- 1Department of Rehabilitation Medicine, International St. Mary's Hospital, Catholic Kwandong University College of Medicine, Incheon, Republic of Korea
- 2International St. Mary's Hospital, Catholic Kwandong University College of Medicine, Incheon, Republic of Korea
Introduction: One of the possible treatment options for patient with cognitive dysfunction is cognitive telerehabilitation. Previous systematic reviews on cognitive telerehabilitation have focused on specific disease groups and the analysis of intervention methods did not differentiate between traditional face-to-face cognition treatment and usual care. In this systematic review, we aim to analyze randomized controlled trials (RCTs) that compare telerehabilitation with face-to-face treatment or usual care for improving cognitive function in elderly individuals with cognitive dysfunction or patients with acquired brain injury.
Methods: We conducted this systematic review following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). In this systematic review, we searched 7 electronic databases (PubMed, Cochrane, EMbase, CINAHL, Web of Science, Scopus, KMbase) to identify relevant studies published through December 10, 2024. We conducted a meta-analysis to assess the quality of the studies and synthesize the evidence. Certainty of evidence was evaluated using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) method.
Results: Finally, 16 studies were included in the analysis. For comparing telerehabilitation with face-to-face cognition treatment, the meta-analysis included 2 RCTs for global cognition (immediate outcome), 2 RCTs for attention (immediate outcome), 2 RCTs for visuospatial function (immediate outcome). For comparing telerehabilitation with usual care, the meta-analysis included 7 RCTs for global cognition (immediate outcome), 3 RCTs for global cognition (persistence outcome), 4 RCTs for attention (immediate outcome), 3 RCTs for executive function (immediate outcome), 3 RCTs for working memory (immediate outcome), 3 RCTs for visuospatial function (immediate outcome).
Discussion: Telerehabilitation has been shown to be more effective than usual care in improving global cognitive function, and its effectiveness is not inferior to that of traditional face-to-face cognitive treatment. By overcoming the limitations of traditional cognition rehabilitation and providing continuous treatment, telerehabilitation can offer effective treatment in specific situations.
Introduction
Cognitive dysfunction is the result of age-related neurodegenerative changes (1). It is also a major complication in acquired brain injury, such as stroke, or traumatic brain injury (TBI) (2, 3). Cognitive dysfunction interferes with functional abilities and activities of daily living (ADLs), thereby reducing people’s quality of life (QOL) and participation in society (4). Mild cognitive impairment (MCI) occurs in 15–20% of elderly, and it is known that 8–15% of those with MCI progress to dementia each year (5). Cognitive dysfunction is reported to occur in 70% of overall stroke survivors, 15% of mild TBI patients, and 65% of moderate-to-severe TBI patients (2, 3).
Cognitive rehabilitation interventions for patients with cognitive dysfunction is essential, with a particular emphasis on early implementation to preserve and enhance individual’s independence in ADLs (6). Cognitive training has also been recognized as an effective intervention strategy for improving or preserving cognitive function in patients with cognitive dysfunction (1). It can address both physiological and pathological neurodegenerative processes by stimulating the brain’s compensatory mechanisms (1). Therefore, appropriately designed cognitive training programs can effectively activate the neural systems involved in sensory and cognitive processing and take advantage of the brain’s plasticity to restore brain and cognitive function to a normal state (7).
However, traditional face-to-face cognition treatment approaches have the disadvantage of accessibility issues (8). Barriers to treatment involve restricted service accessibility, especially post-transition from hospital to home, limited mobility due to physical and cognitive impairments, and reduced levels of participation in the face-to-face cognition rehabilitation programs (9, 10). These accessibility issues interrupt the continuity of treatment. These issues have been exacerbated by the COVID-19 pandemic, underscoring the need for additional training options for maintaining continuity of rehabilitation (1). Telerehabilitation has emerged as a promising approach to address numerous challenges, including dependence on caregivers, financial constraints, insufficient access to local medical resources, and transportation difficulties and has shown high participant satisfaction (10, 11). In the field of cognitive treatment, telerehabilitation is emerging as a promising treatment alternative to traditional face-to-face cognition rehabilitation, through technological advances (12). Telerehabilitation is defined by the American Telemedicine Association as the delivery of rehabilitation services using information and communication technologies, and utilizes telecommunications, remote sensing, and operational technology to deliver medical rehabilitation services remotely (6, 13). This approach improves accessibility, providing more effective treatment opportunities, allowing treatment to continue despite spatial constraints (14).
To the best of our knowledge, previous systematic reviews on cognitive telerehabilitation have focused on specific disease groups and the analysis of intervention methods did not differentiate between traditional face-to-face cognition treatment and usual care. The aim of this systematic review is to analyze and synthesize evidence on the efficacy of cognitive telerehabilitation treatment in patients with cognitive dysfunction and compare it to conventional face-to-face cognition treatment group or usual care group.
Materials and methods
Review question
Does cognitive telerehabilitation improve cognitive function (attention, memory, visuospatial, executive function), activities of daily living, quality of life in patients with cognitive dysfunction?
This literature review aims to assess studies of various forms of telerehabilitation in patients with cognitive dysfunction.
Registration of the study protocol
We conducted this systematic review following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and flow diagram. The protocol of this review was registered in the International Prospective Register of systematic reviews (PROSPERO) under the following registration number CRD 42023454250 and can be accessed in its entirety on the program website.1
Criteria for this review (PICO)
1. Patients (P): Patients with cognitive dysfunction (stroke, traumatic brain injury, neurodegenerative diseases, cognitive dysfunction).
2. Intervention (I): Telerehabilitation.
3. Comparison (C): Face-to-face cognition treatment or Usual care.
4. Outcomes (O): Cognition (memory, attention, executive function, visuospatial function), activities of daily living (ADLs), quality of life (QOL).
Usual care was defined as receiving no treatment, sham treatment, etc., while the face-to-face treatment was defined as receiving traditional therapy provided directly by a therapist. Further details are presented in Table 1.
Search and selection
Publications were searched in PubMed, Cochrane, EMbase, CINAHL, Web of Science (WOS), Scopus, KMbase. For comprehensive literature search, the scope of the search did not specify a start date and the end date was December 10, 2024. The review included publications in English and Korean. Detailed search terms are provided in Supplementary material 1. Two reviewers (H.J, H.H) independently conducted the study selection and data extraction. During the screening phase, when the relevance to the topics is ambiguous based on the title and abstract, a partial review of full text was conducted. Studies meeting the inclusion criteria were included in the review, while those not meeting them were excluded from the review process. A discussion or 3rd reviewer was utilized to resolve conflicts. After screening, the full text was reviewed by two reviewers and excluded studies were described with reasons for their exclusion. Based upon the PRISMA 2020 checklist, the review process was described in the flow chart and the following cases were excluded during the literature screening:
1. Studies published as abstracts only, or those for which the full text is not accessible due to reasons such as being unpublished or inaccessible in the original language (non-English/ non-Korean).
2. Studies that do not correspond to the PICO criteria.
3. Studies that do not match the predefined types of research selected for this study.
Risk of bias (RoB) assessment
Two reviewers (S.P, D.Y.K) independently reviewed the full text for quality assessment. The risk of bias of included studies was assessed using Cochrane revised tool for Risk of Bias in randomized trials (RoB 1.0) to evaluate quality of individual studies.
Data synthesis
Treatment effects were evaluated using Mean Difference (MD) of homogeneous outcome measures or Standardized Mean Difference (SMD) when the outcomes were measured with different scales. For assessing heterogeneity intervention and outcome measures were considered, and data from the most clinically similar trials were combined for analysis. Random-effect model is used to represent an estimate of treatment effect. Subgroups analysis was done according to diagnosis.
Statistical analysis of evidence
We performed a meta-analysis using Reviewer Manager Software 5.4 (Cochrane Collaboration, Oxford, UK). A statistical analysis for continuous variable was conducted. Heterogeneity was estimated using I2, which quantifies the percentage of total variation across studies. An I2 value greater than 50.0% was considered indicative of substantial heterogeneity. The meta-analyses employed a random effects model with the inverse variance method for continuous outcome variables.
Assessment of certainty of evidence
The certainty of evidence was assessed using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) method. This method categorizes the certainty of evidence as high, moderate, low, or very low. Depending on the study design, the certainty of evidence is initially determined as ‘high’, and whether the evidence level can be lowered is determined based on specific criteria. For randomized controlled trails (RCTs), 5 factors are considered: (1) risk of bias, (2) inconsistency, (3) indirectness, (4) imprecision and (5) publication bias, and the certainty of evidence can be lowered by 1 or 2 levels. These evaluations were independently conducted by two authors and then subjected through a consensus process.
Results
Study selection
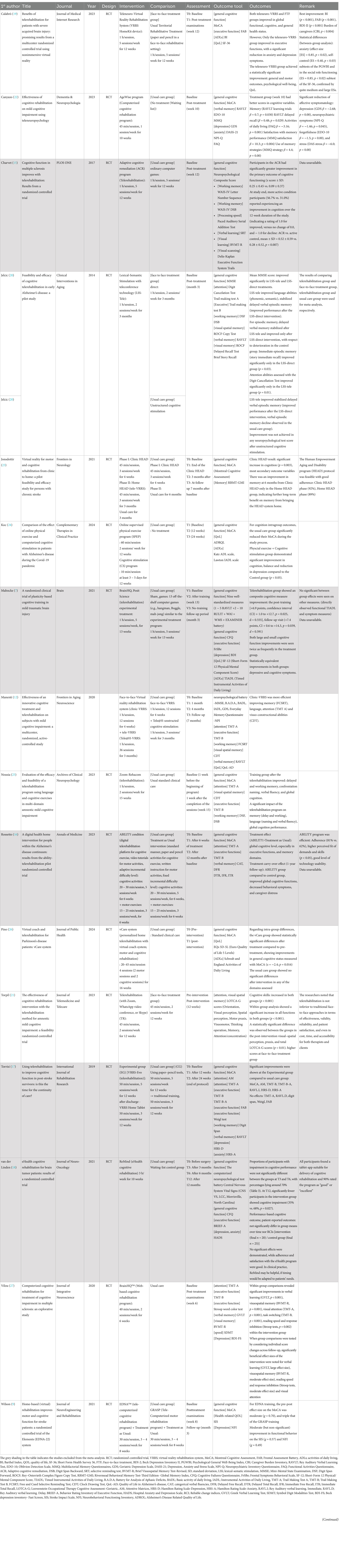
After a comprehensive literature search, 2 reviewers screened 4,338 studies for duplicate and 16 RCTs were finally selected and PRISMA flow is described in Figure 1. A description of the included studies is detailed in Table 1. Of the final selection, Charvet et al. (15), Mahncke et al. (7), Rossetto et al. (16), Torrisi et al. (17) and Van der Linden et al. (18) were excluded from the analysis because data extraction for meta-analysis was not possible. Individual data sharing requests were sent via email to the corresponding authors of these studies, but no responses were received.
