- 1Department of Health and Welfare Occupational Therapy Course, Faculty of Health and Welfare, Prefectural University of Hiroshima, Hiroshima, Japan
- 2Department of Linguistics, Faculty of Humanities, Kyushu University, Fukuoka, Japan
The early detection of cognitive decline in older adults is crucial for preventing dementia. This mini-review focuses on electroencephalography (EEG) markers of early dementia-related precursors, including subjective cognitive decline, subjective memory complaints, and cognitive frailty. We present recent findings from EEG analyses identifying high dementia risk in older adults, with an emphasis on conditions that precede mild cognitive impairment. We also cover event-related potentials, quantitative EEG markers, microstate analysis, and functional connectivity approaches. Moreover, we discuss the potential of these neurophysiological markers for the early detection of cognitive decline as well as their correlations with related biomarkers. The integration of EEG data with advanced artificial intelligence technologies also shows promise for predicting the trajectory of cognitive decline in neurodegenerative disorders. Although challenges remain in its standardization and clinical application, EEG-based approaches offer non-invasive, cost-effective methods for identifying individuals at risk of dementia, which may enable earlier interventions and personalized treatment strategies.
1 Introduction
Rapid aging of the global population has intensified the need to extend healthy life expectancy, and dementia poses an important challenge to this goal. Alzheimer’s disease (AD) and other types of dementia are characterized by cognitive decline that is distinct from that of normal aging, necessitating a deeper understanding of the underlying mechanisms. Recent research has revealed that AD-associated pathophysiological changes can begin more than a decade before the onset of clinical symptoms (Ritchie et al., 2016; Moffat et al., 2022). Although postmortem examination remains the definitive method for diagnosing dementia, important advancements in in vivo assessment techniques have emerged, including cerebrospinal fluid biomarkers, positron emission tomography, and magnetic resonance imaging (MRI) (Clark et al., 2018; Hojjati et al., 2018; Liu et al., 2024). However, these methods present various challenges, such as high cost, invasiveness, and limited clinical accessibility.
Electroencephalography (EEG) offers a non-invasive, cost-effective approach for detecting neurological markers of cognitive decline. Recent reviews have focused on EEG characteristics in AD and mild cognitive impairment (MCI) (Al-Qazzaz et al., 2014; Sanchez-Reyes et al., 2021; Torres-Simon et al., 2022; Wijaya et al., 2023). EEG activity correlates with cognitive decline assessed by the Mini-Mental State Examination (MMSE), and combining these measures improves dementia prediction accuracy (Doan et al., 2021). EEG may detect subtle early functional changes. However, research on EEG markers of early precursors, such as subjective cognitive decline (SCD), subjective memory complaints (SMC), and cognitive frailty (CF), remains scarce.
This mini-review summarizes recent EEG findings used to identify a high risk of dementia in older adults, emphasizing conditions such as SCD, SMC, and CF. We explore EEG-based approaches, including event-related potentials (ERPs), quantitative EEG (qEEG) markers, microstate analysis, and functional connectivity measures. Additionally, we discuss the integration of EEG with artificial intelligence technologies for early diagnosis and prediction of dementia progression.
By focusing on pre-MCI states, we aim to increase knowledge of the early detection of cognitive decline, thus enabling earlier interventions and more effective prevention strategies. Additionally, we highlight the challenges and future directions in this field, emphasizing the need for standardized approaches and larger-scale studies to validate the clinical utility of EEG-based markers in dementia risk assessments.
2 Early cognitive decline: from normal aging to pre-MCI states
The risk of dementia in older adults is influenced by 12 modifiable risk factors (Livingston et al., 2020). Previous studies have pointed out the association between preclinical stages of AD (i.e., SMC and SCD) and these lifestyle risk factors, such as low education and hypertension (Chen et al., 2014), and depression and cigarette smoking (Ahn et al., 2021). The importance of treating these factors before cognitive decline onset or at the subjective complaint stage is increasingly emphasized (Van Der Flier et al., 2023).
The spectrum of cognitive decline ranges from normal aging to dementia, encompassing crucial intermediate stages for early detection and intervention. MCI is a high-risk state for progression to dementia, particularly AD (Arnáiz and Almkvist, 2003), and is characterized by clinical symptoms, minimal assistance needs with daily activities, and potentially reversible cognitive decline (García et al., 2021).
Recent studies have focused on earlier stages of cognitive decline. In SCD and SMC, individuals experience self-perceived cognitive decline but perform within the normal range on objective tests, and exhibit an increased risk of progressing to MCI and dementia (Kryscio et al., 2014; Bessi et al., 2018). CF represents coexisting physical frailty and MCI, and encompasses mild cognitive decline even without a diagnosed neurological disorder (Kelaiditi et al., 2013; Shimada et al., 2018; Facal et al., 2021). Kocagoncu et al. (2022) defined CF as mild cognitive decline without subjective awareness, indicating that the concept of CF is not fully established. CF is linked to increased risks of dementia, care needs, hospitalization, disability, and mortality compared with healthy aging (Lee et al., 2018; Panza et al., 2018).
Distinguishing these early stages from normal aging is challenging because differences can be subtle and not always apparent using standard cognitive assessments. EEG primarily reflects postsynaptic potentials, offering promising avenues for identifying early markers of cognitive decline. EEG may detect subtle changes in postsynaptic fields that potentially underlie cognitive dysfunction in AD and MCI (Arendt, 2009; Targa Dias Anastacio et al., 2022).
3 Contemporary ERP methodologies and their application
While EEG may reflect postsynaptic potentials and neuronal population activity, ERPs are derived from averaging electrical responses to specific stimuli or tasks, enabling identification of components related to perception and cognition. Goodin et al. (1978) first identified the P300 component as a biomarker for dementia, characterized by a positive waveform occurring 200–300 ms after an oddball task event. AD typically results in attenuated P300 amplitude and increased latency compared with normal aging (Pedroso et al., 2012; Hedges et al., 2016; Fruehwirt et al., 2019). The P300 is also sensitive to MCI; reduced P300 amplitude indicates cognitive deterioration in at-risk older adults (Newsome et al., 2013), and its latency may predict MCI progression to AD (Jiang et al., 2015).
