
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 11 April 2025
Sec. Neurocognitive Aging and Behavior
Volume 17 - 2025 | https://doi.org/10.3389/fnagi.2025.1540040
Introduction: The relevance of electroencephalographic (EEG) biomarkers is increasing, as advancements in spectral analysis enable computational decomposition of complex neural signals into quantitative EEG (qEEG) parameters. Especially the differentiation of periodic and aperiodic components can reveal insights into neural function, disease biomarkers, and therapeutic efficacy. The aim of these analyses from real-world clinical routine EEG recordings was to provide normative values of physiological age-related oscillatory (periodic) and non-rhythmic (aperiodic) activity.
Methods: We analyzed 532 physiological EEGs of patients between 8 and 92 years of age. EEG segments were preprocessed, and the power spectrum was computed using a multitaper method. We decomposed the power spectrum into periodic (peak power, frequency, and bandwidth) and aperiodic (intercept and exponent) components. Linear regression models were used to investigate age-related changes in these parameters.
Results: We observed significant global age-related changes in the periodic alpha (−0.015 Hz/year) and gamma (+0.013 to +0.031 Hz/year) peak frequency as well as in the aperiodic exponent (−0.003 to −0.004 μV2/Hz/year). In the other parameters there were solely regional or no significant age-related changes.
Conclusion: Decomposing the power spectrum into periodic and aperiodic components allows for the characterization of age-related changes.
Significance: This study provides the first spectrum-wide normative characterization of age-related changes in periodic and aperiodic activity, relevant for non-invasive brain stimulation with alternating current targeting ongoing oscillatory activity.
Electroencephalography (EEG) provides essential insights into the dynamic changes in neural activity across various life stages. The nuanced age-related alterations in both periodic and aperiodic EEG activity reveal complex patterns of neural development and aging (Donoghue et al., 2020). From early childhood to elderly adulthood, the aperiodic activity diminishes (Hill et al., 2022). These alterations may arise from a shift in the balance between oscillatory coupling and local population spiking (Voytek and Knight, 2015). Likewise, aging alters dynamic network communication, which is primarily reflected by changes in the periodic components of the spectrum (Jauny et al., 2024; Tröndle et al., 2023). A well described phenomenon is the slowing of the center frequency in the alpha range, that is integral to attention and cognition processes (Cesnaite et al., 2023).
Recent methodological advancements offer an apt means to decompose the EEG power spectrum into rhythmic oscillations (periodic component) and non-rhythmic fluctuations (aperiodic component). It helps to dissect complex neural signals (Leroy et al., 2022), enrich our understanding of brain function (Lendner et al., 2020), identify potential biomarkers of disease (Pollak et al., 2024), and assess the effectiveness of a therapy (Kundu et al., 2023; Salvatore et al., 2023). The strength of the approach particularly stems from its reflection of the EEG signal’s two-fold nature, encompassing both its mathematical characteristics in signal analysis and its neurophysiological correlates. The underlying aperiodic activity, distributed in a 1/f-manner, has been linked to the cortical balance of synaptic excitation and inhibition in computational modeling trials (Wang, 2020). In contrast, the superimposed periodic activity involves an interplay of cortical neural networks partly orchestrated by subcortical nodes (Seeber et al., 2019), and is estimated by gaussians centered around the oscillatory peaks.
Quantitative EEG (qEEG) analysis emerged from digital signal analysis and spread itself quickly, due to increasing capacities of commonly used computers (Höller, 2021). In classical, clinical EEG analysis, the recordings are inspected visually by trained experts regarding the occurrence of specific patterns (Zhang et al., 2023). In contrast, qEEG analysis uses computer-based methods to breakdown EEG signals, allowing for the quantification of signal components both at specific channels and between channels (Gavaret et al., 2023). Normative databases of qEEG have been emerging and are essential to develop EEG biomarkers of diseases (Ko et al., 2021; Prichep, 2005).
Therefore, this analysis of clinical data aims to describe the age-related periodic and aperiodic activity in 532 physiological real-world resting-state EEG recordings by decomposing the spectrum into its periodic and aperiodic components. We derive a comprehensive method to estimate the age-adjusted periodic and aperiodic parameters.
This analysis was conducted with EEG data from a German tertiary care university hospital. This study was approved by the local ethics committee (BB 103/20). The quality standard regarding ethical and scientific data collection followed the ICH-GCP guidelines. Formal consent was obtained from the data protection board to allow handling and pseudonymization of clinical routine data.
The analyzed EEG data was recorded between 2004 and 2014. EEG recordings included in this study were obtained as part of routine clinical assessments, often for the diagnostic work-up of transient neurological symptoms such as presyncope, syncope, migraine aura, or transient sensory disturbances. Along with the EEG recording, a brief medical history as well as the interpretation of the neurologist in charge were assessed. The electrodes were placed with a common montage following the 10/20-system at the following 19 electrode positions: Fp1, Fp2, Fz, F3, F4, F7, F8, Cz, C3, C4, T7, T8, Pz, P3, P4, P7, P8, O1, and O2. The EEG was recorded with a commercially available system used in the clinical routine (Galileo.NET, BE Light system, EB Neuro S.p.A., Firenze, Italy) with a sampling frequency of 256 Hz. The reference and ground electrode were placed at A1 and A2, respectively. The EEG recordings were standard clinical resting-state EEGs, acquired following IFCN guidelines (Nuwer et al., 1998). Patients were instructed to close their eyes during the recording to minimize movement artifacts and ensure stable resting-state conditions. While provocation maneuvers (e.g., hyperventilation, photic stimulation) were conducted as part of the routine EEG procedure, these segments were excluded from the analysis.