Study characteristics
The studies comparing the efficacy of cognitive telerehabilitation with face-to-face cognition treatment were Calabrò (19), Jelcic (20), Torpil (21). Studies comparing cognitive telerehabilitation with usual care were Canyazo (22), Jelcic (20), Jonsdottir (23), Koc (24), Manenti (12), Nousia (25), Pino (26), Vilou (27), Wilson (9). There were no available data for meta-analysis on the other outcomes, ADLs and QOL. The Risk of bias for the studies included in the analysis is shown in Figure 2.
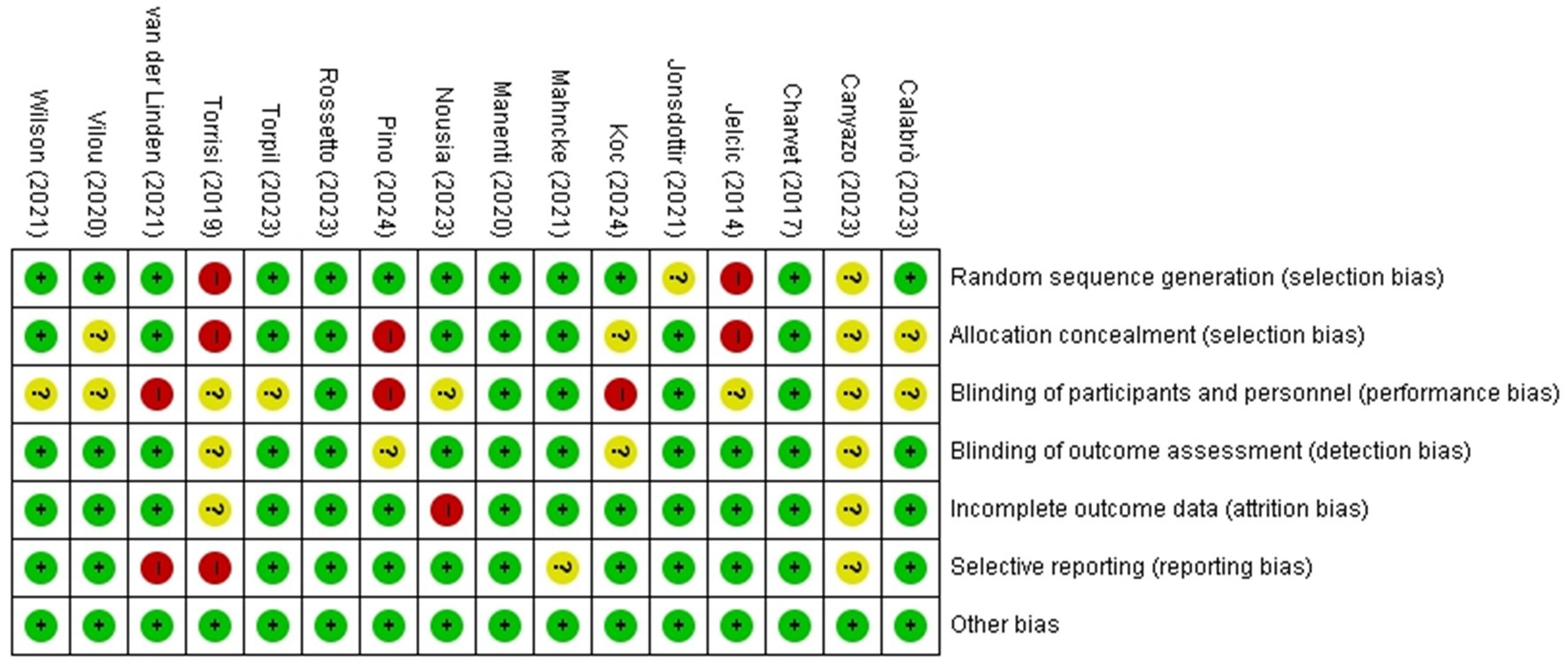
Figure 2. Risk of bias for included studies. The included studies were independently assessed and agreed by 2 reviewers using the Cochrane’s RoB of 1.0. The colors of the symbols represent the risk of bias as follows: green indicates a low risk of bias, yellow indicates an unclear risk of bias, and red indicates a high risk of bias.
Meta-analysis for effects of telerehabilitation
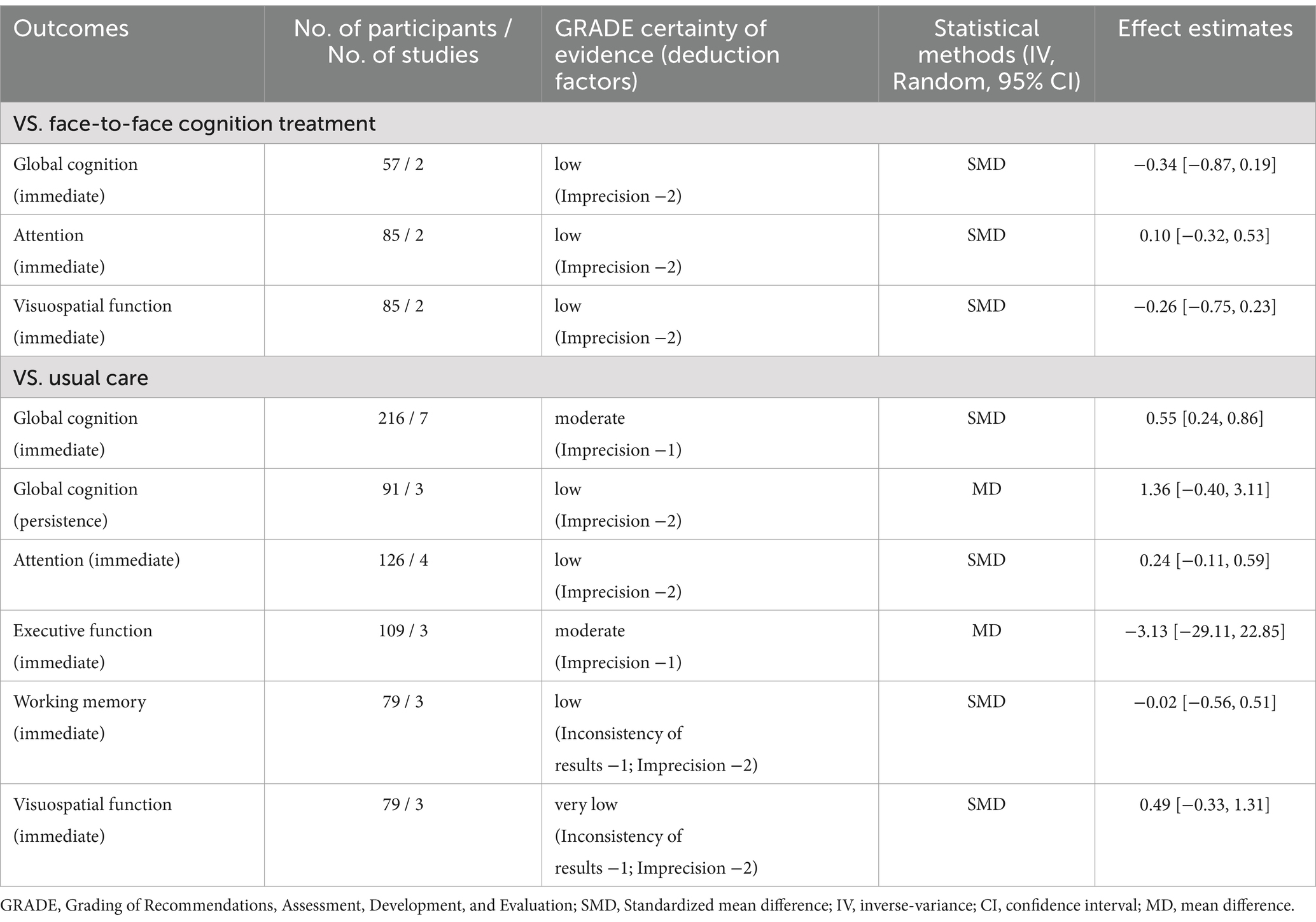
For comparing telerehabilitation with face-to-face cognition treatment, the meta-analysis included 2 RCTs for global cognition (immediate outcome), 2 RCTs for attention (immediate outcome), 2 RCTs for visuospatial function (immediate outcome). For comparing telerehabilitation with usual care, the meta-analysis included 7 RCTs for global cognition (immediate outcome), 3 RCTs for global cognition (persistence outcome), 4 RCTs for attention (immediate outcome), 3 RCTs for executive function (immediate outcome), 3 RCTs for working memory (immediate outcome), 3 RCTs for visuospatial function (immediate outcome). The evidence summaries and GRADE assessments are provided in Table 2, while forest plots of the meta-analyses are presented in Figures 3–11. Across all analyses, the 95% confidence intervals of the MD and SMD for the effectiveness of the cognitive telerehabilitation were distributed including zeros, indicating no significant difference between the interventions. We examined synthesis of evidence for cognitive telerehabilitation, and conducted subgroup analyses based on diagnosis, including stroke, mild cognitive impairment (MCI), Parkinsons’s disease, and multiple sclerosis. Out of the total 16 studies, 11 were included in the meta-analysis and analyzed by subdomain of cognition. The subdomain with the highest number of studies analyzed together was global cognition (immediate outcome), with 7 studies. Therefore, a funnel plot for assessing publication bias was not generated.
