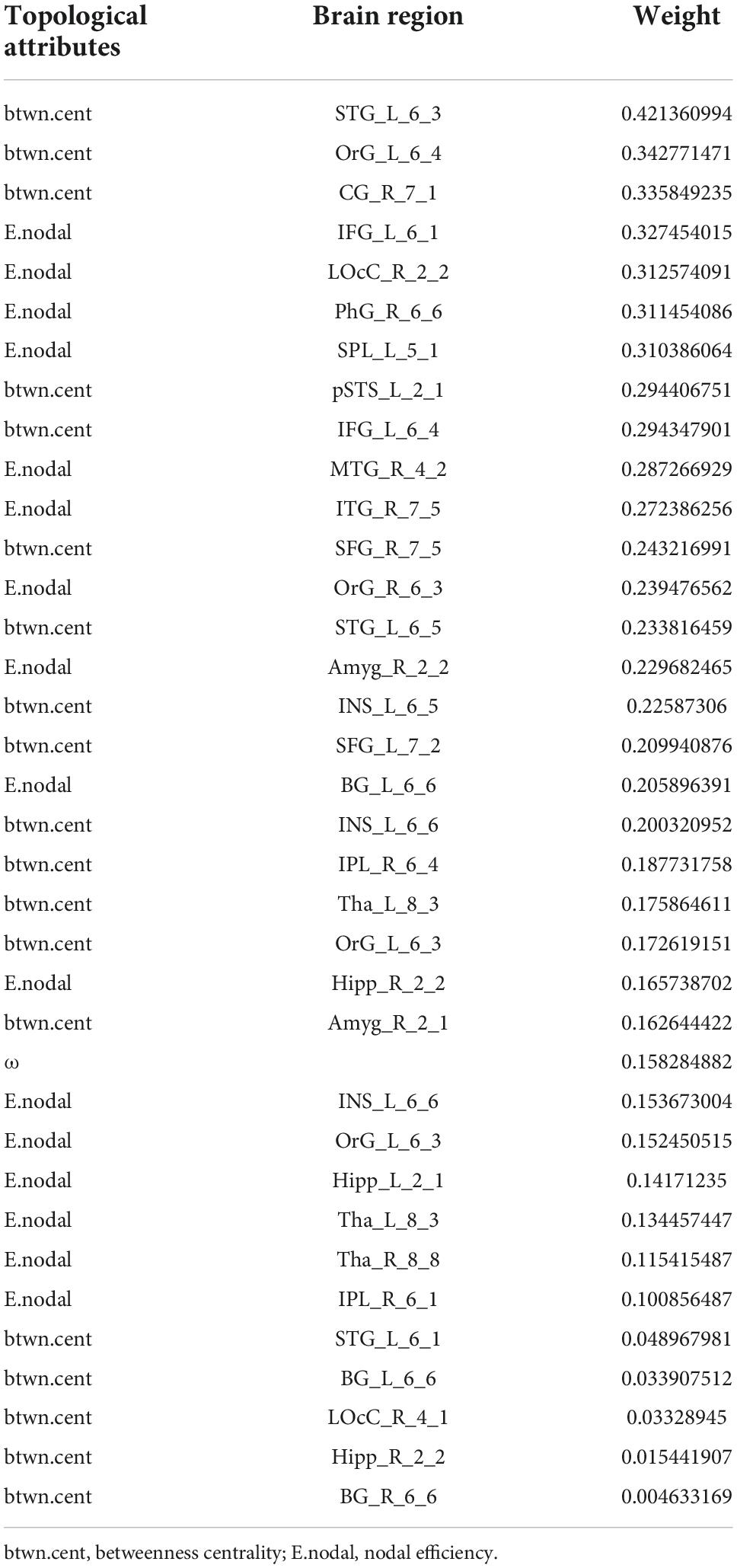
Table 2. The weights (absolute values) of the 36 features in the support vector machine (SVM) model selected by least absolute shrinkage and selection operator (LASSO).
