- 1Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China
- 2Department of Neurology, Tianjin Medical University General Hospital, Tianjin, China
- 3Epilepsy Center, Beijing Fengtai You'anmen Hospital, Beijing, China
- 4Department of Neurology, Tianjin Huanhu Hospital, Tianjin, China
- 5Advanced Center of Stroke, Beijing Institute for Brain Disorders, Beijing, China
- 6Department of China-America Institute of Neuroscience, Xuanwu Hospital, Capital Medical University, Beijing, China
- 7Department of Neurosurgery, Wayne State University School of Medicine, Detroit, MI, United States
- 8Department of Neurosurgery, Xuanwu Hospital, Capital Medical University, Beijing, China
Cerebral venous outflow disturbance (CVOD) has begun to garner the attention of researches owing to a series of clinical symptoms that impose a significant impact on people's quality of life. Herein, we aimed to investigate whether normobaric oxygen (NBO) can ameliorate CVOD-induced neurological symptoms. This was one part of the prospective trial registered in ClinicalTrials.gov (NCT03373292). A total of 37 CVOD patients were divided into the NBO group (5–8 L/min of oxygen inhalation, 1 h per time, 3 times daily, n = 19) and the control group (without oxygen inhalation, n = 18) randomly. The assessments were performed at admission, 1-week hospitalization, and 6-month follow-up. Quantitative electroencephalogram (qEEG) data were recorded prior to and post 1 h of NBO in some patients. R software was used for data analysis. No NBO-related adverse events were observed during the whole NBO intervention process. The 1-week Patient Global Impression of Change (PGIC) scale showed that the symptom improvement occurred in nine patients in the NBO group (47.4%) while none in the control group (p = 0.001). NBO could improve headache evaluated with visual analog scale (pre-NBO vs. post-NBO: 4.70 ± 2.16 vs. 2.90 ± 2.03, p = 0.024) and Headache Impact Test-6 (53.40 ± 12.15 vs. 50.30 ± 13.04, p = 0.041). As for 6-month PGIC follow-up, eight out of 14 cases (57.1%) in the NBO group reported improvement, while only one out of 12 patients in the control group replied mild improvement (p = 0.014). The qEEG revealed that NBO reduced the ratio of theta to alpha power (0.65 ± 0.38 vs. 0.56 ± 0.35, p = 0.030) over the fronto-central electrodes. To sum up, NBO may be a safe and effective approach to attenuate CVOD-related symptoms (especially for headache) by brain functional improvement resulting from increasing oxygen supply to the brain tissues.
Introduction
Recently, a growing body of literature has demonstrated the causative role of impaired venous drainage function or structures on the intractable neurological deficits, in which cerebral venous sinus stenosis (CVSS) and internal jugular venous stenosis (IJVS) are in the majority (1–7). No matter what kinds of the cerebral venous outflow disturbance (CVOD), long-term elevated trans-stenosis pressure gradient leads to venous outflow disturbance and further affects the whole arteriovenous circulation, resulting in cerebral circulation insufficiency (CCI) consequently (1–7). A variety of associated clinical symptoms, such as headache, tinnitus, head noise, insomnia, dizziness, and even high intracranial hypertension (IH), impose a significant impact on people's well-being and quality of life (3, 4, 8, 9). Endovascular stenting may be a promising regimen against CVOD; however, given the technical constraints, it is not very commonly used in clinical settings (10–12). Importantly, most of the patients with osseous impingement-induced venous stenosis are inappropriate for stenting (2–4). Moreover, a proper specific stent for cerebral venous sinus (CVS) and internal jugular veins (IJV) is still lacking, and notably, this method is under exploration period currently. A convenient, safe, and effective adjuvant therapy is warranted to improve the venous anomaly-related symptoms.
Normobaric oxygen (NBO), supplied by a face mask (such as simple mask) with one atmosphere pressure (1 ATA = 101.325 kPa), is a routine adjuvant therapy for various diseases, especially for brain ischemia (13–15). NBO can elevate brain tissue oxygen pressure (pO2), increase cerebral blood flow and volume (CBF and CBV), protect the blood–brain barrier (BBB), attenuate ischemic injury, and ameliorate inflammation in CCI (16–21). Similar to cerebral arterial disease-induced CCI, CVOD also induces CCI by reducing CBF, damaging the BBB, promoting inflammatory factor release, and facilitating neuron injury (1–7), whereby NBO may be capable of yielding some benefits to patients with CVOD as well (2, 3, 7). This study aimed to investigate the safety and efficacy of NBO on correcting CVOD-related neurological symptoms to explore an adjuvant therapy for CVOD.
Materials and Methods
Subjects
This study was an assessor-bind randomized controlled study, which was one part of the prospective trial registered with ClinicalTrials.gov (NCT03373292). It had been approved by the Institutional Ethic Committee of Xuanwu Hospital, Capital Medical University (Beijing, China) based on the guidelines of the 1964 Declaration of Helsinki. Informed consent was obtained from all individuals before any specific interventions or tests performed. It has been acknowledged that 10–20 patients per group are often adequate for determining whether a larger multicenter trial should be conducted (22, 23). Therefore, we aimed to enroll 15–20 patients in the protocol, and the sample size might be changed in accordance with the funding.