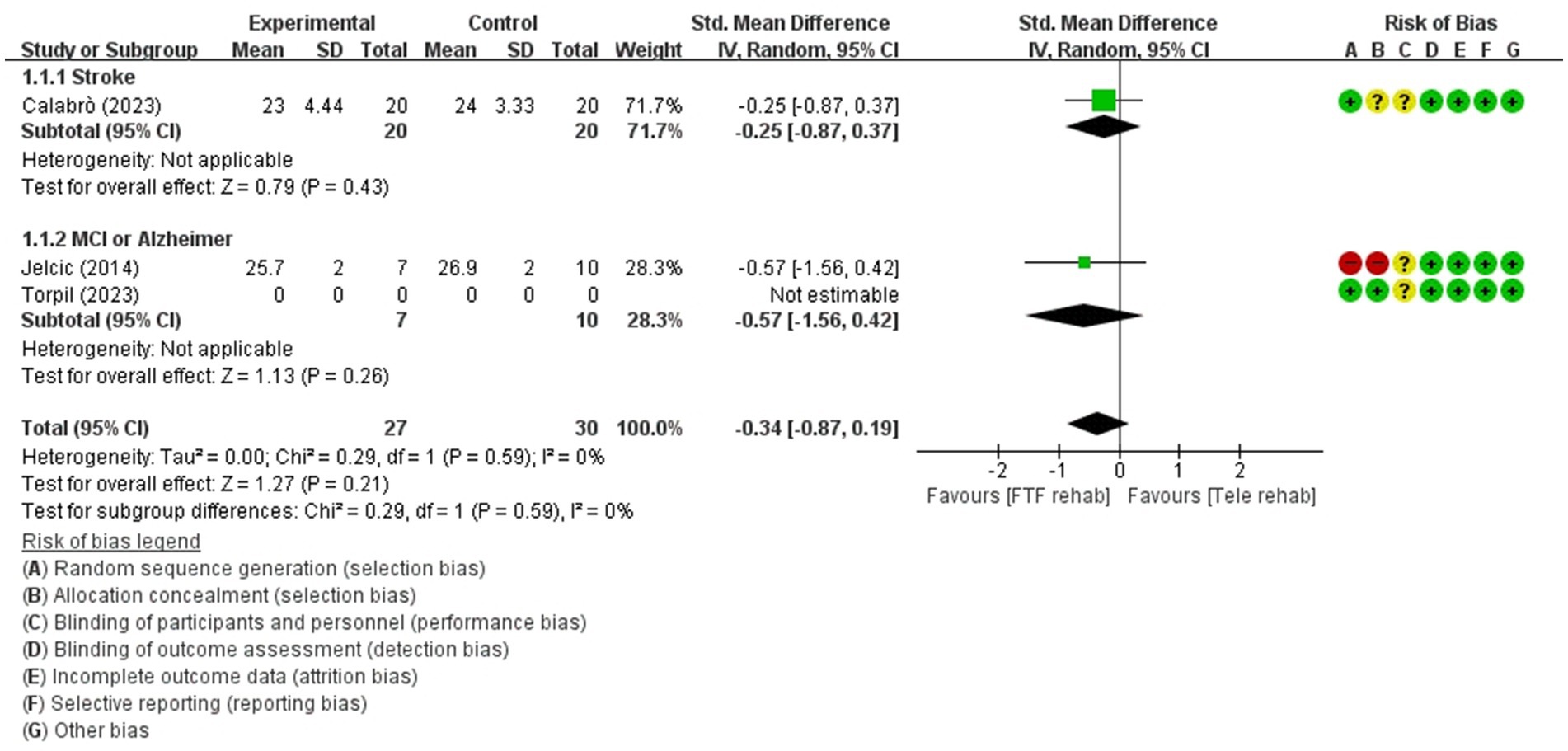
Figure 3. Forest plot of meta-analyses: telerehabilitation versus face-to-face treatment on global cognition (immediate). SD, standard deviation; IV, inverse-variance; CI, confidence interval.
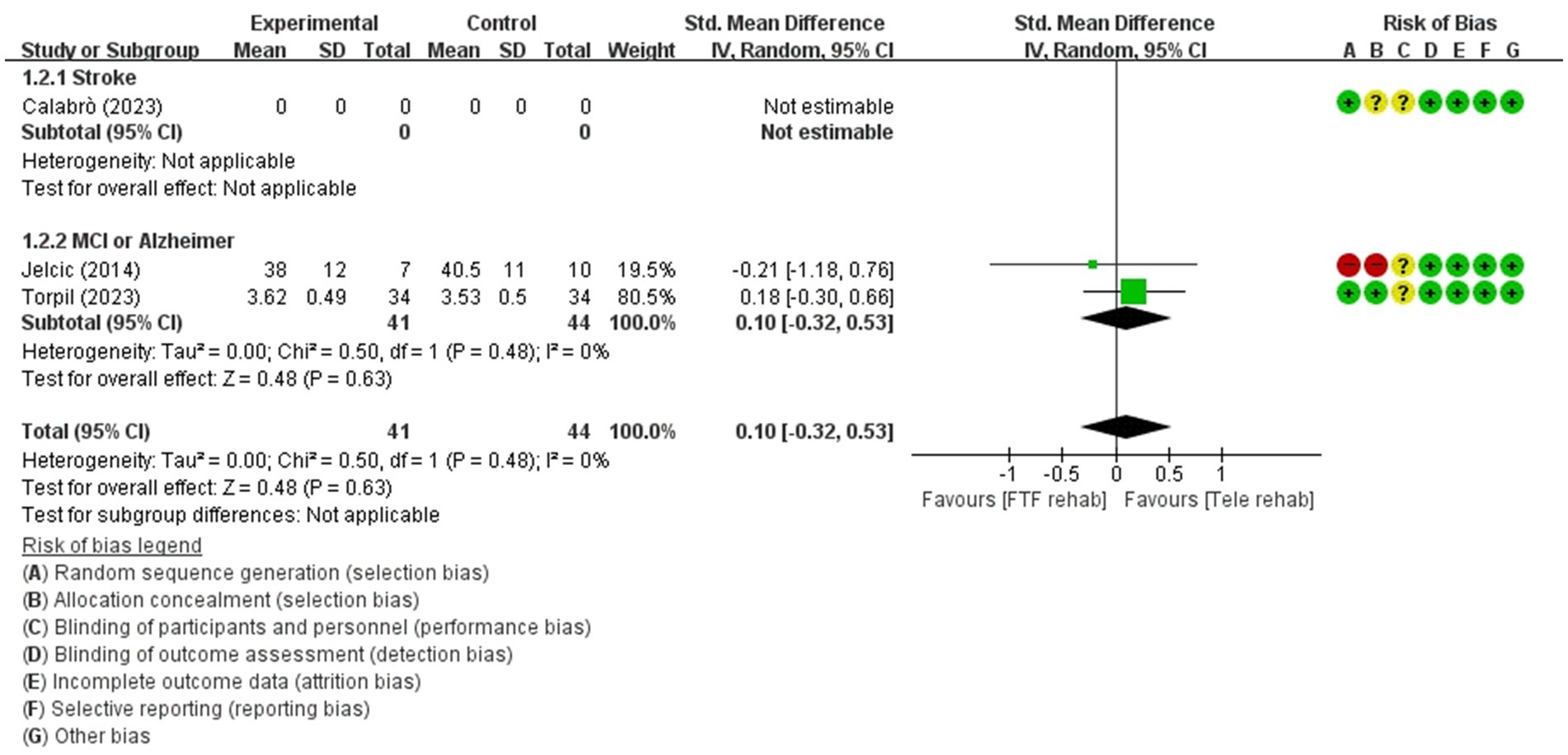
Figure 4. Forest plot of meta-analyses: telerehabilitation versus face-to-face treatment on attention (immediate). SD, standard deviation; IV, inverse-variance; CI, confidence interval.
