
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol., 15 April 2025
Sec. Stroke
Volume 16 - 2025 | https://doi.org/10.3389/fneur.2025.1554583
This article is part of the Research TopicEvaluation of Fitness in Stroke SurvivorsView all 8 articles
 Mingtong Bian1,2
Mingtong Bian1,2 Fuyan Chen1,2,3*
Fuyan Chen1,2,3* Huizhen Su3
Huizhen Su3 Zhiying Li1,2
Zhiying Li1,2 Xiaowei Sun1,2
Xiaowei Sun1,2 Yang Liu1,2
Yang Liu1,2 Jinyuan Shi1,2
Jinyuan Shi1,2 Shuo Liu1,2
Shuo Liu1,2 Ru Rong1,2
Ru Rong1,2Background: Upper limb spasticity is a common and disabling sequela of stroke, which significantly impairing motor function and the capacity to perform activities of daily living (ADL). The relative efficacy of different physical therapies and their combinations compared to monotherapies remains unclear.
Methods: A comprehensive database search was conducted to identify randomized controlled trials (RCTs) published from database inception to 2024 that evaluated physical therapies for post-stroke upper limb spasticity. Data were analyzed using RevMan and STATA/R software with a Bayesian framework for network meta-analysis. Evidence consistency was assessed via node-splitting approaches, and intervention efficacy was ranked using the surface under the cumulative ranking curve (SUCRA). Effect sizes were expressed as mean differences (MD) with 95% confidence intervals (CI), and study quality was evaluated using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) system.
Results: Forty-nine RCTs involving 3,219 patients were included. The combination of physical rehabilitation (PR) with repetitive transcranial magnetic stimulation (rTMS) and electro-acupuncture (EA) demonstrated the highest improvement in Fugl-Meyer Assessment for Upper Extremity (FMA-UE) scores (91.1%), outperforming PR alone (13.2%) or EA monotherapy (30.3%). PR combined with rTMS and body acupuncture (BA) shows the most significant improvement in the Modified Barthel Index (MBI) (83.1%), superior to PR (20.8%) or BA (23.8%) alone. Adverse events (e.g., minor bruising from EA) were infrequent and self-resolving.
Conclusion: Current evidence indicates that synergistic application of PR with rTMS and acupuncture (EA/BA) significantly enhances upper limb motor function and ADL capacity. However, GRADE evaluations rated most evidence as moderate quality, limited by implementation bias, insufficient subgroup analyses, and lack of long-term follow-up data. Future studies should adopt standardized protocols and investigate efficacy variations across stroke subtypes.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42025633289, identifier [CRD42025633289].
Upper limb dysfunction following a stroke represents a significant cause of long-term disability in patients, frequently occurring in conjunction with a range of injuries, including upper limb weakness and spasticity. The principal manifestation of upper limb spastic paralysis is an increase in muscle tone on the affected side, which is characterized by symptoms such as shoulder adduction and internal rotation, elbow flexion and pronation, wrist flexion and ulnar deviation, and finger clenching (1). This can result in a number of adverse effects, including pain, muscle contraction, changes in soft tissue structure, weakness, associated reactions, loss of passive function, limited active function, and a decrease in quality of life. This has a significant impact on the patient’s activities of daily living (2). The pathological mechanism of upper limb spasticity is complex, involving damage to the corticospinal tract, peripheral mechanisms, extensor mechanisms, and potential spastic dystonia, among other factors (3). Furthermore, because the upper limb’s role in more refined and diverse functions, the recovery of its dysfunction is more complex and slow, posing significant challenges to the patient’s daily life and social participation.
Nevertheless, research has demonstrated that spasticity can be effectively managed in the chronic phase of stroke through appropriate intervention, thereby enhancing motor function and facilitating the restoration of limb function (4). It is therefore imperative to identify and investigate efficacious rehabilitation techniques to facilitate enhanced recovery of upper limb function in patients. At present, there is a general consensus on the rehabilitation treatment for this condition, both domestically and internationally. The aforementioned treatments are primarily comprised of physical exercise and occupational therapy. In recent years, the advancement of medical technology and the intensification of clinical research have given rise to a multitude of novel rehabilitation therapies, including acupuncture, massage, proprioceptive neuromuscular facilitation (PNF), repetitive transcranial magnetic stimulation (rTMS), and theta-burst stimulation (TBS). A number of studies (5–8) have demonstrated that these physical therapies can facilitate the improvement of post-stroke spastic paralysis to a certain extent. However, existing research has predominantly focused on monotherapies, with insufficient comparative investigations of multimodal therapeutic regimens, leaving the optimal therapeutic combinations poorly defined.
Network meta-analysis (NMA) overcomes the limitations of traditional pairwise meta-analyses, which are restricted to comparing two interventions at a time, by integrating direct and indirect evidence to systematically evaluate the synergistic effects of complex multimodal rehabilitation strategies within a unified framework (9). This study applied NMA to compare the efficacy of 21 intervention modalities for post-stroke upper limb spasticity, aiming to provide evidence-based insights for personalized, multimodal rehabilitation protocols. A total of 49 randomized controlled trials (RCTs) involving 3,219 participants, published between 2009 and 2024, were included. Outcomes were quantified using the Fugl-Meyer Assessment for Upper Extremity (FMA-UE) for motor function and the Modified Barthel Index (MBI) for activities of daily living (ADL). A Bayesian network meta-analysis (implemented in STATA/R) was conducted to comprehensively assess efficacy differences among rehabilitation therapies, neuromodulation techniques, and integrative traditional Chinese medicine (TCM) regimens. Interventions were ranked via the surface under the cumulative ranking curve (SUCRA). The results elucidated effectiveness hierarchies through probabilistic estimates and established indirect efficacy comparison pathways for interventions lacking direct comparative data. This framework provides clinicians and patients with a scientific foundation for optimizing combined strategies of rehabilitation, neuromodulation, and TCM therapies, while bridging critical evidence gaps in the current literature on post-stroke spasticity management.
The evaluation plan of this system has been registered with the International Prospective Register of Systematic Reviews (PROSPERO) under registration number CRD42024607022. This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Network Meta-Analyses (PRISMA-NMA), as detailed in the Supplementary Appendix S1.
A systematic search of the following databases was conducted in order to identify eligible randomized controlled trials (RCTs): The following databases were searched: PubMed, Embase, Cochrane Library, Web of Science, China National Knowledge Infrastructure (CNKI), China Biomedical Literature Database (CBM), China Science and Technology Journal (VIP) database, and Wanfang Database. The search was conducted from the inception of the databases to October 2024, with the search terms limited to Chinese or English language sources. Furthermore, the reference lists of the retrieved relevant review articles were examined to ascertain whether any additional literature had been overlooked. The search strategy employed the following keywords: (“stroke” OR “cerebrovascular accident” OR “cerebral infarction” OR “cerebral haemorrhage”) AND (“spastic paralysis” OR “rigid paralysis” OR “paralysis, spastic”) AND (“upper extremity”) AND (“acupuncture” OR “massage” OR “rTMS” OR “low-frequency electrical stimulation”). Additionally, the search was conducted in Chinese databases using Chinese characters with the same meanings (shown in Supplementary Appendix S2).
The literature screening and adjustment were conducted in accordance with the inclusion and exclusion criteria set forth in Table 1.
Two researchers (JY-S and S-L) conducted the preliminary search and excluded titles and abstracts that were not pertinent to the subject matter of this review, while also cross-verifying the screening results. Furthermore, two additional researchers (MT-B and XW-S) conducted independent evaluations of the remaining titles and abstracts, obtained the full texts of these studies, and determined whether they met the inclusion criteria. They also cross-checked these results. Only after confirming that the full-text literature met the inclusion criteria was it included in the study, and the relevant Data were analyzed were then extracted. The extracted content comprised the following elements: basic study information (first author, publication year, diagnostic criteria, number of participants), study design (including sample size, specific description of interventions, type of control group, duration of treatment, treatment cycle, frequency), participant characteristics (age, gender, type of stroke and duration of stroke), outcomes, and data on the quality of the studies (randomization method, allocation concealment, implementation of blinding, loss to follow-up or withdrawal, etc.). Subsequently, an additional researcher (FY-C) undertook an independent review of the extracted data. Any discrepancies were resolved through discussion with FY-C.
The methodological quality of each study was evaluated by two researchers (MT-B and XW-S) using the Cochrane Risk of Bias tool (ROB2). The Cochrane tool identifies seven potential areas of bias, including sequence generation, allocation concealment, blinding of participants and personnel, incomplete outcome data, selective outcome reporting, and other biases. The risk of bias and quality of evidence for each domain can be categorized as low risk, unclear risk (insufficient detail or not reported), or high risk of bias. In order to assess the quality of the included literature, the Consolidated Standards of Reporting Trials (CONSORT) guidelines (10) were adopted. Furthermore, the quality of evidence for each outcome measure was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system (11), with ratings classified as high, moderate, low, or very low levels, respectively. Any discrepancies that arose during the assessment process were resolved by a third researcher (FY-C).
Pairwise meta-analyses were conducted utilizing RevMan 5.4 software (Cochrane Collaboration, Oxford, United Kingdom). In the case of continuous data, the mean difference (MD) and its 95% confidence interval (CI) were employed as a means of measuring the effect size. In the case of binary data, the effect size was evaluated using the odds ratio (OR) and its 95% confidence intervals (CIs). The extent of heterogeneity among the included studies was assessed using Cochran’s Q test (p-value) and Higgins’s I2 statistic. If p ≥ 0.05 and I2 ≤ 50%, heterogeneity was considered acceptable, and a fixed-effect model was used. Otherwise, a random-effects model was selected.
