
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 04 January 2023
Sec. Neurological Biomarkers
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.1076898
This article is part of the Research TopicBiomarkers in ischemic strokeView all 11 articles
Aim: To evaluate the relationship between carotid stenosis with variants in genes referred to inflammation and endothelial function.
Methods: There was a multi-center, cross sectional survey in southwestern China. The eight communities were selected at random in southwestern China. The residents aged ≥40 years volunteered to participate in face-to-face survey. Subjects with at least three of the aforementioned eight stroke related risk factors or a history of stroke were classified as high-risk population for stroke. A total of 2,377 subjects were the high-risk population for stroke in the eight communities, and degree of carotid stenosis was assessed by carotid ultrasound. Genotypes of 6 variants in 3 genes related to inflammation and endothelial function were examined. Gene-gene interaction was analyzed by generalized multifactor dimensionality reduction (GMDR).
Results: Carotid stenosis were found in 295 (12.41%) subjects, of whom 51 (17.29%) had moderate or severe stenosis. According to multivariate logistic regression analysis, we found that HABP2rs7923349TT was independent risk factor for carotid stenosis (OR, 1.96, 95% CI: 1.22–3.13, P = 0.005) and ITGA2rs1991013AG and HABP2rs7923349TT were independent risk factors for moderate to severe carotid stenosis (OR, 2.28, 95% CI: 1.28–4.07, P = 0.005; OR, 2.90, 95% CI: 1.19–7.08, P = 0.019). GMDR analysis showed that there was a significant gene-gene interaction between ITGA2 rs4865756 and HABP2 rs7923349, and the high-risk interactive genotype in the two variants was independently associated with a higher risk for carotid stenosis after adjusting the covariates (OR,1. 42, 95% CI 1.10–1.84, P = 0.008).
Conclusions: Prevalence of carotid stenosis was very high in the high-risk stroke population in southwestern China. Variants in genes referred in endothelial function were associated with the carotid stenosis. The high—risk interactive genotype in ITGA2 rs4865756 and HABP2 rs7923349 was independently associated with a higher risk for carotid stenosis.
Stroke is one of the leading causes of disability and mortality in China (1). A report has proven that stroke recurrence or cardiovascular outcomes are influenced by serious carotid stenosis, and it has been well-established in the population with stroke (2, 3). Moreover, severe internal carotid artery stenosis (≥50%) is usually accompanied by carotid atherosclerosis and emboli, thereby reducing blood flow of the brain, which increases the risk of stroke as well (4, 5). It is critical to clarify the etiology of carotid stenosis, particularly in the genetic etiology. Further understanding of the roles of genetic factors in carotid stenosis can provide better insight into the pathogenesis of this disease, to better preventing stroke occurrence.
Atherosclerosis is a process of chronic inflammation (6, 7). Carotid plaque, a phenotype of subclinical atherosclerosis, is an inflammatory lesion associated with stroke (8). Variants in inflammation and endothelial function genes have been reported to play roles in carotid plaques in the Dominican population (8). In addition, several studies have revealed that genes implicated in inflammation and endothelial function are associated with the occurrence, stability and vulnerability of carotid plaques (9–11). However, few studies have focused on genetic mechanism of carotid stenosis. According to the China National Stroke Screening Survey (CNSSS), a total of 2,377 subjects were the high-risk population for stroke in the eight communities in this study. Extracranial carotid artery stenosis was assessed by carotid ultrasound. In following article, extracranial carotid artery stenosis was abbreviated as carotid stenosis. Meanwhile, genotypes of 6 variants in 3 genes related to inflammation and endothelial function were examined. The aim of the present study was to evaluate the morbidity of carotid stenosis in a population with high stroke risk, the association between the 6 variants and carotid stenosis, and the effect of gene-gene interactions among the 6 variants on carotid stenosis according to this community-based study.
This study was part of the CNSSS (Grant No. 2011BAI08B01) which was approved by the Stroke Screening and Prevention Programme of the National Health and Family Planning Commission of China. The survey protocol was approved by the ethics committee of the participating hospitals (The People's Hospital of Deyang City, the Affiliated Hospital of Southwest Medical University and Suining Central Hospital), Written informed consent was obtained from each subject prior to study enrollment.