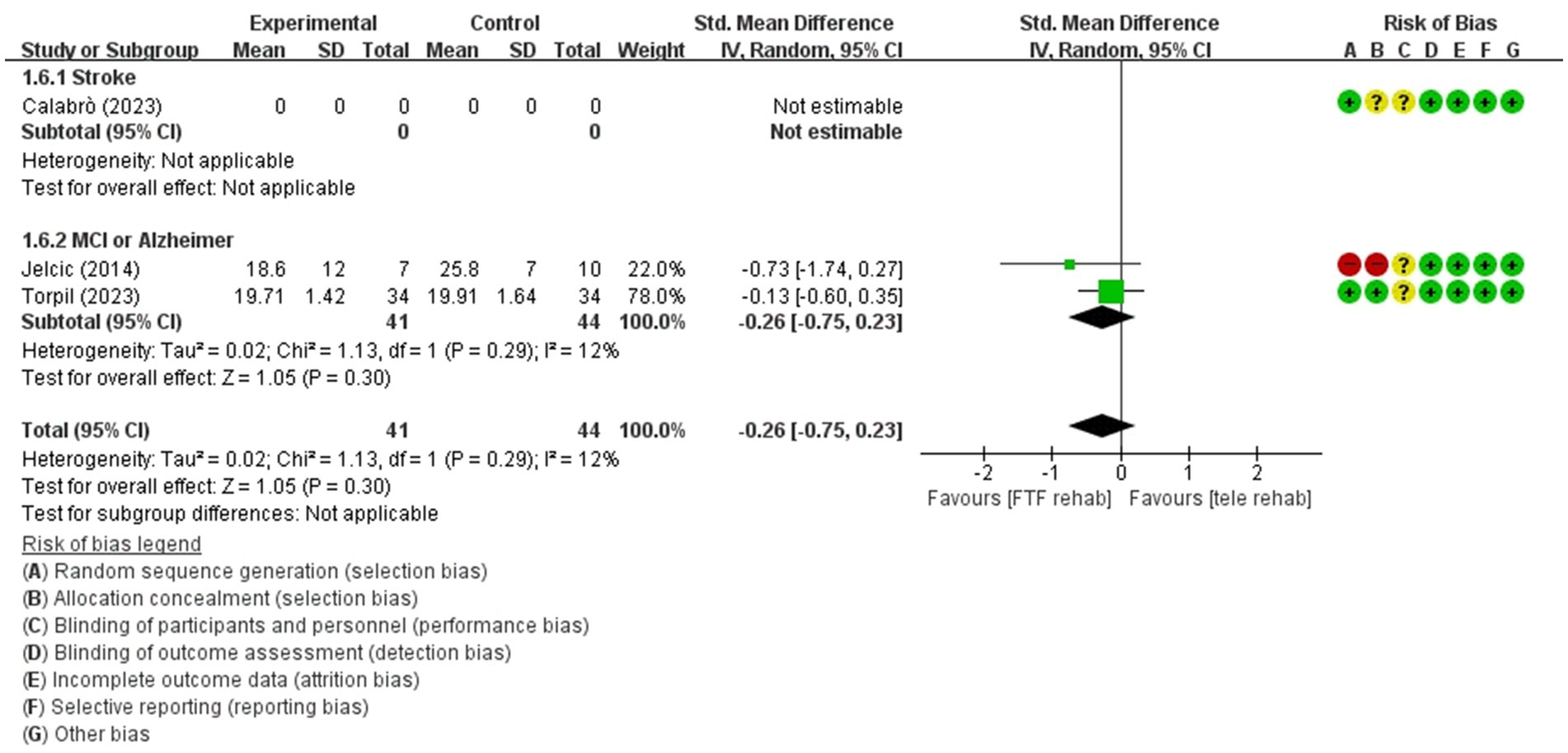
Figure 5. Forest plot of meta-analyses: telerehabilitation versus face-to-face treatment on visuospatial function (immediate). SD, standard deviation; IV, inverse-variance; CI, confidence interval.
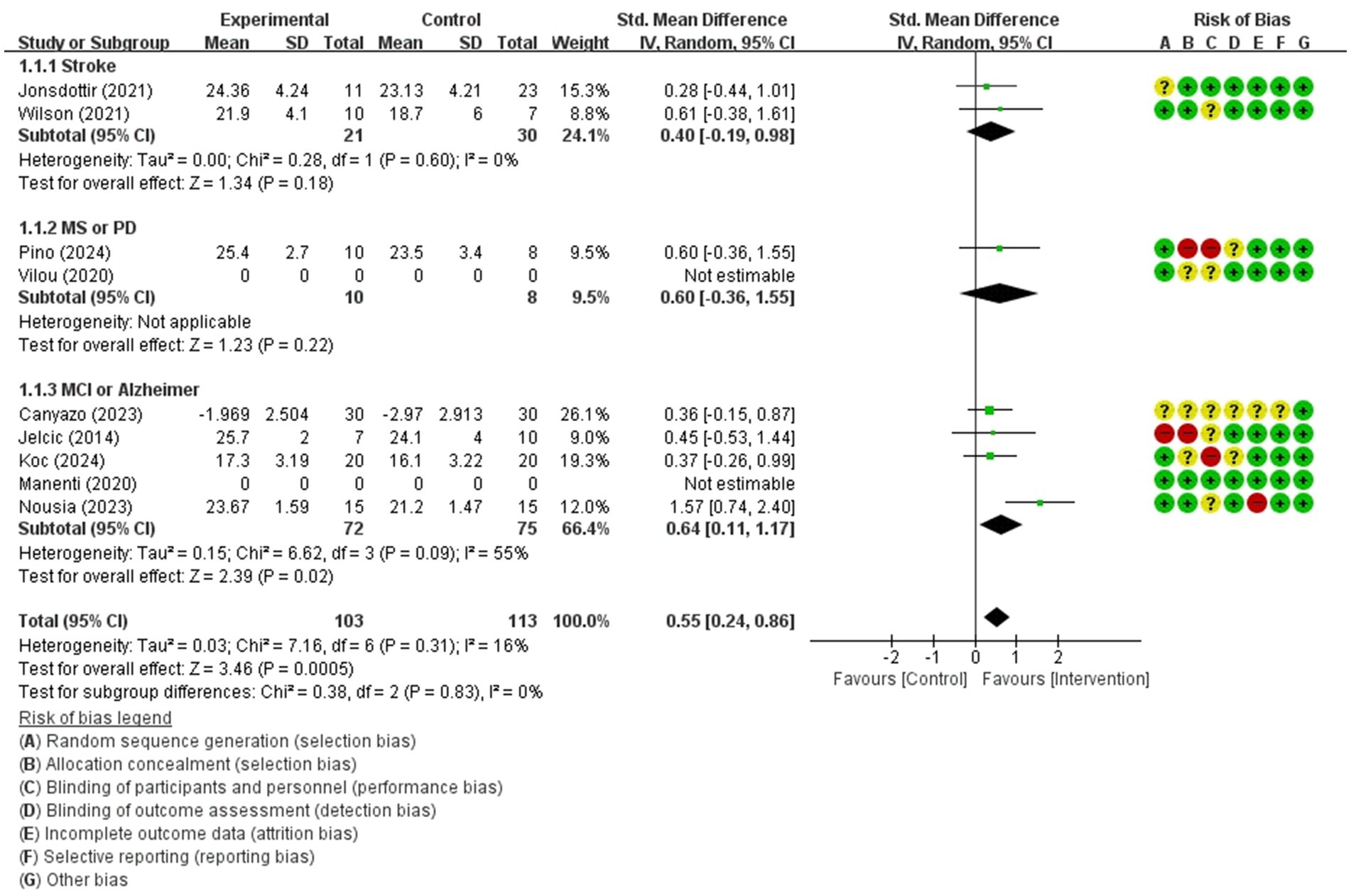
Figure 6. Forest plot of meta-analyses: telerehabilitation versus usual care on global cognition (immediate). SD, standard deviation; IV, inverse-variance; CI, confidence interval.
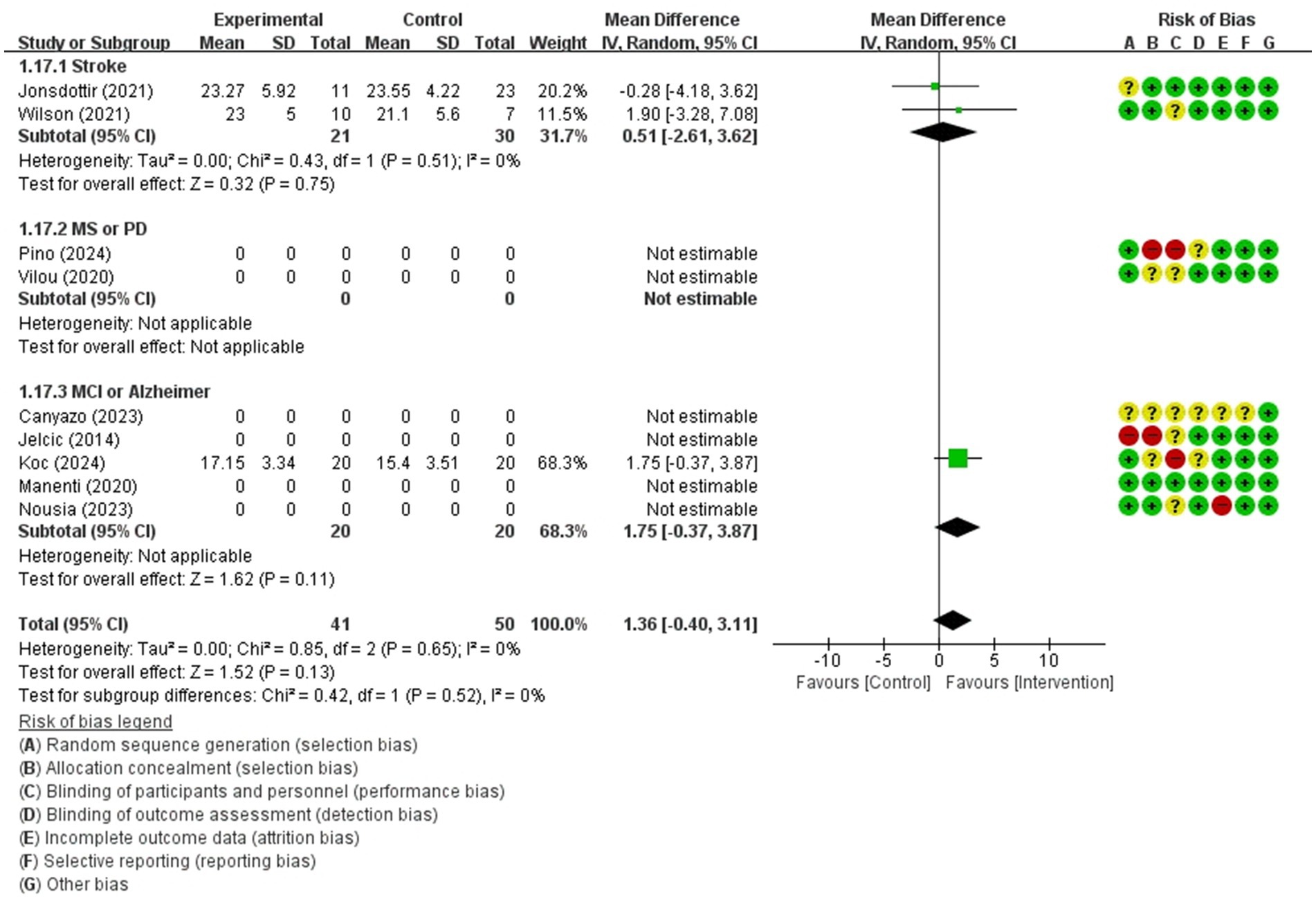
Figure 7. Forest plot of meta-analyses: telerehabilitation versus usual care on global cognition (persistence). SD, standard deviation; IV, inverse-variance; CI, confidence interval.