In conducting the network meta-analysis, the STATA 14.0 (Stata Corp, College Station, Texas, United States) and the R 4.3.3 (maintained by the R Core Team, Vienna, Austria) were employed to perform the requisite analysis within a Bayesian framework. In the event of a closed loop of evidence, an initial assessment of the inconsistency of the evidence was conducted. An inconsistency model was constructed to ascertain whether the p-value exceeded 0.05. If the p-value is greater than 0.05, this indicates that there is no significant inconsistency and that a consistency model should be selected for subsequent effect size estimation. Conversely, if the p-value was less than or equal to 0.05, this indicated significant inconsistency across the studies. In such cases, it was necessary to investigate the sources of inconsistency and consider the use of an inconsistency model or the implementation of sensitivity analyses to assess the potential impact of this inconsistency on the study results. In view of the potential heterogeneity of the included studies, a random-effects model was employed for the synthesis of the data. As the outcome variables of the studies were continuous, the effect size was measured using mean differences (MDs) and 95% confidence intervals (CIs). Markov Chain Monte Carlo (MCMC) methods were employed to estimate the model, with four chains configured, 20,000 iterations, and a burn-in period of 5,000, setting a thinning interval of 1. To confirm model convergence, Brooks-Gelman-Rubin diagnostics plots, chain trace plots, and probability density plots were examined. The node-splitting method was employed to assess the consistency of direct and indirect comparisons. If the resulting p-value was greater than 0.05, it was inferred that there was a higher degree of consistency. In instances where closed-loop comparisons were present, the inconsistency factor (IF) was utilized for evaluation purposes. If the 95% CI encompassed 0, this indicated that there was consistency between the direct and indirect evidence. Furthermore, the Surface Under the Cumulative Ranking (SUCRA) was calculated to probabilistically rank the various treatment interventions, with SUCRA scores ranging from 0 to 100%, where higher scores indicated superior treatment effectiveness. In analyzing the result data, consideration was given to the potential impact of baseline differences by employing a correlation coefficient R value of 0.5 in the following formula (Equations 1, 2) for estimation.
In accordance with the established inclusion criteria, our preliminary search yielded a total of 1,466 published studies. Following the preliminary review, 581 studies were identified as duplicates and subsequently removed. The remaining 885 studies were then subjected to independent examination by two researchers, with their titles and abstracts analyzed. A total of 179 studies were excluded on the basis of their irrelevance to the research question. Subsequently, a comprehensive review was conducted on the 706 selected studies, with a detailed assessment of their study design, participant population, interventions, and outcome measurements. In conclusion, a total of 49 RCTs (12–60) were included in the final analysis. The process of study selection is outlined in detail in the PRISMA flow diagram (shown in Figure 1).
The studies included in the analysis spanned a period of approximately 15 years, from September 2009 to March 2024. The studies were distributed across a number of countries, including mainland China (n = 44), Taiwan, China (n = 2), Brazil (n = 1), Turkey (n = 1) and Iran (n = 1) Of the included trials, 42 RCTs (12, 13, 15–28, 30–35, 37, 38, 41, 42, 44–55, 57–60) were two-arm designs (85.71%), 6 RCTs (14, 29, 36, 39, 40, 56) were three-arm designs (12.24%), and 1 RCTs (43) was a four-arm experiment (2.04%). The studies exhibited considerable variation in terms of sample size, duration of treatment, and intervention measures. In total, the studies recruited 3,219 participants, with 1,697 allocated to the experimental group and 1,522 to the control group. The number of participants ranged from 12 (19) to 204 (45). The baseline characteristics of the participants in the two groups were generally similar, with the average age being 60.24 years (standard deviation 9.15). However, one study (16) did not provide data on the mean age. Seven studies (15, 22, 30, 36, 43, 44, 58) did not report the mean duration of disease. Among the remaining 42 studies, the mean duration of disease ranged from (8.2 ± 6.6) days (27) to (58.9 ± 27.2) months (18). Regarding disease phases, the majority of studies (12–14, 16, 17, 20, 21, 23–26, 28, 29, 31, 33–35, 37, 38, 40–42, 45, 46, 48–57, 59, 60) (75.5%, 37/49) involved patients in the subacute phase, whereas only 4 (18, 19, 32, 39) and 1 studies (27) focused on chronic and acute phases, respectively. With the exception of one study (19) that did not provide gender information, the proportion of male participants among those who had experienced a stroke was 59.34%. Four studies (23, 29, 38, 58) provided data on patient dropout and the specific reasons for this, with the number of dropouts ranging from one to five individuals.
The included RCTs employed seven physical rehabilitation treatment methods, including physical rehabilitation (PR, encompassing exercise training and functional activity training, among others). The remaining treatments were acupuncture therapy (including body acupuncture BA and electro-acupuncture, EA), massage (M), PNF, rTMS, extracorporeal shock wave therapy (ESWT), and TBS (including continuous theta burst stimulation cTBS and intermittent theta burst stimulation iTBS). These physical rehabilitation treatments may be applied either individually or in combination, forming a total of 21 distinct treatment strategies. The core operational parameters of the interventions exhibited limited overall heterogeneity (shown in Supplementary Table S1), with the following modality-specific patterns: In the 16 studies (18–20, 25, 28, 31, 37, 39, 40, 44, 48, 52, 54, 57, 58, 60) that employed rTMS, a low-frequency stimulation pattern of 1 Hz was frequently utilized. However, there was some variation in stimulation intensity thresholds, ranging from 60 to 120% of resting motor threshold (RMT) or active motor threshold (AMT). Furthermore, the target of stimulation in all studies focused on the primary motor cortex (contralesional M1) contralateral to the lesion. Among the 6 studies (43, 46, 47, 55, 56, 59) employing ESWT, five (46, 47, 55, 56, 59) opted for a frequency of 8 Hz, while one (43) selected 5 Hz. The energy intensity was modulated based on anatomical location, with the majority of upper limb treatment parameters ranging from 1.0 to 3.0 bar. For instance, Ai et al. (56) utilized a gradient strategy (1.0–2.0 bar for the upper limb and 2.0–3.0 bar for the elbow-shoulder complex) depending on the site, whereas Chen et al. (59) employed a uniform intensity program (3.0 bar). Of the 4 studies (20, 30, 38, 40) employing TBS, all utilized 80% AMT. In acupuncture treatments, the duration of a single stimulation of BA ranged from 15–30 min, and all studies (13, 16, 21, 23, 24, 27, 29, 32–35, 41–43, 46, 47, 50, 51, 53, 55, 56, 58, 60) using BA focused on upper limb acupoints. The frequency parameters of EA exhibited a bimodal distribution, with a low-frequency group (2–5 Hz) (14, 52) and a high-frequency group (50–100 Hz) (12). Among the 8 studies (15, 22, 26, 35, 36, 41, 45, 49) employing M therapy, the single-session intervention duration ranged from 10 to 40 min, with all protocols exclusively applied to the spastic upper limb.
In terms of outcomes, a total of 41 studies (14, 15, 18, 24, 26, 27, 30, 37, 39, 49, 52, 53, 56, 59–61) employed the Fugl-Meyer Assessment-Upper Extremity (FMA-UE) scale, a tool with a maximum score of 66 points, designed to assess patients’ motor function, balance, joint pain, and range of motion. Furthermore, 34 studies (14, 19, 21–23, 25–28, 31, 32, 34–38, 40–44, 46–49, 51–53, 56–61) employed the Modified Barthel Index (MBI) scale to assess patients’ abilities in activities of daily living. The MBI has a total score of 100 points and encompasses aspects such as self-care ability, mobility, and degree of dependence. In both assessment tools, a higher score indicates superior functional performance of the patient. Additional details regarding the characteristics of the studies are presented in Table 2.
Follow-up outcomes were reported in 7 studies (18, 20, 25, 39, 46, 48, 54), revealing time-dependent therapeutic effects: A study (48) conducted post-intervention revealed that patients in the low-frequency rTMS group exhibited a significantly higher MBI scores at the 2-week follow-up when compared to the conventional group (p < 0.05), thereby suggesting an early effect of enhanced ability in performing ADL. Three studies found that at 4-week follow-up, myotonia modified Ashworth scale (MAS) scores were reduced by ≥1 in the rTMS group by up to 55.5% (18) and that combined cTBS maintained upper limb motor function and improved carpal flexor spasticity (39), but did not significantly enhance ADL independence (25). Three-month follow-up data suggest that combined rTMS with an iTBS regimen resulted in sustained improvements in motor function (20), with a significantly lower relapse rate in the observation group than in the control group (54). Furthermore, Zhang et al. (46) reported a significant improvement in self-assessed outcome (PRO) scores from baseline in both groups (p < 0.05).
The results of the bias risk assessment for the included studies are presented in Supplementary Figure S1. Three studies (23, 29, 56, 58) were classified as exhibiting a high risk of bias, six (18, 25, 30, 34, 39, 43) were deemed to have a low risk of bias, and the remaining studies were situated between these two categories, indicating a certain level of bias risk. While the majority of studies adhered to the fundamental tenets of the CONSORT statement, the absence of certain essential information is a notable shortcoming. For example, deficiencies were identified in the description of intervention similarity, discussion of trial limitations, and assessment of external validity. The reporting of blindness and allocation concealment, two fundamental methods for controlling bias, was inadequate, thereby further undermining the reliability of the trial results. Furthermore, the majority of studies did not indicate whether they had been registered, which restricts the capacity to evaluate the transparency and reliability of the trials. Further detailed assessment information can be found in Supplementary Appendix S3.
