
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 09 December 2021
Sec. Epilepsy
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.794086
Purpose: This retrospective observational study aimed to investigate the self-reported prevalence of seizure clusters (SCs) in patients with epilepsy (PWE) and its relationship with clinical characteristics.
Methods: We retrospectively analyzed data from consecutive PWE from our hospital in northeastern China. Data were collected from the databank of a tertiary epilepsy center. Logistic regression models were employed to investigate the relationships between the individual patient demographic/clinical variables and the occurrence of SC.
Results: In total, 606 consecutive PWE were included in the final analysis, and 268 (44.2%) patients experienced at least one seizure cluster. In multivariate logistic regression models, age (OR: 1.014; 95% CI: 1.002–1.027; p = 0.02), seizure frequency (OR: 2.08; 95% CI: 1.555–2.783; p < 0.001), multiple seizure types (OR: 5.111; 95% CI: 1.737–15.043; p = 0.003), number of current anti-seizure medications (ASM) (OR: 1.533; 95% CI: 1.15–2.042; p = 0.004), drug-resistant epilepsy (OR: 1.987; 95% CI: 1.159–3.407; p = 0.013), and a history of status epilepticus (OR: 1.903; 95% CI: 1.24–2.922; p = 0.003) were independent variables associated with a history of SC in PWE.
Conclusion: Seizure clusters (SCs) are common occurrences at our study center. The occurrence of SC in individuals with epilepsy, to some extent, is determined by the epilepsy severity.
Epilepsy is one of the most common serious brain disorders, affecting more than 70 million patients worldwide (1). Approximately 30% of patients with epilepsy (PWE) reported uncontrolled or poorly controlled seizures despite having appropriate anti-seizure medication (ASM) therapy (2). Seizure clusters (SCs) refer to a closely grouped series of seizures within a defined period (3). SC is a common clinical phenomenon reported by many PWE (4–7). The reported prevalence of SC ranged from 20 to 83% in prior investigations (6, 8, 9). One major reason for the varying prevalence of SC across the studies is the lack of a precise definition of SC (4, 10). Different clinical definitions of SC have been applied previously, including ≥3 seizures within 24 h, >2 seizures within 24 h, and >2 seizures within 6 h (10).
Many researchers have paid attention to the potential factors contributing to SC in PWE and they also estimated the patients' risk of SC (5, 6, 10). Factors associated with SC in PWE include previous head trauma, extratemporal seizure localization, history of status epilepticus, higher frequency of seizures, intractable epilepsy, symptomatic generalized epilepsy, focal epilepsy (vs. idiopathic generalized epilepsy), epilepsy onset at early age, and longer epilepsy duration (5–7, 10, 11). The use of various SC definitions, different study populations, and the inclusion of PWE with varying degrees of severity of epilepsy may lead to a wide range of reported risk factors across the studies. Furthermore, it has been reported that SC is associated with an increased risk of emergency room visits or hospitalization for PWE (3, 12). The negative effect of SC on the patient's quality of life has also been established (13).
The prevalence of SC in Chinese adults with epilepsy has yet to be investigated, and knowledge about factors associated with SC in PWE is also scarce in mainland China. Thus, our study aimed to answer the following questions: (1) How many Chinese adults with epilepsy have experienced a history of SC defined as ≥3 seizures in 24 h? (2) Which demographic or clinical characteristics are associated with a history of SC? (3) Are the potential risk factors for SC vary by epilepsy type?
We conducted a retrospective observational study of PWE in an epilepsy management program at the epilepsy center in the First Hospital of Jilin University. A clinical databank was established for PWE in our center. The databank included demographic and clinical information on the PWE who were treated and followed up at our hospital. A standardized questionnaire was used to collect data during the interview when they visited their neurologist at the center. We recorded the demographic data such as name, age, and gender. The recorded clinical information consisted of the diagnosis, 24-h video electroencephalogram (EEG), neuroimaging, epilepsy type, seizure frequency, and medication profile. The establishment of the databank was approved by the Human Research Ethics Committees of our hospital, and participants provided written informed consent before we collected the data.
We retrospectively reviewed the medical records of patients in the databank from January 2020 to July 2021. Individuals were included in our analysis if they had the following criteria: (1) had a definite diagnosis of epilepsy in accordance with the International League Against Epilepsy (ILAE) (14); (2) had reached the age of 18 years or older; and lastly (3) had been taking a stable dose of an ASM for at least 1 month. We excluded individuals who had non-epileptic seizures, a severe neurological disorder other than epilepsy (e.g., Parkinson's disease), or a serious physical condition (e.g., a significant hepatic, renal, or cardiopulmonary condition). The records were examined by two investigators to ensure that individual data were not repeatedly entered in the analysis.