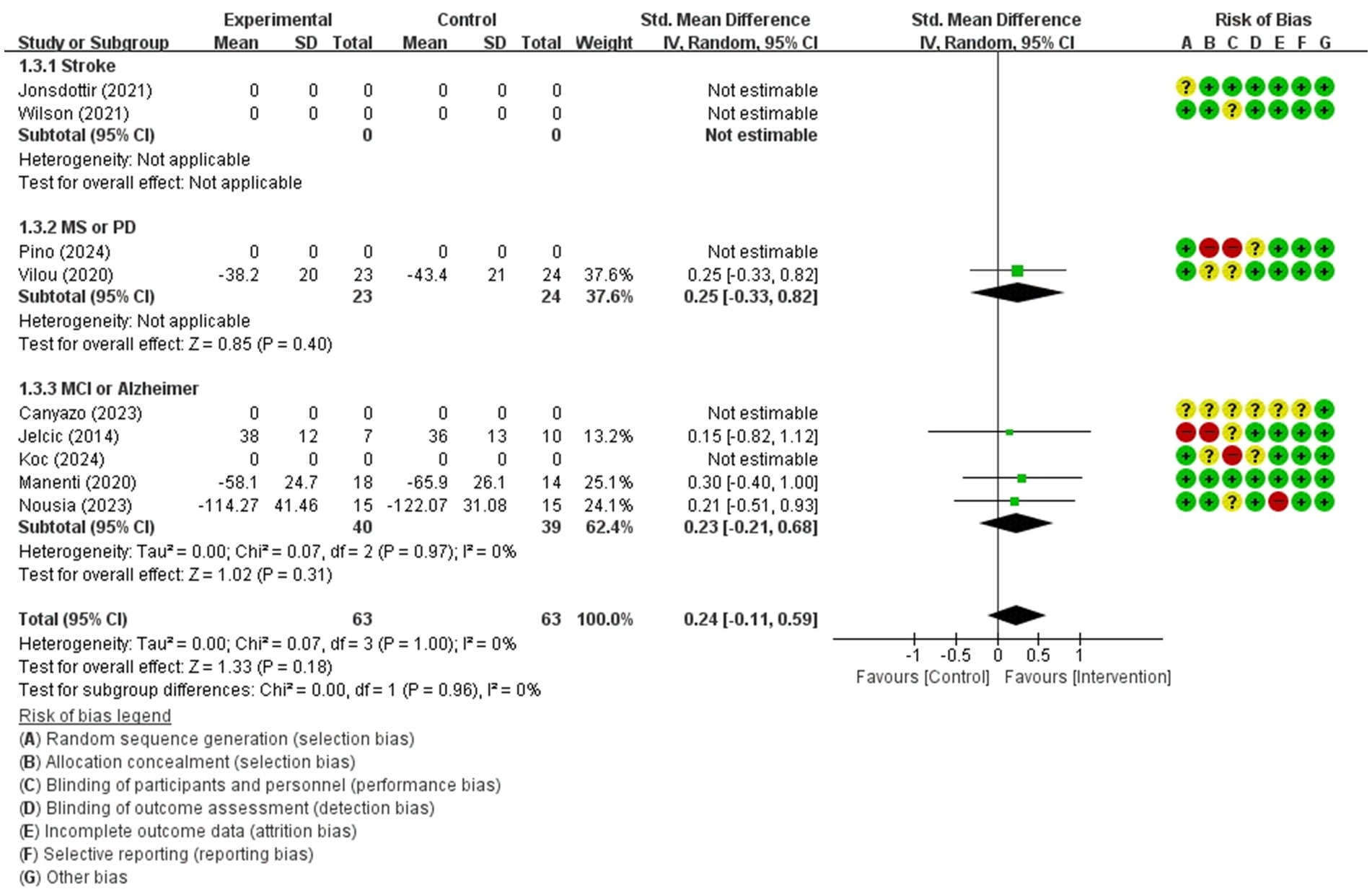
Figure 8. Forest plot of meta-analyses: telerehabilitation versus usual care on attention (immediate). SD, standard deviation; IV, inverse-variance; CI, confidence interval.
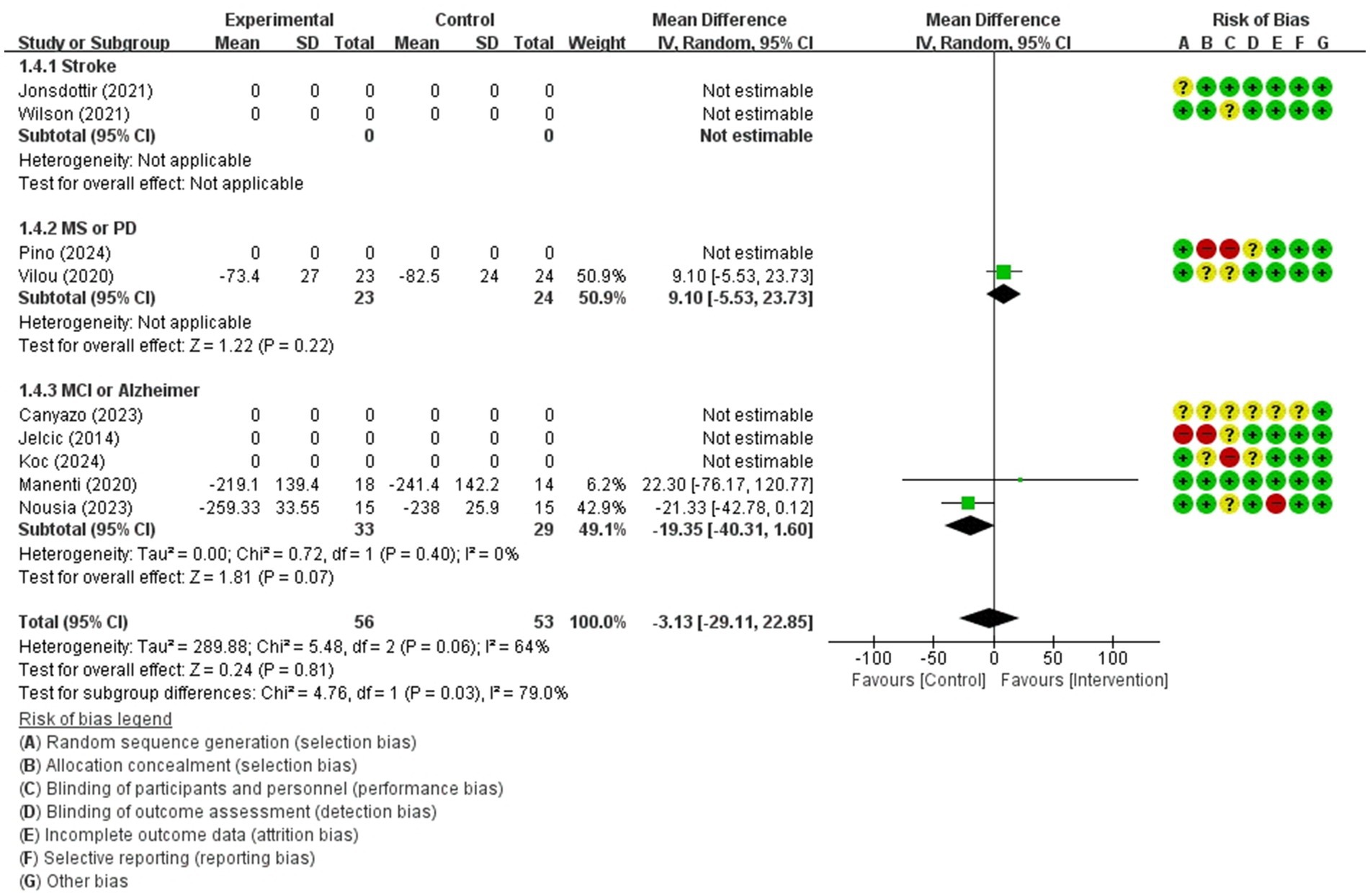
Figure 9. Forest plot of meta-analyses: telerehabilitation versus usual care on executive function (immediate). SD, standard deviation; IV, inverse-variance; CI, confidence interval.