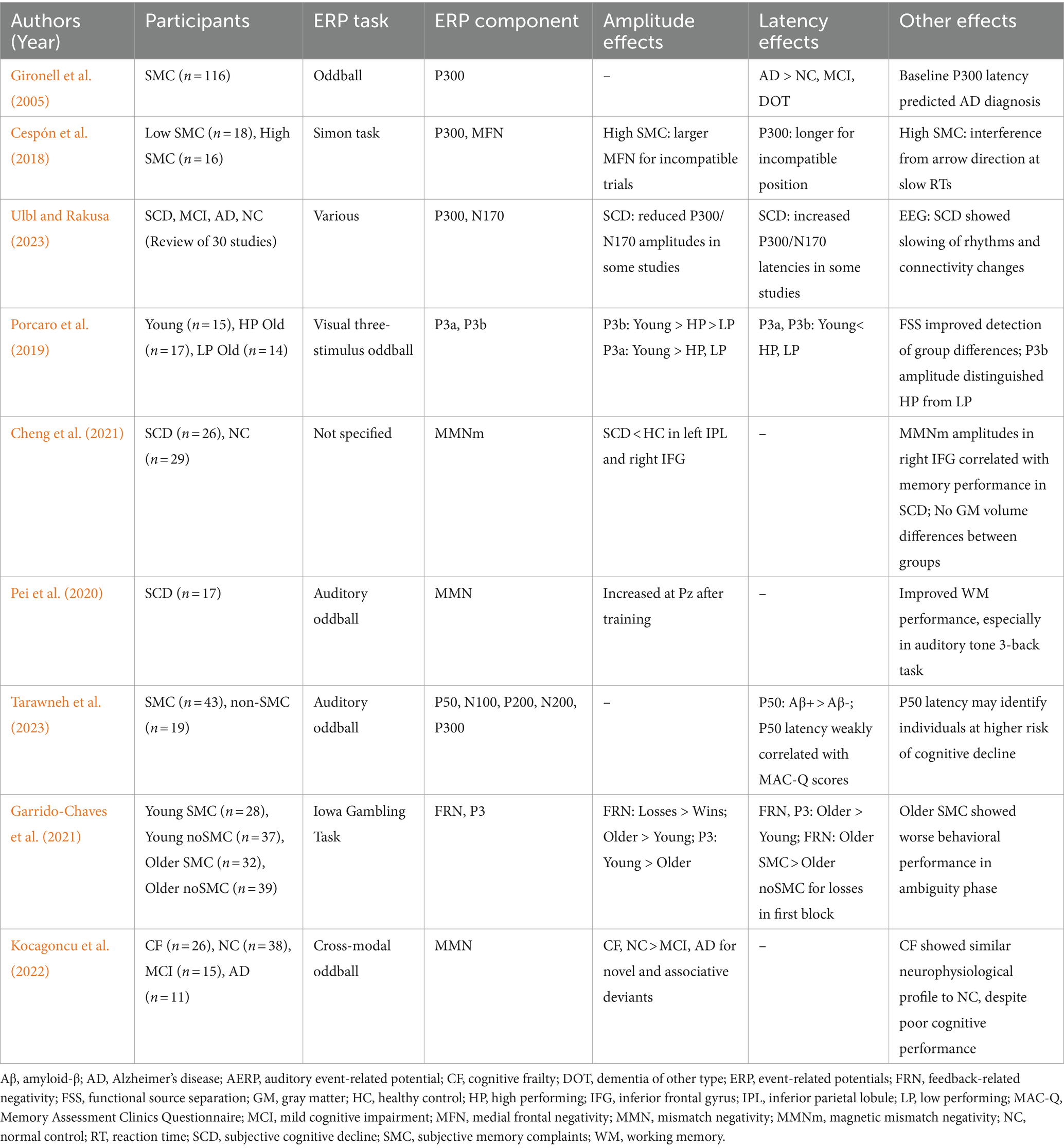
Table 1 summarizes recent ERP studies on early cognitive decline in older adults. Evidence regarding the P300 in SCD and SMC is limited but promising. People with SMC progressing to AD show a prolonged P300 latency before AD onset (Gironell et al., 2005) and in response to stimulus–response incongruence (Cespón et al., 2018). Ulbl and Rakusa (2023) reviewed studies that demonstrated decreased N170 and P300 amplitudes in SCD, although the results across ERP components were inconsistent. The P3b is a later component of the P300, and has exhibited decreased amplitude in cognitively low-performing older adults, suggesting age-independent episodic memory decline (Porcaro et al., 2019). Additionally, P300 peak amplitude correlates with bilateral hippocampal volume in healthy older adults (Devos et al., 2021).
Mismatch negativity (MMN) reflects the automatic detection of sensory input changes. Attenuated MMN is associated with memory and psychosocial deficits (Mowszowski et al., 2012) and is decreased in AD and MCI compared with normal aging (Kazmerski et al., 1997; Papadaniil et al., 2016). The neural sources of MMN show a characteristic migration pattern with AD progression (Papadaniil et al., 2016; Tsolaki et al., 2017). Ruzzoli et al. (2016) reported distinctive patterns of auditory MMN distribution in normal aging, MCI, and AD. In SCD, magnetoencephalography (MEG)-measured MMN revealed that attenuated responses were correlated with memory function (Cheng et al., 2021). Additionally, MMN-based neurofeedback is reportedly effective for working memory training in SCD (Pei et al., 2020).
The N200 component has shown utility for differentiating MCI from AD (Papaliagkas et al., 2009b; Morrison et al., 2018) and predicting progression risk to MCI/AD in healthy older adults (Papaliagkas et al., 2009a; Howe, 2014). Although similar effects in N400 and P600 have been reported (Grieder et al., 2013; Chou et al., 2023), their usefulness remains unclear in the context of SCD, SMC, and CF.
Research on other ERP components has been limited. Tarawneh et al. (2023) reported prolonged P50 latency in amyloid-β-positive participants compared with healthy controls. Changes in ERPs during cognitive tasks have been reported in SMC, including prolonged feedback-related negativity latencies (Garrido-Chaves et al., 2021). Kocagoncu et al. (2022) proposed that CF is part of the normal neurocognitive spectrum, as its MMN responses resemble those of normal aging.
Cognitive function in the normal range, possibly resulting from compensatory neural mechanisms (Sala-Llonch et al., 2015; Wei et al., 2022), may contribute to low sensitivity to ERP components in SMC and SCD. Thus, EEG may offer more sensitive and valuable information regarding early cognitive decline than ERPs in SCD, SMC, and CF.
4 Exploring the frontiers of EEG research: advanced approaches to elucidating early cognitive decline as a risk for dementia
4.1 Quantitative EEG markers during precursor symptoms of AD
qEEG analyzes digital EEG signals using mathematical algorithms (Nuwer, 1997), providing insights into potential early neurobiomarkers of pathological cognitive aging (Keller et al., 2023). Unlike ERPs focusing on time-locked responses, qEEG examines ongoing EEG activity, offering a broader view of brain function. qEEG includes linear techniques, including power spectral analysis, and nonlinear methods, including entropy measurements and fractal dimension analysis (Al-Qazzaz et al., 2014). In AD, EEG typically shows reduced alpha and beta band activity (Wada et al., 1998; Knott et al., 2000), distinct from normal aging (Babiloni et al., 2021).
Recent research focuses on differences between normal aging and prodromal AD pathophysiology, including MCI, SCD, and SMC. A key finding in MCI and AD is “EEG slowing,” in which increased occipital low-frequency power and decreased frontal high-frequency power are correlated with cognitive performance (Farina et al., 2020; Medici et al., 2023). The theta/alpha ratio indicates cognitive decline, showing differences between AD, MCI, and healthy older adults (Meghdadi et al., 2021), with an increased ratio in MCI associated with higher dementia risk (Hamilton et al., 2021).