During May 2015 and September 2015, eight communities in Sichuan were randomly selected. More details on the organization and implementation can be found on the official websites (12) and the literature we published before (13). We only screened all residents aged ≥40 years who lived in the community for more than 6 months. A structured face-to-face questionnaire was used in the initial screening by interviewers.
Information on all the participants was collected, including demographic characteristics (e.g., age, sex), stroke-related behavioral factors (e.g., smoking, exercise habits and diet), personal and family medical history of stroke and chronic diseases (e.g., hypertension, diabetes mellitus, dyslipidemia and atrial fibrillation [AF]), and physical examination (e.g., height, weight).
The eight conventional stroke risk factors were assessed in the CNSSS questionnaire, including hypertension, diabetes, dyslipidemia, AF, overweight/obesity, smoking, physical inactivity, and family history of stroke. The detailed information of the participants was described in our previous article (13). Subjects were classified as the high-risk population for stroke if they experienced at least three of the aforementioned eight stroke related risk factors or a history of stroke. Exclusion criteria included: (i) severe cardiovascular, liver or renal disease, (ii) blood system disease and coagulation dysfunction, (iii) acute and chronic inflammation, malignant tumors and immune system diseases, (iv) transient ischemic attack only, and (v) individuals declined to participate in the study.
Finally, 16,892 valid individual records (including 524 stroke cases [429 ischemic strokes, 95 hemorrhagic strokes]) were enrolled. Among them, 2,893 participants were the high-risk population. However, DNA and carotid ultrasonography information was obtained in 2,377 participants among the 2,893 high-risk population. The detailed data cleaning procedure and quality control is presented in Figure 1.
Color duplex scans (Acuson Sequoia Apparatus type 512, 7.5-MHz probe, Berlin, Germany) were performed to evaluate the severity of extracranial carotid artery stenosis. Then we acquired longitudinal and transverse sections from color duplex imaging and power mode. Carotid stenosis assessment in diameter was combined with peak systolic velocities at the location of the stenosis as well as the internal carotid artery/common carotid artery ratio (14).
A total of 2,377 subjects were classified into carotid stenosis and non-carotid stenosis (< 15% stenosis) groups on the basis of their vascular imaging. According to degree of carotid stenosis, the subjects were further divided into moderate to severe stenosis group (≥50% stenosis) and non-moderate to severe stenosis group (< 50% stenosis) that includes mild stenosis group (15–49% stenosis) and non-carotid stenosis (< 15% stenosis) groups. Carotid ultrasound was conducted by sonographers blinded to the laboratory and questionnaire data.
Six SNPs involved in inflammation and endothelial function were selected (https://www.ncbi.nlm.nih.gov/snp) according to the following criteria: (i) minor allele frequency>0.05; (ii) nonsynonymous variants; (iii) SNPs that have been assessed in previous studies (8). Whole blood (3 ml) collection, genomic DNA extraction, and genotyping were performed as previously described (10, 11, 13). Each SNP was designed with two amplification primers and one extension primer. According to our previous study, genotyping of the 6 variants was performed using the matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) method (10, 11, 13).
All statistical analyses were performed using SPSS 16.0 (SPSS Inc, Chicago, IL, USA). Deviation of Hardy–Weinberg equilibrium for genotype frequencies was analyzed by χ2 test. Continuous variables conforming to a normal distribution are expressed as the mean ± standard deviation, and intergroup differences were evaluated using Student's t-test or analysis of variance. Categorical variables that were not in accord with a normal distribution were expressed as median and Interquartile range, and the rank sum test was used for comparison between groups. Categorical variables are presented as frequencies or percentages; intergroup differences were evaluated using the χ2 test or Fisher's exact test. Multivariate logistic regression analysis was performed to adjust covariate variables (age, gender, hypertension, diabetes, smoking, and hyperlipidemia) and to assess the independent risk factors for carotid stenosis, and odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. All tests were 2 sided, and the threshold level of P < 0.05 denoted statistical significance.
Gene-gene interactions were analyzed using the statistical software GMDR Beta, version 0.7 (15), as described in our previous studies (11, 16). The GMDR computed the maximum likelihood estimates and the scores of all individuals under the null hypothesis. A cumulative score was then calculated within each multifactor cell, which was labeled either as high-risk if the average score met or exceeded a pre-assigned threshold of zero, or as low-risk if the score was less than zero. An exhaustive search of all possible one- to six-locus models was performed for all variants. The model with the minimum prediction error, the maximum cross-validation consistency score, and a P-value of 0.05 or less (derived automatically from the sign test in the GMDR software) was considered as the best model.