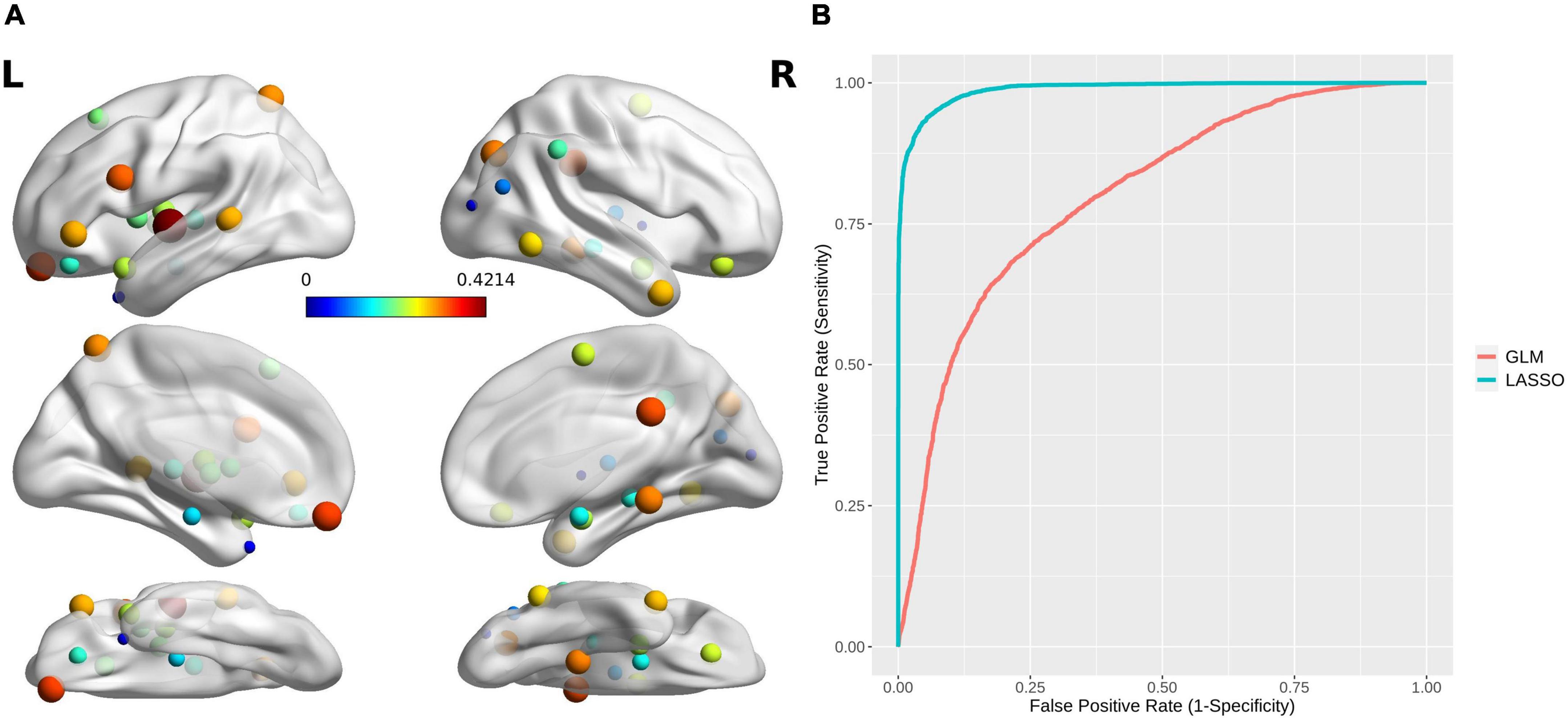
Figure 4. (A) The brain regions with topological attributes selected by Lasso for the construction of an SVM classifier for MA dependence. The size/color of each ball indicates the weight of every brain region with topological attributes. (B) Receiver operating characteristics curves for cross-validated prediction performance of classifiers trained on topological attributes selected using least absolute shrinkage and selection operator (LASSO) and general linear models (GLMs), respectively.
Discussion
To our knowledge, this is the first study to analyze rsFC using Tikhonov regularization, and investigate altered AUC of rsFC-based topological attributes in SUDs. In the present study, NBS analysis on Tikhonov regularization-based rsFC data revealed a subnetwork with increased FC and a subnetwork with decreased FC in MA-dependent patients. The brain regions that comprised the two subnetworks originated from the FP, limbic, visual, VA, and SCGM networks, and most of these brain regions were located in the OFG. By using graph theory on these data, the MA-dependent patients presented altered AUC of global topological attributes, i.e., decreased small-worldness (σ, ω), Cp, Lp, and modularity and increased E.glob and Sp. ω was the only topological attribute that presented significant correlation with HAMA/BPRS scores in the MA group. Moreover, aberrant local topological attributes, i.e., three regions with increased AUC of E.nodal, one region with increased AUC of btwn.cent and one region with decreased AUC of btwn.cent were also discovered in the patients. An SVM trained with features selected by LASSO from AUC of all topological attributes achieved excellent classification of MA-dependent patients with a cross-validated accuracy of 94.00 ± 5.78%.
Intergroup differences in resting-state functional connectivity
Confirmed by diffusion tractography imaging (Hsu et al., 2020), the orbitofrontal cortex (OFC) receives visual inputs directly from the inferior temporal cortex (ITC) and then projects back to the ITC (Rolls, 2000). The responses of the visual neurons in the OFC encode the reward value of visual stimuli. As most increased FC values in the MA-dependent patients existed between the OFC and ITC in this study, it suggests enhanced transmission of information between the two structures and may indicate overactivation of the reward circuit, making patients very sensitive to drug-related visual cues.
As a functionally heterogeneous structure, the OFC can be divided into multiple regions distinguished by cytoarchitecture with strong fiber connections between subregions (Hsu et al., 2020). This structure has been proven to play a critical role in compulsive drug-seeking and drug relapse (Schoenbaum and Shaham, 2008). A number of MRI and metabolic studies have reported altered structure (Daumann et al., 2011; Nakama et al., 2011; Li et al., 2017) and hypofunctionality (Volkow et al., 2001; Paulus et al., 2002, 2003; Sekine et al., 2003; London et al., 2004; Li et al., 2019) of the OFC in MA-dependent patients. However, very few studies have explored FC patterns within the OFC in patients with SUDs. The NBS analysis in this study revealed that in the MA-dependent subjects, all associations with decreased connectivity had one node in the OFG, and half of these connections were with subregions within the OFC. Similarly, by using voxel-mirrored homotopic connectivity (VMHC), an index to measure the strength of the functional connection between a voxel/ROI and its counterpart in the opposite hemisphere, researchers have found significantly reduced VMHC between the bilateral medial OFCs (mOFCs) in patients with internet gaming disorder (Chen et al., 2020) or codeine cough syrup-dependence (Qiu et al., 2017). Ieong and Yuan (2017), by using functional near-infrared spectroscopy in heroin users, also reported weaker functional connections between these structures. These results confirmed the key role of the OFC in the development of MA dependence and indicate widespread MA-related impairment in synchronized spontaneous activity of subregions within the OFC system, which are thought to be implicated in the core characteristics of SUDs, such as biased associations between stimuli and reward responses and deficits in properly inhibiting drug intake and aversive/withdrawal reactions to potentially dangerous situations (Goldstein et al., 2005). However, in addition to a decreased connection between the bilateral lateral OFC, Ma et al. (2010) found stronger rsFC between the left OFC (left lOFC) and right mOFC and between the bilateral mOFC in heroin users. These discrepant results may have stemmed from different data processing methods and different drugs used and require further investigation to uncover the neuromechanisms underlying the altered rsFC circuits within the OFC system.