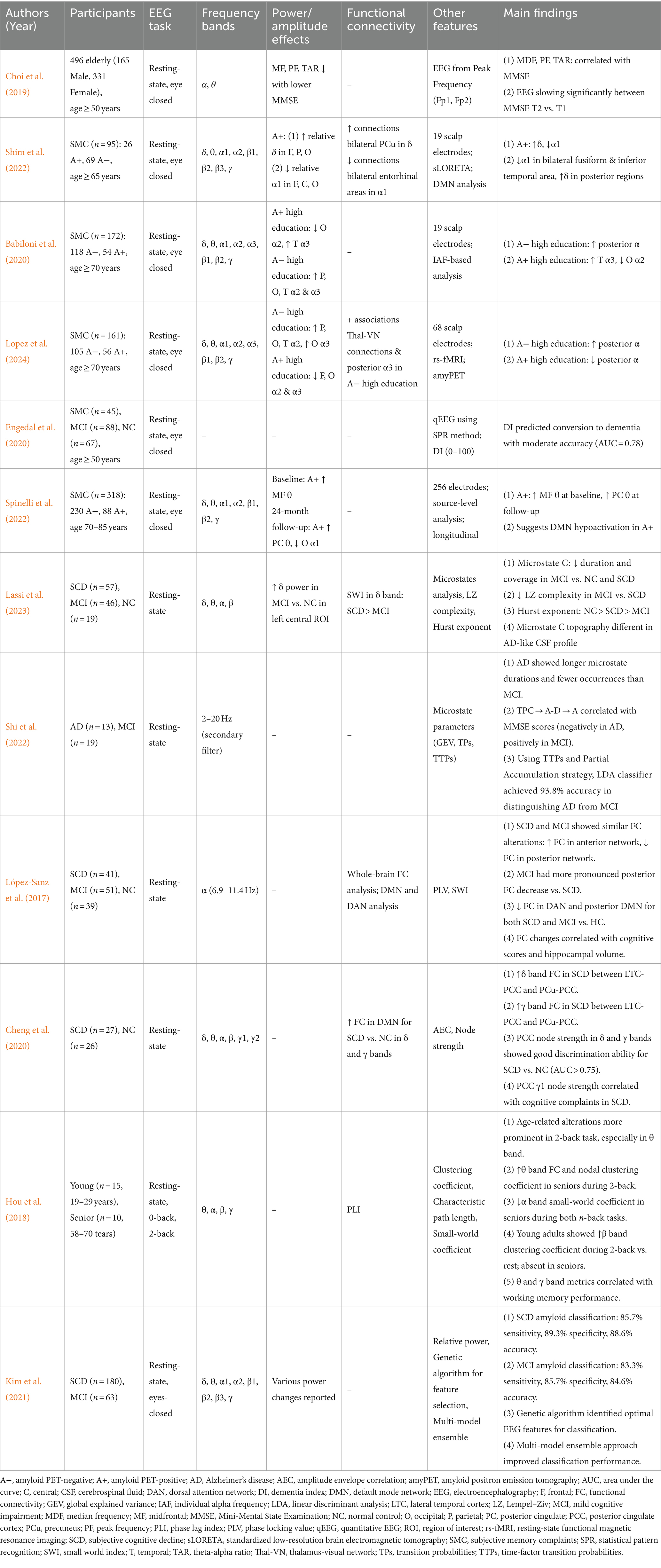
EEG slowing parameters show promise for detecting early cognitive decline in SCD and SMC (Table 2). Previous studies have reported decreased frontal EEG slowing parameters with declining cognitive scores in healthy older adults (Choi et al., 2019), increased theta power and reduced alpha reactivity in SMC (Perez et al., 2022), and altered oscillatory activity in SCD (Shim et al., 2022).
Higher education levels are correlated with higher posterior alpha rhythm amplitudes in SMC (Babiloni et al., 2020) and enhanced neural coupling between posterior alpha rhythm and thalamus-visual networks (Lopez et al., 2024), suggesting a protective role of cognitive reserve.
Several studies have demonstrated qEEG’s potential for predicting progression from preclinical to AD. Engedal et al. (2020) reported moderate accuracy in predicting transition to dementia in SMC and MCI. Associations between qEEG parameters and pathological protein biomarkers suggest that resting-state EEG changes might reflect increased brain amyloid burden in AD progression (Spinelli et al., 2022; Ulbl and Rakusa, 2023).
Nonlinear methods have shown promising results in distinguishing AD patients from healthy older individuals (Abásolo et al., 2006; Pineda et al., 2020), potentially capturing complex brain dynamics not evident in linear analyses. However, studies employing nonlinear techniques for SMC and SCD have been limited, mainly using MEG (e.g., Shumbayawonda et al., 2020). The application of this approach to preclinical dementia stages faces challenges, including high computational costs and complex data interpretation (Vicchietti et al., 2023).
Although EEG biomarkers exist for SCD and SMC, research examining CF remains limited. Some studies have suggested that CF exhibits brain activity patterns related to physical conditions (Suárez-Méndez et al., 2021) linked to cognitive function (Liu et al., 2024). CF characteristics may be discerned through changes in cognitive function-related neural oscillations, microstate analysis, functional connectivity, and phase coherence analysis.
4.2 Integrating microstate and connectivity analyses for the early detection of cognitive decline
Although qEEG provides insights into frequency characteristics of resting-state brain activity, advanced techniques like microstate analysis, functional connectivity assessment, and graph theory approaches offer a deeper understanding of brain network dynamics in cognitive decline. These methods show promise for differentiating normal aging from pathological changes, including AD and prodromal AD symptoms.
Microstate analysis captures functional network dynamics with millisecond-level resolution, revealing distinct characteristics between AD and MCI. EEG microstates, brief periods of quasi-stable scalp electrical patterns typically classified into four topographies (A–D), reflect momentary global brain states and the basic units of cognitive processing (Michel and Koenig, 2018). Significant differences in microstate topographies—particularly A, C, and D—between healthy controls and AD/MCI (Britz et al., 2010; Smailovic et al., 2019; Lian et al., 2021) may reflect dysfunction in key brain networks (e.g., default mode network or frontoparietal network) associated with AD pathology.
Changes in microstate dynamics have been observed in MCI and AD. Musaeus et al. (2019, 2020) reported higher transition probabilities from microstates C and D to A, and increased occurrence frequencies and coverage of microstate A, in AD and MCI compared with healthy controls. Notably, Lassi et al. (2023) found reduced complexity of microstate transitions in MCI and SCD, indicating simpler brain network dynamics even at the SCD stage. Shi et al. (2022) reported that specific microstate transition probabilities (C → A − D → A) correlate with MMSE scores, suggesting applications for identifying potential cognitive impairment and brain activity patterns in the pre-dementia stage.
Functional connectivity analysis provides insights into SCD and MCI pathophysiology without apparent structural changes. López-Sanz et al. (2017) identified anterior network hyper-synchronization and decreased posterior network connectivity in SCD and MCI during the resting state. Cheng et al. (2020) reported increased functional connectivity within the default mode network in the delta and gamma frequency bands in SCD using MEG, potentially representing compensatory mechanisms.
Graph theory approaches have further elucidated changes in brain network organization across the cognitive decline spectrum (Rubinov and Sporns, 2010). Vecchio et al. (2014) applied graph theory to EEG analysis, revealing differences in brain networks between healthy elderly and AD patients. EEG of normal subjects showed high interaction between channels, while AD patients exhibited more random brain network structures, particularly in the alpha band. These changes correlated with cognitive decline, suggesting that EEG-based brain network analysis may be useful for early diagnosis and monitoring of dementia progression.
Task-related functional connectivity analyses have provided additional insights into cognitive decline. During working memory tasks, MCI patients exhibit altered connectivity patterns, including decreased fronto-temporal connectivity and increased fronto-occipital and parieto-occipital connectivity in theta and alpha bands (Jiang et al., 2024). Furthermore, decreased alpha band connectivity and lack of beta band modulation with increasing memory load were observed, resulting in a more centralized network structure (Fodor et al., 2021). These changes may reflect compensatory mechanisms in response to neurodegeneration in the hippocampus and surrounding regions. Table 2 summarizes EEG studies of microstate analysis and functional connectivity.
In healthy older adults, high cognitive load tasks are also associated with decreased alpha band connectivity and increased theta band phase synchronization and connectivity (Hou et al., 2018). These findings suggest that graph theory-based functional connectivity analysis during cognitively demanding tasks may reveal characteristic changes in brain functional networks specific to SCD, SMC, and potentially CF.