Among the 2,377 high-risk population, 295 cases (12.41%) had carotid stenosis, of which 51 (17.29%) subjects had moderate to severe stenosis.
Compared with subjects without carotid stenosis, subjects with carotid stenosis were older and had a higher proportion of men, a history of stroke, cigarette smoking and taking antihypertensive drugs (Table 1). Subjects with moderate to severe carotid stenosis had a higher proportion of men and a history of stroke than subjects without moderate to severe carotid stenosis (Table 1).
The genotype distributions of these 6 variants were in Hardy–Weinberg Equilibrium (all P > 0.05). Univariate analyses revealed that HABP2 rs7923349 was associated with carotid stenosis (P < 0.05, Table 2). The genotypes of ITGA2 rs1991013 and HABP2 rs7923349 were associated with moderate to severe carotid stenosis (P < 0.05, Table 3).
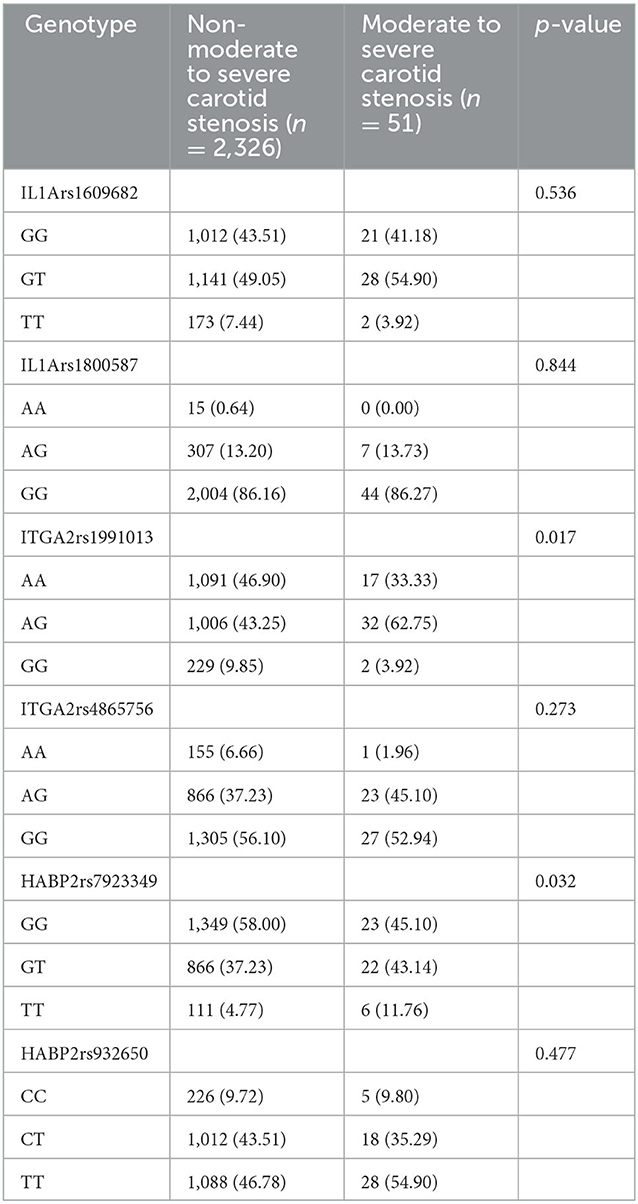
Table 3. Genotype distribution in moderate to severe carotid stenosis group and the group without moderate to severe carotid stenosis (n [%]).
The associations of gene-gene interactions among the 6 variants with carotid stenosis were performed by the GMDR approach (Table 4). The best model for carotid stenosis including ITGA2 rs4865756 and HABP2 rs7923349 scored 8/10 for cross-validation consistency and 10/10 for sign testing. The P-value for prediction error was 0.010 for GMDR using permutation testing.