In order to evaluate the impact of different interventions on the improvement of patients’ upper limb function, a comprehensive analysis was conducted on studies utilizing the same treatment and observing the same outcome indicators. This analysis was employed to facilitate direct paired meta-analyses for the FMA-UE and MBI, with 25 and 22 studies, respectively. For the FMA-UE scores, the following interventions were compared to PR: BA (two RCTs; MD = 5.6, 95% CI: 0.90, 10, = 0.39), PR+rTMS (ten RCTs; MD = 7.2, 95% CI: 4.4, 9.9, < 0.00001), PR+ESWT (two RCTs; MD = 7.2, 95% CI: 2.4, 12, < 0.00001), PR+EA (two RCTs; MD = 12, 95% CI: 5.6, 18, < 0.00001), PR+BA (eleven RCTs; MD = 6.1, 95% CI: 4.0, 8.2, = 0.68), PR+M (five RCTs; MD = 7.2, 95% CI: 3.9, 11, < 0.00001), PR+ESWT+BA (five RCTs; MD = 7.5, 95% CI: 2.8, 12, = 0.15), all showing superior effects to PR. BA+ESWT (one RCT; MD = 11, 95% CI: 3.1, 19, < 0.00001) was more effective than BA alone, EA+rTMS (one RCT; MD = 8.3, 95% CI: 1.0, 16, < 0.00001) was more effective than EA alone, PR+M (one RCT; MD = 16, 95% CI: 5.1, 27, < 0.00001) had a better effect than M alone. In addition, PR+ESWT+BA (one RCT; MD = 4.4, 95% CI: 1.6, 7.2, = 0.51) was more effective than PR+ESWT, and PR+ESWT+BA (four RCTs; MD = 4.6, 95% CI: 1.1, 8.1, < 0.00001) was superior to PR+BA. With regard to the MBI scores, the following interventions were observed to yield enhanced outcomes in comparison to PR: PR+rTMS (eight RCTs; MD = 6.6, 95% CI: 0.074, 13, < 0.00001), PR+BA (eight RCTs; MD = 9.0, 95% CI: 2.9, 15, < 0.00001), PR+M (four RCTs; MD = 18, 95% CI: 9.5, 27, < 0.00001), PR+ESWT+BA (two RCTs; MD = 16, 95% CI: 2.9, 30, = 0.80) all demonstrated statistically significant superiority over PR. Furthermore, no statistically significant differences were identified in the comparisons between the remaining treatment measures. For further details, please refer to Table 3.
The transferability hypothesis was evaluated by means of a comparison of the FMA-UE baseline data. The results demonstrated MD = −0.0609, with 95% CI [−0.2749; 0.1531], and = 0.5772 > 0.05. This indicates that there was no statistically significant difference in the baseline FMA-UE scores among the included studies, and thus no heterogeneity. Similarly, a comparison of the MBI baseline data revealed MD = −0.1220, with 95% CI [−0.5859; 0.3419], and = 0.6063 > 0.05. This indicates that no significant heterogeneity was detected between the MBI baseline data. In light of these findings, it can be concluded that the transferability hypothesis is supported, indicating that the baseline characteristics across different studies are comparable. This provides support for the reliability of the study outcomes.
The inconsistency tests for the FMA-UE and MBI scores yielded -values of 0.7784 and 0.6056, respectively, both greater than 0.05. Consequently, a consistency model was selected for subsequent analysis. To further investigate the potential for internal distribution inconsistency, a node-splitting method was employed for additional testing. The forest plots demonstrate that there are no statistically significant differences between the direct and indirect comparisons at each split node ( > 0.05), indicating that there is no evidence of inconsistency (shown in Supplementary Figure S2). In the closed-loop inconsistency test, all 95% CIs were found to include 0, indicating a high degree of consistency in the closed-loop comparisons (shown in Supplementary Table S2). Furthermore, the Brooks-Gelman-Rubin diagnostic plots indicated that the median and 97.5th percentile of the shrinkage factor exhibited a tendency toward 1 and reached a stable state after 5,000 iterations. Subsequently, the Bayesian model computations were completed with 20,000 iterations, as illustrated in Supplementary Figure S3. Furthermore, the trajectory and density plots of the model were analyzed (shown in Supplementary Figure S4). These results consistently indicate that the model exhibited excellent convergence.
Figures 2A,B present the NMA diagrams for the impact of different treatments on FMA-UE and MBI scores, respectively. The size of the nodes in the diagrams is proportional to the number of participants in each intervention, while the thickness of the lines between nodes is proportional to the number of studies that have been conducted to make the corresponding comparisons. The largest sample sizes were observed for the PR, PR+BA, and PR+M interventions. The most frequently compared pairs were PR vs. PR+rTMS and PR vs. PR+BA.
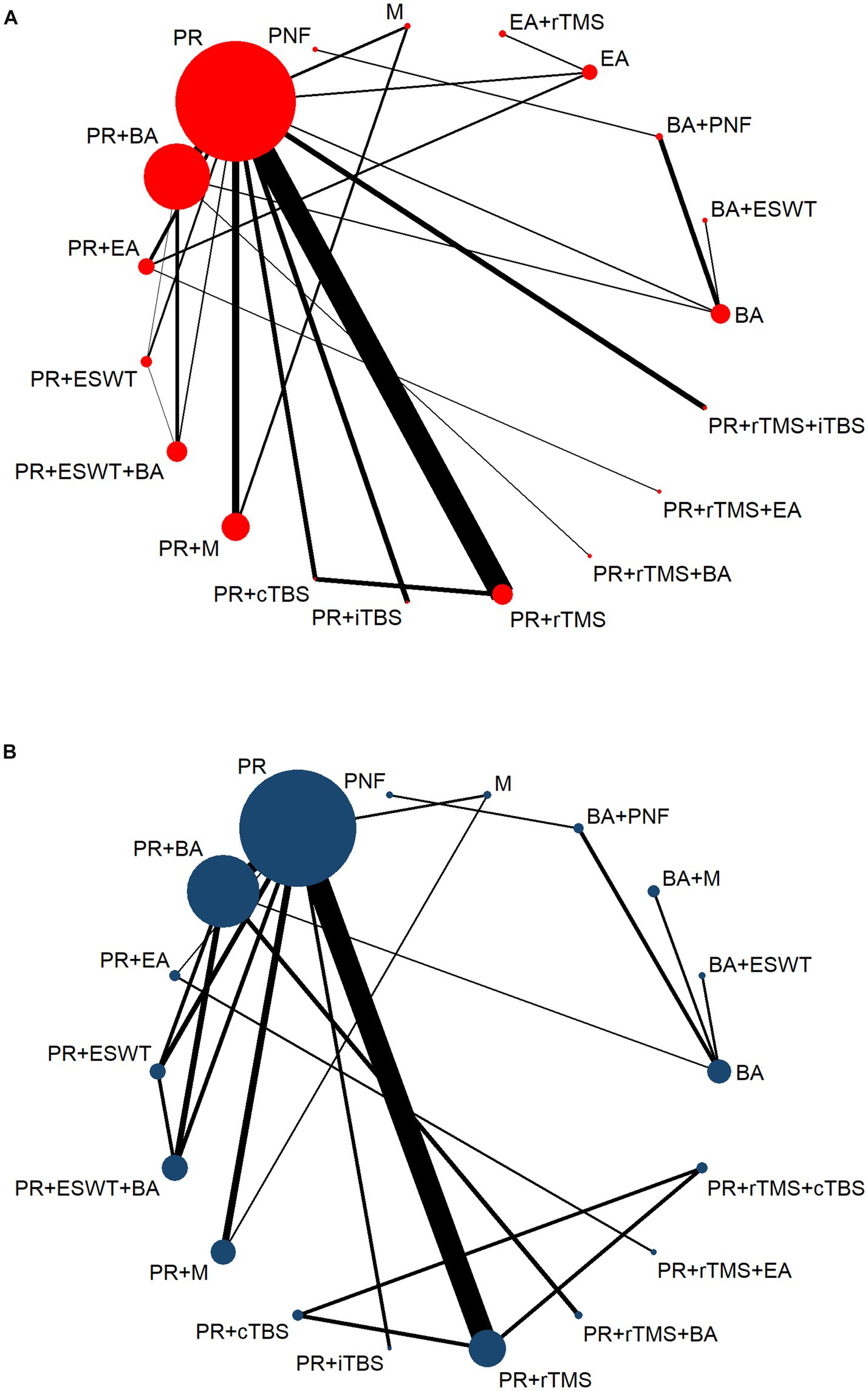
Figure 2. Network evidence diagram. PR, Physical rehabilitation; BA, Body acupuncture; EA, Electro-acupuncture; M, Massage; PNF, Proprioceptive Neuromuscular Facilitation; BA+ESWT, Body acupuncture plus extracorporeal shock wave treatment; BA+PNF, Body acupuncture plus proprioceptive neuromuscular facilitation; BA+M, Body acupuncture plus massage; EA+rTMS, Electro-acupuncture plus repetitive transcranial magnetic stimulation; PT+cTBS, Physical rehabilitation plus continuous theta burst stimulation; PT+iTBS, Physical rehabilitation plus intermittent theta burst stimulation; PT+rTMS, Physical rehabilitation plus repetitive transcranial magnetic stimulation; PT+ESWT, Physical rehabilitation plus extracorporeal shock wave treatment; PT+EA, Physical rehabilitation plus electro-acupuncture; PT+BA, Physical rehabilitation plus body acupuncture; PT+M, Physical rehabilitation plus massage; PT+rTMS+cTBS, Physical rehabilitation plus repetitive transcranial magnetic stimulation plus continuous theta burst stimulation; PT+rTMS+iTBS, Physical rehabilitation plus repetitive transcranial magnetic stimulation plus intermittent theta burst stimulation; PT+rTMS+BA, Physical rehabilitation plus repetitive transcranial magnetic stimulation plus body acupuncture; PT+ESWT+BA, Physical rehabilitation plus extracorporeal shock wave treatment plus body acupuncture; PT+rTMS+EA, Physical rehabilitation plus repetitive transcranial magnetic stimulation plus electro-acupuncture; FMA-UE, The Fugl-Meyer Assessment-Upper Extremity scale; MBI, The Modified Barthel Index scale. (A) The Fugl-Meyer Assessment-Upper Extremity scale (FMA-UE). (B) The Modified Barthel Index scale (MBI).