4.3 Novel EEG methods using machine learning and deep learning algorithms
Integrating artificial intelligence with EEG analysis has emerged as a powerful approach for predicting cognitive decline progression. Machine learning algorithms applied to EEG data have high accuracy for classifying AD patients and predicting progression from MCI to AD. For instance, studies using support vector machines and gradient-boosted trees have achieved impressive classification accuracies, reaching 95% for AD detection (Rossini et al., 2022) and 83% for MCI progression prediction in healthy older adults (Mazzeo et al., 2023a). Al-Hagery et al. (2020) improved the accuracy of AD diagnosis to 96.66% using the random forest algorithm as an ensemble method, representing a significant improvement over the single decision tree algorithm (73.33%). These results demonstrate the potential of machine learning techniques, particularly ensemble methods, in enhancing early diagnosis and prediction of dementia progression. The high accuracy achieved by these models suggests their potential clinical application, potentially enabling earlier interventions and more personalized treatment strategies for patients at risk of cognitive decline.
Multimodal approaches combining EEG with other biomarkers may enhance prediction accuracy. Maestú et al. (2019) demonstrated that integrating EEG data with other biomarkers (e.g., genotypes, cognitive tests, or brain imaging) may provide more accurate AD predictions. Kim et al. (2021) developed a model integrating EEG and apolipoprotein E genotypes to predict amyloid positron emission tomography positivity in SCD and MCI, with high accuracy in both groups (see Table 2). These advancements extend early intervention potential to preclinical stages. Mazzeo et al. (2023b) reported a protocol for a prospective cohort study of SCD patients, aiming to develop a model for predicting AD progression using machine learning by integrating multifaceted data including neuropsychological assessments, genetic analysis, EEG, and ERPs.
However, challenges remain in implementing these approaches for large-scale screening, including cost, generalizability, and invasiveness (Rossini et al., 2022). Many studies face limitations, including small sample sizes, short follow-up periods, and difficulties controlling diverse data in multimodal approaches. The variability and reproducibility of machine learning findings across facilities are also concerns. However, in SCD and SMC contexts, machine learning and deep learning models based on large-scale databases are becoming increasingly crucial for distinguishing between actual cognitive impairment and personal cognitive complaints.
5 Discussion
Herein, we reviewed the clinical implications of EEG approaches for the early screening of dementia risk in cognitively frail individuals.
Resting-state qEEG is a promising biomarker for SCD, SMC, and possibly CF. When adjusted for cognitive reserve factors, EEG slowing may detect frequency pattern changes and correlate with cognitive decline in high-risk individuals. Combining qEEG with AD pathology markers could enhance its predictive potential for AD progression (Spinelli et al., 2022).
Microstate analysis, functional connectivity analyses, and graph theory approaches may serve as early neural markers of dementia, revealing brain network alterations. These methods, especially when combined with cognitive tasks, can identify subtle functional changes before overt impairments manifest. Recent machine-learning approaches have shown promise in classifying amyloid status in SCD and MCI using EEG features (Kim et al., 2021).
ERP components, particularly P300 and MMN, may detect cognitive frailty in older adults when paired with cognitive tasks. However, their effectiveness is limited in pre-MCI states caused by subtle, multidomain cognitive decline. ERPs are more useful in detecting MCI and AD. As reviewed above, numerous studies have identified common EEG/ERP features in MCI and AD. Combining these with neuropsychological tests and AD biomarkers can improve diagnostic accuracy.
With the increase in young-onset dementia (YOD), EEG has shown potential for YOD diagnosis, particularly in early-onset AD and frontotemporal dementia. Studies highlight distinct EEG patterns, such as increased theta and delta activity in YOD, making EEG a valuable, cost-effective tool for early detection and differentiation (Lin et al., 2021; Brown et al., 2023).
However, clinical application of EEG faces methodological challenges. Evidence for EEG alone to predict dementia progression is insufficient compared with established AD biomarkers (Gouw et al., 2017; Jiao et al., 2023). The absence of standardized guidelines for dementia-specific EEG limits the comparability and generalizability of results (Monllor et al., 2021). Gender differences in dementia risk remain underexplored in EEG research on pre-dementia symptoms despite higher risk in women (Hayden et al., 2006; Chêne et al., 2015). Both EEG and fMRI alone show limited efficacy in distinguishing healthy older adults from MCI (Farina et al., 2020), suggesting the need for multimodal integration (Li et al., 2024).
To overcome these limitations, we propose multi-center collaborative research, such as the “Dementia ConnEEGtome” project (Prado et al., 2022). This approach, with 5-year follow-ups incorporating conventional diagnostic approaches, including AD pathology, could advance standardization, address methodological issues, and improve EEG’s reliability as an early AD biomarker.
Author contributions
MT: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. EY: Conceptualization, Methodology, Writing – review & editing. FM: Conceptualization, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by JSPS KAKENHI (Grant Numbers JP18K10807 and JP22K11455).
Acknowledgments
We thank Bronwen Gardner, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript. In this article, we utilized AI tools (Claude 3.5 and ChatGPT-4o) for the initial listing and categorization of relevant literature. The final bibliography was manually verified and revised by our research team.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abásolo, D., Hornero, R., Espino, P., Álvarez, D., and Poza, J. (2006). Entropy analysis of the EEG background activity in Alzheimer's disease patients. Physiol. Meas. 27, 241–253. doi: 10.1088/0967-3334/27/3/003
Ahn, S., Mathiason, M. A., Lindquist, R., and Yu, F. (2021). Factors predicting episodic memory changes in older adults with subjective cognitive decline: a longitudinal observational study. Geriatr. Nurs. 42, 268–275. doi: 10.1016/j.gerinurse.2020.08.016