For each patient, EEG epochs were selected based on the absence of artifacts and provocation maneuvers to ensure signal integrity. A minimum of 20 artifact-free and provocation-free epochs were retained per patient, corresponding to a total of 200 s of usable EEG data per participant. The number of selected epochs was kept consistent across all recordings to allow for uniform comparisons. The selection process was designed to maintain an equal sample size across individuals.
EEG recordings were visually inspected and interpreted by a neurology resident and a senior consultant. The classification as ‘physiological EEG’ was based on the absence of abnormalities, including deviations in ground frequency, slowing, epileptiform discharges, burst suppression, or seizure activity. Only recordings explicitly deemed physiological in the written clinical report were included in the database of clinical recordings. We intentionally did not apply further exclusion criteria like age or medical conditions.
For each patient 20 epochs of 10 s free of artifacts or provocation maneuvers were selected. All analyses were conducted in MATLAB (MATLAB R2023b, 155 Natick, Massachusetts: The MathWorks Inc.; 2023.) with the Chronux toolbox (version 2.12 v03, http://chronux.org/) (Mitra and Bokil, 2008). Analyses were performed separately for every single patient, channel, and epoch. Preprocessing included trendline removal and bandpass filtering (0–45 Hz). The canonical frequency bands were defined as following: delta (δ, 1–4 Hz), theta (θ, 4–7 Hz), alpha (α, 7–12 Hz), low beta (β1, 12–20 Hz), high beta (β2, 20–30 Hz), and gamma (γ, 30–45 Hz).
A multitaper method was applied to estimate the power spectrum with a moving window length of 2 s, a shift of 0.1 s, a time-bandwidth product of 2 and 3 Slepian tapers. We did not conduct a normalization of the power spectrum to be able to assess age-related changes. The power spectrum was decomposed into its periodic and aperiodic components using the FOOOF toolbox with default settings: peak width limit 0.5–12 Hz, infinite maximum number of peaks, minimum peak height of 0 μV2, peak threshold of 2 standard deviations and a fixed aperiodic mode without a knee parameter (Donoghue et al., 2020).
We characterized the aperiodic offset and exponent, respectively the intercept and the slope of the aperiodic activity. The FOOOF toolbox parametrizes the periodic activity as fitted gaussians over the aperiodic slope with the center frequency, the adjusted periodic power, and the bandwidth. Instead of defining the power of the alpha peak as the total power within a canonical band, we searched if a power peak with a center frequency within the range of interest was fitted and the associated adjusted periodic power and bandwidth was assessed (Figure 1). If more than one power peak was comprised within the band of interest, the peak with the highest power was selected. Topographic representations were performed with the topoplot function in EEGlab (version 2023.1) (Delorme and Makeig, 2004).
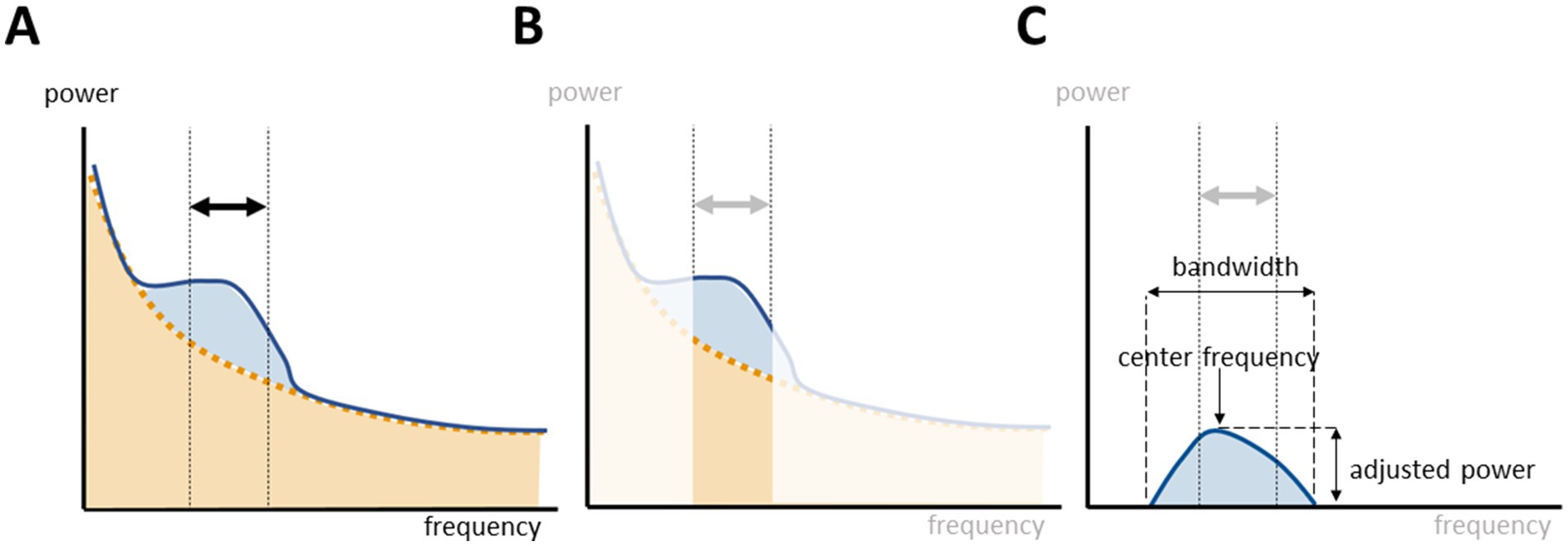
Figure 1. Parametrization of oscillatory activity within a frequency range of interest. (A) Power spectrum decomposition with fitting of the aperiodic (orange) and periodic (blue) components. (B) The power within a canonical band comprises the periodic and aperiodic components of the power spectrum. (C) The FOOOF toolbox parametrizes the periodic activity by fitting gaussians of oscillatory activity over the underlying aperiodic slope.