A league table (Supplementary Table S7) provides a summary of the comparative results of different treatment methods. The table presents the treatment effects based on FMA-UE scores in the lower triangular area and the results related to MBI scores in the upper triangular area. In order to evaluate the efficacy of treatments in improving upper limb motor function, this study compared the effects of standalone PR with various combined treatment. The findings demonstrate that the combined treatments, when compared to the standalone PR, yielded significantly enhanced outcomes in terms of FMA-UE scores. The combined treatments BA+ESWT (MD = −15.15, 95% CI: −23.75, −6.48), EA+rTMS (MD = −12.11, 95% CI: −23.18, −1.07), PR+EA (MD = −11.66, 95% CI: −17.69, −5.56), and PR+rTMS+EA (MD = −17.57, 95% CI: −27.15, −7.97) were able to increase the scores by more than 10 points. Further analysis indicates that, in comparison to the BA treatment alone, the treatments of BA+ESWT (MD = −10.76, 95% CI: −18.7, −3.09) and PR+rTMS+EA (MD = −13.12, 95% CI: −23.51, −2.78) demonstrated more pronounced improvements in FMA-UE scores. Similarly, the combination of PR+ rTMS+EA (MD = −13.68, 95% CI: −24.84, −2.75) also demonstrated superior therapeutic efficacy in comparison to EA treatment alone. Furthermore, the treatments of BA+ESWT (MD = −23.16, 95% CI: −35.43, −10.96), BA+PNF (MD = −21.79, 95% CI: −40.12, −2.79), EA+rTMS (MD = −20.04, 95% CI: −33.92, −6.17), PR+rTMS (MD = −15.11, 95% CI: −24.11, −5.86), PR+rTMS (MD = −14.97, 95% CI: −24.64, −5.16), PR+EA (MD = −19.58, 95% CI: −30.17, −8.97), PR+BA (MD = −14.15, 95% CI: −22.88, −5.13), PR+M (MD = −15.21, 95% CI: −23.7, −6.52), PR+rTMS+BA (MD = −16.69, 95% CI: −27.91, −5.3), PR+ESWT+BA (MD = −17.86, 95% CI: −27.17, −8.45), and PR+rTMS+EA (MD = −25.48, 95% CI: −38.2, −12.58) all showed significant improvements in FMA-UE scores compared to M. Similarly, the combination of PR+rTMS+EA (MD = −10.41, 95% CI: −20.38, −0.45) demonstrated superior efficacy compared to PR+rTMS. The combination of PR+rTMS+EA (MD = −11.36, 95% CI: −21.23, −1.57) demonstrated a superior therapeutic effect compared to PR+BA. The combination of PR+rTMS+EA (MD = −10.3, 95% CI: −20.29, −0.1) demonstrated superior efficacy compared to PR+M, while the combination of PR+rTMS+EA (MD = −20.21, 95% CI: −39.79, −0.81) exhibited enhanced effectiveness compared to PR+rTMS+ITBS. However, when compared to BA, M was observed to have a slightly lesser impact on the total FMA score (MD = 12.33, 95% CI: 2.72 to 21.78). Similarly, PR+BA was less effective in increasing the FMA-UE score than BA+ESWT (MD = 9.01, 95% CI: 0.32 to 17.66).
In terms of enhancing patients’ capacity to perform activities of daily living, combined treatment exhibited greater efficacy than did the use of PR alone. Specifically, PR+M (MD = 18.12, 95% CI: 9.33, 26.7), PR+rTMS+BA (MD = 19.7, 95% CI: 5.77, 33.59), and PR+ESWT+BA (MD = 17.32, 95% CI: 7.32, 27.63) were all significantly more efficacious than PR alone. Further comparison revealed that the PR+M (MD = 27.19, 95% CI: 5.68, 48.22), PR+rTMS+cTBS (MD = 28.88, 95% CI: 0.29, 57.68), PR+rTMS+BA (MD = 28.75, 95% CI: 4.79, 53.01), and PR+ESWT+BA (MD = 26.42, 95% CI: 4.16, 48.75) all demonstrated superiority over the PR+iTBS. Furthermore, the PR+M (MD = 11.51, 95% CI: 0.3, 22.18) exhibited superior outcomes in comparison to the PR+rTMS.
The SUCRA values for each intervention method were calculated in order to facilitate a probabilistic ranking. The specific data can be found in Supplementary Table S3 and Supplementary Figure S5. A probability rank histogram was constructed for the purpose of visually presenting these rankings. As illustrated in Figure 3A, the three most efficacious treatment modalities for enhancing FMA-UE scores were PR+rTMS+EA (91.1%), BA+ESWT (84%), and BA+PNF (74.8%). Figure 3B illustrates that the most efficacious three treatment protocols for enhancing patients’ activities of daily living and increasing MBI scores were PR+rTMS+BA (83.1%), PR+M (80.6%), and PR+rTMS+cTBS (79.0%). Furthermore, probability rank graphs and tables were constructed, and their outcomes corroborated those of the probability rank histograms, thus providing additional validation of the analytical findings.
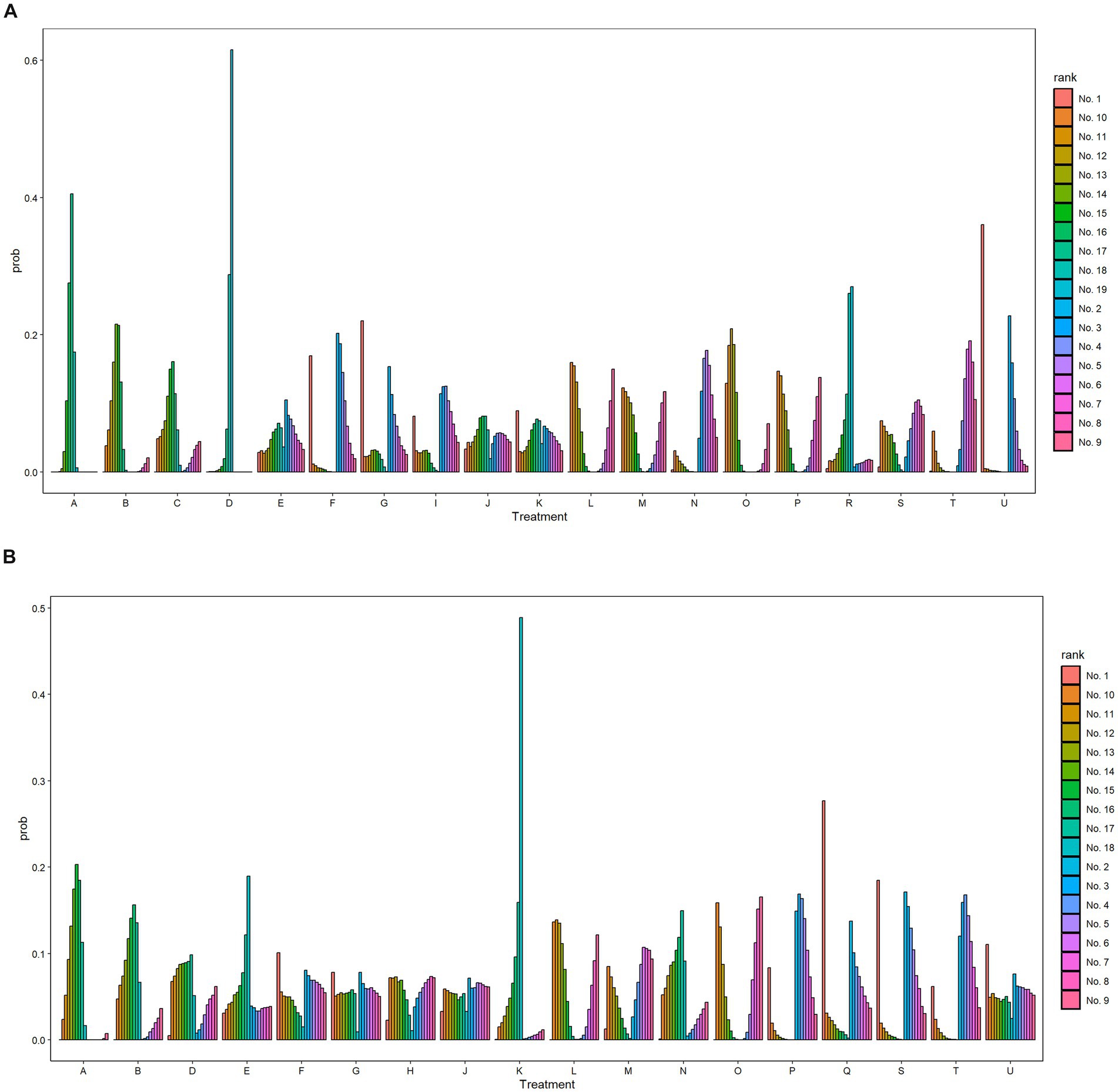
Figure 3. Probability ranking diagram. A, Physical rehabilitation; B, Body acupuncture; C, Electro-acupuncture; D, Massage; E, Proprioceptive Neuromuscular Facilitation; F, Body acupuncture plus extracorporeal shock wave treatment; G, Body acupuncture plus proprioceptive neuromuscular facilitation; H, Body acupuncture plus massage; I, Electro-acupuncture plus repetitive transcranial magnetic stimulation; J, Physical rehabilitation plus continuous theta burst stimulation; K, Physical rehabilitation plus intermittent theta burst stimulation; L, Physical rehabilitation plus repetitive transcranial magnetic stimulation; M, Physical rehabilitation plus extracorporeal shock wave treatment; N, Physical rehabilitation plus electro-acupuncture; O, Physical rehabilitation plus body acupuncture; P, Physical rehabilitation plus massage; Q, Physical rehabilitation plus repetitive transcranial magnetic stimulation plus continuous theta burst stimulation; R, Physical rehabilitation plus repetitive transcranial magnetic stimulation plus intermittent theta burst stimulation; S, Physical rehabilitation plus repetitive transcranial magnetic stimulation plus body acupuncture; T, Physical rehabilitation plus extracorporeal shock wave treatment plus body acupuncture; U, Physical rehabilitation plus repetitive transcranial magnetic stimulation plus electro-acupuncture; FMA-UE, The Fugl-Meyer Assessment-Upper Extremity scale; MBI, The Modified Barthel Index scale. (A) The Fugl-Meyer Assessment-Upper Extremity scale (FMA-UE). (B) The Modified Barthel Index scale (MBI).