Al-Hagery, M. A., Al-Fairouz, E. I., and Al-Humaidan, N. A. (2020). Improvement of Alzheimer disease diagnosis accuracy using ensemble methods. Indones. J. Electr. Eng. Inform. 8, 132–139. doi: 10.52549/ijeei.v8i1.1321
Al-Qazzaz, N. K., Ali, S. H. B. M., Ahmad, S. A., Chellappan, K., Islam, M. S., and Escudero, J. (2014). Role of EEG as biomarker in the early detection and classification of dementia. Sci. World J. 2014, 1–16. doi: 10.1155/2014/906038
Arendt, T. (2009). Synaptic degeneration in Alzheimer’s disease. Acta Neuropathol. 118, 167–179. doi: 10.1007/s00401-009-0536-x
Arnáiz, E., and Almkvist, O. (2003). Neuropsychological features of mild cognitive impairment and preclinical Alzheimer's disease. Acta Neurol. Scand. 107, 34–41. doi: 10.1034/j.1600-0404.107.s179.7.x
Babiloni, C., Ferri, R., Noce, G., Lizio, R., Lopez, S., Lorenzo, I., et al. (2021). Resting state alpha electroencephalographic rhythms are differently related to aging in cognitively unimpaired seniors and patients with Alzheimer’s disease and amnesic mild cognitive impairment. J. Alzheimers Dis. 82, 1085–1114. doi: 10.3233/jad-201271
Babiloni, C., Lopez, S., Del Percio, C., Noce, G., Pascarelli, M. T., Lizio, R., et al. (2020). Resting-state posterior alpha rhythms are abnormal in subjective memory complaint seniors with preclinical Alzheimer's neuropathology and high education level: the INSIGHT-preAD study. Neurobiol. Aging 90, 43–59. doi: 10.1016/j.neurobiolaging.2020.01.012
Bessi, V., Mazzeo, S., Padiglioni, S., Piccini, C., Nacmias, B., Sorbi, S., et al. (2018). From subjective cognitive decline to Alzheimer's disease: the predictive role of neuropsychological assessment, personality traits, and cognitive reserve. A 7-year follow-up study. J. Alzheimers Dis. 63, 1523–1535. doi: 10.3233/JAD-171180
Britz, J., Van De Ville, D., and Michel, C. M. (2010). BOLD correlates of EEG topography reveal rapid resting-state network dynamics. NeuroImage 52, 1162–1170. doi: 10.1016/j.neuroimage.2010.02.052
Brown, C. W., Chen, H.-Y., and Panegyres, P. K. (2023). Electroencephalography in young onset dementia. BMC Neurol. 23:202. doi: 10.1186/s12883-023-03248-w
Cespón, J., Galdo-Álvarez, S., and Díaz, F. (2018). Event-related potentials reveal altered executive control activity in healthy elderly with subjective memory complaints. Front. Hum. Neurosci. 12:445. doi: 10.3389/fnhum.2018.00445
Chen, S. T., Siddarth, P., Ercoli, L. M., Merrill, D. A., Torres-Gil, F., and Small, G. W. (2014). Modifiable risk factors for Alzheimer disease and subjective memory impairment across age groups. PLoS One 9:e98630. doi: 10.1371/journal.pone.0098630
Chêne, G., Beiser, A., Au, R., Preis, S. R., Wolf, P. A., Dufouil, C., et al. (2015). Gender and incidence of dementia in the Framingham heart study from mid-adult life. Alzheimers Dement. 11, 310–320. doi: 10.1016/j.jalz.2013.10.005
Cheng, C.-H., Chang, C.-C., Chao, Y.-P., Lu, H., Peng, S.-W., and Wang, P.-N. (2021). Altered mismatch response precedes gray matter atrophy in subjective cognitive decline. Psychophysiology 58:e13820. doi: 10.1111/psyp.13820
Cheng, C.-H., Wang, P.-N., Mao, H.-F., and Hsiao, F.-J. (2020). Subjective cognitive decline detected by the oscillatory connectivity in the default mode network: a magnetoencephalographic study. Aging 12, 3911–3925. doi: 10.18632/aging.102859
Choi, J., Ku, B., You, Y. G., Jo, M., Kwon, M., Choi, Y., et al. (2019). Resting-state prefrontal EEG biomarkers in correlation with MMSE scores in elderly individuals. Sci. Rep. 9:10468. doi: 10.1038/s41598-019-46789-2
Chou, C. J., Liu, Y. C., and Lee, C. Y. (2023). The predictability and plausibility effects on N400 reflect the language deficit in patients with mild cognitive impairment. Alzheimers Dement. 19:65121. doi: 10.1002/alz.065121
Clark, L. R., Berman, S. E., Norton, D., Koscik, R. L., Jonaitis, E., Blennow, K., et al. (2018). Age-accelerated cognitive decline in asymptomatic adults with CSF β-amyloid. Neurology 90, e1306–e1315. doi: 10.1212/wnl.0000000000005291
Devos, H., Brooks, W., Gustafson, K., Liao, K., Ahmadnezhad, P., Mahnken, J. D., et al. (2021). The relationship between hippocampal volume and P3 event-related potential in cognitively normal older adults without and with elevated amyloid: a pilot study. Alzheimers Dement. 17:52418. doi: 10.1002/alz.052418
Doan, D. N. T., Ku, B., Choi, J., Oh, M., Kim, K., Cha, W., et al. (2021). Predicting dementia with prefrontal electroencephalography and event-related potential. Front. Aging Neurosci. 13:659817. doi: 10.3389/fnagi.2021.659817
Engedal, K., Barca, M. L., Hogh, P., Bo Andersen, B., Winther Dombernowsky, N., Naik, M., et al. (2020). The power of EEG to predict conversion from mild cognitive impairment and subjective cognitive decline to dementia. Dement. Geriatr. Cogn. Disord. 49, 38–47. doi: 10.1159/000508392
Facal, D., Burgo, C., Spuch, C., Gaspar, P., and Campos-Magdaleno, M. (2021). Cognitive frailty: an update. Front. Psychol. 12, 2017–2021. doi: 10.3389/fpsyg.2021.813398
Farina, F. R., Emek-Savas, D. D., Rueda-Delgado, L., Boyle, R., Kiiski, H., Yener, G., et al. (2020). A comparison of resting state EEG and structural MRI for classifying Alzheimer's disease and mild cognitive impairment. NeuroImage 215:116795. doi: 10.1016/j.neuroimage.2020.116795
Fodor, Z., Horváth, A., Hidasi, Z., Gouw, A. A., Stam, C. J., and Csukly, G. (2021). EEG alpha and beta band functional connectivity and network structure mark hub overload in mild cognitive impairment during memory maintenance. Front. Aging Neurosci. 13:680200. doi: 10.3389/fnagi.2021.680200
Fruehwirt, W., Dorffner, G., Roberts, S., Gerstgrasser, M., Grossegger, D., Schmidt, R., et al. (2019). Associations of event-related brain potentials and Alzheimer's disease severity: a longitudinal study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 92, 31–38. doi: 10.1016/j.pnpbp.2018.12.013
García, S., Cuetos, F., Novelli, A., and Martínez, C. (2021). A new and short protocol to achieve the early diagnosis of mild cognitive impairment. Neurol. Sci. 42, 3687–3694. doi: 10.1007/s10072-021-05044-1
Garrido-Chaves, R., Perez, V., Perez-Alarcón, M., Crespo-Sanmiguel, I., Paiva, T. O., Hidalgo, V., et al. (2021). Subjective memory complaints and decision making in young and older adults: an event-related potential study. Front. Aging Neurosci. 13:695275. doi: 10.3389/fnagi.2021.695275
Gironell, A., García-Sánchez, C., Estévez-González, A., Boltes, A., and Kulisevsky, J. (2005). Usefulness of P300 in subjective memory complaints: a prospective study. J. Clin. Neurophysiol. 22, 279–284. doi: 10.1097/01.Wnp.0000173559.60113.Ab