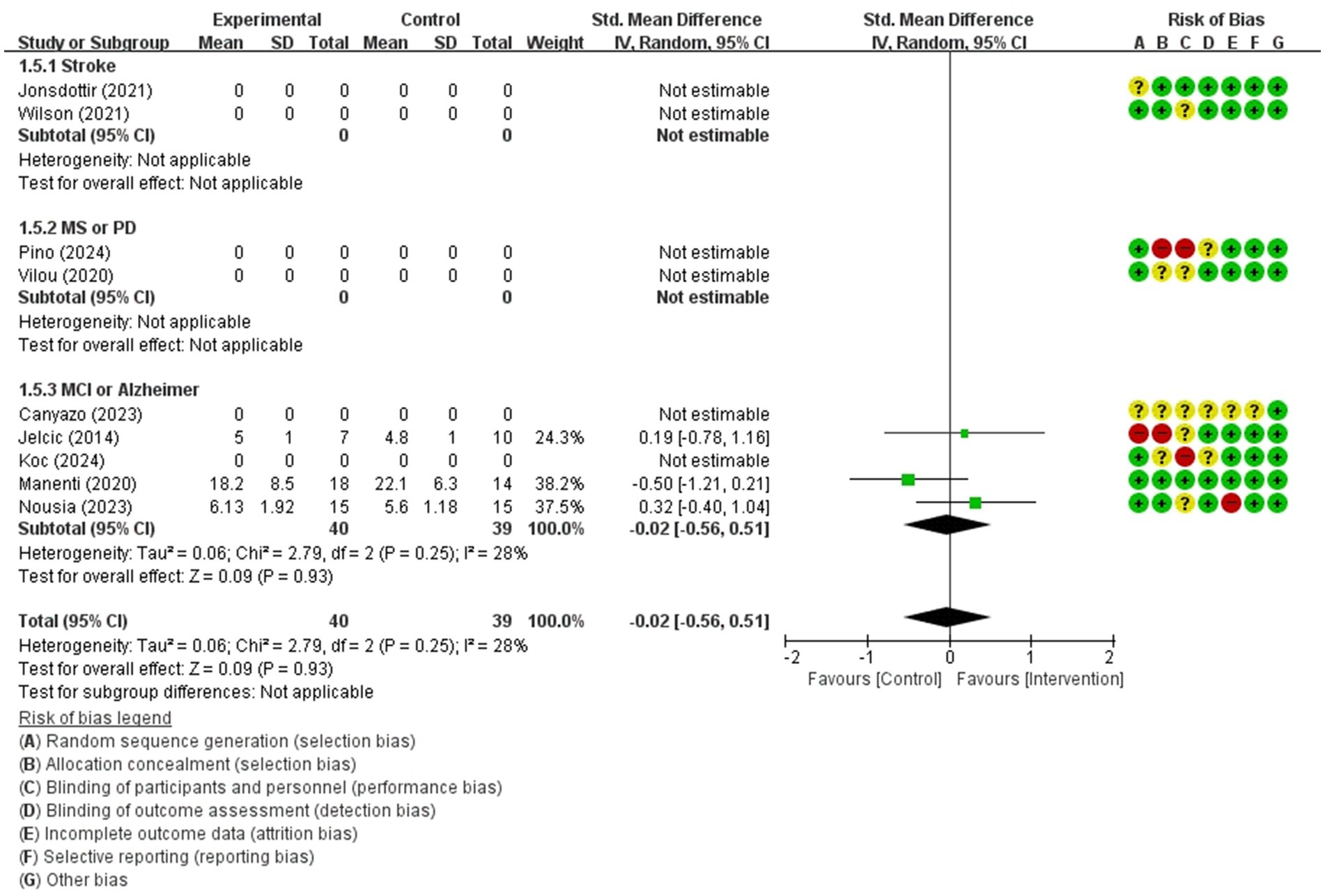
Figure 10. Forest plot of meta-analyses: telerehabilitation versus usual care on working memory (immediate). SD, standard deviation; IV, inverse-variance; CI, confidence interval.
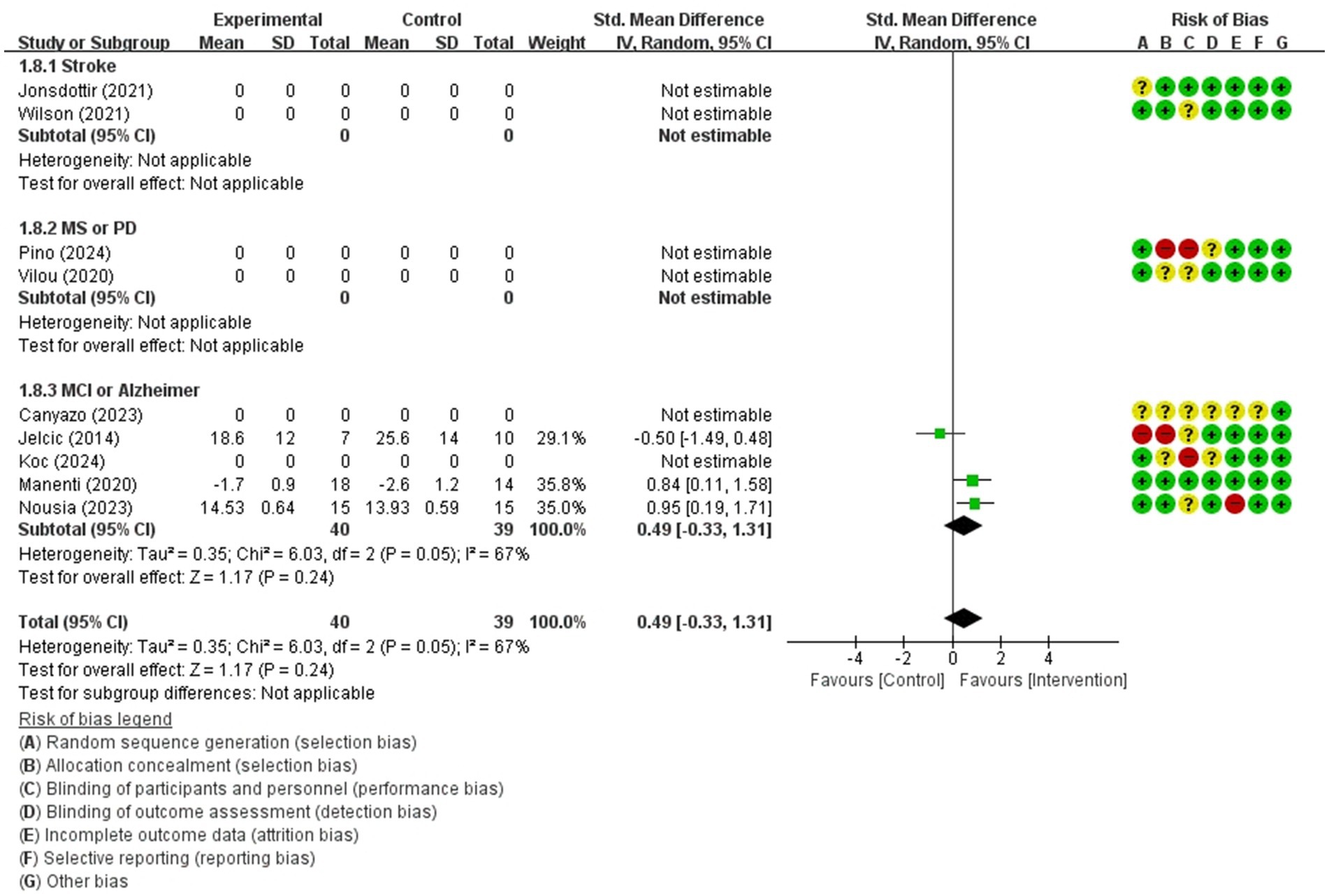
Figure 11. Forest plot of meta-analyses: telerehabilitation versus usual care on visuospatial function (immediate). SD, standard deviation; IV, inverse-variance; CI, confidence interval.
Telerehabilitation versus face-to-face treatment
Global cognition (immediate)
The studies included in the meta-analysis to determine the effects of telerehabilitation versus face-to-face treatment on global cognition (immediate), were a total of 2 studies. The evaluation tools for the outcome measures were Montreal Cognitive Assessment (MoCA) and Mini-Mental State Examination (MMSE). The effect size was calculated using SMD and the result was −0.34 (−0.87, 0.19).
In the stroke subgroup analyses, to evaluate the effects of telerehabilitation versus face-to-face treatment on global cognition (immediate), a total of 1 study was included in the meta-analysis. The evaluation tool for the outcome measures MoCA. The effect size was calculated using SMD and the result was −0.25 (−0.87, 0.37).
In the MCI or Alzheimer’s disease subgroup analyses, to evaluate the effects of telerehabilitation versus face-to-face treatment on global cognition (immediate), a total of 1 study was included in the meta-analysis. The evaluation tool for the outcome measures was MMSE. The effect size was calculated using SMD and the result was −0.57 (−1.56, 0.42) (Figure 3).
Attention (immediate)
For attention (immediate), a total of 2 studies were included in the meta-analysis to determine the effects of telerehabilitation versus face-to-face treatment in patients with MCI or Alzheimer’s disease. The evaluation tools were digital cancelation and Loewenstein Occupational Therapy Cognitive Assessment-Geriatric (LOTCA-G). The effect size was calculated using SMD and the result was 0.10 (−0.32, 0.53) (Figure 4).
Visuospatial function (immediate)
For visuospatial function (immediate), a total of 2 studies were included in the meta-analysis to determine the effects of telerehabilitation versus face-to-face treatment. The evaluation tools were Rey-Osterrieth complex figure copy test (ROCF) and LOTCA-G. The effect size was calculated using SMD and the results were − 0.26 (−0.75, 0.23) (Figure 5).
Telerehabilitation vs usual care group
Global cognition (immediate)
The studies included in the meta-analysis to determine the effects of telerehabilitation versus usual care on global cognition (immediate), were a total of 7 studies. The evaluation tools of the outcome measures were MoCA and MMSE. The effect size was calculated using SMD and the result was 0.55 (0.24, 0.86).
In the stroke subgroup analyses, to evaluate the effects of telerehabilitation versus usual care on global cognition (immediate), a total of 2 studies were included in the meta-analysis. The evaluation tool for the outcome measures was MoCA. The effect size was calculated using SMD and the result was 0.40 (−0.19, 0.98).
In the multiple sclerosis or Parkinson’s disease subgroup analyses, to evaluate the effects of telerehabilitation versus usual care on global cognition (immediate), a total of 1 study was included in the meta-analysis. The evaluation tool for the outcome measures was MoCA. The effect size was calculated using SMD and the result was 0.60 (−0.36, 1.55).
In the MCI or Alzheimer’s disease subgroup analyses, to evaluate the effects of telerehabilitation versus usual care on global cognition (immediate), a total of 4 studies were included in the meta-analysis. The evaluation tools were MoCA and MMSE. The effect size was calculated using SMD and the result was 0.64 (0.11, 1.17) (Figure 6).
Global cognition (persistence)
The studies included the meta-analysis to determine the effects of telerehabilitation versus usual care on global cognition (persistence), were a total of 3 studies. The evaluation tool was MoCA. The effect size was calculated using MD and the result was 1.36 (−0.40, 3.11).
In the stroke subgroup analyses, to evaluate the effects of telerehabilitation versus usual care on global cognition (persistence), a total of 2 studies were included in the meta-analysis. The evaluation tool for the outcome measures was MoCA. The effect size was calculated using MD and the result was 0.51 (−2.61, 3.62).
In the MCI or Alzheimer’s disease subgroup analyses, to evaluate the effects of telerehabilitation versus usual care on global cognition (persistence), a total of 1 study was included in the meta-analysis. The evaluation tool for the outcome measures was MoCA. The effect size was calculated using MD and the result was 1.75 (−0.37, 3.87) (Figure 7).