All statistical analyses were performed in MATLAB. Electrodes were grouped and averaged into five regions: frontal (Fp1, Fp2, Fz, F3, F4, F7, and F8), central (Cz, C3, and C4), temporal (T7 and T8), parietal (Pz, P3, P4, P7, and P8), and occipital (O1 and O2). Linear regressions were fitted for each parameter with the fitlm function. We extracted the residual mean standard error (RMSE)—the spread of the empirical data around the linear fit, the coefficient—the change in value each year, and the intercept—the theoretical frequency at 0 years—for each parameter. The linear models were compared to models with only a constant to test for significance. Intrasubject variability of the peak center frequency was defined as the standard deviation of the center frequency within 20 epochs for each patient within a single EEG recording.
We defined the age-adjusted values of periodic and aperiodic parameters based on the results of the linear regression models. The age-adjusted frequency can be calculated using with the linear formula:
A total of 10.620 EEG epochs from 532 patients were included in this analysis (Table 1 and Figure 2).
An average topographical representation of the center frequency dynamics within all power bands can be found in Figure 2. A global age-related change in the center frequency was solely found in the alpha and gamma range. In the alpha band, there was a significant decrease of around 0.01 Hz per year, while we saw an increase in the gamma peak frequency between 0.01 and 0.03 Hz per year, depending on the region. In the other frequency bands, significant changes were regional and had smaller orders of magnitude. The RMSE depends on the frequency band and is generally larger in the faster oscillatory ranges. A similar dynamic was observed for intrasubject variability (Figure 3 and Supplementary Table S1).
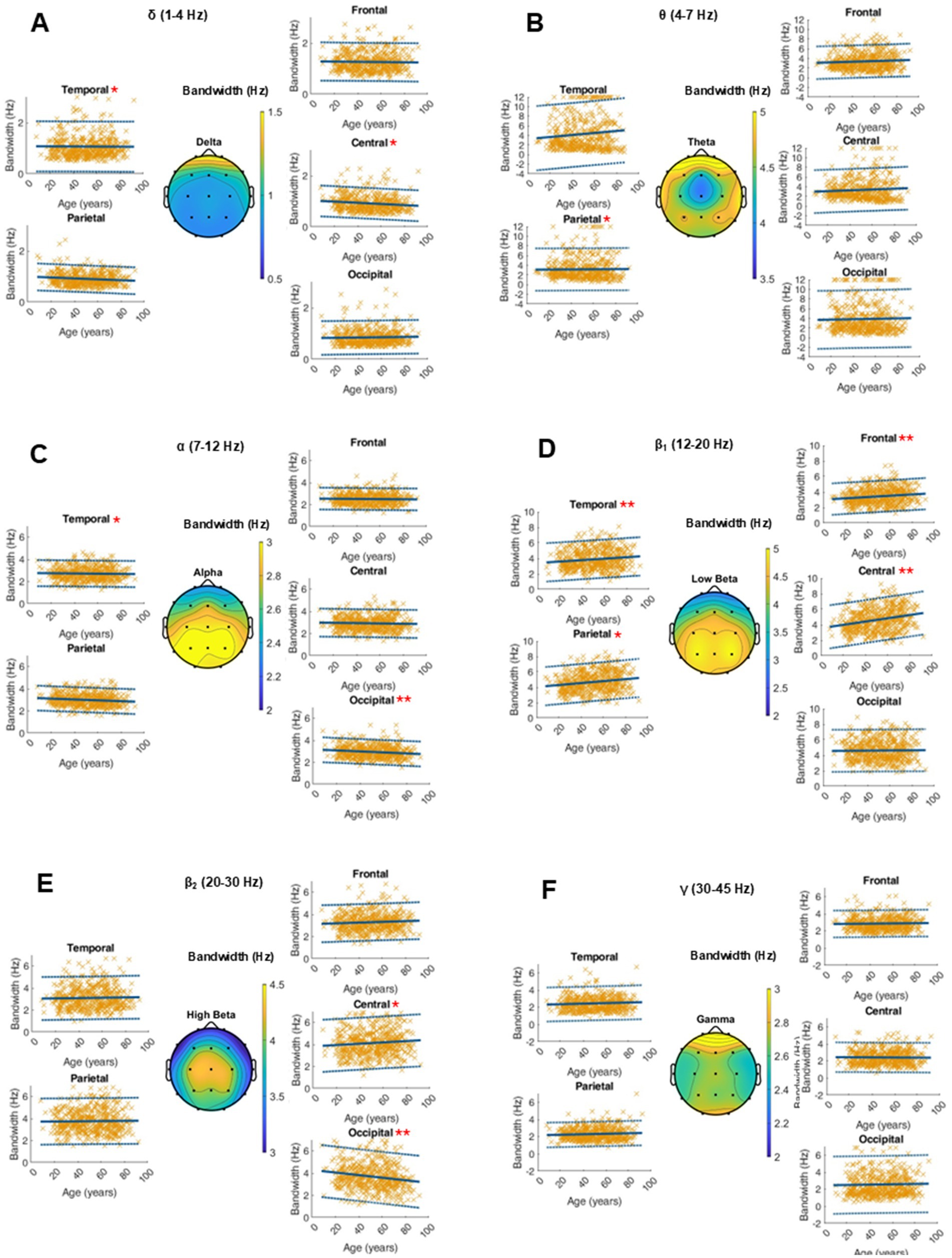
Figure 3. Characterization of peak frequency (Hz) within the frequency bands of interest on the average of 20 single 10-s epochs per patient. For each frequency band, an averaged topographical representation of the center frequency was computed, and the linear regression (thick blue line) as well as the 95% confidence interval (dashed blue lines) was plotted for each parameter. The frequency bands were defined as follows: delta (A, 1–4 Hz), theta (B, 4–8 Hz), alpha (C, 8–12 Hz), low beta (D, 12–20 Hz), high beta (E, 20–30 Hz), and gamma (F, 30–50 Hz). If the p-value was below the significance level of 0.05, the statistical significance was reported with red asterisks (*: p-value 0.05–0.001; **: p-value ≤0.001).