To evaluate the history of SC, we defined SC as three or more seizures occurring within 24 h, which is the habitual pattern that has been widely used by previous studies (5, 6, 15). Data on potential risk factors for SC (i.e., age, sex, age at first seizure onset, duration of epilepsy, family history of epilepsy, history of febrile seizures, seizure frequency over the last 6 months, epilepsy type, multiple seizure types, number of anti-seizure medications (ASM), known etiology of epilepsy, abnormal MRI, drug-resistant epilepsy, and presence of status epilepticus) were collected and recorded from a databank. A family history of epilepsy was defined as epilepsy occurring in a first-degree relative (parent or sibling). Patients had a known etiology of epilepsy if they reported genetic, tumor, vascular, dysplasia, infectious, autoimmune, hippocampal sclerosis, or other defined etiologies. MRI findings were reported by a neuroradiologist, and their reports were subsequently reviewed by a second epileptologist, who made a final classification for this study. MRI was classified as abnormal only when the observed abnormality was considered the cause of epilepsy. Drug-resistant epilepsy was defined as a failure of adequate trials of two tolerated, appropriately chosen, and used ASM schedules (whether as monotherapies or as combination) to achieve sustained seizure freedom (16). SE was defined if the diagnostic time exceeded 5 min of ongoing seizure activity for convulsive SE or 10 min for absence status or focal status with or without impaired consciousness (17).
Data are described as percentages for categorical variables and as medians [interquartile ranges (IQRs)] for continuous variables. For group comparisons, Mann–Whitney U-tests were used for continuous variables, while Chi-square/Fisher's exact tests were used for categorical variables. We employed logistic regression models to investigate the relationships between the individual patient demographic/clinical variables and the history of SC. Variables achieving significance at p < 0.05 in the univariate analyses were entered into multivariate logistic regression models. The results were presented as odds ratios (ORs) with the corresponding 95% CIs. The type of epilepsy was classified as focal epilepsy or non-focal epilepsy, including unknown classification. The same logistic regression analyses were also performed based on epilepsy type. All p-values were two-sided, and statistical significance was set at p < 0.05. All data were analyzed in SPSS 26.0 (SPSS Inc., Chicago, IL, USA).
In total, 606 consecutive PWE were included in the final analysis, with a median age of 35 years and a male percentage of 53.5%. The demographic and clinical characteristics are shown in Table 1. The median age at first seizure onset and epilepsy duration was 25 and 4 years, respectively. A total of 25.9% (157/606) of the patients were seizure-free in 6 months. Focal epilepsy was the most common epilepsy type reported by 452 (74.6%) patients, and 5% of the patients had multiple seizure types. Overall, 268 (44.2%) of the patients experienced at least one seizure cluster.
Patients with a history of SC were more likely to be older (p = 0.021), have a longer epilepsy duration (P < 0.001), reported a family history of epilepsy (p = 0.041), and experienced a higher seizure frequency over the last six months (P < 0.001) than those without a history of SC. Multiple seizure types were more frequent in patients with a history of SC (p < 0.001), and their number of present ASMs were higher (p < 0.001). Patients who experienced SC were more likely to have a known etiology of epilepsy (p = 0.002), abnormal MRI (p = 0.011), and drug-resistant epilepsy (P < 0.001). There was an increased risk of the presence of status epilepticus in the SC group (P < 0.001) for details (see Table 2).