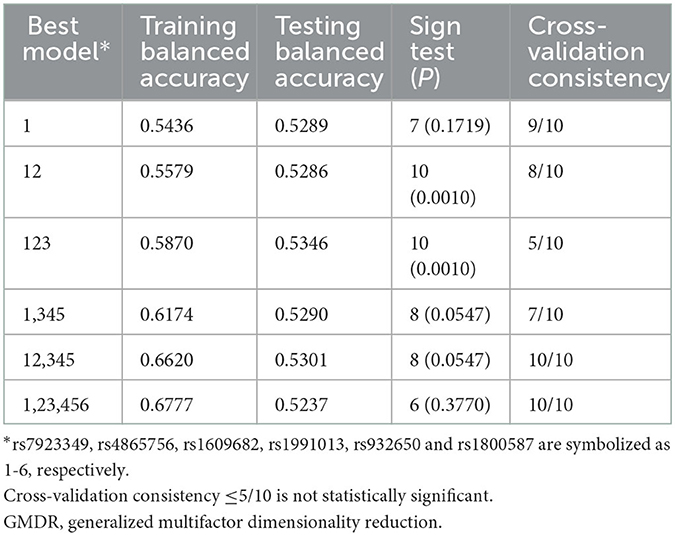
Table 4. Comparison of the best models, prediction accuracies, cross-validation consistencies, and P-values identified by GMDR.
Subsequently, we assessed the associations of different genotype combinations in ITGA2 rs4865756 and HABP2 rs7923349 with the risk of carotid stenosis.
Compared to patients harboring wild type genotypes (rs4865756GG, rs7923349GG), the relative risk of different genotype combinations of rs4865756 and rs7923349 was analyzed. The 5 genotype combinations making larger contributions to carotid stenosis risk were ITGA2rs4865756AA, HABP2rs7923349GG; ITGA2rs4865756AG, HABP2rs7923349GT; ITGA2rs48657 56AG, HABP2rs7923349TT; ITGA2rs4865756GG, HABP2rs7 923349GT and ITGA2rs4865756GG, HABP2rs7923349TT, which were considered as the high-risk interactive genotypes. The other combination genotypes among rs4865756 and rs7923349 did not reach statistical significance (P > 0.05) and were defined as the low-risk interactive genotype.
Multivariate logistic regression analysis was performed to assess the risk of carotid stenosis conferred by different genotype combinations among rs4865756 and rs7923349. The incidence of carotid stenosis was significantly higher in subjects with the high-risk interactive genotype than in those carrying the low-risk interactive genotype [15.14 (160/1,057) vs. 10.23% (135/1,320), χ2 = 13.017, P < 0.001]. The high-risk interactions were assigned as one, and the low-risk interactions were assigned as zero. The other variables that showed a significant association with carotid stenosis (P < 0.05) on univariate analysis were enrolled in the multivariate logistic regression model. The results revealed that the high-risk interactions were independently associated with a higher risk for carotid stenosis after adjustment with covariates (OR, 1.42, 95% CI: 1.10–1.84, P = 0.008, Table 5). Besides, HABP2rs7923349TT was considered be an independent risk factor for carotid stenosis (OR, 1.96, 95% CI: 1.22–3.13, P = 0.005, Table 5).
However, the incidence of moderate to severe carotid stenosis had no statistical differences between subjects with the high-risk interactive genotype and the low-risk interactive genotype [2.55 (27/1,057) vs. 1.82% (24/1,320), χ2 = 1.515, P = 0.218]. Multivariate logistic regression analysis was also performed to determine the risk factors for moderate to severe carotid stenosis using variables with P-values < 0.05 in univariate analysis, and found that ITGA2rs1991013AG and HABP2rs7923349TT were independent risk factors for moderate to severe carotid stenosis (OR, 2.28, 95% CI: 1.28–4.07, P = 0.005; OR, 2.90, 95% CI: 1.19–7.08, P = 0.019, Table 6).
In this study, the relationships among baseline characteristics, genic interactions and carotid stenosis in high-risk stroke population were investigated. We detected a high prevalence (12.41%) of carotid stenosis among the high-risk population for stroke in southwestern China. By multivariate analysis, we found that one endothelium-associated gene loci (HABP2 rs7923349TT) was associated with occurrence of carotid stenosis and two endothelium-associated gene loci (ITGA2 rs1991013AG, HABP2 rs7923349TT) were associated with occurrence of moderate to severe carotid stenosis.
Atherothrombosis is a main pathomechanism in the evolution of vessel stenosis (17). In recent years, increasing evidence suggests that atherosclerosis is an active process akin to the chronic inflammatory process adjusted by multiple genes (6, 7).