Goodin, D. S., Squires, K. C., and Starr, A. (1978). Long latency event-related components of the auditory evoked potential in dementia. Brain 101, 635–648. doi: 10.1093/brain/101.4.635
Gouw, A. A., Alsema, A. M., Tijms, B. M., Borta, A., Scheltens, P., Stam, C. J., et al. (2017). EEG spectral analysis as a putative early prognostic biomarker in nondemented, amyloid positive subjects. Neurobiol. Aging 57, 133–142. doi: 10.1016/j.neurobiolaging.2017.05.017
Grieder, M., Crinelli, R. M., Jann, K., Federspiel, A., Wirth, M., Koenig, T., et al. (2013). Correlation between topographic N400 anomalies and reduced cerebral blood flow in the anterior temporal lobes of patients with dementia. J. Alzheimers Dis. 36, 711–731. doi: 10.3233/jad-121690
Hamilton, C. A., Schumacher, J., Matthews, F., Taylor, J.-P., Allan, L., Barnett, N., et al. (2021). Slowing on quantitative EEG is associated with transition to dementia in mild cognitive impairment. Int. Psychogeriatr. 33, 1321–1325. doi: 10.1017/s1041610221001083
Hayden, K. M., Zandi, P. P., Lyketsos, C. G., Khachaturian, A. S., Bastian, L. A., Charoonruk, G., et al. (2006). Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County study. Alzheimer Dis. Assoc. Disord. 20, 93–100. doi: 10.1097/01.wad.0000213814.43047.86
Hedges, D., Janis, R., Mickelson, S., Keith, C., Bennett, D., and Brown, B. L. (2016). P300 amplitude in Alzheimer's disease: a meta-analysis and meta-regression. Clin. EEG Neurosci. 47, 48–55. doi: 10.1177/1550059414550567
Hojjati, S. H., Ebrahimzadeh, A., Khazaee, A., and Babajani-Feremi, A. (2018). Predicting conversion from MCI to AD by integrating rs-fMRI and structural MRI. Comput. Biol. Med. 102, 30–39. doi: 10.1016/j.compbiomed.2018.09.004
Hou, F., Liu, C., Yu, Z., Xu, X., Zhang, J., Peng, C. K., et al. (2018). Age-related alterations in electroencephalography connectivity and network topology during n-Back working memory task. Front. Hum. Neurosci. 12:484. doi: 10.3389/fnhum.2018.00484
Howe, A. S. (2014). Meta-analysis of the endogenous N200 latency event-related potential subcomponent in patients with Alzheimer's disease and mild cognitive impairment. Clin. Neurophysiol. 125, 1145–1151. doi: 10.1016/j.clinph.2013.10.019
Jiang, S., Qu, C., Wang, F., Liu, Y., Qiao, Z., Qiu, X., et al. (2015). Using event-related potential P300 as an electrophysiological marker for differential diagnosis and to predict the progression of mild cognitive impairment: a meta-analysis. Neurol. Sci. 36, 1105–1112. doi: 10.1007/s10072-015-2099-z
Jiang, Y., Zhang, X., Guo, Z., and Jiang, N. (2024). Altered EEG Theta and alpha band functional connectivity in mild cognitive impairment during working memory coding. IEEE Trans. Neural Syst. Rehabil. Eng. 32, 2845–2853. doi: 10.1109/tnsre.2024.3417617
Jiao, B., Li, R., Zhou, H., Qing, K., Liu, H., Pan, H., et al. (2023). Neural biomarker diagnosis and prediction to mild cognitive impairment and Alzheimer’s disease using EEG technology. Alzheimers Res. Ther. 15:32. doi: 10.1186/s13195-023-01181-1
Kazmerski, V. A., Friedman, D., and Ritter, W. (1997). Mismatch negativity during attend and ignore conditions in Alzheimer's disease. Biol. Psychiatry 42, 382–402. doi: 10.1016/S0006-3223(96)00344-7
Kelaiditi, E., Cesari, M., Canevelli, M., Abellan van Kan, G., Ousset, P. J., Gillette-Guyonnet, S., et al. (2013). Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J. Nutr. Health Aging 17, 726–734. doi: 10.1007/s12603-013-0367-2
Keller, S. M., Reyneke, C., Gschwandtner, U., and Fuhr, P. (2023). Information contained in EEG allows characterization of cognitive decline in neurodegenerative disorders. Clin. EEG Neurosci. 54, 391–398. doi: 10.1177/15500594221120734
Kim, N. H., Yang, D. W., Choi, S. H., and Kang, S. W. (2021). Machine learning to predict brain amyloid pathology in pre-dementia Alzheimer’s disease using QEEG features and genetic algorithm heuristic. Front. Comput. Neurosci. 15:755499. doi: 10.3389/fncom.2021.755499
Knott, V., Mohr, E., Mahoney, C., and Ilivitsky, V. (2000). Electroencephalographic coherence in Alzheimer's disease: comparisons with a control group and population norms. J. Geriatr. Psychiatry Neurol. 13, 1–8. doi: 10.1177/089198870001300101
Kocagoncu, E., Nesbitt, D., Emery, T., Hughes, L. E., Henson, R. N., and Rowe, J. B. (2022). Neurophysiological and brain structural markers of cognitive frailty differ from Alzheimer’s disease. J. Neurosci. 42, 1362–1373. doi: 10.1523/JNEUROSCI.0697-21.2021
Kryscio, R. J., Abner, E. L., Cooper, G. E., Fardo, D. W., Jicha, G. A., Nelson, P. T., et al. (2014). Self-reported memory complaints. Neurology 83, 1359–1365. doi: 10.1212/wnl.0000000000000856
Lassi, M., Fabbiani, C., Mazzeo, S., Burali, R., Vergani, A. A., Giacomucci, G., et al. (2023). Degradation of EEG microstates patterns in subjective cognitive decline and mild cognitive impairment: early biomarkers along the Alzheimer's disease continuum? Neuroimage Clin. 38:103407. doi: 10.1016/j.nicl.2023.103407
Lee, W. J., Peng, L. N., Liang, C. K., Loh, C. H., and Chen, L. K. (2018). Cognitive frailty predicting all-cause mortality among community-living older adults in Taiwan: a 4-year nationwide population-based cohort study. PLoS One 13:e0200447. doi: 10.1371/journal.pone.0200447
Li, J., Li, X., Chen, F., Li, W., Chen, J., and Zhang, B. (2024). Studying the Alzheimer’s disease continuum using EEG and fMRI in single-modality and multi-modality settings. Rev. Neurosci. 35, 373–386. doi: 10.1515/revneuro-2023-0098
Lian, H., Li, Y., and Li, Y. (2021). Altered EEG microstate dynamics in mild cognitive impairment and Alzheimer's disease. Clin. Neurophysiol. 132, 2861–2869. doi: 10.1016/j.clinph.2021.08.015
Lin, N., Gao, J., Mao, C., Sun, H., Lu, Q., and Cui, L. (2021). Differences in multimodal electroencephalogram and clinical correlations between early-onset Alzheimer’s disease and frontotemporal dementia. Front. Neurosci. 15:687053. doi: 10.3389/fnins.2021.687053
Liu, H., Wang, J., Xin, X., Wang, P., Jiang, W., and Meng, T. (2024). The relationship and pathways between resting-state EEG, physical function, and cognitive function in older adults. BMC Geriatr. 24:463. doi: 10.1186/s12877-024-05041-x
Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., et al. (2020). Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet 396, 413–446. doi: 10.1016/S0140-6736(20)30367-6
Lopez, S., Hampel, H., Chiesa, P. A., Del Percio, C., Noce, G., Lizio, R., et al. (2024). The association between posterior resting-state EEG alpha rhythms and functional MRI connectivity in older adults with subjective memory complaint. Neurobiol. Aging 137, 62–77. doi: 10.1016/j.neurobiolaging.2024.02.008