Attention (immediate)
The studies included in the meta-analysis to determine the effects of telerehabilitation versus usual care on attention (immediate), were a total of 4 studies. The evaluation tools for outcome measures were Trail Making Test-A (TMT-A) and digit cancelation. The effect size was calculated using SMD and the result was 0.24 (−0.11, 0.59).
In the multiple sclerosis subgroup analyses, to evaluate the effects of telerehabilitation versus usual care on attention (immediate), a total of 1 study was included in the meta-analysis. The evaluation tool for the outcome measures was TMT-A. The effect size was calculated using SMD and the result was 0.25 (−0.33, 0.82).
In the MCI or Alzheimer’s disease subgroup analyses, to evaluate the effects of telerehabilitation versus usual care on attention (immediate), a total of 3 studies were included in meta-analysis. The evaluation tools were TMT-A and digit cancelation. The effect size was calculated using SMD and the result was 0.23 (−0.21, 0.68) (Figure 8).
Executive function (immediate)
The studies included in the meta-analysis to determine the effects of telerehabilitation versus usual care on executive function (immediate), were a total of 3 studies. The evaluation tool was Trail Making Test-B (TMT-B). The effect size was calculated using MD and the result was −3.13 (−29.11, 22.85).
In the multiple sclerosis subgroup analyses, to evaluate the effects of telerehabilitation versus usual care on executive function (immediate), a total of 1 study was included in meta-analysis. The evaluation tool was TMT-B. The effect size was calculated using MD and the result was 9.10 (−5.53, 23.73).
In the MCI or Alzheimer’s disease group analyses, to evaluate the effects of telerehabilitation versus usual care on executive function (immediate), a total of 2 studies were included in meta-analysis. The evaluation tool was TMT-B. The effect size was calculated using MD and the result was −19.35 (−40.31, 1.60) (Figure 9).
Working memory (immediate)
For working memory (immediate), a total of 3 studies were included in meta-analysis to determine the effects of telerehabilitation versus usual care in patients with MCI or Alzheimer’s disease. The evaluation tools were Free and Cued Selective Reminding test (FCSRT), digital span (Forward). The effect size was calculated using SMD and the result was −0.02 (−0.56, 0.51) (Figure 10).
Visuospatial function (immediate)
For visuospatial function (immediate), a total of 3 studies were included in meta-analysis to determine the effects of telerehabilitation versus usual care in patients with multiple sclerosis. The evaluation tools were Clock drawing test and ROCF. The effect size was calculated using SMD and the result was 0.49 (−0.33, 1.31) (Figure 11).
Discussion
To determine the effectiveness of telerehabilitation, two approaches of meta-analysis were conducted. In this analysis, we categorized and compared different clinical conditions as follows: usual care was defined as no treatment or sham treatment, while face-to-face treatment referred to traditional therapy directly provided by a therapist. Telerehabilitation was defined as treatment delivered using remote devices capable of providing medical rehabilitation services. The first analysis compared cognitive telerehabilitation with traditional face-to-face cognition treatment. The outcomes showed that cognitive telerehabilitation was not significantly inferior to traditional face-to-face treatment in global cognition, attention, and visuospatial function. The second analysis compared cognitive telerehabilitation with the usual care group. Cognitive telerehabilitation demonstrated better outcomes in immediate global cognition compared to usual care. However, no significant differences were observed in persistent global cognition, attention, executive function, working memory, or visuospatial function. The studies analyzing executive function and visuospatial function showed high heterogeneity, with I2 values exceeding 50%. Overall, the meta-analysis results suggest that cognitive telerehabilitation offers significant benefits in improving immediate global cognition in patients with cognitive dysfunction compared to usual care or sham treatment. Additionally, it demonstrates an equivalent level of effectiveness in cognitive function improvement when compared to traditional face-to-face cognition treatment. These results could provide support for the implementation of cognitive telerehabilitation.
The results of the studies that were not included in the meta-analysis because their data could not be used were similar to the findings of the meta-analysis. In a study by Charvet et al. (15), significant improvement in cognitive function was observed in patients with multiple sclerosis when comparing a remotely monitored, supervised-based telerehabilitation group with a computer-based treatment (usual care group). They suggested that cognitive telerehabilitation could serve as an alternative method for cognitive rehabilitation through remote supervision. Mahncke et al. (7) conducted a randomized controlled trial comparing the effects of telerehabilitation versus computer game-based treatment (usual care group) on cognitive function in traumatic brain injury patients. They observed improvements in cognitive function in the telerehabilitation group, as well as improvements in depressive and cognitive symptoms in both groups. However, no significant differences were observed in instrumental activities of daily living between the two groups. Rossetto et al. (16) conducted a randomized controlled trial comparing cognitive telerehabilitation with usual care in patients with mild cognitive impairment and Alzheimer’s disease, and reported significant improvement on global cognitive level, including language, memory domains, and executive functions. It is known that impairments of executive function can have the most devastating impact on activities of daily living because of its super ordinate role in behavioral and cognitive processing (28). In addition, both patients and caregivers responded positively to the system usability scale and caregivers also noted reduced levels of distress associated with caregiving (16). Caregiver burden, defined as a multidimensional response linked to caregiver distress, can be exacerbated by various factors (29). A predictive risk factor that increases caregiver burden are known to include the overall negative experience with formal care and services (29). The psychological well-being of caregivers was linked to the nature of caregiving tasks, their subjective perception of rehabilitation, and the functional recovery of patients (30). A more positive approach by caregivers to rehabilitation was also corrected with an overall beneficial influence on the caregiving process in rehabilitation and improved functional outcomes for patients (30). Torrisi et al. (17) conducted a randomized controlled trial comparing telerehabilitation using virtual reality with usual care (usual care group) in patients with post stroke cognitive dysfunction to assess the efficacy of improving cognitive function. Significant improvements were observed in global cognitive level, attention, memory, and linguistic skills domains. The study also reported that participants perceived consistent attention and maintained a high level of motivation. Furthermore, the study emphasized the positive effects of telerehabilitation, highlighting the importance of longer training sessions facilitated by participant encouragement. Van der Linder et al. (18) conducted a randomized controlled trial comparing telerehabilitation with tablet-based cognitive rehabilitation (usual care group) in patients with brain tumors to assess cognitive function outcomes. While the outcomes did not significantly differ in group, 90% of participants reported positive feedback about the intervention, with 95% indicating that they would recommend the program to others.
The included studies observed limitations of cognitive telerehabilitation. Limitations included difficulties in using telerehabilitation devices, such as reduced user engagement in digital literacy and lack of familiarity with the device. Yi et al. (31) conducted a systematic review on the barriers and facilitators of telerehabilitation for patients with dementia. Barriers included meeting technological requirements and adapting to sensory needs. Technological barriers encompassed the lack of necessary equipment and the older adults’ ability to independently operate technologies. Sensory challenges, such as communication difficulties related to hearing and vision, were highlighted across multiple studies as barriers to the successful adoption of telemedicine. To address these barriers, enabling factors such as assistance of caregivers, pre-training on devices, utilization of captioned services to enhance communication, and the incorporation of electronic magnification and text-to-speech technology on devices were proposed.
Within the included studies, no significant adverse effects related to telerehabilitation were observed. Meanwhile, Gideon A Caplan et al. (32) conducted a randomized controlled trial comparing the incidence of delirium in in-hospital rehabilitation with early discharge rehabilitation in 104 elderly individuals and found that the incidence of delirium was lower during the rehabilitation at home process.