Integrin α2 (integrin alpha2), encoded by ITGA2, is a significant member of the integrin family and is connected with platelet adhesion and aggregation through collagen receptors. The ITGA2 gene polymorphism may be a susceptible predictor of the risk of ischemic stroke (18). However, Georgios K. Nikolopoulos et al. hold a different opinion. In their meta-analysis, there was no evidence to support an association between the C807T polymorphism of the ITGA2 gene and stroke (19). But a study about Dominicans found that ITGA2 rs1991013 is associated with calcified plaque and increases the risk of general atherosclerosis (8). This conclusion is in accord with our result. In our research, ITGA2 rs1991013 is associated with moderate to severe carotid stenosis. But number of moderate to severe carotid stenosis is limited, so further study is need.
HABP2, which encodes an extracellular serine protease involved in coagulation, fibrinolysis and inflammatory pathways, may be a genetic susceptibility locus in early-onset stroke (20). Meanwhile, HABP2 is believed to affect vascular smooth muscle cell proliferation and atherosclerotic plaque instability (21). This study detects that HABP2 rs7923349 SNPs are related to carotid stenosis and moderate to severe carotid stenosis in people who are in high risk for stroke. The reason may be that HABP2 polymorphism is associated with inhibited activation of pro-urokinase and progression of carotid stenosis.
Many studies have explained the relationship between ischemic stroke and genes involved in inflammation and the endothelium. Yi et al. (22) discovered that eleven variants of platelet activation-relevant genes are unrelated to carotid stenosis, but gene-gene interactions among TXA2R rs1131882, P2Y1 rs1371097 and GPIIIa rs2317676 have a synergistic influence on carotid stenosis by GMDR analysis. This indicates that a single-locus analytical approach seemed unfit for the genetic etiology of carotid stenosis. Atherosclerosis may be caused by gene-gene or gene-environment interactions (23, 24). Therefore, the genetic risk of carotid stenosis can be investigated gene-gene interactions via the GMDR approach (15). This study focuses on subclinical carotid stenosis which has received less attention before. According to GMDR analysis, we detected gene-gene interactions at the genetic locus between ITGA2 rs4865756 and HABP2 rs7923349. High-risk interaction genotypes increase the risk of carotid stenosis. However, the specific molecular mechanisms of gene-gene interactions among the 2 variants are still unclear. The reason may be that the two genes are involved in encoding and adjusting enzymes that cause atherosclerosis and refer to endothelial function, thereby resulting in the occurrence and development of arteriostenosis. The function of adhesion and aggregation could be increased by gene polymorphisms in HABP2 rs7923349 and ITGA2 rs4865756. Moreover, vascular integrity and atherosclerotic plaques are regulated by these genes (8). The relationship between genes related to endothelial and inflammatory function and carotid stenosis was investigated in China for the first time. The study discovered gene-gene interactions among HABP2 rs7923349 and ITGA2 rs4865756, and high-risk interaction genotypes increase the risk of carotid stenosis. New targets for preventing and treating carotid stenosis may be found in this research. However, the connection between high-risk interaction genotypes and moderate to severe carotid stenosis haven't been found in univariate analysis or multivariate analysis. The deficiency of number in participants or follow-up studies may be the reason. Therefore, it is meaningful to explore the theory of this research more deeply.
As we know, IL-1α from macrophage is a principal cytokine controlling the development of atherosclerotic plaques (25). But, in our study, genotypes of IL-1α were irrelevant to carotid stenosis. Besides, we found that antihypertensive therapy is positively associated with carotid stenosis in multivariate analysis. It's different from our inertial thinking and experience. The factors that contribute to this situation include that subjects with carotid stenosis have a higher proportion of hypertension than another group (Table 1) and people with carotid stenosis may have better compliance in taking antihypertensive. But further research and follow-up visit are needed.
Several potential limitations need to be considered in our findings. First, our study used a cross-sectional study design and self-report questionnaire; therefore, recalling bias might play roles in affecting the validity of our results. Second, carotid stenosis was assessed by ultrasound in our study. Although ultrasound can recognize carotid stenosis, Magnetic Resonance Angiography (MRA), computed tomography angiography (CTA) and digital subtraction angiography (DSA) may provide more detail about carotid stenosis. Third, we acknowledge that although the current study examined the roles of several important genes related to endothelial and inflammatory responses, other relevant genes were not examined. Fourth, in this study, even though gene-gene interactions between HABP2 rs7923349 and ITGA2 rs4865756 increased the risk of carotid stenosis, the detailed mechanism was unknown. Additionally, further studies are needed to clarify their role in carotid stenosis formation.