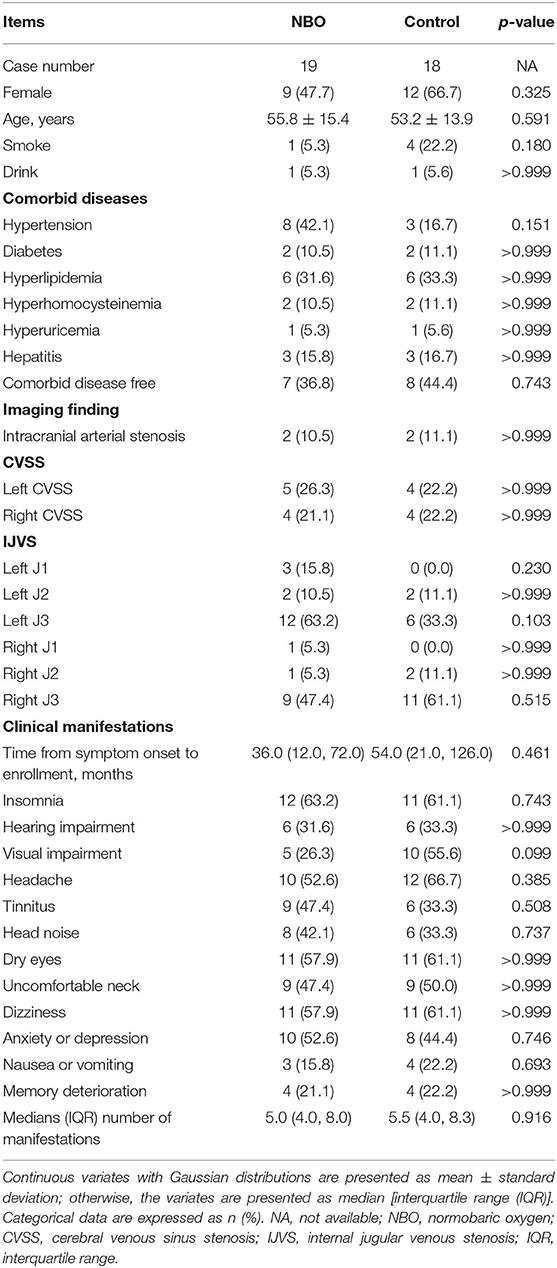
Cerebral venous outflow disturbance consisted of CVSS and IJVS, which referred to the narrowing degree of the inner diameter of IJV or CVS reached ≥50% in respect to the proximal adjacent IJV or CVS segment; meanwhile, with one abnormal collateral vessel, the inner diameter of collateral vessel at least must be ≥50% of the maximal inner diameter of the adjacent IJV, or with two or more abnormal collateral vessels, the inner diameters of these collateral vessels were without special definition, as presented in magnetic resonance venography (MRV) and/or computed tomographic venography (CTV) (2–5, 24, 25). Since representing the severity of the venous outflow insufficiency, abnormal collateral generation is essential for diagnosis of IJVS and CVSS.
All of the patients complied with the inclusion and exclusion criteria and were enrolled after giving their informed consent.
Inclusion criteria were as follows: (1) age range from 18 to 80 years; (2) definite radiographic diagnosis of IJVS or CVSS; (3) suffered focal or non-focal neurological symptoms; (4) inappropriate for cerebral endovascular treatment; and (5) National Institutes of Health Stroke Scale (NIHSS) ≤ 4, modified Rankin scale (mRS) ≤ 2.
Exclusion criteria were as follows: (1) comorbid with other life-threatening diseases; (2) clinical symptoms can be illustrated by other diseases; (3) with a history of cerebral endovascular surgery; (4) confirmed as moderate or severe intracranial/extracranial arterial stenosis or with ischemic/hemorrhagic stroke history; and (5) poor compliance.
In order to identify the electroencephalogram (EEG) features for CVOD, a cohort of healthy volunteers were also incorporated into this study. This population should have confirmed without any cerebral vascular diseases and with no vascular disease-related risk factors (such as hypertension, diabetes, dyslipidemia, and autoimmunity disease).
Interventions
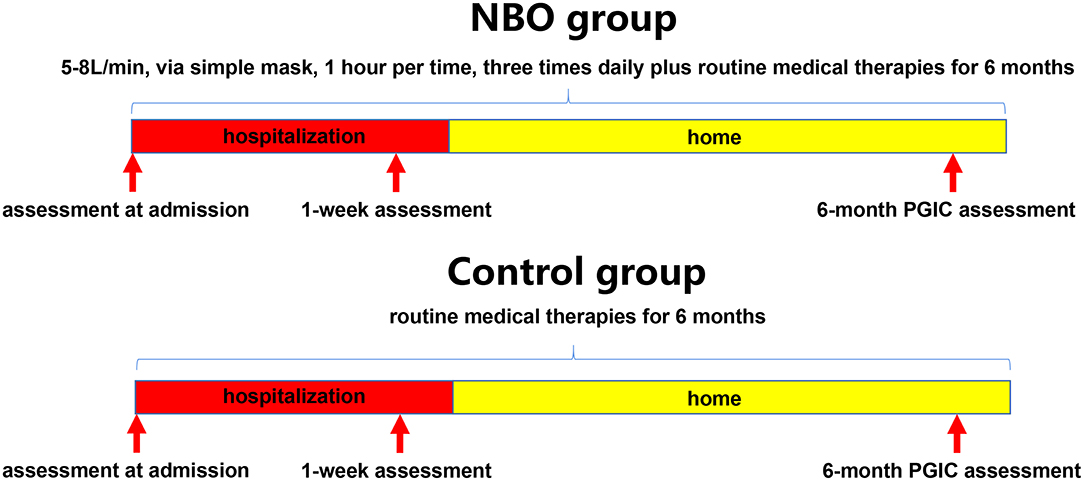
Patients were randomly assigned in a 1:1 ratio to receive routine medical therapies plus oxygen supplement or routine medical therapies alone by means of a random number table; the patients in the NBO group underwent 1 h of 5–8 L/min of oxygen inhaling via a simple mask, by oxygen concentrator each time, three times daily besides routine medical therapies for 6 months (1–2 weeks in a hospital); the patients in the control group only underwent routine medical therapies for 6 months (1–2 weeks in a hospital). Routine medical therapies included neuron nutrition, anti-inflammatory, anti-coagulation, and symptomatic treatments (Figure 1).