Univariate logistic regression analyses were performed to investigate the associations between all demographic/clinical variables and a history of SC. The univariate regression models confirmed that these ten variables were associated with an increased OR for SC: age (OR: 1.012; 95% CI: 1.002–1.022; p = 0.021), duration of epilepsy (OR: 1.031; 95% CI: 1.015–1.048; p < 0.001), family history of epilepsy (OR: 1.758; 95% CI: 1.018–3.037; p = 0.041), seizure frequency (OR: 3.008; 95% CI: 2.37–3.818; p < 0.001), multiple seizure type (OR: 6.952; 95% CI: 2.586–18.153; p < 0.001), number of present ASMs (OR: 2.198; 95% CI: 1.74–2.777; p < 0.001), known etiology of epilepsy (OR: 1.664; 95% CI: 1.196–2.314; p = 0.002), abnormal MRI (OR: 1.525; 95% CI: 1.099–2.114; p = 0.011), drug-resistant epilepsy (OR: 6.042; 95% CI: 4.098–8.906; p < 0.001), and history of status epilepticus (OR: 2.715; 95% CI: 1.882–3.917; p < 0.001). The ten variables that were significant in the unadjusted univariate analysis were entered into the multivariate logistic regression analysis. In the multivariate logistic regression models, age (OR: 1.014; 95% CI: 1.002–1.027; p = 0.02), seizure frequency (OR: 2.08; 95% CI: 1.555–2.783; p < 0.001), multiple seizure type (OR: 5.111; 95% CI: 1.737–15.043; p = 0.003), number of present ASMs (OR: 1.533; 95% CI: 1.15–2.042; p = 0.004), drug-resistant epilepsy (OR: 1.987; 95% CI: 1.159–3.407; p = 0.013), and history of status epilepticus (OR: 1.903; 95% CI: 1.24–2.922; p = 0.003) were independent variables associated with a history of SC in PWE for details (see Table 3).
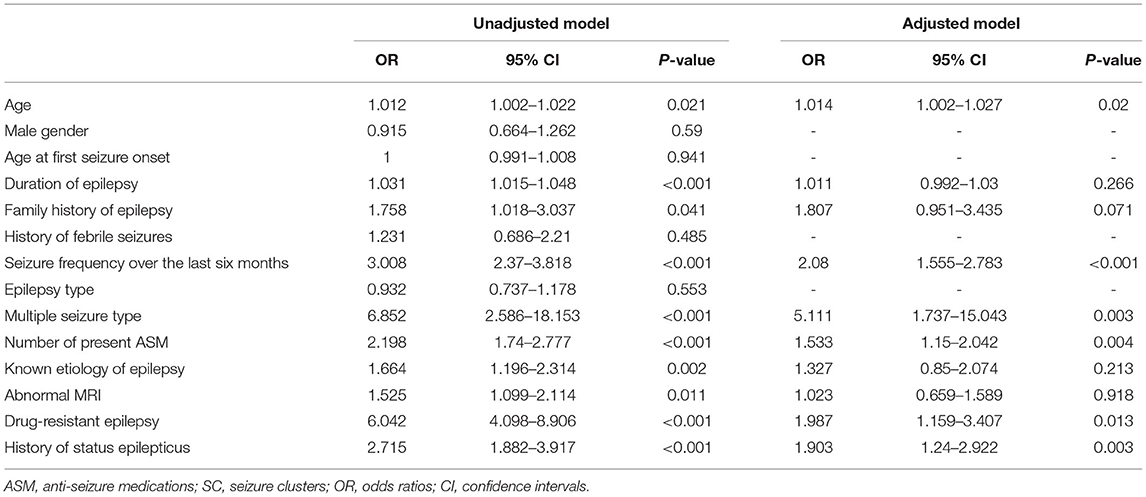
Table 3. Univariate and multivariate logistic regression analysis on factors associated with the occurrence of SC.
Similar multivariate logistic regression analyses were performed for patients with focal epilepsy and non-focal epilepsy. In the focal epilepsy group, four variables were found to be associated with a history of SC: seizure frequency (OR: 1.838; 95% CI: 1.317–2.564; p < 0.001), number of present ASMs (OR: 1.486; 95% CI: 1.082–2.043; p = 0.015), drug-resistant epilepsy (OR: 1.986; 95% CI: 1.094–3.607; p = 0.024), and a history of status epilepticus (OR: 2.194; 95% CI: 1.336–3.602; p = 0.002). In the non-focal epilepsy group, the only variable associated with an increased OR for SC was seizure frequency (OR: 3.088; 95% CI: 1.679–5.678; p < 0.001) for details (see Table 4).