In conclusion, the prevalence of carotid stenosis was high among the high-risk stroke population in southwestern China. We identified variants in genes related to endothelial function that were significantly associated with carotid stenosis risk. Furthermore, we found a significant gene–gene interaction between HABP2 rs7923349 and ITGA2 rs4865756 in affecting the risk of carotid stenosis. This study is expected to identify new targets for the prevention and treatment of carotid artery stenosis and provides a theoretical basis for gene therapy and drug development against new targets in the future.
The original contributions presented in the study are publicly available. This data can be found here: https://datadryad.org/, https://doi.org/10.5061/dryad.k98sf7m9r.
The studies involving human participants were reviewed and approved by the Ethics Committee of the participating hospitals (The People's Hospital of Deyang City, The Affiliated Hospital of Southwest Medical University, and Suining Central Hospital). The patients/participants provided their written informed consent to participate in this study.
LL and XY analyzed the data, contributed to method design, and contributed to writing the manuscript. XY, HL, and MY provided data and disease expertise. All authors read and approved the final manuscript.
This study was supported in part by grants from the Scientific Research Foundation of Sichuan Provincial Health Department (Grant No. 16ZD046). The funding body did not participate in the design of the study; collection, analysis, and interpretation of data; and in writing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Jiang B, Wang WZ, Chen H, Hong Z, Yang QD, Wu SP, et al. Incidence and trends of stroke and its subtypes in China: results from three large cities. Stroke. (2006) 37:63–8. doi: 10.1161/01.STR.0000194955.34820.78
2. Brott TG, Hobson RN, Howard G, Roubin GS, Clark WM, Brooks W, et al. Stenting vs. endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. (2010) 363:11–23. doi: 10.1056/NEJMoa0912321
3. Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg MR, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. (2003) 361:107–16. doi: 10.1016/S0140-6736(03)12228-3
4. Deb P, Sharma S, Hassan KM. Pathophysiologic mechanisms of acute ischemic stroke: an overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology. (2010) 17:197–218. doi: 10.1016/j.pathophys.2009.12.001
5. Inzitari D, Eliasziw M, Gates P, Sharpe BL, Chan RK, Meldrum HE, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. (2000) 342:1693–700. doi: 10.1056/NEJM200006083422302
6. Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. (2012) 32:2045–51. doi: 10.1161/ATVBAHA.108.179705
7. Taleb S. Inflammation in atherosclerosis. Arch Cardiovasc Dis. (2016) 109:708–15. doi: 10.1016/j.acvd.2016.04.002
8. Gardener H, Beecham A, Cabral D, Yanuck D, Slifer S, Wang L, et al. Carotid plaque and candidate genes related to inflammation and endothelial function in Hispanics from northern Manhattan. Stroke. (2011) 42:889–96. doi: 10.1161/STROKEAHA.110.591065
9. Maeno T, Koyama H, Tahara H, Komatsu M, Emoto M, Shoji T, et al. The 807T allele in alpha2 integrin is protective against atherosclerotic arterial wall thickening and the occurrence of plaque in patients with type 2 diabetes. Diabetes. (2002) 51:1523–8. doi: 10.2337/diabetes.51.5.1523
10. Yi XY, Liao DX, Wang C, Cheng W, Fu XQ, Zhang B. Cytochrome P450 genetic variants and their metabolite levels associated with plaque stability in patients with ischemic stroke. J Atheroscler Thromb. (2016) 23:330–8. doi: 10.5551/jat.31120
11. Yi X, Lin J, Luo H, Wang C, Liu Y. Genetic variants of PTGS2, TXA2R and TXAS1 are associated with carotid plaque vulnerability, platelet activation and TXA2 levels in ischemic stroke patients. PLoS ONE. (2017) 12:e0180704. doi: 10.1371/journal.pone.0180704
12. National Center for Stroke Control and Prevention National National Health and Family Planning Commission of the People's Republic of China. The China National Stroke Screening Survey Guidelines [online, in Chinese]. Available at: cnstroke.com/WebManage/InterveneProject/Index (accessed November 18, 2016).