Scale Assessment
Each of the enrolled subjects should fill in a questionnaire at admission to delineate the symptoms they had. They would blind to the proposed effectiveness of each treatment strategy in order to avoid subjective bias. According to the common non-specific neurological symptoms seen in the patients with CVOD, some scales were recorded at admission, that is, visual analog scale (VAS; 0–10 scores) and Headache Impact Test-6 (HIT-6; 36–78 scores) for headache; Athens insomnia scale (AIS; 0–24 scores) and insomnia severity index (ISI; 0–28 scores) for insomnia; tinnitus handicap inventory (THI; 0–100 scores) for tinnitus/head noise; and hospital anxiety and depression scale (HADS; 0–42 scores) for anxiety and depression (26–31). Higher scores mean severe symptoms. All of the above scales were recorded again at 1-week hospitalization. Additionally, the Patient Global Impression of Change (PGIC) scale was also assessed at this time (32). A phone call follow-up was performed in each patient at 6 months, and only PGIC scale was recorded due to the constraints of this follow-up method (Figure 1). The assessors were blind to the assignment when evaluating PGIC scale.
Electroencephalogram Acquisition and Analysis
The EEG data in some patients were recorded with the EEG recording equipment (EEG YAL PN-NET, Beijing Yunshen Science and Technology Corporation). Ag/AgCl electrodes were positioned at Fp1, Fp2, Fpz, F3, F4, F7, F8, Fz, C3, C4, Cz, P3, P4, Pz, T3, T4, T5, T6, O1, O2, Oz, A1, and A2 (23 channels) according to the international 10–20 system, with electrode impedances all <5 kΩ. The sampling rate was 256 Hz for all channels using a 16-bit AD convertor. The healthy volunteers underwent 30-min EEG recording for one time, and the CVOD patients underwent 30-min EEG recording for two times. During the interval between the two times of recording, the patients underwent 1 h of 5–8 L/min of NBO via a simple mask. Firstly, compared with healthy EEG, the CVOD-related EEG anomalies would be determined. Secondly, the EEG datasets prior to and post-NBO treatment would be compared to investigate the impact of NBO on brain functions.
Five minutes of artifact-free data was selected from EEG recordings in each subject at random, and these participants should remain awake and lying quietly with eyes closed as much as possible. Channels A1 and A2 were the references. Visual artifact rejection and data filtering (band pass, 1–30 Hz) were performed in EEGLAB (version 2019.1) with supplementary scripts operating in the MATLAB environment (33). Meanwhile, artifact removal (blink artifacts and electromyogram artifacts) was also performed using BSS algorithm (sobi). The absolute power (AP; with units μV2) of the delta (1–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), and beta (13–20 Hz) frequency bands was computed using fast Fourier transform for each electrode with a 2-s Hamming window length. The relative power (RP; the frequency band of interest power/the total power across the 1- to 20-Hz range), theta/alpha AP ratio (TAR), delta/alpha AP ratio (DAR), and (delta+theta)/(alpha+beta) AP ratio (DTABR) were calculated as well. Besides overall channels, the EEG oscillations over the fronto-central electrodes (C3, C4, Cz, F3, F4, and Fz channels) were also used to evaluate the patient's brain function due to the minimal occipital alpha oscillation contamination.
Outcomes
The primary outcome was 1-week PGIC. Patient Global Impression of Change is a self-reported evaluation of symptom improvement by patients; it consists of a 5-point scale (1 = obvious improvement; 2 = mild improvement; 3 = no change; 4 = mild deterioration; and 5 = significant deterioration) (32).
The secondary outcomes include 1-week VAS, HIT-6, AIS, ISI, THI, and HADS scales, and 6-month PGIC evaluated through phone call follow-up. Meanwhile, the EEG features prior to and post-NBO intervention were also compared in order to assess the transient effect of NBO.
Statistical Analysis
R software (http://www.r-project.org) was used for the analysis in this study. Continuous data following a Gaussian distribution were expressed as mean ± standard deviation (SD) and analyzed with Student's t-test; otherwise as median [interquartile range (IQR)] and analyzed with Mann–Whitney U-test. The comparison between prior to and post-intervention used paired samples t-test and Wilcoxon-test. Categorical data were presented as n (percentage) and preceded by Fisher's exact-tests. p-value < 0.05 was indicative of statistical significance.
Results
Patient Demographics
From January 2018 through June 2019, a total of 37 patients who were confirmed as CVOD in the Department of Neurology, Xuanwu Hospital, Capital Medical University, were recruited in this study, in whom 19 cases were labeled as the NBO group and 18 cases were called the control group. The baseline characteristics between the two groups showed no statistical difference. Details are displayed in Table 1.
Safety Outcomes
No NBO-related adverse events occurred during NBO intervention. All patients tolerated NBO treatment during hospitalization and follow-up time period. No major discomfort as a result of NBO intervention was reported.
Primary Outcome
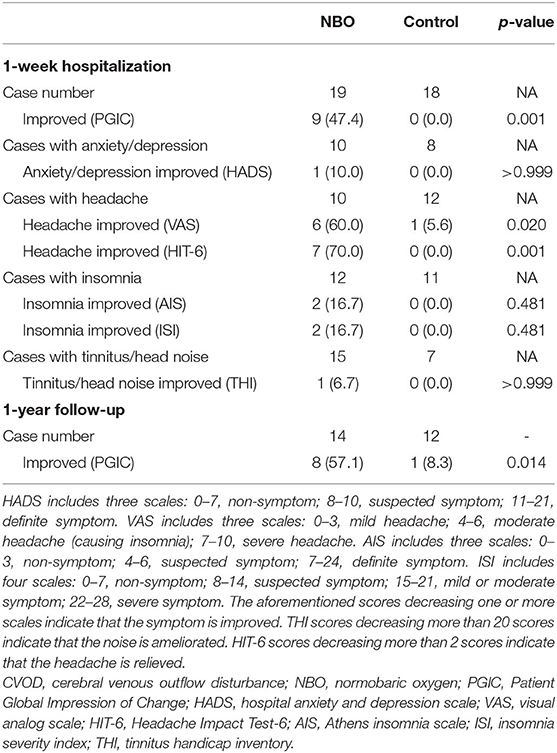
The 1-week PGIC showed that nine out of the 19 patients (47.4%) in the NBO group reported improvement (three cases with remarkable improvement, six cases with mild improvement, seven cases with no change, and three cases with mild deterioration), while no patients in the control group felt that their symptoms improved (11 cases with no change and seven cases with mild deterioration). The difference illustrated a statistical significance (p = 0.001).