A total of 16 studies (32.65%) reported adverse reactions among the 49 included studies. Twelve of the studies indicated that no adverse reactions were observed during the course of treatment. One study reported that a very small number of patients developed mild subcutaneous bruising following electroacupuncture therapy. However, these cases resolved spontaneously without the need for specialized treatment. Additionally, three studies indicated that patients experienced discomfort at the site of treatment during extracorporeal stimulation physical therapy. However, no further adverse reactions of a serious nature were reported (shown in Supplementary Table S5). Overall, extracorporeal stimulation physical therapy appears to have a favorable safety profile. Nevertheless, given the paucity of current research data, a cautious evaluation of its long-term safety and efficacy is still warranted.
To further investigate the potential publication bias and the impact of small sample sizes on the FMA-UE and MBI scores, corresponding funnel plots were constructed for analysis. As can be observed in Figures 4A,B, the adjusted funnel plots for the comparison of the FMA-UE and MBI scales both demonstrate a symmetrical distribution, with the majority of study points situated equidistant from the central guiding line on either side. This suggests that the included studies have moderate sample sizes and a low risk of publication bias.
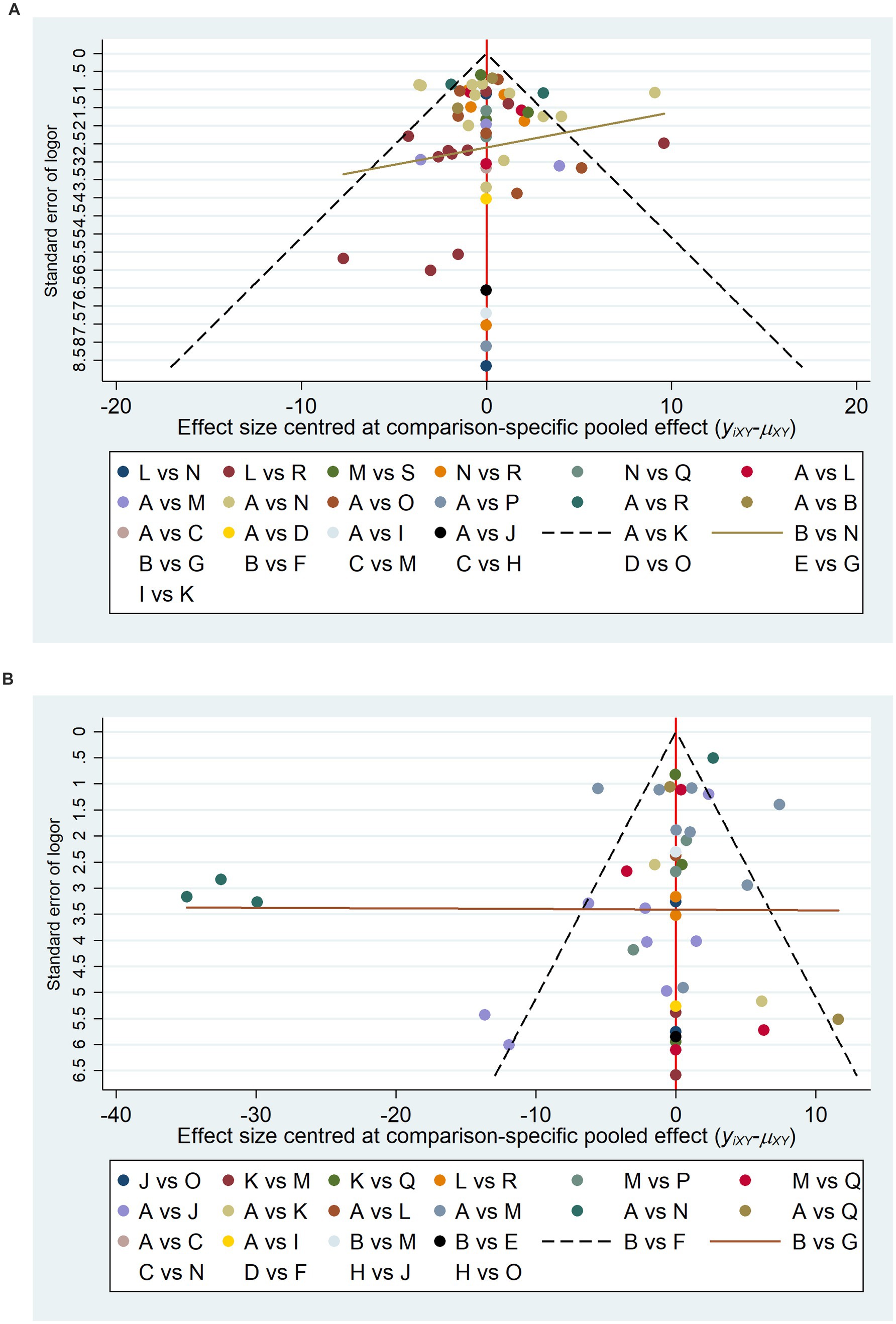
Figure 4. Comparative adjustment funnel plots. A, Physical rehabilitation; B, Body acupuncture; C, Electro-acupuncture; D, Massage; E, Proprioceptive Neuromuscular Facilitation; F, Body acupuncture plus extracorporeal shock wave treatment; G, Body acupuncture plus proprioceptive neuromuscular facilitation; H, Body acupuncture plus massage; I, Electro-acupuncture plus repetitive transcranial magnetic stimulation; J, Physical rehabilitation plus continuous theta burst stimulation; K, Physical rehabilitation plus intermittent theta burst stimulation; L, Physical rehabilitation plus repetitive transcranial magnetic stimulation; M, Physical rehabilitation plus extracorporeal shock wave treatment; N, Physical rehabilitation plus electro-acupuncture; O, Physical rehabilitation plus body acupuncture; P, Physical rehabilitation plus massage; Q, Physical rehabilitation plus repetitive transcranial magnetic stimulation plus continuous theta burst stimulation; R, Physical rehabilitation plus repetitive transcranial magnetic stimulation plus intermittent theta burst stimulation; S, Physical rehabilitation plus repetitive transcranial magnetic stimulation plus body acupuncture; T, Physical rehabilitation plus extracorporeal shock wave treatment plus body acupuncture; U, Physical rehabilitation plus repetitive transcranial magnetic stimulation plus electro-acupuncture; FMA-UE, The Fugl-Meyer Assessment-Upper Extremity scale; MBI, The Modified Barthel Index scale. (A) The Fugl-Meyer Assessment-Upper Extremity scale (FMA-UE). (B) The Modified Barthel Index scale (MBI).
Following an assessment of the pertinent outcomes using the GRADE scoring system, it was determined that the strength of evidence for the two scales under discussion ranges from very low to moderate. The principal factors responsible for the reduction in the quality of the evidence are the limitations of the study design and the considerable statistical heterogeneity. The detailed information can be found in the Supplementary Table S6.
This study used systematic review and meta-analysis methods to thoroughly investigate the impact of different interventions on improving upper limb function in stroke patients. The results showed that a variety of combined treatment regimens significantly outperformed PR alone in improving FMA-UE scores and MBI scores.