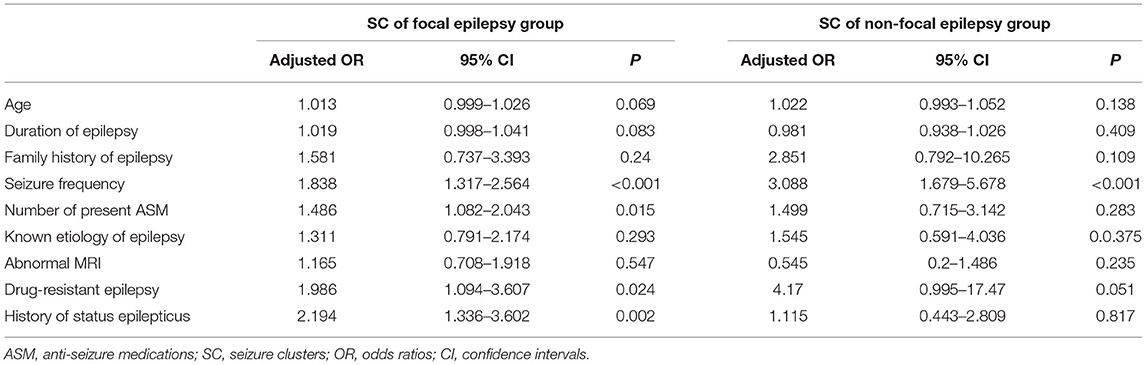
Table 4. Multivariate-adjusted odds ratios for SC stratified by epilepsy type (focal epilepsy vs. non-focal epilepsy).
The present study analyzed the factors associated with the occurrence of SC in a sample of adults with epilepsy in northeastern China. There are three main findings of our study. First, SCs are common occurrences at our study center and were reported by 44.2% of this population of adults with epilepsy. Second, the multivariate analysis identified six risk factors associated with the occurrence of SC in our cohort. Patients with older age, higher seizure frequency, multiple seizure types, more current ASMs, drug-resistant epilepsy, and history of status epilepticus had greater likelihood of having SC. Third, risk factors for the occurrence of SC varied between the focal and non-focal epilepsy groups.
The reported prevalence of SC ranged from 20 to 83% in prior studies (6, 8, 9, 12). Compared with the prevalence rates reported by prior studies, the 44.2% of PWE with a history of SC in this study was higher than the 29.0% reported by Haut et al. (6) and the 14.9% reported by Chen et al. (5), but lower than the 83% reported by Ferastraoaru et al. (8) and the 61.5% reported by Haut et al. (18). The varying reported occurrence rates of SC may be partly explained by the imprecise clinical definition of SC across the studies. Another possible explanation could be the different study populations and the inclusion of PWE with varying degrees of severity of epilepsy across the studies. Additionally, there may be an overestimation of SC prevalence since our data were collected from a tertiary epilepsy center that has more patients with refractory epilepsy.
Significant risk factors for the occurrence of SC reported previously included female gender, drug-resistant epilepsy, head trauma, symptomatic generalized epilepsy, intractable epilepsy (defined as a lack of seizure control/higher seizure frequency/absence of one-year seizure freedom), duration of epilepsy, seizure frequency, localization (extratemporal epilepsy, in particular, frontal lobe epilepsy), MTS, remote symptomatic epilepsy, posttraumatic epilepsy, CNS infection, cortical dysplasia, and status epilepticus (4–6, 15, 18–25). Our findings confirmed the independent relationships between a history of SC and several risk factors, including seizure frequency, drug-resistant epilepsy, and history of status epilepticus. Our novel findings were that older age, multiple seizure types, and more current ASMs were significantly associated with seizure cluster occurrence in this cohort. Chen et al. also reported a link between 2 or more ASMs that did not work in the history of the patient's treatment for epilepsy and seizure cluster occurrence (5). Yao et al. predicted that multiple seizure types were associated with a decreased likelihood of seizure remission in PWE by using supervised machine learning technologies (26). In a recent case-control study of ASM-resistant patients and ASM-responsive controls, Brad et al. found that certain seizure-type combinations, such as a combination of absence, myoclonic, and generalized tonic-clonic seizures, were independent predictors of ASM resistance in PWE (27). The association between ASM resistance and seizure cluster occurrence has been established (10). Furthermore, univariate analyses found associations of a known etiology of epilepsy and abnormal MRI with the risk of SC. However, these associations disappeared in the multivariate analyses. Thus, a higher seizure frequency, more current ASMs, drug-resistant epilepsy, history of status epilepticus, and multiple seizure type—all features of severe epilepsy—appeared to be significantly associated with an increased likelihood of the occurrence of SC. In consideration of these findings, we proposed a hypothesis that the occurrence of SC in persons with epilepsy, to some extent, is determined by epilepsy severity.
To date, it remains unclear why seizures cluster. The neurobiological underpinnings of SC were not addressed in our investigation. However, prior evidence indicated that this may be because endogenous hormone fluctuations can lead to periods of enhanced seizure susceptibility and other periods with greater resistance to seizure recurrences (28). Periods of enhanced seizure susceptibility might result in the occurrence of SC (7).