Secondary Outcome
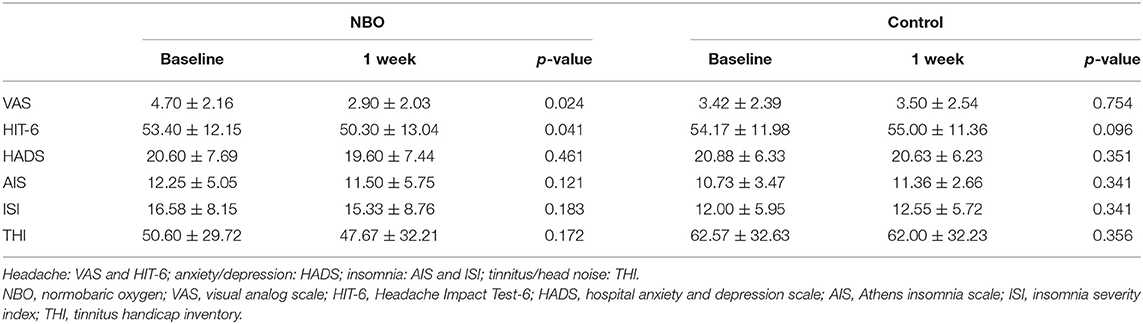
There were 10 patients in the NBO group and 12 patients in the control group complaining of headache. According to the VAS, six patients in the NBO group (60.0%) reported headache ameliorated, while only one patient in the control group (5.6%) felt mildly improved headache (p = 0.020). Compared with the baseline, the VAS in the NBO group was significantly improved at 1-week investigation (4.70 ± 2.16 vs. 2.90 ± 2.03, p = 0.024); however, it did not change at 1 week in the control group (3.42 ± 2.39 vs. 3.50 ± 2.54, p = 0.754). Additionally, there was no difference of VAS between the NBO group and the control group at 1-week hospitalization (p = 0.553). Based on the HIT-6 scale, seven patients (70.0%) in the NBO group reported headache relief, while no one in the control group reported headache attenuation (p = 0.001). The 1-week HIT-6 scale was obviously better than their baseline in the NBO group (53.40 ± 12.15 vs. 50.30 ± 13.04, p = 0.041) but has no changes in the control (54.17 ± 11.98 vs. 55.00 ± 11.36, p = 0.096). However, the difference of the 1-week HIT-6 between the NBO group and the control group did not reach statistical significance (p = 0.377).
Other secondary outcomes including anxiety/depression assessed by HADS scale, insomnia assessed by AIS and ISI scale, and tinnitus/head noise assessed by THI scale showed no statistic difference between the NBO and control groups. The proportions of the improved patients in each group are presented in Table 2, and the scale scores are detailed in Figure 2 and Table 3.
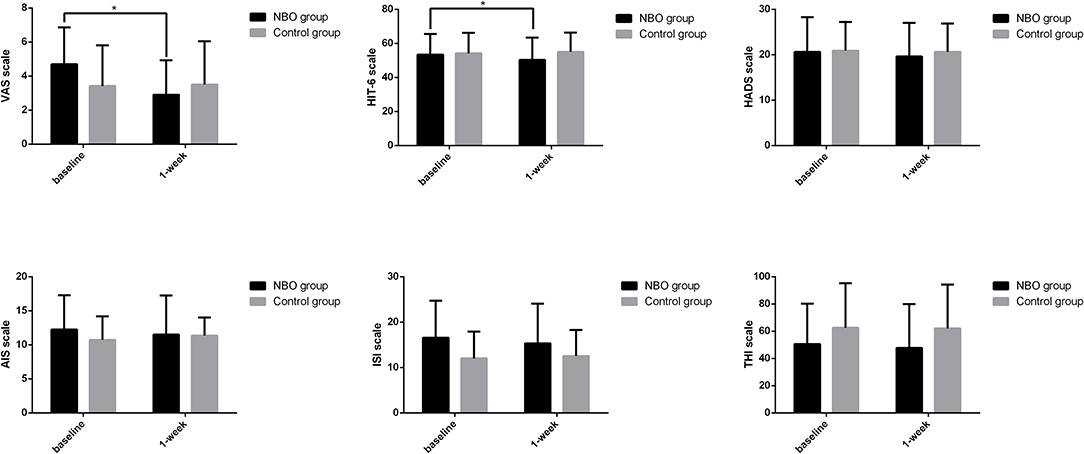
Figure 2. The symptom scales in normobaric oxygen (NBO) and control groups. Visual analog scale (VAS) and Headache Impact Test-6 (HIT-6) scale declined after 1-week NBO performance but remained unchanged after routine medical management. Compared with baseline, 1-week hospital anxiety and depression scale (HADS), Athens insomnia scale (AIS), insomnia severity index (ISI), and tinnitus handicap inventory (THI) improved in the NBO group; however, this change did not reach a statistical significance. *p < 0.05.
6-Month Follow-Up
26 patients (14 in the NBO group and 12 in the control group) finished the follow-up with good compliance. Eight out of 14 cases (57.1%) in the NBO group reported improvement (three remarkable improvement, five mild improvement, four no change, and two mild deterioration) and one out of 12 patients in the control group reported mild improvement (one mild improvement, seven no change, and four mild deterioration), p = 0.014.