In this study, connectivity between the right subgenual anterior cingulate gyrus (sgACC, CG_R_7_7) and the right medial OFG (right mORG, ORG_R_6_3, BA 11) was significantly attenuated in MA-dependent patients. Both of these structures have been verified to play important roles in regulating emotion (Rudebeck et al., 2014). Ramirez-Mahaluf et al. (2018), by applying graph analysis methods to rsFC data, identified a brain network that included the sgACC and mOFG and is associated with sadness processing. Moreover, the stronger the rsFC between these two structures is, the better the clinical response to accelerated intermittent theta burst stimulation (a repetitive transcranial magnetic stimulation protocol) in patients with depression (Baeken et al., 2017). As there is a high prevalence of comorbid anxiety/depression and MA dependence (National Institutes on Drug Abuse, 2020), this disrupted connectivity was presumed to be a brain imaging biomarker for mood disturbance in MA-dependent individuals. London et al. (2004), by using [18F]fluorodeoxyglucose positron emission tomography, revealed MA-related significantly lowered glucose metabolism in the sgACC, which may underlie this weakened connectivity.
By using task-fMRI-based FC analysis, Ross et al. (2013) proved the critical role of connectivity between the lOFC and hippocampus in both working memory and long-term memory when separate representations of overlapping stimuli need to be disambiguated, which is important for social interaction, i.e., enabling us to distinguish between changing contexts and social situations and then to act appropriately. The same connectivity under resting-state conditions, i.e., rsFC between the lateral part of the left OFG (left lOFG, OrG_L_6_6) and the hippocampus (Hipp_R_2_1), was weakened in MA-dependent patients in the current study. Related to these results, MA-induced long-lasting impairments in working memory have been reported in male rats (Braren et al., 2014). Several studies have also yielded a correlation between impaired working memory and MA dependence in both current and abstinent chronic MA users (Guerin et al., 2019). We postulate that this altered connectivity may indicate impaired working memory in patients, which needs further research by analyzing the correlation between this rsFC and measures of working memory.
Intergroup differences in global topological organization in the resting-state functional connectivity network
A healthy human brain is a typical model of a small-world network. The characteristics of small-world networks, i.e., high Cp and short Lp with optimized balance between functional segregation and integration, make it possible to maintain efficient and specialized modular information processing and rapid global information transmission in the network (Rubinov and Sporns, 2010). In the present study, although the nature of small-world architecture was conserved in the MA group, the aberrant AUC for σ and ω in this group indicated impaired small-worldness that, together with decreased Cp (a measure of network functional segregation), Lp (a measure of network integration), and Sp (a measure of network density or the total “wiring cost” of the network) and increased E.glob imply an MA-related shift toward random networks (Jiang et al., 2013; Zhu et al., 2016; Park et al., 2017). The shift in brain functional network configuration toward a random network organization has been exhibited for internet gaming addiction (Park et al., 2017), heroin dependence (Jiang et al., 2013), and other neuropsychiatric disorders, such as schizophrenia (Zhu et al., 2016), depression (Long et al., 2015), and Alzheimer’s disease (AD) (Stam et al., 2009). These kinds of topological alterations suggest less functional segregation, which also lead to a less optimized functional organization, in the MA-dependent brain.
In addition, the MA group in this study demonstrated lower modularity, a global topological attribute measuring the division of a network into separate modules (Rubinov and Sporns, 2010). A module is characterized as having denser connections between nodes within the module but sparser connections with nodes outside the module. Reduced network organization, implied by lower modularity and Cp in MA-dependent brains, suggested poor functional network segregation. Consistent with these results, single-cell whole-brain imaging-based functional networks from male mice administered cocaine/MA/nicotine for 1 week (Kimbrough et al., 2021), as well as white matter structural networks in synthetic cannabinoid users (Celik et al., 2020), have also presented decreased modularity. By using simple computer simulations on the dynamics of modularization in a minimal substrate, Lipson et al. (2002) suggested that modularity can spontaneously arise under changing environments, i.e., higher modularity results in higher adaptability of their behavior to acquire rewards and to avoid punishments (Hu, 2016). As impairments in this ability are one of the main symptoms of addiction, we presume that decreased modularity in MA dependence is closely related to the failure to adapt to environmental changes, which then results in aberrant reward function and behavior.