López-Sanz, D., Bruña, R., Garcés, P., Martín-Buro, M. C., Walter, S., Delgado, M. L., et al. (2017). Functional connectivity disruption in subjective cognitive decline and mild cognitive impairment: a common pattern of alterations. Front. Aging Neurosci. 9:109. doi: 10.3389/fnagi.2017.00109
Maestú, F., Cuesta, P., Hasan, O., Fernandéz, A., Funke, M., and Schulz, P. E. (2019). The importance of the validation of M/EEG with current biomarkers in Alzheimer's disease. Front. Hum. Neurosci. 13:17. doi: 10.3389/fnhum.2019.00017
Mazzeo, S., Lassi, M., Padiglioni, S., Moschini, V., Vergani, A. A., Scarpino, M., et al. (2023a). A machine learning model to predict progression from subjective cognitive decline to mild cognitive impairment and dementia: a 12-year follow-up study. Alzheimers Dement. 19:82276. doi: 10.1002/alz.082276
Mazzeo, S., Lassi, M., Padiglioni, S., Vergani, A. A., Moschini, V., Scarpino, M., et al. (2023b). PRedicting the EVolution of SubjectIvE cognitive decline to Alzheimer’s disease with machine learning: the PREVIEW study protocol. BMC Neurol. 23:300. doi: 10.1186/s12883-023-03347-8
Medici, V., Cassini, P., Rossi, M., Pettinato, L., Negro, G., Profka, X., et al. (2023). Is QEEG an effective biomarker in predicting cognitive decline? Alzheimers Dement. 19:74765. doi: 10.1002/alz.074765
Meghdadi, A. H., Stevanovic Karic, M., McConnell, M., Rupp, G., Richard, C., Hamilton, J., et al. (2021). Resting state EEG biomarkers of cognitive decline associated with Alzheimer's disease and mild cognitive impairment. PLoS One 16:e0244180. doi: 10.1371/journal.pone.0244180
Michel, C. M., and Koenig, T. (2018). EEG microstates as a tool for studying the temporal dynamics of whole-brain neuronal networks: a review. NeuroImage 180, 577–593. doi: 10.1016/j.neuroimage.2017.11.062
Moffat, G., Zhukovsky, P., Coughlan, G., and Voineskos, A. N. (2022). Unravelling the relationship between amyloid accumulation and brain network function in normal aging and very mild cognitive decline: a longitudinal analysis. Brain Commun. 4:fcac282. doi: 10.1093/braincomms/fcac282
Monllor, P., Cervera-Ferri, A., Lloret, M.-A., Esteve, D., Lopez, B., Leon, J.-L., et al. (2021). Electroencephalography as a non-invasive biomarker of Alzheimer’s disease: a forgotten candidate to substitute CSF molecules? Int. J. Mol. Sci. 22:10889. doi: 10.3390/ijms221910889
Morrison, C., Rabipour, S., Knoefel, F., Sheppard, C., and Taler, V. (2018). Auditory event-related potentials in mild cognitive impairment and Alzheimer’s disease. Curr. Alzheimer Res. 15, 702–715. doi: 10.2174/1567205015666180123123209
Mowszowski, L., Hermens, D. F., Diamond, K., Norrie, L., Hickie, I. B., Lewis, S. J. G., et al. (2012). Reduced mismatch negativity in mild cognitive impairment: associations with neuropsychological performance. J. Alzheimers Dis. 30, 209–219. doi: 10.3233/JAD-2012-111868
Musaeus, C. S., Engedal, K., Hogh, P., Jelic, V., Khanna, A. R., Kjaer, T. W., et al. (2020). Changes in the left temporal microstate are a sign of cognitive decline in patients with Alzheimer's disease. Brain Behav. 10:e01630. doi: 10.1002/brb3.1630
Musaeus, C. S., Nielsen, M. S., and Hogh, P. (2019). Microstates as disease and progression markers in patients with mild cognitive impairment. Front. Neurosci. 13:563. doi: 10.3389/fnins.2019.00563
Newsome, R. N., Pun, C., Smith, V. M., Ferber, S., and Barense, M. D. (2013). Neural correlates of cognitive decline in older adults at-risk for developing MCI: evidence from the CDA and P300. Cogn. Neurosci. 4, 152–162. doi: 10.1080/17588928.2013.853658
Nuwer, M. (1997). Assessment of digital EEG, quantitative EEG, and EEG brain mapping: report of the American Academy of Neurology and the American clinical neurophysiology society. Neurology 49, 277–292. doi: 10.1212/wnl.49.1.277
Panza, F., Lozupone, M., Solfrizzi, V., Sardone, R., Dibello, V., Di Lena, L., et al. (2018). Different cognitive frailty models and health-and cognitive-related outcomes in older age: from epidemiology to prevention. J. Alzheimers Dis. 62, 993–1012. doi: 10.3233/jad-170963
Papadaniil, C. D., Kosmidou, V. E., Tsolaki, A., Tsolaki, M., Kompatsiaris, I., and Hadjileontiadis, L. J. (2016). Cognitive MMN and P300 in mild cognitive impairment and Alzheimer's disease: a high density EEG-3D vector field tomography approach. Brain Res. 1648, 425–433. doi: 10.1016/j.brainres.2016.07.043
Papaliagkas, V. T., Anogianakis, G., Tsolaki, N. M., Koliakos, G., and Kimiskidis, K. V. (2009a). Prediction of conversion from mild cognitive impairment to Alzheimer’s disease by CSF cytochrome c levels and N200 latency. Curr. Alzheimer Res. 6, 279–284. doi: 10.2174/156720509788486626
Papaliagkas, V. T., Anogianakis, G., Tsolaki, M. N., Koliakos, G., and Kimiskidis, V. K. (2009b). Progression of mild cognitive impairment to Alzheimer’s disease: improved diagnostic value of the combined use of N200 latency and β-amyloid (1–42) levels. Dement. Geriatr. Cogn. Disord. 28, 30–35. doi: 10.1159/000229023
Pedroso, R. V., Fraga, F. J., Corazza, D. I., Andreatto, C. A. A., Coelho, F. G. D. M., Costa, J. L. R., et al. (2012). Latência e amplitude do P300 auditivo na doença de Alzheimer: uma revisão sistemática. Braz. J. Otorhinolaryngol. 78, 126–132. doi: 10.1590/s1808-86942012000400023
Pei, G., Yang, R., Shi, Z., Guo, G., Wang, S., Liu, M., et al. (2020). Enhancing working memory based on mismatch negativity neurofeedback in subjective cognitive decline patients: a preliminary study. Front. Aging Neurosci. 12:263. doi: 10.3389/fnagi.2020.00263
Perez, V., Garrido-Chaves, R., Zapater-Fajari, M., Pulopulos, M. M., Hidalgo, V., and Salvador, A. (2022). EEG markers and subjective memory complaints in young and older people. Int. J. Psychophysiol. 182, 23–31. doi: 10.1016/j.ijpsycho.2022.09.006
Pineda, A. M., Ramos, F. M., Betting, L. E., and Campanharo, A. S. L. O. (2020). Quantile graphs for EEG-based diagnosis of Alzheimer’s disease. PLoS One 15:e0231169. doi: 10.1371/journal.pone.0231169
Porcaro, C., Balsters, J. H., Mantini, D., Robertson, I. H., and Wenderoth, N. (2019). P3b amplitude as a signature of cognitive decline in the older population: an EEG study enhanced by functional source separation. NeuroImage 184, 535–546. doi: 10.1016/j.neuroimage.2018.09.057
Prado, P., Birba, A., Cruzat, J., Santamaria-Garcia, H., Parra, M., Moguilner, S., et al. (2022). Dementia ConnEEGtome: towards multicentric harmonization of EEG connectivity in neurodegeneration. Int. J. Psychophysiol. 172, 24–38. doi: 10.1016/j.ijpsycho.2021.12.008