Electroencephalogram Analysis
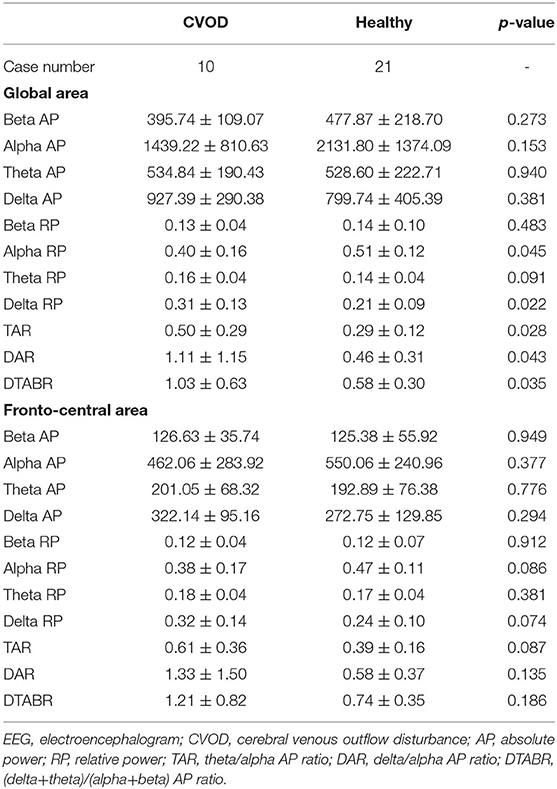
A total of 10 patients (31.25 ± 8.31 years, 2 males and 8 females) and 21 healthy volunteers (30.57 ± 9.31 years, 4 males and 17 females) completed the EEG recording in this study. Visual evaluation showed paroxysmal slow activities in their EEG maps (Ding J. and Liu Y. evaluated the EEG maps in a blind manner). Further quantitative analysis indicated that the slow-frequency activities increased in the CVOD-related EEG, all of which are detailed in Table 4.
The impacts of NBO on the abnormal EEG were evaluated in eight out of 10 CVOD patients; two cases were ruled out due to inevitable artifact in the second EEG recordings. After 1 h of NBO intervention, the global slow activities declined, and the fast activities increased compared with the baseline (prior to vs. post-NBO, delta: 0.32 ± 0.14 vs. 0.31 ± 0.14, p = 0.756; theta: 0.16 ± 0.04 vs. 0.15 ± 0.03, p = 0.098; alpha: 0.39 ± 0.17 vs. 0.40 ± 0.17, p = 0.536; and beta: 0.12 ± 0.04 vs. 0.13 ± 0.04, p = 0.375), despite no significance. The global TAR, DAR, and DTABR were reduced but with no significance as well (TAR: 0.53 ± 0.31 vs. 0.49 ± 0.32, p = 0.242; DAR: 1.25 ± 1.26 vs. 1.25 ± 1.31, p = 0.991; and DTABR: 1.11 ± 0.68 vs. 1.02 ± 0.62, p = 0.492). As for the fronto-central electrodes, the slow activities declined and the fast activities increased after 1-h NBO performance but did not reach statistical significance as well (delta: 0.33 ± 0.16 vs. 0.31 ± 0.13, p = 0.261; theta: 0.18 ± 0.04 vs. 0.17 ± 0.03, p = 0.212; alpha: 0.37 ± 0.18 vs. 0.39 ± 0.17, p = 0.218; and beta: 0.11 ± 0.04 vs. 0.13 ± 0.05, p = 0.168). Notably, the fronto-central TAR was significantly declined after undergoing NBO (0.65 ± 0.38 vs. 0.56 ± 0.35, p = 0.030); the DAR and DTABR also tended to be lower post-NBO performance (DAR: 1.50 ± 1.64 vs. 1.20 ± 1.13, p = 0.207 and DTABR: 1.31 ± 0.88 vs. 1.06 ± 0.58, p = 0.098). All of above are shown in Figure 3.
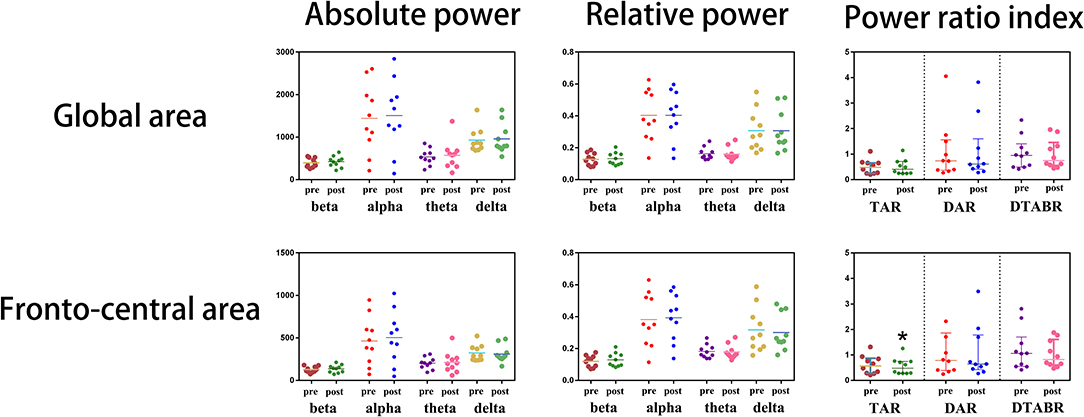
Figure 3. Immediate electroencephalogram (EEG) analysis before and after normobaric oxygen (NBO) intervention.