The age-related changes in the adjusted power of the fitted peaks is shown in Figure 3. Age did not show a globally significant effect on the adjusted power within any frequency band. The RMSE, coefficients, intercept, and p-values as well as the intrasubject variability of the adjusted power within the ranges of interest can be found in the supplementary materials (Figure 4 and Supplementary Table S2).
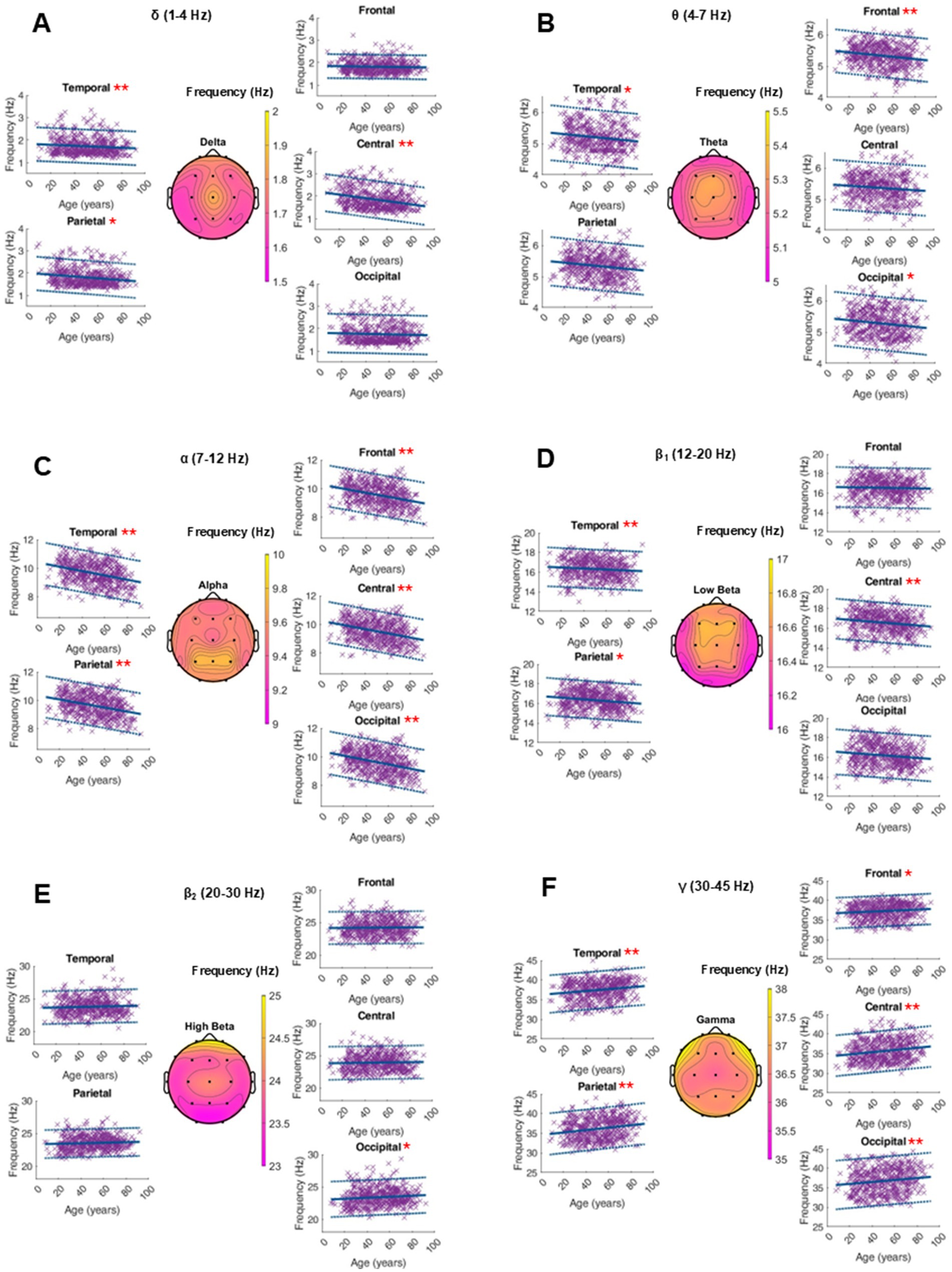
Figure 4. Characterization of peak power (dB) within the frequency bands of interest on the average of 20 single 10-s epochs per patient. For each frequency band, an averaged topographical representation of the peak power was computed, and the linear regression (thick blue line) as well as the 95% confidence interval (dashed blue lines) was plotted for each parameter. The frequency bands were defined as follows: delta (A, 1–4 Hz), theta (B, 4–8 Hz), alpha (C, 8–12 Hz), low beta (D, 12–20 Hz), high beta (E, 20–30 Hz), and gamma (F, 30–50 Hz). If the p-value was below the significance level of 0.05, the statistical significance was reported with red asterisks (*: p-value 0.05–0.001; **: p-value ≤0.001).