13. Yi X, Luo H, Zhou J, Yu M, Chen X, Tan L, et al. Prevalence of stroke and stroke related risk factors: a population based cross sectional survey in southwestern China. BMC Neurol. (2020) 20:5. doi: 10.1186/s12883-019-1592-z
14. Momjian-Mayor I, Kuzmanovic I, Momjian S, Bonvin C, Albanese S, Bichsel D, et al. Accuracy of a novel risk index combining degree of stenosis of the carotid artery and plaque surface echogenicity. Stroke. (2012) 43:1260–5. doi: 10.1161/STROKEAHA.111.634766
15. Lou XY, Chen GB, Yan L, Ma JZ, Zhu J, Elston RC Li MD, et al. generalized combinatorial approach for detecting gene-by-gene and gene-by-environment interactions with application to nicotine dependence. Am J Hum Genet. (2007) 80:1125–37. doi: 10.1086/518312
16. Yi X, Lin J, Wang Y, Zhou J, Zhou Q. Interaction among CYP2C8, GPIIIa and P2Y12 variants increase susceptibility to ischemic stroke in Chinese population. Oncotarget. (2017) 8:70811–20. doi: 10.18632/oncotarget.19991
17. Willeit J, Kiechl S, Weimer T, Mair A, Santer P, Wiedermann CJ, et al. Marburg I polymorphism of factor VII—activating protease: a prominent risk predictor of carotid stenosis. Circulation. (2003) 107:667–70. doi: 10.1161/01.CIR.0000055189.18831.B1
18. Wu G, Xi Y, Yao L, Su L, Yan Y, Li M, et al. Genetic polymorphism of ITGA2 C807T can increase the risk of ischemic stroke. Int J Neurosci. (2014) 124:841–51. doi: 10.3109/00207454.2013.879718
19. Nikolopoulos GK, Tsantes AE, Bagos PG, Travlou A, Vaiopoulos G. Integrin, alpha 2 gene C807T polymorphism and risk of ischemic stroke: a meta-analysis. Thromb Res. (2007) 119:501–10. doi: 10.1016/j.thromres.2006.04.002
20. Cheng YC, Stanne TM, Giese AK, Ho WK, Traylor M, Amouyel P, et al. Genome-wide association analysis of young-onset stroke identifies a locus on chromosome 10q25 near HABP2. Stroke. (2016) 47:307–16. doi: 10.1161/STROKEAHA.115.011328
21. Kanse SM, Parahuleva M, Muhl L, Kemkes-Matthes B, Sedding D, Preissner KT. Factor VII-activating protease (FSAP): vascular functions and role in atherosclerosis. Thromb Haemost. (2008) 99:286–9. doi: 10.1160/TH07-10-0640
22. Yi X, Lin J, Zhou Q, Huang R, Chai Z. The TXA2R rs1131882, P2Y1 rs1371097 and GPIIIa rs2317676 three-loci interactions may increase the risk of carotid stenosis in patients with ischemic stroke. BMC Neurol. (2019) 19:44. doi: 10.1186/s12883-019-1271-0
23. Bevan S, Traylor M, Adib-Samii P, Malik R, Paul NL, Jackson C, et al. Genetic heritability of ischemic stroke and the contribution of previously reported candidate gene and genomewide associations. Stroke. (2012) 43:3161–7. doi: 10.1161/STROKEAHA.112.665760
24. Culverhouse R, Suarez BK, Lin J, Reich T, A. perspective on epistasis: limits of models displaying no main effect. Am J Hum Genet. (2002) 70:461–71. doi: 10.1086/338759
Keywords: stroke, high-risk population, inflammation, carotid stenosis, genetic polymorphism
Citation: Liu L, Yi X, Luo H and Yu M (2023) Inflammation and endothelial function relevant genetic polymorphisms in carotid stenosis in southwestern China. Front. Neurol. 13:1076898. doi: 10.3389/fneur.2022.1076898
Received: 22 October 2022; Accepted: 01 December 2022;
Published: 04 January 2023.
Edited by:
Pedro Ramos-Cabrer, CIC biomaGUNE, SpainReviewed by:
Zheng Dai, Affiliated Wuxi People's Hospital of Nanjing Medical University, ChinaCopyright © 2023 Liu, Yi, Luo and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingyang Yi,  eWl4aW5neWFuZzY0QDEyNi5jb20=
eWl4aW5neWFuZzY0QDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.