Intergroup differences in local topological attributes in the resting-state functional connectivity network
In the patients in this study, the brain regions with significantly increased E.nodal included the right amygdala and hippocampus, and a similar pattern was also discovered in patients with major depressive disorder (Ye et al., 2016). The hippocampus and amygdala are core parts of the affective processing network. Specifically, the amygdala manifests greater activity that lasts longer when depressed patients process negative stimuli, and the hippocampus shows enhanced activity in the recall of negative, not positive, stimuli after encoding in the amygdala (Ye et al., 2016). Therefore, it is plausible that these results provide biomarkers for comorbid depression and MA dependence from the perspective of the local topology.
The insular cortex is known to be involved in interoception, decision making, anxiety, pain perception, cognition, mood, threat recognition, and conscious urges (Ibrahim et al., 2019). In MA users, the left insula has been reported to present volume reduction (Hall et al., 2015), greater activation across risky and safe decisions (Gowin et al., 2014), and decreased glucose metabolism (Vuletic et al., 2018). Consistent with these findings, our results, i.e., the left insula as the only structure with MA-related increment of betweenness centrality and as important features for constructing a high-efficacy classifier, supports the perspective that left insular cortex is involved in key aspects underlying MA-dependence.
It has been suggested that the mOFC is involved in processing stimulus-reward associations and with the reinforcement of behavior (Rolls, 2000). Moreover, in a task-fMRI-based FC and rsFC study on alcohol use disorder that enrolled 1890 subjects, the mOFG was suggested to be a central/core structure in a neural network that may underlie the onset of alcoholism (Jia et al., 2021). In this network, the authors identified two independent regulatory pathways between the mOFC and the dorsal periaqueductal gray (dPAG) in the brainstem contributing to alcohol abuse; in particular, excitatory regulation during the resting state was related to impulsive behavior, and inhibitory regulation upon receiving a non-reward (relative punishment) was related to compulsive alcohol use. In the present study, the left mOFC with a significant reduction in betweenness centrality, which is a more sensitive measure of the importance of a node connecting disparate parts of a network, may indicate dysregulation of this core structure in the substance dependence-related network and hence inevitably lead to dependence on MA.
Support vector machine classifier
The only global topological attribute selected by LASSO to train an SVM was small-worldness ω. The currently accepted definition of a small-world network is that it has clustering comparable to a regular lattice and Lp comparable to a random network. σ is defined by comparing clustering and Lp to that of a comparable random network, which means that networks with very low clustering can also be, and indeed are, defined as small world. To solve this defect, Telesford et al. (2011) introduced, which is defined by comparing network clustering to an equivalent lattice network and Lp to a random network; ω has the following advantages: (a) it provides a more accurate estimation of small-worldness; (b) it is less sensitive to the size of a network and benefits from inherent scaling, which provides a powerful tool for comparing and ranking small-world properties in various systems. From the perspective of machine learning, our results suggested that ω is a better feature for the classification of MA dependence than the other global topological attributes. Moreover, the evident positive associations between the AUC for ω and HAMA/BPRS scores in the MA group indicate that ω may be an ideal brain network marker for the psychiatric symptoms of MA-dependent patients.
Most of the regions with local topological attributes selected by LASSO were from the DMN, limbic, FP, visual, attention, and SCCM networks, covering four addiction-related interacting circuits (reward, motivation/drive, memory and learning, and control), attention circuits and emotion circuits as proposed by Volkow et al. (2003). The excellent classification accuracy of the SVM in the present study indicates that topological attributes derived from rsFC networks may be promising features for constructing a high-efficacy classifier for MA-dependent individuals.
Limitations
Several considerations should be taken into account with respect to the interpretation of the present results. First, the relatively small sample size made it difficult to further analyze the differences in rsFC networks between subgroups (e.g., between male and female patients, between patients without psychosis and, those with psychosis). Moreover, a potential overfitting problem for the classifier can hardly be avoided without establishing an independent test dataset. A larger sample size in future studies would help enhance the generalizability of the present classifier. Second, datasets with other SUDs are needed to tell whether the methods applied in the present study could also be sensitive to discover altered rsFC-based connectome, and could construct a high-efficacy classifier using rsFC-based topological attributes, in patients with other SUDs. Third, the low sampling rate (TR = 2 s) in the present study cannot completely eliminate the effects of physiological noise, such as cardiac and respiratory noise, on BOLD signals, which can affect the calculation of rsFC, especially within the DMN, even after the application of bandpass filtering (Yoshikawa et al., 2020). In future studies, a faster sampling rate or algorithm for removing physiological noise should be applied to address this problem (Liao et al., 2013; Yoshikawa et al., 2020). Fourth, as this is a cross-sectional study and no correlations were found between the topological attributes and duration of MA use/age at first MA use, it is unclear whether the altered topological attributes were the result of MA dependence or a pre-addiction condition, which could be clarified by genetic and longitudinal imaging studies in the future.
In conclusion, the present study demonstrates an abnormal brain Tikhonov regularization-based rsFC connectome in MA-dependent patients from the perspectives of FC and topology. The MA-related alterations in brain rsFC networks present a shift toward random networks. The rsFC-based topological attributes may be promising features for constructing high-efficacy machine-learning-based classifiers for MA-dependent individuals, which needs verification from a larger sample size.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Ningbo Medical Center Lihuili Hospital, Ningbo University, Zhejiang, China. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YL and WZ were responsible for the study concept. YL conducted the literature searches, data analyses, and drafted the manuscript. PC and HD were provided expertise in statistical analyses and multivariate pattern classification. LL performed the MRI data collection. HL and WS were responsible for recruitment, interview of all subjects, and collection of their clinical data. WS provided diagnosis for all patients. WZ provided critical revisions of the manuscript. All authors reviewed the manuscript and approved the final version for publication.