Age-related changes in the bandwidth of the fitted oscillatory peaks are depicted in Figure 4. There were no global dynamics for this parameter. In the low beta range, we saw a significant age-related increase of the peak bandwidth in the frontal, parietal, central and temporal electrodes of 0.01 to 0.02 Hz per year. The RMSE, coefficients, intercept, and p-values as well as the intrasubject variability of the peak bandwidth within the ranges of interest can be found in the supplementary materials (Figure 5 and Supplementary Table S3).
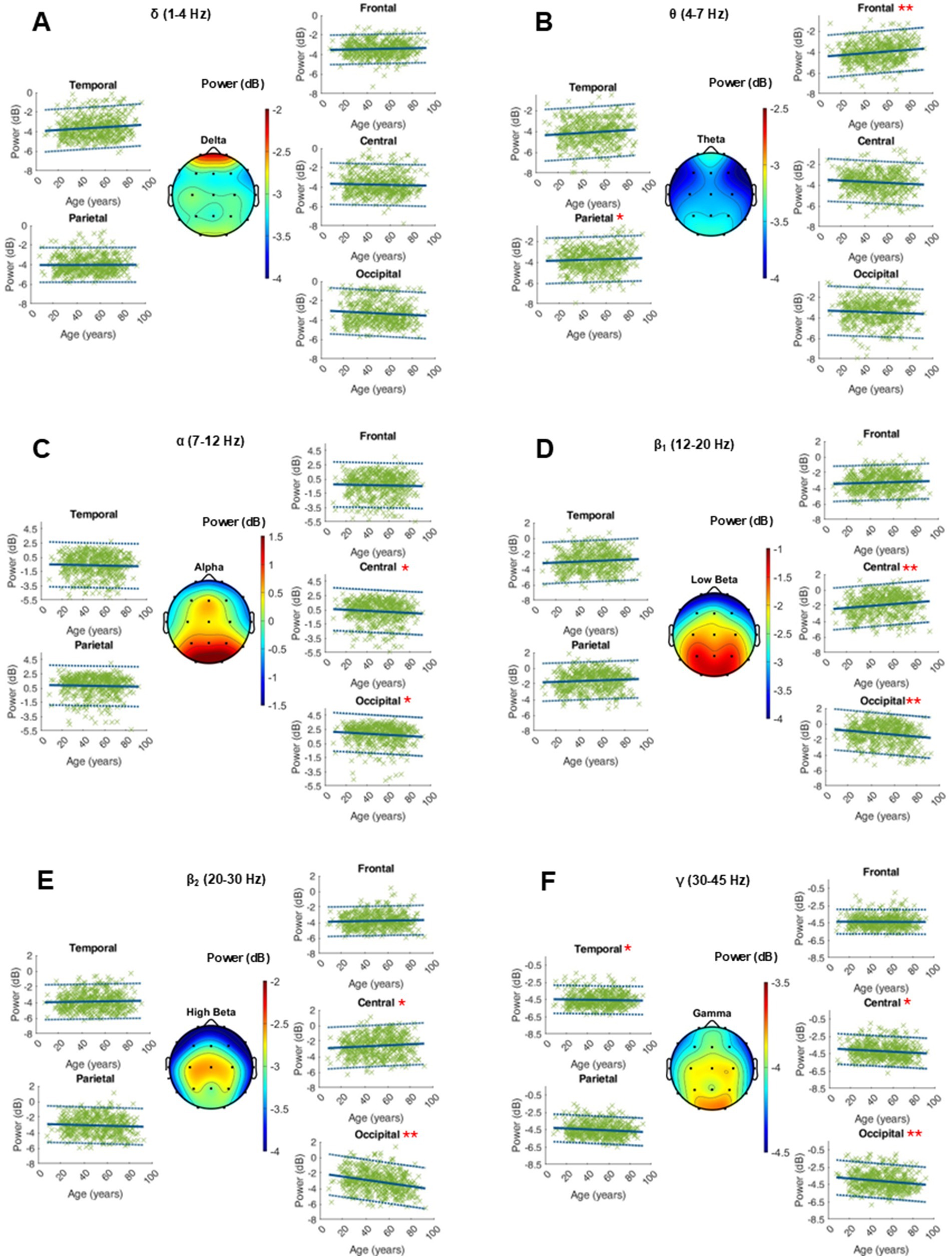
Figure 5. Characterization of peak bandwidth (Hz) within the frequency bands of interest on the average of 20 single 10-s epochs per patient. For each frequency band, an averaged topographical representation of the peak bandwidth was computed, and the linear regression (thick blue line) as well as the 95% confidence interval (dashed blue lines) was plotted for each parameter. The frequency bands were defined as follows: delta (A, 1–4 Hz), theta (B, 4–8 Hz), alpha (C, 8–12 Hz), low beta (D, 12–20 Hz), high beta (E, 20–30 Hz), and gamma (F, 30–50 Hz). If the p-value was below the significance level of 0.05, the statistical significance was reported with red asterisks (*: p-value 0.05–0.001; **: p-value ≤0.001).