The effectiveness of cognitive telerehabilitation, as discussed above, shows better outcomes compared to usual care and comparable effects to face-to-face treatment. Usual care may result in less effective outcomes compared to active cognitive treatment, whereas face-to-face treatment presents spatial and temporal constraints as well as cost issues. Although telerehabilitation may pose challenges related to digital literacy in device usage, its adoption is supported by advantages such as improved accessibility and continuity of rehabilitation. Especially, it can be used while reducing time and space constraints in the individual’s own environment, and these advantages are also beneficial in preventing delirium, which can occur in elderly individuals and patients with acquired brain injuries associated with cognitive dysfunction. Telerehabilitation is not meant to replace the traditional face-to-face treatment but can be applied in a variety of ways depending on patient needs and characteristics. Cognitive telerehabilitation could prove beneficial in addressing chronic diseases with significant social implications and issues related to continuous long-term care, encompassing conditions associated with aging, such as dementia and other neurogenerative disorders. From this perspective and the results of this meta-analysis demonstrating that cognitive telerehabilitation is not inferior to face-to-face treatment and is more effective than usual care in improving general cognition (immediate), it may, in certain circumstances, even be superior to face-to-face treatment, particularly in terms of cost and accessibility.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
HJ: Writing – original draft, Data curation, Formal analysis. DK: Conceptualization, Data curation, Formal analysis, Supervision, Writing – review & editing. S-WP: Data curation, Formal analysis, Supervision, Writing – review & editing. B-SL: Data curation, Formal analysis, Supervision, Writing – review & editing. H-WH: Data curation, Formal analysis, Writing – review & editing. NJ: Data curation, Formal analysis, Writing – review & editing. MKi: Data curation, Formal analysis, Writing – review & editing. MKa: Data curation, Formal analysis, Writing – review & editing. SK: Data curation, Formal analysis, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by a grant of the Korean Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant number: RS-2023-00263587).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1450977/full#supplementary-material
Footnotes
References
1. Bernini, S, Stasolla, F, Panzarasa, S, Quaglini, S, Sinforiani, E, Sandrini, G, et al. Cognitive Telerehabilitation for older adults with neurodegenerative diseases in the COVID-19 era: a perspective study. Front Neurol. (2020) 11:623933. doi: 10.3389/fneur.2020.623933
2. Rost, NS, Brodtmann, A, Pase, MP, van Veluw, SJ, Biffi, A, Duering, M, et al. Post-stroke cognitive impairment and dementia. Circ Res. (2022) 130:1252–71. doi: 10.1161/CIRCRESAHA.122.319951
3. Subbarao, BS, Stokke, J, and Martin, SJ. Telerehabilitation in acquired brain injury. Phys Med Rehabil Clin N Am. (2021) 32:223–38. doi: 10.1016/j.pmr.2021.01.001
4. Perez-Marcos, D, Bieler-Aeschlimann, M, and Serino, A. Virtual reality as a vehicle to empower motor-cognitive Neurorehabilitation. Front Psychol. (2018) 9:2120. doi: 10.3389/fpsyg.2018.02120
5. Petersen, RC. Mild cognitive impairment. Continuum (Minneap Minn). (2016) 22:404–18. doi: 10.1212/CON.0000000000000313
6. Cotelli, M, Manenti, R, Brambilla, M, Gobbi, E, Ferrari, C, Binetti, G, et al. Cognitive telerehabilitation in mild cognitive impairment, Alzheimer's disease and frontotemporal dementia: a systematic review. J Telemed Telecare. (2019) 25:67–79. doi: 10.1177/1357633X17740390
7. Mahncke, HW, DeGutis, J, Levin, H, Newsome, MR, Bell, MD, Grills, C, et al. A randomized clinical trial of plasticity-based cognitive training in mild traumatic brain injury. Brain. (2021) 144:1994–2008. doi: 10.1093/brain/awab202
8. Brown, GC. Living too long: the current focus of medical research on increasing the quantity, rather than the quality, of life is damaging our health and harming the economy. EMBO Rep. (2015) 16:137–41. doi: 10.15252/embr.201439518
9. Wilson, PH, Rogers, JM, Vogel, K, Steenbergen, B, McGuckian, TB, and Duckworth, J. Home-based (virtual) rehabilitation improves motor and cognitive function for stroke patients: a randomized controlled trial of the elements (EDNA-22) system. J Neuroeng Rehabil. (2021) 18:165. doi: 10.1186/s12984-021-00956-7
10. Boulos, ME, Colella, B, Meusel, LA, Sharma, B, Peter, MK, Worthington, T, et al. Feasibility of group telerehabilitation for individuals with chronic acquired brain injury: integrating clinical care and research. Disabil Rehabil. (2024) 46:750–62. doi: 10.1080/09638288.2023.2177357
11. Knepley, KD, Mao, JZ, Wieczorek, P, Okoye, FO, Jain, AP, and Harel, NY. Impact of Telerehabilitation for stroke-related deficits. Telemed J E Health. (2021) 27:239–46. doi: 10.1089/tmj.2020.0019
12. Manenti, R, Gobbi, E, Baglio, F, Macis, A, Ferrari, C, Pagnoni, I, et al. Effectiveness of an innovative cognitive treatment and Telerehabilitation on subjects with mild cognitive impairment: a multicenter, randomized, Active-Controlled Study. Front Aging Neurosci. (2020) 12:585988. doi: 10.3389/fnagi.2020.585988
13. Brennan, D, Tindall, L, Theodoros, D, Brown, J, Campbell, M, Christiana, D, et al. A blueprint for telerehabilitation guidelines. Int J Telerehabil. (2010) 2:31–4. doi: 10.5195/ijt.2010.6063
14. McCue, M, Fairman, A, and Pramuka, M. Enhancing quality of life through telerehabilitation. Phys Med Rehabil Clin N Am. (2010) 21:195–205. doi: 10.1016/j.pmr.2009.07.005
15. Charvet, LE, Yang, J, Shaw, MT, Sherman, K, Haider, L, Xu, J, et al. Cognitive function in multiple sclerosis improves with telerehabilitation: results from a randomized controlled trial. PLoS One. (2017) 12:e0177177. doi: 10.1371/journal.pone.0177177
16. Rossetto, F, Isernia, S, Realdon, O, Borgnis, F, Blasi, V, Pagliari, C, et al. A digital health home intervention for people within the Alzheimer's disease continuum: results from the ability-TelerehABILITation pilot randomized controlled trial. Ann Med. (2023) 55:1080–91. doi: 10.1080/07853890.2023.2185672
17. Torrisi, M, Maresca, G, De Cola, MC, Cannavò, A, Sciarrone, F, Silvestri, G, et al. Using telerehabilitation to improve cognitive function in post-stroke survivors: is this the time for the continuity of care? Int J Rehabil Res. (2019) 42:344–51. doi: 10.1097/MRR.0000000000000369
18. van der Linden, SD, Rutten, GM, Dirven, L, Taphoorn, MJB, Satoer, DD, Dirven, CMF, et al. eHealth cognitive rehabilitation for brain tumor patients: results of a randomized controlled trial. J Neuro-Oncol. (2021) 154:315–26. doi: 10.1007/s11060-021-03828-1
19. Calabrò, RS, Bonanno, M, Torregrossa, W, Cacciante, L, Celesti, A, Rifici, C, et al. Benefits of Telerehabilitation for patients with severe acquired brain injury: promising results from a multicenter randomized controlled trial using nonimmersive virtual reality. J Med Internet Res. (2023) 25:e45458. doi: 10.2196/45458
20. Jelcic, N, Agostini, M, Meneghello, F, Bussè, C, Parise, S, Galano, A, et al. Feasibility and efficacy of cognitive telerehabilitation in early Alzheimer's disease: a pilot study. Clin Interv Aging. (2014) 9:1605–11. doi: 10.2147/CIA.S68145
21. Torpil, B, Pekçetin, E, and Pekçetin, S. The effectiveness of cognitive rehabilitation intervention with the telerehabilitation method for amnestic mild cognitive impairment: a feasibility randomized controlled trial. J Telemed Telecare. (2023):1357633x231189541. doi: 10.1177/1357633X231189541
22. Canyazo, CM, Keller, G, Helou, B, Arruabarrena, M, Corvalán, N, Carello, A, et al. Effectiveness of cognitive rehabilitation on mild cognitive impairment using teleneuropsychology. Dement Neuropsychol. (2023) 17:e20220079. doi: 10.1590/1980-5764-dn-2022-0079
23. Jonsdottir, J, Baglio, F, Gindri, P, Isernia, S, Castiglioni, C, Gramigna, C, et al. Virtual reality for motor and cognitive rehabilitation from clinic to home: a pilot feasibility and efficacy study for persons with chronic stroke. Front Neurol. (2021) 12:601131. doi: 10.3389/fneur.2021.601131
24. Koc, EA, Yazici-Mutlu, Ç, Cinar, N, and Sahiner, T. Comparison of the effect of online physical exercise and computerized cognitive stimulation in patients with Alzheimer's disease during the Covid-19 pandemic. Complement Ther Clin Pract. (2024) 57:101881. doi: 10.1016/j.ctcp.2024.101881
25. Nousia, A, Pappa, E, Siokas, V, Liampas, I, Tsouris, Z, Messinis, L, et al. Evaluation of the efficacy and feasibility of a Telerehabilitation program using language and cognitive exercises in multi-domain amnestic mild cognitive impairment. Arch Clin Neuropsychol. (2023) 38:224–35. doi: 10.1093/arclin/acac078
26. Del Pino, R, de Echevarría, AO, Díez-Cirarda, M, Ustarroz-Aguirre, I, Caprino, M, Liu, J, et al. Virtual coach and telerehabilitation for Parkinson´ s disease patients: vCare system. J Public Health. (2023) 1-14. doi: 10.1007/s10389-023-02082-1
27. Vilou, I, Bakirtzis, C, Artemiadis, A, Ioannidis, P, Papadimitriou, M, Konstantinopoulou, E, et al. Computerized cognitive rehabilitation for treatment of cognitive impairment in multiple sclerosis: an explorative study. J Integr Neurosci. (2020) 19:341–7. doi: 10.31083/j.jin.2020.02.35
28. Dawson, DR, Anderson, ND, Binns, MA, Bottari, C, Damianakis, T, Hunt, A, et al. Managing executive dysfunction following acquired brain injury and stroke using an ecologically valid rehabilitation approach: a study protocol for a randomized, controlled trial. Trials. (2013) 14:306. doi: 10.1186/1745-6215-14-306
29. Lethin, C, Leino-Kilpi, H, Bleijlevens, MH, Stephan, A, Martin, MS, Nilsson, K, et al. Predicting caregiver burden in informal caregivers caring for persons with dementia living at home - a follow-up cohort study. Dementia (London). (2020) 19:640–60. doi: 10.1177/1471301218782502
30. Bivona, U, Villalobos, D, De Luca, M, Zilli, F, Ferri, G, Lucatello, S, et al. Psychological status and role of caregivers in the neuro-rehabilitation of patients with severe acquired brain injury (ABI). Brain Inj. (2020) 34:1714–22. doi: 10.1080/02699052.2020.1812002
31. Yi, JS, Pittman, CA, Price, CL, Nieman, CL, and Oh, ES. Telemedicine and dementia care: a systematic review of barriers and facilitators. J Am Med Dir Assoc. (2021) 22:1396–402.e18. doi: 10.1016/j.jamda.2021.03.015
Keywords: telerehabilitation, cognitive remediation, cognitive dysfunction, neurodegenerative diseases, health services accessibility
Citation: Jeon H, Kim DY, Park S-W, Lee B-S, Han H-W, Jeon N, Kim M, Kang M and Kim S (2025) A systematic review of cognitive telerehabilitation in patients with cognitive dysfunction. Front. Neurol. 15:1450977. doi: 10.3389/fneur.2024.1450977
Edited by:
Silvia Paola Caminiti, University of Pavia, ItalyReviewed by:
Jorge Cancela, Roche, SwitzerlandFrancesca Borgnis, Fondazione Don Carlo Gnocchi Onlus (IRCCS), Italy
Copyright © 2025 Jeon, Kim, Park, Lee, Han, Jeon, Kim, Kang and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Doo Young Kim, a2R5Z2Vub0BnbWFpbC5jb20=
 Hyeonwoo Jeon
Hyeonwoo Jeon Doo Young Kim
Doo Young Kim Si-Woon Park1
Si-Woon Park1 Suebeen Kim
Suebeen Kim