In terms of improving upper limb motor function, the efficacy of combined treatments such as PR in conjunction with rTMS, ESWT, and EA, among others, is superior to that of standalone PR. This indicates that combined treatment strategies have significant advantages in enhancing upper limb motor function in stroke patients. Among these, the PR+rTMS+EA (MD = −17.57, 95% CI: −27.15, −7.97, SUCRA = 91.1%) regimen has demonstrated the most outstanding performance in increasing the FMA-UE score. This regimen incorporates three distinct treatment modalities: PR, rTMS, EA. These three approaches may act on the central and peripheral nervous systems through different mechanisms, resulting in additive or synergistic effects that promote the recovery of upper limb motor function. rTMS can modulate the release and expression of neurotransmitters, regulate the excitability of the cerebral cortex, and improve neural inflammation by modulating the activation and polarization of astrocytes and microglia (61). This may facilitate the reorganization of damaged neural networks and enhance motor control abilities. Electroacupuncture has been demonstrated to enhance the area of cerebral infarction and downregulate the expression of various inflammatory factors by stimulating specific acupoints, thereby further promoting neural repair and regeneration (62). Furthermore, a meta-analysis (63) has demonstrated that the combination of EA and rehabilitation training represents an efficacious approach to the reduction of post-stroke limb spasticity.
The NMA also demonstrated that the combination of PR with rTMS and BA yielded the most favorable outcomes for improving activities of daily living (MD = 19.7, 95% CI: 5.77, 33.59, SUCRA = 83.1%). Concurrently, combined treatment regimens, including PR+M (MD = 18.12, 95% CI: 9.33, 26.7, SUCRA = 80.6%) and PR+rTMS+cTBS (MD = 28.88, 95% CI: 0.29, 57.68, SUCRA = 79.0%), demonstrated remarkable efficacy, exhibiting superior outcomes compared to standalone physical therapy. This may be due to the fact that these combined treatment strategies can facilitate functional recovery through a number of different mechanisms. To illustrate, rTMS has been demonstrated to stimulate the cerebral cortex (61), thereby promoting neural remodeling. Meanwhile, BA has been shown to alleviate muscle spasticity and improve joint mobility (64). M has been demonstrated to enhance blood circulation and relieve muscle tension (65). Furthermore, the combination of rTMS+cTBS has the capacity to stimulate multiple brain regions simultaneously, thereby producing a broader neural effect (66, 67). It can therefore be concluded that these combined treatment strategies can complement each other, generating a synergistic effect and thus more effectively improving activities of daily living.
The results clearly demonstrate that combined treatments have a significant impact on patients’ motor function and also markedly enhance their activities of daily living. The combined treatment strategies have a positive impact on patients through multifaceted functional improvements, including but not limited to increasing muscle strength, improving joint mobility, enhancing coordination and balance, as well as promoting neuroplasticity and functional recovery. In contrast, a standalone physical therapy program may not address all the issues that patients face in a comprehensive manner. It can therefore be concluded that combined treatment regimens, which adopt a more comprehensive approach, represent a superior choice for enhancing patients’ activities of daily living.
It is notable that this study did not identify any significant heterogeneity during the analysis phase. This suggests that the baseline characteristics across the various studies were comparable, thereby reinforcing the reliability of the research findings. Furthermore, inconsistency tests and closed-loop inconsistency tests revealed that the model demonstrated a high degree of consistency, thereby providing additional validation for the stability of the analysis results.
Adverse events were infrequent and generally mild. Only one study reported subcutaneous bruising associated with EA, while three studies utilizing ESWT or rTMS noted transient discomfort at the stimulation site. No serious or persistent adverse events were documented, underscoring the safety of these interventions.
Follow-up outcomes demonstrated short-term rTMS efficacy (improved MBI/MAS at 2–4 weeks) and sustained motor benefits at 3 months with lower relapse rates, though ADL gains were inconsistent; PRO scores improved significantly across groups. The inconsistent ADL improvements may reflect differences in rehabilitation intensity or patient-specific functional goals.
Nevertheless, given the limited nature of the current research data, further investigation is required to evaluate the long-term safety and effectiveness of this approach.
This study has several limitations that warrant consideration. First, the generalizability of findings may be constrained by geographic bias, as over 85% of included trials were conducted in China. Regional variations in rehabilitation paradigms—such as the prevalent integration of acupuncture in Chinese practice (68, 69) versus Western preferences for botulinum toxin or robotic-assisted training (70)—may introduce cultural specificity. Future multinational studies are needed to validate the cross-cultural applicability of these interventions.
Methodological shortcomings in primary studies further limit evidence quality, including inadequate allocation concealment, insufficient blinding of assessors, and incomplete reporting of prospective protocols. To address this, future studies should prioritize rigorous methodologies, including robust allocation concealment, blinded outcome assessment, and adherence to CONSORT guidelines.
Heterogeneity in intervention parameters (e.g., rTMS intensity thresholds, acupuncture session duration) complicates direct comparisons. Standardization of intervention protocols—particularly for multimodal combinations—is critical to enhance reproducibility and cross-study comparability. Additionally, the predominance of short-term interventions (≤4 weeks in 85.7% of trials) and paucity of longitudinal follow-up data (≥6 months) constrain assessment of sustained treatment effects. Prolonged observation periods are essential to evaluate durability of therapeutic benefits and monitor potential late-onset complications.
The rehabilitation process following stroke is categorized into three distinct phases—acute, subacute, and chronic—each necessitating tailored therapeutic strategies. However, this study was unable to conduct stratified analyses across these phases or specific patient subgroups due to insufficient raw data, which may partially explain the observed heterogeneity in outcomes. This limitation likely stems from a paucity of clinical trials investigating personalized, advanced rehabilitation techniques optimized for distinct recovery stages. Future research should address this gap by conducting granular analyses stratified by stroke phase, etiology (ischemic vs. hemorrhagic), and spasticity severity to identify phase-specific optimal interventions. Such stratified investigations are critical for advancing precision rehabilitation protocols in stroke care.
Mechanistically, the neuroplastic effects of combined therapies remain incompletely understood. Multicenter trials integrating advanced neuroimaging techniques (e.g., fMRI, DTI) and biomarker profiling are warranted to map neural reorganization pathways and optimize synergistic mechanisms.
Consequently, a cautious approach should be employed when interpreting the results of this NMA.
The findings of this study suggest that, in comparison to single treatment modalities, combined therapies are more efficacious in improving motor dysfunction and markedly enhancing the daily living abilities of patients with upper limb spasticity following a stroke. The combined physical rehabilitation and rTMS and EA appear to offer a notable advantage in terms of increasing FMA-UE scores and alleviating upper limb spasticity. The combination of physical rehabilitation with rTMS and BA may represent the optimal treatment approach for enhancing functional outcomes as measured by the MBI and improving patients’ daily living abilities. Nonetheless, it is imperative to exercise caution when interpreting these findings, given the limited number and questionable quality of the extant studies. A future imperative is to undertake additional high-quality studies that will facilitate the validation of the findings and the further exploration of the long-term efficacy and safety of combination therapies.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
MB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. FC: Data curation, Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing. HS: Data curation, Methodology, Supervision, Writing – review & editing. ZL: Data curation, Methodology, Supervision, Writing – review & editing. XS: Conceptualization, Writing – review & editing. YL: Data curation, Formal analysis, Writing – review & editing. JS: Validation, Visualization, Writing – review & editing. SL: Validation, Visualization, Writing – review & editing. RR: Validation, Visualization, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by a grant from the National Natural Science Foundation of China (No. 81603684) and the Qinghai Traditional Chinese and Tibetan Medicine Research Project (No. J2024020). The funders’ roles in the study included support for the study design, data collection, data analysis, and manuscript writing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1554583/full#supplementary-material
1. Raghavan, P . Upper limb motor impairment after stroke. Phys Med Rehabil Clin N Am. (2015) 26:599–610. doi: 10.1016/j.pmr.2015.06.008
2. Flinn, SR, and Craven, K. Upper limb casting in stroke rehabilitation: rationale, options, and techniques. Top Stroke Rehabil. (2014) 21:296–302. doi: 10.1310/tsr2104-296
3. Frenkel-Toledo, S, Levin, MF, Berman, S, Liebermann, DG, Baniña, MC, Solomon, JM, et al. Shared and distinct voxel-based lesion-symptom mappings for spasticity and impaired movement in the hemiparetic upper limb. Sci Rep. (2022) 12:10169. doi: 10.1038/s41598-022-14359-8
4. Li, S, Francisco, GE, and Rymer, WZ. A new definition of Poststroke spasticity and the interference of spasticity with motor recovery from acute to chronic stages. Neurorehabil Neural Repair. (2021) 35:601–10. doi: 10.1177/15459683211011214
5. Fan, W, Kuang, X, Hu, J, Chen, X, Yi, W, Lu, L, et al. Acupuncture therapy for poststroke spastic hemiplegia: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Clin Pract. (2020) 40:101176. doi: 10.1016/j.ctcp.2020.101176
6. Xu, P, Huang, Y, Wang, J, An, X, Zhang, T, Li, Y, et al. Repetitive transcranial magnetic stimulation as an alternative therapy for stroke with spasticity: a systematic review and meta-analysis. J Neurol. (2021) 268:4013–22. doi: 10.1007/s00415-020-10058-4
7. Cabanas-Valdés, R, Calvo-Sanz, J, Serra-Llobet, P, Alcoba-Kait, J, González-Rueda, V, and Rodríguez-Rubio, PR. The effectiveness of massage therapy for improving sequelae in post-stroke survivors. A systematic review and Meta-analysis. Int J Environ Res Public Health. (2021) 18:189. doi: 10.3390/ijerph18094424
8. Sharififar, S, Shuster, JJ, and Bishop, MD. Adding electrical stimulation during standard rehabilitation after stroke to improve motor function. A systematic review and meta-analysis. Ann Phys Rehabil Med. (2018) 61:339–44. doi: 10.1016/j.rehab.2018.06.005
9. Zhao, T, Tang, C, Yan, H, Wang, H, and Guo, M. Comparative efficacy and acceptability of non-pharmacological interventions for depression among people living with HIV: a protocol for a systematic review and network meta-analysis. PLoS One. (2023) 18:e0287445. doi: 10.1371/journal.pone.0287445
10. Schulz, KF, Altman, DG, and Moher, D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. (2010) 340:c332. doi: 10.1136/bmj.c332
11. Guyatt, GH, Oxman, AD, Vist, GE, Kunz, R, Falck-Ytter, Y, Alonso-Coello, P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
12. Chu, G, and Yi, X. Clinical observation of electroacupuncture combined with rehabilitation therapy in the treatment of spastic paralysis due to stroke. Hubei J TCM. (2009) 318:13–4. doi: 10.3969/j.issn.1000-0704.2009.08.006
13. Xu, Y, and Jin, J. Effects of acupuncture treatment on post-stroke upper limb spasticity. Chin J Nat Med. (2010) 1201:44–6. doi: 10.16505/j.2095-0136.2010.01.013
14. Bao, Y, Wang, Y, Chu, J, Zhu, G, Wang, C, and Hou, H. Clinical observation on the effect of Electroacupuncture combined with rehabilitation on pain and upper limb motor function improvement in post-stroke hemiplegic patients with shoulder pain. Chinese J Tradit Med Sci Technol. (2012) 1901:59–60.