Funding
This study was supported by the National Natural Science Foundation of China (82071499), National Key Research and Development Program of China (2017YFC1310403), Zhejiang Basic Public Welfare Research Program Project (LGF21H090007), Zhejiang Provincial Medical and Health Science and Technology Program (2018KY708), and Ningbo Public Welfare Technology Plan Project (202002N3166).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
- ^ https://www.unodc.org/unodc/en/data-and-analysis/wdr2021.html
- ^ http://www.nncc626.com/2022-06/23/c_1211659746.htm
- ^ https://github.com/cwatson/brainGraph
- ^ https://cloud.r-project.org/package=glmnet
- ^ https://github.com/topepo/caret/
References
Avants, B. B., Tustison, N. J., Song, G., and Gee, J. C. (2009). ANTS: Open-source tools for normalization and neuroanatomy. HeanetIe 10, 1–11.
Baeken, C., Duprat, R., Wu, G. R., De Raedt, R., and van Heeringen, K. (2017). Subgenual anterior cingulate-medial orbitofrontal functional connectivity in medication-resistant major depression: A neurobiological marker for accelerated intermittent theta burst stimulation treatment? Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2, 556–565. doi: 10.1016/j.bpsc.2017.01.001
Barenholtz, E., Fitzgerald, N. D., and Hahn, W. E. (2020). Machine-learning approaches to substance-abuse research: Emerging trends and their implications. Curr. Opin. Psychiatry 33, 334–342. doi: 10.1097/YCO.0000000000000611
Braren, S. H., Drapala, D., Tulloch, I. K., and Serrano, P. A. (2014). Methamphetamine-induced short-term increase and long-term decrease in spatial working memory affects protein kinase M zeta (PKMzeta), dopamine, and glutamate receptors. Front. Behav. Neurosci. 8:438. doi: 10.3389/fnbeh.2014.00438
Celik, Z. C., Colak, C., Di Biase, M. A., Zalesky, A., Zorlu, N., Bora, E., et al. (2020). Structural connectivity in adolescent synthetic cannabinoid users with and without ADHD. Brain Imaging Behav. 14, 505–514. doi: 10.1007/s11682-018-0023-x
Chen, J., Li, X., Zhang, Q., Zhou, Y., Wang, R., Tian, C., et al. (2020). Impulsivity and response inhibition related brain networks in adolescents with internet gaming disorder: A preliminary study utilizing resting-state fMRI. Front. Psychiatry 11:618319. doi: 10.3389/fpsyt.2020.618319
Daumann, J., Koester, P., Becker, B., Wagner, D., Imperati, D., Gouzoulis-Mayfrank, E., et al. (2011). Medial prefrontal gray matter volume reductions in users of amphetamine-type stimulants revealed by combined tract-based spatial statistics and voxel-based morphometry. Neuroimage 54, 794–801.
Fan, L., Li, H., Zhuo, J., Zhang, Y., Wang, J., Chen, L., et al. (2016). The human brainnetome atlas: A new brain Atlas based on connectional architecture. Cereb. Cortex 26, 3508–3526. doi: 10.1093/cercor/bhw157
Goldstein, R. Z., Alia-Klein, N., Leskovjan, A. C., Fowler, J. S., Wang, G. J., Gur, R. C., et al. (2005). Anger and depression in cocaine addiction: Association with the orbitofrontal cortex. Psychiatry Res. 138, 13–22. doi: 10.1016/j.pscychresns.2004.10.002
Gowin, J. L., Stewart, J. L., May, A. C., Ball, T. M., Wittmann, M., Tapert, S. F., et al. (2014). Altered cingulate and insular cortex activation during risk-taking in methamphetamine dependence: Losses lose impact. Addiction 109, 237–247. doi: 10.1111/add.12354
Guerin, A. A., Bonomo, Y., Lawrence, A. J., Baune, B. T., Nestler, E. J., Rossell, S. L., et al. (2019). Cognition and related neural findings on methamphetamine use disorder: Insights and treatment implications from schizophrenia research. Front. Psychiatry 10:880. doi: 10.3389/fpsyt.2019.00880
Hall, M. G., Alhassoon, O. M., Stern, M. J., Wollman, S. C., Kimmel, C. L., Perez-Figueroa, A., et al. (2015). Gray matter abnormalities in cocaine versus methamphetamine-dependent patients: A neuroimaging meta-analysis. Am. J. Drug Alcohol Abuse 41, 290–299. doi: 10.3109/00952990.2015.1044607
Hsu, C. C. H., Rolls, E. T., Huang, C. C., Chong, S. T., ZacLo, C. Y., Feng, J., et al. (2020). Connections of the human orbitofrontal cortex and inferior frontal gyrus. Cereb. Cortex 30, 5830–5843. doi: 10.1093/cercor/bhaa160
Humphries, M. D., and Gurney, K. (2008). Network ‘small-world-ness’: A quantitative method for determining canonical network equivalence. PLoS One 3:e0002051. doi: 10.1371/journal.pone.0002051