Ritchie, K., Carrière, I., Berr, C., Amieva, H., Dartigues, J. F., Ancelin, M. L., et al. (2016). The clinical picture of Alzheimer's disease in the decade before diagnosis: clinical and biomarker trajectories. J. Clin. Psychiatry 77, e305–e311. doi: 10.4088/JCP.15m09989
Rossini, P. M., Miraglia, F., and Vecchio, F. (2022). Early dementia diagnosis, MCI-to-dementia risk prediction, and the role of machine learning methods for feature extraction from integrated biomarkers, in particular for EEG signal analysis. Alzheimers Dement. 18, 2699–2706. doi: 10.1002/alz.12645
Rubinov, M., and Sporns, O. (2010). Complex network measures of brain connectivity: uses and interpretations. NeuroImage 52, 1059–1069. doi: 10.1016/j.neuroimage.2009.10.003
Ruzzoli, M., Pirulli, C., Mazza, V., Miniussi, C., and Brignani, D. (2016). The mismatch negativity as an index of cognitive decline for the early detection of Alzheimer’s disease. Sci. Rep. 6:33167. doi: 10.1038/srep33167
Sala-Llonch, R., Bartres-Faz, D., and Junque, C. (2015). Reorganization of brain networks in aging: a review of functional connectivity studies. Front. Psychol. 6:663. doi: 10.3389/fpsyg.2015.00663
Sanchez-Reyes, L.-M., Rodriguez-Resendiz, J., Avecilla-Ramirez, G. N., Garcia-Gomar, M.-L., and Robles-Ocampo, J.-B. (2021). Impact of EEG parameters detecting dementia diseases: a systematic review. IEEE Access 9, 78060–78074. doi: 10.1109/access.2021.3083519
Shi, Y., Ma, Q., Feng, C., Wang, M., Wang, H., Li, B., et al. (2022). Microstate feature fusion for distinguishing AD from MCI. Health Inf. Sci. Syst. 10:16. doi: 10.1007/s13755-022-00186-8
Shim, Y., Yang, D. W., Ho, S., Hong, Y. J., Jeong, J. H., Park, K. H., et al. (2022). Electroencephalography for early detection of Alzheimer's disease in subjective cognitive decline. Dement. Neurocogn. Disord. 21, 126–137. doi: 10.12779/dnd.2022.21.4.126
Shimada, H., Doi, T., Lee, S., Makizako, H., Chen, L. K., and Arai, H. (2018). Cognitive frailty predicts incident dementia among community-dwelling older people. J. Clin. Med. 7:7090250. doi: 10.3390/jcm7090250
Shumbayawonda, E., Lopez-Sanz, D., Bruna, R., Serrano, N., Fernandez, A., Maestu, F., et al. (2020). Complexity changes in preclinical Alzheimer's disease: an MEG study of subjective cognitive decline and mild cognitive impairment. Clin. Neurophysiol. 131, 437–445. doi: 10.1016/j.clinph.2019.11.023
Smailovic, U., Koenig, T., Laukka, E. J., Kalpouzos, G., Andersson, T., Winblad, B., et al. (2019). EEG time signature in Alzheimer s disease: functional brain networks falling apart. Neuroimage Clin. 24:102046. doi: 10.1016/j.nicl.2019.102046
Spinelli, G., Bakardjian, H., Schwartz, D., Potier, M.-C., Habert, M.-O., Levy, M., et al. (2022). Theta band-power shapes amyloid-driven longitudinal EEG changes in elderly subjective memory complainers at-risk for Alzheimer’s disease. J. Alzheimers Dis. 90, 69–84. doi: 10.3233/jad-220204
Suárez-Méndez, I., Walter, S., López-Sanz, D., Pasquín, N., Bernabé, R., Castillo Gallo, E., et al. (2021). Ongoing oscillatory electrophysiological alterations in frail older adults: a MEG study. Front. Aging Neurosci. 13:609043. doi: 10.3389/fnagi.2021.609043
Tarawneh, H. Y., Jayakody, D. M. P., Verma, S., Doré, V., Xia, Y., Mulders, W., et al. (2023). Auditory event-related potentials in older adults with subjective memory complaints. J. Alzheimers Dis. 92, 1093–1109. doi: 10.3233/jad-221119
Targa Dias Anastacio, H., Matosin, N., and Ooi, L. (2022). Neuronal hyperexcitability in Alzheimer’s disease: what are the drivers behind this aberrant phenotype? Transl. Psychiatry 12:257. doi: 10.1038/s41398-022-02024-7
Torres-Simon, L., Doval, S., Nebreda, A., Llinas, S. J., Marsh, E. B., and Maestu, F. (2022). Understanding brain function in vascular cognitive impairment and dementia with EEG and MEG: a systematic review. Neuroimage Clin. 35:103040. doi: 10.1016/j.nicl.2022.103040
Tsolaki, A. C., Kosmidou, V., Kompatsiaris, I. Y., Papadaniil, C., Hadjileontiadis, L., Adam, A., et al. (2017). Brain source localization of MMN and P300 ERPs in mild cognitive impairment and Alzheimer's disease: a high-density EEG approach. Neurobiol. Aging 55, 190–201. doi: 10.1016/j.neurobiolaging.2017.03.025
Ulbl, J., and Rakusa, M. (2023). The importance of subjective cognitive decline recognition and the potential of molecular and neurophysiological biomarkers-a systematic review. Int. J. Mol. Sci. 24:10158. doi: 10.3390/ijms241210158
Van Der Flier, W. M., De Vugt, M. E., Smets, E. M. A., Blom, M., and Teunissen, C. E. (2023). Towards a future where Alzheimer’s disease pathology is stopped before the onset of dementia. Nat. Aging 3, 494–505. doi: 10.1038/s43587-023-00404-2
Vecchio, F., Miraglia, F., Marra, C., Quaranta, D., Vita, M. G., Bramanti, P., et al. (2014). Human brain networks in cognitive decline: a graph theoretical analysis of cortical connectivity from EEG data. J. Alzheimers Dis. 41, 113–127. doi: 10.3233/JAD-132087
Vicchietti, M. L., Ramos, F. M., Betting, L. E., and Campanharo, A. (2023). Computational methods of EEG signals analysis for Alzheimer's disease classification. Sci. Rep. 13:8184. doi: 10.1038/s41598-023-32664-8
Wada, Y., Nanbu, Y., Kikuchi, M., Koshino, Y., Hashimoto, T., and Yamaguchi, N. (1998). Abnormal functional connectivity in Alzheimer's disease: intrahemispheric EEG coherence during rest and photic stimulation. Eur. Arch. Psychiatry Clin. Neurosci. 248, 203–208. doi: 10.1007/s004060050038
Wei, Y.-C., Kung, Y.-C., Huang, W.-Y., Lin, C., Chen, Y.-L., Chen, C.-K., et al. (2022). Functional connectivity dynamics altered of the resting brain in subjective cognitive decline. Front. Aging Neurosci. 14:817137. doi: 10.3389/fnagi.2022.817137
Keywords: electroencephalogram (EEG), event-related potentials (ERPs), dementia prevention, neurophysiological biomarker, mild cognitive impairment, subjective cognitive decline, subjective memory complaint, cognitive frailty
Citation: Tanaka M, Yamada E and Mori F (2024) Neurophysiological markers of early cognitive decline in older adults: a mini-review of electroencephalography studies for precursors of dementia. Front. Aging Neurosci. 16:1486481. doi: 10.3389/fnagi.2024.1486481
Edited by:
Takao Yamasaki, Minkodo Minohara Hospital, JapanReviewed by:
Mercedes Atienza, Universidad Pablo de Olavide, SpainJan Kujala, Aalto University, Finland
Sorinel A. Oprisan, College of Charleston, United States
Copyright © 2024 Tanaka, Yamada and Mori. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mutsuhide Tanaka, bXRhbmFrYUBwdS1oaXJvc2hpbWEuYWMuanA=
 Mutsuhide Tanaka
Mutsuhide Tanaka Emi Yamada
Emi Yamada Futoshi Mori1
Futoshi Mori1