Discussion
In this study, we found that NBO might relieve CVOD-related symptoms in a short time, especially for headache, and this effect has potential to be maintained for a long run. This may be due to the correction of CVOD-induced brain dysfunctions presented in the EEG maps afforded by NBO performance. As there are few effective methods to ameliorate CVOD-related symptoms currently, this study provided a novel approach on controlling this refractory disease entity.
After 1-week NBO use, 47.7% of the patients reported that their symptoms attenuated, especially headache relief, whereas at the same period, patients without NBO use complained of symptoms being unchanged or even deteriorated. As previous publications report, CVOD-related non-focal neurological symptoms are classified into three types: the ischemic–hypoxic condition always causes headache, insomnia, dizziness, dry eyes, uncomfortable neck, anxiety, and memory decline; the abnormal collateral vessel nets and the blood outflow turbulence contribute to tinnitus, head noise, and hearing impairment; severe IH leads to massive headache, visual impairment, and fierce vomiting (2–6). NBO is capable of elevating pO2 per volume in the brain tissue immediately, which can improve the ischemic–hypoxic condition, so as to ameliorate relevant non-focal neurological symptoms (16). The pO2 elevation in the brain tissue is the rationale for the protective effect afforded by NBO on CVOD. Tinnitus or head noise is always considered to be mainly caused by abnormal vessel dilation and blood outflow turbulence, whereby transient NBO may not relieve these symptoms effectively. NBO cannot treat CVOD-related IH as well and is less likely being helpful in visual recovery. In contrast, stenting in stenosis segments is useful in reducing high intracranial pressure (7, 10, 34). After long-term continuous NBO treatment, half of the patients in the NBO group reported that their symptoms ameliorated; despite this, a detailed questionnaire cannot be finished due to the constraints of the phone call follow-up.
In general, the subjective assessment outcomes indicated that NBO might yield some benefit to improve hypoxia-related symptoms within a short period of time but could not get rid of the structural anomaly-related symptoms. The structural anomaly could be modified by endovascular interventions as reported in some previous publications (7, 10, 34, 35). Despite that endovascular stenting is able to restore the venous outflow, some patients with osseous impingement or other external compression induced CVOD cannot gain the benefit from stenting (4). Meanwhile, the surgery is not without risk because a proper stent for the intracranial or extracranial veins is not still invented. NBO may be an appropriate and convenient adjuvant method to relieve CVOD-related symptoms.
Because the aforementioned subjective assessment may be not convincing enough, we select EEG as the powerful objective evidence in this study. EEG is a sensitive approach for detecting cerebral ischemia and hypoxia (36). The abnormal enhancement in the delta and theta activities could be always seen in the ischemic EEG maps. Cerebral venous outflow disturbance-induced CCI may also contain the abnormal increased slow activities (36). Compared with the EEG maps in health, CVOD-related EEG had higher delta and theta RP accompanied with lower alpha and beta RP over the global area of the brain. The RP mainly reflects the components of the EEG oscillations; higher slow-frequency RP means more frequent slow-activity release. Moreover, the TAR, DAR, and DTABR, which represent the ratio of fast activities to slow activities, were significantly higher in the CVOD EEG in comparison with healthy EEG. This further provided more convincing evidences that CVOD could promote the slow-frequency oscillation release and retard the fast-frequency activities. The slow-activity RP and the TAR, DAR, and DTABR were considered to be associated with the neurological outcome in acute stroke patients (37–39). These indices are different from those of the healthy EEG in the CVOD-related EEG that represented brain dysfunctions.
After 1-h NBO intervention, both the global and fronto-central slow activities were suppressed, and the fast activities were restored, however without statistical significance. It is noteworthy that the fronto-central TAR was significantly decreased after undergoing NBO, and the DAR and DTABR were also reduced but without significance. These results indicated that NBO has potential to improve CVOD-related brain dysfunctions, which might further provide objective evidences to verify the efficacy of NBO on CVOD. As the short-term brain functional improvement, we hypothesized that NBO can also be used for patient pre-conditioning, especially prior to endovascular stenting or balloon dilating in the cerebral or jugular venous stenosis. Notably, NBO is capable of promoting exogenous erythropoietin (EPO) production, which plays a pivotal role in anti-apoptosis and neuroprotection in patients with cerebral ischemia (40). Brain functional improvement prior to surgery may enlarge the benefits and decrease the complications in a long run.
There are several limitations that need to be addressed. (1) The sample size was small to reach a statistical significance in some indices. (2) Most of the clinical outcome evaluation methods were based on subjective questionnaires. Despite the blinded manner, the subjective assessors may bias the results toward null hypothesis to some extent. (3) A simple mask was used to inhale oxygen in this study; however, more advanced devices should be used in the future, such as a double-trunk mask, which is easy to use and may produce better results (41). (4) We declared that NBO might only relieve some part of symptoms, especially headache. Surgery is still the ultimate method to reopen the stenosed veins and restore the venous outflow, despite that the evidence and experience are still far from enough.
Conclusions
NBO may be a safe and promising adjuvant therapy to ameliorate CVOD-related symptoms, especially for headache. It can also correct CVOD-related EEG anomalies in some degree. Considering the aforementioned limitations in this study, a larger sample size and well-designed prospective clinical trial is needed to further verify our conclusions in the future.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Ethic Committee of Xuanwu Hospital, Capital Medical University (Beijing, China). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JD and YL performed all the statistical analyses with the guidance of the Department of Statistics of Capital Medical University and drafted the manuscript. JD generated the random allocation sequence. XL provided suggestions for the study design and revised the manuscript. ZC, JG, KJ, and ZW selected appropriate subjects and conducted the assessments. RM, YD, and XJ reviewed and edited the manuscript. XJ and RM contributed to the conception and design of this study and proposed the amendments. All authors contributed to the article and approved the submitted version.
Funding
This study was sponsored by the National Key R&D Program of China (2017YFC1308401), the National Natural Science Foundation of China (81371289), and the Project of Beijing Municipal Top Talent for Healthy Work of China (2014-2-015).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank all patients and doctors who participated in this study for their cooperation.