Ibrahim, C., Rubin-Kahana, D. S., Pushparaj, A., Musiol, M., Blumberger, D. M., Daskalakis, Z. J., et al. (2019). The insula: A brain stimulation target for the treatment of addiction. Front. Pharmacol. 10:720. doi: 10.3389/fphar.2019.00720
Ieong, H. F., and Yuan, Z. (2017). Abnormal resting-state functional connectivity in the orbitofrontal cortex of heroin users and its relationship with anxiety: A pilot fNIRS study. Sci. Rep. 7:46522. doi: 10.1038/srep46522
Ipser, J. C., Uhlmann, A., Taylor, P., Harvey, B. H., Wilson, D., and Stein, D. J. (2018). Distinct intrinsic functional brain network abnormalities in methamphetamine-dependent patients with and without a history of psychosis. Addict. Biol. 23, 347–358. doi: 10.1111/adb.12478
Jia, T., Xie, C., Banaschewski, T., Barker, G. J., Bokde, A. L. W., Buchel, C., et al. (2021). Neural network involving medial orbitofrontal cortex and dorsal periaqueductal gray regulation in human alcohol abuse. Sci. Adv. 7:eabd4074. doi: 10.1126/sciadv.abd4074
Jiang, G., Wen, X., Qiu, Y., Zhang, R., Wang, J., Li, M., et al. (2013). Disrupted topological organization in whole-brain functional networks of heroin-dependent individuals: A resting-state FMRI study. PLoS One 8:e82715. doi: 10.1371/journal.pone.0082715
Kimbrough, A., Kallupi, M., Smith, L. C., Simpson, S., Collazo, A., and George, O. (2021). Characterization of the brain functional architecture of psychostimulant withdrawal using single-cell whole-brain imaging. eNeuro 8:ENEURO.0208-19.2021. doi: 10.1523/ENEURO.0208-19.2021
Kohno, M., Okita, K., Morales, A. M., Robertson, C. L., Dean, A. C., Ghahremani, D. G., et al. (2016). Midbrain functional connectivity and ventral striatal dopamine D2-type receptors: Link to impulsivity in methamphetamine users. Mol. Psychiatry 21, 1554–1560. doi: 10.1038/mp.2015.223
Li, Y., Cui, Z., Liao, Q., Dong, H., Zhang, J., Shen, W., et al. (2019). Support vector machine-based multivariate pattern classification of methamphetamine dependence using arterial spin labeling. Addict. Biol. 24, 1254–1262. doi: 10.1111/adb.12705
Li, Y., Dong, H., Li, F., Wang, G., Zhou, W., Yu, R., et al. (2017). Microstructures in striato-thalamo-orbitofrontal circuit in methamphetamine users. Acta Radiol. 58, 1378–1385. doi: 10.1177/0284185117692170
Liao, X. H., Xia, M. R., Xu, T., Dai, Z. J., Cao, X. Y., Niu, H. J., et al. (2013). Functional brain hubs and their test-retest reliability: A multiband resting-state functional MRI study. Neuroimage 83, 969–982. doi: 10.1016/j.neuroimage.2013.07.058
Lipson, H., Pollack, J. B., and Suh, N. P. (2002). On the origin of modular variation. Evolution 56, 1549–1556. doi: 10.1111/j.0014-3820.2002.tb01466.x
London, E. D., Simon, S. L., Berman, S. M., Mandelkern, M. A., Lichtman, A. M., Bramen, J., et al. (2004). Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch. Gen. Psychiatry 61, 73–84. doi: 10.1001/archpsyc.61.1.73
Long, Z., Duan, X., Wang, Y., Liu, F., Zeng, L., Zhao, J. P., et al. (2015). Disrupted structural connectivity network in treatment-naive depression. Prog. Neuro Psychopharmacol. Biol. Psychiatry 56, 18–26. doi: 10.1016/j.pnpbp.2014.07.007
Ma, N., Liu, Y., Li, N., Wang, C. X., Zhang, H., Jiang, X. F., et al. (2010). Addiction related alteration in resting-state brain connectivity. Neuroimage 49, 738–744. doi: 10.1016/j.neuroimage.2009.08.037
Malina, M., Keedy, S., Weafer, J., Van Hedger, K., and de Wit, H. (2021). Effects of methamphetamine on within- and between-network connectivity in healthy adults. Cereb. Cortex Commun. 2:tgab063. doi: 10.1093/texcom/tgab063
Mansoory, M. S., Oghabian, M. A., Jafari, A. H., and Shahbabaie, A. (2017). Analysis of resting-state fMRI topological graph theory properties in methamphetamine drug users applying box-counting fractal dimension. Basic Clin. Neurosci. 8, 371–385. doi: 10.18869/nirp.bcn.8.5.371
Mete, M., Sakoglu, U., Spence, J. S., Devous, M. D. Sr., Harris, T. S., and Adinoff, B. (2016). Successful classification of cocaine dependence using brain imaging: A generalizable machine learning approach. BMC Bioinformatics 17:357.
Nakama, H., Chang, L., Fein, G., Shimotsu, R., Jiang, C. S., and Ernst, T. (2011). Methamphetamine users show greater than normal age-related cortical gray matter loss. Addiction 106, 1474–1483. doi: 10.1111/j.1360-0443.2011.03433.x
National Institutes on Drug Abuse (2020). Common comorbidities with substance use disorders research report. Bethesda, MD: National Institutes on Drug Abuse (US).