The aperiodic exponent showed a global significant decrease with age of around 0.003 μV2/Hz per year. We did not see age-dependent significant changes in the aperiodic offset (Supplementary Table S4).
This analysis provides the first spectrum-wide normative characterization of the age-related changes in oscillatory (periodic) and non-rhythmic (aperiodic) activity within physiological resting-state EEGs. While these processes have been partially described in some frequency bands or within age-cohorts, a parametrization of these dynamics over the life span was lacking. We found age-related changes in both periodic and aperiodic EEG parameters. Regarding the periodic activity, especially the center frequency of the alpha peak significantly decreased with age, while the gamma peak frequency increased. The aperiodic exponent describing the slope of the aperiodic activity decreased with age, but no significant changes were observed in the offset.
By focusing on real-world clinical EEG data, we aim to offer reference values that are directly applicable in clinical and research settings. While previous studies have examined periodic and aperiodic activity in distinct patient populations, our study provides a foundational dataset that can serve as a comparative baseline for both healthy aging and disease-related alterations.
We provided a normative characterization of ongoing oscillatory activity in the resting-state EEG in six frequency bands by isolating the periodic from the aperiodic components in the power spectrum. In line with previous studies, the aperiodic slope showed a ubiquitous decrease with age, significantly affecting the total power (Donoghue et al., 2020). Conventional decomposition of EEG spectra into canonical frequency bands does neither account for this, nor for frequency shifts across rigid band limits (Scally et al., 2018). For instance, we confirmed previous evidence that the major part of age-related differences in the alpha power can be explained by the flattening of the aperiodic slope and a shift towards the theta range, rather than a loss of oscillatory activity (Tröndle et al., 2023; Cesnaite et al., 2023; Merkin et al., 2023). Similarly, the loss of theta power associated with increasing age and deterioration of cognitive status can be explained from the flattening of the aperiodic slope (Cesnaite et al., 2023; Caplan et al., 2015).
The observed slowing in the alpha center frequency by approximately 0.01 Hz per year corresponds to previous findings and reinforces the notion that the individual alpha frequency (IAF) alters as the brain ages and cognition deteriorates (Cesnaite et al., 2023; Merkin et al., 2023). We observed an acceleration of the center frequency in the gamma range amounting to 0.01 to 0.03 Hz per year depending on the brain region. The acceleration of frequencies in the gamma range has been proposed as a compensatory mechanism to counteract for declining nerve conduction velocities (Hong and Rebec, 2012). It has been postulated that this mechanism contributes to the flattening of the aperiodic slope and is also reflected in the acceleration of the center frequency in the gamma oscillations (Voytek et al., 2015). For the other frequency bands, we show that there is no overall age-related trend in the center frequency and present normative, age-independent values. Intrasubject variability was assessed across all analyzed parameters and was generally higher in faster oscillatory bands, particularly in the gamma range.
This analysis was originally conceived to inform a protocol for individualized transcranial alternating current stimulation (tACS) in an elderly population (Leroy et al., 2025). Non-invasive brain stimulation techniques such as transcranial alternating stimulation (tACS) are increasingly used to entrain oscillatory brain activity in elderly subjects and numerous neurological and psychiatric disorders, including but not limited to Parkinson’s disease (Madrid and Benninger, 2021), dementia (Manippa et al., 2023) and depression (Lee et al., 2022). The tACS is intended to interact with ongoing oscillatory activity, yet the frequency of these oscillations changes in the aging brain, resulting in possible mismatches (Fröhlich et al., 2015). Recent work showed that tACS in the alpha range modulates the periodic but not the aperiodic components of the power spectrum, underlying the importance of this distinction in the context of brain stimulation (Kasten et al., 2024). Age-adjusted stimulation frequencies in the alpha range, could provide a practical alternative that balances standardization and efficacy, circumventing the complexity of closed-loop EEG-synchronized setups (Zrenner and Ziemann, 2024; Nasr et al., 2022). This approach offers a reproducible and clinically feasible method to refine tACS protocols and addresses variability in neurophysiological characteristics.
A limitation of this analysis lies in its cross-sectional design, which does not allow for the tracking of individual aging processes over time. Consequently, longitudinal studies would offer a more nuanced understanding of how EEG parameters evolve with age in the same subjects. The study’s interpretations of frequency changes, especially in the gamma bands, may also be impacted by external variables such as minor physical movements or muscle artifacts, not accounted for during EEG recordings. Moreover, the analysis did not implement corrections for multiple testing since the primary goal was to describe physiological phenomena, rather than to introduce or validate new hypotheses.