Prior researchers identified focal epilepsy as a risk factor for SC in PWE (5). However, we could not replicate the previously reported relationship between epilepsy type and SC. When examining SCs by epilepsy type, Chen and his colleagues reported the differences in the prevalence rates and the related risk factors in patients across the different epilepsy types (5). Our results also supported this finding, and the risk factors for the occurrence of SC varied between patients with focal and non-focal epilepsy. Higher seizure frequency was the only consistent risk factor associated with a higher likelihood of developing SC in patients with focal and non-focal epilepsy.
Several limitations should be acknowledged in the current study. First, this study is limited by its retrospective observational nature. Data were collected based on patient self-reports, and thus, there may be recall bias. Second, the data were restricted to PWE in a tertiary epilepsy center, which may also cause a strong sampling bias toward people with refractory epilepsy. Another inherent bias of this study is that patients who died from SC cannot be included. Third, some factors that might be associated with the occurrence of SC in epilepsy [e.g., psychiatric symptoms, sleep quality, or extratemporal epilepsy (6)] were not available and were not investigated in this sample. It is well-known that patients with temporal lobe epilepsy usually have fewer SCs than extratemporal, especially with frontal lobe epilepsy. Finally, we did not differentiate between patients who had a single SC and those who had multiple SCs in this study.
Seizure clusters are common occurrences at our study center and were reported by 44.2% of this PWE population. The occurrence of SC in persons with epilepsy, to some extent, is determined by epilepsy severity. Factors associated with the occurrence of SC included older age, higher seizure frequency, multiple seizure types, more current ASMs, drug-resistant epilepsy, and history of status epilepticus.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Human Research Ethics Committees of the First Hospital of Jilin University. The patients/participants provided their written informed consent to participate in this study.
RZ and WL conceived of and designed the study. RZ, QC, and XZ were involved in data acquisition. RZ and QC analyzed the data and wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by a grant from the Programme of Jilin University First Hospital Clinical Cultivation Fund (LCPYJJ2017006).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank all of the participants for their valuable information, cooperation, and participation.
1. Thijs RD, Surges R, O'Brien TJ, Sander JW. Epilepsy in adults. Lancet. (2019) 393:689–701. doi: 10.1016/S0140-6736(18)32596-0
2. Sheng J, Liu S, Qin H, Li B, Zhang X. Drug-resistant epilepsy and surgery. Curr Neuropharmacol. (2018) 16:17–28. doi: 10.2174/1570159X15666170504123316
4. Detyniecki K, O'Bryan J, Choezom T, Rak G, Ma C, Zhang S, et al. Prevalence and predictors of seizure clusters: A prospective observational study of adult patients with epilepsy. Epilepsy Behav. (2018) 88:349–56. doi: 10.1016/j.yebeh.2018.09.035
5. Chen B, Choi H, Hirsch LJ, Katz A, Legge A, Wong RA, et al. Prevalence and risk factors of seizure clusters in adult patients with epilepsy. Epilepsy Res. (2017) 133:98–102. doi: 10.1016/j.eplepsyres.2017.04.016
6. Haut SR, Shinnar S, Moshe SL. Seizure clustering: risks and outcomes. Epilepsia. (2005) 46:146–9. doi: 10.1111/j.0013-9580.2005.29004.x
7. Asadi-Pooya AA, Nei M, Sharan A, Sperling MR. Seizure clusters in drug-resistant focal epilepsy. Epilepsia. (2016) 57:e187–90. doi: 10.1111/epi.13465
8. Ferastraoaru V, Schulze-Bonhage A, Lipton RB, Dumpelmann M, Legatt AD, Blumberg J, et al. Termination of seizure clusters is related to the duration of focal seizures. Epilepsia. (2016) 57:889–95. doi: 10.1111/epi.13375
9. Haut SR, Lipton RB, LeValley AJ, Hall CB, Shinnar S. Identifying seizure clusters in patients with epilepsy. Neurology. (2005) 65:1313–5. doi: 10.1212/01.wnl.0000180685.84547.7f