15. Lei, M, Lv, Z, Tan, W, Huang, D, Lu, B, Wu, M, et al. The influence of Acupoint massage combined with rehabilitation therapy on the recovery of upper limb function in stroke patients with hemiplegia. Guangxi Med J. (2012) 3412:1613–1615+1618.
16. Ni, H, Cui, X, Hu, Y, Wu, Y, Huang, D, Qu, P, et al. Therapeutic observation on acupuncture plus rehabilitation for upper-limb spasticity after cerebral apoplexy. Shanghai J Acu-mox. (2012) 3111:789–91.
17. Sun, Y, and Yao, Q. Upper limb spastic hemiplegia following cerebral infarction treated by antagonistic acupuncture combined with rehabilitation. JCAM. (2013) 2905:36–8.
18. Barros Galvão, SC, Costa, B, dos Santos, R, Borba dos Santos, P, Cabral, ME, and Monte-Silva, K. Efficacy of coupling repetitive transcranial magnetic stimulation and physical therapy to reduce upper-limb spasticity in patients with stroke: a randomized controlled trial. Arch Phys Med Rehabil. (2014) 95:222–9. doi: 10.1016/j.apmr.2013.10.023
19. Motamed Vaziri, P, Bahrpeyma, F, Firoozabadi, M, Forough, B, Hatef, B, Sheikhhoseini, R, et al. Low frequency repetitive transcranial magnetic stimulation to improve motor function and grip force of upper limbs of patients with hemiplegia. Iran Red Crescent Med J. (2014) 16:e13579. doi: 10.5812/ircmj.13579
20. Wang, CP, Tsai, PY, Yang, TF, Yang, KY, and Wang, CC. Differential effect of conditioning sequences in coupling inhibitory/facilitatory repetitive transcranial magnetic stimulation for poststroke motor recovery. CNS Neurosci Ther. (2014) 204:355–63. doi: 10.1111/cns.12221
21. Zhang, L, and Zhang, L. Acupuncture combined with rehabilitation training for the treatment of upper limb spastic paralysis after stroke: a clinical observation study. Modern J Integrat Tradit Chinese Western Med. (2015) 13:1406–8. doi: 10.3969/j.issn.1008-8849.2015.13.014
22. Hao, J, and Wang, Y. Clinical observation on antagonistic muscle massage combined with Bobath in treating spastic hemiplegia of stroke. J Hubei Univ Chinese Med. (2016) 1803:98–100.
23. Xu, S, and Gu, J. Clinical observation of Jin’s three-needle acupuncture plus rehabilitation for post-stroke spastic hemiplegia. Shanghai J Acu-mox. (2016) 352:153–6. doi: 10.13460/j.issn.1005-0957.2016.02.0153
24. Li, B, Hou, Q, and Wang, X. Observation on the effect of antagonistic acupuncture combined with modified constraint -induced movement therapy in upper limb spasticity after acute cerebral infarction. China Med Herald. (2017) 1422:97–100.
25. Yang, NY, Fong, KN, Li-Tsang, CW, and Zhou, D. Effects of repetitive transcranial magnetic stimulation combined with sensory cueing on unilateral neglect in subacute patients with right hemispheric stroke: a randomized controlled study. Clin Rehabil. (2017) 31:1154–63. doi: 10.1177/0269215516679712
26. Gu, Y, Shi, J, Sun, P, and Qi, R. Clinical efficacy observation of massage and acupoint pressure in the treatment of spastic hemiplegia of upper limb after cerebral infarction. Shanghai J Tradit Chinese Med. (2018) 5206:50–2. doi: 10.16305/j.1007-1334.2018.06.016
27. Lin, F, Zheng, W, Zhang, W, Wu, Y, Liu, Z, and Teng, C. Clinical observation of acupuncture at governor vessel points and Jiaji (EX-B2) points in intervening early-stage post-stroke spastic hemiplegia. Shanghai J Acu-mox. (2018) 3706:614–8. doi: 10.13460/j.issn.1005-0957.2018.06.0614
28. Liu, Y, Wang, X, Zhang, C, Huang, D, Guo, X, Xiao, H, et al. Effects of low-frequency repetitive transcranial magnetic stimulation on upper limb spasticity after stroke: a task-state functional magnetic resonance study. Chinese J Rehabil Theory Practice. (2018) 247:828–33.
29. Wang, J, Bao, Y, Xiang, Y, Hao, C, and Hou, Z. Observation of curative effect of acupuncture of unblocking meridians and releasing spasticity plus rehabilitation on upper limb spastic paralysis after stroke. Modern J Integrat Tradit Chinese Western Med. (2018) 277:699–702. doi: 10.3969/j.issn.1008-8849.2018.07.005
30. Chen, YJ, Huang, YZ, Chen, CY, Chen, CL, Chen, HC, Wu, CY, et al. Intermittent theta burst stimulation enhances upper limb motor function in patients with chronic stroke: a pilot randomized controlled trial. BMC Neurol. (2019) 19:69. doi: 10.1186/s12883-019-1302-x
31. Liu, S, Li, Z, and Guo, G. Clinical research on the influence of low-frequency repetitive transcranial magnetic stimulation on spasticity and motor function of patients after stroke. Chinese J Rehabil Med. (2019) 3411:1328–32.
32. Shi, J, Yuan, A, Yang, J, Liu, Z, Pan, B, Gu, G, et al. Clinical effect of acupuncture combined with proprioceptive neuromuscular facilitation in treatment of upper limb spastic hemiplegia after stroke. J Anhui Univ Chinese Med. (2019) 383:16. doi: 10.3969/j.issn.2095-7246.2019.03.016
33. Zhou, P, Dong, X, Deng, Y, Wang, X, and Jie, W. Clinical study on the treatment of upper limb spastic hemiplegia after stroke with acupuncture combined with rehabilitation training. J External Ther TCM. (2019) 281:50–2. doi: 10.3969/j.issn.1006-978X.2019.01.029
34. Dang, Y . The impact of acupuncture combined with rehabilitation training on spasticity and quality of life in treating upper limb spastic paralysis after stroke. J Pract Tradit Chinese Med. (2020) 361:100–1.
35. Ma, J, Xu, J, and Chu, J. Clinical observation on spastic paralysis of upper limbs in ischemic stroke treated by acupuncture combined with meridian tendon massage. Tianjin J TCM. (2020) 374:434–7. doi: 10.11656/j.issn.1672-1519.2020.04.18
36. Wen, D, Yuan, H, Shi, G, Dai, Y, Qin, X, and Ma, X. Effect of mirror therapy combined with meridian massage on upper limb function in patients with cerebral infarction and hemiplegia. Chinese J Pract Nerv Dis. (2020) 2306:524–8. doi: 10.12083/SYSJ.2020.06.033
37. Chen, Q, Huang, H, and Chen, Z. Effects of low- frequency repetitive transcranial magnetic stimulation combined with MOTOmed gracile on upper limb spasticity after stroke. Chinese J Rehabil Med. (2021) 364:437–42.
38. Chen, Y, Wei, QC, Zhang, MZ, Xie, YJ, Liao, LY, Tan, HX, et al. Cerebellar intermittent Theta-burst stimulation reduces upper limb spasticity after subacute stroke: a randomized controlled trial. Front Neural Circuits. (2021) 15:655502. doi: 10.3389/fncir.2021.655502
39. Kuzu, Ö, Adiguzel, E, Kesikburun, S, Yaşar, E, and Yılmaz, B. The effect of sham controlled continuous Theta burst stimulation and low frequency repetitive transcranial magnetic stimulation on upper extremity spasticity and functional recovery in chronic ischemic stroke patients. J Stroke Cerebrovasc Dis. (2021) 30:105795. doi: 10.1016/j.jstrokecerebrovasdis.2021.105795
40. Li, D, Cheng, A, Zhang, Z, Sun, Y, and Liu, Y. Effects of low-frequency repetitive transcranial magnetic stimulation combined with cerebellar continuous theta burst stimulation on spasticity and limb dyskinesia in patients with stroke. BMC Neurol. (2021) 21:369. doi: 10.1186/s12883-021-02406-2
41. Lin, B . Observation on the effect of acupuncture combined with Meridian and muscle massage in treating upper limb spastic paralysis in patients after ischemic stroke. Chinese Health Care. (2021) 3912:7–8.