The findings of this study are inherently limited by both intra- and intersubject variability, which is a common characteristic of resting-state EEG. While intersubject variability is a key consideration when designing individualized stimulation protocols, intrasubject variability should also be taken into account, particularly in the context of tACS. While the real-world nature of this dataset enhances its generalizability to clinical practice, it inherently includes patients with diverse medical backgrounds. Eventually, non-neurological disorders such as syncopes or unspecific transient symptoms are frequent results of a physiological EEG during diagnostic work-up. This said, patients did not necessarily suffer a neurological disorder just because they underwent EEG examination.
The findings from this analysis of clinical routine data highlight the intricate dynamics of age-related changes in resting-state EEG signals, which may have crucial implications for the understanding of the aging brain and the tailoring of neurotherapeutic interventions.
The data analyzed in this study will be made available upon reasonable request. Requests to access these datasets should be directed to cm9iZXJ0LmZsZWlzY2htYW5uQHVuaS1ncmVpZnN3YWxkLmRl.
The studies involving humans were approved by Ethikkommission an der Universitätsmedizin Greifswald. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
SL: Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. VB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. FD: Supervision, Writing – review & editing, Methodology, Project administration. DA: Methodology, Project administration, Supervision, Writing – review & editing. RF: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the European Fund for Regional Development (EFRE, GHS-19-0015).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2025.1540040/full#supplementary-material
Caplan, J. B., Bottomley, M., Kang, P., and Dixon, R. A. (2015). Distinguishing rhythmic from non-rhythmic brain activity during rest in healthy neurocognitive aging. NeuroImage 112, 341–352. doi: 10.1016/j.neuroimage.2015.03.001
Cesnaite, E., Steinfath, P., Jamshidi Idaji, M., Stephani, T., Kumral, D., Haufe, S., et al. (2023). Alterations in rhythmic and non-rhythmic resting-state EEG activity and their link to cognition in older age. NeuroImage 268:119810. doi: 10.1016/j.neuroimage.2022.119810
Delorme, A., and Makeig, S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21. doi: 10.1016/j.jneumeth.2003.10.009
Donoghue, T., Haller, M., Peterson, E. J., Varma, P., Sebastian, P., Gao, R., et al. (2020). Parameterizing neural power spectra into periodic and aperiodic components. Nat. Neurosci. 23, 1655–1665. doi: 10.1038/s41593-020-00744-x
Fröhlich, F., Sellers, K. K., and Cordle, A. L. (2015). Targeting the neurophysiology of cognitive systems with transcranial alternating current stimulation. Expert. Rev. Neurother. 15, 145–167. doi: 10.1586/14737175.2015.992782
Gavaret, M., Iftimovici, A., and Pruvost-Robieux, E. (2023). EEG: current relevance and promising quantitative analyses. Rev. Neurol. 179, 352–360. doi: 10.1016/j.neurol.2022.12.008
Hill, A. T., Clark, G. M., Bigelow, F. J., Lum, J. A. G., and Enticott, P. G. (2022). Periodic and aperiodic neural activity displays age-dependent changes across early-to-middle childhood. Dev. Cogn. Neurosci. 54:101076. doi: 10.1016/j.dcn.2022.101076
Höller, Y. (2021). Quantitative EEG in Cognitive Neuroscience. Brain Sci. 11:517. doi: 10.3390/brainsci11040517
Hong, S. L., and Rebec, G. V. (2012). A new perspective on behavioral inconsistency and neural noise in aging: compensatory speeding of neural communication. Front Aging Neurosci. 4:27. doi: 10.3389/fnagi.2012.00027
Jauny, G., Mijalkov, M., Canal-Garcia, A., Volpe, G., Pereira, J., Eustache, F., et al. (2024). Linking structural and functional changes during aging using multilayer brain network analysis. Commun. Biol. 7:239. doi: 10.1038/s42003-024-05927-x
Kasten, F. H., Lattmann, R., Strüber, D., and Herrmann, C. S. (2024). Decomposing the effects of α-tACS on brain oscillations and aperiodic 1/f activity. Brain Stimulat. 17, 721–723. doi: 10.1016/j.brs.2024.05.015
Ko, J., Park, U., Kim, D., and Kang, S. W. (2021). Quantitative electroencephalogram standardization: a sex- and age-differentiated normative database. Front. Neurosci. 15:766781. doi: 10.3389/fnins.2021.766781
Kundu, B., Charlebois, C. M., Anderson, D. N., Peters, A., and Rolston, J. D. (2023). Chronic intracranial recordings after resection for epilepsy reveal a “running down” of epileptiform activity. Epilepsia 64, e135–e142. doi: 10.1111/epi.17645
Lee, A. R. Y. B., Yau, C. E., Mai, A. S., Tan, W. A., Ong, B. S. Y., Yam, N. E., et al. (2022). Transcranial alternating current stimulation and its effects on cognition and the treatment of psychiatric disorders: a systematic review and meta-analysis. Ther. Adv. Chronic Dis. 13:20406223221140390. doi: 10.1177/20406223221140390