10. Gidal B, Klein P, Hirsch LJ. Seizure clusters, rescue treatments, seizure action plans: Unmet needs and emerging formulations. Epilepsy Behav. (2020) 112:107391. doi: 10.1016/j.yebeh.2020.107391
11. Jafarpour S, Hirsch LJ, Gainza-Lein M, Kellinghaus C, Detyniecki K. Seizure cluster: Definition, prevalence, consequences, and management. Seizure. (2019) 68:9–15. doi: 10.1016/j.seizure.2018.05.013
12. Haut SR. Seizure clusters: characteristics and treatment. Curr Opin Neurol. (2015) 28:143–50. doi: 10.1097/WCO.0000000000000177
13. Komaragiri A, Detyniecki K, Hirsch LJ. Seizure clusters: A common, understudied and undertreated phenomenon in refractory epilepsy. Epilepsy Behav. (2016) 59:83–6. doi: 10.1016/j.yebeh.2016.02.030
14. Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. (2017) 58:522–30. doi: 10.1111/epi.13670
15. Sillanpaa M, Schmidt D. Seizure clustering during drug treatment affects seizure outcome and mortality of childhood-onset epilepsy. Brain. (2008) 131:938–44. doi: 10.1093/brain/awn037
16. Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen HW, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. (2010) 51:1069–77. doi: 10.1111/j.1528-1167.2009.02397.x
17. Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus–Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. (2015) 56:1515–23. doi: 10.1111/epi.13121
18. Haut SR, Swick C, Freeman K, Spencer S. Seizure clustering during epilepsy monitoring. Epilepsia. (2002) 43:711–5. doi: 10.1046/j.1528-1157.2002.26401.x
19. Sinha S, Satishchandra P, Kalband BR, Thennarasu K. New-onset status epilepticus and cluster seizures in the elderly. J Clin Neurosci. (2013) 20:423–8. doi: 10.1016/j.jocn.2012.02.050
20. Milton JG, Gotman J, Remillard GM, Andermann F. Timing of seizure recurrence in adult epileptic patients: a statistical analysis. Epilepsia. (1987) 28:471–8. doi: 10.1111/j.1528-1157.1987.tb03675.x
21. Balish M, Albert PS, Theodore WH. Seizure frequency in intractable partial epilepsy: a statistical analysis. Epilepsia. (1991) 32:642–9. doi: 10.1111/j.1528-1157.1991.tb04703.x
22. Bauer J, Burr W. Course of chronic focal epilepsy resistant to anticonvulsant treatment. Seizure. (2001) 10:239–46. doi: 10.1053/seiz.2000.0499
23. Fogarasi A, Janszky J, Faveret E, Pieper T, Tuxhorn I. A detailed analysis of frontal lobe seizure semiology in children younger than 7 years. Epilepsia. (2001) 42:80–5. doi: 10.1046/j.1528-1157.2001.43799.x
24. Bauer J, Ghane Y, Flugel D, Wildt L, Stefan H. [Etiology, follow-up and therapy of seizure clusters in temporal lobe epilepsy and catamenial epileptic seizures]. Schweiz Arch Neurol Psychiatr. (1992) 143:117–34.
25. Laskowitz DT, Sperling MR, French JA, O'Connor MJ. The syndrome of frontal lobe epilepsy: characteristics and surgical management. Neurology. (1995) 45:780–7. doi: 10.1212/WNL.45.4.780
26. Yao L, Cai M, Chen Y, Shen C, Shi L, Guo Y. Prediction of antiepileptic drug treatment outcomes of patients with newly diagnosed epilepsy by machine learning. Epilepsy Behav. (2019) 96:92–7. doi: 10.1016/j.yebeh.2019.04.006
27. Kamitaki BK, Janmohamed M, Kandula P, Elder C, Mani R, Wong S, et al. Clinical and EEG factors associated with antiseizure medication resistance in idiopathic generalized epilepsy. Epilepsia. (2021). doi: 10.1111/epi.17104. [Epub ahead of print].
Keywords: seizure clusters, epilepsy severity, seizure frequency, multiple seizure type, more current ASMs, drug-resistant epilepsy, history of status epilepticus
Citation: Zhong R, Chen Q, Zhang X and Lin W (2021) The Occurrence of Seizure Clusters in Patients With Epilepsy Is Partly Determined by Epilepsy Severity: A Single-Center Retrospective Observational Study. Front. Neurol. 12:794086. doi: 10.3389/fneur.2021.794086
Received: 13 October 2021; Accepted: 10 November 2021;
Published: 09 December 2021.
Edited by:
Kette D. Valente, Universidade de São Paulo, BrazilReviewed by:
Silvia Vincentiis, Universidade de São Paulo, BrazilCopyright © 2021 Zhong, Chen, Zhang and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weihong Lin, bGlud2hAamx1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.