References
1. Zivadinov R, Chung CP. Potential involvement of the extracranial venous system in central nervous system disorders and aging. BMC Med. (2013) 11:260. doi: 10.1186/1741-7015-11-260
2. Zhou D, Ding JY, Ya JY, Pan LQ, Yan F, Yang Q, et al. Understanding jugular venous outflow disturbance. CNS Neurosci Ther. (2018) 24:473–82. doi: 10.1111/cns.12859
3. Zhou D, Ding J, Asmaro K, Pan L, Ya J, Yang Q, et al. Clinical characteristics and neuroimaging findings in internal jugular venous outflow disturbance. Thromb Haemost. (2019) 119:308–18. doi: 10.1055/s-0038-1676815
4. Ding JY, Zhou D, Pan LQ, Ya JY, Liu C, Yan F, et al. Cervical spondylotic internal jugular venous compression syndrome. CNS Neurosci Ther. (2020) 26:47–54. doi: 10.1111/cns.13148
5. Ding J, Guan J, Rajah G, Iii DD, Li W, Wang Z, et al. Clinical and neuroimaging correlates among cohorts of cerebral arteriostenosis, venostenosis and arterio-venous stenosis. Aging (Albany NY). (2019) 11:11073–83. doi: 10.18632/aging.102511
6. Bai C, Xu Y, Zhou D, Ding J, Yang Q, Ding Y, et al. The comparative analysis of non-thrombotic internal jugular vein stenosis and cerebral venous sinus stenosis. J Thromb Thrombolysis. (2019) 48:61–7. doi: 10.1007/s11239-019-01820-1
7. Ding J, Guan J, Ji X, Meng R. Cerebral venous sinus stenosis may cause intracranial arterial hypoperfusion. Clin Neuroradiol. (2020) 30:409–11. doi: 10.1007/s00062-019-00833-w
8. Ahmed RM, Wilkinson M, Parker GD, Thurtell MJ, Macdonald J, McCluskey PJ, et al. Transverse sinus stenting for idiopathic intracranial hypertension: a review of 52 patients and of model predictions. Am J Neuroradiol. (2011) 32:1408–14. doi: 10.3174/ajnr.A2575
9. De Simone R, Ranieri A, Bonavita V. Advancement in idiopathic intracranial hypertension pathogenesis: focus on sinus venous stenosis. Neurol Sci. (2010) 31(Suppl. 1):S33–9. doi: 10.1007/s10072-010-0271-z
10. Li K, Ren M, Meng R, Ding Y, Rajah GB, Wang F, et al. Efficacy of stenting in patients with cerebral venous sinus thrombosis-related cerebral venous sinus stenosis. J Neurointerv Surg. (2019) 11:307–12. doi: 10.1136/neurintsurg-2018-014328
11. Puffer RC, Mustafa W, Lanzino G. Venous sinus stenting for idiopathic intracranial hypertension: a review of the literature. J Neurointerv Surg. (2013) 5:483–6. doi: 10.1136/neurintsurg-2012-010468
12. Satti SR, Leishangthem L, Chaudry MI. Meta-analysis of CSF diversion procedures and dural venous sinus stenting in the setting of medically refractory idiopathic intracranial hypertension. Am J Neuroradiol. (2015) 36:1899–904. doi: 10.3174/ajnr.A4377
13. Ding J, Zhou D, Liu C, Pan L, Ya J, Ding Y, et al. Normobaric oxygen: a novel approach for treating chronic cerebral circulation insufficiency. Clin Interv Aging. (2019) 14:565–70. doi: 10.2147/CIA.S190984
14. Rusyniak DE, Kirk MA, May JD, Kao LW, Brizendine EJ, Welch JL, et al. Hyperbaric oxygen therapy in acute ischemic stroke: results of the hyperbaric oxygen in acute ischemic stroke trial pilot study. Stroke. (2003) 34:571–4. doi: 10.1161/01.STR.0000050644.48393.D0
15. Ding J, Zhou D, Sui M, Meng R, Chandra A, Han J, et al. The effect of normobaric oxygen in patients with acute stroke: a systematic review and meta-analysis. Neurol Res. (2018) 40:433–44. doi: 10.1080/01616412.2018.1454091
16. Liu S, Shi H, Liu W, Furuichi T, Timmins GS, Liu KJ. Interstitial pO2 in ischemic penumbra and core are differentially affected following transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. (2004) 24:343–9. doi: 10.1097/01.WCB.0000110047.43905.01
17. Dunn AK, Bolay H, Moskowitz MA, Boas DA. Dynamic imaging of cerebral blood flow using laser speckle. J Cereb Blood Flow Metab. (2001) 21:195–201. doi: 10.1097/00004647-200103000-00002
18. Liu S, Liu W, Ding W, Miyake M, Rosenberg GA, Liu KJ. Electron paramagnetic resonance-guided normobaric hyperoxia treatment protects the brain by maintaining penumbral oxygenation in a rat model of transient focal cerebral ischemia. J Cereb Blood Flow Metab. (2006) 26:1274–84. doi: 10.1038/sj.jcbfm.9600277
19. Shi S, Qi Z, Ma Q, Pan R, Timmins GS, Zhao Y, et al. Normobaric hyperoxia reduces blood occludin fragments in rats and patients with acute ischemic stroke. Stroke. (2017) 48:2848–54. doi: 10.1161/STROKEAHA.117.017713
20. Dong W, Qi Z, Liang J, Shi W, Zhao Y, Luo Y, et al. Reduction of zinc accumulation in mitochondria contributes to decreased cerebral ischemic injury by normobaric hyperoxia treatment in an experimental stroke model. Exp Neurol. (2015) 272:181–9. doi: 10.1016/j.expneurol.2015.04.005
21. Poli S, Veltkamp R. Oxygen therapy in acute ischemic stroke—experimental efficacy and molecular mechanisms. Curr Mol Med. (2009) 9:227–41. doi: 10.2174/156652409787581619
22. Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health. (2008) 31:180–91. doi: 10.1002/nur.20247
23. Dobkin BH. Progressive staging of pilot studies to improve phase III trials for motor interventions. Neurorehabil Neural Repair. (2009) 23:197–206. doi: 10.1177/1545968309331863
24. Dolic K, Siddiqui AH, Karmon Y, Marr K, Zivadinov R. The role of noninvasive and invasive diagnostic imaging techniques for detection of extra-cranial venous system anomalies and developmental variants. BMC Med. (2013) 11:155. doi: 10.1186/1741-7015-11-155
25. Traboulsee AL, Knox KB, Machan L, Zhao Y, Yee I, Rauscher A, et al. Prevalence of extracranial venous narrowing on catheter venography in people with multiple sclerosis, their siblings, and unrelated healthy controls: a blinded, case-control study. Lancet. (2014) 383:138–45. doi: 10.1016/S0140-6736(13)61747-X
26. Aitken RC. Measurement of feelings using visual analogue scales. Proc R Soc Med. (1969) 62:989–93. doi: 10.1177/003591576906201005
27. Kosinski M, Bayliss MS, Bjorner JB, Ware JE Jr, Garber WH, Batenhorst A, et al. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res. (2003) 12:963–74. doi: 10.1023/A:1026119331193
28. Soldatos CR, Dikeos DG, Paparrigopoulos TJ. The diagnostic validity of the athens insomnia scale. J Psychosom Res. (2003) 55:263–7. doi: 10.1016/S0022-3999(02)00604-9
29. Bastien CH, Vallieres A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. (2001) 2:297–307. doi: 10.1016/S1389-9457(00)00065-4
30. Kam AC, Cheung AP, Chan PY, Leung EK, Wong TK, van Hasselt CA, et al. Psychometric properties of the Chinese (Cantonese) Tinnitus Handicap inventory. Clin Otolaryngol. (2009) 34:309–15. doi: 10.1111/j.1749-4486.2009.01946.x
31. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. (1983) 67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x
32. Hurst H, Bolton J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J Manipulative Physiol Ther. (2004) 27:26–35. doi: 10.1016/j.jmpt.2003.11.003
33. Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. (2004) 134:9–21. doi: 10.1016/j.jneumeth.2003.10.009
34. Zhou D, Meng R, Zhang X, Guo L, Li S, Wu W, et al. Intracranial hypertension induced by internal jugular vein stenosis can be resolved by stenting. Eur J Neurol. (2018) 25:365-e13. doi: 10.1111/ene.13512
35. Yan F, Rajah G, Ding Y, Hua Y, Zhang H, Jiao L, et al. Safety and efficacy of intravascular ultrasound as an adjunct to stenting for cerebral venous sinus stenosis-induced idiopathic intracranial hypertension: a pilot study. J Neurosurg. (2019) 1–6. doi: 10.3171/2018.11.JNS181885
36. Finnigan S, van Putten MJ. EEG in ischaemic stroke: quantitative EEG can uniquely inform (sub-)acute prognoses and clinical management. Clin Neurophysiol. (2013) 124:10–9. doi: 10.1016/j.clinph.2012.07.003
37. Finnigan SP, Walsh M, Rose SE, Chalk JB. Quantitative EEG indices of sub-acute ischaemic stroke correlate with clinical outcomes. Clin Neurophysiol. (2007) 118:2525–32. doi: 10.1016/j.clinph.2007.07.021
38. Leon-Carrion J, Martin-Rodriguez JF, Damas-Lopez J, Barroso y Martin JM, Dominguez-Morales MR. Delta-alpha ratio correlates with level of recovery after neurorehabilitation in patients with acquired brain injury. Clin Neurophysiol. (2009) 120:1039–45. doi: 10.1016/j.clinph.2009.01.021
39. Sheorajpanday RV, Nagels G, Weeren AJ, De Surgeloose D, De Deyn PP. Additional value of quantitative EEG in acute anterior circulation syndrome of presumed ischemic origin. Clin Neurophysiol. (2010) 121:1719–25. doi: 10.1016/j.clinph.2009.10.037
40. Rocco M, D'Itri L, De Bels D, Corazza F, Balestra C. The “normobaric oxygen paradox”: a new tool for the anesthetist. Minerva Anestesiol. (2014) 80:366–72.
Keywords: normobaric oxygen, cerebral venous outflow disturbance, brain dysfunction, EEG, neurological impairment
Citation: Ding J, Liu Y, Li X, Chen Z, Guan J, Jin K, Wang Z, Ding Y, Ji X and Meng R (2020) Normobaric Oxygen May Ameliorate Cerebral Venous Outflow Disturbance-Related Neurological Symptoms. Front. Neurol. 11:599985. doi: 10.3389/fneur.2020.599985
Received: 28 August 2020; Accepted: 13 October 2020;
Published: 13 November 2020.
Edited by:
Massimiliano Valeriani, Bambino Gesù Children Hospital (IRCCS), ItalyReviewed by:
Costantino Balestra, Haute École Bruxelles-Brabant (HE2B), BelgiumMichela Ada Noris Ferilli, Bambino Gesù Children Hospital (IRCCS), Italy
Copyright © 2020 Ding, Liu, Li, Chen, Guan, Jin, Wang, Ding, Ji and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ran Meng, cmFubWVuZzIwMTFAcGt1Lm9yZy5jbg==
†These authors have contributed equally to this work and share first authorship
 Jiayue Ding1,2†
Jiayue Ding1,2† Zhiying Chen
Zhiying Chen Zhongao Wang
Zhongao Wang Yuchuan Ding
Yuchuan Ding Ran Meng
Ran Meng