Lendner, J. D., Helfrich, R. F., Mander, B. A., Romundstad, L., Lin, J. J., Walker, M. P., et al. (2020). An electrophysiological marker of arousal level in humans. eLife 9:e55092. doi: 10.7554/eLife.55092
Leroy, S., Bublitz, V., Grittner, U., Fleischmann, R., von Dincklage, F., and Antonenko, D. (2025). Modulating delirium through stimulation (MoDeSt): study protocol for a randomized, double-blind, sham-controlled trial assessing the effect of postoperative transcranial electrical stimulation on delirium incidence. Trials 26:4. doi: 10.1186/s13063-024-08699-1
Leroy, S., Major, S., Bublitz, V., Dreier, J. P., and Koch, S. (2022). Unveiling age-independent spectral markers of propofol-induced loss of consciousness by decomposing the electroencephalographic spectrum into its periodic and aperiodic components. Front. Aging Neurosci. 14:1076393. doi: 10.3389/fnagi.2022.1076393
Madrid, J., and Benninger, D. H. (2021). Non-invasive brain stimulation for Parkinson’s disease: clinical evidence, latest concepts and future goals: a systematic review. J. Neurosci. Methods 347:108957. doi: 10.1016/j.jneumeth.2020.108957
Manippa, V., Palmisano, A., Nitsche, M. A., Filardi, M., Vilella, D., Logroscino, G., et al. (2023). Cognitive and neuropathophysiological outcomes of gamma-tACS in dementia: a systematic review. Neuropsychol. Rev. 34, 338–361. doi: 10.1007/s11065-023-09589-0
Merkin, A., Sghirripa, S., Graetz, L., Smith, A. E., Hordacre, B., Harris, R., et al. (2023). Do age-related differences in aperiodic neural activity explain differences in resting EEG alpha? Neurobiol. Aging 121, 78–87. doi: 10.1016/j.neurobiolaging.2022.09.003
Nasr, K., Haslacher, D., Dayan, E., Censor, N., Cohen, L. G., and Soekadar, S. R. (2022). Breaking the boundaries of interacting with the human brain using adaptive closed-loop stimulation. Prog. Neurobiol. 216:102311. doi: 10.1016/j.pneurobio.2022.102311
Nuwer, M. R., Comi, G., Emerson, R., Fuglsang-Frederiksen, A., Guérit, J. M., Hinrichs, H., et al. (1998). IFCN standards for digital recording of clinical EEG. Electroencephalogr. Clin. Neurophysiol. 106, 259–261
Pollak, M., Leroy, S., Röhr, V., Brown, E. N., Spies, C., and Koch, S. (2024). EEG biomarkers from anesthesia induction to identify vulnerable patients at risk for postoperative delirium. Anesthesiology 159:e31. doi: 10.1016/j.clinph.2023.12.078
Prichep, L. S. (2005). Use of normative databases and statistical methods in demonstrating clinical utility of QEEG: importance and cautions. Clin. EEG Neurosci. 36, 82–87. doi: 10.1177/155005940503600207
Salvatore, S. V., Lambert, P. M., Benz, A., Rensing, N. R., Wong, M., Zorumski, C. F., et al. (2023). Periodic and aperiodic changes to cortical EEG in response to pharmacological manipulation. J Neurophysiol. 131, 529–40. doi: 10.1101/2023.09.21.558828
Scally, B., Burke, M. R., Bunce, D., and Delvenne, J. F. (2018). Resting-state EEG power and connectivity are associated with alpha peak frequency slowing in healthy aging. Neurobiol. Aging 71, 149–155. doi: 10.1016/j.neurobiolaging.2018.07.004
Seeber, M., Cantonas, L. M., Hoevels, M., Sesia, T., Visser-Vandewalle, V., and Michel, C. M. (2019). Subcortical electrophysiological activity is detectable with high-density EEG source imaging. Nat. Commun. 10:753. doi: 10.1038/s41467-019-08725-w
Tröndle, M., Popov, T., Pedroni, A., Pfeiffer, C., Barańczuk-Turska, Z., and Langer, N. (2023). Decomposing age effects in EEG alpha power. Cortex 161, 116–144. doi: 10.1016/j.cortex.2023.02.002
Voytek, B., and Knight, R. T. (2015). Dynamic network communication as a unifying neural basis for cognition, development, aging, and disease. Biol. Psychiatry 77, 1089–1097. doi: 10.1016/j.biopsych.2015.04.016
Voytek, B., Kramer, M. A., Case, J., Lepage, K. Q., Tempesta, Z. R., Knight, R. T., et al. (2015). Age-related changes in 1/f neural electrophysiological noise. J. Neurosci. 35, 13257–13265. doi: 10.1523/JNEUROSCI.2332-14.2015
Wang, X. J. (2020). Macroscopic gradients of synaptic excitation and inhibition in the neocortex. Nat. Rev. Neurosci. 21, 169–178. doi: 10.1038/s41583-020-0262-x
Zhang, H., Zhou, Q. Q., Chen, H., Hu, X. Q., Li, W. G., Bai, Y., et al. (2023). The applied principles of EEG analysis methods in neuroscience and clinical neurology. Mil. Med. Res. 10:67. doi: 10.1186/s40779-023-00502-7
Keywords: EEG, brain oscillations, aging, periodic activity, aperiodic activity
Citation: Leroy S, Bublitz V, von Dincklage F, Antonenko D and Fleischmann R (2025) Normative characterization of age-related periodic and aperiodic activity in resting-state real-world clinical EEG recordings. Front. Aging Neurosci. 17:1540040. doi: 10.3389/fnagi.2025.1540040
Received: 05 December 2024; Accepted: 24 March 2025;
Published: 11 April 2025.
Edited by:
Chiara Spironelli, University of Padua, ItalyCopyright © 2025 Leroy, Bublitz, von Dincklage, Antonenko and Fleischmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sophie Leroy, c29waGllLmxlcm95QG1lZC51bmktZ3JlaWZzd2FsZC5kZQ==
†These authors share first authorship
‡These author share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.