42. Liu, Q . Influence of Tiao Shen Jie Jing acupuncture combined with PNF therapy on the rehabilitation of upper limb spastic hemiplegia patients after stroke. J Med Theor Prac. (2021):3410.
43. Wei, C, Wang, D, and Wei, R. Effect of extracorporeal shock wave therapy combined with acupuncture on stroke patients with upper limb flexor spasticity. Neural Injury Funct Reconstruct. (2021) 1609:515–517+549. doi: 10.16780/j.cnki.sjssgncj.20210657
44. Yang, X, Zhang, C, Liu, M, Guo, X, Liu, X, and Qin, Y. Observation of the efficacy of rTMS combined with modern rehabilitation exercise therapy in treating upper limb spastic paralysis after stroke. World Latest Med Informat. (2021) 2140:138. doi: 10.3969/j.issn.1671-3141.2021.40.049
45. Zhang, Q . Analysis of the effectiveness of Acupoint massage therapy combined with rehabilitation in treating upper limb spastic hemiplegia after cerebral infarction. Chinese Manipulat Rehabil Med. (2021) 1209:1–3. doi: 10.19787/j.issn.1008-1879.2021.09.001
46. Zhang, X, Xia, W, Chen, Y, and Liu, L. Effect of extracorporeal shock wave therapy combined with acupuncture on spasticity of upper limb in stroke patients neural injury and functional reconstruction. Neural Injury Funct Reconstruct. (2021) 1603:150–3. doi: 10.16780/j.cnki.sjssgncj.20191255
47. Zhao, H, and Wang, L. Clinical observation of acupuncture combined with extracorporeal shock wave therapy for upper limb spasticity after stroke. Modern J Integrat Tradit Chinese Western Med. (2021) 3028:3162–5. doi: 10.3969/j.issn.1008-8849.2021.28.019
48. Zhao, J . Low-frequency repetitive transcranial magnetic stimulation for the treatment of hemiplegic upper limb spasticity and motor function improvement in stroke patients: a therapeutic efficacy observation. Clin Res. (2021) 2902:83–5.
49. Li, Z, Zhang, P, and Li, L. Effect of sling-massage exercise on upper limb spasticity for stroke patients at recovery stage. Chinese J Rehabil Theory Pract. (2022) 2811:1252–8.
50. Ma, A, and Xing, Y. Effects of acupuncture combined with rehabilitation training on the Electromyographic physiological indicators and motor function rehabilitation in patients with Poststroke plastic hemiplegia. Shanghai J Acu-mox. (2022) 4103:213–8. doi: 10.13460/j.issn.1005-0957.2022.03.0213
51. Tong, J, Du, H, Li, R, Zhao, S, Jin, X, You, Y, et al. Efficacy analysis of tendon-bone three-needle therapy combined with Bobath therapy in treating post-stroke spastic paralysis of upper limb. JCAM. (2022) 383:30–3. doi: 10.19917/j.cnki.1005-0779.022050
52. Jiang, Y, Wang, J, Wang, M, Jia, H, Ma, K, Chang, B, et al. The effect of Electroacupuncture combined with low-frequency repetitive transcranial magnetic stimulation on upper limb spasticity and motor function in stroke patients with hemiplegia. Guangxi J TCM. (2023) 4602:37–40.
53. Liu, H, Pi, T, and Rong, T. Clinical observation on upper limb spastic hemiplegia after stroke by using Xingnao Kaiqiao needling and rehabilitation. Acta Neuropharmacol. (2023) 133:45. doi: 10.3969/j.issn.2095-1396.2023.03.007
54. Liu, S, Ye, T, Shen, J, Yang, J, and Chen, C. The effect of repetitive transcranial magnetic stimulation combined with electroacupuncture on upper limb motor function and serum BDNF, NGF in patients with stroke hemiplegia during recovery period. Chin J Gerontol. (2023) 4311:2578–81. doi: 10.3969/j.issn.1005-9202.2023.11.005
55. Sun, X, Ma, X, Liu, H, and Ai, Y. Observation on the clinical effect of extracorporeal shock wave therapy combined with acupuncture in the treatment of flexor spasm of upper limb after stroke. CJGMCM. (2023) 3805:916–8. doi: 10.3969/j.issn.1003-8914.2023.05.035
56. Ai, Y, Liu, H, Ma, X, Sun, X, and Cheng, W. Effect of acupuncture combined with shock wave therapy on post-stroke upper-limb dysfunction. Shanghai J Acu-mox. (2023) 4210:1042–7. doi: 10.13460/j.issn.1005-0957.2023.10.1042
57. Qin, Y, Liu, X, Zhang, Y, Wu, J, and Wang, X. Effects of transcranial combined with peripheral repetitive magnetic stimulation on limb spasticity and resting-state brain activity in stroke patients. Front Hum Neurosci. (2023) 17:992424. doi: 10.3389/fnhum.2023.992424
58. Xie, W, Ye, W, Cheng, F, Zang, Q, and Chen, Y. Combining low-frequency transcranial magnetic stimulation with acupuncture in treating upper limb motor dysfunction after a stroke. Chinese J Phys Med Rehabil. (2023) 4510:888–92.
59. Chen, D, Chen, Z, Chen, S, Zhou, L, and Liu, C. Influence of extracorporeal shock wave therapy combined with exercise training on upper limb motor function and spasticity in elderly stroke patients with hemiplegia. Chin J Gerontol. (2024) 4415:3651–4. doi: 10.3969/j.issn.1005-9202.2024.15.018
60. Lei, J, Fan, X, Zang, S, Zhang, C, and Tang, J. Effect of acupuncture combined with repetitive transcranial magnetic stimulation and deep muscle stimulation on upper limb spasm after stroke and its effect on cortical blood oxygen. Shandong J TCM. (2024) 4303:231–238+247. doi: 10.16295/j.cnki.0257-358x.2024.03.003
61. Sheng, R, Chen, C, Chen, H, and Yu, P. Repetitive transcranial magnetic stimulation for stroke rehabilitation: insights into the molecular and cellular mechanisms of neuroinflammation. Front Immunol. (2023) 14:1197422. doi: 10.3389/fimmu.2023.1197422
62. Ren, X, Gao, X, Li, Z, Ding, Y, Xu, A, Du, L, et al. Electroacupuncture ameliorates neuroinflammation by inhibiting TRPV4 channel in ischemic stroke. CNS Neurosci Ther. (2024) 30:e14618. doi: 10.1111/cns.14618
63. Cai, Y, Zhang, CS, Liu, S, Wen, Z, Zhang, AL, Guo, X, et al. Electroacupuncture for poststroke spasticity: a systematic review and meta-analysis. Arch Phys Med Rehabil. (2017) 98:2578–2589.e4. doi: 10.1016/j.apmr.2017.03.023
64. Liu, X, Bao, C, and Dong, G. Using acupoint-to-acupoint penetrative needling to treat post-stroke spastic paralysis: a clinical progress review. J Tradit Chin Med. (2014) 34:609–15. doi: 10.1016/s0254-6272(15)30071-6
65. Yunhui, X, Hao, GU, Qing, Z, Dejie, LI, Ying, G, Junxing, X, et al. Efficacy of meridian massage for motor function after a stroke: a systematic review and Meta-analysis. J Tradit Chin Med. (2022) 42:321–31. doi: 10.19852/j.cnki.jtcm.2022.03.001
66. Boddington, LJ, Gray, JP, Schulz, JM, and Reynolds, JNJ. Low-intensity contralesional electrical theta burst stimulation modulates ipsilesional excitability and enhances stroke recovery. Exp Neurol. (2020) 323:113071. doi: 10.1016/j.expneurol.2019.113071
67. Bai, Z, Zhang, J, and Fong, KNK. Effects of transcranial magnetic stimulation in modulating cortical excitability in patients with stroke: a systematic review and meta-analysis. J Neuroeng Rehabil. (2022) 191:19–24. doi: 10.1186/s12984-022-00999-4
68. Zhong, LL, Zheng, Y, Lau, AY, Wong, N, Yao, L, Wu, X, et al. Would integrated Western and traditional Chinese medicine have more benefits for stroke rehabilitation? A systematic review and meta-analysis. Stroke Vasc Neurol. (2022) 7:77–85. doi: 10.1136/svn-2020-000781
69. Zhao, Q, Guo, R, Fan, Z, Hu, L, Hu, Z, and Liu, Y. Medical conditions and preference of traditional Chinese medicine: results from the China healthcare improvement evaluation survey. Patient Prefer Adherence. (2023) 17:227–37. doi: 10.2147/ppa.S398644
Keywords: spasticity, stroke, rehabilitation, review, network meta-analysis
Citation: Bian M, Chen F, Su H, Li Z, Sun X, Liu Y, Shi J, Liu S and Rong R (2025) Comparison of the effects of different physical stimulation therapies on reducing upper limb spastic paralysis and motor dysfunction in stroke survivors after stroke: a network meta-analysis of randomized controlled trials. Front. Neurol. 16:1554583. doi: 10.3389/fneur.2025.1554583
Received: 13 January 2025; Accepted: 24 March 2025;
Published: 15 April 2025.
Edited by:
Arthur Sá Ferreira, University Center Augusto Motta, BrazilReviewed by:
Yundong Shen, Fudan University, ChinaCopyright © 2025 Bian, Chen, Su, Li, Sun, Liu, Shi, Liu and Rong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fuyan Chen, ZWNmeV8yMDA1QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.