Noble, W. S. (2006). What is a support vector machine? Nat. Biotechnol. 24, 1565–1567. doi: 10.1038/nbt1206-1565
Overall, J. E., and Gorham, D. R. (1962). The brief psychiatric rating scale. Psychol. Rep. 10, 799–812. doi: 10.2466/pr0.1962.10.3.799
Pandria, N., Kovatsi, L., Vivas, A. B., and Bamidis, P. D. (2018). Resting-state abnormalities in heroin-dependent individuals. Neuroscience 378, 113–145. doi: 10.1016/j.neuroscience.2016.11.018
Park, C. H., Chun, J. W., Cho, H., Jung, Y. C., Choi, J., and Kim, D. J. (2017). Is the internet gaming-addicted brain close to be in a pathological state? Addict. Biol. 22, 196–205. doi: 10.1111/adb.12282
Paulus, M. P., Hozack, N., Frank, L., Brown, G. G., and Schuckit, M. A. (2003). Decision making by methamphetamine-dependent subjects is associated with error-rate-independent decrease in prefrontal and parietal activation. Biol. Psychiatry 53, 65–74. doi: 10.1016/s0006-3223(02)01442-7
Paulus, M. P., Hozack, N. E., Zauscher, B. E., Frank, L., Brown, G. G., Braff, D. L., et al. (2002). Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology 26, 53–63. doi: 10.1016/S0893-133X(01)00334-7
Pervaiz, U., Vidaurre, D., Woolrich, M. W., and Smith, S. M. (2020). Optimising network modelling methods for fMRI. Neuroimage 211:116604. doi: 10.1016/j.neuroimage.2020.116604
Qiu, Y. W., Lv, X. F., Jiang, G. H., Su, H. H., Ma, X. F., Tian, J. Z., et al. (2017). Larger corpus callosum and reduced orbitofrontal cortex homotopic connectivity in codeine cough syrup-dependent male adolescents and young adults. Eur. Radiol. 27, 1161–1168. doi: 10.1007/s00330-016-4465-5
R Core Team (2018). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Ramaekers, J. G., Mason, N. L., Toennes, S. W., Theunissen, E. L., and Amico, E. (2022). Functional brain connectomes reflect acute and chronic cannabis use. Sci. Rep. 12:2449. doi: 10.1038/s41598-022-06509-9
Ramirez-Mahaluf, J. P., Perramon, J., Otal, B., Villoslada, P., and Compte, A. (2018). Subgenual anterior cingulate cortex controls sadness-induced modulations of cognitive and emotional network hubs. Sci. Rep. 8:8566.
Rolls, E. T. (2000). The orbitofrontal cortex and reward. Cereb. Cortex 10, 284–294. doi: 10.1093/cercor/10.3.284
Ross, R. S., LoPresti, M. L., Schon, K., and Stern, C. E. (2013). Role of the hippocampus and orbitofrontal cortex during the disambiguation of social cues in working memory. Cogn. Affect. Behav. Neurosci. 13, 900–915. doi: 10.3758/s13415-013-0170-x
Rubinov, M., and Sporns, O. (2010). Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 52, 1059–1069. doi: 10.1016/j.neuroimage.2009.10.003
Rudebeck, P. H., Putnam, P. T., Daniels, T. E., Yang, T., Mitz, A. R., Rhodes, S. E., et al. (2014). A role for primate subgenual cingulate cortex in sustaining autonomic arousal. Proc. Natl. Acad. Sci. U.S.A. 111, 5391–5396. doi: 10.1073/pnas.1317695111
Rusyniak, D. E. (2013). Neurologic manifestations of chronic methamphetamine abuse. Psychiatr. Clin. North Am. 36, 261–275. doi: 10.1016/j.psc.2013.02.005
Schoenbaum, G., and Shaham, Y. (2008). The role of orbitofrontal cortex in drug addiction: A review of preclinical studies. Biol. Psychiatry 63, 256–262. doi: 10.1016/j.biopsych.2007.06.003
Sekine, Y., Minabe, Y., Ouchi, Y., Takei, N., Iyo, M., Nakamura, K., et al. (2003). Association of dopamine transporter loss in the orbitofrontal and dorsolateral prefrontal cortices with methamphetamine-related psychiatric symptoms. Am. J. Psychiatry 160, 1699–1701. doi: 10.1176/appi.ajp.160.9.1699
Sjoerds, Z., Stufflebeam, S. M., Veltman, D. J., Van den Brink, W., Penninx, B. W., and Douw, L. (2017). Loss of brain graph network efficiency in alcohol dependence. Addict. Biol. 22, 523–534. doi: 10.1111/adb.12346
Stam, C. J., de Haan, W., Daffertshofer, A., Jones, B. F., Manshanden, I., van Walsum, A. M. V. C., et al. (2009). Graph theoretical analysis of magnetoencephalographic functional connectivity in Alzheimer’s disease. Brain 132, 213–224. doi: 10.1093/brain/awn262
Sun, Y., Wang, G. B., Lin, Q. X., Lu, L., Shu, N., Meng, S. Q., et al. (2017). Disrupted white matter structural connectivity in heroin abusers. Addict. Biol. 22, 184–195. doi: 10.1111/adb.12285
Sun, Y., Zhang, Y., Zhang, D., Chang, S., Jing, R., Yue, W., et al. (2018). GABRA2 rs279858-linked variants are associated with disrupted structural connectome of reward circuits in heroin abusers. Transl. Psychiatry 8:138. doi: 10.1038/s41398-018-0180-0
Telesford, Q. K., Joyce, K. E., Hayasaka, S., Burdette, J. H., and Laurienti, P. J. (2011). The ubiquity of small-world networks. Brain Connect. 1, 367–375. doi: 10.1089/brain.2011.0038
Volkow, N. D., Chang, L., Wang, G. J., Fowler, J. S., Ding, Y. S., Sedler, M., et al. (2001). Low level of brain dopamine D2 receptors in methamphetamine abusers: Association with metabolism in the orbitofrontal cortex. Am. J. Psychiatry 158, 2015–2021. doi: 10.1176/appi.ajp.158.12.2015
Volkow, N. D., Fowler, J. S., and Wang, G. J. (2003). The addicted human brain: Insights from imaging studies. J. Clin. Invest. 111, 1444–1451. doi: 10.1172/JCI18533
Vriend, C., van den Heuvel, O. A., Berendse, H. W., van der Werf, Y. D., and Douw, L. (2018). Global and subnetwork changes of the structural connectome in de novo Parkinson’s disease. Neuroscience 386, 295–308. doi: 10.1016/j.neuroscience.2018.06.050
Vuletic, D., Dupont, P., Robertson, F., Warwick, J., Zeevaart, J. R., and Stein, J. (2018). Methamphetamine dependence with and without psychotic symptoms: A multi-modal brain imaging study. Neuroimage Clin. 20, 1157–1162. doi: 10.1016/j.nicl.2018.10.023
Wetherill, R. R., Rao, H., Hager, N., Wang, J., Franklin, T. R., and Fan, Y. (2019). Classifying and characterizing nicotine use disorder with high accuracy using machine learning and resting-state fMRI. Addict. Biol. 24, 811–821. doi: 10.1111/adb.12644
Xia, M., and He, Y. (2017). Functional connectomics from a “big data” perspective. Neuroimage 160, 152–167. doi: 10.1016/j.neuroimage.2017.02.031
Yan, C., Yang, X., Yang, R., Yang, W., Luo, J., Tang, F., et al. (2021). Treatment response prediction and individualized identification of short-term abstinence methamphetamine dependence using brain graph metrics. Front. Psychiatry 12:583950. doi: 10.3389/fpsyt.2021.583950
Yan, C. G., Wang, X. D., Zuo, X. N., and Zang, Y. F. (2016). DPABI: Data processing & analysis for (resting-state) brain imaging. Neuroinformatics 14, 339–351. doi: 10.1007/s12021-016-9299-4
Ye, M., Qing, P., Zhang, K., and Liu, G. (2016). Altered network efficiency in major depressive disorder. BMC Psychiatry 16:450. doi: 10.1186/s12888-016-1053-9
Yeo, B. T., Krienen, F. M., Sepulcre, J., Sabuncu, M. R., Lashkari, D., Hollinshead, M., et al. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 1125–1165. doi: 10.1152/jn.00338.2011
Yip, S. W., Scheinost, D., Potenza, M. N., and Carroll, K. M. (2019). Connectome-based prediction of cocaine abstinence. Am. J. Psychiatry 176, 156–164. doi: 10.1176/appi.ajp.2018.17101147
Yoshikawa, A., Masaoka, Y., Yoshida, M., Koiwa, N., Honma, M., Watanabe, K., et al. (2020). Heart rate and respiration affect the functional connectivity of default mode network in resting-state functional magnetic resonance imaging. Front. Neurosci. 14:631. doi: 10.3389/fnins.2020.00631
Zalesky, A., Fornito, A., and Bullmore, E. T. (2010). Network-based statistic: Identifying differences in brain networks. Neuroimage 53, 1197–1207. doi: 10.1016/j.neuroimage.2010.06.041
Zhang, J., Wang, J., Wu, Q., Kuang, W., Huang, X., He, Y., et al. (2011). Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol. Psychiatry 70, 334–342. doi: 10.1016/j.biopsych.2011.05.018
Zhang, S., Zhu, F., Yu, Q., and Zhu, X. (2021). Identifying DNA-binding proteins based on multi-features and LASSO feature selection. Biopolymers 112:e23419. doi: 10.1002/bip.23419
Zhang, Y., Tian, J., Yuan, K., Liu, P., Zhuo, L., Qin, W., et al. (2011). Distinct resting-state brain activities in heroin-dependent individuals. Brain Res. 1402, 46–53. doi: 10.1016/j.brainres.2011.05.054
Keywords: methamphetamine, resting-state, connectome, graph theory, machine-learning
Citation: Li Y, Cheng P, Liang L, Dong H, Liu H, Shen W and Zhou W (2022) Abnormal resting-state functional connectome in methamphetamine-dependent patients and its application in machine-learning-based classification. Front. Neurosci. 16:1014539. doi: 10.3389/fnins.2022.1014539
Received: 09 August 2022; Accepted: 04 November 2022;
Published: 17 November 2022.
Edited by:
Dahua Yu, Inner Mongolia University of Science and Technology, ChinaReviewed by:
Yajing Meng, Sichuan University, ChinaKai Yuan, Xidian University, China
Jiang Du, Shanghai Jiao Tong University, China
Copyright © 2022 Li, Cheng, Liang, Dong, Liu, Shen and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yadi Li, bGl5YWRpMjAxMEAxMjYuY29t; Wenhua Zhou, d2h6aG91QHZpcC4xNjMuY29t
 Yadi Li
Yadi Li Ping Cheng
Ping Cheng Liang Liang1
Liang Liang1 Wenhua Zhou
Wenhua Zhou